| 1 New infection: by type of challenge Show forest plot | 3 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 1.1 Experimental challenge | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.35, 0.90] |
| 1.2 Natural challenge | 2 | 723 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.87, 1.06] |
| 2 New infection: by age group Show forest plot | 3 | 747 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.86, 1.04] |
|
| 2.1 Adults | 2 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.79, 1.17] |
| 2.2 Children | 1 | 417 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.85, 1.04] |
| 3 Clinical malaria episodes Show forest plot | 2 | 1911 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.66, 0.88] |
|
| 3.1 Adults | 1 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.63, 1.23] |
| 3.2 Children | 1 | 1605 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.63, 0.87] |
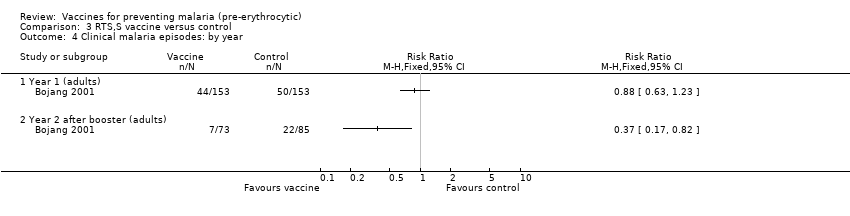
| 4 Clinical malaria episodes: by year Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
|
| 4.1 Year 1 (adults) | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Year 2 after booster (adults) | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe malaria Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
|
| 6 Malaria requiring admission Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
|
| 7 Admission to hospital for any cause Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
|
| 8 Prevalence of parasitaemia Show forest plot | 2 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 8.1 After 6 months | 2 | 2022 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.62, 0.89] |
| 8.2 After 18 months | 2 | 1794 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.65, 0.99] |
| 9 Anaemia (packed cell volume < 25%) Show forest plot | 2 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 9.1 After 6 months | 2 | 2022 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.42, 1.12] |
| 9.2 After 18 months | 1 | 1442 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.14] |
| 10 Adverse events Show forest plot | 5 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 10.1 Injection site pain | 5 | 7502 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.28, 1.45] |
| 10.2 Arm pain | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.80, 3.89] |
| 10.3 Swelling | 4 | 7329 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.85 [6.61, 14.67] |
| 10.4 Induration | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.26 [0.50, 136.74] |
| 10.5 Warmth | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.94 [0.67, 36.25] |
| 10.6 Tenderness | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.83, 4.01] |
| 10.7 Erythema | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.82 [0.68, 11.68] |
| 10.8 Arm motion limitation | 3 | 1272 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.32 [2.95, 6.33] |
| 10.9 Myalgia | 2 | 1003 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.74, 1.80] |
| 10.10 Arthralgia | 2 | 1003 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.87, 1.83] |
| 10.11 Axillary adenopathy | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [0.26, 17.00] |
| 10.12 Headache | 3 | 1272 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.21, 1.78] |
| 10.13 Fever | 3 | 1527 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.68, 1.46] |
| 10.14 Feverishness (subjective) | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.26 [0.50, 136.74] |
| 10.15 Chills | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.30, 3.70] |
| 10.16 Drowsiness | 1 | 392 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.53, 2.58] |
| 10.17 Loss of appetite | 1 | 392 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.57, 1.88] |
| 10.18 Nausea | 2 | 1134 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.99, 2.51] |
| 10.19 Malaise | 3 | 1360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.30, 2.14] |
| 10.20 Irritability/fussiness | 1 | 392 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.83, 2.98] |
| 10.21 General (fever, irritability, drowsiness, anorexia) | 1 | 5838 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.38 [1.47, 3.86] |
| 10.22 Any event preventing normal activity | 1 | 1876 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [1.02, 1.16] |
| 11 Severe adverse events Show forest plot | 1 | 3815 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.62, 0.85] |
|
| 11.1 Up to 6 months | 1 | 1876 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.61, 0.85] |
| 11.2 6 to 18 months | 1 | 1939 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.53, 1.07] |