早産の検出するための在宅子宮モニタリング
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised controlled trial | |
| Participants | 168 women who had been discharged and sent home after hospitalisation for threatened preterm labour (44%) and women at high risk for preterm labour (56%). Women enrolled between 24 and 34 weeks of pregnancy | |
| Interventions | Intervention: home uterine monitoring (twice daily), daily telephone contact with midwife, and home visit once a week from midwife from the Tokos centre (supplier of device) Control group: standard care (home visits once or twice a week from a community midwife) Women with persistent symptoms or contractions outside baseline frequency were sent to outpatient clinic or the inpatient ward | |
| Outcomes | Primary outcomes: perinatal mortality rate Secondary outcomes: | |
| Funding | Unclear: authors state that desired sample size not possible as the supplier of the home uterine monitoring device (Tokos Medical Corporation) was no longer funded in France. Tokos Medical Corporation provided the home uterine monitoring care system | |
| Notes | Provides other outcome measures not included in review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence not described. 2 groups of women (discharged after hospitalisation for threatened preterm labour, and women at high risk for preterm labour) included. "Allocated...to the monitored and control groups by randomization with sealed envelopes." Demographic characteristics of experimental and control groups comparable; authors note that the proportion of women with some risk factors smaller in the monitored (experimental) group |
| Allocation concealment (selection bias) | Unclear risk | "randomization with sealed envelopes." |
| Blinding of participants and personnel (performance bias) | High risk | Midwives and participants knew allocation group |
| Blinding of outcome assessment (detection bias) | High risk | Midwives referred women to hospital for treatment. Authors state doctors knew results of the monitoring, so not all prenatal outcomes were objective |
| Incomplete outcome data (attrition bias) | Low risk | 3 withdrawals from intervention group, 1 from control group. Analysis by authors of 167 records (out of 168 recruited) |
| Selective reporting (reporting bias) | Low risk | No evidence that reporting incomplete. Author has published related papers |
| Other bias | High risk | Claims that 900 women required for suitable power (10% difference in groups), making the study underpowered. 13 in control group had no home visits |
| Methods | Randomised controlled trial | |
| Participants | Of 343 women treated with parenteral tocolytic therapy for preterm labour who met study criteria, 186 were enrolled initially, and 162 cases available for analysis (n = 82 experimental, n = 80 control). Study criteria included Medicaid coverage, recruitment between 24 and 34 weeks of pregnancy. \ Setting: Indiana, USA, between 1 July 1991 and 1 October 1996 | |
| Interventions | Intervention: home uterine monitoring in addition to prenatal care of socio‐economically disadvantaged women who had received inpatient treatment for preterm labour. Experimental group transmitted monitor strip twice daily by telephone. Both experimental and control group had daily contact with perinatal nurse, and both groups on maintenance dose of oral terbutaline. Both groups received education in self‐palpation and were given instructions on how and when to call for further assistance if preterm labour was suspected | |
| Outcomes | Primary outcomes: see notes. | |
| Funding | Tokos Medical Corporation provided the monitor support. Indiana Office of Medicaid Policy and Planning supported the study | |
| Notes | Preterm birth < 35 weeks, and 35 to 37‐week births measured. Measured compliance with home uterine monitoring for < 35 and greater or equal to 35‐week deliveries | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence not described. "The random assignment process used sealed opaque envelopes to determine whether a patient would be in the monitored or control group." Maternal demographic and risk factors not statistically different between experimental and control groups |
| Allocation concealment (selection bias) | Low risk | "Sealed opaque envelopes." |
| Blinding of participants and personnel (performance bias) | High risk | Participants and perinatal nurse in daily contact with both groups were aware of allocation |
| Blinding of outcome assessment (detection bias) | High risk | Perinatal nurse in daily contact with both groups was aware of allocation; unclear whether hospital physicians aware, but perinatal nurse advised participant on management. Therefore some outcomes (unscheduled hospital observations, tocolysis) were not objective |
| Incomplete outcome data (attrition bias) | Unclear risk | 186 initially enrolled, for 24 of these circumstances changed |
| Selective reporting (reporting bias) | Low risk | No evidence that reporting incomplete |
| Other bias | Low risk | Power calculation based on reducing the risk of preterm delivery at < 37 weeks' gestation from 40% to 20%. Authors indicate that 82 women required for each group |
| Methods | Randomised controlled trial (double blinded) | |
| Participants | From 1355 recruited women between 24 and 36 weeks' gestation, and at high risk for preterm labour or birth, 1292 randomised, 1165 given device, active (n = 574) or sham (n = 591). | |
