Quimioterapia o radioterapia en combinación con la cirugía para el carcinosarcoma ovárico
Resumen
Antecedentes
El carcinosarcoma ovárico, también conocido como tumor mülleriano mixto maligno, es un tumor ginecológico maligno poco frecuente que constituye cerca del 1% o menos de todos los cánceres de ovario. En más del 80% de los casos, hay diseminación intraabdominal extraovárica en el momento del diagnóstico. El tratamiento primario fue tradicionalmente la citorreducción quirúrgica seguida de la radioterapia y quimioterapia o la quimioterapia sola. Los regímenes han incluido el cisplatino solo, una combinación de doxorrubicina, ifosfamida, dacarbazina, ciclofosfamida, dacarbazina, ciclofosfamida, taxol, y otras combinaciones varias. La efectividad de estos diversos regímenes es diversa. Por lo tanto, es necesario aclarar si hay un tratamiento adyuvante o neoadyuvante óptimo después de la citorreducción quirúrgica para este tumor poco frecuente. Además, es importante abordar los temas sobre la calidad de vida (CdV) relacionados con el tratamiento, en particular la toxicidad, ya que el pronóstico general parece ser malo.
Objetivos
Evaluar la efectividad y la seguridad de diversas quimioterapias adyuvantes y neoadyuvantes, y las opciones de radioterapia o quimioterapia sola en combinación con la cirugía en el tratamiento del carcinosarcoma ovárico.
Métodos de búsqueda
Se hicieron búsquedas en el registro de ensayos del Grupo Cochrane de Cáncer Ginecológico (Cochrane Gynaecological Cancer Group), en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials, CENTRAL), en MEDLINE y EMBASE, hasta febrero de 2012. También se realizaron búsquedas en registros de ensayos clínicos, resúmenes de reuniones científicas, listas de referencias de artículos de revisión y se estableció contacto con expertos en el campo.
Criterios de selección
Se buscaron los ensayos controlados aleatorizados (ECA) que compararon la quimioterapia adyuvante o la neoadyuvante y la radioterapia, o la quimioterapia sola, en las pacientes con carcinosarcoma ovárico (sarcoma mülleriano mixto maligno de ovario). A falta de ECA, también se revisaron los estudios no aleatorizados (ENA) para la discusión.
Obtención y análisis de los datos
Dos autores de la revisión evaluaron de forma independiente si los estudios relevantes cumplían los criterios de inclusión. No se identificaron ensayos y, por lo tanto, no hubo datos para el análisis.
Resultados principales
La estrategia de búsqueda identificó 297 referencias únicas, las cuales fueron todas excluidas.
Conclusiones de los autores
No se halló evidencia para informar las decisiones acerca de la quimioterapia adyuvante y la neoadyuvante y los regímenes de radioterapia, o la quimioterapia sola, para las pacientes con carcinosarcoma ovárico . En condiciones ideales, se necesita un ECA multicéntrico o multinacional, o estudios no aleatorizados bien diseñados que usen el análisis multifactorial para ajustar los desequilibrios iniciales para comparar las modalidades de tratamiento y mejorar el conocimiento actual. Los estudios de investigación adicionales sobre las vías de señalización genéticas y moleculares quizá mejoren la comprensión de este subtipo tumoral.
PICO
Resumen en términos sencillos
Quimioterapia, radioterapia o ambas después de la cirugía para el tratamiento de un tumor de ovario poco frecuente
El carcinosarcoma ovárico (tumor mülleriano mixto maligno) es un tumor ginecológico maligno poco frecuente que comprende alrededor del 1% o menos de todos los cánceres de ovario. Estos tumores contienen tejido carcinomatoso (tejido epitelial que reviste las cavidades y superficies de las estructuras en todo el cuerpo) y tejido sarcomatoso (tejido conectivo) dentro de ellos. Este tumor presenta generalmente una fase avanzada y tiene una tasa de supervivencia baja a pesar del tratamiento. En general se trata con una combinación de cirugía y quimioterapia, y algunas veces radioterapia. Diversos tipos de agentes quimioterapéuticos se usaron para tratar a las mujeres antes y después de la cirugía (contextos adyuvantes y neoadyuvantes).
