Intervenciones domiciliarias para el blanqueamiento de dientes en adultos
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Stratified, matched‐pair, clinical study. Change assessed at baseline, 2 weeks, 3 months, and 6 months from baseline. | |
| Participants | 61 adult subjects. Age = 18‐65 years. Groups were matched by age, sex, and gingival health. Maxillary anterior teeth shade A3 or darker. | |
| Interventions | Group 1: placebo with 0% CP, gel applied in a tray. Group 2: NUPRO Gold, with 10% CP, gel applied in a tray. | |
| Outcomes | 10% CP had statistically significant improvement when compared to 0% (P < 0.01). | |
| Notes | Vita Shade Guide. Supported by Dentsply Preventive Care. Each member of a pair was 'allocated' to a group. It is not clear at which point the examiner knew which subjects were in each group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised, controlled clinical study. Change assessed at baseline and at 2 weeks and 6 months from baseline. | |
| Participants | 95 subjects (47 group 1, 48 group 2), males and females, ranging in age from 18‐70 years. Minimum Vita Shade A3 on at least 1 upper central incisor. | |
| Interventions | Group 1: Colgate Simply White, 18% CP, paint‐on gel. Group 2: Colgate Simply White, 16.4% CP, paint‐on gel. | |
| Outcomes | No significant difference between 18% CP and 16.4% CP in teeth whitening. Significant reduction in gingival scores P < 0.001. Transient tooth and gingival sensitivity reported by 15% of participants. | |
| Notes | Vita Shade Guide. Supported by Colgate Palmolive. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Stratified, randomised, parallel group, clinical study. Follow up: 2 weeks. | |
| Participants | 59 adult subjects, age ranged from 20‐60 years old. 83% of participants were female and 90% were white. Vitapan shade A2 or more on at least 2 maxillary incisors. Group 1: n = 29, ages 20‐60, 86% female, 90% white; group 2: n = 30, ages 28‐57, 80% female, 90% white (3 smokers, 2 in group 1). | |
| Interventions | Group 1: used 6% HP, applied as gel in a strip. Group 2: used 18% CP. Brush‐applied liquid gel. | |
| Outcomes | 6% HP gel in a strip had statistically significant improvement in whitening compared with 18% CP gel paint‐on. (Vita Shade ‐ P = 0.005; Shade Vision ‐ P = 0.001). | |
| Notes | 1) Vita Shade Guide and 2) Chroma (Shade Vision). 1 subject claimed increased sensitivity when using HP. Supported by Pfizer. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Stratified, randomised, examiner‐blind, 4‐group clinical study. Follow up: 2 weeks. Assessment was using digital image and computer analysis. | |
| Participants | Respectively n = 10, ages 24‐57, 90% female, 100% white; n = 10, ages 29‐52, 90% female, 90% white; n = 11, ages 25‐47, 82% female, 91% white; and n = 5, ages 31‐51, 60% female, 100% white in the 4 groups. Only 1 participant (group 4) smoked. | |
| Interventions | Group 1: Crest Whitestrips, gel in strips, 5.3% HP applied 2x30 min daily. Group 2: Opalescence, 10% CP, gel applied in a tray 2 hr daily. Group 3: Opalescence, 15% CP + F, gel applied in a tray 2 hr daily. Group 4: Opalescence, 20% CP + F, gel applied in a tray 2 hr daily. | |
| Outcomes | Group 4 showed significantly greater composite colour change when compared with other groups (P = 0.005, P < 0.05, P < 0.01 versus groups 1, 2, and 3). Groups 2, 3, and 4 transient tooth sensitivity (0‐60%) and minor oral irritation (27‐30%), more common in group 4. | |
| Notes | Chroma Meter. Supported by Procter and Gamble. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised, examiner‐blind, parallel group clinical trial. Follow up: 2 weeks. Assessment was using digital image and computer analysis. | |
| Participants | Group 1: n = 10, ages 25‐48, 70% female, 90% white; group 2: n = 10, ages 26‐56, 70% female, 90% white (2 smokers). 3 subjects lost from group 2. | |
| Interventions | Group 1: Crest Whitestrips, 6.0%, HP, gel in strips, 30 min before brushing daily. Group 2: Rembrandt Superior Plus Bleaching System, 10%, CP, gel in tray, 20‐30 min after brushing daily then whitening rinse. | |
| Outcomes | Combined colour change was significantly greater for group 1 than group 2 (P < 0.001). Tooth sensitivity reported in 40% of group 1 and 10% of group 2. Oral irritation was reported in 70% of group 2. | |
| Notes | Chroma Meter. Supported by Procter and Gamble. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Stratified, randomised, placebo‐controlled, double‐blind clinical trial. Follow up: 2 weeks and 6 months with assessment by digital image analysis. | |
| Participants | Group 1: n = 28, ages 19‐61, 75% female, 89% white; group 2: n = 29, ages 18‐71, 79% female, 83% white. 4 smokers in each group. 5 subjects were lost from group 1 and 3 from group 2. | |
| Interventions | Group 1: used Crest Whitestrips, with 5.3% HP gel 30 min 2x daily. Group 2: Placebo Strip, 0% HP gel 30 min 2x daily. Both mandibular and maxillary teeth were treated. | |
| Outcomes | Combined colour change was significantly greater for group 1 than group 2 (P < 0.001). Tooth sensitivity reported in 10% of group 1 and 3% of group 2. Oral irritation was reported in 3 participants, 2 in group 1 and the other in 2. | |
| Notes | Chroma Meter. Supported by Procter and Gamble. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Stratified, randomised, examiner‐blind, 2‐group clinical study. Follow up: 2 weeks with assessment by digital image analysis. | |
| Participants | Group 1: n = 10, ages 22‐49, 50% female, 80% white; group 2: n = 10, ages 24‐59, 40% female, 90% white. 10% were smokers. | |
| Interventions | Group 1: used Crest Professional Whitestrips, with 6.5% HP, gel 2x30 min daily. Group 2: used Nite White Excel 2, with 10% CP, gel applied in a tray 1x2 hr daily. | |
| Outcomes | Combined colour change was significantly greater for group 1 than group 2 (P < 0.05). Tooth sensitivity reported in 20% of group 1 and 10% of group 2. Gingival irritation was reported in 30% of participants in group 1, only. | |
| Notes | Chroma Meter (ShadeEyeEx). Supported by Procter and Gamble. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Stratified, randomised clinical study. Follow up: 2 weeks with assessment by digital image analysis. | |
| Participants | 57 healthy adults with 4 maxillary teeth Vitapan shade A2 or darker. Mean age was 40.3, 56% were female, and 14% were smokers. 30 participants were in group 1 (1 drop out) and 27 were in group 2. | |
| Interventions | Group 1: used Crest Night Effects 19% SP and group 2: used Colgate Simply White Night paint‐on gel containing 8.7% HP. Each was applied at night after brushing and left in place until morning. | |
| Outcomes | Combined colour change was significantly greater for group 1 than group 2 (P < 0.001). 1 participant from each group reported tooth sensitivity and 1 in group 1 reported mild oral irritation. | |
| Notes | Chroma Meter. Supported by Procter and Gamble. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Stratified, randomised, parallel group. Follow up: 2 weeks with assessment by digital image analysis. | |