| Interventions | Intervention group sent twice daily home uterine monitoring transmissions of 1 hour duration to base station and successful transmissions acknowledged by nurses. Control group also sent transmissions but the uterine activity data were not seen by nurses ‐ authors state they were "electronically buried". All participant interactions with base station nurses followed a scripted protocol, similar for both groups, whether for remonitoring, alerting of physicians or referral to hospitals | |
| Outcomes | Primary outcomes: preterm birth ≤ 34 weeks Secondary outcomes: 1) infant; admission to neonatal ICU, birthweight < 2500 g; 2) prenatal, unscheduled emergency visits, antepartum admissions, use of tocolysis. | |
| Funding | The study was supported by Caremark Inc. (supplier of the monitoring device used) | |
| Notes | Provides other outcome measures not included in review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation, with blocked random‐number sequences used. Both experimental and control groups similar in demographic and risk factors, and authors state that subgroups were also similar (and withdrawals) |
| Allocation concealment (selection bias) | Low risk | "Computer generated randomization scheme prepared for each investigational site was used to assign patients consecutively without regard to specific risk factor. The identity of group assignment was blinded to patients and their care givers through the completion of the entire study." |
| Blinding of participants and personnel (performance bias) | Low risk | Authors state "identity of group assignment blinded to patients and their caregivers through the completion of the entire study". Those who were 'unblinded' initially were withdrawn from study |
| Blinding of outcome assessment (detection bias) | Low risk | Group allocation unknown to caregivers |
| Incomplete outcome data (attrition bias) | Unclear risk | Of 1165 who were given devices, 842 completed (29.4%, n = 169 of experimental group, 25.7%, n = 152 of control group withdrew). All participants enrolled and monitored followed up until delivery, including women who were noncompliant or who withdrew voluntarily. Authors state analysis conducted on both per‐protocol (completed, n = 842) and on ITT basis (but this only includes those who started monitoring), and that both analyses showed comparable results. It is unclear what happened to the 63 experimental and 64 control women who were randomised but not subsequently monitored with a device |
| Selective reporting (reporting bias) | Low risk | Data summaries show "intent‐to‐treat" data. Authors state other analyses conducted (analysis of variance or logistic models) for all variables, and terms for site and group by site interaction effects included |
| Other bias | Low risk | Power calculation based on 80% power with 2‐tailed alpha of 0.05 for a group difference of 1 cm (variance 1.4 cm) in change of cervical dilatation from previous visit when preterm labour diagnosed. Authors state minimum enrolment of 310 participants for any individual risk factor subgroup to obtain the 62 patients for evaluation, required by power analysis. Trial failed to recruit sufficient multiple gestations for these subgroups. Authors note that preterm labour management varied among the sites, but claim that both arms of the study received the same or similar tocolytic treatment at any particular site |
| Methods | Randomised controlled trial | |
| Participants | Women at risk of preterm labour in 3 hospital sites in USA, recruited between 26 and 32 weeks of gestation. From 2316 women screened, 432 were approached for informed consent, and 339 women with singleton gestations and 38 women with twin gestations agreed to participate (n = 198 experimental, n = 179 control) | |
| Interventions | Intervention group received standard high‐risk obstetric care, and in addition used a home uterine monitoring device, twice daily for an hour, sending transmissions to the centre, where the receiver reported back the number of contractions to the participant. No nursing contact was provided. Both intervention and control group participants received education in self‐palpation, and were instructed to contact their physician if they suspected preterm labour. Control group received standard high‐risk obstetric care. Minimum care scheduled was a visit to obstetric facility once every 4 weeks until 30 weeks, at least every 2 weeks (30 to 36 weeks) and at least weekly thereafter | |
| Outcomes | Primary outcomes: see notes. | |
| Funding | Study designed for a Food and Drug Administration Premarket approval application, and supported by Matria Healthcare, supplier of the device | |
| Notes | Study analysed relative risk reduction for various adverse outcomes (early delivery, low birthweight categories). Data drawn from several publications, the Corwin paper essentially reworking the earlier papers | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation conducted separately for singleton and twin gestations, by "study personnel without direct patient care responsibilities". Different random‐number sequence used for each site. "Group assignment was made by means of opening consecutively numbered envelopes that randomized patients with a table of random numbers." Authors state that no statistically significant differences in demographic and risk factors between groups were detected (although some missing values noted) |
| Allocation concealment (selection bias) | Low risk | "Group assignment was made by means of opening consecutively numbered envelopes that randomized patients with a table of random numbers." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants aware, but were asked not to reveal their group allocation to caregivers. Caregivers were aware they were seeing a study participant |
