戒烟后预防体重增加的干预措施
摘要
研究背景
大多数吸烟者在戒烟后体重增加。这可能会阻碍部分吸烟者尝试戒烟,并抵消戒烟带来的部分健康好处。预防体重增加的干预措施可以改善健康结局,但有人担忧该类干预措施可能会削弱戒烟效果。
研究目的
系统地评价以下干预措施的效果:(1)针对戒烟后体重增加的干预措施(称为“第一部分”),和(2)针对帮助戒烟且可能会影响戒烟后体重增加的干预措施(称为“第二部分”)。
检索策略
第1部分‐我们检索了Cochrane烟草成瘾小组专业注册库(Cochrane Tobacco Addiction Group's Specialized Register);最新检索日期为2020年10月16日。
第2部分‐我们检索了于2020年第10期Cochrane图书馆(the Cochrane Library)中发表,被纳入以下“总集”的Cochrane系统综述中的戒烟干预研究:尼古丁替代疗法(NRT)、抗抑郁药、尼古丁受体部分激动剂、电子烟和的运动干预。我们更新了关于尼古丁受体部分激动剂综述的注册库检索。
纳入排除标准
第1部分‐以戒烟后体重增加为目标,并在戒烟后6个月或更长时间的随访点或戒烟时测量了体重、或戒烟、或两者都测量的干预试验。
第2部分‐包括在选定的总集Cochrane系统综述中的在任何时间点报告体重变化的试验。
资料收集与分析
遵循标准Cochrane方法筛选和提取资料。体重变化指仅在试验组的戒烟人群中报告的从基准测量到随访期间体变化重差异。戒烟使用风险比(RR)表示。在适当的情况下,我们对体重逆方差方法,对吸烟使用Mantel‐Haenszel检验进行meta分析。
主要结果
第一部分:我们共纳入了37项已完成的研究;其中21项为此次新增。我们判断五项研究的偏倚风险较低,17项风险不明确,其余风险较高。
与如何避免体重增加的教育相比,包括免费提供的全餐替代和伴有强化营养师支持的间歇性极低热量饮食(VLCD)显著降低了治疗结束时的体重增加(平均差异(MD))=−3.70 kg,95%置信区间(CI)[−4.82, −2.58];1项研究,121名受试者),但没有证据表明在12个月时有效(MD)=−1.30 kg,95%CI [−3.49, 0.89];1项研究,62名受试者)。VLCD增加了12个月时的戒烟几率(RR=1.73,95%CI [1.10, 2.73];1项研究,287名受试者)。然而,第二项研究发现,没有人完成VLCD干预或实现戒烟。
旨在提高对体重增加接受度的干预措施在治疗结束、6个月和12个月时报告了参差不齐的效果,置信区间包括与没有建议或健康教育组相比的体重增加和减少。由于高度异质性,我们没有合并数据。这些干预措施增加了6个月时的戒烟率(RR=1.42,95%CI [1.03, 1.96];4项研究,619名受试者;I2=21%),但在12个月时没有证据(RR=1.25,95%CI [0.76, 2.06];2项研究,496名受试者;I2=26%)。
一些用于限制戒烟后体重增加(PCWG)的药物干预措施减少了治疗结束时的体重增加(右氟苯丙胺、苯丙醇胺、纳曲酮)。麻黄碱和咖啡因联合、氯酪蛋白和铬的效果太不精确,无法对治疗效果做出有效估计。有极低质量的证据表明,个性化体重管理支持减少治疗结束时的体重增加(MD)=−1.11kg,95%CI [−1.93, −0.29];3项研究,121名受试者;I2=0%),但在长期的12个月内没有证据(MD=−0.44kg,95%CI [−2.34, 1.46];4项研究,530名受试者;I2=41%)。有低到极低质量的证据表明,没有个性化评价、计划和反馈的详细体重管理教育并不能减少体重增加,并可能降低戒烟率(12个月:MD=−0.21kg,95%CI [−2.28, 1.86];2项研究,61名受试者;I2=0%;戒烟的RR=0.66,95%CI [0.48, 0.90];2项研究,522名受试者;I2=0%)。
第二部分:我们纳入了83项已完成的研究,其中27项是本次更新中的新研究。
与治疗结束时的标准护理相比,运动干预很少甚至不能导致体重减轻(MD=−0.25 kg,95%CI [−0.78, 0.29];4项研究,404名受试者;I2=0%)。然而,12个月时体重有所减轻(MD −2.07 kg,95%置信区间 −3.78至 −0.36;3项研究,182名受试者; I2 =0%)。
安非他酮和氟西汀都限制了治疗结束时的体重增加(安非他酮MD=−1.01 kg,95%CI [−1.35, −0.67];10项研究,1098名受试者;I2=3%);(氟西汀MD=−1.01kg,95%CI [−1.49, −0.53];2项研究,144名受试者;I2=38%;分别为低质量和极低质量证据)。没有证据表明安非他酮在12个月时有效,但估计值不精确(安非他酮MD=−0.26kg,95%CI [−1.31, 0.78];7项研究,471名受试者;I2=0%)。没有研究提供氟西汀12个月时的数据。
NRT在治疗结束时减少体重有中等质量证据(MD=−0.52kg,95%CI [−0.99, −0.05];21项研究,2784名受试者;I2=81%),在12个月时有中等质量的相似效果(MD=−0.37kg,95%CI [−0.86, 0.11];17项研究,1463名受试者;I2=0%),然而估计值太不精确,无法评价长期效益。
伐尼克兰对体重影响的证据参差不齐,高质量的证据表明治疗结束时体重变化非常轻微(MD=−0.23kg,95%CI [‐0.53, 0.06];1项研究,2566名受试者;I2=32%);低质量估计给出了12个月时较高体重的不精确估计(MD=1.05kg,95%CI [−0.58, 2.69];3项研究,237名受试者;I2=0%)。
作者结论
总体而言,没有任何干预措施对长期体重增加的临床有效性具有中等质量证据。也没有中等或高质量的证据表明,旨在限制体重增加的干预措施降低了人们戒烟的机会。
PICO
简语概要
戒烟后预防体重增加的干预措施
戒烟后避免体重增加的最佳方法是什么?
关键信息
我们不确定哪些干预方案或治疗最能帮助人们在戒烟后长期(最多12个月)避免体重增加,亦不确定它们如何影响成功戒烟。这是因为证据显示对体重增加的影响不一致且不清楚。进一步的研究应该继续关注如何限制戒烟者的体重增加。未来对帮助人们戒烟的新药的研究也应该测量戒烟者体重的变化。
戒烟和体重增加
如果您吸烟,为您的健康所能做的最好的事情就是戒烟。但是人们在戒烟后,通常会增加体重,且通常是在戒烟的最初几个月。体重增加可能会抵消戒烟带来的某些好处,并可能会影响部分吸烟者尝试戒烟的动力。
我们想要了解什么?
一些帮助人们戒烟的计划专门针对体重控制。其他帮助人们戒烟的方法也可能影响他们的体重;这些措施包括:运动干预、服药和使用尼古丁替代疗法(NRT)。
我们想找出戒烟时防止体重增加的最佳方案。
我们做了什么?
在此次已发表系统综述的更新中,我们检索了检测以下干预措施的研究:
‐戒烟时控制体重的具体方案;
‐其他帮助吸烟者戒烟的方案,方案内容同时包含了体重变化测量。
我们感兴趣的是:
‐6个月或12个月时有多少人戒烟;
‐受试者在治疗结束时、6个月后和12个月后的体重。
我们发现了什么?
我们共检索到116项研究:
其中37项关于戒烟者体重控制具体方案的研究(本次更新21项新研究);83项关于帮助人们戒烟的其他方法的研究(本次更新27项新研究)。其中四项研究包含以上两方面的研究内容。
针对特定方案的37项研究检测了对11,514名尝试戒烟的受试者使用包括饮食管理的行为干预方案以控制体重。部分行为干预方案是受试者自愿参与的,包括学习自我节制的技巧(例如,如何处理突如其来的渴望),以帮助他们维持减肥所需的行为。大多数研究(27项)在美国进行,其他研究在澳大利亚、加拿大、中国和欧洲进行。
其中83项关于其他戒烟方法的研究共纳入46248名受试者,评价内容包括:
运动干预;使用NRT;服用药物伐尼克兰(用于帮助人们戒烟);或者服用药物氟西汀(用于治疗抑郁症)。
在这些研究中,39项是在美国进行的,其余的则是在世界其他国家/地区进行的。 几乎没有关于研究副作用的相关报告。
本系统综述的主要结果是什么?
旨在预防体重增加的方案
与没有干预或仅提供简短建议相比,个性化体重管理计划可以减少戒烟治疗结束时、6个月后和12个月后的体重增加。然而,没有个性化评价、计划和反馈的体重管理计划可能不会减少体重增加,并可能减少戒烟人数。
与没有干预相比,自愿参与的体重管理计划:
可以帮助更多的人在6个月和12个月后戒烟;但可能对他们的体重增加几乎没有影响.
其他可能影响体重的方案和治疗
与不参与运动计划相比,参加一项有助于戒烟的运动计划可以减少12个月后的体重增加。
与不使用NRT相比,使用NRT可能会在12个月后略微减少体重增加。
服用伐尼克兰对治疗结束时的体重增加影响不大,并且对6个月或12个月后的体重可能几乎没有影响。
服用氟西汀可能会减少戒烟治疗结束时的体重增加,但它对6个月或12个月后体重增加的影响尚不清楚。
证据的局限性是什么?
我们确信使用伐尼克兰戒烟治疗结束时的体重增加没有差异,进一步的研究不太可能改变这一结果。然而, 我们对其他研究证据的信心是有限的,主要是因为可比较的研究数量较少,这研究纳入的受试者也较少。研究结果的差异很大,没有足够的研究让我们确定结果。如果有进一步的证据,我们对结论的信心可能会改变。
证据时效性如何?
证据检索截至2020年10月。
Authors' conclusions
Summary of findings
| Behavioural weight management interventions compared to brief advice or no intervention for post‐cessation weight control for preventing weight gain after smoking cessation | ||||||
| Patient or population: People wanting to quit smoking | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with advice or no intervention for post‐cessation weight control# | Risk with behavioural weight management interventions | |||||
| Mean weight change (kg) at end of treatment ‐ Weight management education versus no weight intervention | 1.45 kg | 1.41 kg (0.88 to 1.95) | MD 0.04 kg lower | 140 | ⊕⊕⊝⊝ | ‐ |
| Mean weight change (kg) at 6 months ‐ Weight management education verses no weight intervention | 0.97 kg | 1.86 kg (0.19 to 3.52) | MD 0.89 kg higher | 81 | ⊕⊕⊝⊝ | ‐ |
| Mean weight change (kg) at 12 months ‐ Weight management education versus no weight intervention | 0.46 kg | 0.25 kg (‐1.82 to 2.32) | MD 0.21 kg lower | 61 | ⊕⊝⊝⊝ | ‐ |
| Smoking cessation at 6 months ‐ Weight management education versus no intervention | 275 per 1000 | 280 per 1000 | RR 1.02 | 660 | ⊕⊕⊝⊝ | ‐ |
| Smoking cessation at 12 months ‐ Weight management education versus no intervention | 294 per 1000 | 194 per 1000 | RR 0.66 | 522 | ⊕⊕⊝⊝ | ‐ |
| Mean weight change (kg) at end of treatment ‐ Personalised weight management support versus no weight intervention | 1.45 kg | 0.34 kg (‐0.48 to 1.16) | MD 1.11 kg lower | 121 | ⊕⊕⊝⊝ | ‐ |
| Mean weight change (kg) at 6 months ‐ Personalised weight management support versus no weight intervention | 0.97 kg | 0.01 kg (‐1.21 to 1.22) | MD 0.96 kg lower | 816 | ⊕⊝⊝⊝ | ‐ |
| Mean weight change (kg) at 12 months ‐ Personalised weight management support versus no weight intervention | 0.46 kg | 0.02 kg (‐1.88 to 1.92) | MD 0.44 kg lower | 530 | ⊕⊝⊝⊝ | ‐ |
| Smoking cessation at 6 months ‐ Personalised weight management support versus no intervention | 188 per 1000 | 178 per 1000 | RR 0.95 | 5517 | ⊕⊕⊝⊝ | ‐ |
| Smoking cessation at 12 months ‐ Personalised weight management support versus no intervention | 475 per 1000 | 308 per 1000 | RR 0.65 | 3441 | ⊕⊕⊝⊝ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level due to risk of bias. All studies judged to be at unclear or high risk of bias. Results not sensitive to removal of studies at high risk of bias. hDowngraded one level due to imprecision. Wide CIs incorporate clinically meaningful difference in favour of control group, as well as no clinically significant difference. | ||||||
| Acceptance interventions for weight concern compared to no weight management intervention for preventing weight gain after smoking cessation | ||||||
| Patient or population: People wanting to quit smoking | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with no weight management intervention# | Risk with acceptance interventions for weight concern | |||||
| Mean weight change (kg) at end of treatment | see comment | see comment | see comment | 169 | ⊕⊝⊝⊝ | Substantial unexplained statistical heterogeneity precluded meta‐analysis. Of the 3 studies, 1 had wide CIs encompassing both benefit and harm, 1 suggested benefit with CIs excluding no difference, and 1 suggested harm with CIs excluding no difference. |
| Mean weight change (kg) at 6 months | 3.5 kg | 3.5 kg (1.97 to 5.03) | MD 0 kg | 106 | ⊕⊕⊝⊝ | ‐ |
| Mean weight change (kg) at 12 months | 4.39 kg | 3.69 kg (1.44 to 5.95) | MD 0.7 kg lower | 76 | ⊕⊝⊝⊝ | ‐ |
| Smoking cessation at 6 months | 218 per 1000 | 309 per 1000 | RR 1.42 | 619 | ⊕⊕⊝⊝ | ‐ |
| Smoking cessation at 12 months | 143 per 1000 | 179 per 1000 | RR 1.25 | 496 | ⊕⊝⊝⊝ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level due to risk of bias. All studies judged to be at unclear or high risk of bias. Results not sensitive to removal of studies at high risk of bias. | ||||||
| Exercise interventions for smoking cessation compared to no exercise intervention for preventing weight gain after smoking cessation | ||||||
| Patient or population: People who have quit smoking | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with no exercise intervention# | Risk with Exercise interventions for smoking cessation | |||||
| Mean weight change (kg) at end of treatment | 2.85 kg | 2.6 kg (2.07 to 3.14) | MD 0.25 kg lower | 404 | ⊕⊕⊝⊝ | ‐ |
| Mean weight change (kg) at 12 months | 4.67 kg | 2.6 kg (0.89 to 4.31) | MD 2.07 kg lower | 182 | ⊕⊕⊝⊝ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level due to imprecision. CIs encompass clinically significant benefit as well as no clinically significant difference. | ||||||
| Nicotine replacement therapy for smoking cessation compared to placebo for preventing weight gain after smoking cessation | ||||||
| Patient or population: People who have quit smoking | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with placebo# | Risk with nicotine replacement therapy for smoking cessation | |||||
| Mean weight change (kg) at end of treatment | 2.85 kg | 2.33 kg (1.86 to 2.8) | MD 0.52 kg lower | 2784 | ⊕⊕⊕⊝ | ‐ |
| Mean weight change (kg) at 6 months | 4.23 kg | 4.15 kg (3.72 to 4.58) | MD 0.08 kg lower | 1021 | ⊕⊕⊕⊝ | ‐ |
| Mean weight change (kg) at 12 months | 4.67 kg | 4.3 (3.81 to 4.67) | MD 0.37 kg lower | 1463 | ⊕⊕⊕⊝ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level due to risk of bias. All studies at high or unclear risk. Results not sensitive to removal of studies at high risk of bias. | ||||||
| Varenicline for smoking cessation compared to placebo for preventing weight gain after smoking cessation | ||||||
| Patient or population: People who have quit smoking | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with placebo for smoking cessation# | Risk with varenicline | |||||
| Mean weight change (kg) at end of treatment | 2.85 kg | 2.62 kg (2.32 to 2.91) | MD 0.23 kg lower | 2566 | ⊕⊕⊕⊕ | ‐ |
| Mean weight change (kg) at 6 months | 4.23 kg | 4.14 kg (3.14 to 5.13) | MD 0.09 kg lower | 384 | ⊕⊕⊝⊝ | ‐ |
| Mean weight change (kg) at 12 months | 4.67 kg | 5.72 kg (4.09 to 7.36) | MD 1.05 kg higher | 237 | ⊕⊕⊝⊝ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded two levels due to imprecision. CIs incorporate clinically significant benefit as well as clinically significant harm. | ||||||
| Fluoxetine compared to placebo for smoking cessation for preventing weight gain after smoking cessation | ||||||
| Patient or population: People who have quit smoking | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with placebo for smoking cessation# | Risk with all types of antidepressant | |||||
| Mean weight change (kg) at end of treatment | 2.85 kg | 1.84 kg (1.36 to 2.32) | MD 1.01 kg lower | 144 | ⊕⊕⊝⊝ | ‐ |
| Mean weight change (kg) at 6 months | 4.67 kg | 3.66 kg (0.29 to 7.04) | MD 1.01 kg lower | 124 | ⊕⊕⊝⊝ | no studies followed up participants at 12 months |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level due to risk of bias. One study at unclear risk, one at high risk. | ||||||
Background
Description of the condition
Although smoking cessation is associated with substantial health benefits, it is often accompanied by weight gain (Aubin, 2012; Audrain‐McGovern 2011). Increases in weight are most marked in the first months after quitting (Aubin, 2012), and longer‐term studies show further moderate increases in weight in quitters for a number of years compared with continuing smokers (2 Lycett 2011). Reports vary about the amount gained, but on average sustained quitters are likely to gain an additional two to five kilograms (kgs) in the first five years (Chiolero 2008; Klesges 1997; Tian 2015; Veldheer 2015; Williamson 1991), and up to seven kgs after eight years (2 Lycett 2011). It is important to note, however, that average weight gain masks a substantial variability at the individual level, with evidence suggesting some quitters may lose weight (Aubin, 2012; Bossé 1980), whereas around 10% to 15% gain 10 kgs or more (Aubin, 2012; Tian 2015; Williamson 1991). Factors that have been consistently associated with greater weight gain after smoking cessation include female sex, higher cigarettes smoked per day/nicotine dependency and higher body mass index (BMI) at quitting (Komiyama 2013; Scherr 2015; Veldheer 2015; Williamson 1991).
Description of the intervention
Interventions included in this review are discussed in two parts, described below.
Some smoking cessation interventions have been developed to promote smoking cessation and simultaneously control weight gain. They include behavioural interventions, such as exercise and energy restriction or healthy‐eating advice. Some pharmacological treatments with known or potential efficacy for reducing weight have also been tested. Interventions may combine both behavioural components and pharmacological treatments, or these may be tested individually. They are often delivered concurrently with smoking cessation support during the first few months of quitting, when the rate of weight gain is at its highest. The effects of these interventions on both smoking abstinence and weight are included inPart 1 of this review.
Several treatments for smoking cessation have been developed independently of concerns about weight gain, with the sole aim of assisting smoking cessation. Some of these, such as nicotine replacement therapy, e‐cigarettes, antidepressants, varenicline and exercise might plausibly influence weight gain as well as smoking cessation. The effects of these interventions on smoking cessation are evaluated in the relevant Cochrane Reviews, but the effects on weight gain are summarised only in the exercise intervention review (Ussher 2019). The effects of these medications on weight gain are included in Part 2 of this review.
How the intervention might work
Smoking (and quitting smoking) is likely to exert its effect on weight through the biological actions of nicotine and also through the influence of smoking on eating behaviours. Nicotine increases metabolic rate (Collins 1994; Dallosso 1984), and withdrawal of nicotine when people quit smoking results in a decrease in rate (Hofstetter 1986)
Nicotine may also be an appetite suppressant, and people may replace eating with smoking (Chiolero 2008). Particularly during the initial period of withdrawal from nicotine when quitting smoking, some people may eat more in order to deal with nicotine cravings/withdrawal, to satiate increases in appetite and replace the hand‐to‐mouth action of smoking (Audrain‐McGovern 2011; Ward 2001). Through these mechanisms, it is likely that an energy imbalance is created favouring weight gain.
Behavioural interventions that limit energy intake (i.e. dieting or calorie restriction) or increase energy expenditure (i.e. exercise) may serve to reduce any energy imbalance that occurs during quitting, and thus reduce the amount of weight gained (Cheskin 2005; Gritz 1988; Hall 1986; 1 Hall 1992; Hughes 1991).
Pharmacological treatments may limit weight gain through several mechanisms. Appetite suppressants may offset the increased appetite that accompanies cessation, limiting any increase in calorie consumption after quitting to satiate increased hunger. Pharmacotherapies that replace nicotine included in Part 2 (nicotine replacement therapies or e‐cigarettes) may reduce nicotine withdrawal effects on metabolism and appetite. Varenicline is a partial agonist of nicotinic receptors in the brain and so may also work through these mechanisms. Depressed mood is a recognised withdrawal symptom of nicotine, and antidepressants are known to affect bodyweight (Serretti 2010), although some increase and some decrease it.
The availability of interventions that limit weight gain might encourage smokers who are concerned about weight gain to try to stop smoking (Filozof 2004), and limiting weight gain may prevent people from returning to smoking to avoid an increase in weight. However, it is possible that some interventions that aim to limit energy intake might undermine the success of a quit attempt (1 Hall 1992). There is evidence that hunger and cigarette cravings are related, that hunger can undermine quit efforts (1 Hall 1992) and that hunger increases urges to smoke in current smokers (Cheskin 2005). There is evidence that early weight gain is associated with successful cessation (Gritz 1988; Hall 1986; Hughes 1991). It is important to investigate further the impact of these interventions on weight gain, and also to evaluate the effect on abstinence rates.
Why it is important to do this review
Among smokers there is a high prevalence of concerns about post‐cessation weight gain, and it has been cited as a primary reason for putting off quit attempts, especially in women (Clark 2004; Klesges 1989; Klesges 1992). Weight consciousness has been found to predict current smoking (Weekley 1992), and weight gain experienced during or after smoking cessation has been associated with relapse (Klesges 1988; Klesges 1989; Klesges 1992). However there is inconsistent evidence that fear of weight gain or actual weight gain after quitting does lead to relapse, with some studies finding associations (1 Copeland 2006; Clark 2006; Meyers 1997; Pomerleau 2001) and others no associations (Fidler 2009; Hutter 2006; Killen 1996; Mizes 1998); methodological differences make it hard to draw a conclusion.
Post‐cessation weight gain can have health consequences, although these do not outweigh the benefit of quitting smoking. In the shorter term, the incidence of diabetes is higher in people who quit smoking than in those who continue with it, an effect that appears to be explained by weight gain (Davey Smith 2005; Yeh 2010), although some evidence suggests in the longer term (more than 10 years) the risk is no higher in those who quit than in those who continued smoking (Luo 2013). Hypertension can also be mediated by post‐cessation weight gain (Gratziou 2009). Additionally, at least one study has suggested that cessation‐related weight gain (more than 5 kgs) may attenuate reductions in cancer risk (Kim 2019). The extent to which these associations are clinically meaningful is unclear. The temporarily increased risk of type 2 diabetes amongst smokers who quit smoking with associated weight gain, is not associated with increased cardiovascular and all‐cause mortality compared to non‐quitters (Hu 2018). Weight gain after smoking cessation has been found not to modify its protective effect on heart attack and stroke (Clair 2013; Kim 2018).
