Мониторирование состояния матки на дому для выявления преждевременных родов
Abstract
Background
To reduce the morbidity and mortality associated with preterm birth, home uterine activity monitoring aims for early detection of increased contraction frequency, and early intervention with tocolytic drugs to inhibit labour and prolong pregnancy. However, the effectiveness of such monitoring is disputed.
Objectives
To determine whether home uterine activity monitoring is effective in improving the outcomes for women and their infants considered to be at high risk of preterm birth, when compared with care that does not include home uterine activity monitoring.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register (30 June 2016), CENTRAL (Cochrane Library 2016, Issue 5), MEDLINE (1966 to 28 June 2016), Embase (1974 to 28 June 2016), CINAHL (1982 to 28 June 2016), and scanned reference lists of retrieved studies.
Selection criteria
Randomised control trials of home uterine activity monitoring, with or without patient education programmes, for women at risk of preterm birth, compared with care that does not include home uterine activity monitoring.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risks of bias, extracted data and checked them for accuracy. We did not attempt to contact authors to resolve queries. We assessed the evidence using the GRADE approach.
Main results
There were 15 included studies (6008 enrolled participants); 13 studies contributed data. Women using home uterine monitoring were less likely to experience preterm birth at less than 34 weeks (risk ratio (RR) 0.78, 95% confidence interval (CI) 0.62 to 0.99; three studies, 1596 women; fixed‐effect analysis) (GRADE high). This difference was not evident when we carried out a sensitivity analysis, restricting the analysis to studies at low risk of bias based on study quality (RR 0.75, 95% CI 0.57 to 1.00; one study, 1292 women). There was no difference in the rate of perinatal mortality (RR 1.22, 95% CI 0.86 to 1.72; two studies, 2589 babies) (GRADE low).
There was no difference in the number of preterm births at less than 37 weeks (average RR 0.85, CI 0.72 to 1.01; eight studies, 4834 women; random‐effects, Tau2 = 0.03, I2 = 68%) (GRADE very low). Infants born to women using home uterine monitoring were less likely to be admitted to neonatal intensive care unit (average RR 0.77, 95% CI 0.62 to 0.96; five studies, 2367 babies; random‐effects, Tau2 = 0.02, I2 = 32%) (GRADE moderate). This difference was not maintained when we restricted the analysis to studies at low risk of bias (RR 0.86, 95% CI 0.74 to 1.01; one study, 1292 babies). Women using home uterine monitoring made more unscheduled antenatal visits (mean difference (MD) 0.48, 95% CI 0.31 to 0.64; two studies, 1994 women) (GRADE moderate). Women using home uterine monitoring were also more likely to have prophylactic tocolytic drug therapy (average RR 1.21, 95% CI 1.01 to 1.45; seven studies, 4316 women; random‐effects, Tau2 = 0.03, I2 = 62%), but this difference was no longer evident when we restricted the analysis to studies at low risk of bias (average RR 1.22, 95% CI 0.90 to 1.65; three studies, 3749 women; random‐effects, Tau2 = 0.05, I2 = 76%) (GRADE low). The number of antenatal hospital admissions did not differ between home groups (RR 0.91, 95% CI 0.74 to 1.11; three studies, 1494 women (GRADE low)). We found no data on maternal anxiety or acceptability.
Authors' conclusions
Home uterine monitoring may result in fewer admissions to a neonatal intensive care unit but in more unscheduled antenatal visits and tocolytic treatment; the level of evidence is generally low to moderate. Important group differences were not evident when we undertook sensitivity analysis using only trials at low risk of bias. There is no impact on maternal and perinatal outcomes such as perinatal mortality or incidence of preterm birth.
PICOs
Резюме на простом языке
Мониторирование беременных женщин на дому для выявления преждевременных родов
В чем суть проблемы?
Младенцы, родившиеся слишком рано, с большей вероятностью заболеют или умрут. Если выявляется начало преждевременных родов, то можно начать лечение, чтобы замедлить или остановить роды. Это также дает время для лечения, чтобы улучшить дыхание младенца при рождении. Увеличение темпа схваток может быть признаком преждевременных родов.
Почему это важно?
Многие женщины не распознают такие сокращения вовремя, чтобы начать лечение. Беременные женщины, подверженные риску преждевременных родов, могли бы использовать устройство для мониторинга в домашних условиях. Это позволило бы отправлять данные в больницу и помогло бы врачам и акушеркам в выявлении и лечении преждевременных родов.
Какие доказательства мы обнаружили?
Мы провели поиск доказательств, имеющихся на 28 июня 2016 года, и обнаружили 15 рандомизированных исследований, в которых приняли участие 6008 женщин. Тринадцать из этих исследований содержали данные, которые мы могли использовать. Качество результатов варьировало от очень низкого до высокого (GRADE). В большинстве исследований имели место ограничения (недостатки) дизайна, которые в некоторых случаях были серьезными. В большинстве исследований сравнивали женщин, которых научили, как проверять себя на предмет наличия признаков преждевременных родов, с женщинами, которым также предоставили домашний монитор активности матки. В некоторых исследованиях монитор активности матки использовали в обеих группах, однако в одной из них применяли имитационный монитор, который на самом деле не посылал данных лечащим врачам. Использование монитора активности на дому почти не повлияло на многие из исходов для матери или ребенка, хотя не во всех исследованиях оценивали все исходы. Женщины, использующие мониторы, были не меньше подвержены риску развития преждевременных родов на сроке менее 37 или 32 недель беременности (доказательства очень низкого качества GRADE). У женщин, использующих мониторы, преждевременные роды на сроке до 34 недель беременности происходили с меньшей вероятностью, но когда мы проанализировали исследования только высокого качества, значимых различий между группами более не было (доказательства высокого качества GRADE). Дети, рожденные от женщин, использовавших монитор, имели меньшую степень вероятности поступления в отделение интенсивной терапии новорожденных, однако случаев смерти меньше не было (доказательства среднего качества GRADE). Женщины, использовавшие монитор, имели меньшую вероятность незапланированных визитов к врачу в дородовой период (доказательства среднего качества GRADE), однако число госпитализаций [поступлений в стационар] в дородовой период не различалось (доказательства низкого качества GRADE). Женщинам, использующим мониторы, с большей вероятностью назначался токолиз (лечение с целью остановки родов) (доказательства низкого качества GRADE), однако когда мы рассмотрели исследования только высокого качества, четких различий выявлено не было. Мы не нашли данных, чтобы оценить мнение самих женщин, хотя в одном крупном клиническом испытании сообщили о низкой комплаентности в отношении использования мониторов. В некоторых исследованиях женщины в группах с использованием мониторов чаще контактировали с акушерками или медсестрами, однако неясно, какое влияние это могло оказать.
Что это значит?
Домашний мониторинг активности матки может привести к уменьшению числа госпитализаций в отделение интенсивной терапии новорожденных, но к увеличению числа незапланированных визитов к врачу в дородовой период и случаев лечения преждевременных родов. Уровень доказательств в основном был от низкого до умеренного (среднего).
Authors' conclusions
Summary of findings
| Home uterine monitoring for preventing preterm birth | ||||||
| Patient or population: women undergoing home monitoring for preventing preterm birth versus women receiving standard care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Home uterine monitoring | |||||
| Perinatal mortality | Study population | RR 1.22 | 2589 | ⊕⊕⊝⊝ | ||
| 46 per 1000 | 56 per 1000 | |||||
| Preterm birth less than 34 weeks' gestation | Study population | RR 0.78 | 1596 | ⊕⊕⊕⊕ | Sensitivity analysis included 1 study at low risk of bias (1292 women) and did not show any difference in results | |
| 166 per 1000 | 130 per 1000 | |||||
| Antenatal hospital admissions | Study population | RR 0.91 | 1494 | ⊕⊕⊝⊝ | ||
| 186 per 1000 | 169 per 1000 | |||||
| Preterm birth less than 37 weeks' gestation | Study population | RR 0.85 | 4834 | ⊕⊝⊝⊝ | ||
| 364 per 1000 | 310 per 1000 | |||||
| Admission to NICU | Study population | RR 0.77 | 2367 | ⊕⊕⊕⊝ | Evidence not downgraded for moderate heterogeneity (I² = 32%) | |
| 290 per 1000 | 223 per 1000 | |||||
| Number of unscheduled antenatal visits | The mean number of days ranged across control groups from approximately 1 to 2 days | The mean number of days in the monitored group was approximately half a day higher MD 0.48 (0.31 to 0.64) | 1994 | ⊕⊕⊕⊝ | Variation in protocol and healthcare delivery structures make it difficult to generalise from 1 large study contributing 65% of the weight for this outcome | |
| Use of tocolysis | Study population | RR 1.21 | 4316 | ⊕⊕⊝⊝ | This outcome may no longer be useful, due to changes in clinical practice. Sensitivity analysis including only 3 studies at low risk of bias (3749 women) did not show any clear difference in results. | |
| 188 per 1000 | 228 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1All studies contributing data with design limitations (‐1). | ||||||
Background
Description of the condition
Preterm birth is a major cause of perinatal mortality and morbidity. Lockwood 2001 considers various methods used to predict women at high risk of preterm labour, noting problems with sensitivity and specificity of traditional tests, and problems with accuracy of some biochemical and biophysical tests. Home uterine activity monitoring is one of the methods that has been used to try to predict preterm birth in the belief that early detection of increased contraction frequency would allow early intervention with tocolytic drugs to inhibit labour and prolong pregnancy (Maxwell 2001). The rationale has been that many women do not recognise their contractions in time for tocolytic therapy to be applied to inhibit labour. No study so far has demonstrated that tocolysis has any role other than to allow time for the fetus to benefit from maternal steroid administration. One possible reason is that the tocolytic is being administered too late.