Actualmente, no existe evidencia para determinar si alguna forma de quimioterapia o radioterapia, o ambas, en combinación con la cirugía es mejor o peor para prolongar la supervivencia y mejorar la calidad de vida o la toxicidad. La revisión destaca la necesidad de estudios de buena calidad que comparen diversos regímenes de quimioterapia, antes o después de la cirugía, con o sin radioterapia. Se necesitan estudios de buena calidad multicéntricos, multinacionales y colaborativos para investigar esta enfermedad poco frecuente.
Authors' conclusions
Background
Description of the condition
Ovarian carcinosarcoma is a rare malignant gynaecological tumour comprising around 1% or less of all ovarian carcinomas (Russell 1992). Ovarian carcinosarcomas differ from epithelial ovarian tumours in that they contain both carcinomatous (arising from the epithelial tissue, which lines the cavities and surfaces of structures throughout the body) and sarcomatous (mesenchymal, arising from the connective tissue) tissue. The histology of the carcinomatous components is similar to conventional epithelial ovarian carcinoma (serous, endometroid etc.). The sarcomatous component can arise from or resemble the mesenchymal tissue of the ovary (homologous) or can be different to the mesenchymal tissue of the ovary and resemble that found in extra‐ovarian sites (heterologous). Special immunohistochemical staining (an investigative tool that provides supplemental information to the routine morphological assessment of tissues) helps in confirming the subtypes mentioned above (George 1991). There are various hypotheses which have been examined to explain the presence of carcinomatous and sarcomatous cell types within this tumour. The combination theory suggests that a common stem cell (cell which is capable of giving rise to all cell types) gives rise to both the epithelial and mesenchymal components (Guarino 1998; Jin 2003; Sonoda 2000); the conversion theory suggests an origin from a common epithelial clone and an epithelial‐to‐mesenchymal transformation‐based mechanism (otherwise called epithelial de‐differentiation) (Guarino 1998; Schipf 2008). In over 80% of cases, there is extra‐ovarian intra‐abdominal spread at diagnosis (Russell 1992). The diagnosis is confirmed by histology with the presence of malignant epithelial and mesenchymal components. The standard International Federation of Gynaecology and Obstetrics (FIGO) staging for ovarian epithelial tumour is applied to ovarian carcinosarcoma (Benedet 2000) and the prognosis is generally poor (Ariyoshi 2000; Hanjani 1983).
Description of the intervention
The primary treatment has traditionally been surgical cytoreduction (surgery attempting to remove as much of the tumour as possible) followed by radiotherapy and chemotherapy (Carlson 1983; Chang 1995) or chemotherapy alone (Bicher 1995). Treatment guidelines are extrapolated from epithelial ovarian carcinoma. Due to the rarity of this tumour, chemotherapy regimes have often changed over the course of time (Brown 2004). Optimal cytoreduction followed by adjuvant platinum‐based chemotherapy has been practiced based on case series and prospective trials (Morrow 1986; Sutton 1994; Tate Thigpen 2004). Combination platinum chemotherapy in combination with paclitaxel, doxorubicin, ifosfamide and other agents has been used with varying response rates (Duska 2002; Morrow 1986; Prendiville 1994; Simon 1991). The results of these various regimens appear to be mixed.