| Blinding of outcome assessment (detection bias) | Low risk | Caregivers not informed whether suspected uterine contractions were detected by the monitor or the participant. No nursing support provided to experimental group women as part of the monitoring package |
| Incomplete outcome data (attrition bias) | Low risk | Of 339 recruited (174 experimental, 165 control), 14 (6 versus 8) did not complete, 7 fetal deaths prior to observation, available cases 164 versus 154 |
| Selective reporting (reporting bias) | Unclear risk | Twin gestation outcome data not reported (38 women); authors state group too small for analysis |
| Other bias | Unclear risk | Authors calculated that 320 women would need to be enrolled to have sufficient power (80%, with alpha = 0.05, beta = 0.20) to allow detection of improvement from 30% to 60% in the proportion of women with preterm labour with early diagnosis 8 < 2 cm cervical dilatation) |
| Methods | Randomised controlled trial, with retrospective standard care group also included in study | |
| Participants | Women receiving care at Kaiser Permanente, before 28 weeks' gestation, and of these 251 gave consent. 138 (n = 68 experimental, n = 70 control) were singleton gestations, and 109 were twin (n = 57 experimental, n = 52 control), with 2 withdrawals from each arm of the study. | |
| Interventions | Intervention and control groups received home uterine activity monitors, but only in the intervention group were the uterine activity data used in care. All participants were asked initially to monitor for an hour every day, and transmit daily ‐ this was later changed to twice daily monitoring and transmission. Both groups received education in self‐palpation and asked to record presence or absence of signs of preterm labour and number of contractions. Both groups were contacted at least 5 days a week by the study nurse, to discuss such signs and for the intervention group to review monitoring data. Tocolysis conducted according to protocol | |
| Outcomes | Primary outcomes: perinatal mortality rate (twin only), preterm birth < 34 weeks. Secondary outcomes: | |
| Funding | Supported in part by the Community Service Program of Kaiser Foundation Hospitals. Monitoring devices provided by Advanced Medical Systems | |
| Notes | Study reports findings for standard care group comparison (not included in review analyses) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence not described. "patients were randomized into two groups, the home uterine monitoring group...and the education‐palpation group". Singleton and twin gestations not randomised separately. No comparisons of demographic data provided |
| Allocation concealment (selection bias) | High risk | No details provided. Nurses may have been aware of some group assignment because they were asked to "not analyze or respond to" errant transmissions made by women in the education‐palpation group |
| Blinding of participants and personnel (performance bias) | Low risk | For the control group "home uterine monitoring tracings were not analyzed or used in patient management". Participants not aware of group assignment. Nurses only aware which participants had transmitted and could not analyse uterine activity data for the control group (unless participants accidentally transmitted to a different monitor, in which case the nurse did not respond to the tracing) "The charts of all patients in the (control) group who experienced preterm labour were reviewed and in no case did it appear that a patient in the (control) group was referred by a nurse for increased uterine activity detected by one of these accidentally unblinded tracings" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Both groups discussed possible signs and symptoms of preterm labour with nurses and monitoring (experimental) group additionally discussed activity tracings with nurses. 1 outcome (number of unscheduled visits) therefore not objective. |
| Incomplete outcome data (attrition bias) | Low risk | Outcomes obtained for 247 of the 251 women who consented. Singleton and twin gestation data reported separately. |
| Selective reporting (reporting bias) | Unclear risk | Some data also provided about number of unscheduled visits, but for singletons only |
| Other bias | High risk | Figures for EP group, neonatal outcome unclear for singleton gestations. Authors state 16.4%, but this equates to 11.5 infants(?). Unclear whether other data flaws exist |
| Methods | Randomised controlled trial with 3 arms | |
| Participants | Women receiving prenatal care at Kaiser Permanente clinics (n = 8), judged to be high risk. Enrolment between 24 and 30 weeks' gestation for pre‐existing risk factors, and before 33 weeks for risk factors developed during pregnancy. 2422 women enrolled, including 844 women with twins. | |
| Interventions | Intervention group received daily contact with a nurse plus home uterine monitoring device for use twice daily for an hour each session. Data were reviewed immediately after transmission and the woman contacted if her threshold frequency was exceeded. All women in trial received education about symptoms and self‐palpation, and asked to record symptoms. Women in the weekly contact group were asked to assess themselves twice daily, and if persistent symptoms of preterm labour were detected, they were to call for professional advice. A nurse from the perinatal service centre called the women weekly to review their logs. For the daily contact group, the procedures were the same, but the nurse called daily | |