Given the uncertainty of the risks surrounding cessation‐related weight gain, and that concerns about weight gain are cited as a common reason for not attempting quitting, people who smoke, their healthcare providers, and policy‐makers are keen to understand the effects of interventions that could potentially minimize post‐cessation weight gain whilst not adversely affecting cessation. The 2012 version of this review found insufficient evidence to recommend any one type of intervention targeting post‐cessation weight gain. Since then, considerable further literature has been published and new smoking cessation treatments have come onto the market. This updated review considers the entirety of the evidence to date on possible ways to limit post‐cessation weight gain in people abstinent from smoking, and the impact of these interventions on smoking cessation as well as weight.
Objectives
To systematically review the effects of: (1) interventions targeting post‐cessation weight gain on weight change and smoking cessation (referred to as 'Part 1') and (2) interventions designed to aid smoking cessation that may also plausibly affect weight on post‐cessation weight change (referred to as 'Part 2').
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials.
Types of participants
Adults who smoke and are attempting to quit smoking, excluding pregnant women.
In Part 1, we examine smoking cessation in everyone enrolled. In Part 1 and Part 2, we examine the effect of interventions on weight gain in people who have successfully quit smoking only. This is for several reasons. Firstly, if we included those who were not abstinent, mean weight gain would be reduced. This is because people who attempt quitting but fail after a few days do not gain weight, as those who relapse to smoking often lose the weight they gained previously (2 Lycett 2011; O'Hara 1998). Thus the average weight gain of a mixed population of abstinent and non‐abstinent smokers would not reflect the weight gain of either. Secondly, this effect could bias results. If an intervention increased abstinence rates, it is very likely that it would appear to increase weight gain, regardless of whether it actually suppressed weight gain or had no effect. Thirdly, those who return to smoking tend not to attend clinics for follow‐up. Authors typically only report weight data in abstinent smokers, and imputing missing data on weight in those who have relapsed to smoking is problematic. We have little data on the weight trajectory of people who try and fail to achieve abstinence. It is likely that the weight will depend on time since relapse and that imputing data using last observation carried forward or baseline observation carried forward is likely to be misleading. For these reasons, we eschew the intention‐to‐treat approach which is typically used in the Tobacco Addiction Review Group's reviews. This issue has been discussed elsewhere (Parsons 2009b; Parsons 2011; Spring 2011a; Spring 2011b).
Types of interventions
Part 1 ‐ Interventions that are designed specifically to limit post‐cessation weight gain.
Part 2 ‐ Smoking cessation interventions that are not designed primarily to limit post‐cessation weight gain but which might plausibly influence it, i.e. antidepressants, exercise, nicotine replacement therapy (NRT), electronic cigarettes and varenicline, but excluding trials in which all arms receive the same medication, regardless of differences in dose and schedule.
Types of outcome measures
There are two primary outcome measures:
-
Smoking status at six and 12 months
-
Mean (SD) change in body weight (kgs) at end of treatment, six, and 12 months.
Both outcomes are fully examined for studies that fit the criteria for Part 1. For Part 2 studies, effects of these interventions on smoking are reported in the parent Cochrane Reviews and we only report the effects of interventions on weight change.
For Part 1 studies of pharmacotherapies, we also evaluate adverse events and serious adverse events. We do not evaluate adverse and serious adverse events for behavioural interventions, following standard Cochrane Tobacco Addiction Group guidance. Information on adverse and serious adverse events for Part 2 studies can be found in the Cochrane Reviews on the relevant interventions (see Search methods for identification of studies).
Search methods for identification of studies
Electronic searches
Part 1 ‐ For the most recent update, we searched the Cochrane Tobacco Addiction Group's Specialized Register (latest search 16 October 2020), using the following search terms in title, abstract or keywords: food, calorie restrict*, intake, diet*, body mass index (BMI), Quetelet, waist‐hip ratio (WHR), weight, body‐weight, weight‐changes. At the search date the specialized register included the results of searches of the Cochrane Central Register of Controlled trials (CENTRAL), issue 9, 2020; MEDLINE (including in‐process and Epub ahead of print, via OVID) to update 202000928; Embase (via OVID) to week 2020040; and PsycINFO (via OVID) to update 20200921, as well as online registers of controlled trials.
Searching other resources
Part 2 ‐ We searched the following Cochrane Reviews: Antidepressants for smoking cessation (last updated 2020; Howes 2020); Exercise interventions for smoking cessation (last updated 2019; Ussher 2019); Nicotine replacement therapy for smoking cessation (last updated 2018; Hartmann‐Boyce 2018); Different doses, durations and modes of delivery of nicotine replacement therapy for smoking cessation (last updated 2019; Lindson 2019); Electronic cigarettes for smoking cessation (last updated 2020; Hartmann‐Boyce 2020), and Nicotine receptor partial agonists for smoking cessation (last updated 2016; Cahill 2016). We searched the text of references listed as included studies. For reviews last updated prior to 2019, we also searched the Cochrane Tobacco Addiction Group's Specialized Register on 16 October 2020, using the search terms outlined in the individual reviews.
Data collection and analysis
Selection of studies
For the 2020 searches, two review authors (for this update from: JHB, AT, LK, LH, AH and MS) independently screened all titles and abstracts obtained from the search using a piloted screening checklist. Full‐text versions were then independently screened for inclusion. We also ran a 2016 search in which titles and abstracts of records identified were screened in singular by the group's Information Specialist (Lindsay Stead [LS]). Full‐text records were then screened by AF and LS. Any discrepancies about eligibility throughout this update were resolved by discussion or with a third review author. Reasons for exclusion of key studies that required in‐depth discussion are listed in Characteristics of excluded studies.
Data extraction and management
For this update, two review authors (from: JHB, AT, LK, LH, AH, MS, AF, PA, PH, LLJ, DL) independently undertook data extraction using a piloted form. Extractions were then compared and a final version agreed upon following discussion, or with referral to a third review author, when necessary. We extracted the following data for each study:
-
Study design
-
Study start and end date
-
Recruitment
-
Setting*
-
Country
-
Inclusion and exclusion criteria
-
Summary of study participant characteristics
-
Total number randomized
-
Number per arm
-
Total percentage female
-
Mean age, baseline BMI, baseline weight, cigarettes per day (cpd), Fagerström Test for Nicotine Dependence (FTND) score
-
-
Summary of intervention and comparative conditions
-
Definition of smoking abstinence and type of biochemical validation (if any)
-
How weight was measured
-
Number of participants who were abstinent at end of treatment (EOT), 6 months, 12 months and/or at longest follow‐up
-
Mean weight change (kg) from baseline in abstinent smokers
-
Abstinence rates (Part 1 studies only)
-
Adverse events (AEs) and serious adverse events (SAEs) (Part 1 studies only)
-
Risk of bias in domains specified below
-
Study funding statement
-
Author declarations of interest
-
Any other notes
*studies identified during the 2020 search only.
One review author then entered the data into Review Manager 5 software for analyses (JHB), and another checked them (AT).
Assessment of risk of bias in included studies
Two review authors (for this update from: JHB, AT, LK, LH, MS, AF, PA, PH, LLJ, DL) independently assessed the risks of bias for each included study, using the Cochrane risk of bias tool v1 (Higgins 2011). This approach uses a domain‐based evaluation that addresses different areas: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment (smoking and weight); incomplete outcome data; and other potential sources of bias.
Specific considerations about judgements for individual domains for this review are outlined below:
-
Blinding of participants and personnel: This domain was not evaluated for studies investigating behavioural interventions for smoking cessation where blinding was not possible; this is in accordance with standard guidance from the Cochrane Tobacco Addiction Group.
-
Blinding of outcome assessments (detection bias) were evaluated separately for smoking and weight outcomes, given different considerations for each outcome. Risk of detection bias ratings were based on assessments of both weight and smoking outcomes.
-
Following standard methods of the Cochrane Tobacco Addiction Review Group, we rated studies at high risk of attrition bias if loss to follow‐up was greater than 50% overall or if there was a difference in follow‐up rates of more than 20% between study arms.
We assigned a grade (low, high, or unclear) for risk of bias for each domain and resolved any disagreements by discussion or by consulting with a third review author. We judged studies to be at high risk of bias overall if they were rated at high risk in at least one domain, and at low risk of bias overall if they were judged to be at low risk across all domains evaluated. We judged the remaining studies to be at unclear risk of bias overall.
Measures of treatment effect
For Part 1, where possible we extracted smoking outcomes as continuous biochemically‐confirmed abstinence, but we accepted less strict definitions if confirmed continuous abstinence was not available. Abstinence rates and their corresponding risk ratio (95% CI) were reported at six and 12 months of follow‐up. We extracted adverse and serious adverse events for pharmacotherapy studies in Part 1 as the number of people experiencing an event, where these data were available. For studies in Part 2, we extracted data on weight change only.
We used the absolute mean (SD) difference in body weight (kgs) from baseline to follow‐up by trial arm as a summary statistic for the treatment effect on weight. We estimated mean weight change only in those abstinent from smoking.
In some studies in Part 1 and 2, more than one trial arm had been compared with a control arm. Where this was the case, we took one of two approaches: where it was inappropriate to combine arms, we analyzed results separately and report them as such. Where appropriate, to create one comparison intervention arm we combined outcome data. For smoking we added together the numerator and denominator from each arm. We calculated weight outcomes from more than one trial arm using the following formulas:
Meanc = ((Mean1*n1)+(Mean2*n2))/(n1+n2)
Standard deviation = √varc
√varc= (sumsqc ‐ (nc * (Meanc2)))/(nc‐1)
sumsqc= (((n1‐1)*(var1 + ((n1/n1‐1))*(mean12) + ((n2‐1)*(var2 + ((n2/n2‐1))*(mean22))
Key: Meanc= Combined mean; sumsq = sum of squares; var = variance
Unit of analysis issues
The cluster‐randomized trial included reported results adjusted for intra‐class correlation; we use these adjusted estimates in our analysis. All other trials were individually randomized and hence we did not encounter issues with unit of analysis.
Dealing with missing data
We checked that, for smoking abstinence estimates, participants lost to follow‐up were coded as continuing to smoke and therefore all randomized participants were included in the denominator; if not, we corrected abstinence rates for this. Where weight gain had been measured but not reported at all or in full, we contacted authors or sponsors for clarification. If insufficient data were available for meta‐analysis, we reported results narratively. As outlined below, where possible, we converted data for use in our meta‐analysis.
In some studies mean (SD) weight change by trial arm was not reported in full. When the standard deviations for the changes in body weight were not present, we used several methods to calculate them using standard formulas, depending on the information available. This was mainly derived from confidence intervals and standard errors. To calculate standard deviations of the changes in weight from their associated confidence intervals for studies with a large sample size, we used the following formula:
SD = (√(n) x (upper limit ‐ lower limit)) /standard error
For studies with 95% confidence intervals for difference in means we divided by 3.92 standard errors wide. If sample size was less than 60, the 3.92 standard error wide was replaced with numbers specific to both the t‐distribution and the group sample size minus 1.
To calculate standard deviation from standard error we used the following formula:
SD = SE x √(n)
When the absolute mean differences in body weight were not reported explicitly, we calculated them by subtracting the baseline mean weights from the post‐intervention mean weights for the intervention and control groups. We calculated SDs by using an estimated correlation coefficient of 0.99, which describes how similar the baseline and finishing weight were across participants. This was estimated in abstinent smokers from raw data that we have collected from a trial to prevent weight gain on smoking cessation (Parsons 2009a) and from any other included studies that report standard deviations for mean weight at baseline, final measurement, and changes in means. To estimate the correlation coefficient for the intervention and control groups from other studies reporting starting and finishing means with SDs, we used the following formula:
r = (SD (B)2 + SD (F)2 ‐ SD (C)2) / (2 X SD(B) X SD (F))
(where r = correlation coefficient, SD = standard deviation for the changes in means, B = baseline, F = final measurement, and C = change in mean weight measurement).
The imputed correlation coefficient was used to calculate the missing standard deviations for changes in means for the intervention and control groups by using the following formula:
SD (C) = √((SD (B)2 + SD (F)2) ‐ (2 X r X SD (B) X SD (F))
Assessment of heterogeneity
We used the I2 statistic to investigate statistical heterogeneity, given by the formula [(Q‐df)/Q] x 100%], where Q is the Chi2 statistic and df is its degrees of freedom.
Assessment of reporting biases
We created funnel plots to visually investigate possible publication bias for meta‐analyses with 10 or more studies.
Data synthesis
Smoking cessation outcome data are given based on the number of quitters in the treatment and control groups divided by the total number of participants receiving treatment. Adverse and serious adverse event data are given as the number of people experiencing an event divided by the total number of participants followed up at the relevant time point. Results for both are reported as risk ratios (RRs) with 95% confidence intervals (CIs). A risk ratio greater than 1.0 indicates that more people quit in the treatment group than in the control group, or that more people experienced an adverse event. We used the Mantel‐Haenszel random‐effects method for smoking cessation and adverse event outcomes where appropriate. Weight change outcome data are given as the difference in mean weight change between the intervention and control arms, and we combined estimates using the inverse variance method where appropriate.
Subgroup analysis and investigation of heterogeneity
Studies were subgrouped based on intervention type.
Sensitivity analysis
Following the standard approach of the Cochrane Tobacco Addiction Group, we conducted sensitivity analyses removing studies at high risk of bias.
Summary of findings and assessment of the certainty of the evidence
Following standard Cochrane methodology, we created summary of findings tables for the following comparisons, agreed prior to beginning this update, using GRADEpro GDT:
-
Weight management education versus no weight intervention
-
Personalized weight management support versus no weight intervention
-
Interventions to allay concerns about weight gain versus no weight intervention
-
Exercise interventions versus no exercise intervention for smoking cessation
-
Nicotine replacement therapy versus placebo for smoking cessation
-
Varenicline versus placebo for smoking cessation
-
Fluoxetine versus placebo for smoking cessation
We selected these comparisons a priori as being the most clinically relevant. In the summary of findings tables, we present data on our primary outcomes (cessation and weight change) for these main comparisons. Also following standard Cochrane methodology, we used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of the body of evidence for each outcome, and to draw conclusions about the certainty of evidence within the text of the review.
Results
Description of studies
Studies are described by part below. Part 1 represents studies of interventions specifically designed to address post‐cessation weight gain. Part 2 represents studies of smoking cessation interventions not specifically designed to address post‐cessation weight gain.
Results of the search
Part 1
For Part 1 of this update, our 2020 searches identified 379 non‐duplicate references (Figure 1). All references were then screened and 70 full‐text articles were retrieved. For this update, we identified 21 new included studies and 10 new ongoing studies (Characteristics of ongoing studies). In total, we now include 37 studies in Part 1, i.e. 21 new included studies and 16 included studies identified in the 2012 review.
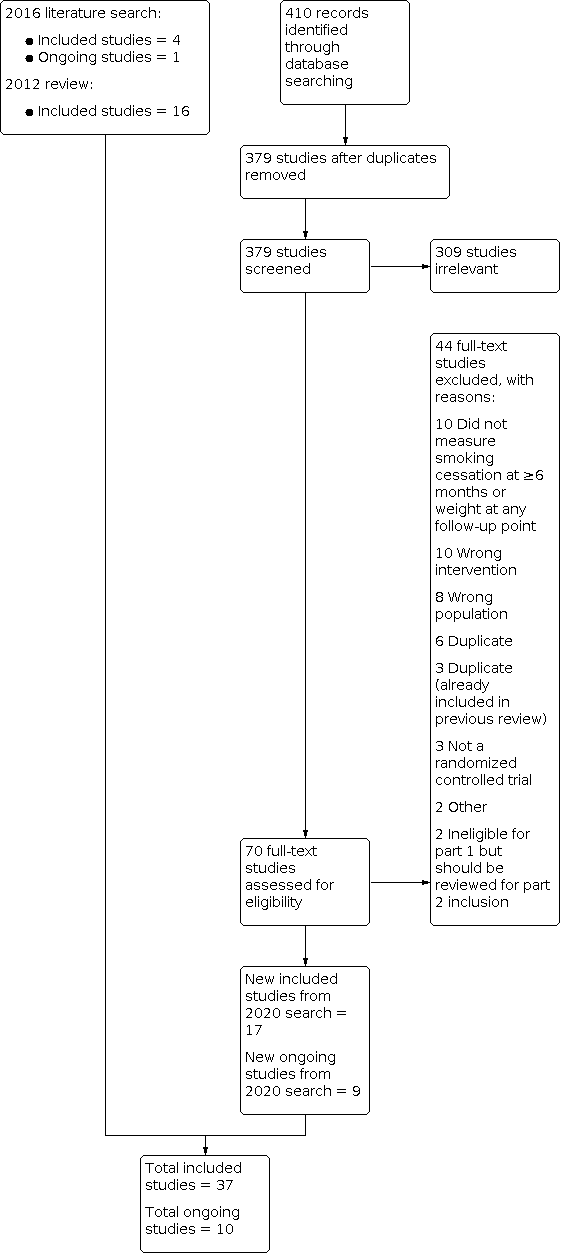
Study flow diagram for Part 1 2020 search plus studies from 2016 search and the 2012 review
Part 2
For Part 2 of this update, our 2020 searches identified 1099 non‐duplicate references (Figure 2). All references were then screened and 433 full‐text articles were retrieved. We identified 27 new included studies and 10 new ongoing studies for this update (Characteristics of ongoing studies). In total, we now include 83 studies in Part 2. Four trials are also included in Part 1 that contributed data to Part 2 (1 Cooper 2005 (also Part 2); 1 Levine 2010 (also Part 2); 1 Pirie 1992 (also Part 2); 1 Spring 1995 (also Part 2))

