Interventions for childhood apraxia of speech
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Parallel‐group randomised controlled trial | |
| Participants | Sample size: 26 children Dropouts/withdrawals: 1 child in the NDP‐3 group dropped out mid‐treatment yet was included in the analysis using intention‐to‐treat analysis Sex: 18 males, 8 females Mean age: 5 years and 6 months (SD = 25 months) Inclusion criteria
No information was collected on race, ethnicity or socioeconomic status | |
| Interventions | Process
| |
| Outcomes | Timing of outcome assessment Primary outcomes
Outcomes were measured based on a 292‐item experimental probe of treated and untreated stimuli. 162 items from NDP‐3 assessment and 80 pseudo words from ReST treatment, and an additional 50 untreated 1‐, 2‐ and 3‐syllable real word stimuli were used to test for generalisation of treatment effects in both groups. The probe assessed impairment level speech outcomes for simultaneous accuracy for articulation and prosody. For further detail on scoring, see Murray 2015. Secondary outcomes
Comparisons
| |
| Notes | Funding Conflicts of interest: none known Study start date: January 2010 Study end date: July 2012 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
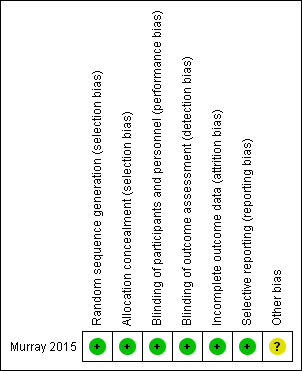
| Random sequence generation (selection bias) | Low risk | Clarification was sought from the corresponding author by phone who confirmed that each envelope had a note within it specifying the treatment condition to which the child was allocated (Murray 2015). The authors could not see through the envelopes. Envelopes were placed in a container and an independent person (corresponding author's husband) not involved in the study selected an envelope that was then given a participant number (P1, P2, etc.) until all participants were allocated to an arm of the study. Allocation was not revealed until after the pre‐treatment evaluation |
| Allocation concealment (selection bias) | Low risk | Clarification was sought from corresponding author (Murray 2015), who confirmed via email that envelopes were sequentially numbered based on the random order in which they were selected from a container (i.e. randomised and not based on any identifying variable). |
| Blinding of participants and personnel (performance bias) | Low risk | SLP could not be blinded to type of intervention |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded, independent assessors |
| Incomplete outcome data (attrition bias) | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | All outcome measures reported in the original protocol, Murray 2012, were reported. A lexical stress measure was added in final outcome ratings but not mentioned in protocol but this was an addition and not a failure to report |
| Other bias | Unclear risk |
Qualified SLPs who had not seen the children previously conducted the 1 week, 1 month and 4 month post‐assessments. In some cases, final‐year undergraduate SLP students (4th‐year students) conducted post‐assessments. The same SLP or student SLP must not have seen/rated the children before. One researcher performed all of the pre‐assessments, including probes, before allocation was revealed |
CAS: childhood apraxia of speech;CELF‐IV: Clinical Evaluation of Language Fundamentals ‐ Fourth Edition; CELF‐P2: Clinical Evaluation of Language Fundamentals ‐ Preschool 2; DEAP: Diagnostic Evaluation of Articulation and Phonology; GFTA‐2: Goldman‐Fristoe Test of Articulation 2;NDP‐3: Nuffield Dyspraxia Programme ‐ Third Edition; ReST: Rapid Syllable Transitions Treatment; SD: standard deviation; SLP: speech language pathologist
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not RCT or quasi‐RCT (case study) | |
| Not RCT or quasi‐RCT | |
| Not RCT or quasi‐RCT (case study) | |
| Not RCT or quasi‐RCT (case study series) | |
| Not RCT or quasi‐RCT (case study) | |
| Not RCT or quasi‐RCT (case study) | |
| Not RCT or quasi‐RCT (case study) | |
| Not RCT or quasi‐RCT (case study series) | |
| Not RCT or quasi‐RCT (case study series) | |
| No experimental treatment data included in study | |
| Not RCT or quasi‐RCT (single case [ABA] design) | |
| Not RCT or quasi‐RCT (case series) | |
| Not RCT or quasi‐RCT (case series) | |
| Not RCT or quasi‐RCT (case study) | |
| Study examined adult participant with AAOS | |
| Not RCT or quasi‐RCT | |
| Study focuses on children with speech disorder, not specifically DAS. No experimental treatment data included in study | |
| No experimental treatment data included in study | |
| Study examined adult participant with AAOS | |
| Not RCT or quasi‐RCT (case study) | |
| Not RCT or quasi‐RCT (longitudinal case study) | |
| Not RCT or quasi‐RCT (case study) | |
| Study uses a hypothetical treatment case only. No experimental treatment data | |
| Study focuses on intervention for a group of participants with a range of speech disorders without dissociating between participants with subtypes of speech disorders. Does not report treatment efficacy specific to participants with DAS | |
| Not RCT or quasi‐RCT (case study series) | |
| Not RCT or quasi‐RCT (case study) | |
| Study does not specify whether child has diagnosis of DAS or only some features of dyspraxia | |
| Not RCT or quasi‐RCT (case study series) | |
| Study examined adult participants with AAOS | |
| Not RCT or quasi‐RCT (case study series) | |
| Study focused on articulation disorders, not specifically DAS | |
| No experimental treatment data included in study | |
| Not RCT or quasi‐RCT (case study) | |
| Not RCT or quasi‐RCT (case study) | |
| Study examined adult participant with AAOS | |
| Not RCT or quasi‐RCT (case study) | |
| Not RCT or quasi‐RCT (case study) | |
| Not RCT or quasi‐RCT (case study) | |
| Not RCT or quasi‐RCT (case study) | |
| Not RCT or quasi‐RCT | |
| Not RCT or quasi‐RCT (case study series) | |
| Not RCT or quasi‐RCT (case study) | |
| Not RCT or quasi‐RCT (case series) | |
| Not RCT or quasi‐RCT (case study) | |
| Not RCT or quasi‐RCT (case study series) | |
| Not RCT or quasi‐RCT (case study series) | |
| Not RCT or quasi‐RCT (case study) | |
| Not RCT or quasi‐RCT (case study series) | |
| Not RCT or quasi‐RCT (pre‐post group design) | |
| Not RCT or quasi‐RCT | |
| Not RCT or quasi‐RCT (case study) | |
| Not RCT or quasi‐RCT (case study) | |
| Study examined adult participant with AAOS | |
| Study focus on motor dyspraxia or developmental coordination disorder not apraxia of speech | |
| Not RCT or quasi‐RCT (case study) | |
| Study combined a number of treatment methods and grouped individuals. Could not determine individual participant outcomes related to specific treatment methods | |
| Not RCT or quasi‐RCT (case study) | |
| No experimental treatment data included in study | |
| Not RCT or quasi‐RCT (case study) | |
| Not RCT or quasi‐RCT (case study) | |
| Not RCT or quasi‐RCT (case series) | |
| Not RCT or quasi‐RCT (case study) | |
| Not RCT or quasi‐RCT (case study) | |
| Not RCT or quasi‐RCT (case study) | |
| Not RCT or quasi‐RCT | |
| Not RCT or quasi‐RCT | |
| Not RCT or quasi‐RCT (case series) | |
| Not RCT or quasi‐RCT | |
| Not RCT or quasi‐RCT (case study) |
AAOS: acquired apraxia of speech.
ABA: applied behaviour analysis
DAS: developmental apraxia of speech.
RCT: randomised controlled trial.

Study flow diagram
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| Nuffield Dyspraxia Programme ‐ Third Edition (NDP‐3) versus Rapid Syllable Transition Treatment (ReST) for Childhood Apraxia of Speech | |||||
| Patient or population: children aged 4 to 12 years with CAS of unknown cause Settings: University of Sydney Communication Disorders Treatment and Research Clinic Intervention: NDP‐3 Comparison: ReST | |||||
| Outcomes | Summary of MD findings | Absolute MD | Number of participants (studies) | Quality of the evidence | Comments |
| Primary outcomes | |||||
| Accuracy of production on treated items Measured by: counting the number of real words produced correctly (/x) Follow‐up: pre‐intervention to 1 month post‐intervention | NDP‐3 MD of 36.0 was greater than the ReST MD of 33.9 | 2.1 | 26 (1 trial) | ⊕⊕⊕⊝ | — |
| Accuracy of production on non‐treated items Measured by: counting the number of real words produced correctly (/x) Follow‐up: pre‐intervention to 1 month post‐intervention | ReST MD of 18.3 was minimally greater than the NDP‐3 MD of 18.2 | 0.1 | 26 (1 trial) | ⊕⊕⊕⊝ | — |
| Secondary outcomes | |||||
| Speech production consistency Measured by: calculating the number of inconsistent productions of 25 words produced 3 times using the DEAP inconsistency subtestb Follow‐up: pre‐intervention to 1 month post‐intervention | NDP‐3 MD of 11.1 was greater than the ReST MD of 10.9 | 0.2 | 26 (1 trial) | ⊕⊕⊕⊝ | — |
| Accuracy of connected speech Measured by: counting the number of correct imitations of 3 word phrases (/x) Follow‐up: pre‐intervention to 1 month post‐intervention | NDP‐3 MD of 14.3 was greater than the ReST MD of 11.5 | 2.8 | 26 (1 trial) | ⊕⊕⊕⊝ | — |
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. | |||||
| CAS: childhood apraxia of speech; DEAP: Diagnostic Evaluation of Articulation and Phonology; MD: mean difference; NDP‐3: Nuffield Dyspraxia Programme ‐ Third Edition;ReST: Rapid Syllable Transition Treatment (ReST) for Childhood Apraxia of Speech | |||||
| aWe downgraded the quality of evidence by one level, to moderate, for imprecision, as there was only one study for comparison. | |||||
| Study | Participants | Methodology/paper type | Intervention | Intervention approach | Intervention intensity and duration | Outcome measures | Treatment outcomes | Timing of outcome measures | Methodological considerations |
| 1 male aged 12.8 years with CAS and charge syndrome | Not quasi‐/RCT (Single case (AB) design) | Dynamic Temporal and Tactile Cueing | Motor | Phase I and II: sessions 4 × per week; Phase III: weekly therapy. Study over 25 months. Home practice not reported | Articulation accuracy on 2‐item scale for treated items; speech rate | Phase I (core vocabulary): change on 4/6 targets. Maintained at last probe. Phase II (core vocabulary): reached 100% accuracy for 3/5 words. Reduction of stereotypies. Phase III: decreased speech rate from 94 to 71 SPM | Baseline and during treatment. No longer‐term follow‐up data | Lack of experimental control, multiple baselines, control, longer‐term follow‐up or generalisation data. Clinical file data used. No replication across participants. Assessors, participants, therapists not blinded | |
| 3 siblings (2 males, 1 female) aged 7.8 and 10.10 years with CAS | Not quasi‐/RCT (Single subject multiple baseline design across behaviours and participants) | Rapid Syllable Transition Treatment (ReST) | Motor | 60‐minute sessions (100‐120 trials per session), 4 × per week for 12 sessions. Home practice not reported | Reading aloud 10 treated and 10 non‐treated non‐word strings; real word generalisation data; perceptual analysis of prosodic pattern and acoustic analysis using pairwise variability index | 3/3 had significant gains in treated items and generalisation to same level of treated complexity. 2/3 generalised to lower and higher complexity non‐word items. Minimal generalisation to real words | Baseline data taken at beginning of every 4th session and at 4 weeks post‐treatment | No long‐term follow‐up data. Limited participants for generalisation of outcomes. No blinding of assessors, participants or therapists. No stimulus generalisation measures | |
| 1 female aged 3 years with CAS | Not quasi‐/RCT (Case description) | Music therapy | Other (alternative interventions) | 30‐minute sessions over 9 months. 24 sessions in total | Descriptive data only | Commenced non‐verbal. At end, had 11 phonemes in inventory | Pre‐treatment and post‐treatment. No follow‐up data | Lack of experimental control, multiple baselines or control data. CAS diagnosis unclear and not replicable. No replicable outcome measures. No statistical analysis. No blinding of assessors, participants or therapists. No follow‐up or generalisation data. Unclear which aspect of treatment provided outcomes or affect of maturation, schooling, etc. No replication across participants. No long‐term follow‐up data | |
| 2 males aged 4.2 and 4.4 years with CAS and language disorder | Not quasi‐/RCT (Single case multiple baseline across participants) | Aided AAC Modeling | Augmentative and alternative communication | 15‐minute sessions, 1 to 3 × per week for 10 to 15 sessions | Frequency of use of multi‐symbol messages in play scenarios | Significantly more frequent use of multi‐symbol messages using aided AAC as well as different types of messages. Maintained and generalised gains. Increased participation | Baseline × 3, every 2nd treatment session, and at 2, 4 and 8 weeks post‐treatment | CAS diagnosis unclear and not replicable. Limited outcome measures. No blinding of assessors. No response generalisation data taken (only stimulus generalisation) | |
| 1 female (Latino) aged 3.4 years with CAS and suspected velocardiofacial syndrome | Not quasi‐/RCT (Single case multiple baseline across participants) | Aided AAC Modeling | Augmentative and alternative communication | 10‐minute sessions, 1 to 3 × per week for 10 to 15 sessions | Frequency of use of multi‐symbol messages in play scenarios | Significantly more frequent use of multi‐symbol messages using aided AAC. Parental response to training excellent. Maintained and generalised gains | Baseline × 3, every 2nd treatment session, and at 2, 4 and 8 weeks post‐treatment | CAS diagnosis unclear and not replicable. No blinding of assessors. No response generalisation data taken (only stimulus generalisation) | |
| 1 female aged 6 years with CAS and language disorder | Not quasi‐/RCT (Single case multiple baseline across behaviours) | Aided AAC Modeling | Augmentative and alternative communication | 15‐minute sessions, 1 to 3 × per week for 10 to 15 sessions | Frequency of use of grammatical morphemes | Significantly more frequent use of grammatical morphemes using aided AAC. 2nd intervention period needed for 2/3 targets. Maintained gains | Baseline × 3, every treatment session, and 2, 4 and 8 weeks post‐treatment | CAS diagnosis unclear and not replicable. No blinding of assessors. No response generalisation data taken (only stimulus generalisation) | |
| 1 male aged 6.6 years with CAS, hemiplegia and seizures | Not quasi‐/RCT (Single case (ABA) design) | Voice output devices (Macaw) | Augmentative and alternative communication | 60‐minute sessions for 2 sessions (training). Home practice focus | Frequency of appropriate responses to questions in structured discourse | Mother provided greater frequency and type of questions. Frequency of appropriate responses increased | 2 × baseline, 2 × practice period, 1 × post‐treatment, and 4 weeks post‐treatment | Lack of experimental control, multiple baselines or control data. | |
| 1 male and 1 female aged 12 and 8 years respectively diagnosed with CAS. Additional 8 children (7 males) aged 4 to 7 years with persistent articulation errors | Not quasi‐/RCT (Case series ‐ single group study) | Electropalatography (EPG) on /t, d, k, g, s, z/ | Motor | 30‐minute sessions, 1 × per week for 10 weeks | Per cent consonants correct (PCC) and Probe Scoring System (PSS) on probe of 43 words | Significant difference noted for PSS for whole group. PCC scores improved in percentage | Pre‐treatment (baseline first session) and post‐treatment | Lack of experimental control, multiple baselines or control data. CAS diagnosis unclear and not replicable. No follow‐up or generalisation data. No blinding of assessors | |
| 1 female aged 8 years with CAS and intellectual disability | Not quasi‐/RCT (Single case (ABA) design) | Partners in Augmentative Communication Training (PACT) | Augmentative and alternative communication | 30 to 90‐minute sessions daily after 3 days of intensive training. Home practice focus | Ratio of parent vs participant messages; ratio of successful/intelligible messages from child | Participant had greater frequency of messages compared to parent, and slightly higher frequency of successful measures (high baseline accuracy). Increased participation | Pre‐treatment and 2 months post‐treatment | Lack of experimental control, multiple baselines or control data. CAS diagnosis unclear and not replicable. No statistical analysis. Limited outcome measures. | |
| 2 females and 1 male aged 3.4, 8 and 12.9 years respectively, with CAS (2 with intellectual disability and 1 with submucous cleft) | Not quasi‐/RCT (3 case studies/reports) | Combined communication boards and voice output devices | Augmentative and alternative communication | 3.4‐year‐old: 2 to 3 × per week for 12 weeks 8‐year‐old: daily for 6 months 12‐year‐old: not reported | 3.4‐year‐old: MLU. 8‐year‐old: assessment of phonological processes; communication repairs. 12‐year‐old: description of functional communication | 3.4‐year old: minimal speech improvement, MLU increased to WNL 8‐year old: no change in speech, parent report of greater communication repairs, and less frustration 12‐year old: supplemented natural speech to initiate, maintain and repair communication | Pre‐assessment and treatment descriptions | Lack of experimental control, multiple baselines or control data. CAS diagnosis unclear and not replicable. No statistical analysis. Limited outcome measures. | |
| 3 males and 1 female aged 3.6 to 6 years diagnosed with CAS | Not quasi‐/RCT (Single subject (ABB or ABC) design) | Prompts for Restructuring Oral Muscular Phonetic Targets (PROMPT) ‐ full programme (FP) for 8 weeks versus PROMPT without tactile‐kinaesthetic‐proprioceptive cueing for 4 weeks and FP for 4 weeks | Motor | 50‐minute session, 2 × per week for 8 weeks | Trained words on probe, untrained words. Pre‐post testing on the DEAP, TOCS+, VMPAC focal motor and sequencing subtests and Vineland socialization scales | 2/4 improved on DEAP. 4/4 improved on TOCS+, VMPAC subtests and Vineland. All 4 showed greater improvement on easier targets and majority maintained to 3 months post‐treatment. Generalisation to untrained items noted | Probe words: baseline × 3, treatment × 4, post‐treatment, and 3 months post‐treatment | Lack of experimental control as control data changed and interpreted as generalisation but no other control used (e.g. multiple baselines). CAS diagnosis concerning prosody unclear. Blinded assessors for only some outcomes. No withdrawal period between treatment phases and participant differences made comparison between conditions difficult. All measures not taken at consistent times | |
| 2 males aged 6.2 and 3.4 years with CAS (1 case with repaired cleft lip and palate and language disorder) | Not quasi‐/RCT (Single case (AB) design) | Integral Stimulation (Dynamic Temporal and Tactile Cueing) | Motor | Varied across participants. 40‐minute sessions (15 minutes each condition plus probes). 1 case: 3 × per week for 11 weeks. | Probe data on targeted phonemes (articulation) in words for each participant. 1 phoneme targeted with high production frequency = 100 trials and another with moderate production frequency = 60 trials. Articulation and language sample taken at 2 weeks post‐treatment | Large effect sizes for high production frequency and moderate for moderate production frequency. Improvement in PCC and phoneme inventory post‐treatment. Some generalisation | Baseline × 3, each treatment session, and 1 probe post‐treatment | Lack of experimental control, multiple baselines or control data. No long‐term follow‐up data. No blinding of assessors. Accuracy based on if target phoneme was correct (including cognate pair substitution) not if whole word was correct | |
| 1 female aged 9 years with mild CAS (followed until 12 years) | Not quasi‐/RCT (Case study/report) | Articulation therapy, motor‐programming remedial model | Motor | 5 school semesters | Templin‐Darley Tests of Articulation | Remediation of all 31 items for /r/, /ɝ/ and /ɚ/ | Test completed each semester | Lack of experimental control, multiple baselines or control data. CAS diagnosis unclear and not replicable. No statistical analysis. Limited outcome measures. | |
| 1 male aged 5 years with CAS and language disorder | Not quasi‐/RCT (Multiple baseline across discourse contexts) | Computer‐based AAC | Augmentative and alternative communication | 4‐minute sessions, 2 × per week for 22 sessions over 4 months | Frequency of noun/verb phrases in reciprocal book reading and structured discourse | Improvement in both contexts but more so in book reading than discourse. Some generalisation | Baseline, treatment, and withdrawal probes | CAS diagnosis unclear and not replicable. No statistical analysis. Limited outcome measures. No follow‐up data. No blinding of assessors. No replication across participants | |
| 3 children (2 males, 1 female) aged 2.9 to 8 years with CAS | Not quasi‐/RCT (Case study series) | Melodic Intonation therapy (MIT) | Linguistic and motor | Varied. 37 to 71 sessions | Varied. Description of skills, consonant inventories, sequencing error rates and intelligibility compared to typical development | Child 1: all consonants in inventory Child 2: spoke in complex sentences, poor intelligibility, and articulation errors present. Child 3: sequencing error rate dropped from 75% to 22%. 13/18 consonant sounds improved | Pre‐ and post‐treatment | No experimental control. Lack of information on diagnosis of CAS. Primarily descriptive measures ‒ not reliable or tested using statistics. No control, maintenance or generalisation data | |
| 4 children (2 males, 2 females) aged 3.7 to 6.10 years with CAS | Not quasi‐/RCT (Single case design) | Stimulability (STP) and modified Core Vocabulary (mCVT) used concurrently | Linguistic and motor | 55‐minute sessions (10 minutes STP, 45 minutes mCVT), 2 × per week for 20 sessions. No home practice | Per cent phonemes correct, phonetic inventory and inconsistency | PCC increased on average 20% after combined therapy (range 9% to 32%). Inventory gained 5 phones on average (range 1 to 10). 3/4 had greater consistency on CSIP and ISP after therapy; 1 had greater inconsistency | Pre‐ and post‐ treatment | Poor experimental control as stable baseline not established, lack of control data. CAS diagnosis unclear and not replicable. No statistical analysis. No blinding of assessors. No immediate post‐treatment data or generalisation data | |
| 1 male aged 5.5 years with "some dyspraxic features" (CAS diagnosis not explicit) | Not quasi‐/RCT (Case study) | Sensory integrative therapy and speech therapy | Motor | Daily sessions for 2 months | (SP only) Illinois Test of Psycholinguistic Abilities | Test not completed post‐treatment. Observation of greater self‐monitoring and correction of speech | Pre‐treatment only | Lack of experimental control, multiple baselines or control data. CAS diagnosis unclear and not replicable. No statistical analysis. Limited outcome measures and no post‐treatment data. | |
| 14 children (9 males, 5 females) aged 3 to 6 years with diagnosed CAS (compared to 14 age‐matched controls) | Not quasi‐/RCT (Case series pre‐post design) | Prompts for Restructuring Oral Muscular Phonetic Targets (PROMPT) | Motor | 2 × per week for 8 weeks (16 sessions in total) | GFTA2, HCAPP, VMPAC, MRI | Significant gains as a group for all speech measures | 1‐week pre‐treatment (baseline), 1‐week post‐treatment | CAS diagnosis unclear and not replicable. Age‐matched control group older than CAS group. Limited information on PROMPT targets selected for replication. No blinding of assessors. No stimulus generalisation measures | |
| 3 males aged 4.1, 5.8 and 8.6 years diagnosed with CAS. 1 of the 3 diagnosed with Opitz FG syndrome and another with PDD‐NOS | Not quasi‐/RCT (Single subject multiple baseline across participants design) | Integrated Multimodal Intervention (structured book reading, drill and play activities with AAC devices present and speech encouraged) | Augmentative and alternative communication | 1‐hour sessions, 2 × per week for 3 to 6 weeks | Category (e.g. vocalisation, AAC or both), type of word and accuracy targets. Case 1: final consonants. Case 2: initial /s/ clusters then /f/. Case 3: initial /s/ clusters | Increases in vocalisations/spoken speech noted for 3/3. Speech accuracy improved on targets for 1/3 cases but all showed some generalisation to more accurate everyday speech | Baseline probes, probes every 2nd treatment session, 1‐month post‐treatment | Poor experimental control for case 1 and some change on control data noted. CAS diagnosis unclear and not replicable. No statistical analysis. Limited outcome measures. | |
| 1 female aged 5.6 years with CAS | Not quasi‐/RCT (Case description) | Adapted Cueing Technique | Motor | 30 minutes of therapy per day for 6 months | Number of single words/utterances | From 2 to 4 words to 12 words and several carrier phrases. After 6 months began to produce novel sentences | Description of progress during treatment | Lack of experimental control, multiple baselines or control data. CAS diagnosis unclear and not replicable. No statistical analysis. Limited outcome measures. | |
| 2 males aged 5 and 6 years diagnosed with CAS | Not quasi‐/RCT (Single case (ABAA) design) | Concurrent Melodic Intonation Therapy (MIT) and traditional therapy (20% and 80% of sessions respectively) | Linguistic and motor | 2 × per week over 2‐month period | Pre‐ and post‐treatment gains on word‐morpheme usage, auditory comprehension, naming, describing function, sentence completion, imitation of word phrases and articulation. Tested using language sampling and Porch Index of Communicative Ability in Children | Significant gains were found in phrase length (MLU), picture naming, and verbal imitation tasks. Little change in articulation | Pre‐treatment, post‐traditional therapy, and post‐MIT therapy | Lack of experimental control, multiple baselines or control data. CAS diagnosis unclear and not replicable. | |
| 2 males aged 5 and 6 years with suspected CAS | Not quasi‐/RCT (Single case (AB) design) | Melodic Intonation Therapy (MIT) compared to 'traditional speech‐language therapy' | Linguistic and motor | Ongoing 1 × per week speech therapy (traditional articulation sessions) and 40‐minute MIT music sessions over 4 weeks (both treatments concurrent) | GFTA2; KLPA2 and speech production on stimulable sounds in 1‐ or 2‐syllable words | Case 1 made greater gains in MIT sessions (but only 2% gain). Case 2 made greater gains on traditional articulation therapy (15% gain) | Pre‐ and post‐ treatment | Lack of experimental control, multiple baselines or control data. CAS diagnosis unclear and not replicable. No statistical analysis. Limited outcome measures. | |
| 1 German‐speaking male aged 2.7 years with severe CAS | Single case design (A‐B design with 3 follow‐up assessments post‐treatment with some treatment sessions between assessments) | Speech Generating Devices ‒ fixed display (Gotalk 20+) and dynamic display (DynaVox V) | Augmentative and alternative communication | 45‐minute sessions × 50 treatment sessions. Treatment sessions 2 to 28 days apart | Means of communication (oral versus SGD), intelligibility of speech productions, consistency of speech productions, lexical development, and grammatical development | Significantly more communication initially with SGD than speech; significant increase in speech intelligibility; consistency (however reduced data in baseline period); amount of words used; and increased MLU and inflections after 8 to 9 sessions | Baseline × 3, every 2nd treatment session, and 2, 4 and 8 weeks post‐treatment | Lack of baseline data for consistency. CAS diagnosis unclear and not replicable. No blinding of assessors. No clear withdrawal phase after treatment with SGDs for control and no generalisation data | |
| 1 female aged 5.1 years with CAS | Not quasi‐/RCT (Single case cross‐over design) | Intra‐oral stimulation and electropalatography | Motor | 25‐minute sessions (5 minutes intra‐oral stim, 20 minutes EPG); daily at home, total of 195 sessions in 12 months | Per cent consonants correct, per cent phonemes correct, per cent words correct, intelligibility, visual deviancy | Significant treatment outcomes on all measures | Pre‐testing, A1 (baseline), B (intervention: oral stimulation therapy), A2 (withdrawal for 3 months), B (intervention: EPG), and A3 (follow‐up) | Cross‐over design, no control group or data taken to control for maturation. No replication across participants. No long‐term follow‐up or generalisation data taken | |
| 4 children (2 males, 2 females) aged 5.4 to 8.4 years with CAS (2 also with dysarthria and a third with language disorder); 3 also in Maas 2012b, as below | Not quasi‐/RCT (Single case alternating treatments design with multiple baselines across behaviours over | Dynamic Temporal and Tactile Cueing (high versus moderate feedback frequency in cross‐over design) | Motor | 50‐minute sessions 3 × per week for 3 participants but 1 had 60‐minute sessions 2 × per week | Per cent accuracy on 2‐point scale of segmental and suprasegmental aspects of target words and phrases with 2 words | 2 responded better to low frequency feedback, 1 to high frequency feedback, and 1 to no condition. No generalisation effects | Weekly probes: 3 to 4 × baseline, 4 × treatment. Phase 1: 4 to 5 × withdrawal, 4 × treatment. Phase 2: 2 × withdrawal and 1 month post‐treatment | Small sample size with heterogeneity. Cross‐over conditions made comparison difficult regarding targets chosen. No control group. Effect sizes used not interpretable or comparable to others. Different doses across all participants. Treatment fidelity < 80%. No stimulus generalisation measures | |
| 4 children (2 males and 2 females) aged 5.0 to 7.9 years with CAS. 2 cases had additional dysarthria diagnoses. 1 other case had multiple co‐occurring disorders | Not quasi‐/RCT (Single case alternating treatments design with multiple baselines across behaviours over | Dynamic Temporal and Tactile Cueing (random versus blocked practice compared in cross‐over design) | Motor | 2 × 4 week blocks of therapy | Per cent accuracy on 2‐point scale of segmental and suprasegmental aspects of entire target words and phrases with 2 words | 3/4 responded to both conditions. 2 responded relatively better to blocked practice, 1 to random practice, and 1 to no condition. 2/4 demonstrated generalization | Weekly probes: 3 to 4 × baseline, 4 × treatment. Phase 1: 4 to 5 × withdrawal, 4 × treatment. Phase 2: 2 × withdrawal and 1 month post‐treatment | Small sample size with heterogeneity. Cross‐over conditions made comparison difficult regarding targets chosen. No control group. Effect sizes used not interpretable or comparable to others. Treatment fidelity < 80%. No stimulus generalisation measures | |
| 1 female aged 4.7 years with CAS | Not quasi‐/RCT (Multiple baseline across behaviours ‐ cross‐over treatment design) | Combined Melodic Intonation Therapy (MIT) and Touch Cue Method (TCM) | Motor and linguistic | 3 sessions for 6 weeks for 18 sessions for MIT. 6 weeks no therapy. 3 sessions for 6 weeks for 18 sessions for TCM | Articulation accuracy: PVC, PCC. Also, overall word accuracy scores: PMLU, PWP, PWC. All calculated from responses to 46 picture cards | 1/5 measures significant post‐MIT (per cent vowels correct). Per cent consonants correct also reduced. 3/5 significant post‐TCM (PVC, PCC, PMLU). PVC, PCC and PMLU maintained. Greater changes for both therapies after withdrawal. PCC and PMLU only significant after MIT withdrawn | Beginning and end of 6‐week baseline, beginning and end of both treatment phases, 12 weeks after TCM withdrawn | Lack of experimental control of other factors. Cross‐over design makes comparison of both treatments difficult as many changes only noted after withdrawal of MIT (accumulation effects). Limited outcome data. Lack of generalisation data No blinded assessors. No replication across participants | |
| 12 children (sex unknown) aged 3 to 10 years with CAS (11 with co‐occurring conditions) | Case series (pre and post design) | DuBard Association Method®. It is a multimodal, phonetic therapy which works from accurate sounds in isolation | Motor | Daily in small groups in a school programme for an 11‐month period | Articulation, mean length of utterance (MLU), and intelligibility on Arizona Articulation Proficiency | Significant changes in articulation, intelligibility and MLU, and some resilience measures over 2‐year period | Pre‐ and post‐ treatment | Lack of experimental control regarding maturation effects (despite using the Intervention Efficiency Index and Proportional Change Index) and lack of control of covariate, including other potential intervention over the same period. No control group. No follow‐up or generalisation data | |
| 4 males aged 5.5 to 8.6 years with CAS. 2 children had additional auditory processing impairments | Not quasi‐/RCT (Single case (AB) design with 1 month follow‐up) | Rapid Syllable Transition Treatment (ReST) | Motor | 60‐minute session, 4 × per week for 3 weeks (12 sessions in total). Minimum of 1200 trials per session | Articulation, prosodic and simultaneous articulation and prosodic accuracy on trained and untrained probe pseudo words; PCC, PVC and per cent lexical stress matches from connected speech; PPVT‐4 as control data | All 4 participants increased perceptual accuracy. 1/4 participants showed change in untreated items. All participants showed change in prosody (average prosody gain 58%, 3/4 in PVC and 2/4 in PCC; average gain 79%). Control data (receptive vocabulary on PPVT‐IV) changed minimally | Baseline × 2, probes in treatment × 2, 1 month follow‐up | There was no immediate post‐treatment data taken to determine treatment effects, the follow‐up data was 1 month post‐treatment and included a withdrawal phase. There was no statistical analysis of connected speech data. 1 participant reached ceiling. No blinding of assessors. No stimulus generalisation measures | |
| 12 children (9 males, 3 females) aged 4.2 to 7.6 years with CAS | Not quasi‐/RCT (Case series design) | Integrated Phonological Awareness Intervention | Linguistic | 45‐minute session; 2 × per week for 6 weeks in 2 blocks with 6‐week withdrawal between blocks. Total of 245 sessions | Trained speech accuracy and phonological awareness accuracy on a probe. Generalisation‐ BTOPP and first trial of DEAP inconsistency subtest for PVC, PVC and inconsistency score. PIPA for 4‐year‐olds. TOPA for 5 to 7‐year‐olds. Burt Word Reading Test for non‐word reading and informal non‐word reading probe (Gillon 2000). Per cent grapheme correct score in spelling 10 words from DEAP inconsistency subtest | Speech: 9/12 children improved on trained items. Phonological awareness: 8/12 children improved in 1 or both intervention blocks. Generalisation for 8/12 on all measures except Burt Word Reading Test | Pre‐ and post‐treatment | Lack of experimental control, control group or control data. CAS diagnosis unclear regarding prosody. Limited information provided on each participant. Limited treatment phase data. No maintenance data. No blinding of assessors | |
| 2 male identical twins aged 4.5 years with CAS (deletion at 10q21.2‐22.1) | Not quasi‐/RCT (Single case design) | Integrated Phonological Awareness intervention | Linguistic | 45‐minute session; 2 × per week for 6 weeks in 2 blocks with 6‐week withdrawal between blocks. Total of 245 sessions | PPC, PVC on BTOPP, and DEAP inconsistency percentage. PIPA, PhonRep, Burt Word Reading, Non‐word Reading, Neale accuracy and comprehension | PCC and PVC improved at post‐treatment and follow‐up. Reduced inconsistency. Sound‐letter knowledge increased from 0 to 7 at post‐treatment. Reading WNL and spelling demonstrated use of strategies at final follow‐up | Pre‐ and post‐ treatment, and 6‐month follow‐up | Lack of experimental control, control group or control data. CAS diagnosis unclear regarding prosody. Limited information provided on each participant. Limited treatment phase data. No maintenance data. No blinding of assessors. No stimulus generalisation measures | |
| 12 children (9 males, 3 females) aged 4.2 to 7.6 years diagnosed with CAS | Not quasi‐/RCT (12‐month follow‐up to 2009 case series) | Integrated Phonological Awareness intervention | Linguistic | As per McNeill 2009a | BBTOP and 1st trial of DEAP yielding PPC. PIPA for 4‐year‐olds & TOPA for 5 to 7‐year‐olds. Decoding measures (Burt Word Reading Test and Non‐word Reading Task) and spelling measures (probe of 10 words from the DEAP inconsistency subtest) were completed for participants at least 6 years of age at the beginning of the study. The NARA was administered for participants aged 5 years, 6 months and up | Significant difference for CAS group from pre‐ to post‐treatment on letter knowledge, non‐word reading probe, spelling, PCC, TOPA and Burt Non‐Word Reading. 3/7 improved on NARA to age‐appropriate level | 1‐year follow‐up to McNeill 2009a | 7/12 of original participants followed up. Whole group data ‒ case series. No control group or control data for experimental control or maturation effects | |
| 3 children (2 males, 1 female) aged 6.3, 6.10 and 7.3 years with CAS | Not quasi‐/RCT (Single case multiple baseline design across behaviours) | Integrated Phonological Awareness Intervention | Linguistic | 45‐minute sessions 3 × per week for 3 weeks | PPC on probe, phoneme segmentation probe, phoneme manipulation probes, initial sound identification probes, letter‒sound knowledge subtest from the PIPA, non‐word reading tasks | 2/3 significantly increased PPC, 2/3 significantly improved phonological awareness skills on probes, letter‒sound knowledge, and non‐word reading. Limited transfer to untreated words | Baseline and post‐treatment (3 probes each) | Lack of control group and control data. CAS diagnosis unclear regarding prosody. Lack of multiple baseline data throughout treatment. No long‐term follow‐up. No blinding of assessors | |
| 12 children (9 males, 3 females) aged 3 to 6 years with speech sound disorders | Not quasi‐/RCT (Case series pre‐post design) | Prompts for Restructuring Oral Muscular Phonetic Targets (PROMPT) | Motor | 45‐minute session 2 × per week for 8 weeks | GFTA2, HCAPP, VMPAC focal motor and sequencing subtests, Children's Speech Intelligibility Measure | Significant gains as a group for all speech measures | Baseline 1 week prior to treatment, and 1 week post‐treatment | Lack of experimental control, control group, multiple baseline or control data. No blinding of assessors. No blinding of assessors. No long‐term follow‐up | |
| 37 children (28 males, 9 females) aged 2.6 to 4.5 years with CAS | Not quasi‐/RCT (pre‐postgroup design) | Motor Speech Treatment Protocol (MSTP) | Motor | Intense treatment group: 45‐minute session, 2 × per week × 10 weeks = 20 sessions. Less intense group: 45‐minute session, 1 × per week × 10 weeks = 10 sessions | GFTA‐2 sounds in words subtest; speech intelligibility using Children's Speech Intelligibility Measure (CSIM) at word level, and Beginner's Intelligibility Test (BIT) at sentence level. Functional Outcomes for Children Under Six (FOCUS) scale | Intense group had greater changes in articulation and functional communication compared to the less intense group with large effect sizes. Mixed results were found for intelligibility: at word‐level (CSIM), both the less intense and 1/2 intense groups made a significant and large change. At sentence level, 1/2 intense groups made a significant change | Pre‐ and post‐ treatment | No control group or control data. Participants were not directly randomised; however, no between‐group differences were found at baseline. There were missing data (dealt with using intention‐to‐treat analysis). No information on session trials was obtained, which is important for intensity calculations | |
| 6 males aged 9 to 15 years with CAS. 1 child had additional ADHD and another child had additional dysarthria | Not quasi‐/RCT (Single case multiple baseline across behaviours across participants) | Ultrasound biofeedback (targeting articulation on clusters and CV or VC sequences of inaccurate phones) | Motor (instrumentally based) | 60 minute sessions, 2 × per week × 18 sessions (at least 150 trials per session) | Probe of whole‐word accuracy of treated and untreated items | U002 and U007 had significant gains on 2/4 treated combinations, U005 for 3/4, and U008, U009 and U012 had significant gains on all treated combinations. All exhibited some generalisation (target‐dependant). U005, U007, U008, U009, U012 demonstrated maintenance above pre‐treatment levels | Probes at baseline × 3, every treatment session, post‐treatment, and 2 months post‐treatment | No control group or comparison treatment. No blinding of assessors. Untreated items were not clearly selected as control or generalisation data with some showing change and others not | |
| 3 male children aged 11 to 13 years diagnosed with CAS and poor expressive language and phonological processing. 1 participant had additional flaccid dysarthria, ADHD, language and learning difficulties | Not quasi‐/RCT (Single case multiple baseline across behaviours (syllable positions)) | Ultrasound biofeedback (using structured chaining and principles of motor learning) | Motor (instrumentally based) | 1 hour sessions × 14 sessions. Sessions 1 to 7 addressed target 1 and sessions 8 to 14 addressed target 2 with randomly assigned prosody or no prosody conditions | Treatment acquisition data, generalisation probe of untreated words, maintenance to 2 months post‐treatment | 2/3 participants acquired accurate articulation. 0/3 demonstrated generalisation or maintenance | 3 × baseline probes, midway therapy probe, post‐therapy probe (within 1 week after treatment), and 2‐month follow‐up | No control group. Greater within‐treatment probes and post‐treatment probes would have allowed for greater statistical analysis. No control data. No blinding of assessors. No stimulus generalisation measures | |
| 3 males aged 11 to 14 years with CAS | Not quasi/RCT (Single case (ABA) design) | Ultrasound biofeedback (using structured chaining and principles of motor learning.) | Motor (Instrumentally based) | 2 × 1‐hour articulation treatment a day for 2 weeks. 16 hours of therapy in total. Over 100 trials per session | Treatment acquisition of /ɹ/, /s/ or /ʧ/. Generalisation to untrained items using a probe and sentence imitation task, and maintenance 1 to 3 weeks post‐treatment (audio‐samples submitted) | Case 1 had acquisition, generalisation, and maintenance of targets. Case 2 had some acquisition in the 2nd week of therapy and no generalisation and maintenance. Case 3 showed acquisition, limited generalisation to words and not phrases, and no maintenance | Probe conducted 1 × before treatment, at the end of the first week, and at the end of the second week (post‐treatment) | Lack of experimental control, multiple baselines or control data. No blinding of assessors. No long‐term follow‐up data. No stimulus generalisation measures | |
| 1 adult with CAS and class III malocclusion. Another 5 adults aged 18 to 23 years with persistent articulation disorders | Not quasi‐/RCT (Case series) | Orofacial myofunctional therapy | Motor (Instrumentally based) | 45‐minute session, 1 × per week for 6 weeks | Dworkin‐Culatta Oral Mechanism Examination for oral postures and intelligibility in single words, sentences, and spontaneous speech | All improved lips and tongue postures. 5/6 participants increased intelligibility. No improvement in intelligibility for person with DVD | Pre‐ and post‐treatment | Lack of experimental control, multiple baselines or control data. No treatment data or follow‐up reported. CAS diagnosis unclear and not replicable. No statistical analysis. Limited outcome measures. | |
| 1 female aged 9 years with CAS | Not quasi‐/RCT (Case study) | Intensive, systematic drill motor therapy | Motor | 22 sessions over 3 months | 20‐item probe of /r/ (target), ineligibility in spontaneous speech | /r/ improved from 0 to 20 correct in probe. Intelligibility judged by unfamilar listeners improved | Treatment sessions | Lack of experimental control, multiple baselines or control data. CAS diagnosis unclear and not replicable. No follow‐up data. Only ancedotal generalisation data. No statistical analysis. No reliability of judgments reported. No replication across participants | |
| 4 children (3 males, 1 female) aged 10‐14 years diagnosed with CAS | Not quasi‐/RCT (Single subject (ABAB) design) | Rate Control Therapy | Linguistic and motor | 20‐minute session per reading passage. No further information available | Articulation accuracy (words read correctly) | Improved to 85% accuracy at 50% habitual rate and maintained in therapy as rate was slowly increased. Limited generalisation to conversation ‐ therapy implemented | Reading rate in 5‐minute intervals | Lack of control and follow‐up data. CAS diagnosis unclear and not replicable. No statistical analysis. No blinding of assessors. No stimulus generalisation measures. No report of data reliability | |
| 3 children (2 males, 1 female) aged 4 to 6 years diagnosed with CAS | Not quasi‐/RCT (Single case multiple baseline design across participants) | Concurrent treatment (using randomised variable practice) | Motor | Therapy until target sounds reached 80% accuracy. P1 had 26, P2 had 12 and P3 had 28 sessions. 2 × per week, 30 minutes per session and on average 100 to 115 trials per session | Per cent correct productions on /s, z, f, v/ trained targets during baseline and treatment; generalisation probes to untrained words and 3‐word phrases | All children reached 80% accuracy on target sounds. Moderate to large generalisation effects at word and 3‐word phrases levels (70% to 100% accuracy) | 3 × baseline probes, probes every 5 therapy sessions | No post‐treatment or follow‐up/maintenance data. No blinded assessors. No stimulus generalisation data. P3 continued regular school therapy during the study so could be a confounding factor. No stimulus generalisation measures | |
| 1 male aged 7 years with residual CAS | Not quasi‐/RCT (Single case (ABA) design) | Articulation with facilitative vowel contexts | Linguistic | 45‐ to 55‐minute session, 3 × per week for 3 weeks. 60+ trials per session. Home practice provided | Accuracy on 'sh' sound in word initial probe, 'tr' as control | Significant improvement in 'sh' articulation accuracy in trained and untrained words. No change in control words with 'tr' initial | Pre‐treatment, mid‐therapy × 2 (after sessions 3 and 6), post‐treatment, and maintenance (2 weeks post‐treatment) | Participant did not meet current CAS criteria. Lack of generalisation data beyond 'sh' sound. No blinded assessors. No replication across participants. No long‐term follow‐up data. No reliability of data reported | |
| 1 female aged 5 years with "severe motor planning deficits but no dysarthria" (CAS) | Not quasi‐/RCT (Single case multiple baseline design) | Integral stimulation | Motor | 30‐ to 50‐minute session, 3 to 5 × per week (1 to 2 × per day) for 10 to 16 sessions. No home practice | Articulation accuracy ratings on a 2‐point scale | Improvement from 0.25 to 0.80 on 2‐point scale. 4/5 treatment stimuli achieved rating of 2/2 by end of therapy | Treated stimuli at start of each session, control stimuli twice a week | No statistical analysis. Limited outcome measures. | |
| 4 males aged 5.5 to 6.1 years with CAS (2 with dysarthria and 1 with mild intellectual disability) | Not quasi‐/RCT (Single case multiple baseline across participants) | Dynamic Temporal and Tactile Cueing | Motor | 30‐minute sessions, 2 × per day for 5 days a week for 38 to 50 sessions | Articulation accuracy on a 3‐point scale | Treatment gains for 3/4 participants maintained by 2/4 | Baseline × 4 (or more, staggered baseline), 20+ treatment probes | No follow‐up or generalisation data. CAS diagnosis unclear and not replicable. No statistical analysis. Limited outcome measures | |
| 4 children (2 males, 2 females) aged 4.8 to 8 years with CAS | Not quasi‐/RCT (Single case multiple baseline across participants and behaviours) | Rapid Syllable Transition Treatment (ReST) | Motor | 50 minute sessions 2 × per week for 6 weeks. 100 trials per session | Accuracy on imitated (a) treated words, (b) untreated pseudo words, (c) untreated real words and control words | Significant improvement on treated words and untreated real words. Significant improvement for 2/4 participants on untreated pseudo words. No change in control items | Baseline × 3 to 6, treatment × 3, and 1 day, 1 month and 4 months post‐treatment | Use of GFTA2 for control items. No stimulus generalisation data | |
| 5 children (4 males, 1 female) aged 5 to 11 years with CAS (3 with mild or moderate receptive language disorder) | Not quasi‐/RCT | Rapid Syllable Transition Treatment (ReST) | Motor (instrumentally based ‐ telehealth) | 60‐minute session, 4 times a week for 3 weeks (12 sessions in total). Minimum of 1200 trials per session | Accuracy on treated pseud‐word items, generalisation to untreated non‐words and real words, and control items (articulation of rhotics) on a probe; client/family satisfaction with telehealth treatment | 5/5 participants demonstrated significant change in treated items. 4/5 maintained gains to 4 months post‐treatment. 4/5 had significant generalisation to untrained non‐words and real words, and 1/5 demonstrated change in control data (articulation errors of rhotics or /s/). Families very satisfied and motivated by telehealth treatment | At least 3 baseline probes, 3 therapy probes (sessions 5, 9 and 1 day post‐treatment). Follow‐up at 1 week, 4 weeks & 4 months post‐treatment | Missing data for some participants at certain time points in Table 3. Problems with change in control data. Some internet issues (dropouts, port sound quality, etc.) were observed in 61% of sessions; however, significant outcomes were found. No stimulus generalisation data | |
| 1 male aged 3 years with CAS and fine motor delay | Not quasi‐/RCT (Single case design; descriptive) | Multimodal therapy: Signed Exact English sign language, Sarah Rosenfeld Johnson's oro‐motor programme and Kaufman Speech Praxis Program | Augmentative and alternative communication | Clinic‐based sessions 45 minutes 1 to 2 × per week and home‐based sessions for 60 minutes 1 × per week | Language assessment; observations and Kaufman Speech Praxis Test; Verbal Motor | Receptive and expressive language consistently in average range but receptive relatively better than expressive language. By 3.6 years of age receptive and expressive language same level. Marked drooling and limited inventory and sequencing at 18 months, yet skills on Kaufman & VMPAC in average range at 3 years, 9 months. Discharged from therapy | Language assessment at 1.1 year, 3 years and 3.6 years. Kaufman test or observations at 1.6, 3 and 3.9 years. VMPAC at 3 years, 9 months | Lack of experimental control, multiple baselines or control data. CAS diagnosis unclear and not replicable regarding prosody and drooling. No statistical analysis. | |
| 1 male aged 14 years with severe CAS and limb/motor apraxia and obsessive compulsive disorder | Not quasi‐/RCT (Case study) | Verbal Motor Learning (with Dynamic Distal Stabilization Technique (DDST)) | Motor | 1 × 30‐minute clinic session and 6 × home practice sessions a week for 4 weeks | (1) Producing highest pitch using /I/ sound with and without DDST, to determine minimum and maximum frequency and length using Speech Analyser 1.5 (2) Imitation of 18 words to analyse word length, maximum loudness, maximum and minimum frequency | Significant t‐test results for (1) increase in maximum frequency and length of pitch after DDST, no change in minimum frequency, and (2) decrease in word length (word said faster), maximum loudness, and maximum frequency | Pre‐ and post‐ treatment | Lack of experimental control, multiple baselines or control data. CAS diagnosis unclear and not replicable. No statistical analysis. Limited outcome measures. | |
| 1 female aged 10 years with CAS and ASD | Not quasi‐/RCT (Case study) | Verbal Motor Learning (Initial Phoneme Cue (IPC) technique) | Motor | 2 × 1 hour sessions, 2 weeks apart (participant had initial therapy: 1‐hour session weekly for 1 year prior to this study) | Imitation accuracy of CVCV treated words either (a) with IPC or (b) without IPC | Imitation of CVCV was 0% to 22% accuracy and imitation with IPC was 96% to 100% accuracy | Pre‐ and post‐ treatment | Lack of experimental control, multiple baselines or control data. CAS diagnosis unclear and not replicable. No statistical analysis. Limited outcome measures. No statistical analysis. No blinding of assessors. No follow‐up or generalisation data. No replication across participants | |
| 10 children (no information on gender reported) aged 6 to 11 years with moderate to severe DAS | Not quasi‐/RCT (Case descriptions/file audit) | School‐based intervention | Motor | 25 to 307 hours of therapy | Articulation, polysyllable words and connected speech in speech samples. Intelligibility rated on a 9‐point scale | Significant improvement on articulation. Minimal generalisation to polysyllable words and connected speech. Intelligibility improved by at least 0.5 points | Pre‐ and post‐ treatment | Lack of experimental control, multiple baselines or control data. CAS diagnosis unclear and not replicable. No statistical analysis. | |
| 1 female aged 11.6 years with CAS, intellectual disability and language disorder | Not quasi‐/RCT (Single case design) | Phonological awareness (phoneme‒grapheme mapping, reading comprehension, 'Basics' programme). Speech ‐ PROMPT and Moving Across Syllables | Linguistic | Between 6.0 and 11.6 ongoing weekly treatments ‐ 1 hour × 1:1 sessions and PROMPT institute over summer | Per cent accuracy on phonological awareness and decoding | Improvement seen in phoneme‒grapheme mapping, segmentation and short vowel identification. Some improvement in decoding | Ongoing 1 × per week sessions from 6.0 to 11.6 years | Lack of experimental control, multiple baselines or control data. CAS diagnosis unclear and not replicable. No statistical analysis. Limited outcome measures. | |
| Participants: All participants are English speakers unless otherwise reported. AOS: apraxia of speech; BBTOP: Bankson‐Bernthal Test of Phonology; CAS: childhood apraxia of speech; CSIP: consonant substitute inconsistency percentage; DAS: developmental apraxia of speech; DEAP: Diagnostic Evaluation of Articulation and Phonology; DVD: developmental verbal dyspraxia; GDD: global developmental delay; GFTA‐2: Goldman Fristoe Test of Articulation 2; HCAPP: Hodson Computerized Analysis of Phonological Patterns; ISP: inconsistency severity percentage; KLPA‐2: Khan‐Lewis Phonological Analysis, Second Edition; NARA: Neale Analysis of Reading Ability; PCC: percentage consonants correct; PDD‐NOS: pervasive developmental disorder ‐ not otherwise specified; PMLU: phonological mean length of utterance; PVC: percentage vowels correct; PWC: percentage words correct; PWP: proportion of whole‐word proximity; PIPA: Preschool and Primary Inventory of Phonological Awareness; RCT: randomised control trial; SSD: speech sound disorder; TOCS+: Test of Children's Speech Plus; TOPA: Test of Phonological Awareness; VMPAC: Verbal Motor Production Assessment for Children | |||||||||