Description of the intervention
The various care packages developed to predict preterm birth include hospital admission to enhance clinical surveillance, and educational packages to help women identify the signs of early labour, with or without the use of electronic home uterine monitoring devices. Mothers are taught to use these devices at home for one‐ to two‐hour periods each day, and the data stored in the device are transmitted by modem to a base centre for interpretation by a midwife or doctor, with appropriate response if the level appeared abnormal. The ICSI 2002 committee report found that home uterine activity monitoring was a safe procedure, but noted that its effectiveness was not proven. One of the identified difficulties in assessing the effectiveness of home uterine activity monitoring is the different types of care packages used, and the difficulty of assessing whether it is the home uterine activity monitoring or the increased nursing support that is responsible for the changes in outcomes. Existing randomised controlled trials have often used different control groups because such intensive monitoring may only be aimed at women deemed at risk of preterm birth, and the risk profiles may differ.
How the intervention might work
The rationale for home uterine monitoring was that early detection of uterine activity is a sensitive and specific diagnostic test for the onset of preterm labour, but studies (for example, Iams 2002) suggest that the relationship between the maximum frequency of contractions and preterm delivery is weak. More recent research (De Lau 2013; Vinken 2009) on the electrohysterogram (EHG) indicates that it may be possible to distinguish physiological uterine activity from uterine contractions that will lead to preterm labour, but more computer modelling of uterine activation is likely to be necessary (Sharp 2013). Monitoring at home could allow mothers to avoid prolonged or additional hospital admissions, and to be cared for at home. On the other hand, some mothers might become more anxious during the monitoring, particularly if they were remote from hospital, and greater awareness might in itself lead to more frequent presentation at hospital. Reducing unnecessary hospital admissions may decrease antenatal healthcare costs but these savings could be offset by the increased costs associated with poor neonatal outcome.
The rationale for home uterine monitoring also requires that the tocolytic drugs that could be administered to prolong pregnancy are effective, but there is no clear evidence for the effectiveness of long‐term tocolysis (Duley 2011). Various types of tocolytic agents exist. Cochrane systematic reviews indicate that the possible adverse effects of the betamimetics should be weighed against the advantages of delayed delivery (Neilson 2014), and that calcium channel blockers may be as effective as betamimetics but with fewer adverse effects (Flenady 2014). Oral betamimetics for maintenance therapy after threatened preterm labour are not advised (Dodd 2012). The evidence on different dosing regimens for magnesium sulphate (McNamara 2015) as single‐agent tocolytic therapy is very limited. However, administration of magnesium sulphate to women considered at risk of preterm birth does reduce the risk of cerebral palsy (Doyle 2009). Nifedipine and atosiban (an oxytocin receptor agonist) have comparable effectiveness but the latter is expensive and is only available intravenously (Duley 2011). The evidence on different dosing regimens for magnesium sulphate (McNamara 2015) as single‐agent tocolytic therapy is very limited. A Health Technology Assessment review of screening to prevent spontaneous preterm birth (Honest 2009) concluded that non‐steroidal anti‐inflammatory agents were the most effective tocolytic agents in reducing spontaneous preterm birth and prolongation of pregnancy in symptomatic women, but there were doubts over their safety and they are not in routine use. Antenatal corticosteroids helped reduce the incidence of respiratory distress syndrome and the risk of intraventricular haemorrhage.
An overview (Piso 2014) of Cochrane systematic reviews on antenatal interventions to reduce preterm birth noted that a few interventions have been found effective (e.g. progesterone for some groups of women, to improve infant health (Dodd 2013)) and a small number appear harmful. For around half the interventions evaluated the evidence did not warrant recommendations for clinical practice (Piso 2014). The accuracy of tests to predict preterm birth has been judged generally poor (Honest 2009), although transvaginal cervical‐length screening has been advocated as a means of identifying women at risk of preterm birth (Berghella 2013; Conde‐Agudelo 2015). Fetal fibronectin testing and similar bed‐side tests on cervical secretions have also been used for screening (Berghella 2008; Sanchez‐Ramoz 2009; Vis 2009). Their benefit is that they have a greater than 95% negative predictive value for delivery within seven days, and studies have looked at combining transvaginal cervical‐length screening with fibronectin testing (e.g. Hadži‐Legal 2016) with variable results.
Why it is important to do this review
There is a lack of consensus on the effectiveness of home uterine monitoring (Reichmann 2008). There are a number of reasons for this, including the variability in study design and the uncertain benefits of early detection of uterine activity. There were two aspects to be explored in the review: (1) is home uterine monitoring effective at detecting uterine activity? and (2) is it worthwhile finding out if women have uterine activity?
Objectives
To determine whether home uterine activity monitoring is effective in improving the outcomes for women and their infants considered to be at high risk of preterm birth, when compared with care that does not include home uterine activity monitoring.
Methods
Criteria for considering studies for this review
Types of studies
Published and unpublished randomised and quasi‐randomised controlled trials in which the use of home uterine activity monitoring is compared with care that does not include home uterine activity monitoring.
Types of participants
Women considered by their obstetricians to be at risk for preterm birth. We included women with multiple pregnancies in the review, but treated them as a subgroup (where possible).
Types of interventions
The emphasis is on identifying the value of home uterine activity monitoring, which may be used as part of a care package to reduce the need for hospital admission or monitoring, or both, to reduce the need for additional educational support for the woman, or reduce the need for additional nursing contact. We also considered trials where home uterine monitoring is compared with a different form of extra surveillance for women defined as being at risk by their obstetricians.
Comparisons:
-
Care including home uterine monitoring versus routine care (without home uterine monitoring or with placebo or 'sham' home monitoring).
-
Care including home uterine monitoring versus care with an alternative form of additional surveillance.
Types of outcome measures
Primary outcomes
Infant outcomes
-
Perinatal mortality rate.
-
Preterm birth at less than 34 weeks' gestation.
Prenatal outcomes
-
Number of days in hospital antenatally.
Secondary outcomes
Infant outcomes
-
Preterm birth (less than 37 weeks).
-
Very preterm birth delivery (less than 32 weeks).
-
Extremely preterm birth delivery (less than 28 weeks).
-
Air leak syndrome.
-
Necrotising enterocolitis.
-
Patent ductus arteriosus requiring treatment.
-
Chronic lung disease.
-
Retinopathy of prematurity.
-
Use of antenatal corticosteroids.
-
Respiratory distress syndrome.
-
Neuropathology on ultrasound (intraventricular haemorrhage all grades, severe grades three or four, periventricular leukomalacia).
-
Use of mechanical ventilation.
-
Admission to neonatal intensive care unit (NICU).
-
Mode of delivery.
Prenatal outcomes
-
Number of antenatal visits.
-
Number of antenatal hospital admissions.
-
Number of midwife/nurse home visits.
-
Use of tocolysis.
Maternal outcomes
-
Maternal anxiety.
-
Maternal acceptability of home uterine monitoring.
Search methods for identification of studies
The following Methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (30 June 2016).
The Register is a database containing over 22,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE (Ovid);
-
weekly searches of Embase (Ovid);
-
monthly searches of CINAHL (EBSCO);
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Two people screen the search results are screened and review the full text of all relevant trial reports identified through the searching activities described above. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the relevant review sections (Included studies; Excluded studies).