Study flow diagram for Part 2 2020 search plus studies from 2016 search and the 2012 review
Included studies
Main features of studies included in Part 1 and Part 2 are summarized below, and further details on each included study can be found in the Characteristics of included studies table.
Participants
Part 1
A total of 11,514 participants were enrolled in the 37 included studies. Three‐quarters of the studies (27 studies) were undertaken in the USA, three were conducted in England, and one each in Australia, Canada, China, Denmark, Norway, Scotland, and Sweden. A median age of 45.5 years was reported across 27 of the 37 included studies, and a median body mass index (BMI) of 28.5 kg/m2 was reported across the 20 studies which provided these data at baseline. The median percentage of women across 35 studies reporting it was 75.4%, with 14 studies recruiting only women (1 Bloom 2020; 1 Cooper 2005 (also Part 2); 1 Copeland 2006; 1 Copeland 2015; 1 Danielsson 1999; 1 Klesges 1990; 1 Levine 2010 (also Part 2); 1 NCT03528304 2016; 1 Oncken 2019; 1 Perkins 2001; 1 Pirie 1992 (also Part 2); 1 Prapavessis 2018; 1 Spring 1995 (also Part 2); 1 Spring 2004). Participants smoked a median of 20 cigarettes per day at baseline, as reported across 26 studies. A median of 5.2 was scored on the Fagerström Test for Nicotine Dependence across the 12 studies that reported it.
Part 2
A total of 46,248 participants were enrolled in the 83 studies included in Part 2 of this review. Nearly half of the studies (39 studies) were conducted in the USA, and a further 15 studies were conducted across multiple countries. Four studies each were conducted in Sweden and England, three each in Switzerland and France and two each in Australia and Denmark. A single study was conducted each in Canada, China, Finland, Greece, India, Iceland, New Zealand, Japan, South Africa, Spain and Turkey.
Of the 83 included studies, 64 reported age, median 43 years, and 14 studies reported baseline BMI, median 27.2 kg/m2.The median percentage of women was 55% as reported across 71 studies, with eight of these studies recruiting only women. Participants smoked a median of 22 cigarettes per day at baseline (across 57 studies) and a median of 5.6 was scored on the Fagerström Test for Nicotine Dependence (22 studies).
Interventions and comparators
Part 1
Behavioural interventions
Twenty‐three studies assessed the effects of a behavioural intervention to prevent weight gain after a smoking cessation attempt.
Three trials examined an intervention consisting of education on weight management (1 Hall 1992; 1 Hankey 2009; 1 Pirie 1992 (also Part 2)) against standard smoking cessation support.
Seven trials examined the effects of personalized weight management against usual smoking cessation care (1 Bush 2012; 1 Bush 2018; 1 Johnson 2017; 1 Lycett 2020; 1 Sobell 2017; 1 Spring 2004; 1 Hall 1992). One study tested the efficacy of a very low calorie diet (VLCD), meaning food replacements providing less than 800 kcal/day, where participants in the intervention and control groups both received the weight management education as well as usual smoking cessation support. Both groups were advised to follow a 1600 kcal diet, while the intervention group received two two‐week blocks of a VLCD provided free of charge. Treatment took place in a specialist obesity treatment centre (1 Danielsson 1999). One study compared 16 face‐to‐face one‐hour motivational interviewing and cognitive behaviour therapy (CBT) counselling sessions, given mostly by telephone (1 Baker 2018).
Four studies compared the use of CBT to promote acceptance of moderate weight gain to no behavioural weight advice. (1 Bloom 2020; 1 Levine 2010 (also Part 2); 1 Perkins 2001; 1 White 2019). One of these studies was a three‐arm RCT (1 Perkins 2001) and was also included in the aforementioned comparison on personalized weight management support versus no weight intervention.
Three studies new to this update directly compared behavioural weight management interventions. 1 Prapavessis 2018 tested an exercise maintenance condition with contact control to contact control alone, which included a behavioural weight management component, while 1 Heggen 2016 compared a low‐carbohydrate diet to a moderately reduced‐fat diet. 1 Copeland 2015 compared a minimally‐tailored group intervention which provided information on smoking and weight with a highly tailored, multidisciplinary individual approach, but could not be included in the statistical analysis as no measures of variance were reported alongside weight‐change data.
Five more studies could not be included in the statistical analyses. 1 Oncken 2019 tested the effect of 30 supervised exercise group sessions compared to relaxation group sessions. 1 Vander Weg 2016 compared a Quitline referral to a tailored tobacco intervention in which eligible participants who were worried about weight gain were offered support for weight management. Trial arms of interest in 1 NCT03528304 2016 compared a 16‐week culturally‐tailored contingency management intervention for smoking abstinence and weight loss to no intervention. 1 Lycett 2010 compared a VLCD to an individual dietary and activity‐planning intervention either begun at baseline or at eight weeks post‐quit. Finally, one study compared the effect of group to individual relapse‐prevention follow‐up sessions on smoking cessation and weight change after a two‐week smoking cessation programme (1 Copeland 2006). Results from this study are also reported narratively.
Pharmacological interventions
Fourteen studies compared the effects of pharmacological interventions to placebo on smoking cessation and post‐cessation weight change.
Pharmacological interventions included 8.33 mg phenylpropanolamine gum 16 pieces/day for 8 weeks (1 Cooper 2005 (also Part 2)), 9 pieces/day for 2 weeks (1 Klesges 1990) and up to 10 pieces/day for 4 weeks (1 Klesges 1995); 20 mg ephedrine plus 200 mg caffeine 3/day for 12 weeks (1 Norregaard 1996), 100, 50 and 25 mg/day naltrexone for 6 weeks (1 O'Malley 2006), 50 mg/day naltrexone for 12 weeks (1 King 2012), 25 mg/day naltrexone for 26 weeks (1 Toll 2010), 100 mg/day topiramate (up‐titrated over 5 weeks) for 10 weeks (1 Oncken 2014), 400 mg/day chromium polynicotinate for 14 weeks (1 Parsons 2009) and 30 mg/day dexfenfluramine for 12 weeks (1 Spring 1995 (also Part 2)). This study also examined the efficacy of 40 mg/day of fluoxetine for preventing weight gain (1 Spring 1995 (also Part 2)). As the other fluoxetine studies were included in Part 2 of the reviews, this comparison is described in Part 2.
1 Rose 2019 provided two weeks of lorcaserin or placebo during the two‐week pre‐quit period, followed by an identical treatment regimen of lorcaserin plus a nicotine patch for 12 weeks post‐quit date. Lorcaserin was also tested at 10 mg once or twice daily for 12 weeks in both 1 Shanahan 2017 and 1 Wilcox 2016, but data on the latter study were limited due to extraction from a conference abstract. Finally, 1 Lyu 2018 investigated a combination of adjunctive naltrexone (25 mg/day) and bupropion (300 mg/day) treatment for 24 weeks.
Part 2
Of the 83 included studies, 71 provided sufficient data to include in the meta‐analysis. The outstanding studies were new to this update and measured weight at eligible time points, but data were not provided in a form that we could meta‐analyze.
Of the 71 studies, 34 tested interventions with NRT, 19 with antidepressants, 17 with varenicline, four with exercise and two with electronic cigarettes. Five of these studies included multiple intervention arms or a combination of these interventions.
Nicotine replacement therapy was delivered in various forms, with most studies using a nicotine patch, while other studies delivered nicotine in the form of gum, lozenges, sublingual tablets, inhalers and intranasal spray. Some studies tested patches with varying nicotine dosing regimens, which were assessed separately.
Nineteen studies on antidepressants were included in this review, three of which compared bupropion to varenicline as well as placebo (2 Gonzales 2006; 2 Jorenby 2006; 2 Nides 2006). Overall, 14 studies compared weight change in participants treated with bupropion to placebo (2 Gonzales 2006; 2 Hurt 1997; 2 Jorenby 2006; 2 Nides 2006;2 Piper 2007; 2 Rigotti 2006; 2 Simon 2004; 2 Simon 2009; 2 Uyar 2007; 2 Zellweger 2005; 2 Cox 2012; 2 Eisenberg 2013; 2 Piper 2009; 1 Levine 2010 (also Part 2)). One of these studies (2 Simon 2004) provided a nicotine patch to both the bupropion and placebo study arms, and two additional studies compared varenicline and bupropion to varenicline and placebo (2 Rose 2014; 2 Ebbert 2014). A further two studies compared fluoxetine to placebo (2 Niaura 2002; 2 Saules 2004). 2 Saules 2004 tested fluoxetine versus placebo; both intervention and control arms used NRT, but we included it in the analyses with other fluoxetine‐versus‐placebo studies. One other study examined the efficacy of fluoxetine versus placebo (1 Spring 1995 (also Part 2)). It was not included in the parent Cochrane Review because smoking cessation at six months was not reported, but was identified and included here. All bupropion studies administered 300 mg/day and 2 Hurt 1997 also included a 100 mg/day and 150 mg/day arm. For the main comparison, we use the 300 mg/day arm for 2 Hurt 1997 and we use the lower‐dose arms to compare to the standard 300 mg/day treatment to the lower‐dose arms. Two fluoxetine studies compared two dosing levels (30 mg and 60 mg/day (2 Niaura 2002) and 20 mg and 40 mg/day (2 Saules 2004)) which we combined for the main comparison, while the lower doses and higher doses were compared in a separate comparison to examine for a dose‐dependent effect. One other study examined 40 mg fluoxetine versus placebo (1 Spring 1995 (also Part 2)).
Fourteen studies included in this review compared varenicline to placebo, with some studies including additional intervention components (e.g. patch) which were balanced between study arms. Once again, some studies compared different dosing regimens which were assessed in additional analyses. One study compared 2 mg/daily varenicline to a 21 mg patch tapering to 7 mg (2 Aubin 2008). As mentioned above, 2 Gonzales 2006; 2 Jorenby 2006; 2 Nides 2006 also compared varenicline with bupropion, while 2 Ioakeimidis 2018 compared varenicline with electronic cigarette (12 mg/ml nicotine) use for 12 weeks.
We found no new studies on exercise interventions for smoking cessation for inclusion in this update. In the four original studies, all participants in the treatment arm received an exercise component in parallel with cognitive behavioural treatment for smoking cessation, which was supplemented with nicotine replacement therapy in 2 Ussher 2003 and 2 Bize 2010. The exercise component included supervised exercise in three studies. 2 Marcus 1999 tested three supervised exercise sessions/week for 12 weeks, 30 to 40 minutes resting heart rate plus 60% to 85% heart reserve; 2 Marcus 2005 tested one supervised, four unsupervised exercise sessions/week for eight weeks, at least 30 minutes at resting heart rate plus 45% to 59% heart reserve; and 2 Bize 2010 tested moderate‐intensity (40% to 60% of maximal aerobic power) group‐based cardiovascular (CV) activity under the supervision of a trained monitor for 45 minutes weekly for nine weeks. In contrast, 2 Ussher 2003 compared the effect of seven weeks of exercise counselling to participants receiving a smoking cessation intervention with brief health education.
Finally, two new studies investigated the use of electronic cigarettes. 2 Walker 2020 was a three‐arm RCT comparing a nicotine patch versus nicotine‐containing electronic cigarette plus patch versus nicotine‐free electronic cigarette plus patch. 2 Ioakeimidis 2018, mentioned above,compared varenicline with electronic cigarette (12 mg/ml nicotine) use for 12 weeks.
Further information on dose or length or both of all interventions outlined above are available in the Characteristics of included studies table. Weight change from baseline in all of the studies included in the second part of the review was measured in abstainers only. Definition of abstinence varied between studies as in Part 1, and is also noted in the Characteristics of included studies table. Data for some time points were received following requests made to study authors, which is also noted in the Characteristics of included studies table.
Outcomes
Part 1
Of the 37 included studies:
-
28 reported data on weight change at end of treatment (EOT), six months, 12 months and/or at the longest follow‐up time point (data for three studies described narratively)
-
25 reported data on abstinence at EOT, six months, 12 months and/or at the longest follow‐up time point (data for one study are described narratively)
-
10 pharmacotherapy trials reported adverse or serious adverse outcomes, or both, six of which are described narratively.
Part 2
Of the 83 included studies:
-
71 reported data on weight change at EOT, six months or 12 months in sufficient detail to be included in the meta‐analysis.
Excluded studies
We list 200 studies excluded at full‐text stage along with reasons in Characteristics of excluded studies. The most common reasons for exclusion in the 2020 search were not measuring any of our outcomes (10 studies) or testing ineligible interventions (10 studies).
Risk of bias in included studies
Part 1: Overall, of the 37 included studies, we judged five studies to be at low risk of bias, 17 to be at unclear risk and the remaining 15 studies at high risk.
Part 2: Of the 83 studies not designed to address post‐cessation weight gain included in Part 2, we judged overall risk of bias to be low for 13 studies, unclear for 46 studies, and high for 24 studies.
Details of risk of bias judgements for each domain of each included study can be found in the Characteristics of included studies table and are illustrated in Figure 3.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
For Part 1. we judged 10 studies to be at low risk of selection bias, and 27 studies to be at unclear risk of bias mainly due to limited information on allocation concealment.
A similar risk of selection bias for all studies included in Part 2. We judged 27 studies to be at low risk, 55 studies to be at unclear risk of bias, and one study at high risk of bias (2 Jodar‐Sanchez 2018). Most studies were again rated at unclear risk of bias due to insufficient information about allocation concealment.
Blinding
Of the 37 studies included in Part 1, we judged 11 out of 14 pharmacological studies to be at low risk of both performance and detection bias and the remaining three studies at unclear risk of bias. We judged 19 of the 23 behavioural studies to be at low risk of detection bias, one at unclear risk and three at high risk of detection bias; performance bias was not assessed in included studies on behavioural interventions.
Of the 83 studies included in Part 2, 74 tested a pharmacological intervention. Of these 74 studies, 29 were judged to be at low risk of both performance and detection bias, 34 at unclear risk and 11 at high risk. Many of these studies were rated at unclear or high risk of bias due to a lack of blinding when blinding was possible or due to insufficient information about blinding, mainly by not specifying who within the study were blinded. As with Part 1, performance bias was not assessed in the remaining nine behavioural studies included in Part 2. Of these nine studies, eight were judged to be at low risk of detection bias, while one study was rated at unclear risk because it did not specify how weight was measured.
Incomplete outcome data
We rated 16 out of the 37 studies included in Part 1 at low risk of attrition bias. We rated 14 studies with greater than 50% loss to follow‐up overall or a follow‐up difference of more than 20% between study arms, or both, at high risk of attrition bias. The remaining seven studies were judged to at unclear risk.