| Participants | Respectively n = 14, ages 18‐69, 71% female; n = 14, ages 18‐56, 50% female; n = 15, ages 19‐70 in the 3 groups. There were 2 smokers in each of groups 1 and 3. 1 participant was lost to follow up in group 2. | |
| Interventions | Group 1: used dentifrice, with 1% HP and manganese gluconate activator for 2 min 2x daily both arches. Group 2: used a paint‐on gel with 18% CP 2x daily both arches, and group 3: bleaching paste containing 5% CP in custom tray overnight. | |
| Outcomes | Combined colour change did not differ significantly between groups 1 and 2 but was significantly greater for group 3 compared to group 1 (P < 0.005) and group 2 (P < 0.01). Tooth sensitivity was reported in 33% of group 3 and oral irritation in 21% of group 1 and 33% of group 3. There were no reports of either in group 2. | |
| Notes | Chroma Meter. Supported by Procter and Gamble. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised, double‐blind, clinical study. Follow up: 1 and 2 weeks. | |
| Participants | 38 healthy adults aged 18‐50, 61% female. Does not indicate how many in each group but 1 lost from group 1. | |
| Interventions | Group 1: used Crest Whitestrips Supreme, with 14% HP 30 min x2 daily; group 2: used Crest Whitestrips, with 6% HP 30 min x2 daily. | |
| Outcomes | Combined colour change was significantly greater for group 1 than group 2 (P < 0.005). 42% of group 1 and 26% of group 2 reported sensitivity and 11% of each reported oral irritation. | |
| Notes | Chroma Meter. Supported by Procter and Gamble. Short report no details. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Stratified, randomised clinical trial. Follow up at 1, 2, and 3 weeks. Assessment was using digital images. | |
| Participants | Group 1: n = 35, ages 18‐65, 74% female, 87% white, 11% smokers; group 2: n = 34, ages 19‐57, 74% female, 82% white, 8% smokers. Minimum 4 maxillary anterior teeth shade A2 or darker. 3 subjects lost from group 2. | |
| Interventions | Stain removal for all. Group 1: used Professional Crest Whitestrips with 6.5% HP gel in a strip 30 min 2x daily; group 2: used Nite White Excel 2, with a 10% CP gel in a tray 2 hr per day. | |
| Outcomes | Combined colour change was significantly greater for group 1 than group 2 (P < 0.001). 26% of group 1 and 15% of group 2 reported sensitivity and 14% of group 1 and 24% of group 2 reported oral irritation. | |
| Notes | Chroma Meter. Supported by Procter and Gamble. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Stratified, randomised clinical trial. Treatment for 14 days with assessment, using digital images, at baseline, 1, and 2 weeks. | |
| Participants | Group 1: n = 29, ages 19‐52, 72% female, 97% white, 24% smokers; group 2: n = 28, ages 18‐60, 86% female, 93% white, 11% smokers. | |
| Interventions | Group 1: used Crest Whitening strip, with 6% HP 2x 30 min daily. Group 2: used tray based whitening/dentifrice/rinse, with a 10% CP 2x 20‐30 min daily. | |
| Outcomes | Combined colour change was significantly greater for group 1 than group 2 (P < 0.001). 17% of group 1 and 11% of group 2 reported sensitivity and 31% of group 1 and 50% of group 2 reported oral irritation. | |
| Notes | Chroma Meter. Supported by Procter and Gamble. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Stratified, randomised clinical trial. Assessed at baseline, 1, and 2 weeks plus 2 weeks post‐treatment. | |
| Participants | 57 adults aged 18‐65 with anterior maxillary teeth A3 or darker, paired by age and sex. | |
| Interventions | Intial professional prophylaxis for all participants. Group 1: NUPRO Gold, 10% CP. Gel applied in a tray. Group 2: NUPRO Gold, 15% CP. Both groups instructed to wear tray 4 hr‐o/n. | |
| Outcomes | Colour shade change was significantly greater for group 2 than group 1 (P < 0.05). Sensitivity, on a 20‐point scale, was reported as 2.78 ± 2.44 for group 1 and 4.19 ± 4.58 for group 2 (P > 0.05). | |
| Notes | Vita Shade Guide. Sponsored by Dentsply Caulk. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Blinded, parallel clinical study. Products used for 14 days, assessment by reflectance spectroscopy at baseline, 1, and 2 weeks. | |
| Participants | 50 healthy adults with teeth assessed as A3 or darker on the Vita Shade Guide. | |
| Interventions | Group 1: Colgate Platinum Pro, 10% CP, gel applied in tray, 1 hr x2 daily. Group 2: Rembrandt Lighten, 10% CP, gel applied in tray, 1 hr x2 daily. | |
| Outcomes | Combined colour change was significantly greater for group 1 than group 2 (P < 0.001). No adverse reactions were noted. | |
| Notes | Chroma Meter. Supported by Colgate Palmolive. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Single‐blind, randomised, parallel, clinical study. Colour measurements taken on mid‐facial aspects of upper central incisors. Treatment continued for 2 weeks with assessment at start and 1 and 2 weeks. | |
| Participants | 75 healthy adults with teeth assessed as A3 or darker on the Vita Shade Guide aged 23‐68 including 30 males, 40 females, and 5 not detailed. | |
| Interventions | Initial professional prophylaxis for all participants. Group 1: Colgate Platinum Pro, 10% CP, gel applied in a tray. Group 2: Rembrandt Gel Plus, 10% CP, applied in a tray. Group 3: placebo, 0% CP, gel applied in tray. All treatments 1 hr x2 daily for 14 days. | |
| Outcomes | Combined colour change was significantly greater for group 1 than group 2 (P < 0.001) and for group 1 than group 3 (P < 0.001). Authors did not present significance of increased colour change between group 2 and 3. Details of sensitivity and irritation were not presented. | |
| Notes | Chroma Meter. Supported by Colgate Palmolive. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised, placebo‐controlled trial. Up to 6 maxillary incisors or canines assessed at baseline and 2‐week follow up. | |
| Participants | 70 adults, 3 or more shade A2 or darker maxillary teeth. Group 1: n = 35, ages 18‐66, 57% female, 77% white; group 2: n = 35, ages 22‐56, 51% female, 86% white. 2 in each group lost to follow up. | |
| Interventions | Group 1: Polyethylene film with gel containing 5.3% HP gel 2x 30 min daily; group 2: Polyethylene film with gel without HP gel 2x 30 min daily. | |
| Outcomes | Colour shade change was significantly greater for group 1 than group 2 (P < 0.001). No exacerbation of baseline pathology was noted and plaque and gingivitis scores did not differ significantly between groups though both experienced significant reductions in these measures due to inclusion in the trial (P < 0.001). | |
| Notes | Vita Shade Guide. Supported by Procter and Gamble. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | A 3‐group, randomised, parallel, clinical study. Whiteness was assessed by shade comparison and by Chroma Meter at baseline and at 3, 7, 14, and 21 (18 group 1) days. | |
| Participants | 90 adults with 4 or more shade A3 or darker (Vitapan Classical) maxillary incisors: respectively, n = 30, ages 25‐67, 63% female; n = 30, ages 26‐64, 70% female, and n = 30, ages 25‐59, 67% female. 1, 3, and 4 subjects were lost from the study. | |