In addition, we searched CENTRAL (Cochrane Library 2016, Issue 5), MEDLINE (1966 to 28 June 2016), Embase (1974 to 28 June 2016), CINAHL (1982 to 28 June 2016) (See:Appendix 1)
Searching other resources
We also scanned the reference lists of articles identified.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeUrquhart 2012.
For this update, the search identified one new report for our consideration (NCT02379351). We used the following methods, which are based on a standard template used by the Cochrane Pregnancy and Childbirth Group, to assess the 15 studies already included in the review.
Selection of studies
Two review authors independently assessed for inclusion all the studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third review author. We entered data into Review Manager 5 software (RevMan 2014) and checked them for accuracy.
When information regarding any of the above was unclear, we had planned to contact authors of the original reports to provide further details, but we did not do this because all the studies are over 15 years old. We consulted some other reviews (ICSI 2002; Keirse 1993).
Assessment of risk of bias in included studies
Two review authors independently assessed risks of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
-
low risk of bias (any truly random process, e.g. random‐number table; computer random‐number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment.
We assessed the methods as:
-
low risk of bias (e.g. telephone or central randomisation; consecutively‐numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcome.
We assessed the methods as:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcome.
We assessed methods used to blind outcome assessment as:
-
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcome, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers or reasons for missing data unbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
-
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
-
low risk of bias (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
-
high risk of bias (where not all the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that we would have expected to have been reported);
-
unclear risk of bias.
(6) Other potential bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
-
low risk of other bias;
-
high risk of other bias;
-
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses (see Sensitivity analysis).
Assessment of the quality of the evidence using the GRADE approach
For this update we assessed the quality of the evidence using the GRADE approach, as outlined in the GRADE handbook. We assessed evidence relating to the following outcomes for the comparison home uterine monitoring versus standard care.
-
Perinatal mortality
-
Preterm birth less than 34 weeks' gestation
-
Number of antenatal hospital admissions
-
Preterm birth less than 37 weeks' gestation
-
Admission to neonatal intensive care unit (NICU)
-
Number of unscheduled antenatal visits
-
Use of tocolysis
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5 (RevMan 2014), in order to create a ’Summary of findings’ table. We produced a summary of the intervention effect and a measure of quality for each of the above outcomes, using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
We presented results as a summary risk ratio (RR) with a 95% confidence interval (CI).
Continuous data
We used the mean difference (MD) if outcomes were measured in the same way between trials. We planned to use the standardised mean difference (SMD) to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We have not included cluster‐randomised trials in this review. If relevant for the next update, we will include cluster‐randomised trials in the analyses along with individually‐randomised trials. We will adjust their sample sizes using the methods described in the Handbook (Section 16.3.4 or 16.3.6), using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and if we consider that the interaction between the effect of intervention and the choice of randomisation unit is unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform sensitivity or subgroup analysis to investigate the effects of the randomisation unit.
Cross‐over trials
We have not included cross‐over trials in this review and do not plan to do so in future updates. This design is not relevant to our review question.
Other unit of analysis issues
We have included trials involving women with multiple pregnancies in this review. Where possible, we have analysed multiple pregnancy in subgroups (see analyses for the outcomes 'Preterm birth less than 34 weeks'; 'Preterm birth less than 37 weeks'; and 'Number of antenatal visits'), but only one study for each of the outcomes provided subgroup data.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, if more eligible studies are included, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we conducted analyses as far as possible on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes we knew to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau2, I2 and Chi2 statistics. We regarded heterogeneity as substantial if an I2 was greater than 30% and either the Tau2 was greater than zero, or there was a low P value (less than 0.10) in the Chi2 test for heterogeneity. If we identified substantial heterogeneity (above 30%) for primary outcomes, we explored it by prespecified subgroup analysis.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager 5 software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect, i.e. where trials were examining the same intervention, and we judged the trials’ populations and methods to be sufficiently similar.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if we detected substantial statistical heterogeneity, we used random‐effects meta‐analysis to produce an overall summary, if we considered an average treatment effect across trials was clinically meaningful. We treated the random‐effects summary as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. Where we used random‐effects analyses, we present the results as the average treatment effect with a 95% confidence interval, and the estimates of Tau2 and I2.
Subgroup analysis and investigation of heterogeneity
Where we identified substantial heterogeneity, we investigated it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, we used random‐effects analysis to produce it.
We carried out the following subgroup analyses:
-
singleton gestation versus twin gestation.
We had also planned to carry out the following subgroup analyses:
-
gestational age at which home uterine activity monitoring (HUAM) began;
-
type of HUAM used;
-
reason HUAM was used.
The outcomes to be used in the subgroup analysis were:
-
preterm birth less than 34 weeks;
-
perinatal mortality.
We assessed subgroup differences by interaction tests available within Review Manager 5 (RevMan 2014). We reported the results of subgroup analyses, quoting the Chi2 statistic and P value, and the interaction test I2 value.
We conducted additional subgroup analyses (singleton/twin) for the following outcomes:
-
preterm birth less than 37 weeks;
-
respiratory distress syndrome;
-
number of unscheduled antenatal visits.
Sensitivity analysis
We undertook sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, excluding poor‐quality studies from the analyses, to assess whether this made any difference to the overall result.
Results
Description of studies
Results of the search
We identified 15 studies (6008 enrolled participants); 13 studies contributed data. All 15 included studies were randomised controlled trials, apart from one quasi‐randomised trial (Morrison 1987). The earliest trials, according to recruitment details in the studies, started in 1986 (Dyson 1991; Iams 1987), while the last ones (Brown 1999; Dyson 1998) completed recruiting in 1996. See also: Description of studies and Figure 1

Study flow diagram.
Two included studies did not contribute to the data analysis: Porto 1987 (no relevant outcomes); Scioscia 1988 (problems with back‐calculation from percentages provided). For Hill 1990a, we have relied on the Keirse 1993 re‐analysis to identify the trial reports associated with this study, as sites appear to have reported separately.
From the updated search in May 2016, we retrieved one report from the Pregnancy and Childbirth Group Register (NCT02379351). We found no further trial reports in CENTRAL (the Cochrane Library), and one each in MEDLINE, Embase and CINAHL. We screened out these three at title and abstract as not being within in the scope of this review.
Included studies
Setting
All trials except one (Blondel 1992, conducted in France) were conducted in the USA.
Sample size
The smallest trials had fewer than 100 participants (e.g. Iams 1990; Lyons 1990; Morrison 1987; Nagey 1993); the largest trials had over 1000 participants (e.g. CHUMS 1995; Dyson 1998). In total, we collected data from 6008 enrolled and randomised participants.
Participants
Participants were women who had successfully been treated for preterm labour in the current pregnancy (Brown 1999; Hill 1990a; Iams 1990; Nagey 1993) or who were judged to be at risk (without prior treatment for preterm labour). One study (Blondel 1992) included both categories. The risk factors used in the studies varied. Some studies included twin and singleton gestations within the sample (e.g. Blondel 1992), others specified that only singleton gestations were included (e.g. Brown 1999), others included twin and singleton gestations but separated the groups for analysis (e.g. Dyson 1991; Dyson 1998), and one report (Hill 1990a) only studied twin gestations. Several trials focused on prenatal care for socially disadvantaged women, and two trials specified Medicaid coverage as one of the criteria for inclusion (Brown 1999; Morrison 1987). Other criteria for inclusion include possession of a telephone, and ability to use the monitoring device. There were differences among studies between the number of those judged eligible on medical and social demographic criteria and those randomised. For example, in CHUMS 1995, a large 18‐centre trial, 1355 women were enrolled and 1292 were randomised (95% of those enrolled). In a trial among 30 Kaiser Permanente clinics in northern California (Dyson 1998), 3455 women were identified as eligible, 2480 were enrolled, and 2422 eventually randomised (to one of three treatment groups) (97% of those enrolled). Corwin 1996 used the Creasy risk score; of those judged eligible (n = 509) on criteria including a Creasy risk score of greater than or equal to 10, 377 were enrolled and randomised (74% of those enrolled). In screening, many women did not meet the criterion of a Creasy risk score greater than or equal to 10, and of 509 who met initial screening criteria, 37 (7.3%) did not possess a telephone. One small study (Lyons 1990) examined military dependents.
Interventions
The type of interventions may be categorised into:
-
home uterine monitoring plus perinatal nursing contact versus standard care, with perinatal nursing contact varying from acknowledgement of receipt of transmissions (e.g. Corwin 1996; Wapner 1995) through to discussion on preterm labour management (Dyson 1998; Iams 1987);
-
home uterine monitoring (active versus sham device), with scripted protocol used for re‐monitoring (e.g. CHUMS 1995) or more general discussion between the monitoring centre perinatal nurses and participants about other records of signs of preterm labour (Dyson 1991).