Similarly, of the 83 studies included in Part 2, most (49 studies) were judged to be at low risk of attrition bias. Twenty‐one studies were rated at unclear risk and 13 studies at high risk.
Other potential sources of bias
No studies in Part 1 were judged to be at unclear or high risk of other bias.
2 Bernard 2015 from Part 2 was rated at unclear risk of other bias as participants in the control group may have participated in exercise (contamination effect).
Effects of interventions
See: Summary of findings 1 Behavioural weight management interventions compared to brief advice or no intervention for post‐cessation weight control; Summary of findings 2 Acceptance interventions for weight concern compared to no weight management intervention for post‐cessation weight control; Summary of findings 3 Exercise interventions for smoking cessation compared to no exercise intervention for preventing weight gain after smoking cessation; Summary of findings 4 Nicotine replacement therapy for smoking cessation compared to placebo for preventing weight gain after smoking cessation; Summary of findings 5 Varenicline for smoking cessation compared to placebo for preventing weight gain after smoking cessation; Summary of findings 6 Fluoexetine compared to placebo for smoking cessation for preventing weight gain after smoking cessation
Part 1
Effects of pharmacological interventions to prevent post‐cessation weight gain versus placebo
Weight change
For three pharmacotherapies, there was evidence of reduced weight gain at end of treatment (EOT), and confidence intervals (CIs) excluded no difference (Analysis 1.1):
-
Dexfenfluramine: mean difference (MD) −2.50 kg, 95% confidence interval (CI) −2.98 to −2.02; 1 study at high risk of bias, 33 participants
-
Phenylpropanolamine (PPA): MD −0.50 kg, 95% CI −0.80 to −0.20; I2 = 0%; 3 studies, 112 participants; results not sensitive to removal of one study at high risk of bias
-
Naltrexone: MD −0.91 kg, 95% CI ‐1.49 to ‐0.34; I2 = 0%; 3 studies, 254 participants; results not sensitive to removal of one study at high risk of bias
For a further three pharmacotherapies, point estimates suggested reduced weight gain at end of treatment, but CIs included the possibility of no difference (Analysis 1.1):
-
Ephedrine + caffeine: MD −1.30 kg, 95% CI −2.87 to 0.27; 1 study at high risk of bias, 40 participants
-
Lorcaserin: MD −1.14 kg, 95% CI −3.65 to 1.37; 1 study at low risk of bias, 41 participants
-
Chromium: MD −0.81 kg, 95% CI −3.05 to 1.43; 1 study at high risk of bias, 15 participants
The one study of topiramate did not have any quitters in the placebo arm and hence an effect estimate could not be calculated (1 Oncken 2014). A further study comparing different treatment regimens of lorcaserin did not provide data by treatment arm (1 Rose 2019).
Four pharmacotherapies were tested in trials that reported at six (Analysis 1.2) and 12 months (Analysis 1.3). All had point estimates suggesting benefit, but only one study contributed data at each time point and in all cases CIs were wide and included no difference:
-
PPA 6 months: MD −2.06, 95% CI −5.56 to 1.44; 12 months MD −1.04, 95% CI −5.03 to 2.95; 38 participants; at unclear risk of bias
-
Ephedrine + caffeine 6 months: MD −0.70 kg, 95% CI −2.72 to 1.32; 32 participants; 12 months MD 1.20 kg, 95% CI −1.84 to 4.24; 24 participants; at unclear risk of bias
-
Chromium 6 months only: MD −3.87 kg, 95% CI −12.01 to 4.27; 9 participants; at unclear risk of bias
-
Naltrexone 6 months: MD −0.29 kg, 95% CI −2.23 to 1.65; 68 participants; 12 months MD −2.30 kg, 95% CI −4.92 to 0.32; 61 participants; at unclear risk of bias
Smoking cessation
For studies which measured smoking cessation at six (Analysis 1.4) or 12 months (Analysis 1.5), or both, CIs for all comparisons were wide and included no difference:
-
PPA 6 months: RR 1.38, 95% CI 0.76 to 2.53; 12 months RR 1.48, 95% CI 0.80 to 2.73; 1 study at unclear risk of bias, 295 participants
-
Ephedrine + caffeine 6 months: RR 1.06, 95% CI 0.53 to 2.11; 12 months RR 1.44, 95% CI 0.60 to 3.48; 1 study at unclear risk of bias, 225 participants
-
Naltrexone 6 months: RR 1.02, 95% CI 0.79 to 1.32; I2 = 0%; 3 studies, 890 participants; removing one at high risk did not impact results; 12 months: RR 1.25, 95% CI 0.67 to 2.31; 1 study at unclear risk of bias, 385 participants
-
Chromium 6 months only: RR 0.48, 95% CI 0.12 to 1.84; 1 study at unclear risk of bias,143 participants
-
Naltrexone and bupropion 6 months only: not estimable due to no quitters in either arm; 1 study at unclear risk of bias, 22 participants
Adverse and serious adverse events (SAEs)
For the most part, data were sparsely and heterogeneously reported, often precluding pooled analyses.
In both studies of lorcaserin, adverse events (AEs) were higher in the intervention arms, but CIs were wide and incorporated no difference: Analysis 1.6; compared to placebo: RR 1.12, 95% CI 0.95 to 1.32; 1 study at low risk of bias, 401 participants; longer duration compared to shorter duration: RR 1.41, 95% CI 0.88 to 2.25; 1 study at high risk of bias, 83 participants. The latter study was the only one of lorcaserin to also report measuring SAEs, with one event occurring in the control arm (Analysis 1.7).
One study of topiramate, judged to be at high risk of bias, measured SAEs, with none reported in either arm (38 participants, Analysis 1.7). In the same study (1 Oncken 2014), authors report that when examining the frequency of all adverse events, only paraesthesia was reported by more participants in the topiramate group than in the placebo group. No participants using placebo reported paraesthesia, compared to 47% (9 of 19) participants in the topiramate group (P = 0.011).
One study of naltrexone measured SAEs and found no difference: RR 0.98, 95% CI 0.14 to 6.89; 1 study at unclear risk of bias, 333 participants; Analysis 1.7. 1 King 2012 reported a higher incidence of dizziness and nausea in those randomized to naltrexone compared to placebo; these data could not be used in our statistical analysis. 1 O'Malley 2006 found no statistically significant between‐group differences in adverse events by type. 1 Toll 2010 found that "the percentage of unique participants reporting non‐serious adverse events rated moderate or severe with a prevalence of ≥5% differed by treatment group for depression and decreased appetite [in each case there were 4 (5%) naltrexone participants vs 0 (0%) placebo participants, Chi2 = 4.21, P = 0.04].”
One study of PPA reported "only one side effect"; heartburn in the placebo group (1 Klesges 1990).
In their study of dexfenfluramine and fluoxetine, 1 Spring 1995 (also Part 2) detected no differences in number of participants stopping treatment due to adverse events.
1 Norregaard 1996, (ephedrine + caffeine) reported a higher incidence of palpitations, sweating, dizziness and nausea in the intervention than in the control group.
No other studies reported data on adverse or serious adverse events.
Effects of behavioural interventions to prevent post‐cessation weight gain
Compared to no support
There was no evidence at any follow‐up that weight management education alone reduced weight gain: At EOT MD −0.04 kg, 95% CI −0.57 to 0.50; I2 = 0%; 2 studies, 140 participants; at 6 months MD 0.89, 95% CI −0.78 to 2.55; I2 = 0%; 2 studies, 81 participants; and 12 months MD −0.21 kg, 95% CI −2.28 to 1.86; I2 = 0%; 2 studies, 61 participants (Analysis 2.1; Analysis 2.2; Analysis 2.3). Results were not sensitive to removing the one study at high risk of bias. At six months, there was no evidence of a difference in quit rates, but CIs incorporated clinically significant benefit and clinically significant harm: RR 1.02, 95% CI 0.78 to 1.33; I2 = 9%; 3 studies, 660 participants; results not sensitive to exclusion of one study at high risk of bias; Analysis 2.4. However, at 12 months there were fewer quitters in the weight‐management education group, with CIs excluding no difference: RR 0.66, 95% CI 0.48 to 0.90; I2 = 0%; 2 studies, 522 participants; results not sensitive to removal of one study at high risk of bias; Analysis 2.5.
Personalized weight‐management support programmes reduced weight gain at end of treatment: MD −1.11 kg, 95% CI −1.93 to −0.29; I2 = 0%; 3 studies, 121 participants; results not sensitive to the removal of the two studies at high risk of bias; Analysis 2.1, with CIs excluding no difference. Weight gain was also reduced relative to control at six months: MD −0.96 kg, 95% CI −2.18 to 0.25; I2 = 46%; 5 studies, 816 participants; results not sensitive to removal of two studies at high risk of bias; Analysis 2.2; and at 12 months: MD −0.44 kg, 95% CI −2.34 to 1.46; I2 = 41%; 4 studies, 530 participants; results not sensitive to exclusion of two studies at high risk of bias; Analysis 2.3, but CIs incorporated no difference. As with weight‐management education, at six months there was no evidence of difference in quit rates: RR 0.95, 95% CI 0.82 to 1.10; I2 = 33%; 7 studies, 5517 participants; results not sensitive to removal of five studies at high risk of bias; Analysis 2.4, but significantly fewer people had quit in the intervention arm at 12 months: RR 0.65, 95% CI 0.45 to 0.92; I2 = 89%; 5 studies, 3441 participants; removal of three studies at high risk of bias widened CIs to include no difference but did not substantially alter the point estimate; Analysis 2.5. 1 Sobell 2017 did not report data in a way that could be used in our analyses, but reported no differences in weight between groups. In 1 Vander Weg 2016, participants in the intervention arm could choose to engage in a weight‐management intervention; results were not reported by randomized group.
Comparisons between weight‐management interventions
The within‐study comparison from 1 Hall 1992 suggested that personalized weight management support is more effective than educating participants about weight management at the end of smoking cessation treatment: MD −1.12 kg, 95% CI −2.17 to −0.07; Analysis 3.1, and at 12 months: MD −2.49 kg, 95% CI −5.51 to 0.53; Analysis 3.3. There was no difference in smoking cessation at six or 12 months, although again CIs were wide (Analysis 3.4; Analysis 3.5).
Data from 1 Danielsson 1999 (unclear risk of bias) showed the benefit of VLCD at end of treatment: MD −3.70, 95% CI −4.82 to −2.58; 121 participants 121; Analysis 3.1; and at 12 months: MD −1.30 kg, 95% CI −3.49 to 0.89; 62 participants; Analysis 3.3; although in the latter case CIs included no difference. This intervention improved abstinence at 12 months with CIs excluding no difference: RR 1.73, 95% CI 1.10 to 2.73, Analysis 3.5.
One study compared providing weight‐management support at the start of a quit attempt versus some weeks after attaining abstinence. Confidence intervals were wide and included no difference in weight at end of treatment: 1 Spring 2004; unclear risk of bias; Analysis 3.1; and at six months Analysis 3.2. Cessation not measured. One study compared 16 x one‐hour counselling sessions comprising motivational interviewing and CBT with 16 x 10‐minute telephone calls aiming to reduce weight gain after cessation (1 Baker 2018; low risk of bias). There was no evidence of benefit for weight but outcomes were imprecisely estimated; Analysis 3.3; Analysis 3.5. One study compared advice to follow a low‐carbohydrate diet with advice to follow a low‐fat one and found no evidence of a difference in weight: 1 Heggen 2016, low risk of bias, Analysis 3.1; Analysis 3.2; Analysis 3.4.
1 Prapavessis 2018 (high risk of bias, 41 participants) randomized participants to an intensive exercise programme or no exercise support. There was no evidence of differences in cessation (Analysis 3.4; Analysis 3.5); weight was not measured. We include this trial in Part 1 as the intervention was delivered specifically in the context of reducing weight gain. 1 Copeland 2006 and 1 Copeland 2015 randomized menopausal women to either group‐based education on CBT principles for weight management or to CBT based on individualized counselling based on questionnaire scores. They found no evidence that weight differed by end of treatment. :A further study compared a very low calorie diet to an individualized diet and exercise plan which began either at baseline or eight weeks post quit‐date (step‐by‐step group), but all participants in two of the three study arms had relapsed or dropped out of the study by end of treatment (1 Lycett 2010). Average weight change in abstinent smokers in the step‐by‐step study arm was 1.15 (SD 2.22) kg (4 participants) at end of treatment and no one in the other groups was abstinent and provided weight data .
Effects of acceptance interventions for weight concern
There was mixed evidence that CBT to support acceptance of weight gain affected weight after stopping smoking. Statistical heterogeneity for weight at all time points precluded statistical synthesis (I2 > 70% in all cases; Analysis 4.1; Analysis 4.2; Analysis 4.3). This heterogeneity was driven by the two studies in which no pharmacotherapy was provided; 1 Levine 2010 (also Part 2) (42 participants) found a point estimate favouring control at all time points, and 1 Perkins 2001 (63 participants) found a point estimate favouring the intervention at all time points; both excluded no difference at end of treatment (Analysis 4.1). Removing the one study at high risk of bias (1 Perkins 2001) did not meaningfully reduce statistical heterogeneity at end of treatment. However, at six months and 12 months, removing this study reduced statistical heterogeneity to 0%. At both time points, results from sensitivity analyses favoured control arms but CIs were wide and for 12 months incorporated no difference: six months: MD 0.89, 95% CI 0.39 to 1.40; 2 studies, 77 participants, at unclear risk of bias; Analysis 4.2; MD 0.37, 95% CI −0.50 to 1.24; 1 study, 54 participants at unclear risk of bias; Analysis 4.3. One further study (1 White 2019) did not provide data in a way that could be incorporated into the meta‐analysis, but weight gain was reduced in the intervention compared to control group.
There was some evidence that interventions that promoted acceptance of weight gain increased smoking abstinence at six and 12 months. At six months the RR was 1.42, 95% CI 1.03 to 1.96; I2 = 21%; 4 studies, 619 participants; removing two at unclear risk of bias decreased the point estimate and led to CIs incorporating no difference; Analysis 4.4. At 12 months, the RR was 1.25, 95% CI 0.76 to 2.06; ; I2 = 26%; 2 studies, 496 participants; removing one at unclear risk of bias decreased the point estimate; Analysis 4.5.
Part 2
Effect of antidepressants on post‐cessation weight gain
Bupropion (300 mg/day) limited post‐cessation weight gain compared with placebo at the end of treatment, with CIs excluding no difference: MD −1.01 kg, 95% CI −1.35 to −0.67; I2 = 3%; 10 studies, 1098 participants; results not sensitive to removal of one study at high risk of bias; Analysis 5.1. A funnel plot showed no evidence of asymmetry (Figure 4). At six and 12 months the reduction in weight was lower than at end of treatment and CIs incorporated no difference: six months: MD −0.38 kg, 95% CI −1.19 to 0.44; I2 = 0%; 7 studies, 420 participants; results not sensitive to removal of two studies at high risk of bias; Analysis 5.3; 12 months: MD −0.26 kg, 95% CI −1.31 to 0.78; I2 = 0%, 7 studies, 471 participants; results not sensitive to removal of two studies at high risk of bias; Analysis 5.5. There was no evidence of a dose‐dependent response for bupropion at end of treatment, six or 12 months (Analysis 5.2, Analysis 5.4, Analysis 5.6), with wide CIs consistent with both benefit and harm. In a further study of bupropion, 2 Ebbert 2014 (low risk of bias), all participants received varenicline. At end of treatment (243 participants), the point estimate favoured bupropion and CIs excluded no difference; at six and 12 months the point estimate still favoured bupropion but CIs included no difference (Analysis 5.1; Analysis 5.3; Analysis 5.5).

Funnel plot of comparison: 5 All types of antidepressant versus placebo for smoking cessation, outcome: 5.1 Mean weight change (kg) at end of treatment.
The one study comparing post‐cessation weight gain in bupropion versus NRT measured weight change at 12 months and detected no evidence of difference (2 Piper 2009, unclear risk of bias, 115 participants; Analysis 7.1).
Fluoxetine reduced weight gain at end of treatment: MD −1.01 kg, 95% CI −1.49 to −0.53; I2 = 38%; 2 studies, 144 participants; results not sensitive to removal of one study at high risk of bias, Analysis 5.1. At six months, statistical heterogeneity was substantial (I2 = 76%) and hence we do not present pooled results, but results from the two studies contributing data (both at unclear risk of bias) had CIs incorporating no difference (Analysis 5.3), Two studies of fluoxetine randomized participants to higher and lower doses as well as to placebo (2 Niaura 2002 to 60 mg and 30 mg and 2 Saules 2004 to 40 mg or 20 mg). There was no evidence that higher doses were more effective at six months and in fact people randomized to 60 mg had significantly greater weight gain at six months than people randomized to 30 mg, an effect not seen in the 40 mg versus 20 mg comparison (Analysis 5.4).
Effect of exercise interventions on post‐cessation weight gain
Neither individual nor pooled data for the four trials of exercise programmes showed any reduction in weight gain at the end of the programme (Analysis 6.1), with a summary estimated mean difference of MD −0.25, 95% CI −0.78 to 0.29; I2 = 0%; 4 studies, 404 participants; results not sensitive to removal of one study at high risk of bias. However, three studies (none at high risk of bias) provided data at 12 months follow‐up which when pooled showed a significant reduction in weight gain favouring treatment (Analysis 6.2), with a summary estimate of MD −2.07 kg, 95% CI −3.78 to −0.36; I2 = 0%; 3 studies, 182 participants.
Effect of nicotine replacement therapy (NRT) on post‐cessation weight gain
Participants taking any type of NRT gained less weight than placebo referents at the end of treatment, with CIs excluding no difference: MD −0.52 kg, 95% CI −0.99 to −0.05; I2 = 81%; 21 studies, 2784 participants; results not sensitive to removal of eight studies at high risk of bias; Analysis 8.1. Statistical heterogeneity was substantial due to one study (2 Abelin 1989), which showed a 4.3 kg difference between weight gain in the treatment and control arms. When this study was removed, statistical heterogeneity reduced to 0% and the overall estimate decreased but CIs continued to exclude no difference: MD −0.41 kg, 95% CI −0.60 to −0.22; I2 = 0%; 20 studies, 2667 participants; Analysis 8.1. A funnel plot showed some asymmetry, suggesting that smaller studies with less weight gain in intervention groups may be missing (Figure 5). Overall, weight gain was less for those taking NRT at six and 12 months although the point estimate was reduced compared to end of treatment, and CIs included no difference: six months: MD −0.08 kg, 95% CI −0.51 to 0.35; I2 = 0%; 11 studies, 1021 participants; Analysis 8.2; and 12 months: MD −0.37 kg, 95% CI −0.86 to 0.11; I2 = 0%; 17 studies, 1463 participants; Analysis 8.3. Whereas six‐month results were not sensitive to removing the four studies at high risk of bias, removing the six studies at high risk of bias from the pooled 12‐month estimate increased the magnitude of benefit, with CIs no longer excluding no difference: MD −0.77, 95% CI −1.45 to −0.08; I2 = 0%; 11 studies, 630 participants. A funnel plot with data at six months showed some asymmetry but this time in the opposite direction to the end‐of‐treatment plot (Figure 6); at 12 months there was no asymmetry present (Figure 7). In 2 Lycett 2011 data were only available at eight‐year follow‐up, and weight change was similar between arms.