| Interventions | Initial professional prophylaxis for all participants. Group 1: Crest Professional Whitestrips, 6.5% HP gel, 2x 30 min daily; group 2: Day White 2 with 7.5% HP gel applied in a tray, 2x 30 min daily; group 3: Nite White Excel Mint with 16% CP equivalent gel applied in a tray, o/n. | |
| Outcomes | Shades were assessed by 2 examiners independently. The average shade for group 3 was significantly less than the other 2 groups (P < 0.5) which were comparable with each other. Chroma Meter values agreed with this result. Self reported gingival irritation varied between 40.7 and 62.3% through the period for group 2, 7%‐23% for group 1, and 0 to 12% for group 3. Sensitivity was reported as 12‐19%, 25‐41%, and 8‐24% respectively for the 3 groups. | |
| Notes | Vita Shade Guide and Chroma Meter. Supported by Discus Dental (manufacturer of Day White and Nite White). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Stratified, randomised, 3‐group, clinical trial. Clinical assessment at baseline and 1, 2, and 3 weeks. | |
| Participants | 120 adults with all maxillary anterior teeth present and classifiable as shade A3 or darker (Vitapan Classical). 3 groups: respectively n = 40, ages 18‐65, 69% female; n = 40, ages 20‐65, 71% female; n = 40, ages 19‐65, 62% female. 16 drop outs not included in these values. | |
| Interventions | All groups used paint‐on 18% CP gel. Group 1: 2x daily, no air drying, and no eating/drinking for 15 min; group 2: 3x daily, 30 sec air‐drying, 30 min no eating/drinking; group 3: 4x daily, 30 sec air‐drying, 30 min no eating/drinking. | |
| Outcomes | Groups 2 and 3 showed significantly higher mean shade change from baseline than group 1 (P < 0.05). The values for groups 2 and 3 did not differ significantly from each other. 1 participant only (group 3) reported mild sensitivity at 7‐day examination but not at further time points. No significant differences occurred in changes in GI scores. | |
| Notes | Vita Shade Guide. Supported by Colgate Palmolive. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Split‐mouth design, double blind, randomised trial with treatment for 14 days. Colorimetric assessments made at baseline, 3 days, and 1, 2, 3, and 6 weeks. | |
| Participants | 25 adults with complete maxillary anterior dentition not lighter than B54 or darker than B85 (Trubyte Shade Guide from Dentsply) or discoloured by tetracycline staining. 8 males and 17 females aged 26‐73 were randomised 12 to group 1 and 13 to group 2. All completed the study. | |
| Interventions | Initial professional prophylaxis for all participants. Group 1: 10% CP, gel applied to right anterior maxillary teeth in a custom mouthguard o/n for up to 14 days and group 2: same but with 15% CP. Both asked to maintain good oral hygiene. | |
| Outcomes | Colour shade change was significantly greater for group 2 than group 1 at 3 weeks (P < 0.001) but the difference was not significant at 6 weeks. Slightly higher sensitivity was noted with group 2 but this difference was not significant. | |
| Notes | Chroma Meter. Supported by Ultradent Products. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Twin strand study. Double‐blind, split‐mouth design. Teeth assessed by visually live and from photographs and Colorimetrically with computer processing at baseline and 1, 2, 3, 6, and 12 weeks. | |
| Participants | 25 adult non‐smokers with complete maxillary anterior dentition not lighter than B54 or darker than B85 (Trubyte Shade Guide from Dentsply) or discoloured by tetracycline staining, aged 28‐80. The 14 females and 11 males were separated into those with teeth B95 or darker and those with teeth judged B94 or paler. | |
| Interventions | Initial professional prophylaxis for all participants. Absolutely unclear. Appears both blocks based on initial shade were each asked to use both treatments on 1 side of their maxilla with placement being assigned by some randomisation process. The 2 treatments were 20% CP and 7.5% HP applied in a custom tray for 2 1 hr periods, 1 in the morning and 1 at night. | |
| Outcomes | For the purpose of analysis, all groups were combined. No significant differences were seen between teeth whitened with either reagent. Sensitivity was not reported in terms of numbers of repartees but no significant difference between products was noted. | |
| Notes | Minolta Chroma Meter 321 and Vita Shade Guide. Supported by Ultradent and Discus Dental Inc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Stratified blinded, randomised, parallel clinical study. Change in tooth colour measured by reflectance spectroscopy (Minolta) at baseline and 1 and 2 weeks. | |
| Participants | 40 adults with tooth discolouration of Vita Shade A3 or darker. No other details given. | |
| Interventions | Product was used 30 min morning and 30 min evening for 14 days. Group 1: Colgate Platinum Pro 10% CP gel; group 2: Rembrandt Lighten, 10% CP gel. | |
| Outcomes | Colour shade change was significantly greater for group 1 than group 2 (P < 0.001). Statistical significance only quantified for 1 week, though absolute difference was greater after 2 weeks. | |
| Notes | Chroma Meter. Sponsored by Colgate Palmolive. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Stratified, randomised, parallel group, clinical study. Vita Shade scores assessed at baseline and after 2 and 3 weeks by closest match of facial surface of teeth. | |
| Participants | 80 adults with full set of maxillary anterior teeth Vita Shade A3 or darker. Not counting drop outs (3), the age range was 18‐58 and 60% of participants were female. | |
| Interventions | Participants brushed teeth, applied gel with brush, air dried 30 sec, and refrain from food/drink 30 min. Group 1: Colgate Simply White Clear Whitening Gel, 18% CP, paint‐on gel; group 2: placebo, 0% CP, paint‐on gel. | |
| Outcomes | Colour shade change was significantly greater for group 1 than group 2 (P < 0.05). No mention was made of irritation or sensitivity. | |
| Notes | Vita Shade Guide. Sponsored by Colgate Palmolive. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Stratified, randomised, parallel group, clinical study. Vita Shade scores assessed at baseline and at 2 and 3 weeks by closest match of facial surface of teeth. | |
| Participants | 59 adults with full set of maxillary anterior teeth Vita Shade A3 or darker aged 18‐70. | |
| Interventions | Before retiring, participants brushed teeth, applied gel with brush tooth by tooth, and refrain from food/drink 15 min. Group 1: Colgate Simply White Clear Whitening 25% CP paint‐on gel; group 2: Colgate Simply White Night Clear Whitening 8.7% HP paint‐on gel. | |
| Outcomes | No significant differences in colour shade were seen between teeth whitened with either product (P > 0.05). | |
| Notes | Vita Shade Guide. Sponsored by Colgate Palmolive. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Split‐mouth design with 1 product on right side and other on left, randomly assigned. Assessment using shade guide (Trubyte) and Chroma Meter at baseline, 3 days, and 1, 2, 3, and 6 weeks. | |
| Participants | 25 adult non‐smokers with complete maxillary anterior dentition not lighter than B54 or darker than B85 (Trubyte Shade Guide from Dentsply) or discoloured by tetracycline staining, ages 31‐73, 56% female. | |