In most studies, authors described how both experimental and control groups received education in self‐palpation, signs and symptoms of preterm labour, instructions on when to call for further professional advice as required (Brown 1999; Corwin 1996; Hill 1990a; Morrison 1987; Nagey 1993; Wapner 1995), and the following additionally asked both groups to make their own records (Dyson 1991; Dyson 1998; Iams 1987; Iams 1990).
All included studies were randomised controlled trials, apart from one quasi‐randomised trial (Morrison 1987). The earliest trials, according to recruitment details in the studies, started in 1986 (Dyson 1991; Iams 1987) and the last ones (Brown 1999; Dyson 1998) completed recruiting in 1996. See also: Description of studies.
Two included studies did not contribute to the data analysis: (Porto 1987) (no relevant outcomes) and Scioscia 1988 (problems with back‐calculation from percentages provided). For one included study (Hill 1990a), we have relied on the Keirse 1993 re‐analysis to identify the trial reports associated with this study, as sites appear to have reported separately.
We did not identify any trials comparing home monitoring with an alternative form of surveillance.
Excluded studies
The following excluded studies contained insufficient clinical data for analysis, or indeed for confirmation that the trials were truly in scope: Ogburn 1993; Tõrõk 1994. We did not identify any further reports of these studies. In Birnie 2000; Blondel 1988; Dawson 1999; Goulet 1999; Goulet 2001; Iedema 1994; Iedema‐Kuiper 1996; Monincx 1997; Monincx 2001; Reece 1992; Spira 1981; Spira 1986, Su 2002, the intervention was out of scope. Merkatz 1991 was a general review, as was Blondel 1990.
Risk of bias in included studies
Allocation
We rated four studies at low risk of bias for sequence generation. The CHUMS 1995 and Dyson 1998 trials used computer‐generated randomisation sequence allocation schemes and were assessed as being at low risk of bias for sequence generation. Corwin 1996 and Wapner 1995 used external randomisation services. Wapner 1995 used separate blocked randomisation at each site; Corwin 1996 used different random‐number sequences for each site; Dyson 1998 and Corwin 1996 stratified by gestation status (singleton or twin) and by site. We rated two studies at high risk of bias for sequence generation: Morrison 1987 used hospital numbers and Iams 1987 did not describe how the sequence was generated and reported an unbalanced sample. We judged the remaining studies to be at unclear risk, as no or unclear information was provided on sequence generation.
We assessed six studies at low risk of bias for allocation concealment (Brown 1999; CHUMS 1995; Corwin 1996; Hill 1990a; Nagey 1993; Wapner 1995); these studies used external randomisation services or sealed opaque envelopes for allocation concealment. In Morrison 1987 and Dyson 1991 staff carrying out randomisation may have been aware of allocation and we rated these studies at high risk of bias for these domains. In the remaining studies there was insufficient information or the method used to conceal allocation at the point of randomisation was not described at all. (see Table 1)
| Methodological item | Adequate | Inadequate |
| Generation of random sequence | Computer‐generated sequence, random‐number tables, lot drawing, coin‐tossing, shuffling cards, throwing dice | Case number, date of birth, date of admission, alternation |
| Concealment of allocation | Central randomisation, coded drug boxes, sequentially‐sealed opaque envelopes | Open allocation sequence, any procedure based on inadequate generation |
Blinding
Only in the two studies that used active versus sham devices (CHUMS 1995; Dyson 1991) were the participants, and monitoring centre staff unaware of the group allocation. Some studies mention specific instructions to participants not to inform caregivers of their group allocation on admission to hospital, or checks to ensure that caregivers were not informed of group allocation. There was an attempt at blinding staff caring for women and we rated these studies as unclear for performance bias (Corwin 1996; Dyson 1998; Morrison 1987; Wapner 1995). Many studies provided few details or indicated that caregivers were aware of at least some of the allocations; we rated these studies at high risk of bias for performance blinding (Figure 2).

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
We judged four studies to be at low risk of detection bias, as either a sham device was used or we considered that the outcomes reported were objective and unlikely to be affected by lack of blinding (CHUMS 1995; Corwin 1996; Nagey 1993; Porto 1987). In the remaining studies the risk of detection bias was either unclear, or at high risk of bias due to lack of blinding and the type of outcomes reported.
Incomplete outcome data
Four of the 13 studies had fewer than 5% withdrawals (Blondel 1992; Dyson 1991; Dyson 1998; Morrison 1987), while one study (Corwin 1996) had between 5% and 9.9% attrition (fetal deaths prior to observation, and noncompletion), and we rated these studies at low risk of attrition bias. The largest study in this group (Dyson 1998) notes that all those randomised completed the study, but 93 women (4%) did not receive the surveillance to which they were assigned. The statistical analysis presented is based on intention‐to‐treat, with the results on a completion‐of‐protocol basis stated to be similar. It seems that Lyons 1990 may have had no withdrawals, although this was not explicitly reported and we rated this study as being at unclear risk.
We judged two studies to be at high risk of bias due to high attrition or loss that may have related to outcomes (Hill 1990a; Scioscia 1988). Hill 1990a notes withdrawals and fetal/medical complications. Hill 1990a presents data for women who experienced preterm labour after entry to the trial, but there are discrepancies among the various reports of this study (Keirse 1993), and it is difficult to back‐calculate with certainty from the data provided. In the Scioscia 1988 trial five of the 72 women randomised were removed from the analysis post randomisation; it wasn't clear how many of these women were lost from the intervention and control groups, and so group denominators weren't clear, and we were unable to include data from this study.
We rated the remaining studies as unclear for attrition bias. Four of them (Brown 1999; Iams 1987; Iams 1990; Wapner 1995) had between 10% and 19.9% attrition. Brown 1999 states the main reason for attrition was a change in circumstances; Iams 1987 notes problems over noncompliance with the protocol, with women who did not monitor as requested for more than three days deemed to be noncompliant. The differences in noncompliance between years one and two of this study were statistically significant. A companion project (Iams 1990) also found noncompliance with the protocol to be a problem; Wapner 1995 notes a variety of reasons for withdrawals.
Nagey 1993 reports that only one woman in the control group was lost to follow‐up, but two women were excluded from the analysis on medical grounds, and four women in the experimental group never left hospital and thus did not receive the intervention.
Two studies (CHUMS 1995; and one contributory report to Hill 1990a (Knuppel 1990a)) had an attrition rate exceeding 20%, although CHUMS 1995 (one of the largest trials) followed up all women who started monitoring, including noncompliant ones, and withdrawals until delivery. Participants who did not transmit for over 48 hours were deemed noncompliant (unless the reason concerned hospital admission or delivery). The results are presented on an intention‐to‐treat basis (but only for those who started monitoring); the authors state that the per‐protocol results are similar. No details are provided for the 127 women who were randomised but did not start monitoring.
Selective reporting
In six studies we did not find evidence of outcome reporting bias and we rated them at low risk of bias (Blondel 1992; Brown 1999; CHUMS 1995; Dyson 1998; Iams 1987; Morrison 1987).
We assessed four studies at high risk of reporting bias. Iams 1990 states that all births before 37 completed weeks were reviewed in detail, but data are only provided for births before 36 weeks, and the end point is different from the companion trial (Iams 1987). Wapner 1995 does not provide the total number of preterm births (mean gestational age only), and outcomes are mostly reported for women diagnosed with preterm labour, a subset of the participants. The Watson 1990 trial report for Hill 1990a presents preterm birth data for the 34 of 86 participants with recurrent preterm labour, but there are data missing for the entire group of participants. It is unclear from the reports for Hill 1990a how the subgroups were formed, whether different sites followed the same or slightly different protocols, or how reporting was organised among the sites (Keirse 1993). Many of the end points in the included studies and outcome measurements (e.g. changes in cervical dilatation) do not map to those selected for the review. In Scioscia 1988 there were insufficient data to support results.
In the remaining six studies it was unclear whether there was outcome reporting bias; for example, Corwin 1996 does not report twin gestation outcome data, and Dyson 1991 includes the number of unscheduled visits for the twin data, but does not report the singleton data separately (reports "all women").
Other potential sources of bias
We assessed five studies to be at high risk of other bias, due to lack of lack of power calculations, being underpowered, protocol deviations or for poor reporting (Blondel 1992; Dyson 1991; Hill 1990a; Iams 1990; Nagey 1993). We rated three studies at low risk of other bias, and seven were at unclear risk.