Why it is important to do this review
A review of published cases indicated a high one‐year mortality rate of 78% (Hanjani 1983) in this rare tumour irrespective of stage. A more recent publication suggested 40% survival at one year (Harris 2003). Advanced surgical stage did not affect the median overall survival (OS) in one study (Terada 1989), but was associated with a poor prognosis in a more recent study (Ariyoshi 2000). More recent studies have indicated that median survival could be improved with platinum‐based chemotherapy (Bicher 1995; Duska 2002). One of the largest reported data series of 47 cases over 10 years (Harris 2003) indicates that maintaining consistency in treatment plans is difficult and the tumour remains more aggressive than epithelial ovarian cancer (Barnholtz‐Sloan 2004). A case series which compared carcinosarcoma of the ovary with epithelial carcinoma of the ovary showed an inferior response with platinum‐based chemotherapy in the former group (Brown 2004). There is, therefore, a need to clarify if there is an optimum therapy after surgical cytoreduction or in the neoadjuvant setting for this rare tumour. Also, it is important to address quality of life (QoL) issues related to treatment, particularly toxicity related to treatment, as the overall prognosis appears to be poor.
Objectives
To assess the effectiveness and safety of chemotherapy (both adjuvant and neoadjuvant) regimens, with or without radiotherapy, in combination with surgery in the management of ovarian carcinosarcoma (malignant mixed Mullerian tumour).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Women of any age with a diagnosis of ovarian carcinosarcoma (malignant mixed Mullerian tumour of the ovary) at any FIGO stage.
Types of interventions
Interventions
We considered direct comparisons between any of the following interventions:
-
adjuvant chemotherapy with or without radiotherapy (surgery followed by chemotherapy with or without radiotherapy);
-
adjuvant radiotherapy and combination chemotherapy;
-
adjuvant single drug chemotherapy versus combination chemotherapy;
-
neoadjuvant chemotherapy and radiotherapy (chemotherapy with or without radiotherapy followed by surgery).
Additionally, we considered any of the above interventions in comparison with the following:
-
surgery alone.
Types of outcome measures
Primary outcomes
-
Overall survival (OS), survival until death from all causes (survival from the time when women were randomised)
-
Disease‐free survival (DFS), defined as time to recurrence
Secondary outcomes
-
Quality of life (QoL), measured using a scale that has been validated through reporting of norms in a peer‐reviewed publication.
-
Cost effectiveness.
-
Adverse events classified according to CTCAE 2006:
-
direct surgical morbidity, death within 30 days, haemorrhage (intraoperative and postoperative), intraoperative organ injury (bladder, ureter, bowel and vessels etc.), febrile morbidity and postoperative site specific infection (surgical site, pelvic, urinary, bowel), postoperative complications related to the urinary and gastrointestinal (GI) tract (obstruction, fistulae, incontinence), unexpected return to theatre, delayed discharge due to complication,
-
surgical related systemic morbidity, vascular and thromboembolic (thrombosis, embolism, coagulopathy), pulmonary (infection, atelectesis etc.), lymphatics (lymphaedema, lymphocele), metabolic (diabetic complications, renal failure etc.), cardiac events (cardiac ischaemia, failure), hepatobiliary, cerebrovascular accidents, sexual dysfunction, chronic pain and psychological morbidity,
-
radiotherapy toxicity,
-
chemotherapy toxicity.
-
Grades of toxicity relating to radiotherapy and chemotherapy were extracted and grouped as:
-
-
haematological (leucopenia, anaemia, thrombocytopenia, neutropenia, haemorrhage),
-
gastrointestinal (nausea, vomiting, anorexia, diarrhoea, liver, proctitis),
-
genitourinary (cystitis, incontinence),
-
skin (stomatitis, mucositis, alopecia, allergy),
-
neurological (peripheral and central),
-
pulmonary,
-
lymphatics (lymphocele, lymphedema),
-
psychosexual,
-
other.
-
Search methods for identification of studies
Papers in all languages were sought and translations carried out when necessary.
Electronic searches
The following electronic databases were searched:
-
Cochrane Gynaecological Cancer Review Group Trials Register,
-
Cochrane Central Register of Controlled Trials (CENTRAL) issue 2, 2012,
-
MEDLINE (to February 2012),
-
EMBASE (to February 2012).
The CENTRAL, MEDLINE and EMBASE search strategies based on terms related to the review topic are presented in Appendix 1, Appendix 2 and Appendix 3 respectively.
Databases were searched from 1950 until February 2012.
All relevant articles found were identified on PubMed and, using the 'related articles' feature, a further search was carried out for newly published articles.
Searching other resources
Unpublished and grey literature
Metaregister, Physicians Data Query, www.controlled‐trials.com/rct, www.clinicaltrials.gov, www.cancer.gov/clinicaltrials and Gynaecologic Oncologists of Canada (http://www.g‐o‐c.org) were searched for ongoing trials.
Handsearching
Reports of conferences were handsearched in the following sources:
-
Gynecologic Oncology (Annual Meeting of the American Society of Gynecologic Oncologists),
-
International Journal of Gynecological Cancer (Annual Meeting of the International Gynecologic Cancer Society),
-
British Journal of Cancer,
-
British Gynaecological Cancer Society (BGCS),
-
British Cancer Research Meeting,
-
Annual Meeting of European Society of Medical Oncology (ESMO),
-
Annual Meeting of the American Society of Clinical Oncology (ASCO),
-
BioMed (open text publisher),
-
American Association for Cancer Research (AACR) conferences,
-
European Society of Gynaecological Oncology (ESGO) conference.
We additionally searched the Journal of Ovarian Research: http://www.ovarianresearch.com/home/.
Data collection and analysis
Selection of studies
Two review authors (TS or RA and AB) downloaded all the titles and abstracts retrieved by electronic searching to the reference management database EndNote, duplicates were removed and the titles and abstracts of the remaining references were examined independently. There were a number of case reports and retrospective studies on interventions (mainly combination chemotherapy) for ovarian carcinosarcoma however none was of sufficient quality or appeared to use statistical adjustment to minimise selection bias, so we did not alter our inclusion criteria to accommodate non‐randomised studies (NRS) (see Agreements and disagreements with other studies or reviews for details of these studies). All were excluded at this stage as they clearly did not meet the inclusion criteria. We did not identify any ongoing RCTs which met our inclusion criteria from our searches of the grey literature. In future updates of the review, we will employ the methods found in the Differences between protocol and review.
Results
Description of studies
Results of the search
The search strategy identified 186 references in MEDLINE, 207 in EMBASE, 13 in CENTRAL and three in the Specialised Register. When the search results were merged into EndNote and duplicates were removed there were 297 unique references. The abstracts of these were read independently by two review authors and all were excluded (Figure 1).
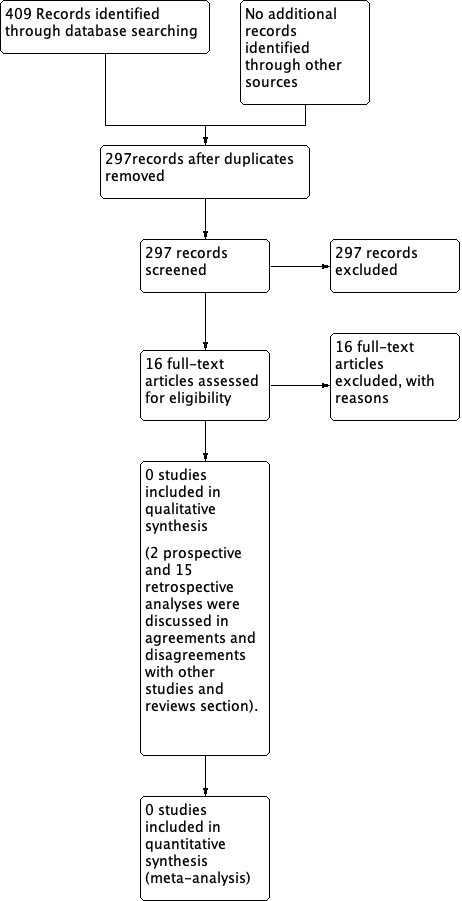
PRISMA 2009 flow diagram.
Two review authors (RA and AB) independently searched the grey literature; these searches also did not identify any relevant studies.
Risk of bias in included studies
No trials were found and therefore the risk of bias tool was not applied.
Effects of interventions
No data were available.
Discussion
Summary of main results
We did not identify any trials that compared the effectiveness and safety of neoadjuvant or adjuvant chemotherapy with or without radiotherapy in combination with surgery for women with ovarian carcinosarcoma. Therefore, there is currently no evidence to determine whether any form of chemotherapy, with or without radiotherapy, in combination with surgery is better or worse in terms of prolonging survival, QoL or toxicity.
We specified OS and DFS as the primary outcomes of interest. Quality of life should perhaps be the main focus if future trials are conducted since treatment related morbidity is likely to degrade the quality of the time that women live. This is especially important in women with ovarian carcinosarcoma where a number of retrospective studies and case reports have shown very poor survival rates (Andersen 1989; Brown 2004).
Quality of the evidence
No studies met the inclusion criteria for this review, so there is no evidence to assess.
Potential biases in the review process
Two review authors independently carried out a comprehensive search, including a thorough search of the grey literature, and all references were sifted. We were restrictive in our inclusion criteria with regards to types of studies as we planned to only include RCTs, as we suspected that some of the NRS designs were dubious and would have been prone to selection bias. No relevant NRSs appeared to use appropriate statistical adjustment or were of adequate quality. Therefore, we attempted to ensure that we did not overlook any relevant evidence by searching a wide range of sources and ensuring the review was not based on poor quality evidence by excluding case reports and poor quality retrospective studies. We felt it was better to highlight the need for RCTs, or at the very least good quality NRSs, rather than report the results of low quality studies that are very likely to be misleading.
The greatest threat to the validity of the review is likely to be publication bias, that is studies that did not find the treatment to have been effective may not have been published. We were unable to assess this possibility as we did not find any studies that met the inclusion criteria.
Agreements and disagreements with other studies or reviews
Two prospective Gynecologic Oncology Group (GOG) studies looked at the outcomes of women diagnosed with ovarian carcinosarcoma, but these studies included too few women in order to make reliable comparisons (Morrow 1986; Tate Thigpen 2004).
Morrow 1986 registered 15 women with a diagnosis of ovarian carcinosarcoma who were treated with combinations of surgery, chemotherapy and radiotherapy depending on the stage of the disease. A combination of vincristine, dactinomycin and cyclophosphamide was used in some women. Survival was better in women with early stage disease or with a smaller residual tumour volume.
Tate Thigpen 2004 studied the cytotoxic effect of platinum in women diagnosed with ovarian carcinosarcoma. The median progression‐free interval was 5.2 months and the median OS was 11.7 months (n = 130). Forty‐four women were evaluable for response and showed an overall response of 20%. Survival was favourable for women with non‐measurable disease and also in women who responded to platinum. Adverse effects were not uncommon. We agree that this is one of the largest prospective series of women with carcinosarcoma ovary studied so far. The recruitment took 20 years, which makes it difficult to compare various cytotoxic drugs along with platinum. Despite the platinum response being similar to uterine carcinosarcoma, it is still lower than the conventional epithelial ovarian tumours, which means that platinum alone as a cytotoxic drug may not be effective in improving survival in ovarian carcinosarcoma. This was confirmed by Brown 2004 who compared epithelial ovarian tumours with carcinosarcoma of the ovary in their retrospective case series and found an inferior response to platinum‐based chemotherapy in the carcinosarcoma group.
A recent case‐control study (Rauh‐Hain 2011) reviewed 50 cases of ovarian carcinosarcoma of the ovary, each case was matched to two women with serous ovarian epithelial carcinoma. Shorter time to recurrence, increased platinum resistance, poorer prognosis with suboptimal cytoreduction and poorer OS were noticed in women with ovarian carcinosarcoma compared to serous epithelial ovarian cancer. Jonson 2006 looked at 17 cases of ovarian carcinosarcoma and 87 women with uterine carcinosarcoma and found no difference in survival between the two groups.
The Chun KC, Cicen 2008 and Duska 2002 studies reported a retrospective analysis of 40, 26 and 28 women diagnosed with ovarian carcinosarcoma, respectively, and showed improved survival with optimal debulking and platinum‐based combination treatment. A platinum combination with taxanes was used in two studies (Chun KC; Duska 2002) and a combination of platinum and Ifosfamide was used in the Cicen 2008 study. Recruitment took 15 to 20 years with various chemotherapeutic agents being used over that period of time, which makes it difficult to compare various regimens. A retrospective series of 31 women (Rutledge 2006) showed improved survival with optimal debulking and the use of ifosfamide and cisplatin chemotherapy compared to a carboplatin and taxol combination. The median OS was 21 months for the whole group.
Signorelli 2009 reviewed 41 women with ovarian carcinosarcoma over a period of 11 years. The women underwent surgery and were given platinum‐based combination chemotherapy. The overall survival was 20 months. The response to the platinum‐based combination (anthracycline, alkylating agent) was good but was associated with high toxicity and the numbers in the two chemotherapeutic regimens were small. Prendiville 1994 reported a series of 20 women over a 10‐year period. The analysis showed a median survival of 14 months and suggested that platinum and cyclophosphamide may be useful therapy following surgery. Chang 1995 studied 37 women with ovarian carcinosarcoma and found stage to be an independent prognostic factor and single agent platinum to be effective to some extent. Sutton 1994, in their phase II trial, reviewed 32 women who were previously treated with platinum‐based chemotherapy for ovarian carcinosarcoma and were administered ifosfamide; they found ifosfamide to have activity.
Harris 2003 retrospectively reviewed 40 women diagnosed with ovarian carcinosarcoma. Eighty per cent presented with advanced stage disease. More than 50% had bulky disease, associated with worse prognosis, and the majority received platinum‐based chemotherapy following surgery. Overall, the one‐ and five‐year survival rates were 40% and 7.5% respectively, which is much lower than for serous epithelial ovarian carcinoma. Leiser 2007 reviewed 30 women with a diagnosis of ovarian carcinosarcoma. All the women had stage III or IV ovarian carcinosarcoma except one who had stage II disease. Fifty‐seven per cent had optimal cytoreduction and all women received platinum and taxane as first‐line chemotherapy. The three‐ and five‐year survival rates were 53% and 30% respectively. Even though there was a trend towards a better response rate with optimal cytoreduction, it did not affect OS. Muntz 1995 reviewed 27 women with a diagnosis of ovarian carcinosarcoma and found advanced stage to be a significant prognostic factor. Less than 50% of women underwent optimal cytoreduction. The majority received platinum‐based chemotherapy. Few women were treated with postoperative radiotherapy. There was a trend towards improved survival with optimal cytoreduction and platinum‐based chemotherapy but it was difficult to interpret because of the small numbers.
All the above mentioned studies consistently confirmed that the OS is poor in women with ovarian carcinosarcoma as compared to epithelial serous ovarian carcinoma. Most studies have confirmed that platinum has some activity in this tumour type. Other variables such as suboptimal cytoreduction, feasibility for cytoreduction and increased platinum resistance have been commented upon by some studies. A major improvement in survival has not been seen in this group of women despite extrapolating cytoreduction and platinum‐based combination therapy. All the above studies had inadequate or no comparison groups for various adjuvant regimens and most of the studies were retrospective case series, and hence inferences cannot be made scientifically. The difficulty in recruiting women for a prospective trial, which has limitations because of the rarity of the tumour, is obvious.

PRISMA 2009 flow diagram.

