Quimioterapia o radioterapia en combinación con la cirugía para el carcinosarcoma ovárico
Information
- DOI:
- https://doi.org/10.1002/14651858.CD006246.pub2Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 28 February 2013see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Gynaecological, Neuro-oncology and Orphan Cancer Group
- Copyright:
-
- Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
TS, RA and AB searched for relevant trials. TS, RA and AB determined the relevance of trials for the review. AB drafted methodological and statistical sections of the review as well as various sections of the discussion. TS drafted clinical sections of the review.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
Department of Health, UK.
NHS Cochrane Collaboration programme Grant Scheme CPG‐10/4001/12
Declarations of interest
None known
Acknowledgements
We thank Jo Morrison for clinical and editorial advice, Jane Hayes for designing the search strategy, and Gail Quinn and Clare Jess for their contribution to the editorial process. We thank Katherine Deane for providing methodological advice at the protocol stage of the review and being a co‐author on the protocol. We also thank Keith Godfrey for providing clinical advice and being a co‐author of the protocol.
Version history
| Published | Title | Stage | Authors | Version |
| 2013 Feb 28 | Chemotherapy and/or radiotherapy in combination with surgery for ovarian carcinosarcoma | Review | T S Shylasree, Andrew Bryant, Ram Athavale | |
| 2006 Oct 18 | Chemotherapy and/or radiotherapy after surgery for ovarian carcinosarcoma | Protocol | Ram Athavale, Keith Godfrey, Katherine Deane | |
Differences between protocol and review
We had initially decided to run the searches with an RCT filter but ultimately ran them without as the number of hits was low. We also decided not to include NRSs when no RCTs were identified due to the problem of selection bias and the fact that no studies appeared to use satisfactory statistical adjustment for important prognostic factors. We had initially stated the following in the protocol:
"It is likely that there are no RCTs of treatment for ovarian MMT. If no such trials are identified, the search strategy will be re‐run without the RCT filter so that any other papers containing prospective or retrospective data can be collated and listed in excluded studies. A systematic discussion of observational studies, case‐controlled studies and studies with historic controls will be considered if no RCTs are identified."
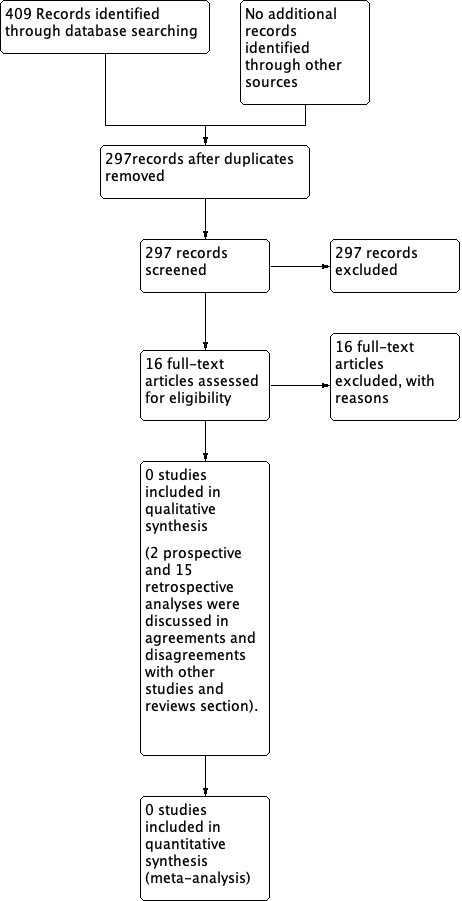
Results of NRSs are discussed in the Agreements and disagreements with other studies or reviews section.
Selection of studies
Copies of the full text of relevant references will be obtained. Two review authors (AB, TS) will assess the eligibility of retrieved papers. We will resolve disagreements by discussion. Reasons for exclusion will be documented.
Data extraction and management
For included trials, data will be abstracted as recommended in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We will extract data independently and will include:
-
author, year of publication and journal citation (including language);
-
country;
-
setting;
-
inclusion and exclusion criteria;
-
study design, methodology;
-
study population (participant characteristics, age, stage and postoperative residual disease);
-
number of participants in each arm of the trial;
-
total number of intervention groups;
-
ovarian carcinosarcoma details (FIGO stage, histology, tumour grade);
-
type of intervention (chemotherapy agents and radiotherapy, dosage and timing of administration relative to surgery);
-
length of follow‐up;
-
withdrawals from treatment protocol;
-
number of participants who experienced delays in treatment or received all, part or none of the proposed treatment;
-
risk of bias in study (see below);
-
outcomes OS, PFS, QoL and adverse events:
-
for each outcome, outcome definition,
-
unit of measurement (if relevant),
-
for scales, upper and lower limits, and whether high or low score is good,
-
results, number of participants allocated to each intervention group,
-
for each outcome of interest, sample size, missing participants.
-
Data on outcomes will be extracted as below
-
For time to event (e.g. overall survival) data, we will extract the log of the hazard ratio [log(HR)] and its standard error from trial reports; if these are not reported, we will attempt to estimate them from other reported statistics using the methods of Parmar 1998.
-
For dichotomous outcomes (e.g. adverse events, or deaths if it was not possible to use a HR), we will extract the number of women in each treatment arm who experienced the outcome of interest and the number of women assessed at endpoint in order to estimate a risk ratio (RR).
-
For continuous outcomes (e.g. QoL measures), we will extract the final value and standard deviation of the outcome of interest and the number of women assessed at the endpoint in each treatment arm at the end of follow‐up in order to estimate the mean difference (if trials measured outcomes on the same scale) or standardised mean differences (if trials measured outcomes on different scales) between treatment arms and its standard error.
Both unadjusted and adjusted statistics will be extracted, if reported.
Where possible, all data extracted will be relevant to an intention‐to‐treat analysis in which participants are analysed in the groups to which they were assigned.
The time points at which outcomes were collected and reported will be noted.
Two review authors will abstract data independently onto a data abstraction form specially designed for the review. Differences between review authors will be resolved by discussion.
Assessment of risk of bias in included studies
The risk of bias in included RCTs will be assessed using the Cochrane Collaboration's tool and the criteria specified in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This will include an assessment of:
-
sequence generation;
-
allocation concealment;
-
blinding (of participants, healthcare providers and outcome assessors);
-
incomplete outcome data, where we will code a satisfactory level of loss to follow‐up for each outcome as:
-
Yes, if fewer than 20% of women are lost to follow‐up and reasons for loss to follow‐up were similar in both treatment arms,
-
No, if more than 20% of women are lost to follow‐up or reasons for loss to follow‐up differed between treatment arms,
-
Unclear, if loss to follow‐up is not reported;
-
-
selective reporting of outcomes;
-
other possible sources of bias.
The risk of bias tool will be applied independently by two review authors (TS, AB) and differences will be resolved by discussion. Results will be presented in the risk of bias tables and also in both a risk of bias graph and a risk of bias summary plot. Results of the meta‐analyses will be interpreted in light of the findings with respect to risk of bias.
Measures of treatment effect
We will use the following measures of the effect of treatment.
-
For time to event data, we will use the HR.
-
For dichotomous outcomes, we will use the RR.
-
For continuous outcomes, we will use the mean difference between treatment arms (if trials measured outcomes on the same scale) or standardised mean differences (if trials measured outcomes on different scales).
Dealing with missing data
We will not impute missing outcome data for the primary outcome. If data are missing or only imputed data are reported, we will contact trial authors to request data on the outcomes only among participants who were assessed.
Assessment of heterogeneity
Heterogeneity between studies will be assessed by visual inspection of forest plots, by estimation of the percentage heterogeneity between trials which cannot be ascribed to sampling variation (Higgins 2003), by a formal statistical test of the significance of the heterogeneity (Deeks 2001) and, if possible, by subgroup analyses (see below). If there is evidence of substantial heterogeneity, the possible reasons for this will be investigated and reported.
Assessment of reporting biases
We will not assess the potential for small study effects such as the potential for publication bias because, given the rarity of ovarian carcinosarcoma, there will be an insufficient number of included studies in which to make this assessment. We did not identify any included trials from 1950 to October 2010 so future updates in the short term are unlikely to yield many trials in this area.
Data synthesis
If sufficient clinically similar studies are available, their results will be pooled in meta‐analyses. Adjusted summary statistics will be used, if available; otherwise unadjusted results will be used.
-
For time to event data, HRs will be pooled using the generic inverse variance facility of RevMan 5.
-
For dichotomous outcomes, the RR will be calculated for each study and these will then be pooled.
-
For continuous outcomes, the mean differences between the treatment arms at the end of follow‐up will be pooled if all trials measured the outcome on the same scale, otherwise standardised mean differences will be pooled.
Random‐effects models with inverse variance weighting will be used for all meta‐analyses (DerSimonian 1986).
Subgroup analysis and investigation of heterogeneity
Subgroup analyses will be performed, grouping the trials by:
-
disease‐free interval (DFI).
Factors such as age, stage, length of follow‐up, adjusted or unadjusted analysis will be considered in the interpretation of any heterogeneity.
Sensitivity analysis
Sensitivity analyses will be performed excluding trials which did not report: (i) concealment of allocation, and (ii) blinding of the outcome assessor.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Female; Humans;