Several studies report power calculations, but there is little consistency in the estimation assumptions. CHUMS 1995 (one of the largest trials) was designed to have 80% power, for a group difference of 1 cm (variance 1.4 cm) in change of cervical dilatation from the previous visit, when preterm labour was diagnosed, and assumed a 20% occurrence rate of preterm labour. Dyson 1998 used similar assumptions. Other studies (e.g. Brown 1999; Iams 1987; Nagey 1993) based their power calculations on a percentage reduction in preterm deliveries before 37 weeks. Other studies (e.g. Corwin 1996) did a power calculation based on the proportion of early detection of preterm labour possible, and assumed 30% would develop preterm labour.
Effects of interventions
See: Summary of findings for the main comparison Home uterine monitoring for preventing preterm birth
Primary outcomes
Infant primary outcomes
Perinatal mortality rate
Only two studies (Blondel 1992; Dyson 1998) with 2589 women, reported this outcome (Analysis 1.1) and although home uterine monitoring was associated with higher perinatal mortality (risk ratio (RR) 1.22, 95% confidence interval (CI) 0.86 to 1.72), the confidence interval was wide and crossed the line of no effect. No subgroup analysis was possible for singleton/twin pregnancy.
Preterm birth at less than 34 weeks' gestation
There were fewer preterm births at less than 34 weeks' gestation in the home uterine monitoring group compared with controls (RR 0.78, 95% CI 0.62 to 0.99; three studies; n = 1596 (Analysis 1.2)). A fourth study (Scioscia 1988) provided no usable data. Of the three studies (CHUMS 1995; Dyson 1991; Nagey 1993) that measured this outcome, two of them (contributing over 93% by weight) compared active versus sham home uterine monitoring. The largest study (CHUMS 1995) provided data for those both randomised and monitored, and the authors state that the findings for the "subset who completed the protocol" were similar. If 36 women in a 1000 are likely to experience preterm birth at less than 34 weeks (CDC 2007), then home uterine monitoring might reduce the number at risk to between 21 and 36.
However, in a sensitivity analysis, the largest trial (CHUMS 1995: 72% contribution to this outcome) has low risk of bias for allocation, blinding, and selective reporting. The other two contributing trials (Dyson 1991; Nagey 1993) were at greater risk of bias (Figure 3). Excluding the data from the two trials at higher risk of bias, results show a slight difference in the upper confidence interval (changed from 0.99 to 1.00) but still favouring the home uterine monitoring group (RR 0.75, 95% CI 0.57 to 1.00; P = 0.05). Using the same scenario as above (36 women in a 1000 are likely to experience preterm birth at less than 34 weeks), then home uterine monitoring might change the number at risk to between 19 and 36. Only one study (Dyson 1991) at higher risk of bias provided singleton and twin data separately for preterm birth at less than 34 weeks' gestation. Home uterine monitoring was not associated with a decrease in the number of preterm twin births at less than 34 weeks' gestation (RR 0.55, 95% CI 0.26 to 1.17; one study, n = 109). Similarly, there was no statistically significant difference in the number of preterm singleton births at less than 34 weeks' gestation (RR 1.12, 95% CI 0.55 to 2.27; one study, n = 138); seeAnalysis 1.3.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Prenatal primary outcomes
Number of days in hospital antenatally
Lyons 1990 reports that women using home uterine monitoring spent 59 days in hospital antenatally (mean 1.9 days), compared with the control group that spent 169 days (mean 5.4 days). No other data are given.
Secondary outcomes
Infant secondary outcomes
Preterm birth (less than 37 weeks)
Nine studies (out of 15) assessed this outcome, but it was not possible to use the Scioscia 1988 data, and the data were analysed differently among the studies (see footnotes in Analysis 2.1). Women using home uterine monitoring were not less likely to experience preterm birth at less than 37 weeks (average RR 0.85, 95% CI 0.72 to 1.01; eight studies, n = 4834; random‐effects, Tau2 = 0.03, I2 = 68% (Analysis 2.1)). However, there was substantial heterogeneity. In Hill 1990a, data analysis focused on the women who experienced preterm labour, and the data presented in Analysis 2.1 are based on a back‐calculation of figures presented in the discussion. Given the difficulties of assessing how many women were actually included in this study (Keirse 1993), the figures presented are only a best estimate. Excluding the two studies at high risk of bias (Hill 1990a; Morrison 1987) does not change the findings (average RR 0.94, 95% CI 0.82 to 1.06; six studies, n = 4521; random‐effects, T2 = 0.01, I2 = 39%). Limiting the analysis to the studies at lower risk of bias (Blondel 1992; CHUMS 1995; Dyson 1998) suggests no clear difference (average RR 0.97, 95% CI 0.84 to 1.11; three studies, n = 3881; random‐effects, Tau2 = 0.031, I2 = 49%).
A small group of studies included data for twin gestations. One report of Hill 1990a apparently presents some data for the twin gestations, focusing on the 30 women who experienced preterm labour. The authors indicate that for the group "in general", 50% of the monitored women delivered preterm compared with 81% of the controls, but it is unclear what denominator is being used. Dyson 1998 presented singleton and twin gestation data. The analysis indicates that women using home uterine monitoring with twin gestation were no less likely to experience preterm birth before 37 weeks (RR 0.96, 95% CI 0.71 to 1.30; n = 844). Dyson 1998 showed no difference in the number of women with singleton gestation using home uterine monitoring who experienced birth before 37 weeks (RR 0.95, 95% CI 0.62 to 1.45; one study, n = 2422). SeeAnalysis 2.2.
Very preterm birth delivery less than 32 weeks
Women using home uterine monitoring were no less likely to experience preterm birth at less than 32 weeks (average RR 0.76, 95% CI 0.31 to 1.85; three studies, n = 2550; random‐effects, Tau2 = 0.36, I2 = 56%), seeAnalysis 2.3. Only Dyson 1998, Morrison 1987 and Nagey 1993 assessed this outcome.
Extremely preterm birth delivery less than 28 weeks
No data available.
Air leak syndrome
No data available.
Necrotising enterocolitis
No data available.
Patent ductus arteriosus requiring treatment
No data available.
Chronic lung disease
No data available.
Retinopathy of prematurity
No data available.
Use of antenatal corticosteroids
Brown 1999 reports that the use of antenatal corticosteroids was similar in both groups (56 women, 69.1% versus 54 women, 67.5%; RR 1.01, 95% CI 0.82 to 1.25; one study; n = 162 (Analysis 2.4)).
Respiratory distress syndrome (RDS)
Dyson 1998 reports that for women with singleton gestations, and less than 34 weeks' gestation, five out of 19 infants from the home uterine monitoring group developed RDS, compared with four out of 19 in the control group (RR 1.25, 95% CI 0.40 to 3.95; one study, n = 38 (Analysis 2.5)), no difference.
Similarly, we found no difference for twins at less than 34 weeks' gestation where four out of 44 infants from women using home uterine monitoring developed RDS, compared with 10 out of 42 in the control group (with sham device) (RR 0.38, 95% CI 0.13 to 1.12; n = 86 (Analysis 2.5)).
Neuropathology on ultrasound (intraventricular haemorrhage all grades, severe grades three or four, periventricular leukomalacia)
No data available.
Use of mechanical ventilation
Two relatively small studies assessed this outcome (Brown 1999; Corwin 1996), the latter providing singleton data only, for women with preterm labour. Infants from the monitored group were not significantly less likely to need mechanical ventilation (average RR 0.31, 95% CI 0.04 to 2.38; two studies, n = 539; random‐effects, T2 = 0.86, I2 = 37% (Analysis 2.6)).
Admission to neonatal intensive care unit
Infants born to women in the home uterine monitoring group were less likely to be admitted to a neonatal intensive care unit than infants born to control group women (average RR 0.77, 95% CI 0.62 to 0.96; five studies, n = 2367; random‐effects, Tau2 = 0.02, I2 = 32% (Analysis 2.7)). Five studies measured this outcome (Brown 1999; CHUMS 1995; Corwin 1996; Dyson 1991; Wapner 1995), but the reporting is incomplete (Wapner 1995), limited to singleton gestations (Corwin 1996), or apparently flawed (Dyson 1991).
CHUMS 1995, the study with the lowest risk of bias in the group, states that 28.5% of all infants born to women in the monitored group were admitted to neonatal intensive care, compared with 32% of all infants born to control group women. However, a sensitivity analysis, excluding the studies at higher risk of bias, leaves CHUMS 1995, which did not find such a big difference (RR 0.86, 95% CI 0.74 to 1.01; n = 1292).
Mode of delivery
Brown 1999 found the same level of caesarean delivery (7/82 in monitored group, 7/80 in controls) (RR 0.98, 95% CI 0.36 to 2.66; one study, n = 162 (Analysis 2.8)).
(2) Secondary outcomes (prenatal)
Number of antenatal visits (unscheduled)
Blondel 1992 cites the average number of "visits to the outpatient clinic" as 3 ± 2.3 for the monitored group (n = 84), 3 ± 1.9 (n = 83) for the control group, in a care delivery system that relied on home visiting by midwives, and three scheduled visits to the outpatient clinic. CHUMS 1995 states that 3% of both the experimental and control groups made unscheduled emergency visits.
Five other studies (Dyson 1991; Dyson 1998; Hill 1990a; Morrison 1987; Wapner 1995) measured the mean number of unscheduled visits to an obstetrician but standard deviations were not available for three studies (Dyson 1991; Morrison 1987; Wapner 1995). Consequently data from only two studies (Dyson 1998; Hill 1990a) contributed to the analysis. The mean number of unscheduled visits was higher among the home uterine monitoring group than in the control group (mean difference (MD) 0.48, 95% CI 0.31 to 0.64; two studies, n = 1994 (Analysis 3.1)).
A review (Reichmann 2009) examined whether home uterine monitoring might be more effective for multiple gestations. One study (Dyson 1998) provided data on twin pregnancies separately: for the twin pregnancies the mean number of unscheduled visits among the home uterine monitoring group is higher (MD 0.60, 95% CI 0.24 to 0.96; one study; n = 564), comparing the daily contact and home uterine monitoring groups. The data for singleton gestations were not reported separately, but have been estimated, with the mean number of unscheduled visits among the home uterine monitoring group higher (MD 0.40, 95% CI 0.15 to 0.65; one study; n = 1060) Analysis 3.3)).
Number of antenatal hospital admissions
Three studies (Blondel 1992; Brown 1999; CHUMS 1995) assessed this outcome. There is no statistically significant difference between the number of antenatal hospital admissions in the monitoring and control groups (RR 0.91, 95% CI 0.74 to 1.11; three studies, n = 1494 (Analysis 3.2)).
Number of midwife/nurse home visits
This was not a variable measured in most of the studies. In Blondel 1992, the control group received home visits by community midwives, whereas the experimental group received weekly visits from the monitoring centre midwife. In other studies, home uterine monitoring was an addition to standard high‐risk care (Brown 1999; Corwin 1996; Hill 1990a; Iams 1987; Iams 1990; Morrison 1987; Nagey 1993; Wapner 1995). The pattern of visits was determined by the protocol. In the studies that compared the active versus sham monitoring device (CHUMS 1995; Dyson 1991), protocols dictated the scope and frequency of interactions with monitoring centre perinatal nurses. Dyson 1998 used a three‐group design to compare: 1) monitoring with daily contact; 2) daily contact (control); 3) weekly contact (control) with the perinatal monitoring centre.
Use of tocolysis
Use of prophylactic tocolytic drug therapy was higher for the home uterine monitoring group compared with the control group (average RR 1.21, 95% CI 1.01 to 1.45; seven studies, n = 4316; random‐effects,Tau2 = 0.03, I2 = 62% (Analysis 3.4)). However, we observed substantial heterogeneity.
A sensitivity analysis, excluding studies at higher risk of bias from the analysis of the use of tocolysis, leaves three trials (Blondel 1992; CHUMS 1995; Dyson 1998). A random‐effects meta‐analysis showed no difference in the use of tocolysis among the women using home uterine monitoring (average RR 1.22, 95% CI 0.90 to 1.65, random‐effects, Tau2 = 0.05, I2 = 76%). CHUMS 1995 reports that tocolysis use was 31% in both monitored and control groups at any time during pregnancy, and 60% for participants diagnosed with preterm labour after enrolment.
(3) Secondary outcomes (maternal)
Maternal anxiety
No data provided.
Maternal acceptability of home uterine monitoring
No studies assessed maternal acceptability directly. One of the largest trials (Dyson 1998) notes that women in the home uterine monitoring group complied with the requirement of at least one daily session of monitoring 86% of the time. In another large trial (active versus sham device), CHUMS 1995 found that noncompliance was 12.5% in the experimental group and 12.7% in the control (noncompliance was assessed as failure to transmit for more than 48 hours).
Discussion
Summary of main results
Primary outcomes
Home uterine monitoring was not associated with a difference in perinatal mortality (on the basis of two studies). Home uterine monitoring was associated with fewer preterm births at 34 weeks (based on three studies). However, this difference was no longer apparent when we conducted a sensitivity analysis based on trial quality, restricting the analysis to a single study graded as being at low risk of bias. One study reported that women using home uterine monitoring spent fewer days in hospital than the control group.
Secondary outcomes
Women using home uterine monitoring were not less likely to experience preterm birth at less than 37 weeks (on the basis of eight contributing studies). Infants born to women in the home uterine monitoring group were less likely to be admitted to a neonatal intensive care unit (NICU) than infants born to control group women, although this difference was no longer apparent when we included only those studies assessed as being at lower risk of bias. There was no difference between the number of antenatal hospital admissions for the monitoring and control groups. The number of unscheduled hospital visits appeared to be higher among the monitored women. Use of prophylactic tocolytic drug therapy was higher among the home uterine monitoring group than the control group. However, the difference was no longer apparent when we restricted our analysis to high‐quality studies.
Overall completeness and applicability of evidence
The trials were clinically heterogeneous. Risk factors for preterm labour were assessed differently, and the delivery of the home uterine monitoring intervention varied. Some trials (e.g. Corwin 1996) used non‐professional call centre staff to receive the transmissions sent by the pregnant women, while in others the contact was more active (e.g. Dyson 1991; Dyson 1998; Iams 1987; Iams 1990), and CHUMS 1995 used a scripted protocol. There appears to be no consensus from these trials on the extent of professional midwifery support deemed appropriate. We do not know what the women thought of their care regimen in the trials where the centre staff receiving the transmissions only acted as conduits, giving no advice directly. It is possible that the lower withdrawal rates in Dyson 1991 and Dyson 1998 could be attributed to the more intensive and personal nursing or midwifery contact, but the organisational setting (a Health Maintenance Organization) for those two trials was different from the settings in the other trials. Only one study (Morrison 1987) included costings, reporting the financial incentive in this case to reduce the number of hospital days associated with a preterm birth among pregnant women receiving Medicaid public assistance. Although a home uterine monitoring system might be appropriate for women in socio‐economically disadvantaged groups, only Brown 1999 targeted this group, and other studies excluded those who could not speak English, or who did not have a telephone.
Many telemedicine trials assess the acceptability of the system to participants and providers, but none of the trials presented data directly on this, and only one related trial (Blondel 1990) presents data on mothers' views of prenatal care with a home visiting system, with later overview of three trials involving home visiting. Arguably, the system architectures for home uterine monitoring involve choices between a) objective monitoring (checking up on education provided) or empowering women to monitor themselves, asking advice as necessary, or b) maintaining or enhancing participant contact (Urquhart 2010). Analysis of the included trials provides few clues on the best way to implement a care delivery system, including how monitoring should be organised, although the topic has been considered (e.g. Merkatz 1991).
All the included studies are based on the premise that increased uterine activity can be used as a predictor of possible preterm labour, and thus the effectiveness of home uterine monitoring as a screening tool is being assessed here. Pregnancy outcomes then depend on the effectiveness of the management of diagnosed preterm labour. Determining the contribution of home uterine monitoring to outcomes is therefore complex, especially in multicentre studies and studies in different countries and with different populations, where approaches to the management of preterm labour may differ. ‘Usual care’ for control groups was not the same in all studies, and some authors set out to investigate the effects of intensive nursing care compared with, or combined with, home uterine monitoring. In most of the studies the women in the home uterine monitoring group also had daily contact with the nurses monitoring their transmitted results, but in, for example, Wapner 1995, the daily nurse contact was withheld from the monitored group. The role of intensive nursing support was not the primary intervention being assessed in any of these studies, and although some authors have attempted to assess its role alongside, or instead of, home uterine monitoring, this is not possible from the data presented. The use of intensive antenatal care (from midwives and others) in the emotional and social support of pregnant women has been the subject of another Cochrane Review (Hodnett 2010) and demonstrates the difficulty of assessing the precise contribution of nursing care to pregnancy outcomes. The home uterine monitoring study authors who conclude that intensive nursing care may be as effective as home uterine monitoring and may provide other benefits for women at risk, are right to suggest that further targeted research in this area is required.
Quality of the evidence
The main reason for downgrading the quality of the evidence was design limitations in the studies contributing data. In the studies that were not testing sham versus real home uterine monitoring devices, it would have been impossible to blind the participants to their allocation, and, depending on the way care was organised, some of the caregivers might easily learn the allocation. This could affect, for example, the use of tocolytic drugs. For objective outcomes, the fact that many of the trials scored 'unclear' for blinding is not a major concern.
One of the main difficulties with the meta‐analyses conducted for the review was the low number of studies that contributed to any particular outcome measurement, and subgroup analysis for any outcome was only possible for one study within each outcome group. It was therefore impossible to clarify whether home uterine monitoring might be more effective for twin gestations, as considered in one review (Reichmann 2009). In addition, if we exclude studies at higher risk of bias from some of the meta‐analyses there is no clear evidence of any difference between the experimental and control groups (Figure 2).
For the primary outcome of perinatal mortality, the two contributing studies are of equal quality scores (Figure 3). The inadequate blinding should not affect this outcome, but the quality of evidence is low (GRADE).
For the primary outcome of the number of preterm births at 34 weeks or less, the largest trial (CHUMS 1995, 75% contribution to this outcome) is one of the higher‐quality studies for allocation, blinding, and selective reporting. Relying solely on the findings from this trial very marginally reduces the size of effect. The overall quality of evidence is high (GRADE).
For secondary outcomes, excluding the studies at high risk of bias from the meta‐analysis of the data for NICU admission reduced the strength of the evidence of the difference between monitoring and control groups. Evidence for admission to NICU was graded overall as of moderate quality (GRADE). The analysis of the two contributing studies (Dyson 1998; Hill 1990a) on unscheduled antenatal visits indicates that the monitored women made more visits, but another study (CHUMS 1995) which used the number of women as the unit of analysis indicates that there was no clear difference between the groups. We rated the evidence for the outcome of number of unscheduled antenatal visits as of moderate quality (GRADE). There were no group differences in the numbers of women admitted to hospital during the antenatal period, and we rated this evidence as low quality (GRADE).
In the analysis of the use of tocolysis, there was no strong evidence of effect when we excluded studies at high risk of bias from the analysis. Clinical protocols for use of tocolysis very likely varied from study to study. The overall quality of evidence is low (GRADE). Women using home uterine monitoring were not less likely to experience preterm birth at less than 37 weeks, and the quality of evidence was very low (GRADE). We cannot determine the role of tocolysis from these studies.
We selected outcomes that we considered best reflected the potential benefits and harms of home uterine monitoring. We included infant outcomes that were covered in a recently‐published core outcome set for studies on preterm birth prevention (Van't Hooft 2015). Our infant outcomes include those relating to gestational age at delivery (birth before 37, 34, 32 and 28 weeks' gestation), and we also included infant mortality and morbidity outcomes. Our key maternal outcomes relate specifically to home uterine monitoring: maternal anxiety, and acceptance of home monitoring rather than to more general maternal morbidity outcomes which form part of the core outcome set. We will consider including these outcomes in future updates.
Potential biases in the review process
We attempted to reduce bias in the review process by ensuring that at least two of the review authors assessed all study reports. Two review authors independently assessed risks of bias in the individual trial reports. Although we took steps to try to minimise bias, we are aware that evaluation of risk of bias and evidence quality is partly a matter of judgement, and accept that a different review team may have made different judgements.
Agreements and disagreements with other studies or reviews
The ICSI 2002 committee report found that home uterine activity monitoring was a safe procedure, but noted that its effectiveness was not proven. Reichmann 2008 reviewed home uterine monitoring studies that included women in current preterm labour at enrolment and concluded that home uterine activity monitoring was not proven to be effective. Reichmann 2009 also concluded that home uterine monitoring was not useful for women with multiple gestations, and cited a study that showed that uterine contractions did not indicate preterm birth in twins. An earlier review (Keirse 1993) re‐analysed data from Hill 1990a, and concluded that this set of studies on the "Term Guard" system appeared to be flawed in design and execution. In addition, the studies provided no data on infant morbidity and mortality. Grimes 1992 also concludes that there were serious methodologic deficiencies in the published trials. On the other hand, Newman 2005 reports in a review of trials that "home uterine contraction monitoring with or without frequent perinatal nursing contact can reduce the risk of preterm birth and improve perinatal outcomes and that both are independently superior to standard preterm birth prevention education and care". Newman 2005 mentions another meta‐analysis by Colton 1995 that included the same studies as in Grimes 1992, plus some other studies. One reason for the discrepancies in the conclusions of these various meta‐analyses is that, as Newman 2005 acknowledges, the benefits become insignificant when the denominator is changed from the women in preterm labour to the entire randomised cohort. The meta‐analysis by Colton 1995 (in favour of home uterine monitoring) was partially supported by one of the device manufacturers.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Comparison 1 Home uterine monitoring versus standard care ‐ primary outcomes, Outcome 1 Perinatal mortality.
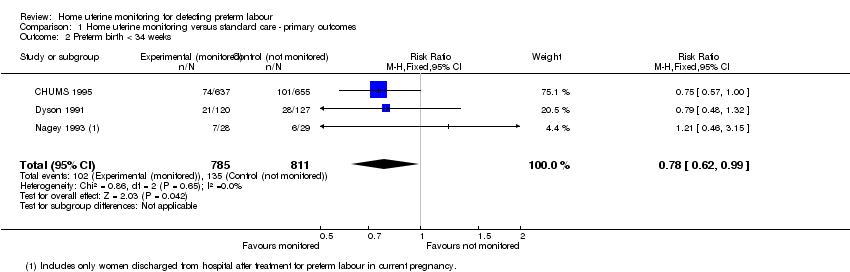
Comparison 1 Home uterine monitoring versus standard care ‐ primary outcomes, Outcome 2 Preterm birth < 34 weeks.

Comparison 1 Home uterine monitoring versus standard care ‐ primary outcomes, Outcome 3 Preterm birth < 34 weeks (Subgroup analysis).
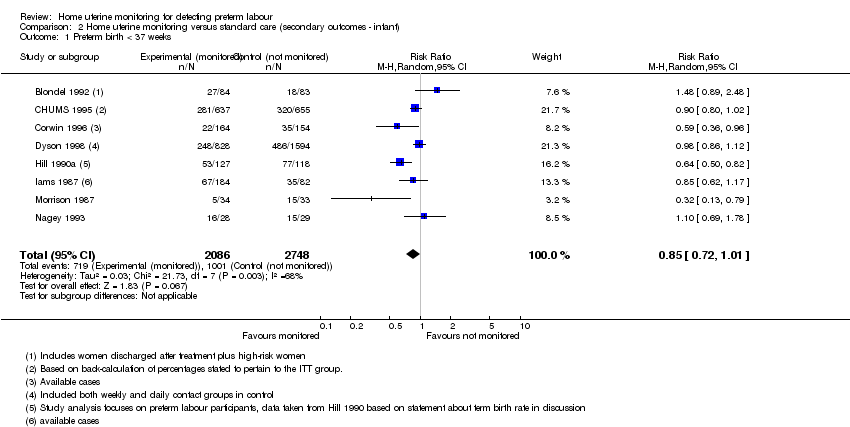
Comparison 2 Home uterine monitoring versus standard care (secondary outcomes ‐ infant), Outcome 1 Preterm birth < 37 weeks.

Comparison 2 Home uterine monitoring versus standard care (secondary outcomes ‐ infant), Outcome 2 Preterm birth < 37 weeks (Subgroup analysis).

Comparison 2 Home uterine monitoring versus standard care (secondary outcomes ‐ infant), Outcome 3 Preterm birth < 32 weeks.
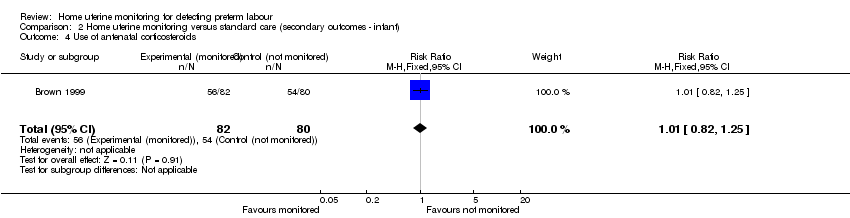
Comparison 2 Home uterine monitoring versus standard care (secondary outcomes ‐ infant), Outcome 4 Use of antenatal corticosteroids.