| Outcomes | Primary outcomes: perinatal mortality rate | |
| Funding | Supported in part by a grant (01 41 9032) from the Sidney Garfield Memorial Fund | |
| Notes | Primary end point of the study was incidence of birth at less than 35 weeks' gestation, secondary end points (not included in the review) included cervical status | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Women were assigned to one of three treatment groups in a ratio of 1:1:1 with the use of a computer‐generated randomization sequence." Randomisation stratified according to status (twin or at‐risk singleton) and according to treatment centre "to control for possible differences in treatment philosophy" (there were 8 tertiary centres for preterm labour care). Authors state no statistically significant differences in the demographic and risk factors for the 3 groups |
| Allocation concealment (selection bias) | Unclear risk | Unclear whether a central randomisation office was used, no details of the implementation of randomisation at different centres |
| Blinding of participants and personnel (performance bias) | Unclear risk | Women and perinatal nurses aware of allocation but "instructed not to inform the obstetricians of the group assignments. The women and the perinatal service nurses were also instructed not to divulge the method of detecting uterine activity when they reported increased uterine activity to the obstetricians". |
| Blinding of outcome assessment (detection bias) | Unclear risk | Perinatal nurse contact could affect prenatal outcomes (unscheduled visits, and also, therefore, tocolysis). 3 outcomes objective |
| Incomplete outcome data (attrition bias) | Low risk | All 2422 women assigned completed the study but 93 (4% of those randomised) did not receive the surveillance to which they had been assigned |
| Selective reporting (reporting bias) | Low risk | Not evident |
| Other bias | Low risk | Authors state the study has a power of more than 95% to detect a 1 cm difference between groups in cervical dilatation at the time of preterm labour diagnosis for all study participants Women in home uterine monitoring, and daily contact groups complied with at least 1 daily session 86% of the time, weekly contact compliance less at 79% of the time |
| Methods | Randomised controlled trial | |
| Participants | 299 women at risk for preterm labour enrolled from 4 tertiary care centres in the USA (n = 155 experimental, n = 144 control). Women were between 20 and 34 weeks' gestational age at entry. Participants in Watson 1990 (n = 86) had been successfully treated for preterm labour. Knuppel 1990a participants (n = 45) were twin gestations from 4 centres (presumably the same as the Hill 1990b report). Knuppel 1990b mentions enrolment of 385 women at risk for preterm labour | |
| Interventions | Intervention group used home uterine monitoring device, twice daily for an hour, and transmitted data to the centre. Perinatal nurses contacted the women daily, women also encouraged to call if an emergency problem was suspected. Both intervention and control groups received education in symptoms of preterm labour and self‐palpation. The control group were instructed to contact their physician if they became aware of any persistent sign of premature labour. The description of the Hill 1990a and Knuppel 1990b protocol appears similar In the Watson 1990 report, the control group received standard home management for the institution | |
| Outcomes | Primary outcomes: see notes | |
| Funding | Supported in part by a medical service grant from Tokos Medical Corporation and Vicksburg Hospital Medical Foundation | |
| Notes | Study examined other outcomes not included in review, e.g. cervical status at first episode of preterm labour. Several reports associated with this study (e.g.Bentley 1990 and Martin 1990 discuss rationale for methods used), but it is unclear about extent of subgroup analysis. Keirse 1993 provides some additional details on procedures based on information obtained after publication of the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided; "patients were assigned randomly". No comparison of demographic or risk factors in tables (apart from Watson 1990), authors state these were similar between the groups |
| Allocation concealment (selection bias) | Low risk | No details provided on implementation of randomisation procedures in the main study report, although Keirse 1993 mentions that one of the main investigators for Hill 1990a stated that sealed opaque envelopes were used and that randomisation was conducted separately for women, with and without an episode of preterm labour in the current pregnancy |
| Blinding of participants and personnel (performance bias) | High risk | Women aware of their assignment. Nurses in the monitoring centre also aware. Unclear whether hospital staff were aware |
| Blinding of outcome assessment (detection bias) | High risk | Perinatal nurses in the monitoring centre could influence prenatal outcomes (unscheduled visits) |
| Incomplete outcome data (attrition bias) | High risk | Analysis (Hill 1990a) excluded 25 (13 experimental, 12 control) withdrawals, and participants delivered for fetal or maternal medical complications (15 experimental, 14 control). Also excluded from some of the analyses were 13 participants in the experimental group who did not comply fully with the monitoring regimens. Women ceased to participate in the study when they reached 37 weeks' gestation Similarly Knuppel 1990a only provides data on the women who experienced preterm labour, and 13 of 58 enrolled were excluded from data analysis. Knuppel 1990b excluded 71 of 385 enrolled |
| Selective reporting (reporting bias) | High risk | Very unclear how the various reports for this study relate to one another, with discrepancies in the data |
| Other bias | High risk | No power calculation is reported. Authors (Hill 1990a) state the distribution of risk factors and demographic characteristics across both groups, but no tables are provided Participating physicians used the preterm labour protocol in place at the respective institution. This may account for some differences among the reports included within this multi‐site study, but it is unclear how many women in total were enrolled in the multi‐site study, and how the subgroup analysis was organised and reported |
| Methods | Randomised controlled trial, in 2 parts | |
| Participants | Women at risk of preterm labour (n = 157 year 1, n = 152 year 2) were recruited from area physicians in prenatal clinic (n = 205 experimental, n = 104 control). All women were between 20 and 34 weeks' gestational age at entry, none had experienced preterm labour prior to enrolment. Area physicians recruited 240, OSU 69 women | |
| Interventions | Intervention group used home uterine monitoring device, and a perinatal nurse from the monitoring centre called daily to transmit and interpret uterine activity data. The control group received education in self‐palpation, and were asked to record contractions of 1 hour twice daily. They were contacted on weekdays by a perinatal nurse from the monitoring centre to discuss recorded contractions. Both groups were instructed to seek professional support if they experienced persistent symptoms above their baseline | |
| Outcomes | Primary outcomes: see notes. | |
| Funding | Supported by a grant from the Tokos Medical Corporation (supplier of the monitoring device) and by March of Dimes Birth Defects Foundation Grant no 2‐1987/C‐185 | |
| Notes | Study measured other outcomes not included in protocol, e.g. preterm birth < 35 weeks. Study end point was number of women reaching 35 and 37 weeks at delivery | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequence not described. Authors comment " unfortunately did not stratify random assignment...within risk factors". A 2 to 1 (experimental:control) scheme applied. More women with multiple gestations allocated to the control group in both years of the study |
| Allocation concealment (selection bias) | Unclear risk | No details of the implementation of the randomisation procedures provided. Author comment "physicians who enrolled their patients in the study often forgot which study group the patient was in, suggesting they perceived similarly the care received by both groups" (Iams 1987) |
| Blinding of participants and personnel (performance bias) | High risk | Women aware of allocation. Authors comment that "monitoring centre staff were aware of the crude preterm birth rates for both groups as the study progressed". Primary perinatal nurses aware of group allocation, and had participants in both groups. Authors comment that participating physicians were visited at least once to reinforce protocols. There was an apparent learning curve phenomenon among nursing staff over the course of the study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Preterm birth an objective outcome, but use of tocolysis not objective if perinatal nurses aware of group allocation, as they could influence visits to physicians |
| Incomplete outcome data (attrition bias) | Unclear risk | Authors report significant differences in noncompliance between years 1 and 2 of the study. In year 1 there were 15 withdrawals (8.4% of experimental, 12% of controls) from a total of 157 enrolled, while in year 2 there were 28 withdrawals (12.2% of experimental, 29.6% of controls) from a total of 152 enrolled. Of the 309 women recruited, 266 completed the study (n = 184 experimental, n = 82 control) |
| Selective reporting (reporting bias) | Low risk | No gaps evident |
| Other bias | Unclear risk | Authors report a statistically significant decline in preterm births < 37 weeks overall from year 1 to year 2, which is not apparently correlated with changes in risk factors or demography or physician behaviour. Authors suggest that "something the nurses do in the course of their contact with the patient may actually inhibit the development of preterm labour" Authors report estimating that to detect a 30% reduction in deliveries < 37 weeks, 230 participants were required in each group to achieve power of 80%, 1‐tailed P of 0.05 |
| Methods | Randomised controlled trial | |
| Participants | Women with singleton gestations (n = 76) who had been successfully treated for preterm labour were recruited from private, transport and clinic populations served by the Centre. 2 to 1 allocation used with 46 in experimental group and 21 in control. | |
| Interventions | Intervention group used the home uterine activity monitoring device to record contractions twice daily for 1 hour. Staff at the monitoring centre contacted the women daily for transmission and reporting of activity data. Both groups received education in signs and symptoms of preterm labour from the centre staff. The control group performed self‐palpation for 1 hour twice daily. Centre staff phoned every weekday for reports and, if needed, at weekends. Both groups were instructed to seek professional support if they experienced persistent symptoms above their baseline. Nursing staff at the centre also contacted physicians sometimes | |
| