Mitomicina C versus 5‐fluorouracilo para la cicatrización de heridas en la cirugía de glaucoma
Resumen
Antecedentes
La presión intraocular elevada es un factor de riesgo de glaucoma. Una opción de tratamiento es la cirugía de drenaje del glaucoma (trabeculectomía). Los antimetabolitos se utilizan durante la cirugía para reducir la cicatrización posoperatoria durante la curación de la herida. Dos agentes de uso frecuente son la mitomicina C (MMC) y el 5‐fluorouracilo (5‐FU).
Objetivos
Evaluar los efectos de la MMC en comparación con 5‐FU como un antimetabolito complementario en la trabeculectomía.
Métodos de búsqueda
Se realizaron búsquedas en CENTRAL (que contiene el registro de ensayos del Grupo Cochrane de Trastornos de los Ojos y la Visión) (2015, número 9), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (de enero de 1946 a octubre de 2015), EMBASE (de enero de 1980 a octubre de 2015), Latin American and Caribbean Health Sciences Literature Database (LILACS) (de enero de 1982 a octubre de 2015), el registro ISRCTN (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov) y la Plataforma de Registros Internacionales de Ensayos Clínicos (ICTRP) de la Organización Mundial de la Salud (www.who.int/ictrp/search/en). No se aplicó ninguna restricción de fecha o idioma en las búsquedas electrónicas de ensayos. Se buscó por última vez en las bases de datos electrónicas el 2 de octubre 2015.
Criterios de selección
Se incluyeron los ensayos controlados aleatorios donde la cicatrización de la herida se modificó con MMC en comparación con 5‐FU.
Obtención y análisis de los datos
Dos revisores de forma independiente seleccionaron los ensayos y obtuvieron los datos. El resultado primario fue fracaso de una trabeculectomía funcionante al año después de la cirugía. Los resultados secundarios incluyeron presión intraocular media al año. Se consideraron tres subgrupos: alto riesgo de fracaso de la trabeculectomía (pacientes con cirugía previa por glaucoma, cirugía por catarata extracapsular, origen africano y pacientes con glaucoma secundario o glaucoma congénito); riesgo medio de fracaso de la trabeculectomía (pacientes a los que se les realizó trabeculectomía con cirugía por catarata extracapsular) y bajo riesgo de fracaso de la trabeculectomía (pacientes que no han recibido cirugías oculares previas).
Resultados principales
Se identificaron 11 ensayos con 687 ojos de 679 participantes. Los estudios se realizaron en los Estados Unidos, Europa, Asia y África. Cinco estudios reclutaron participantes con bajo riesgo de fracaso de la trabeculectomía, cinco estudios reclutaron participantes con alto riesgo de fracaso y un estudio reclutó pacientes con riesgo de fracaso alto y bajo. Ninguno de los ensayos incluidos reclutó participantes con cirugía combinada de trabeculectomía / catarata.
Se consideró que un estudio tuvo bajo riesgo de sesgo en todos los dominios, seis estudios tuvieron alto riesgo de sesgo en uno o más dominios y los cuatro estudios restantes tuvieron riesgo incierto de sesgo en todos los dominios.
El riesgo de fracaso de la trabeculectomía al año después de la cirugía fue menor en los participantes que recibieron MMC en comparación con los que recibieron 5‐FU; sin embargo, los intervalos de confianza fueron amplios y son compatibles con ningún efecto (cociente de riesgos [CR] 0,54; intervalo de confianza [IC] del 95%: 0,30 a 1,00; estudios = 11; I2= 40%). No hubo pruebas de ninguna diferencia entre los grupos con alto y bajo riesgo de fracaso (prueba para las diferencias de subgrupos p = 0,69).
Como promedio, los pacientes tratados con MMC tuvieron menor presión intraocular al año (diferencia de medias [DM] ‐3,05 mmHg; IC del 95%: ‐4,60 a ‐1,50), pero los estudios fueron inconsistentes (I2 = 52%). El tamaño del efecto fue mayor en el grupo de alto riesgo (DM ‐4,18 mmHg; IC del 95%: ‐6,73 a ‐1,64) en comparación con el grupo de bajo riesgo (DM ‐1,72 mmHg; IC del 95%: ‐3,28 a ‐0,16), pero nuevamente la prueba de interacción no fue estadísticamente significativa (p = 0,11).
Proporciones similares de ojos tratados con MMC perdieron 2 o más líneas de agudeza visual un año después de la cirugía en comparación con 5‐FU, pero los intervalos de confianza fueron amplios (CR 1,05; IC del 95%: 0,54 a 2,06).
Los eventos adversos ocurrieron relativamente con poca frecuencia y generalmente las estimaciones del efecto fueron imprecisas. Hubo algunas pruebas de menos epiteliopatía en el grupo de MMC (CR 0,23; IC del 95%: 0,11 a 0,47) y menos hifema en el grupo de MMC (CR 0,62; IC del 95%: 0,42 a 0,91).
Ninguno de los estudios informó la calidad de vida.
En general, la calidad de las pruebas se calificó como baja, debido en gran parte al riesgo de sesgo de los estudios incluidos y la imprecisión en la estimación del efecto.
Conclusiones de los autores
Se encontraron pruebas de baja calidad de que la MMC puede ser más eficaz para lograr una presión intraocular inferior a largo plazo que el 5‐FU. Se necesitan estudios de investigación comparativos adicionales sobre MMC y 5‐FU para mejorar la fiabilidad y la validez de los resultados mostrados en esta revisión. Además, sería valioso el desarrollo de agentes nuevos que controlen la formación de tejido cicatrizal posoperatorio sin efectos secundarios, y se justifica por los resultados de esta revisión.
PICO
Resumen en términos sencillos
Mitomicina C versus 5‐fluorouracilo para la cicatrización de heridas en la cirugía de glaucoma
Pregunta de la revisión
¿La mitomicina C (MMC) ofrece alguna ventaja en comparación con 5‐fluorouracilo (5‐FU) como antimetabolito utilizado para mejorar la cirugía por glaucoma (trabeculectomía)? ¿La MMC ayuda a lograr tasas inferiores de fracaso de la trabeculectomía que el 5‐FU al año después de la cirugía?
Antecedentes
La presión intraocular elevada es un factor de riesgo de glaucoma. Una opción de tratamiento es la cirugía de drenaje del glaucoma (trabeculectomía) para ayudar a bajar la presión intraocular. Los antimetabolitos son fármacos administrados durante la cirugía para ayudar a reducir la cicatrización después de la cirugía durante la curación de la herida. Si ocurre cicatrización puede provocar el fracaso del tratamiento porque el canal de drenaje deja de funcionar. Dos agentes en que se utilizan habitualmente son MMC y 5‐FU.
Fecha de la búsqueda
Las pruebas están actualizadas hasta octubre 2015.
Características de los estudios
En esta revisión se incluyeron 11 ensayos controlados aleatorios realizados en los Estados Unidos, Europa, Asia y África. En general, a 687 ojos de 679 participantes se les realizó trabeculectomía habitual para el control del glaucoma. Algunos participantes tuvieron un mayor riesgo de fracaso que otros, por ejemplo si habían recibido cirugía previa por glaucoma, eran de origen africano o si presentaban glaucoma secundario. Cinco estudios reclutaron participantes con bajo riesgo de fracaso de la trabeculectomía, cinco estudios reclutaron participantes con alto riesgo de fracaso y un estudio reclutó pacientes con riesgo de fracaso alto y bajo. Ninguno de los ensayos incluidos reclutó participantes con cirugía combinada de trabeculectomía / catarata.
Resultados clave
Esta revisión indicó que el riesgo de fracaso de la trabeculectomía al año después de la cirugía fue ligeramente menor en los participantes tratados con MMC en comparación con 5‐FU. Todos los ensayos controlados aleatorios incluidos contribuyeron a este resultado, con una población de estudio mixta de participantes con alto y bajo riesgo y una metodología de aplicación del antimetabolito que varió. No se detectaron diferencias significativas entre los subgrupos de participantes con bajo y alto riesgo de fracaso, pero el poder estadístico de este análisis fue bajo.
No se identificaron diferencias entre los resultados visuales del grupo que recibió MMC y el grupo que recibió 5‐FU al año después de la cirugía, así como tampoco en el número de gotas utilizadas después de la cirugía. Sin embargo, se encontraron pruebas que indican que la MMC fue más eficaz en la disminución de la presión intraocular que el 5‐FU en los participantes de alto y bajo riesgo, y al año se logró una presión intraocular media inferior después de la cirugía en comparación con los participantes tratados con 5‐FU. Este efecto pareció ser mayor en las poblaciones de alto riesgo.
La evaluación de las complicaciones generales entre todos los estudios mostró una tendencia leve a favor del uso de MMC, en particular en la incidencia de epiteliopatía e hifema. Hubo una tendencia hacia pérdidas de la bula, pérdidas por la herida, hipotonía tardía y formación de cataratas en el grupo tratado con MMC.
Ninguno de los estudios informó la calidad de vida.
Calidad de la evidencia
La calidad de las pruebas se calificó de baja, principalmente debido al riesgo de sesgo en los estudios incluidos. Habitualmente se encontró un sesgo que provino de las diferentes técnicas de administración del antimetabolito, lo que dificultó ocultar el fármaco que se administraba. Además, la mayoría de los estudios solamente informaron algunas complicaciones, lo que significó que hubo escasos números generales para incluir en el análisis de las complicaciones.
Authors' conclusions
Summary of findings
| MMC compared to 5‐FU for wound healing in glaucoma surgery | ||||||
| Patient or population: wound healing in glaucoma surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants/eyes | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| 5‐FU | MMC | |||||
| Failure of functioning trabeculectomy at 1 year | Study population | Low‐risk population RR 0.65 (95% CI 0.19 to 2.20) High‐risk population RR 0.49 (95% CI 0.22 to 1.08) | 634 | ⊕⊕⊝⊝ | ||
| Low‐risk population: 74 per 1000 High‐risk population: 272 per 1000 | Low‐risk population: 50 per 1000 High‐risk population: 137 per 1000 | |||||
| Intraocular pressure at 1 year | The mean intraocular pressure at 1 year ranged across 5‐FU groups. Low‐risk population: 10.9 to 14.3 mmHg High‐risk population: 14.8 to 16.3 mmHg | The mean intraocular pressure at 1 year in the MMC groups had a range of values. Low‐risk population: 9.9 to 11.6 mmHg High‐risk population: 8.6 to 13.7 mmHg | ‐ | 386 | ⊕⊕⊝⊝ | |
| Loss of 2 or more lines of Snellen visual acuity at 1 year | Study population | Low‐risk population RR 2.00 (95% CI 0.53 to 7.59) High‐risk population RR 0.81 (95% CI 0.36 to 1.80) | 328 | ⊕⊕⊝⊝ | ||
| Low‐risk population: 47 per 1000 High‐risk population: 115 per 1000 | Low‐risk population: 94 per 1000 High‐risk population: 96 per 1000 | |||||
| Postoperative complications: late hypotony | Study population | RR 1.37 (95% CI 0.41 to 4.63) | 211 | ⊕⊕⊝⊝ | ||
| 37 per 1000 | 59 per 1000 | |||||
| Postoperative complications: choroidal detachment | Study population | RR 0.86 (95% CI 0.45 to 1.63) | 494 | ⊕⊕⊝⊝ | ||
| 68 per 1000 | 70 per 1000 | |||||
| Postoperative complications: endophthalmitis | Study population | RR 3.89 (95% CI 0.44 to 34.57) | 315 | ⊕⊕⊝⊝ | ||
| 0 per 1000 | 19 per 1000 | |||||
| Quality of life at 1 year | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded for risk of bias: only one study at low risk of bias in all domains 2Downgraded for imprecision: wide confidence intervals 3Downgraded for inconsistency: I2 = 60% 4Downgraded for risk of bias: no study at low risk of bias in all domains | ||||||
Background
Description of the condition
Glaucoma is a chronic, progressive optic neuropathy characterised by a progressive loss of ganglion cells that leads to a characteristic visual function loss. Intraocular pressure (IOP) is often considered to be a major risk factor for glaucoma, and it is the only factor that can be modified to try to change the course of the condition. The publication of a series of randomised controlled trials (RCTs) has established the evidence for treating glaucoma with IOP reduction (AGIS 1998; CNTGS 1998; Heijl 2002; Kass 2002; Maier 2005; Vass 2007).
Glaucoma drainage surgery remains an important treatment option for the control of IOP despite the addition of several new IOP‐lowering drugs. Some evidence suggests that trabeculectomy is more effective than either medicine or laser treatment alternatives (Migdal 1994). However, a Cochrane systematic review from 2012 found that visual field deterioration up to five years is not significantly different whether treatment is initiated with medication or trabeculectomy (Burr 2012).
Optimum success rates are achieved when the eye has been exposed to no previous interventions, either surgical or medical, although this is not the usual situation in high‐income countries. Risk factors for trabeculectomy failure are thought to be those that increase the scarring response and include previous exposure to topical medication, previous surgical manipulation of the conjunctiva or other injury, young age, African origin, a history of uveitis and neovascular glaucoma (EGS 2003).
Presentation and diagnosis
The diagnosis of glaucoma is made by the identification of a progressive optic neuropathy or a characteristic visual field defect. There are subgroups of glaucoma, primary open angle glaucoma being most common in European and African populations. A person with primary open angle glaucoma is often unaware of any symptoms until the late stages of the disease, making early diagnosis essential.
Description of the intervention
Treatment is usually initiated with topical treatment, and surgical options are considered if topical treatment fails to prevent progression of the disease. The trabeculectomy produces a guarded fistula between the anterior chamber and the subconjunctival space. There have been numerous modifications since its first description (Cairns 1968), including the use of antimetabolites to reduce fibroblast activity and postoperative scarring at the site of the scleral flap and the subconjunctival space.
How the intervention might work
Once trabeculectomy has been selected, the treatment decisions are whether to augment the surgery with antiscarring agents such as antimetabolites. Antimetabolites are applied to the surgical site to inhibit fibroblast activity and reduce postoperative scarring; the two agents commonly in use are mitomycin C (MMC) and 5‐Fluorouracil (5‐FU). Due to reported side effects such as increased risk of bleb leak, hypotony and endophthalmitis (DeBry 2002), there is concern that use of these agents should be restricted to high‐risk cases only. A number of RCTs have reported the use of MMC (Andreanos 1997; Carlson 1997; Cohen 1996; Costa 1996; Martini 1997; Robin 1997; Shin 1995; Shin 1998; Wu 1996). A Cochrane systematic review concluded that compared to placebo, MMC reduces mean IOP at 12 months in all groups of participants (Wilkins 2010). Apart from increase in cataract formation, there was insufficient power to detect any increase in other serious side effects. Postoperative 5‐FU injections to augment trabeculectomy have also been assessed with RCTs (FFSSG 1989; Goldenfeld 1994; Ophir 1992; Ruderman 1987), and also confer an improvement in IOP control at one year compared to placebo (Green 2014). Clinically, MMC and 5‐FU can be applied intraoperatively on a sponge placed for one to five minutes between the conjunctiva and sclera at the start of the operation. Alternatively, 5‐FU may be given as one or more postoperative subconjunctival injections. There is marked variation in the concentrations of both drugs used, the time of intraoperative application and the position and volume of postoperative injections.
Why it is important to do this review
The results of two Cochrane reviews comparing MMC, in Wilkins 2010, and 5‐FU, in Green 2014, to placebo suggest a similar effect for the two agents in inhibiting scarring after trabeculectomy. However, there is no direct comparative evidence to influence which antimetabolite a surgeon should choose. The purpose of this review was to systematically summarise the RCTs in which MMC was compared to 5‐FU in an attempt to clearly identify treatment benefits of one agent over the other.
Objectives
To assess the effects of MMC compared to 5‐FU as an antimetabolite adjunct in trabeculectomy surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs where wound healing had been modified with one of the antimetabolites in one group of people undergoing trabeculectomy, compared to the other antimetabolite in the other group.
Types of participants
There were three separate subgroup populations:
-
High risk of trabeculectomy failure: people with previous glaucoma or extracapsular cataract surgery, people of African origin and people with secondary glaucoma or congenital glaucoma.
-
Medium risk of trabeculectomy failure: (combined surgery) people undergoing trabeculectomy with extracapsular cataract surgery.
-
Low risk of trabeculectomy failure: (primary trabeculectomy): people who have received no previous surgical eye intervention. People who underwent previous laser procedures may be included in this group.
For the purpose of this review, there were no restrictions regarding age or gender.
Types of interventions
We included the following interventions:
-
Use of intraoperative MMC versus intraoperative 5‐FU.
-
Use of intraoperative MMC versus postoperative 5‐FU.
-
Use of intraoperative MMC versus intraoperative and postoperative 5‐FU.
-
Use of intraoperative MMC and postoperative MMC versus intraoperative and postoperative 5‐FU.
Types of outcome measures
Primary outcomes
The primary outcome was failure of a functioning trabeculectomy at one year from surgery (dichotomous).
We used the following definitions:
-
Success: adequate pressure control (< 22 mmHg) without additional treatment.
-
Failure: need for repeat filtration surgery or uncontrolled IOP (= or > 22 mmHg).
Secondary outcomes
-
Survival analysis (time to event) for the previously given definition of failure
-
Mean IOP for each group at one year from surgery
-
Quality‐of‐life measures
-
Economic data
Adverse outcomes
Adverse events in either group with reference to choroidal detachment, hypotony and late endophthalmitis were reported. Adverse events were reported at any time during the follow‐up period.
We used the following definitions:
-
Bleb leakage: presence of a positive Seidel test (visible aqueous flow with the tear film stained with fluorescein).
-
Hypotony: IOP below 5 mmHg and/or associated with complications such as macular oedema and sight loss or choroidal detachments.
-
Endophthalmitis: an infection of the globe contents that even with prompt aggressive treatment results in substantial loss of visual function.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2015 Issue 9), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to October 2015), EMBASE (January 1980 to October 2015), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to October 2015), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 2 October 2015.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), LILACS (Appendix 4), ISRCTN (Appendix 5), ClinicalTrials.gov (Appendix 6) and the ICTRP (Appendix 7).
Searching other resources
We searched the reference lists of identified trial reports to find additional trials. We contacted investigators as necessary to identify additional published and unpublished studies.
Data collection and analysis
Selection of studies
Three review authors (JC/EC/JE) independently reviewed the titles and abstracts resulting from the searches. We obtained full copies of any report referring to possibly or definitely relevant trials and assessed them according to the definitions in the Criteria for considering studies for this review section. We assessed only trials meeting these predefined criteria for methodological quality. We resolved any disagreements by discussion.
Data extraction and management
Three review authors (JC/EC/JE) independently extracted data with relation to the outcome measures outlined above. We resolved discrepancies by discussion. One review author entered the data into Review Manager (RevMan 2014), and the other review authors checked the data entry.
Assessment of risk of bias in included studies
Three review authors (JC/EC/JE) independently assessed risk of bias according to methods set out in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We considered six domains: random sequence generation, allocation concealment, masking, incomplete outcome data, selective reporting and any other identified bias. We graded each domain as low risk of bias, high risk of bias, or unclear risk of bias. For example, in allocation concealment the grading was low risk if there was central randomisation of subjects, high risk if there was simple alternating methods used to allocate subjects and unclear if there was no real qualifying statement. We resolved disagreements by discussion. Review authors were not masked to trial details during the assessment. We excluded trials scoring 'high risk' on allocation concealment. In cases where missing or confusing data did not permit a clear grading of the trial, we contacted the study authors in order to obtain further information.
Measures of treatment effect
We measured the effect of dichotomous data by risk ratio; continuous data by difference in means; and time to event data by hazard ratio.
Unit of analysis issues
All studies were parallel‐group RCTs. In the majority of studies, one eye per person was enrolled, and therefore there were no unit of analysis issues. In Lamping 1995, WuDunn 2002 and Xinyu 2001, both eyes of some participants were enrolled, but in most cases this was less than 10%, and overall less than 5% of the data would be affected by this. None of the trials took into account the potential correlation between eyes, and we have analysed the data from the trials as reported.
Dealing with missing data
We did an available case analysis. This assumes that data are missing at random. We assessed whether this assumption was reasonable by collecting data from each included trial on the number of participants excluded or lost to follow‐up and reasons for loss to follow‐up by treatment group, if reported. We collected this information as part of the assessment of attrition bias.
Assessment of heterogeneity
We examined the overall characteristics of the studies, in particular the types of participants and interventions, in order to assess the extent to which the studies were similar enough to make pooling study results sensible.
We looked at the forest plot of study results to see how consistent the studies were, in particular looking at the size and direction of effects.
We calculated I2, which is the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance) (Higgins 2002). We considered I2 values over 50% to indicate substantial inconsistency or heterogeneity. We also considered Chi2 P values; when the number of studies was few we used P less than 0.1 to indicate statistical significance of the Chi2 test.
Assessment of reporting biases
We planned to do a 'funnel plot' to investigate reporting (publication) bias, but there were not enough included trials (fewer than 10 in each meta‐analysis) to make this possible.
Data synthesis
If there was inconsistency between individual study results such that a pooled result may not have been a good summary of the individual trial results, for example the effects were in different directions, or I2 was greater than 50% and P less than 0.1, we did not pool the data but did describe the pattern of the individual study results.
If I2 was greater than 50%, but all the effect estimates were in the same direction such that a pooled estimate would seem to have provided a good summary of the individual trial results, we did pool the data.
If there was inconsistency between individual study results such that a pooled result may not have been a good summary of the individual trial results, for example the effects were in different directions, or I2 was greater than 50% and P less than 0.1, we did not pool the data but did describe the pattern of the individual study results.
If I2 was greater than 50%, but all the effect estimates were in the same direction such that a pooled estimate would have provided a good summary of the individual trial results, we did pool the data.
Subgroup analysis and investigation of heterogeneity
We compared the effect of intervention in a pre‐planned analysis comparing effects in groups at high and low risk of failure.
Sensitivity analysis
We conducted sensitivity analyses to determine the impact of risk of bias on effect size. We repeated the analyses excluding trials at high risk of bias in one or more domains.
Results
Description of studies
Results of the search
The electronic searches yielded a total of 446 references (Figure 1). The Trials Search Co‐ordinator scanned the search results, removed 58 duplicates and then removed 298 references that were not relevant to the scope of the review. We screened the remaining 90 reports and discarded 69 reports as not relevant. After assessing the reports, we identified a further two studies studies for potential inclusion in the review (Oh 1994; Uva 1996). In total, we obtained 23 full‐text reports for potential inclusion in the review. After consideration of each report, we included a total of 13 reports of 11 studies in the final review; see Characteristics of included studies and excluded seven studies; see Characteristics of excluded studies for reasons. We have categorised three studies as awaiting assessment, two of which we are unable to source copies of the reports and one is awaiting a response from the authors regarding information on methods of randomisation (Liu 2015).

Study flow diagram
Included studies
Design
We included a total of 11 studies in this Cochrane Review and summarised them in the Characteristics of included studies. All 11 studies were designed as a prospective RCTs. One study in this review was a multicentre study (Singh 2000); the rest were single‐centre.
Setting
Four studies were based in the United States (Katz 1995; Lamping 1995; Singh 2000; WuDunn 2002), two in Italy (Sisto 2007; Uva 1996) and the remainder in Ghana (Singh 1997), Japan (Kitazawa 1991), China (Xinyu 2001), Israel (Zadok 1995) and Iran (Mostafaei 2011). All research was carried out in clinical ophthalmic institutes.
Participants and sample sizes
In total, 687 eyes of 679 participants underwent routine trabeculectomy for glaucoma control. The smallest study was of 20 eyes of 20 participants (Zadok 1995), and the largest study included 115 eyes of 103 people (WuDunn 2002). Five studies included high‐risk cases only (Katz 1995; Kitazawa 1991; Lamping 1995; Singh 1997; Sisto 2007), one study enrolled both high‐ and low‐risk cases (Xinyu 2001), and the participants in the remaining five studies were low risk. Participants across the studies were a mixture of male and female; the percentage female ranged from 19% to 67%. The average age in the studies ranged from 47 years to 71 years, with a median average age of 62 years. One study had a significant age difference (P = 0.01), with a mean age of 41.2 in the MMC group and 54.2 in the 5‐FU group (Kitazawa 1991).
Interventions
We have summarised the interventions in Table 1.
| Study | MMC* | 5‐FU | ||||||
| Dose | Duration (minutes) | Location | Intraoperative or postoperative | Dose | Number of injections | Duration | Location | |
| 0.5 mg/ml | 5 | Between the conjunctiva and the episclera | Postoperative | 5 mg | 10 (daily for 1 week, 3 times following week) | NA (injection) | Subconjunctival injection | |
| 0.4 mg/ml | 5 | Between the conjunctival and scleral flap | Postoperative | 5 mg | 10 (each day for 1 week and every other day for the following week) | NA (injection) | Subconjunctival injections, 90 to 180 degrees away from the surgical site | |
| 0.4 mg/ml | 2.5 | Between the conjunctival and scleral flap | Postoperative | 5 mg | 10 (first 10 days) | NA (injection) | Subconjunctival injection, 180 degrees from operating site | |
| 0.02 mg | not stated | Subconjunctival injection, 180 degrees away from operating site | Intraoperative | 5 mg | NA | Not stated | Subconjunctival injection | |
| 0.5 mg/ml | 3.5 | Between scleral flap and conjunctiva | Intraoperative | 50 mg/ml | NA | 5 | Between scleral flap and conjunctiva | |
| 0.4 mg/ml | 2 | Not stated | Intraoperative | 50 mg/ml | NA | 5 | Not stated | |
| 0.2 mg/ml | 2 | Between the sclera and the Tenon's capsule | Postoperative | 0.1 ml of 50 mg/ml | 10 (starting on day 7, 2 injections per week for 2 weeks and then 1 injection per week for 6 weeks | NA (injection) | Subconjunctival injections near the bleb | |
| 0.2 mg/ml | 2 | Between the sclera and the Tenon's capsule | Intraoperative | 50 mg/ml | NA | 5 | Between the sclera and the Tenon's capsule | |
| 0.2 mg/ml | 2 | Not stated | Intraoperative | 50 mg/ml | NA | 5 | Not stated | |
| 0.2 mg/ml | 5 | Not stated | Postoperative | 5 mg | 6 to 8 (alternate days, starting on day 3) | NA (injection) | Subconjunctival, 180 degrees away from the site of scleral flap | |
| 0.2 mg/ml | 5 | Between the conjunctiva and episclera | Postoperative | 5 mg (0.5 ml of 10 mg/ml solution) | 7 (once daily up to 7 times in the first week after surgery) | NA (injection) | Subconjunctival, 180 degrees from site of surgery | |
NA: not applicable
* All MMC only one intraoperative application
The majority of trials applied MMC using an intraoperative sponge; the exception was Mostafaei 2011, where 0.02mg MMC was applied by intraoperative subconjunctival injection. Subconjunctival application of MMC is not consistent with current practice (Dhingra 2009). The MMC dose given by intraoperative sponge varied between studies:
-
Two studies used 0.5 mg/ml applied for 5 minutes, in Katz 1995, or 3.5 minutes, in Singh 1997.
-
Three studies used 0.4 mg/ml applied for 5 minutes (Kitazawa 1991), 2.5 minutes (Lamping 1995), or 2 minutes (Singh 2000).
-
Five studies used 0.2 mg/ml applied for 5 minutes, in Xinyu 2001 and Zadok 1995, or 2 minutes, in Sisto 2007, Uva 1996 and WuDunn 2002.
The method of administration of the 5‐FU varied between studies: four studies used an intraoperative sponge technique similar to that of MMC application (Singh 1997; Singh 2000; Uva 1996; WuDunn 2002), six trials used a series of postoperative subconjunctival injections (Katz 1995; Kitazawa 1991; Lamping 1995; Sisto 2007; Xinyu 2001; Zadok 1995), and one study used intraoperative subconjunctival 5‐FU 5 mg (Mostafaei 2011). All four studies with a group receiving intraoperative sponge‐applied 5‐FU used 50 mg/ml for 5 minutes, which is consistent with current practice (Dhingra 2009).
Different dosing regimens were used for the postoperative injections.
-
Four studies used 10 postoperative injections
-
daily for 1 week, 3 times the following week (Katz 1995);
-
each day for 1 week, every other day for the following week (Kitazawa 1991);
-
first 10 days (Lamping 1995);
-
starting on day 7, 2 injections per week for 2 weeks and then 1 injection per week for 6 weeks (Sisto 2007).
-
-
Two studies used approximately 7 postoperative injections
-
once daily up to 7 times in the first week after surgery (Zadok 1995);
-
6 to 8 (alternate days, starting on day 3) (Xinyu 2001)
-
Outcomes
All of the 11 included studies stated an optimal postoperative IOP to achieve in order to accept success: five studies used a level of below 21 mmHg as desirable (Kitazawa 1991; Singh 1997; Singh 2000; Sisto 2007; Zadok 1995), two studies used equal to or less than 21 mmHg (Lamping 1995; WuDunn 2002), two used equal to or less than 12 mmHg (Katz 1995; Uva 1996), one used less than 21.06 mmHg (Xinyu 2001), and one study used 6 to 22 mmHg (Mostafaei 2011). Each study group reported their findings either as a percentage success or mean IOP.
Excluded studies
We excluded seven studies from the review: Ashworth 2003; Dreyer 1995; Li 2001; Membrey 2000; Membrey 2001; Rodriguez‐Bermejo 1993; Oh 1994. For further details please see Characteristics of excluded studies.
Risk of bias in included studies

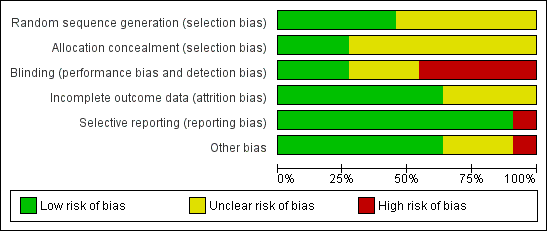
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
The review authors individually assessed the risk of bias. If the relative point was addressed in a study’s manuscript, then a true assessment of ‘high’ or ‘low’ risk was carried out. If we deemed the risk as unclear, then this indicated we could make no true assessment because the required information was not given either in the published manuscript or after making contact with the lead author.
Allocation
Five studies reported adequate methods to generate a random allocation sequence: Singh 1997 tossed a coin in the operating theatre to allocate participants; Uva 1996 used a table of random numbers; and the remaining three studies used computer‐generated allocation sequences (Singh 2000; Sisto 2007; WuDunn 2002).
It was unclear how the allocation schedule was generated in the remaining six studies.
Five studies reported adequate methods of allocation concealment (Kitazawa 1991; Singh 1997; Singh 2000; Uva 1996; WuDunn 2002)
Blinding
In four of the included studies, 5‐FU was administered using a different technique to that of MMC, and no report was given about whether or not the follow‐up information was gathered from masked assessors. We classified all these studies as high risk of performance and detection bias (Katz 1995; Kitazawa 1991; Lamping 1995; Xinyu 2001).
In one study, the method of 5‐FU administration was the same as for MMC, but information gathered in the follow‐up period was not from masked assessors. We therefore classified this study as high risk (Singh 1997).
Two studies used different techniques for antimetabolite administration but assessors were masked during the follow‐up period. We graded these two studies as low risk of performance and detection bias for the primary outcome of this review (Sisto 2007; Zadok 1995).
Only one study used a placebo to mask allocation, which we graded as at low risk of perfomance and detection bias (WuDunn 2002).
We graded the other three studies as unclear because the surgical administration of the antimetabolites was the same, but there was no mention of masking during follow‐up (Mostafaei 2011; Singh 2000; Uva 1996).
Incomplete outcome data
Four studies did not comment on the exclusion or inclusion of participants in their analysis (Kitazawa 1991; Mostafaei 2011; Singh 1997; Xinyu 2001); we classified these as unclear risk. We classified the other seven studies as low risk as participants were clearly identified as included or not. No studies raised any concern over their intention to include or exclude participants.
Selective reporting
Singh 1997 did not specify in the methods of the paper what outcomes they considered, thus we cannot be certain that all the intended outcomes were addressed; we highlighted this as high risk. All other studies commented on all stated outcomes.
Other potential sources of bias
The only other sources of bias identified were that of postoperative care with regard to what other care or medications participants received and the varied amount of 5‐FU a participant would receive with an incomplete postoperative regimen. This was highlighted in the study by Katz 1995. Other studies had no other clear identifiable bias.
Effects of interventions
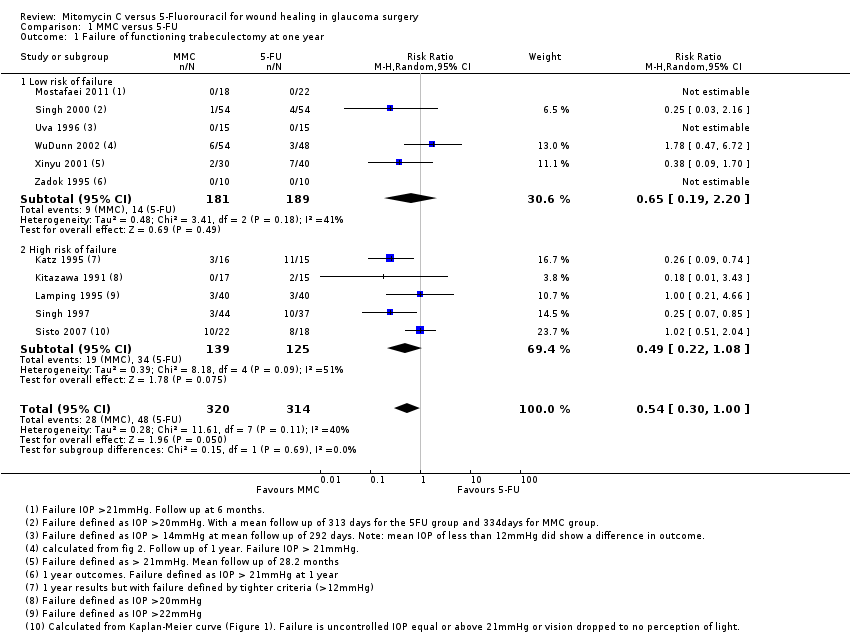
Failure of a functioning trabeculectomy at one year from surgery (primary outcome)
All 11 studies reported failure of a functioning trabeculectomy at approximately one year, which was defined as IOP above (approximately) 22 mmHg or more (Analysis 1.1).
The risk of failure of trabeculectomy at one year after surgery was lower in those treated with MMC compared to 5‐FU (risk ratio (RR) 0.54, 95% confidence interval (CI) 0.30 to 1.00; studies = 11; I2 = 40%). However, the confidence intervals of the studies were wide, and we cannot exclude important differences.
There was no evidence for any difference between groups at high and low risk of failure (test for subgroup differences P = 0.69), but with only a few trials in each group, the power of the analysis to detect any differences was low.
The dose of MMC varied across the studies included in the review, and consequently we performed a dose‐response analysis. We identified a trend showing that studies increasingly favoured the use of MMC rather than 5‐FU as the intraoperative exposure to MMC increased (Analysis 1.2). Overall exposure was calculated by multiplying the concentration of MMC by the duration of exposure for each study. We then listed the studies in descending order of MMC exposure to view the overall effect. We excluded one study that administered the MMC by subconjunctival injection from this analysis.
When considering the method of 5‐FU administration as in Analysis 1.3, there was no significant effect on the overall outcome whether the 5‐FU was administered by postoperative subconjunctival injections or by the more current method of intraoperative sponge application (subgroup difference P = 0.93)
Time to failure of functioning trabeculectomy
No trial reported this outcome.
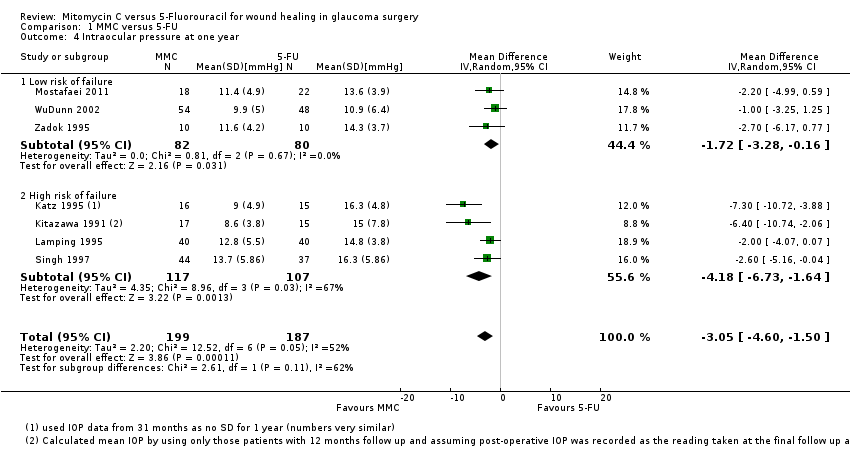
Mean IOP one year from surgery
Seven studies reported mean IOP at 12 months (range 6 to 18 months). On average, people treated with MMC had lower IOP at one year (mean difference (MD) ‐3.05 mmHg, 95% CI ‐4.60 to ‐1.50) Analysis 1.4. There was inconsistency between trials (I2 = 52%), the MD showing a large range in the studies.
The size of the effect was greater in the high‐risk group (MD ‐4.18 mmHg, 95% CI ‐6.73 to ‐1.64) compared to the low‐risk group (MD ‐1.72 mmHg, 95% CI ‐3.28 to ‐0.16), but the test for interaction was not statistically significant (P = 0.11).
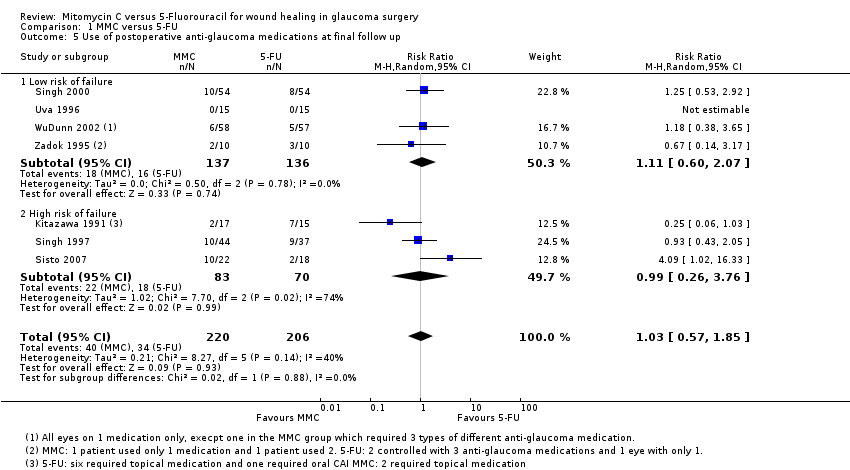
Postoperative use of antiglaucoma medications
Seven studies reported on the frequency of postoperative use of antiglaucoma medications. Similar proportions of people treated with MMC and 5‐FU required postoperative medication to control pressure (RR 1.03, 95% CI 0.57 to 1.85) (Analysis 1.5). There was no evidence for any difference in effect between high‐risk and low‐risk groups (P = 0.88). The low‐risk group trials were consistent (I2 = 0%), but we saw different results in the three higher‐risk group trials (I2 = 74%).
Four studies reported the mean number of antiglaucoma medications used. On average, people receiving MMC used fewer antiglaucoma medications (MD ‐0.33, 95% CI ‐0.70 to 0.05), but the effect was uncertain (CIs include 0.00), and the studies were inconsistent (I2 = 71%) (Analysis 1.6). The inconsistency in the trials came from those with a higher risk of failure (I2 = 62%). However, there was a difference between the trials including participants at high risk of failure and those including participants at low risk of failure with a greater relative effect of MMC in the higher‐risk groups (test for interaction P = 0.06). The main caveat was that there were only two trials in each group of the analysis.
Reduction in visual acuity
Five studies reported postoperative visual acuity. The proportion of eyes treated with MMC that lost 2 or more lines of visual acuity one year after surgery was similar to that of 5‐FU, but the CIs were wide (RR 1.05, 95% CI 0.54 to 2.06) Analysis 1.7.
Quality of life
No trial reported this outcome.
Economic data
No trial reported this outcome.
Adverse outcomes
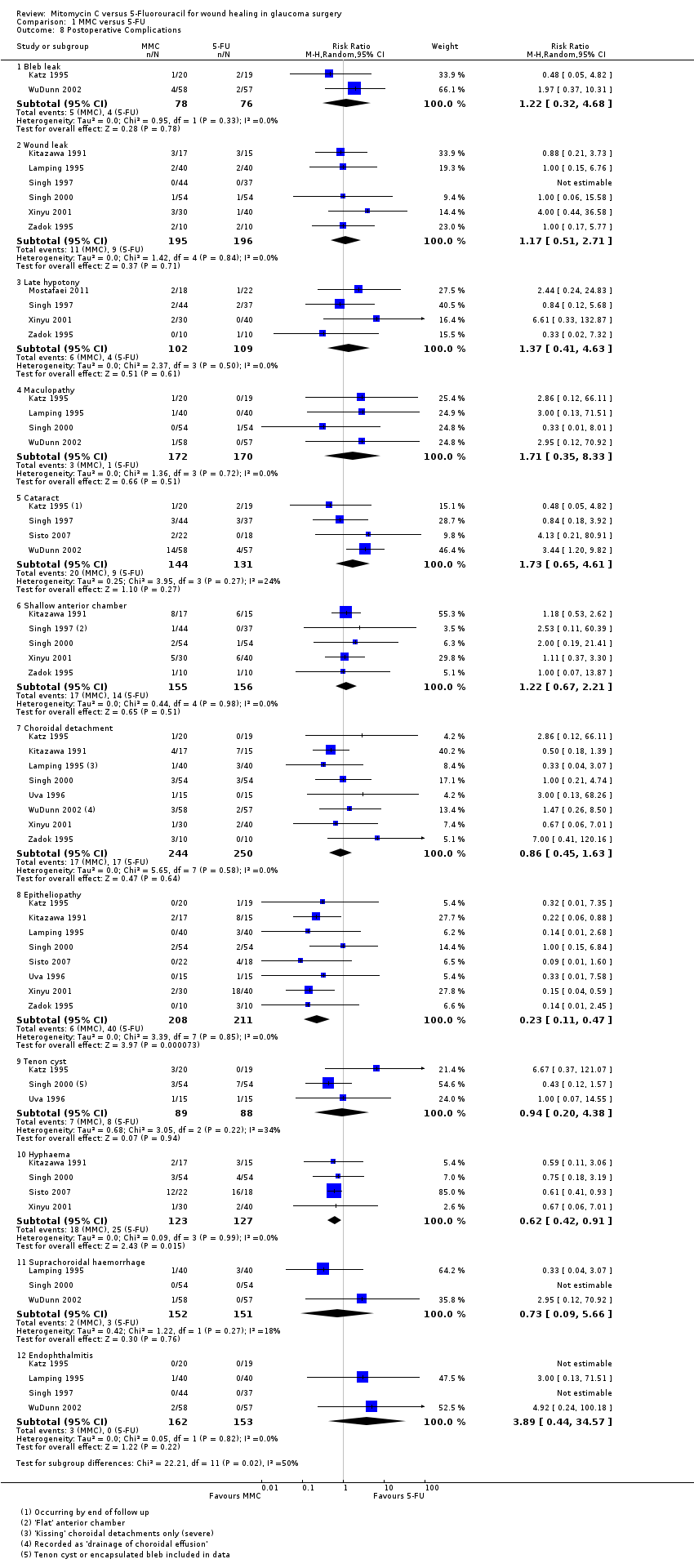
Bleb leak
Two studies reported bleb leak as a complication encountered following trabeculectomy. Participants receiving MMC were more likely to have a postoperative bleb leak, although the CI was wide, and only two studies reported this outcome (RR 1.22, 95% CI 0.32 to 4.68; I2 = 0%).
Six studies used the term 'wound leak' rather than 'bleb leak' in their assessment of postoperative complications. These studies also showed, with similar statistics, that participants receiving MMC were more likely to have a postoperative wound leak than those receiving 5‐FU, although the CI was wide (RR 1.17, 95% CI 0.51 to 2.71; I2 = 0%).
Late hypotony
Five studies reported hypotony post‐trabeculectomy. Participants receiving MMC were more likely to have postoperative hypotony compared to those participants who received 5‐FU, however the effect was uncertain with wide CIs compatible with no effect or increased hypotony in the 5‐FU group (RR 1.37, 95% CI 0.41 to 4.63; I2 = 0%).
Maculopathy
Four studies reported maculopathy following trabeculectomy. Participants receiving MMC were more likely to encounter maculopathy postoperatively than those receiving 5‐FU, but the effect was uncertain and CI compatible with no effect or increased maculopathy in the 5‐FU group (RR 1.71, 95% CI 0.35 to 8.33; I2 = 0%).
Cataract
Four studies reported the incidence of postoperative cataract development. Participants receiving MMC were more likely to develop cataract than those receiving 5‐FU, but again the CIs include 1 (null effect) (RR 1.73, 95% CI 0.65 to 4.61; I2 = 24%).
Shallow anterior chamber
Five studies noted postoperative shallowing of the anterior chamber. Those participants receiving MMC were more likely to present with a shallow anterior chamber than those who received 5‐FU. The statistical analysis showed a wide CI (RR 1.22, 95% CI 0.67 to 2.21; I2 = 0%).
Choroidal detachment
Nine studies (549 eyes) reported a choroidal detachment as a postoperative complication following trabeculectomy. Three studies (303 eyes) reported the same event as a 'suprachoroidal haemorrhage'. The former group of studies found no difference in the rate of events between those participants who received MMC and those who received 5‐FU (RR 0.86, 95% CI 0.45 to 1.63; I2 = 0%). The latter group of studies favoured those participants who received MMC, although the CI was wide (RR 0.73, 95% CI 0.09 to 5.66; I2 = 18%).
Epitheliopathy
Nine studies (474 eyes) reported this complication following trabeculectomy. Those participants who received MMC were less likely to have an epitheliopathy following surgery than those who received 5‐FU, which is most likely a result of the differences in the technique of antimetabolite application (RR 0.23, 95% CI 0.11 to 0.47; I2 = 0%).
Tenon's cyst
Four studies (232 eyes) reported Tenon's cysts in their postoperative complication analysis. Those participants who received MMC were less likely to have a Tenon's cyst following surgery, although the CI was wide (RR 0.94, 95% CI 0.20 to 4.38; I2 = 34%).
Hyphaema
Four studies (250 eyes) documented postoperative hyphaema during their follow‐up of participants. Participants who received MMC were less likely to have a postoperative hyphaema than those who received 5‐FU, which may be a consequence of antimetabolite application differences (RR 0.62, 95% CI 0.42 to 0.91; I2 = 0%).
Endophthalmitis
Four studies (315 eyes) published rates of postoperative endophthalmitis. Participants receiving MMC were more likely to have endophthalmitis following trabeculectomy than those who received 5‐FU. The CI was wide (RR 3.89, 95% CI 0.44 to 34.57; I2 = 0%).
Sensitivity analyses (excluding studies at high risk of bias)
An interesting feature of these analyses was that the trials at high risk of bias were also the trials recruiting participants at high risk of failure. In general, excluding these studies improved the consistency (reduced I2). Although the estimate of effect changed in these analyses, in general the conclusions (of uncertainty in most cases) did not.
| Outcome | Name | All trials | Excluding trials at high risk of bias in 1 or more domains |
| Failure of functioning trabeculectomy at 1 year | RR 0.54, 95% CI 0.30 to 1.00 | RR 1.02, 95% CI 0.50 to 2.04 | |
| Mean intraocular pressure at 1 year | MD ‐3.05 mmHg, 95% CI ‐4.60 to ‐1.50 | MD ‐1.72 mmHg, 95% CI ‐3.28 to ‐0.16 | |
| Use of postoperative medication at 1 year | RR 1.03, 95% CI 0.57 to 1.85 | RR 1.39, 95% CI 0.75 to 2.57 | |
| Mean number of postoperative medications at 1 year | MD ‐0.33, 95% CI ‐0.70 to 0.05 | MD ‐0.08, 95% CI ‐0.27 to 0.11 | |
| Loss of 2 or more lines of visual acuity at 1 year | RR 1.05, 95% CI 0.54 to 2.06 | RR 2.00, 95% CI 0.53 to 7.59 |
CI: confidence interval; MD: mean difference; RR: risk ratio
* The trials at high risk of bias were also the trials of the subgroup at high risk of failure.
** Two trials with a high risk of failure and one trial with a low risk of failure were excluded.
Discussion
Summary of main results
We have summarised the results in the summary of findings Table for the main comparison.
We identified 11 trials conducted in the United States, Europe, Asia and Africa. Five studies enrolled participants at low risk of trabeculectomy failure, five studies enrolled participants at high risk of failure, and one study enrolled people with both high and low risk of failure. None of the included trials enrolled participants with combined trabeculectomy/cataract surgery.
We considered one study to be at low risk of bias in all domains, six studies at high risk of bias in one or more domains, and the remaining four studies at an unclear risk of bias.
Our review showed that the risk of failure of trabeculectomy at one year after surgery was lower in those participants treated with MMC compared to those treated with 5‐FU. However, the estimate of effect was imprecise, and we cannot exclude important differences. All 11 RCTs contributed to this finding with an overall mixed study population and varied methodology of antimetabolite application. Although MMC appeared to have a greater success and more of an IOP‐lowering effect in the higher‐risk populations, we detected no significant difference between the subgroups of participants at low and high risk of failure in these analyses. We identified no difference between the visual outcomes of the people receiving MMC or 5‐FU at one year postoperatively nor in the number of drops used postoperatively.
Evaluation of postoperative complications showed that there was a higher incidence of epitheliopathy and hyphaema when using 5‐FU compared to MMC. However, we found those participants who received MMC to have more reported bleb leaks, wound leaks, late hypotony and cataract formation than those who received 5‐FU. The quality of the evidence was low given that in general adverse outcomes were rare, and hence estimates of effect were imprecise. Although there were trends, any real significance cannot be determined from this review alone.
None of the studies reported quality of life.
Overall completeness and applicability of evidence
This review is limited owing to the small numbers of and large variability between studies, for example in participant demographics, methodology, masking of participants and varied follow‐up. Some of the included studies had only 20 or 30 participants in their study population, which contrasts with the largest study, which had 115 participants.
After many years of widespread use of antimetabolite agents, uncertainty remains about the relative benefits and harms of their use in trabeculectomy surgery. Newer agents and techniques may be developed and evaluated to then eventually take over the role of antimetabolites.
The majority of studies were carried out in the United States, although we included studies from European, Asian, African and Middle Eastern countries. Two of the included papers, one in Chinese and one in Italian, were translated. The analysis has taken into account the risk of failure of each study population and reported the risk as high or low. This is important when interpreting the results in a clinical setting in order to reflect the practice population. However, results showed a similar trend between high‐ and low‐risk participants, which perhaps may be due to the inclusion of poor‐quality evidence as discussed.
Quality of the evidence
Overall, we graded the quality of the evidence as low, in most cases because of risk of bias in the included studies and imprecision in the estimate of effect. One commonly encountered bias came from the difficulty in masking participants and surgeons owing to the different techniques of antimetabolite administration. All studies included in the review were RCTs, but the variability in outcome reporting reduced the quality of the evidence for some outcomes. Each study group reported few complications, which subsequently led to small numbers being incorporated into the analysis of complications.
Potential biases in the review process
We identified no obvious bias from the review process.
Agreements and disagreements with other studies or reviews
Fendi 2013 completed a meta‐analysis that showed significantly higher success rates with the use of MMC when compared with 5‐FU. This analysis included only five studies with participants who had recieved previous surgical treatment. Lin 2012 found in an analysis of eight studies that MMC achieved a significantly lower postoperative IOP than 5‐FU, but MMC and 5‐FU were comparable in achieving success. Likewise, Abdu 2010 found similar results to both Lin 2012 and this review with little difference between the two antimetabolites at achieving success. Abdu 2010 also also found that there was no difference in the mean postoperative IOP between participants who received MMC and participants who received 5‐FU and suggest that further research in this area would enhance results to determine any true superiority of either MMC or 5‐FU.

Study flow diagram

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Comparison 1 MMC versus 5‐FU, Outcome 1 Failure of functioning trabeculectomy at one year.

Comparison 1 MMC versus 5‐FU, Outcome 2 Failure of functioning trabeculectomy at one year in descending order of MMC exposure (dose x duration).

Comparison 1 MMC versus 5‐FU, Outcome 3 Failure of functioning trabeculectomy at one year depending on 5‐FU administration technique.
Comparison 1 MMC versus 5‐FU, Outcome 4 Intraocular pressure at one year.
Comparison 1 MMC versus 5‐FU, Outcome 5 Use of postoperative anti‐glaucoma medications at final follow up.

Comparison 1 MMC versus 5‐FU, Outcome 6 Mean number of postoperative anti‐glaucoma medications.

Comparison 1 MMC versus 5‐FU, Outcome 7 Loss of 2 or more lines of Snellen visual acuity postoperatively.
Comparison 1 MMC versus 5‐FU, Outcome 8 Postoperative Complications.
| MMC compared to 5‐FU for wound healing in glaucoma surgery | ||||||
| Patient or population: wound healing in glaucoma surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants/eyes | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| 5‐FU | MMC | |||||
| Failure of functioning trabeculectomy at 1 year | Study population | Low‐risk population RR 0.65 (95% CI 0.19 to 2.20) High‐risk population RR 0.49 (95% CI 0.22 to 1.08) | 634 | ⊕⊕⊝⊝ | ||
| Low‐risk population: 74 per 1000 High‐risk population: 272 per 1000 | Low‐risk population: 50 per 1000 High‐risk population: 137 per 1000 | |||||
| Intraocular pressure at 1 year | The mean intraocular pressure at 1 year ranged across 5‐FU groups. Low‐risk population: 10.9 to 14.3 mmHg High‐risk population: 14.8 to 16.3 mmHg | The mean intraocular pressure at 1 year in the MMC groups had a range of values. Low‐risk population: 9.9 to 11.6 mmHg High‐risk population: 8.6 to 13.7 mmHg | ‐ | 386 | ⊕⊕⊝⊝ | |
| Loss of 2 or more lines of Snellen visual acuity at 1 year | Study population | Low‐risk population RR 2.00 (95% CI 0.53 to 7.59) High‐risk population RR 0.81 (95% CI 0.36 to 1.80) | 328 | ⊕⊕⊝⊝ | ||
| Low‐risk population: 47 per 1000 High‐risk population: 115 per 1000 | Low‐risk population: 94 per 1000 High‐risk population: 96 per 1000 | |||||
| Postoperative complications: late hypotony | Study population | RR 1.37 (95% CI 0.41 to 4.63) | 211 | ⊕⊕⊝⊝ | ||
| 37 per 1000 | 59 per 1000 | |||||
| Postoperative complications: choroidal detachment | Study population | RR 0.86 (95% CI 0.45 to 1.63) | 494 | ⊕⊕⊝⊝ | ||
| 68 per 1000 | 70 per 1000 | |||||
| Postoperative complications: endophthalmitis | Study population | RR 3.89 (95% CI 0.44 to 34.57) | 315 | ⊕⊕⊝⊝ | ||
| 0 per 1000 | 19 per 1000 | |||||
| Quality of life at 1 year | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded for risk of bias: only one study at low risk of bias in all domains 2Downgraded for imprecision: wide confidence intervals 3Downgraded for inconsistency: I2 = 60% 4Downgraded for risk of bias: no study at low risk of bias in all domains | ||||||
| Study | MMC* | 5‐FU | ||||||
| Dose | Duration (minutes) | Location | Intraoperative or postoperative | Dose | Number of injections | Duration | Location | |
| 0.5 mg/ml | 5 | Between the conjunctiva and the episclera | Postoperative | 5 mg | 10 (daily for 1 week, 3 times following week) | NA (injection) | Subconjunctival injection | |
| 0.4 mg/ml | 5 | Between the conjunctival and scleral flap | Postoperative | 5 mg | 10 (each day for 1 week and every other day for the following week) | NA (injection) | Subconjunctival injections, 90 to 180 degrees away from the surgical site | |
| 0.4 mg/ml | 2.5 | Between the conjunctival and scleral flap | Postoperative | 5 mg | 10 (first 10 days) | NA (injection) | Subconjunctival injection, 180 degrees from operating site | |
| 0.02 mg | not stated | Subconjunctival injection, 180 degrees away from operating site | Intraoperative | 5 mg | NA | Not stated | Subconjunctival injection | |
| 0.5 mg/ml | 3.5 | Between scleral flap and conjunctiva | Intraoperative | 50 mg/ml | NA | 5 | Between scleral flap and conjunctiva | |
| 0.4 mg/ml | 2 | Not stated | Intraoperative | 50 mg/ml | NA | 5 | Not stated | |
| 0.2 mg/ml | 2 | Between the sclera and the Tenon's capsule | Postoperative | 0.1 ml of 50 mg/ml | 10 (starting on day 7, 2 injections per week for 2 weeks and then 1 injection per week for 6 weeks | NA (injection) | Subconjunctival injections near the bleb | |
| 0.2 mg/ml | 2 | Between the sclera and the Tenon's capsule | Intraoperative | 50 mg/ml | NA | 5 | Between the sclera and the Tenon's capsule | |
| 0.2 mg/ml | 2 | Not stated | Intraoperative | 50 mg/ml | NA | 5 | Not stated | |
| 0.2 mg/ml | 5 | Not stated | Postoperative | 5 mg | 6 to 8 (alternate days, starting on day 3) | NA (injection) | Subconjunctival, 180 degrees away from the site of scleral flap | |
| 0.2 mg/ml | 5 | Between the conjunctiva and episclera | Postoperative | 5 mg (0.5 ml of 10 mg/ml solution) | 7 (once daily up to 7 times in the first week after surgery) | NA (injection) | Subconjunctival, 180 degrees from site of surgery | |
| NA: not applicable * All MMC only one intraoperative application | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure of functioning trabeculectomy at one year Show forest plot | 11 | 634 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.30, 1.00] |
| 1.1 Low risk of failure | 6 | 370 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.19, 2.20] |
| 1.2 High risk of failure | 5 | 264 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.22, 1.08] |
| 2 Failure of functioning trabeculectomy at one year in descending order of MMC exposure (dose x duration) Show forest plot | 10 | 594 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.30, 1.00] |
| 3 Failure of functioning trabeculectomy at one year depending on 5‐FU administration technique Show forest plot | 10 | 594 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.30, 1.00] |
| 3.1 5‐FU by postoperative injections | 6 | 273 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.27, 1.15] |
| 3.2 5‐FU by intraoperative sponge application | 4 | 321 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.13, 2.08] |
| 4 Intraocular pressure at one year Show forest plot | 7 | 386 | Mean Difference (IV, Random, 95% CI) | ‐3.05 [‐4.60, ‐1.50] |
| 4.1 Low risk of failure | 3 | 162 | Mean Difference (IV, Random, 95% CI) | ‐1.72 [‐3.28, ‐0.16] |
| 4.2 High risk of failure | 4 | 224 | Mean Difference (IV, Random, 95% CI) | ‐4.18 [‐6.73, ‐1.64] |
| 5 Use of postoperative anti‐glaucoma medications at final follow up Show forest plot | 7 | 426 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.57, 1.85] |
| 5.1 Low risk of failure | 4 | 273 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.60, 2.07] |
| 5.2 High risk of failure | 3 | 153 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.26, 3.76] |
| 6 Mean number of postoperative anti‐glaucoma medications Show forest plot | 4 | 342 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.70, 0.05] |
| 6.1 Low risk of failure | 2 | 223 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.27, 0.11] |
| 6.2 High risk of failure | 2 | 119 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐1.34, ‐0.09] |
| 7 Loss of 2 or more lines of Snellen visual acuity postoperatively Show forest plot | 5 | 328 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.54, 2.06] |
| 7.1 Low risk of failure | 2 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.53, 7.59] |
| 7.2 High risk of failure | 3 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.36, 1.80] |
| 8 Postoperative Complications Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Bleb leak | 2 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.32, 4.68] |
| 8.2 Wound leak | 6 | 391 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.51, 2.71] |
| 8.3 Late hypotony | 4 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.41, 4.63] |
| 8.4 Maculopathy | 4 | 342 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [0.35, 8.33] |
| 8.5 Cataract | 4 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [0.65, 4.61] |
| 8.6 Shallow anterior chamber | 5 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.67, 2.21] |
| 8.7 Choroidal detachment | 8 | 494 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.45, 1.63] |
| 8.8 Epitheliopathy | 8 | 419 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.11, 0.47] |
| 8.9 Tenon cyst | 3 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.20, 4.38] |
| 8.10 Hyphaema | 4 | 250 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.42, 0.91] |
| 8.11 Suprachoroidal haemorrhage | 3 | 303 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.09, 5.66] |
| 8.12 Endophthalmitis | 4 | 315 | Risk Ratio (M‐H, Random, 95% CI) | 3.89 [0.44, 34.57] |

