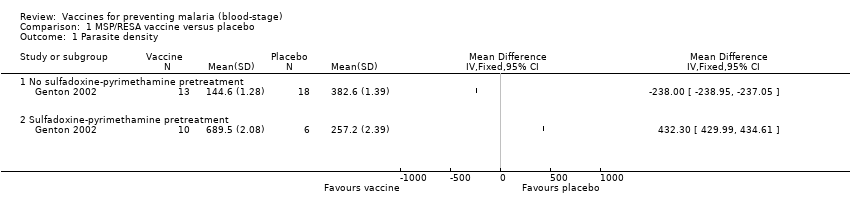
| 1 Parasite density Show forest plot | 1 | | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
|
| 1.1 No sulfadoxine‐pyrimethamine pretreatment | 1 | | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Sulfadoxine‐pyrimethamine pretreatment | 1 | | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Clinical malaria episodes Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
|
| 2.1 No sulfadoxine‐pyrimethamine pretreatment | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Sulfadoxine‐pyrimethamine pretreatment | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 New malaria infection, by MSP2 type Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
|
| 3.1 3D7 | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 FC27 | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
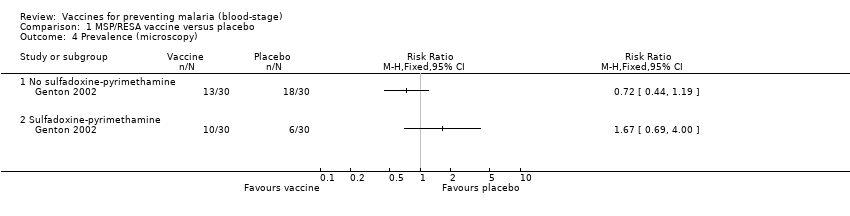
| 4 Prevalence (microscopy) Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
|
| 4.1 No sulfadoxine‐pyrimethamine | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Sulfadoxine‐pyrimethamine | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Prevalence (PCR), by MSP2 type Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
|
| 5.1 3D7 | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 FC27 | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Adverse events (any severity) Show forest plot | 3 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 6.1 Pain at injection site | 3 | 295 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.82, 1.20] |
| 6.2 Limping gait | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.41, 1.61] |
| 6.3 Firmness/nodule at injection site | 3 | 297 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.65, 2.79] |
| 6.4 Swelling/induration at injection site | 3 | 297 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.67, 3.74] |
| 6.5 Fever | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.46, 1.45] |
| 6.6 Cough | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.38, 3.83] |
| 6.7 Headache | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.34, 4.54] |
| 6.8 Pain | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.11, 3.92] |
| 6.9 Vomiting | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.45, 35.27] |
| 6.10 Swelling | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.76] |
| 6.11 Conjunctivitis | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.91] |
| 6.12 Diarrhoea | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.10] |
| 6.13 Difficulty hearing | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.10] |
| 6.14 Earache | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.10] |
| 6.15 Nausea | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.80] |
| 6.16 Running nose | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.10] |
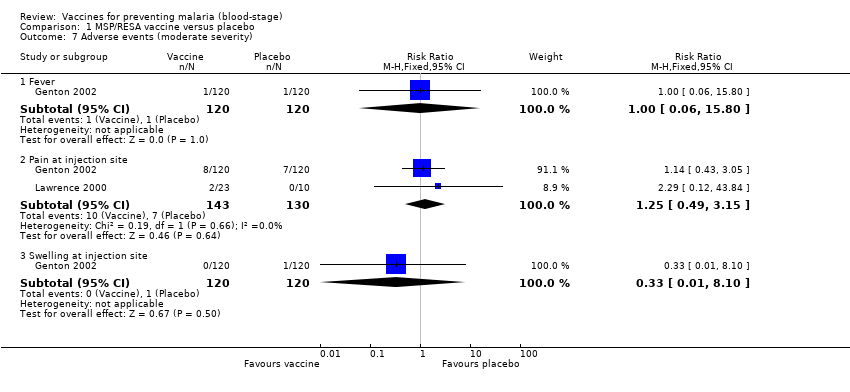
| 7 Adverse events (moderate severity) Show forest plot | 2 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 7.1 Fever | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.80] |
| 7.2 Pain at injection site | 2 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.49, 3.15] |
| 7.3 Swelling at injection site | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.10] |