Comparison 2 Home uterine monitoring versus standard care (secondary outcomes ‐ infant), Outcome 5 Respiratory distress syndrome.
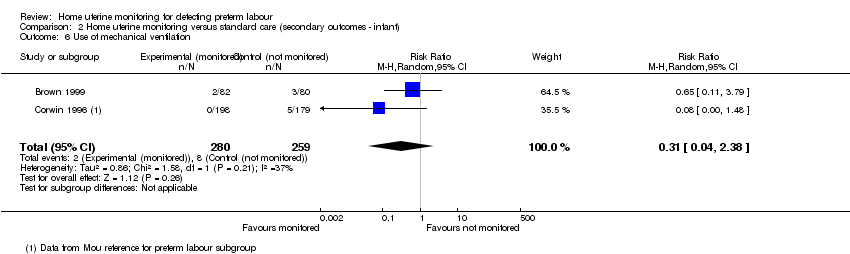
Comparison 2 Home uterine monitoring versus standard care (secondary outcomes ‐ infant), Outcome 6 Use of mechanical ventilation.
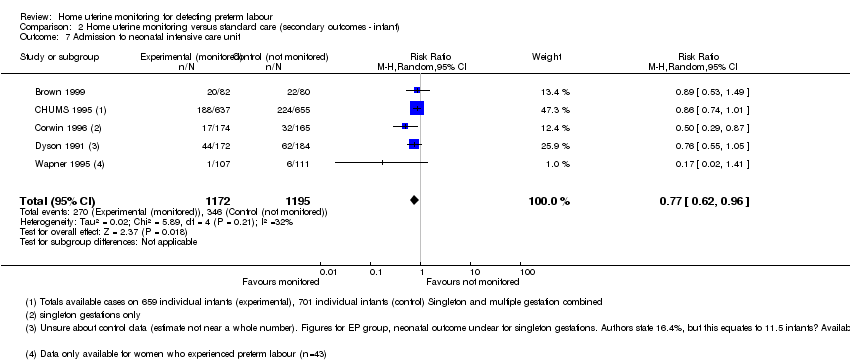
Comparison 2 Home uterine monitoring versus standard care (secondary outcomes ‐ infant), Outcome 7 Admission to neonatal intensive care unit.
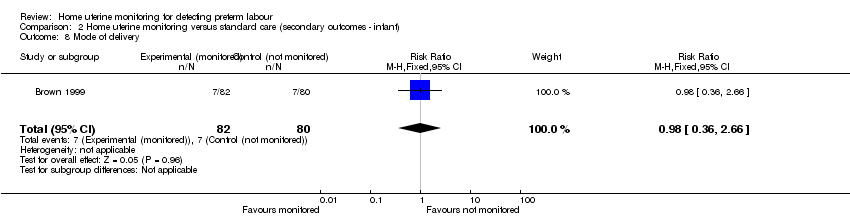
Comparison 2 Home uterine monitoring versus standard care (secondary outcomes ‐ infant), Outcome 8 Mode of delivery.
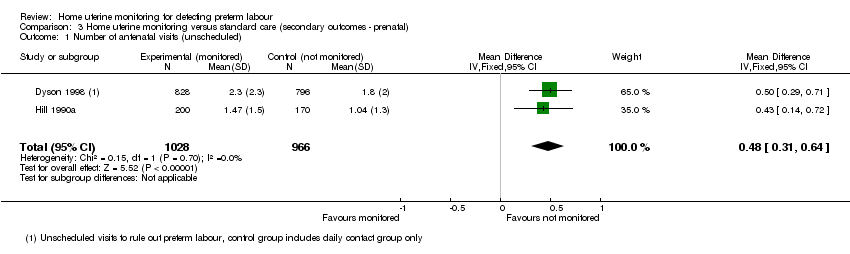
Comparison 3 Home uterine monitoring versus standard care (secondary outcomes ‐ prenatal), Outcome 1 Number of antenatal visits (unscheduled).

Comparison 3 Home uterine monitoring versus standard care (secondary outcomes ‐ prenatal), Outcome 2 Number of antenatal hospital admissions.

Comparison 3 Home uterine monitoring versus standard care (secondary outcomes ‐ prenatal), Outcome 3 Number of antenatal visits (unscheduled) (Subgroup analysis).

Comparison 3 Home uterine monitoring versus standard care (secondary outcomes ‐ prenatal), Outcome 4 Use of tocolysis.
| Home uterine monitoring for preventing preterm birth | ||||||
| Patient or population: women undergoing home monitoring for preventing preterm birth versus women receiving standard care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Home uterine monitoring | |||||
| Perinatal mortality | Study population | RR 1.22 | 2589 | ⊕⊕⊝⊝ | ||
| 46 per 1000 | 56 per 1000 | |||||
| Preterm birth less than 34 weeks' gestation | Study population | RR 0.78 | 1596 | ⊕⊕⊕⊕ | Sensitivity analysis included 1 study at low risk of bias (1292 women) and did not show any difference in results | |
| 166 per 1000 | 130 per 1000 | |||||
| Antenatal hospital admissions | Study population | RR 0.91 | 1494 | ⊕⊕⊝⊝ | ||
| 186 per 1000 | 169 per 1000 | |||||
| Preterm birth less than 37 weeks' gestation | Study population | RR 0.85 | 4834 | ⊕⊝⊝⊝ | ||
| 364 per 1000 | 310 per 1000 | |||||
| Admission to NICU | Study population | RR 0.77 | 2367 | ⊕⊕⊕⊝ | Evidence not downgraded for moderate heterogeneity (I² = 32%) | |
| 290 per 1000 | 223 per 1000 | |||||
| Number of unscheduled antenatal visits | The mean number of days ranged across control groups from approximately 1 to 2 days | The mean number of days in the monitored group was approximately half a day higher MD 0.48 (0.31 to 0.64) | 1994 | ⊕⊕⊕⊝ | Variation in protocol and healthcare delivery structures make it difficult to generalise from 1 large study contributing 65% of the weight for this outcome | |
| Use of tocolysis | Study population | RR 1.21 | 4316 | ⊕⊕⊝⊝ | This outcome may no longer be useful, due to changes in clinical practice. Sensitivity analysis including only 3 studies at low risk of bias (3749 women) did not show any clear difference in results. | |
| 188 per 1000 | 228 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1All studies contributing data with design limitations (‐1). | ||||||
| Methodological item | Adequate | Inadequate |
| Generation of random sequence | Computer‐generated sequence, random‐number tables, lot drawing, coin‐tossing, shuffling cards, throwing dice | Case number, date of birth, date of admission, alternation |
| Concealment of allocation | Central randomisation, coded drug boxes, sequentially‐sealed opaque envelopes | Open allocation sequence, any procedure based on inadequate generation |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Perinatal mortality Show forest plot | 2 | 2589 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.86, 1.72] |
| 2 Preterm birth < 34 weeks Show forest plot | 3 | 1596 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.62, 0.99] |
| 3 Preterm birth < 34 weeks (Subgroup analysis) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Singleton gestations | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.55, 2.27] |
| 3.2 Twin gestations | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.26, 1.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Preterm birth < 37 weeks Show forest plot | 8 | 4834 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.72, 1.01] |
| 2 Preterm birth < 37 weeks (Subgroup analysis) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Singleton gestations | 1 | 2422 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.62, 1.45] |
| 2.2 Twin gestations | 1 | 844 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.71, 1.30] |
| 3 Preterm birth < 32 weeks Show forest plot | 3 | 2550 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.31, 1.85] |
| 4 Use of antenatal corticosteroids Show forest plot | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.82, 1.25] |
| 5 Respiratory distress syndrome Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Singleton gestations | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.40, 3.95] |
| 5.2 Twin gestations | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.13, 1.12] |
| 6 Use of mechanical ventilation Show forest plot | 2 | 539 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.04, 2.38] |
| 7 Admission to neonatal intensive care unit Show forest plot | 5 | 2367 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.62, 0.96] |
| 8 Mode of delivery Show forest plot | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.36, 2.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of antenatal visits (unscheduled) Show forest plot | 2 | 1994 | Mean Difference (IV, Fixed, 95% CI) | 0.48 [0.31, 0.64] |
| 2 Number of antenatal hospital admissions Show forest plot | 3 | 1494 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.74, 1.11] |
| 3 Number of antenatal visits (unscheduled) (Subgroup analysis) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Singleton gestations | 1 | 1060 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [0.15, 0.65] |
| 3.2 Twin gestations | 1 | 564 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [0.24, 0.96] |
| 4 Use of tocolysis Show forest plot | 7 | 4316 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.01, 1.45] |