| Interventions | Each participant used both reagents by applying 1 to each side of a custom tray and wearing for 2x 30 min daily. Product 1: Day White 5.5% HP, in gel form; product 2: Opalescence F 15% CP, in gel form. | |
| Outcomes | Combined colour change did not significantly differ between products when assessed by Chroma Meter at 6 weeks (P = 0.94) or by shade comparison (P = 0.33). Tooth and gum sensitivity did not differ significantly between mouth sides. | |
| Notes | Chroma Meter. Supported by Ultradent and Discus Dental Inc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Stratified, randomised, parallel group, clinical study. Tooth shade assessments at baseline and at 2 and 3 weeks. Group 1 was additionally assessed at 6 months. | |
| Participants | 75 adults with full set of maxillary anterior teeth with minimum Vita Shade A3. Group 1 ages 19‐70, 63% female and group 2 ages 18‐68, 70% female. | |
| Interventions | Group 1: Colgate Simply White Clear Whitening Gel. 25% CP paint‐on gel. Participants brushed teeth, applied gel with brush, air dried 30 sec, and refrain from food/drink 30 min. Group 2: Colgate standard toothpaste, with 0% peroxide paste. | |
| Outcomes | Colour shade change was significantly greater for group 1 than group 2 (P < 0.05). No mention was made of irritation or sensitivity. | |
| Notes | Vita Shade Guide. Supported by Colgate Palmolive. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
CP = Carbamide peroxide; HP = Hydrogen peroxide; SP = Sodium percarbonate.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| No data were reported on whitening of teeth. | |
| No mean values were reported (only the median and per cent improvement). | |
| No mean values were reported (only the median). | |
| Evaluation of soft tissues and no data were reported on whitening of teeth. | |
| No data on Delta E were reported. | |
| Before and after design; no control group. | |
| Only percentage differences in shade guides were reported. | |
| Data were reported after 3 and 6 weeks post‐treatment. | |
| Data on Delta E were not reported. | |
| Measurements were carried out at 7 days in one group and 14 days in the second group. | |
| Only percentages of subjects with improvement in colour were reported. | |
| Duration of follow up was 1 week post‐treatment. | |
| Data were reported after 3 weeks post‐treatment. | |
| Data were reported after 22 days post‐treatment and not after 2 weeks, which was the time point used in this review. | |
| Only data on individual shade scores per subject were reported. | |
| No relevant data were presented on the 2‐week follow up. Evaluation of tooth colour was carried out using a guide with 24 shade tabs while the Vita Shade Guide in all included studies has 16 shade tabs. | |
| Data were presented separately for specific teeth; hence, incomparable with the other included studies. | |
| Data were not reported for differences in shade guides between experimental and control groups. | |
| Data on Delta E could not be estimated from the reported graphs. | |
| No mean values were reported (median shade change only). | |
| Data were reported after 4 and 22 weeks of follow up but not after 2 weeks. | |
| Study evaluated the effect of adding reservoirs to trays. | |
| A duplicate study of an included master thesis. | |
| No mean values were reported (median shade change). | |
| Data on Delta E were not reported. | |
| Follow up was overnight and 3 hours after bleaching. | |
| Data could not be estimated from the reported figures. | |
| No means or standard deviations were reported. | |
| Insufficient data published. | |
| A densitometer was used to assess the change in colour; not comparable with the other included studies. | |
| No relevant data were reported for the purposes of this review. | |
| No means or standard deviations were reported. | |
| Only frequencies of individuals by each shade code were reported. | |
| Only 3‐week results were reported. | |
| Not a randomised controlled trial and no relevant data were reported. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gel in tray versus placebo (change in shade baseline to 2 weeks) Show forest plot | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐6.29 [‐7.82, ‐4.76] |
| Analysis 1.1  Comparison 1 Whitening product versus placebo/no treatment ‐ Vita Shade, Outcome 1 Gel in tray versus placebo (change in shade baseline to 2 weeks). | ||||
| 2 Paint‐on film versus placebo/no treatment (shade change baseline to 2 weeks) Show forest plot | 2 | 152 | Mean Difference (IV, Random, 95% CI) | ‐2.52 [‐2.86, ‐2.19] |
| Analysis 1.2  Comparison 1 Whitening product versus placebo/no treatment ‐ Vita Shade, Outcome 2 Paint‐on film versus placebo/no treatment (shade change baseline to 2 weeks). | ||||
| 2.1 Colgate Simply White 18% CP versus paint‐on placebo | 1 | 77 | Mean Difference (IV, Random, 95% CI) | ‐2.54 [‐2.89, ‐2.19] |
| 2.2 Colgate Simply White 25% CP versus placebo | 1 | 75 | Mean Difference (IV, Random, 95% CI) | ‐2.38 [‐3.43, ‐1.33] |
| 3 Whitening strip versus placebo (shade change baseline to 2 weeks) Show forest plot | 1 | 66 | Mean Difference (IV, Random, 95% CI) | ‐2.83 [‐3.65, ‐2.01] |
| Analysis 1.3  Comparison 1 Whitening product versus placebo/no treatment ‐ Vita Shade, Outcome 3 Whitening strip versus placebo (shade change baseline to 2 weeks). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gel in tray versus placebo (change in compounded colour baseline to 2 weeks) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Whitening product versus placebo/no treatment ‐ Colorimetric, Outcome 1 Gel in tray versus placebo (change in compounded colour baseline to 2 weeks). | ||||
| 1.1 Colgate Platinum versus placebo | 1 | 46 | Mean Difference (IV, Random, 95% CI) | 3.61 [2.45, 4.77] |
| 1.2 Rembrandt versus placebo | 1 | 46 | Mean Difference (IV, Random, 95% CI) | 2.01 [1.25, 2.77] |
| 2 Whitening strip versus placebo (change in compounded colour baseline to 2 weeks) Show forest plot | 1 | 52 | Mean Difference (IV, Random, 95% CI) | 2.1 [1.71, 2.49] |
| Analysis 2.2  Comparison 2 Whitening product versus placebo/no treatment ‐ Colorimetric, Outcome 2 Whitening strip versus placebo (change in compounded colour baseline to 2 weeks). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paint‐on gel versus paint‐on gel (change in shade baseline to 2 weeks) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Whitening product versus whitening product ‐ Vita Shade, Outcome 1 Paint‐on gel versus paint‐on gel (change in shade baseline to 2 weeks). | ||||
| 1.1 Colgate Simply White 18% CP versus 16.4% CP | 1 | 93 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐1.42, 0.62] |
| 1.2 Colgate Simply White 18% CP 3 times a day versus twice daily | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.67, ‐0.27] |
| 1.3 Colgate Simply White 25% CP versus Colgate Simply White 8.7% HP | 1 | 59 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.83, 0.19] |
| 2 Strip versus paint‐on gel (change in shade baseline to 2 weeks) Show forest plot | 1 | 59 | Mean Difference (IV, Random, 95% CI) | ‐1.47 [‐2.50, ‐0.44] |
| Analysis 3.2  Comparison 3 Whitening product versus whitening product ‐ Vita Shade, Outcome 2 Strip versus paint‐on gel (change in shade baseline to 2 weeks). | ||||
| 3 Strip versus gel in tray (change in shade baseline to 2 weeks) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.3  Comparison 3 Whitening product versus whitening product ‐ Vita Shade, Outcome 3 Strip versus gel in tray (change in shade baseline to 2 weeks). | ||||
| 3.1 Crest Strip versus Day White gel in tray | 1 | 56 | Mean Difference (IV, Random, 95% CI) | 0.66 [‐0.56, 1.88] |
| 3.2 Crest Strip versus Nite White gel in tray | 1 | 56 | Mean Difference (IV, Random, 95% CI) | 2.39 [1.26, 3.52] |
| 4 Tray application versus tray application (shade comparison baseline and 2 weeks) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.4  Comparison 3 Whitening product versus whitening product ‐ Vita Shade, Outcome 4 Tray application versus tray application (shade comparison baseline and 2 weeks). | ||||
| 4.1 10% CP versus 15% CP | 1 | 52 | Mean Difference (IV, Random, 95% CI) | ‐1.73 [‐3.20, ‐0.26] |
| 4.2 7.5% HP versus 16% CP | 1 | 54 | Mean Difference (IV, Random, 95% CI) | ‐1.81 [‐2.79, ‐0.83] |
| 4.3 7.5% HP versus 20% CP | 1 | 48 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐1.36, 1.54] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paint‐on gel versus custom tray (change in compounded colour baseline to 2 weeks) Show forest plot | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 1.25 [0.41, 2.09] |
| Analysis 4.1  Comparison 4 Whitening product versus whitening product ‐ Colorimetric, Outcome 1 Paint‐on gel versus custom tray (change in compounded colour baseline to 2 weeks). | ||||
| 2 Paint‐on gel versus paint‐on gel (change in compounded colour baseline to 2 weeks) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 4.2  Comparison 4 Whitening product versus whitening product ‐ Colorimetric, Outcome 2 Paint‐on gel versus paint‐on gel (change in compounded colour baseline to 2 weeks). | ||||
| 2.1 Crest Night Effects (19% SP ˜ equiv. 5.3% HP) versus Colgate Simply White Night (8.7% HP) | 1 | 56 | Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.90, ‐0.38] |
| 3 Strip (6% HP) versus paint‐on gel (18% CP) (change in compounded colour baseline to 2 weeks) Show forest plot | 1 | 59 | Mean Difference (IV, Random, 95% CI) | ‐1.87 [‐2.53, ‐1.21] |
| Analysis 4.3  Comparison 4 Whitening product versus whitening product ‐ Colorimetric, Outcome 3 Strip (6% HP) versus paint‐on gel (18% CP) (change in compounded colour baseline to 2 weeks). | ||||
| 4 Strip versus strip (change in compounded colour baseline to 2 weeks) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 4.4  Comparison 4 Whitening product versus whitening product ‐ Colorimetric, Outcome 4 Strip versus strip (change in compounded colour baseline to 2 weeks). | ||||
| 4.1 Crest Whitestrips 14% HP versus 6% HP | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐2.74, ‐0.66] |
| 5 Strip versus gel in tray (change in compounded colour baseline to 2 weeks) Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 4.5  Comparison 4 Whitening product versus whitening product ‐ Colorimetric, Outcome 5 Strip versus gel in tray (change in compounded colour baseline to 2 weeks). | ||||
| 5.1 Crest Whitestrips HP versus Opalescence 10% CP | 5 | 178 | Mean Difference (IV, Random, 95% CI) | ‐1.82 [‐3.38, ‐0.26] |
| 5.2 Crest Whitestrips HP versus 15/16% CP | 2 | 73 | Mean Difference (IV, Random, 95% CI) | 1.31 [‐1.22, 3.85] |
| 5.3 Crest Whitestrips 5.3% HP versus Opalescence 20% | 1 | 14 | Mean Difference (IV, Random, 95% CI) | 1.55 [0.66, 2.44] |
| 6 Gel in tray versus gel in tray (change in compounded colour baseline to 2 weeks) Show forest plot | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 4.6  Comparison 4 Whitening product versus whitening product ‐ Colorimetric, Outcome 6 Gel in tray versus gel in tray (change in compounded colour baseline to 2 weeks). | ||||
| 6.1 Opalescence 10% CP versus Opalescence 15% CP | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.92, 0.80] |
| 6.2 Opalescence 10% CP versus Opalescence 20% CP | 1 | 13 | Mean Difference (IV, Random, 95% CI) | 1.42 [0.45, 2.39] |
| 6.3 Opalescence 15% CP versus Opalescence 20% CP | 1 | 15 | Mean Difference (IV, Random, 95% CI) | 1.48 [0.58, 2.38] |
| 6.4 Colgate Platinum 10% CP versus Rembrandt Gel + 10% CP | 3 | 136 | Mean Difference (IV, Random, 95% CI) | ‐1.86 [‐2.70, ‐1.01] |
| 6.5 Day White 7.5% HP versus Nite White Excel 16% CP | 1 | 52 | Mean Difference (IV, Random, 95% CI) | 2.97 [1.55, 4.39] |
| 6.6 10% CP versus 15% CP assessed 4 weeks post‐treatment | 1 | 25 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.24, 0.30] |
| 6.7 Opalescence 20% CP versus Day White 7.5% HP | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐1.39 [‐3.06, 0.28] |
| 6.8 Opalescence (F) 15% CP versus Day White 5.5% HP | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐1.49, 1.61] |

Comparison 1 Whitening product versus placebo/no treatment ‐ Vita Shade, Outcome 1 Gel in tray versus placebo (change in shade baseline to 2 weeks).

Comparison 1 Whitening product versus placebo/no treatment ‐ Vita Shade, Outcome 2 Paint‐on film versus placebo/no treatment (shade change baseline to 2 weeks).

Comparison 1 Whitening product versus placebo/no treatment ‐ Vita Shade, Outcome 3 Whitening strip versus placebo (shade change baseline to 2 weeks).
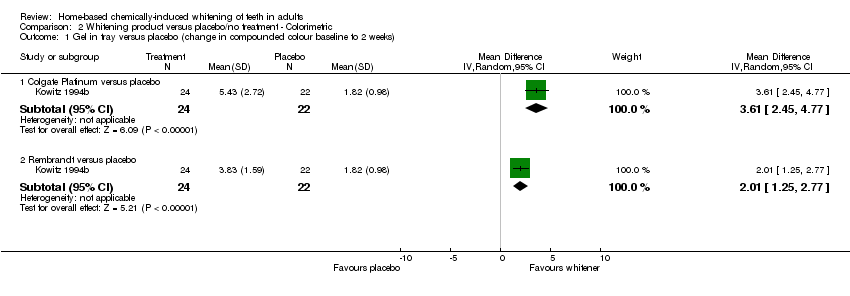
Comparison 2 Whitening product versus placebo/no treatment ‐ Colorimetric, Outcome 1 Gel in tray versus placebo (change in compounded colour baseline to 2 weeks).

Comparison 2 Whitening product versus placebo/no treatment ‐ Colorimetric, Outcome 2 Whitening strip versus placebo (change in compounded colour baseline to 2 weeks).

Comparison 3 Whitening product versus whitening product ‐ Vita Shade, Outcome 1 Paint‐on gel versus paint‐on gel (change in shade baseline to 2 weeks).

Comparison 3 Whitening product versus whitening product ‐ Vita Shade, Outcome 2 Strip versus paint‐on gel (change in shade baseline to 2 weeks).

Comparison 3 Whitening product versus whitening product ‐ Vita Shade, Outcome 3 Strip versus gel in tray (change in shade baseline to 2 weeks).
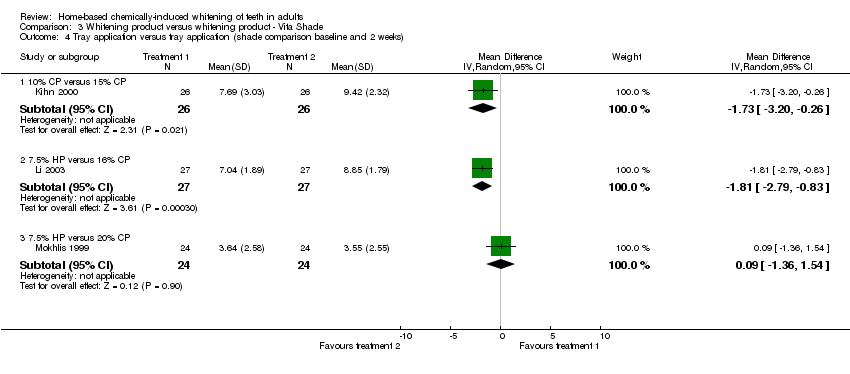
Comparison 3 Whitening product versus whitening product ‐ Vita Shade, Outcome 4 Tray application versus tray application (shade comparison baseline and 2 weeks).
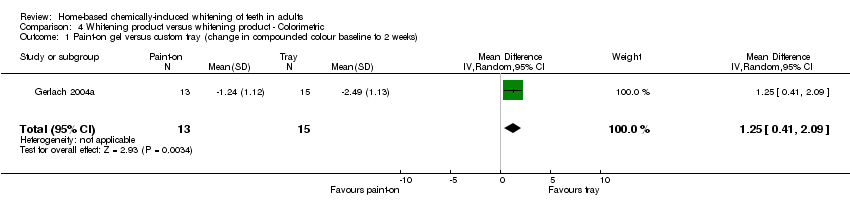
Comparison 4 Whitening product versus whitening product ‐ Colorimetric, Outcome 1 Paint‐on gel versus custom tray (change in compounded colour baseline to 2 weeks).

Comparison 4 Whitening product versus whitening product ‐ Colorimetric, Outcome 2 Paint‐on gel versus paint‐on gel (change in compounded colour baseline to 2 weeks).
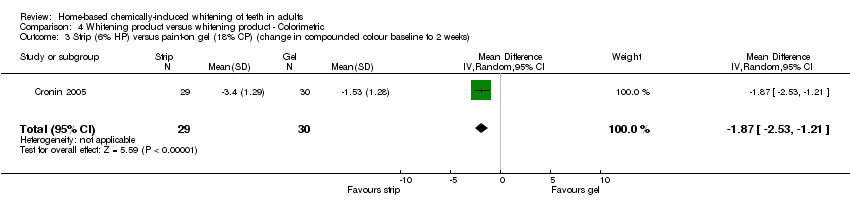
Comparison 4 Whitening product versus whitening product ‐ Colorimetric, Outcome 3 Strip (6% HP) versus paint‐on gel (18% CP) (change in compounded colour baseline to 2 weeks).

Comparison 4 Whitening product versus whitening product ‐ Colorimetric, Outcome 4 Strip versus strip (change in compounded colour baseline to 2 weeks).

Comparison 4 Whitening product versus whitening product ‐ Colorimetric, Outcome 5 Strip versus gel in tray (change in compounded colour baseline to 2 weeks).

Comparison 4 Whitening product versus whitening product ‐ Colorimetric, Outcome 6 Gel in tray versus gel in tray (change in compounded colour baseline to 2 weeks).
| Study | Measurement method | Inclusion/exclusion |
| Cronin 2005 | The CIE L*a*b* System used in digital colour analysis expresses 3 referenced colour co‐ordinate values: L, a, and b. L indicates lightness or brightness, and the co‐ordinates a and b indicate varying amounts of red/green and yellow/blue, respectively. Quantifying colour through the use of devices with this digital technology is possible. More importantly, it offers an objective way of determining the differences in tooth colour and studying the effects of whitening agents. | Exclusion criteria: subjects with anterior restorations covering more than 25% of the facial surfaces of the maxillary anterior teeth, orthodontics, previously bleached teeth within the last 6 months. |
| Gerlach 2003 | Digital images of the facial surfaces of the 6 maxillary anterior teeth were collected at baseline and day 15. | Inclusion criteria: healthy adult with 4 maxillary anterior teeth with shade of "A2" or darker. Exclusion criteria: prior bleaching, current tooth sensitivity, or extensive restorative dentistry or orthodontics appliances involving the facial surfaces of the maxillary anterior teeth. |
| Gerlach 2002b | Digital image analysis: evaluation of teeth whiteness was carried out using Chroma Meter (Shade Eye‐EX) (Shofu Dental Corp, Menlo Park, CA 94025). Delta | Inclusion criteria: healthy adults who desired to have their teeth whitened. |
| Karpinia 2002 | Digital image analysis: to measure teeth whiteness this study used Chroma Meter (Shade Eye‐EX) (Shofu Dental Corp, Menlo Park, CA 94025). | Inclusion criteria: healthy adults with at least 4 maxillary anterior teeth with a tooth shade of A2 or darker as measured using a 16‐tab shade guide. |
| Gerlach 2002a | Digital image analysis (Sh‐HC1000 CCD). | Inclusion criteria: adults 18 years or older with no prior bleaching history, no tooth sensitivity, no restoration on facial of anterior teeth, no prophylactic for at least 3 months. |
| Gerlach 2000 | Digital image analysis. | Exclusion criteria: sensitive teeth, past tooth whitening, or restorations involving anterior teeth. |
| Gerlach 2001 | Digital image analysis. | Subjects were assigned after balancing for tooth colour, coffee or tea drinking, and tobacco use, and demographics. |
| Kowitz 1994a | Reflectance Spectroscopy: baseline colour measurements were taken on the mid‐facial aspects to each upper central incisor with a small area colorimeter using a custom fabricated silicone jigs to ensure repeatable positioning of the meter. Each reading was repeated 3 times to obtain a mean baseline colour before treatment. | Inclusion criteria: healthy subjects with discolouration equal to or darker than A3 on the Vita Shade Guide. |
| Kowitz 1994b | Reflectance Spectroscopy: baseline colour measurements were taken on the mid‐facial aspects to each upper central incisor with a small area colorimeter using a custom fabricated silicone jigs to ensure repeatable positioning of the meter. Each reading was repeated 3 times to obtain a mean baseline colour before treatment start. | Inclusion criteria: healthy subjects with discolouration equal to or darker than A3 on the Vita Shade Guide. |
| Matis 2000 | Digital image analysis: Chroma Meter measures L*, a*, b* colour spaces. | Inclusion criteria: all 6 maxillary anterior teeth had to be present. None of the maxillary anterior teeth could have more than 1/6 of the labial surface covered with a restoration, and the location of a restoration could not interfere with colorimeter placement. The patient had to be at least 18 years of age. The patient had to be willing to refrain from use of tobacco products during the study period. |
| Mokhlis 1999 | Matching with a shade guide, comparing right and left sides of clinical photographs, using colorimeter. | All subjects had tooth cleaning performed by a hygienist prior to the study. |
| Panich 2001 | Degree of teeth whiteness was measured using Chroma Meter 321. Eichhold positioning system was used to ensure the colorimeter could return to its predetermined position at baseline. | Inclusion criteria: adult subjects must have all 6 maxillary anterior teeth. None of the teeth have more than 1/6 of the labial surface covered with restorations. Subjects should be willing to sign a consent form and are able to return for recall examination and refrain from the use of tobacco. |
| Nathoo 1994 | Reflectance Spectroscopy: Minolta Chroma Meter. All readings were carried out by the same investigator and under colour balanced operatory light to avoid error. | Inclusion criteria: Shade of teeth A3 or darker. |
| Li 2003 | Chroma Meter evaluation was carried out using Minolta CR‐221, calibrated according to the manufacturer's instructions. The measurements were taken directly from the facial surfaces of 6 anterior teeth. | Inclusion criteria: subjects were required to have all maxillary anterior teeth present with minimum shade of A3 or darker. Exclusion criteria: subjects wearing orthodontic appliances; subjects with crowned anterior teeth or had periodontal disease, 5 or more carious lesions, pregnant or lactating, previously used tooth whiteners, or had oral pathologies. |
| Gerlach 2004 | Digital image analysis: subjects were positioned in a chin rest, then images of the anterior facial tooth surfaces were captured using a high‐resolution digital camera (Fuji HC1000 CCD, Fuji Photo Film Co., Tokyo, Japan) and motorized zoom lens under standard polarized lighting conditions. | Inclusion criteria: healthy adult volunteers with no history of vital bleaching or dental prophylaxis within the past month. Exclusion criteria: individuals with tooth sensitivity, extensive restorative dentistry involving the anterior dentition, or fixed or orthodontic devices on the maxillary anterior teeth. |
| Karpinia 2003 | Digital image analysis. | Inclusion criteria: healthy adults volunteers who consented to have their teeth whitened, or evidence of tooth sensitivity, previous tooth whitening, or restorative dentistry on the facial surfaces of the anterior teeth. Exclusion criteria: not reported. |
| Gerlach 2004 | Digital image analysis: objective colour measurement using the L* a* b* colour scale of the Commission International del'Eclairage. | Inclusion criteria: healthy adults. Exclusion criteria: not reported. |
| Study ID | Measurement method | Inclusion/exclusion |
| Barnes 1998 | Vita Shade Guide. | Adults with good general and dental health and with good oral hygiene. |
| Brunton 2004 | Vita Shade Guide with 16 tabs. 1 examiner recorded the shade values of the labial surfaces of the maxillary anterior teeth. | Inclusion criteria: healthy adults with at least maxillary anterior teeth present with a minimum shade of A3 and available for the 6 months of the study. Exclusion criteria: adults with orthodontic appliances, soft tissue pathology, moderate or advanced periodontal disease, 5 or more carious lesions, pregnant or lactating, history of allergy to any whitening products, or restorations of teeth to be scored. |
| Cronin 2005 | Tooth shades of the 4 maxillary incisors was visually graded using the Vita Shade Guide. The assessment was made under standardized lighting conditions immediately after wiping the teeth dry. | Exclusion criteria: subjects with anterior restorations covering more than 25% of the facial surfaces of the maxillary anterior teeth, orthodontics, previously bleached teeth within the last 6 months. |
| Kihn 2000 | Value‐oriented Vita Lumen (Vita Zahnfabrik, Germany). | Healthy adults with at least A3 or darker anterior tooth colour as recorded using Vita Lumin. In good general and dental health. |
| Kugel 2000 | Vita Shade Guide was used to measure whiteness of the 6 maxillary anterior teeth. B1 represented a score of 1, while C4 represented a score of 16. A decrease in Vita Shade Guide score represented an increase in tooth whiteness. | Exclusion criteria: active caries, hypersensitivity, tetracycline stain, fluorosis, and previous bleaching of teeth. |
| Li 2003 | Vita Shade Guide was used to measure whiteness of the 6 maxillary anterior teeth. | Inclusion criteria: subjects were required to have all maxillary anterior teeth present with minimum shade of A3 or darker. Exclusion criteria: subjects wearing orthodontic appliances; subjects with crowned anterior teeth or had periodontal disease, 5 or more carious lesions, pregnant or lactating, previously used tooth whiteners, or had oral pathologies. |
| Li 2004 | Same as Li 2003. | Same as Li 2003. |
| Mokhlis 1999 | Trubyte Bioform Colour Ordered Shade Guide. | All subjects had tooth cleaning performed by a hygienist prior to the study. |
| Nathoo 2003 | Vita Shade Guide with 16 tabs. | Inclusion criteria: subjects with all maxillary anterior teeth; free from large restorations; and with Vita Shade score of A3 or darker. Exclusion: similar to Li 2004. |
| Nathoo 2002 | Same as Nathoo 2003. | Same as Nathoo 2003. |
| Sielski 2003 | Vita Shade Guide. | Inclusion criteria: same as Li 2003. |
| Author, year | Masking | Randomisation | Assessor training | Follow up | Degree of bias |
| Barnes 1998 | 1. Yes | Paired lists of names were supplied to the manufacturer, who assigned 1 member to the control group and 1 to treatment group Concealed: yes | Calibrated clinicians | Yes (at least 80% ) (No < 80%) | Moderate |
| Brunton 2004 | 1. Yes | Randomised into 2 groups | Not reported | Yes | High |
| Cronin 2005 | 1. Yes | Subjects were stratified according to baseline Vitapan Classical shade scores and gender | Training and reliability of the examiner was not reported | Yes | High |
| Gerlach 2004 | 1. Yes | Not reported | Training and reliability of the examiner was not reported | Yes | High |
| Gerlach 2002b | 1. Yes | Not reported | Training and reliability of the examiner was not reported | Yes | High |
| Gerlach 2000 | 1. Yes | Protocol: not reported | Not reported | Yes | High |
| Gerlach 2001 | 1. Yes | Protocol: not reported | Not reported | Yes | High |
| Gerlach 2002a | 1. Yes | After balancing for baseline colour, subjects were randomised | Not reported | Yes | High |
| Gerlach 2004 | 1. Yes | Subjects were randomly assigned Concealed: not reported | Training and reliability of the examiner was not reported | Yes | High |
| Gerlach 2003 | 1. Yes | Protocol: not reported | Training and reliability of the examiner was not reported | Yes | High |
| Karpinia 2002 | 1. No | The study group was randomised in blocks of 4, balancing for initial tooth colour | Not reported | Yes | High |
| Karpinia 2003 | 1. No | Protocol: not reported | Training and reliability of the examiner was not reported | Yes | High |
| Kihn 2000 | 1. Yes | The subjects were randomly assigned by the company | Calibrated clinician | Yes | Moderate |
| Kowitz 1994a | 1. Yes | Protocol: not reported | Not reported | Yes | High |
| Kowitz 1994b | 1. Yes | After balancing for baseline colour, subjects were randomised to either group Concealed: not reported | Not reported | Yes | High |
| Kugel 2000 | 1. Yes | Randomised with a block‐randomisations protocol using Vita Shade Guide score at baseline and gender Concealed: not reported | A standardized, trained, and calibrated examiner was used | Yes | High |
| Li 2003 | 1. Yes | Using randomisation scheme generated by computer software and balanced with average Vitapan Classical shade and age | Training: not reported | Yes | High |
| Li 2004 | 1. Yes | Subjects were stratified according to baseline Vitapan Classical shade scores and age, and were randomly assigned within each strata to 1 of the 3 groups Concealed: not reported | Trained and experienced examiner | Yes | High |
| Matis 2000 | 1. Yes | The subjects were randomised into groups, according to baseline shade, by an assistant not directly involved in the study. Concealed: yes | 1 examiner: experienced | Yes | Moderate |
| Mokhlis 1999 | 1. Yes | Randomised according to the baseline shade guide into 2 groups by a study monitor not directly involved in the study. Concealed: yes | Evaluators were calibrated using colour slides, if differences existed consensus was reached using clinical photographs comparisons. | Yes | Moderate |
| Nathoo 1994 | 1. Yes | Protocol: not reported | Not reported | Yes | High |
| Nathoo 2003 | 1. Yes | Qualifying subjects were stratified according to gender, age, and baseline VITA Shade Guide scores, and were randomly assigned within strata to 1 or 2 of the 2 study treatments. Concealed: not reported | A single, experienced, trained examiner performed all of the clinical examination | Yes | High |
| Nathoo 2002 | 1. Yes | Qualifying subjects were stratified according to gender, age, and baseline VITA Shade Guide scores, and were randomly assigned within strata to 1 of the study treatment groups. Concealed: not reported | 1 experienced and trained examiner performed all clinical examinations | Yes | High |
| Panich 2001 | 1. Yes | The patients were placed into 2 groups, according to baseline shade B56 or lighter and B77 or darker. A staff person not associated with the study did the assignment. Concealed: yes | 2 evaluators calibrated in a previous study | Yes | High |
| Sielski 2003 | 1. Yes | Qualifying subjects were stratified according to gender, age, and baseline VITA Shade Guide scores, and were randomly assigned within strata to one of the 2 study treatment groups. Concealed: not reported | 1 experienced and trained examiner performed all clinical examinations | Yes | High |
| Study ID | Side effects |
| Barnes 1998 | There was no significant difference in average sensitivity between the active whitener group and the placebo group at the end of 2 weeks. There were no significant changes in gingival condition for either groups between baseline and 2 weeks. |
| Brunton 2004 | Gingivitis scores were reduced from 0.91 to 0.44 (statistically significant). |
| Cronin 2005 | Not reported. |
| Gerlach 2004 | Tooth sensitivity was more common in the experimental group at 42% while the control group had a prevalence of 26%. 11% of subjects in both groups had oral irritation. |
| Gerlach 2002b | Tooth sensitivity: 20% in the strip group; 10 % in the tray group. Gingival irritation: 30% in strip and 0 % in the tray. |
| Gerlach 2000 | Tooth sensitivity and gingival irritation were mild to moderate in severity and were transient. The higher the concentration of the peroxide the higher was the sensitivity. The 20% CP product caused 60% tooth sensitivity compared to 9% and 10% in the 10% CP and 15% CP groups. |
| Gerlach 2001 | Tooth sensitivity was more common in the strip group while gingival irritation was more common in the tray system. |
| Gerlach 2002 | A total of 4 subjects (13%) of the study population reported tooth sensitivity at some time during the trial. 3 subjects reported gingival irritation. |
| Gerlach 2004 | Oral irritation and tooth sensitivity were the most common adverse events in the study with 30% of the subjects experiencing one or both of these conditions sometimes during treatment. |
| Gerlach 2003 | 2 subjects reported tooth sensitivity, 1 in each treatment group. 1 subject in the 19% SP reported mild oral irritation. All adverse events were mild in severity, and no subject discontinued treatment because of product related adverse events. |
| Karpinia 2002 | Tooth sensitivity and oral irritation were the most common adverse events reported. Tooth sensitivity was more common in the strip group at 26% compare to 15% in the tray group. |
| Karpinia 2003 | Transient tooth sensitivity and oral irritation were the most common findings. A total of 29 subjects (51%) reported oral irritation or tooth sensitivity and 10 subjects (18%) had treatment related oral irritation. Tooth sensitivity was more common in the strip group, whereas oral irritation was more common in the combination system group. Both were mild in severity. |
| Kihn 2000 | There was a significant difference in tooth sensitivity associated with use of 10% CP versus use of 15% CP (10% = 2.44 and 15% = 4.58). Incidence of tooth sensitivity was equal between the 2 groups. |
| Kowitz 1994a | No adverse reactions were clinically evident or reported. |
| Kowitz 1994b | Tooth sensitivity was reported in 1 subject who dropped out of the study. |
| Kugel 2000 | In the placebo group, 1 patient had slight cervical inflammation over 1 tooth. 1 patient developed a minor superficial cervical lesion on 1 tooth and a second patient a swollen papilla between 2 adjacent teeth. Tooth sensitivity was unremarkable in both groups. |
| Li 2003 | No tooth sensitivity was detected in any subject during clinical examination. Tooth sensitivity reported by the subjects ranged from 11.5% to 19.2% for Crest Whitestrips, 25%‐41.4% for Day White, 8%‐24% for Nite White. |
| Li 2004 | Tooth sensitivity: 1 subject reported having mild tooth sensitivity. |
| Matis 2000 | No statistically significant difference between products was found for gingival sensitivity. Although there was a trend toward greater tooth sensitivity with the 15% product, the difference between products was not statistically significant at the 5% level. |
| Mokhlis 1999 | Both products caused tooth sensitivity and gingival sensitivity. There was no difference between the 2 products. |
| Nathoo 1994 | No adverse reactions were clinically evident or reported. |
| Nathoo 2003 | No adverse reaction reported by the subjects or noted by the examiner. |
| Nathoo 2002 | Throughout the study no adverse effects were associated with the use of either of the 2 treatments. |
| Panich 2001 | CP caused more gum sensitivity than HP. CP and HP were equal in causing tooth sensitivity, and statistically not significant. |
| Sielski 2003 | No adverse effects on the oral hard or soft tissue were observed by the examiner or reported by the subjects when questioned. |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gel in tray versus placebo (change in shade baseline to 2 weeks) Show forest plot | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐6.29 [‐7.82, ‐4.76] |
| 2 Paint‐on film versus placebo/no treatment (shade change baseline to 2 weeks) Show forest plot | 2 | 152 | Mean Difference (IV, Random, 95% CI) | ‐2.52 [‐2.86, ‐2.19] |
| 2.1 Colgate Simply White 18% CP versus paint‐on placebo | 1 | 77 | Mean Difference (IV, Random, 95% CI) | ‐2.54 [‐2.89, ‐2.19] |
| 2.2 Colgate Simply White 25% CP versus placebo | 1 | 75 | Mean Difference (IV, Random, 95% CI) | ‐2.38 [‐3.43, ‐1.33] |
| 3 Whitening strip versus placebo (shade change baseline to 2 weeks) Show forest plot | 1 | 66 | Mean Difference (IV, Random, 95% CI) | ‐2.83 [‐3.65, ‐2.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gel in tray versus placebo (change in compounded colour baseline to 2 weeks) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Colgate Platinum versus placebo | 1 | 46 | Mean Difference (IV, Random, 95% CI) | 3.61 [2.45, 4.77] |
| 1.2 Rembrandt versus placebo | 1 | 46 | Mean Difference (IV, Random, 95% CI) | 2.01 [1.25, 2.77] |
| 2 Whitening strip versus placebo (change in compounded colour baseline to 2 weeks) Show forest plot | 1 | 52 | Mean Difference (IV, Random, 95% CI) | 2.1 [1.71, 2.49] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paint‐on gel versus paint‐on gel (change in shade baseline to 2 weeks) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Colgate Simply White 18% CP versus 16.4% CP | 1 | 93 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐1.42, 0.62] |
| 1.2 Colgate Simply White 18% CP 3 times a day versus twice daily | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.67, ‐0.27] |
| 1.3 Colgate Simply White 25% CP versus Colgate Simply White 8.7% HP | 1 | 59 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.83, 0.19] |
| 2 Strip versus paint‐on gel (change in shade baseline to 2 weeks) Show forest plot | 1 | 59 | Mean Difference (IV, Random, 95% CI) | ‐1.47 [‐2.50, ‐0.44] |
| 3 Strip versus gel in tray (change in shade baseline to 2 weeks) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Crest Strip versus Day White gel in tray | 1 | 56 | Mean Difference (IV, Random, 95% CI) | 0.66 [‐0.56, 1.88] |
| 3.2 Crest Strip versus Nite White gel in tray | 1 | 56 | Mean Difference (IV, Random, 95% CI) | 2.39 [1.26, 3.52] |
| 4 Tray application versus tray application (shade comparison baseline and 2 weeks) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 10% CP versus 15% CP | 1 | 52 | Mean Difference (IV, Random, 95% CI) | ‐1.73 [‐3.20, ‐0.26] |
| 4.2 7.5% HP versus 16% CP | 1 | 54 | Mean Difference (IV, Random, 95% CI) | ‐1.81 [‐2.79, ‐0.83] |
| 4.3 7.5% HP versus 20% CP | 1 | 48 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐1.36, 1.54] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paint‐on gel versus custom tray (change in compounded colour baseline to 2 weeks) Show forest plot | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 1.25 [0.41, 2.09] |
| 2 Paint‐on gel versus paint‐on gel (change in compounded colour baseline to 2 weeks) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Crest Night Effects (19% SP ˜ equiv. 5.3% HP) versus Colgate Simply White Night (8.7% HP) | 1 | 56 | Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.90, ‐0.38] |
| 3 Strip (6% HP) versus paint‐on gel (18% CP) (change in compounded colour baseline to 2 weeks) Show forest plot | 1 | 59 | Mean Difference (IV, Random, 95% CI) | ‐1.87 [‐2.53, ‐1.21] |
| 4 Strip versus strip (change in compounded colour baseline to 2 weeks) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Crest Whitestrips 14% HP versus 6% HP | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐2.74, ‐0.66] |
| 5 Strip versus gel in tray (change in compounded colour baseline to 2 weeks) Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Crest Whitestrips HP versus Opalescence 10% CP | 5 | 178 | Mean Difference (IV, Random, 95% CI) | ‐1.82 [‐3.38, ‐0.26] |
| 5.2 Crest Whitestrips HP versus 15/16% CP | 2 | 73 | Mean Difference (IV, Random, 95% CI) | 1.31 [‐1.22, 3.85] |
| 5.3 Crest Whitestrips 5.3% HP versus Opalescence 20% | 1 | 14 | Mean Difference (IV, Random, 95% CI) | 1.55 [0.66, 2.44] |
| 6 Gel in tray versus gel in tray (change in compounded colour baseline to 2 weeks) Show forest plot | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Opalescence 10% CP versus Opalescence 15% CP | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.92, 0.80] |
| 6.2 Opalescence 10% CP versus Opalescence 20% CP | 1 | 13 | Mean Difference (IV, Random, 95% CI) | 1.42 [0.45, 2.39] |
| 6.3 Opalescence 15% CP versus Opalescence 20% CP | 1 | 15 | Mean Difference (IV, Random, 95% CI) | 1.48 [0.58, 2.38] |
| 6.4 Colgate Platinum 10% CP versus Rembrandt Gel + 10% CP | 3 | 136 | Mean Difference (IV, Random, 95% CI) | ‐1.86 [‐2.70, ‐1.01] |
| 6.5 Day White 7.5% HP versus Nite White Excel 16% CP | 1 | 52 | Mean Difference (IV, Random, 95% CI) | 2.97 [1.55, 4.39] |
| 6.6 10% CP versus 15% CP assessed 4 weeks post‐treatment | 1 | 25 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.24, 0.30] |
| 6.7 Opalescence 20% CP versus Day White 7.5% HP | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐1.39 [‐3.06, 0.28] |
| 6.8 Opalescence (F) 15% CP versus Day White 5.5% HP | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐1.49, 1.61] |