Funnel plot of comparison: 8 All types of NRT versus placebo for smoking cessation, outcome: 8.1 Mean weight change (kg) at end of treatment.

Funnel plot of comparison: 8 All types of NRT versus placebo for smoking cessation, outcome: 8.2 Mean weight change (kg) at 6 months.

Funnel plot of comparison: 8 All types of NRT versus placebo for smoking cessation, outcome: 8.3 Mean weight change (kg) at 12 months.
There was no evidence from direct comparisons (Analysis 9.1; Analysis 9.2; Analysis 9.3) or from subgroup analyses that results differed by type of NRT (I2 < 10% for all tests of subgroup differences), with the exception of one study. In 2 Pack 2008 (high risk of bias, 54 participants), weight gain was less in lozenge compared to gum groups, with CIs excluding no difference at end of treatment: MD −2.45 kg, 95% CI −4.43 to −0.47; Analysis 9.1.
Pooled data from two studies (both at high risk of bias) comparing longer courses of NRT with 15 mg or 25 mg patches to shorter courses resulted in a point estimate favouring longer courses at 12 months, but CIs incorporated no difference: MD −0.24 kg, 95% CI −0.97 to 0.48; I2 = 0%; 1 study, 404 participants; Analysis 9.4. Point estimates for pooled data of studies comparing higher versus lower doses of NRT were close to no difference, with CIs including no difference at both time points with data available: end of treatment: MD 0.22 kg, 95% CI −0.04 to 0.48; I2 = 0%; 4 studies, 1038 participants; not sensitive to removal of four studies at high risk of bias; Analysis 9.5; 12 months: MD 0.27 kg, 95% CI −0.41 to 0.96; I2 = 0%; 3 studies, 554 participants; not sensitive to removal of two studies at high risk of bias; Analysis 9.6.
Effect of varenicline on post‐cessation weight gain
At end of treatment and six months, pooled data on the effect of varenicline on post‐cessation weight gain had point estimates close to no difference and CIs incorporating no difference. At end of treatment, the MD was −0.23 kg, 95% CI −0.53 to 0.06; I2 = 32%; 14 studies, 2566 participants; a funnel plot did not show asymmetry (Figure 8), and results were not sensitive to removal of two studies at high risk of bias (Analysis 10.1). At six months, the MD was −0.09 kg, 95% CI −1.09 to 0.90; I2 = 19%; 3 studies, 384 participants; unclear risk, 1 low risk; Analysis 10.2. At 12 months, the point estimate favoured the intervention but CIs were wide and incorporated no difference: MD 1.05 kg, 95% CI −0.58 to 2.69; I2 = 0%; 3 studies, 237 participants; at unclear risk of bias; Analysis 10.3.

Funnel plot of comparison: 10 Varenicline versus placebo for smoking cessation, outcome: 10.1 Mean weight change (kg) at end of treatment.
Three studies (two at low risk of bias, one at unclear risk) compared treatment with bupropion to varenicline and provided usable data. Participants taking varenicline gained more weight at the end of treatment. although CIs narrowly included no difference: MD 0.53 kg, 95% CI 0.00 to 1.05; I2 = 29%; 3 studies, 598 participants; Analysis 11.1. One further study (2 Rose 2014) did not provide data in a form we could analyse.
There was no evidence that weight gain differed in the one trial of varenicline versus NRT (2 Aubin 2008, high risk of bias, Analysis 12.1).
Effect of e‐cigarettes on post‐cessation weight gain
Two studies of e‐cigarettes (one as an adjunct to NRT, the other compared to varenicline) both had very wide CIs at all time points, encompassing clinically significant weight loss and weight gain (Analysis 13.1; Analysis 14.1; Analysis 14.2; Analysis 15.1; Analysis 15.2; Analysis 16.1).
Discussion
Summary of main results
Since the previous version of this review, published in 2012, we have found 21 additional, completed trials fitting criteria for Part 1 (interventions targeting post‐cessation weight gain on weight change and smoking cessation), so there are now 37 trials of interventions specifically designed to limit post‐cessation weight gain. Although a range of pharmacological interventions were tested, none showed evidence that weight gain was prevented in the longer term (beyond six months), but most trials followed up participants only in the short term, primarily at end of treatment, and newer medications have since come on the market. Although some behavioural interventions suggested benefit, certainty in the evidence was limited across all comparisons. There was low‐ to very low‐certainty evidence that personalized weight‐management support, which included weight‐management education with both feedback on personal goals and a personal energy prescription, reduced weight gain at end of treatment, at six, and 12 months (summary of findings Table 1). There was low‐ to very low‐certainty evidence that detailed weight‐management education without personalized assessment, planning and feedback did not reduce weight gain and may have reduced smoking cessation rates (summary of findings Table 1). There was very low‐certainty evidence that acceptance‐based interventions for weight concern had no impact on weight, and low‐certainty evidence that these interventions increased cessation rates (summary of findings Table 2).
We identified 27 new, completed trials during the update that fitted the criteria for Part 2 (interventions designed to aid smoking cessation that plausibly affect post‐cessation weight gain) of this review, bringing the total to 83 trials included in this part. In total, we examined evidence for six different interventions used to support smoking cessation that might incidentally reduce weight gain on cessation. There was low‐certainty evidence that exercise interventions did not impact weight at end of treatment but that they reduced weight gain at 12 months by approximately 2 kg (summary of findings Table 3). There was also moderate‐certainty evidence that nicotine replacement therapy reduced weight at 12 months relative to control, although here the reduction was very modest (approximately 0.4 kg) (summary of findings Table 4). There was low‐certainty evidence that varenicline made little difference to weight at any time point (summary of findings Table 5). Studies of fluoxetine showed low‐certainty evidence of benefit at end of treatment and very low‐certainty evidence of benefit at six months; no studies provided data at 12 months (summary of findings Table 6). Bupropion appeared to limit weight gain at end of treatment, but there was no evidence of this at six or 12 months. There was insufficient evidence to draw any conclusions about the potential role of electronic cigarettes in limiting post‐cessation weight gain.
Overall completeness and applicability of evidence
As described below, the major limitation to the evidence base was imprecision, meaning there are many places in which the evidence is too uncertain to assess whether interventions have worthwhile benefits on weight gain or pose a risk to attaining abstinence from smoking. Studies in Part 2 are probably broadly applicable to the general population of people motivated to quit smoking; these studies were of cessation that measured weight as a secondary outcome. Studies in Part 1, however, may not be as generalizable, with many selecting study populations based on weight concern. In addition, some of the pharmacotherapies tested in Part 1 are no longer widely available due to safety and tolerability concerns, and newer weight‐loss medications have yet to be tested in the context of smoking cessation.
Certainty of the evidence
Part 1
We rated all outcomes in Part 1 to be of low to very low certainty, primarily due to imprecision (small numbers of studies and participants, resulting in wide CIs) and risk of bias (summary of findings Table 1; summary of findings Table 2). Although sensitivity analyses removing studies at high risk of bias did not affect results, in many cases all of the studies contributing to a given outcome were judged to be at high or unclear risk of bias. A further limitation to Part 1 studies is that most enrolled people who were weight‐concerned in some way; such participants may have been more likely to default from the control programme than when allocated the active intervention that they presumably wanted, especially in studies such as 1 Danielsson 1999 and 1 Spring 2004, where this included free meals. The open‐label design is unavoidable in this field, but it is important to note that it could bias the smoking abstinence results in favour of the intervention and decrease the difference in weight at follow‐up.
Part 2
At end of treatment and six months, there was low‐ and very low‐certainty evidence of benefit from fluoxetine, due to issues with serious imprecision (end of treatment) and very serious imprecision (six months) (summary of findings Table 6). There were no data on fluoxetine at 12 months. Certainty of the evidence in the longer‐term impact of nicotine replacement therapy on weight change was downgraded to moderate, due to issues with imprecision (summary of findings Table 3). Further studies may increase certainty in these estimates. Evidence on exercise showed low‐certainty evidence of no benefit at end of treatment, but benefit at 12 months (summary of findings Table 4). In most trials of interventions in weight management, the difference between intervention and control is most marked at end of treatment and declines over follow‐up. In this context, it is puzzling that there was no evidence of effectiveness of exercise on weight at end of programme, but there was at 12‐month follow‐up. This might either represent a chance finding or reflect the fact that the programme encouraged people to go on exercising after it had finished. Further evidence is required before we can be confident that physical activity programmes provide an effective intervention.
Evidence of no difference in weight at end of treatment with varenicline was high certainty, meaning we think further studies are unlikely to meaningfully change the estimate of no clinically significant difference. Longer‐term outcomes (six and 12 months) were low certainty for varenicline, due to imprecision and to the fact that all studies contributing data at these time points were judged to be at unclear risk of bias (summary of findings Table 5).
Potential biases in the review process
Several aspects of our methodological approach warrant consideration.
Firstly, in Part 2, we used existing Cochrane Reviews of interventions for smoking cessation to identify relevant studies. In effect, this means that to be included in Part 2, studies must have measured cessation at six months or longer, as this was an inclusion criterion of the parent review. This means that some studies reporting weight data at earlier than six months may have been missed. For example, we encountered studies of fluoxetine in Part 1 and Part 2 of the review. The study of fluoxetine in Part 1 was excluded from the parent Cochrane Review because it did not incorporate at least a six‐month follow‐up. It was included in the Part 1 search because the aim was to reduce weight gain. This means that it is possible that we did not include some other studies of fluoxetine that were not specifically aimed at reducing weight gain and did not incorporate a six‐ or 12‐month follow‐up. There is, however, no reason to imagine that excluding them would create a bias. Moreover, it is long‐term weight gain that is relevant to health and all such studies would have been included. Second, in this and the 2012 update, but not in the original version of our review, we include 2 Tonstad 2006 in our main analysis of the effect of varenicline on weight gain. In this trial, participants had taken 12 weeks of varenicline before the abstinent participants were randomized to a further 12 weeks or placebo. Thus this study examines weight gain in months three to six of a quit attempt, not from months zero to three as in the other studies. Weight gain is less rapid in months three to six (O'Hara 1998), meaning any effect of varenicline in preventing weight gain is likely to be lower, slightly biasing the effect downwards.
Thirdly, we split the behavioural interventions for weight control in Part 1 of the review into two categories: those that provided weight‐management education only, and those that provided personalized weight‐management support. This split was chosen based on meta‐analysis evidence that healthy eating and physical activity interventions combining self‐monitoring with at least one other technique derived from control theory, (such as specific goal setting, feedback on progress or review of goals set) are significantly more effective than those that do not (Michie 2009).
Finally, as noted in the Methods, the data here relate to weight gain in abstinent smokers only. It is practically difficult to follow up non‐abstinent smokers as they have no motive to attend smoking cessation clinics and thus authors do not usually provide data on continuing smokers. However, most people gain weight on cessation and most people make repeated attempts to quit. It is possible that this leads to incremental weight gain and it would be useful if data could be collected on this.
Agreements and disagreements with other studies or reviews
English smoking cessation guidelines from the National Institute for Health and Care Excellence (NICE) make no specific recommendations about preventing post‐cessation weight gain. However, the public‐facing NHS website suggests exercise, varenicline, nicotine replacement, bupropion and dieting as options (NHS 2021). Similarly, US guidance recommends either bupropion, NRT, or exercise as interventions. A common perception is that concurrent behavioural treatment for smoking and weight undermines smoking cessation, and the advice is to establish smoking cessation before tackling weight (McEwen 2006). Some of the reason for this is the evidence from laboratory studies which show increased urges to smoke during periods of food restriction (Cheskin 2005; Leeman 2010).
Our review produced mixed evidence of this, with some data at 12 months suggesting that perhaps it undermines abstinence. However, data earlier in the quit attempt show no evidence of this and are rather more precise. Given that the putative mechanism relates to the similarity of hunger pangs and smoking urges, it is perhaps most likely that the somewhat lower abstinence in those who had weight‐management interventions could be chance findings. However, other interpretations are possible, and only more data will clarify the issue.
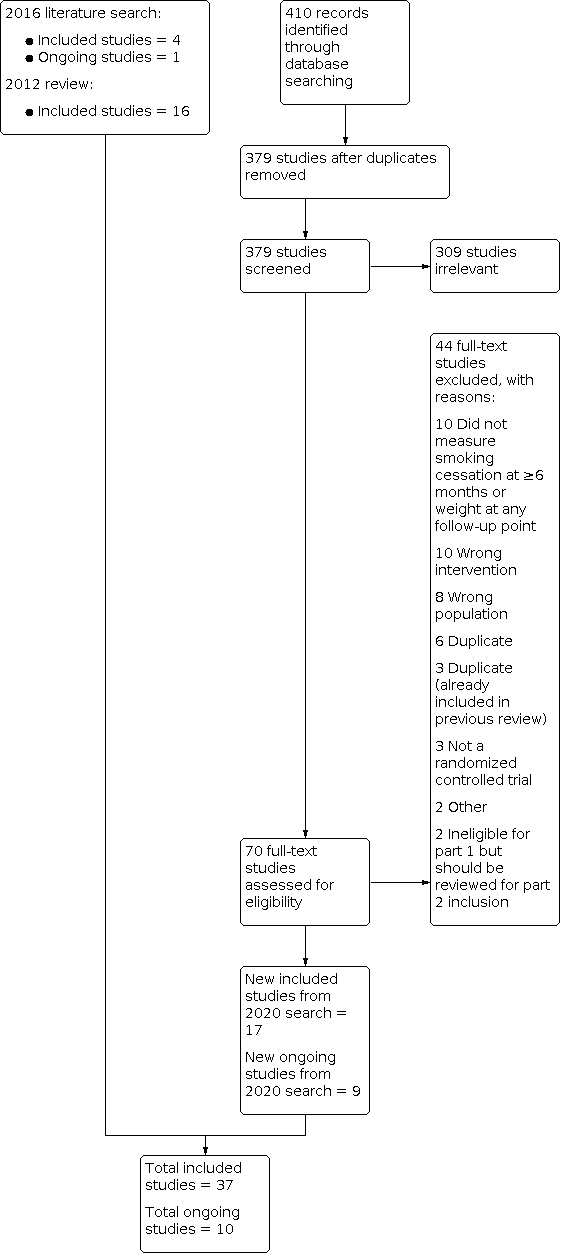
Study flow diagram for Part 1 2020 search plus studies from 2016 search and the 2012 review
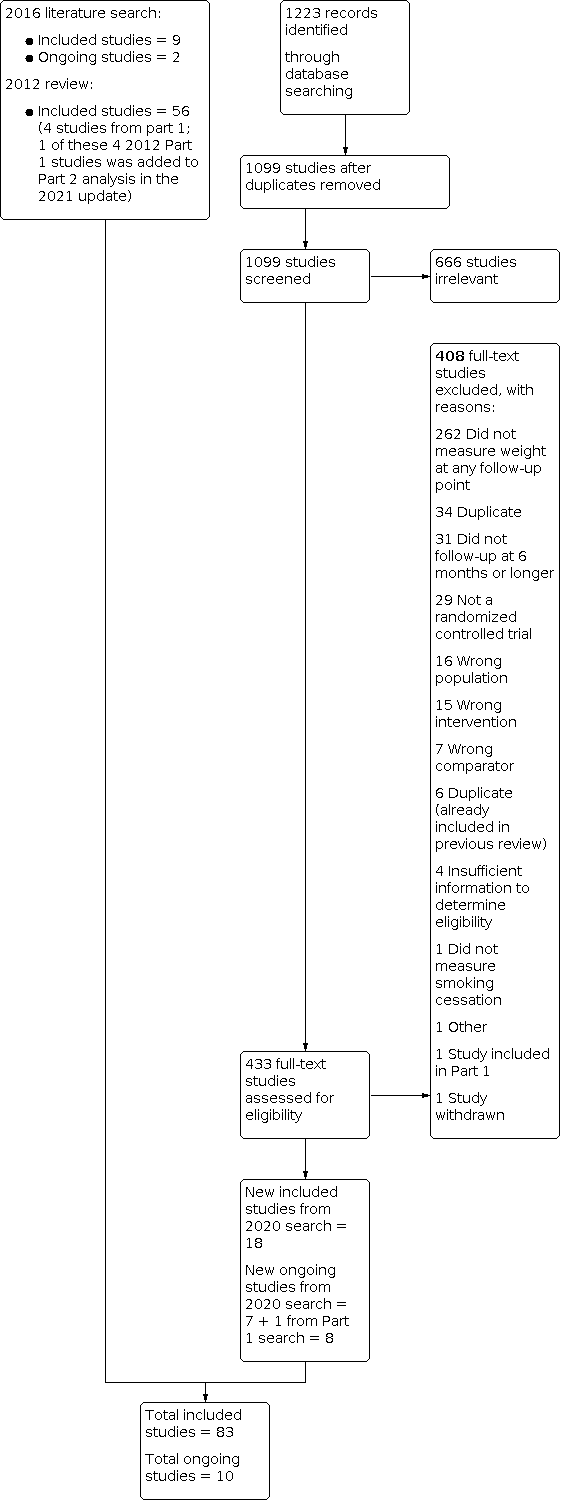
Study flow diagram for Part 2 2020 search plus studies from 2016 search and the 2012 review

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Funnel plot of comparison: 5 All types of antidepressant versus placebo for smoking cessation, outcome: 5.1 Mean weight change (kg) at end of treatment.

Funnel plot of comparison: 8 All types of NRT versus placebo for smoking cessation, outcome: 8.1 Mean weight change (kg) at end of treatment.

Funnel plot of comparison: 8 All types of NRT versus placebo for smoking cessation, outcome: 8.2 Mean weight change (kg) at 6 months.

Funnel plot of comparison: 8 All types of NRT versus placebo for smoking cessation, outcome: 8.3 Mean weight change (kg) at 12 months.

Funnel plot of comparison: 10 Varenicline versus placebo for smoking cessation, outcome: 10.1 Mean weight change (kg) at end of treatment.

Comparison 1: Pharmacological interventions versus placebo for post cessation weight control, Outcome 1: Mean weight change (kg) at end of treatment

Comparison 1: Pharmacological interventions versus placebo for post cessation weight control, Outcome 2: Mean weight change (kg) at 6 months

Comparison 1: Pharmacological interventions versus placebo for post cessation weight control, Outcome 3: Mean weight change (kg) at 12 months

Comparison 1: Pharmacological interventions versus placebo for post cessation weight control, Outcome 4: Smoking cessation at 6 months

Comparison 1: Pharmacological interventions versus placebo for post cessation weight control, Outcome 5: Smoking cessation at 12 months

Comparison 1: Pharmacological interventions versus placebo for post cessation weight control, Outcome 6: Adverse events

Comparison 1: Pharmacological interventions versus placebo for post cessation weight control, Outcome 7: Serious adverse events

Comparison 2: Behavioural weight management interventions versus advice or no intervention for post‐cessation weight control, Outcome 1: Mean weight change (kg) at end of treatment

Comparison 2: Behavioural weight management interventions versus advice or no intervention for post‐cessation weight control, Outcome 2: Mean weight change (kg) at 6 months

Comparison 2: Behavioural weight management interventions versus advice or no intervention for post‐cessation weight control, Outcome 3: Mean weight change (kg) at 12 months

Comparison 2: Behavioural weight management interventions versus advice or no intervention for post‐cessation weight control, Outcome 4: Smoking cessation at 6 months

Comparison 2: Behavioural weight management interventions versus advice or no intervention for post‐cessation weight control, Outcome 5: Smoking cessation at 12 months

Comparison 3: Direct comparisons between behavioural weight management interventions, Outcome 1: Mean weight change (kg) at end of treatment

Comparison 3: Direct comparisons between behavioural weight management interventions, Outcome 2: Mean weight change (kg) at 6 months

Comparison 3: Direct comparisons between behavioural weight management interventions, Outcome 3: Mean weight change (kg) at 12 months

Comparison 3: Direct comparisons between behavioural weight management interventions, Outcome 4: Smoking cessation at 6 months

Comparison 3: Direct comparisons between behavioural weight management interventions, Outcome 5: Smoking cessation at 12 months

Comparison 4: Acceptance interventions for weight concern, Outcome 1: Mean weight change (kg) at end of treatment

Comparison 4: Acceptance interventions for weight concern, Outcome 2: Mean weight change (kg) at 6 months

Comparison 4: Acceptance interventions for weight concern, Outcome 3: Mean weight change (kg) at 12 months

Comparison 4: Acceptance interventions for weight concern, Outcome 4: Smoking cessation at 6 months

Comparison 4: Acceptance interventions for weight concern, Outcome 5: Smoking cessation at 12 months

Comparison 5: All types of antidepressant versus placebo for smoking cessation, Outcome 1: Mean weight change (kg) at end of treatment

Comparison 5: All types of antidepressant versus placebo for smoking cessation, Outcome 2: Mean weight change (kg) at end of treatment: dose response

Comparison 5: All types of antidepressant versus placebo for smoking cessation, Outcome 3: Mean weight change (kg) at 6 months

Comparison 5: All types of antidepressant versus placebo for smoking cessation, Outcome 4: Mean weight change (kg) at 6 months: dose response

Comparison 5: All types of antidepressant versus placebo for smoking cessation, Outcome 5: Mean weight change (kg) at 12 months

Comparison 5: All types of antidepressant versus placebo for smoking cessation, Outcome 6: Mean weight change (kg) at 12 months: dose response

Comparison 6: Exercise interventions versus no exercise for smoking cessation, Outcome 1: Mean weight change (kg) at end of treatment

Comparison 6: Exercise interventions versus no exercise for smoking cessation, Outcome 2: Mean weight change (kg) at 12 months

Comparison 7: Bupropion versus NRT, Outcome 1: Weight change (kg) at 12 months

Comparison 8: All types of NRT versus placebo for smoking cessation, Outcome 1: Mean weight change (kg) at end of treatment

Comparison 8: All types of NRT versus placebo for smoking cessation, Outcome 2: Mean weight change (kg) at 6 months

Comparison 8: All types of NRT versus placebo for smoking cessation, Outcome 3: Mean weight change (kg) at 12 months

Comparison 9: Direct comparisons between NRT types for smoking cessation, Outcome 1: Mean weight change (kg) at end of treatment: comparisons by type

Comparison 9: Direct comparisons between NRT types for smoking cessation, Outcome 2: Mean weight change (kg) at 6 months: comparisons by type

Comparison 9: Direct comparisons between NRT types for smoking cessation, Outcome 3: Mean weight change (kg) at 12 months: comparisons by type

Comparison 9: Direct comparisons between NRT types for smoking cessation, Outcome 4: Mean weight change (kg) at 12 months: longer course vs. shorter

Comparison 9: Direct comparisons between NRT types for smoking cessation, Outcome 5: Mean weight change (kg) at end of treatment: dose response

Comparison 9: Direct comparisons between NRT types for smoking cessation, Outcome 6: Mean weight change (kg) at 12 months: dose response

Comparison 10: Varenicline versus placebo for smoking cessation, Outcome 1: Mean weight change (kg) at end of treatment

Comparison 10: Varenicline versus placebo for smoking cessation, Outcome 2: Mean weight change (kg) at 6 months

Comparison 10: Varenicline versus placebo for smoking cessation, Outcome 3: Mean weight change (kg) at 12 months

Comparison 11: Varenicline versus bupropion, Outcome 1: Mean weight change (kg) at end of treatment

Comparison 12: Varenicline versus NRT, Outcome 1: Mean weight change (kg) at end of treatment

Comparison 13: Nicotine EC + patch vs patch, Outcome 1: Weight (kg) at EOT

Comparison 14: Nicotine EC + patch vs nicotine‐free EC + patch, Outcome 1: Weight (kg) at EOT

Comparison 14: Nicotine EC + patch vs nicotine‐free EC + patch, Outcome 2: Weight (kg) at 6 months

Comparison 15: Nicotine‐free EC + patch vs patch, Outcome 1: Weight (kg) at EOT

Comparison 15: Nicotine‐free EC + patch vs patch, Outcome 2: Weight (kg) at 6 months

Comparison 16: Nicotine EC versus varenicline, Outcome 1: Weight change (kg) at EOT
| Behavioural weight management interventions compared to brief advice or no intervention for post‐cessation weight control for preventing weight gain after smoking cessation | ||||||
| Patient or population: People wanting to quit smoking | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with advice or no intervention for post‐cessation weight control# | Risk with behavioural weight management interventions | |||||
| Mean weight change (kg) at end of treatment ‐ Weight management education versus no weight intervention | 1.45 kg | 1.41 kg (0.88 to 1.95) | MD 0.04 kg lower | 140 | ⊕⊕⊝⊝ | ‐ |
| Mean weight change (kg) at 6 months ‐ Weight management education verses no weight intervention | 0.97 kg | 1.86 kg (0.19 to 3.52) | MD 0.89 kg higher | 81 | ⊕⊕⊝⊝ | ‐ |
| Mean weight change (kg) at 12 months ‐ Weight management education versus no weight intervention | 0.46 kg | 0.25 kg (‐1.82 to 2.32) | MD 0.21 kg lower | 61 | ⊕⊝⊝⊝ | ‐ |
| Smoking cessation at 6 months ‐ Weight management education versus no intervention | 275 per 1000 | 280 per 1000 | RR 1.02 | 660 | ⊕⊕⊝⊝ | ‐ |
| Smoking cessation at 12 months ‐ Weight management education versus no intervention | 294 per 1000 | 194 per 1000 | RR 0.66 | 522 | ⊕⊕⊝⊝ | ‐ |
| Mean weight change (kg) at end of treatment ‐ Personalised weight management support versus no weight intervention | 1.45 kg | 0.34 kg (‐0.48 to 1.16) | MD 1.11 kg lower | 121 | ⊕⊕⊝⊝ | ‐ |
| Mean weight change (kg) at 6 months ‐ Personalised weight management support versus no weight intervention | 0.97 kg | 0.01 kg (‐1.21 to 1.22) | MD 0.96 kg lower | 816 | ⊕⊝⊝⊝ | ‐ |
| Mean weight change (kg) at 12 months ‐ Personalised weight management support versus no weight intervention | 0.46 kg | 0.02 kg (‐1.88 to 1.92) | MD 0.44 kg lower | 530 | ⊕⊝⊝⊝ | ‐ |
| Smoking cessation at 6 months ‐ Personalised weight management support versus no intervention | 188 per 1000 | 178 per 1000 | RR 0.95 | 5517 | ⊕⊕⊝⊝ | ‐ |
| Smoking cessation at 12 months ‐ Personalised weight management support versus no intervention | 475 per 1000 | 308 per 1000 | RR 0.65 | 3441 | ⊕⊕⊝⊝ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level due to risk of bias. All studies judged to be at unclear or high risk of bias. Results not sensitive to removal of studies at high risk of bias. hDowngraded one level due to imprecision. Wide CIs incorporate clinically meaningful difference in favour of control group, as well as no clinically significant difference. | ||||||
| Acceptance interventions for weight concern compared to no weight management intervention for preventing weight gain after smoking cessation | ||||||
| Patient or population: People wanting to quit smoking | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with no weight management intervention# | Risk with acceptance interventions for weight concern | |||||
| Mean weight change (kg) at end of treatment | see comment | see comment | see comment | 169 | ⊕⊝⊝⊝ | Substantial unexplained statistical heterogeneity precluded meta‐analysis. Of the 3 studies, 1 had wide CIs encompassing both benefit and harm, 1 suggested benefit with CIs excluding no difference, and 1 suggested harm with CIs excluding no difference. |
| Mean weight change (kg) at 6 months | 3.5 kg | 3.5 kg (1.97 to 5.03) | MD 0 kg | 106 | ⊕⊕⊝⊝ | ‐ |
| Mean weight change (kg) at 12 months | 4.39 kg | 3.69 kg (1.44 to 5.95) | MD 0.7 kg lower | 76 | ⊕⊝⊝⊝ | ‐ |
| Smoking cessation at 6 months | 218 per 1000 | 309 per 1000 | RR 1.42 | 619 | ⊕⊕⊝⊝ | ‐ |
| Smoking cessation at 12 months | 143 per 1000 | 179 per 1000 | RR 1.25 | 496 | ⊕⊝⊝⊝ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level due to risk of bias. All studies judged to be at unclear or high risk of bias. Results not sensitive to removal of studies at high risk of bias. | ||||||
| Exercise interventions for smoking cessation compared to no exercise intervention for preventing weight gain after smoking cessation | ||||||
| Patient or population: People who have quit smoking | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with no exercise intervention# | Risk with Exercise interventions for smoking cessation | |||||
| Mean weight change (kg) at end of treatment | 2.85 kg | 2.6 kg (2.07 to 3.14) | MD 0.25 kg lower | 404 | ⊕⊕⊝⊝ | ‐ |
| Mean weight change (kg) at 12 months | 4.67 kg | 2.6 kg (0.89 to 4.31) | MD 2.07 kg lower | 182 | ⊕⊕⊝⊝ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level due to imprecision. CIs encompass clinically significant benefit as well as no clinically significant difference. | ||||||
| Nicotine replacement therapy for smoking cessation compared to placebo for preventing weight gain after smoking cessation | ||||||
| Patient or population: People who have quit smoking | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with placebo# | Risk with nicotine replacement therapy for smoking cessation | |||||
| Mean weight change (kg) at end of treatment | 2.85 kg | 2.33 kg (1.86 to 2.8) | MD 0.52 kg lower | 2784 | ⊕⊕⊕⊝ | ‐ |
| Mean weight change (kg) at 6 months | 4.23 kg | 4.15 kg (3.72 to 4.58) | MD 0.08 kg lower | 1021 | ⊕⊕⊕⊝ | ‐ |
| Mean weight change (kg) at 12 months | 4.67 kg | 4.3 (3.81 to 4.67) | MD 0.37 kg lower | 1463 | ⊕⊕⊕⊝ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level due to risk of bias. All studies at high or unclear risk. Results not sensitive to removal of studies at high risk of bias. | ||||||
| Varenicline for smoking cessation compared to placebo for preventing weight gain after smoking cessation | ||||||
| Patient or population: People who have quit smoking | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with placebo for smoking cessation# | Risk with varenicline | |||||
| Mean weight change (kg) at end of treatment | 2.85 kg | 2.62 kg (2.32 to 2.91) | MD 0.23 kg lower | 2566 | ⊕⊕⊕⊕ | ‐ |
| Mean weight change (kg) at 6 months | 4.23 kg | 4.14 kg (3.14 to 5.13) | MD 0.09 kg lower | 384 | ⊕⊕⊝⊝ | ‐ |
| Mean weight change (kg) at 12 months | 4.67 kg | 5.72 kg (4.09 to 7.36) | MD 1.05 kg higher | 237 | ⊕⊕⊝⊝ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded two levels due to imprecision. CIs incorporate clinically significant benefit as well as clinically significant harm. | ||||||
| Fluoxetine compared to placebo for smoking cessation for preventing weight gain after smoking cessation | ||||||
| Patient or population: People who have quit smoking | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with placebo for smoking cessation# | Risk with all types of antidepressant | |||||
| Mean weight change (kg) at end of treatment | 2.85 kg | 1.84 kg (1.36 to 2.32) | MD 1.01 kg lower | 144 | ⊕⊕⊝⊝ | ‐ |
| Mean weight change (kg) at 6 months | 4.67 kg | 3.66 kg (0.29 to 7.04) | MD 1.01 kg lower | 124 | ⊕⊕⊝⊝ | no studies followed up participants at 12 months |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level due to risk of bias. One study at unclear risk, one at high risk. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Mean weight change (kg) at end of treatment Show forest plot | 11 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1.1 Dexfenfluramine versus placebo | 1 | 33 | Mean Difference (IV, Random, 95% CI) | ‐2.50 [‐2.98, ‐2.02] |
| 1.1.2 Phenylpropanolamine versus Placebo | 3 | 112 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.80, ‐0.20] |
| 1.1.3 Ephedrine + Caffeine versus Placebo | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐2.87, 0.27] |
| 1.1.4 Lorcaserin versus placebo | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐1.14 [‐3.65, 1.37] |
| 1.1.5 Chromium versus placebo | 1 | 15 | Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐3.05, 1.43] |
| 1.1.6 Naltrexone versus placebo | 3 | 254 | Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.49, ‐0.34] |
| 1.1.7 Topiramate versus placebo | 1 | 6 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 1.2 Mean weight change (kg) at 6 months Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.2.1 Phenylpropanolamine versus Placebo | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.2.2 Ephedrine + caffeine versus placebo | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.2.3 Chromium versus placebo | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.2.4 Naltrexone versus placebo | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.3 Mean weight change (kg) at 12 months Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.3.1 Phenylpropanolamine versus placebo | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.3.2 Ephedrine + Caffeine versus placebo | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.3.3 Naltrexone versus placebo | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.4 Smoking cessation at 6 months Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.4.1 Phenylpropanolamine gum versus placebo | 1 | 295 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.76, 2.53] |
| 1.4.2 Ephedrine + Caffeine versus placebo | 1 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.53, 2.11] |
| 1.4.3 Naltrexone versus placebo | 3 | 890 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.79, 1.32] |
| 1.4.4 Chromium versus placebo | 1 | 143 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.12, 1.84] |
| 1.4.5 Naltrexone & bupropion versus placebo | 1 | 22 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.5 Smoking cessation at 12 months Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.5.1 Phenylpropanolamine gum versus placebo | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.5.2 Ephedrine + Caffeine versus Placebo | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.5.3 Naltrexone versus placebo | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.6 Adverse events Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.6.1 Lorcaserin versus placebo | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.6.2 Lorcaserin (longer duration) versus placebo + lorcaserin (shorter duration) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.7 Serious adverse events Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.7.1 Topiramate versus placebo | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.7.2 Naltrexone versus placebo | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.7.3 Lorcaserin (longer duration) versus placebo + lorcaserin (shorter duration) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Mean weight change (kg) at end of treatment Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1.1 Weight management education versus no weight intervention | 2 | 140 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.57, 0.50] |
| 2.1.2 Personalised weight management support versus no weight intervention | 3 | 121 | Mean Difference (IV, Random, 95% CI) | ‐1.11 [‐1.93, ‐0.29] |
| 2.2 Mean weight change (kg) at 6 months Show forest plot | 7 | 897 | Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.55, 0.53] |
| 2.2.1 Weight management education verses no weight intervention | 2 | 81 | Mean Difference (IV, Random, 95% CI) | 0.89 [‐0.78, 2.55] |
| 2.2.2 Personalised weight management support versus no weight intervention | 5 | 816 | Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐2.18, 0.25] |
| 2.3 Mean weight change (kg) at 12 months Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.3.1 Weight management education versus no weight intervention | 2 | 61 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐2.28, 1.86] |
| 2.3.2 Personalised weight management support versus no weight intervention | 4 | 530 | Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐2.34, 1.46] |
| 2.4 Smoking cessation at 6 months Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.4.1 Weight management education versus no intervention | 3 | 660 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.78, 1.33] |
| 2.4.2 Personalised weight management support versus no intervention | 7 | 5517 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.82, 1.10] |
| 2.5 Smoking cessation at 12 months Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.5.1 Weight management education versus no intervention | 2 | 522 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.48, 0.90] |
| 2.5.2 Personalised weight management support versus no intervention | 5 | 3441 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.45, 0.92] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Mean weight change (kg) at end of treatment Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1.1 Low carb diet versus reduced fat diet | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1.2 Personalised weight management support versus weight management education | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1.3 VLCD + advice versus advice | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1.4 Early versus late personalised weight management support | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.2 Mean weight change (kg) at 6 months Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.2.1 Low carb diet versus reduced fat diet | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.2.2 Early versus late personalised weight management support | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.3 Mean weight change (kg) at 12 months Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.3.1 Personalised weight management support versus weight management education | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.3.2 VLCD + advice versus advice | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.3.3 Motivational interviewing + CBT versus telephone support | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.4 Smoking cessation at 6 months Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.4.1 Exercise maintenance condition + standard care versus standard care (incl. behavioural weight management) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.4.2 Low carb diet versus reduced fat diet | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.4.3 Personalised weight management support versus weight management education | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.5 Smoking cessation at 12 months Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.5.1 Exercise maintenance condition + standard care versus standard care (incl. behavioural weight management) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.5.2 Personalised weight management support versus weight management education | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.5.3 VLCD + advice versus advice | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.5.4 Motivational interviewing + CBT versus telephone support | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Mean weight change (kg) at end of treatment Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1.1 Acceptance intervention, no additional pharmacotherapy | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1.2 Acceptance intervention, with bupropion in both arms | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1.3 Acceptance intervention, with NRT in both arms | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.2 Mean weight change (kg) at 6 months Show forest plot | 3 | 106 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐1.53, 1.53] |
| 4.2.1 CBT to accept moderate weight gain versus no behavioural weight advice, no additional pharmacotherapy | 2 | 55 | Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐5.55, 3.45] |
| 4.2.2 CBT to accept moderate weight gain versus no behavioural weight advice, with bupropion | 1 | 46 | Mean Difference (IV, Random, 95% CI) | 0.86 [0.30, 1.42] |
| 4.2.3 Acceptance intervention versus health education, with NRT | 1 | 5 | Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐5.72, 4.66] |
| 4.3 Mean weight change (kg) at 12 months Show forest plot | 2 | 76 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐2.95, 1.56] |
| 4.3.1 CBT to accept moderate weight gain versus no behavioural weight advice, no additional pharmacotherapy | 2 | 44 | Mean Difference (IV, Random, 95% CI) | ‐2.15 [‐7.54, 3.24] |
| 4.3.2 CBT to accept moderate weight gain versus no behavioural weight advice, with bupropion | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.57, 1.33] |
| 4.4 Smoking cessation at 6 months Show forest plot | 4 | 619 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.03, 1.96] |
| 4.4.1 CBT to accept moderate weight gain versus no behavioural weight advice (no additional pharmacotherapy) | 3 | 355 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.92, 2.55] |
| 4.4.2 CBT to accept moderate weight gain versus no behavioural weight advice (with bupropion) | 1 | 195 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.85, 2.16] |
| 4.4.3 ACT for weight concern versus health education | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.22, 2.35] |
| 4.5 Smoking cessation at 12 months Show forest plot | 2 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.76, 2.06] |
| 4.5.1 CBT to accept moderate weight gain versus no behavioural weight advice (no additional pharmacotherapy) | 2 | 301 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.58, 3.59] |
| 4.5.2 CBT to accept moderate weight gain versus no behavioural weight advice (with bupropion) | 1 | 195 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.62, 1.81] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Mean weight change (kg) at end of treatment Show forest plot | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1.1 Bupropion versus placebo | 10 | 1098 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐1.35, ‐0.67] |
| 5.1.2 Fluoxetine versus placebo | 2 | 144 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐1.49, ‐0.53] |
| 5.1.3 Bupropion + varenicline vs placebo + varenicline | 1 | 243 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐2.18, ‐0.62] |
| 5.2 Mean weight change (kg) at end of treatment: dose response Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.2.1 Bupropion: 300mg/day v 150mg/day placebo | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.2.2 Bupropion: 300mg/day v 100mg/day placebo | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.3 Mean weight change (kg) at 6 months Show forest plot | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.3.1 Bupropion versus placebo | 7 | 420 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐1.19, 0.44] |
| 5.3.2 Fluoxetine versus placebo | 2 | 124 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐4.38, 2.37] |
| 5.3.3 Bupropion + varenicline vs placebo + varenicline | 1 | 162 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐1.71, 0.91] |
| 5.4 Mean weight change (kg) at 6 months: dose response Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.4.1 Bupropion: 300mg/day v 150mg/day | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.4.2 Bupropion: 300mg/day v 100mg/day | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.4.3 Fluoxetine: 40mg v 20mg | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.4.4 Fluoxetine: 60mg v 30mg | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.5 Mean weight change (kg) at 12 months Show forest plot | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.5.1 Bupropion versus placebo | 7 | 471 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐1.31, 0.78] |
| 5.5.2 Bupropion + varenicline vs placebo + varenicline | 1 | 140 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐3.18, 0.78] |
| 5.6 Mean weight change (kg) at 12 months: dose response Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.6.1 Bupropion: 300mg/day v 150mg/day | 1 | 33 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐4.81, 5.21] |
| 5.6.2 Bupropion: 300mg/day v 100mg/day | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐2.00 [‐8.04, 4.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Mean weight change (kg) at end of treatment Show forest plot | 4 | 404 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.78, 0.29] |
| 6.1.1 Exercise + SC versus SC only | 4 | 404 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.78, 0.29] |
| 6.2 Mean weight change (kg) at 12 months Show forest plot | 3 | 182 | Mean Difference (IV, Random, 95% CI) | ‐2.07 [‐3.78, ‐0.36] |
| 6.2.1 Exercise + SC versus SC only | 3 | 182 | Mean Difference (IV, Random, 95% CI) | ‐2.07 [‐3.78, ‐0.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 7.1 Weight change (kg) at 12 months Show forest plot | 1 | 115 | Mean Difference (IV, Random, 95% CI) | 0.48 [‐1.46, 2.42] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 8.1 Mean weight change (kg) at end of treatment Show forest plot | 21 | 2784 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.99, ‐0.05] |
| 8.1.1 Gum versus placebo | 4 | 345 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.02, ‐0.13] |
| 8.1.2 Patch versus placebo | 11 | 1666 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.51, 0.19] |
| 8.1.3 Inhaler versus placebo | 2 | 111 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐1.19, 0.45] |
| 8.1.4 Sub‐lingual tablet versus placebo | 2 | 478 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐1.11, 0.12] |
| 8.1.5 Intranasal spray versus placebo | 1 | 47 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐1.54, 3.34] |
| 8.1.6 Lozenge versus placebo | 1 | 137 | Mean Difference (IV, Random, 95% CI) | 0.48 [‐0.57, 1.52] |
| 8.2 Mean weight change (kg) at 6 months Show forest plot | 11 | 1021 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.51, 0.35] |
| 8.2.1 Gum versus placebo | 2 | 103 | Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐2.35, 0.69] |
| 8.2.2 Patch versus placebo | 4 | 282 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐1.16, 0.49] |
| 8.2.3 Inhaler versus placebo | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.98, 0.78] |
| 8.2.4 Sub‐lingual tablet versus placebo | 2 | 329 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐1.09, 0.72] |
| 8.2.5 Patch + varenicline versus placebo + varenicline | 1 | 113 | Mean Difference (IV, Random, 95% CI) | 0.96 [‐0.53, 2.45] |
| 8.2.6 Lozenge versus placebo | 1 | 137 | Mean Difference (IV, Random, 95% CI) | 0.48 [‐0.57, 1.52] |
| 8.3 Mean weight change (kg) at 12 months Show forest plot | 17 | 1463 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.86, 0.11] |
| 8.3.1 Gum versus placebo | 1 | 49 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐3.07, 2.93] |
| 8.3.2 Patch versus placebo | 7 | 812 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.89, 0.44] |
| 8.3.3 Intranasal spray versus placebo | 3 | 122 | Mean Difference (IV, Random, 95% CI) | ‐1.55 [‐3.09, ‐0.00] |
| 8.3.4 Inhaler versus placebo | 2 | 90 | Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐2.23, 0.17] |
| 8.3.5 Sub‐lingual tablet versus placebo | 3 | 303 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.99, 1.54] |
| 8.3.6 Lozenge versus placebo | 1 | 87 | Mean Difference (IV, Random, 95% CI) | 0.97 [‐1.82, 3.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 9.1 Mean weight change (kg) at end of treatment: comparisons by type Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1.1 Patch versus spray | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1.2 Lozenge versus gum | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.2 Mean weight change (kg) at 6 months: comparisons by type Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.2.1 Lozenge versus gum | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.2.2 Patch versus spray | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.3 Mean weight change (kg) at 12 months: comparisons by type Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.3.1 Loxenge versus gum | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.3.2 Lozenge versus patch | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.4 Mean weight change (kg) at 12 months: longer course vs. shorter Show forest plot | 1 | 404 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.97, 0.48] |
| 9.4.1 22 weeks vs 8 weeks 25mg patch | 1 | 222 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐1.46, 0.46] |
| 9.4.2 22 weeks vs 8 weeks 15mg patch | 1 | 182 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐1.00, 1.20] |
| 9.5 Mean weight change (kg) at end of treatment: dose response Show forest plot | 4 | 1038 | Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.04, 0.48] |
| 9.5.1 4mg vs 2mg gum | 1 | 161 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.61, 0.41] |
| 9.5.2 22mg vs 11mg patch | 1 | 15 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐2.65, 1.85] |
| 9.5.3 44mg vs 22mg patch | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.99, 1.59] |
| 9.5.4 25mg patch vs 15mg patch‐ 8 week treatment course | 1 | 497 | Mean Difference (IV, Random, 95% CI) | 0.40 [0.04, 0.76] |
| 9.5.5 25mg patch vs 15mg patch‐ 22 weeks treatment | 1 | 299 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.57, 0.97] |
| 9.5.6 15x2mg gum vs 7x2mg gum | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 1.59 [‐0.27, 3.45] |
| 9.5.7 30x2mg gum vs 15x2mg gum | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐1.83, 1.29] |
| 9.6 Mean weight change (kg) at 12 months: dose response Show forest plot | 3 | 554 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.41, 0.96] |
| 9.6.1 22mg patch vs 11mg | 1 | 7 | Mean Difference (IV, Random, 95% CI) | ‐3.90 [‐10.74, 2.94] |
| 9.6.2 44mg patch vs 11mg | 1 | 12 | Mean Difference (IV, Random, 95% CI) | ‐2.20 [‐10.12, 5.72] |
| 9.6.3 25mg patch vs 15mg‐ 8 week treatment course | 1 | 198 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐0.43, 1.63] |
| 9.6.4 25mg patch vs 15mg‐ 22 weeks treatment course | 1 | 206 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐1.04, 1.04] |
| 9.6.5 Patch + lozenge vs lozenge | 1 | 131 | Mean Difference (IV, Random, 95% CI) | 0.59 [‐1.50, 2.68] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 10.1 Mean weight change (kg) at end of treatment Show forest plot | 14 | 2566 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.53, 0.06] |
| 10.1.1 1mg versus placebo | 2 | 230 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.64, 0.85] |
| 10.1.2 2mg versus placebo | 13 | 2308 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.63, 0.02] |
| 10.1.3 1mg + NRT versus placebo + NRT | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 0.54 [‐2.07, 3.15] |
| 10.2 Mean weight change (kg) at 6 months Show forest plot | 3 | 384 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐1.09, 0.90] |
| 10.3 Mean weight change (kg) at 12 months Show forest plot | 3 | 237 | Mean Difference (IV, Random, 95% CI) | 1.05 [‐0.58, 2.69] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 11.1 Mean weight change (kg) at end of treatment Show forest plot | 3 | 598 | Mean Difference (IV, Random, 95% CI) | 0.53 [0.00, 1.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 12.1 Mean weight change (kg) at end of treatment Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 13.1 Weight (kg) at EOT Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 14.1 Weight (kg) at EOT Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 14.2 Weight (kg) at 6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 15.1 Weight (kg) at EOT Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15.2 Weight (kg) at 6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 16.1 Weight change (kg) at EOT Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |

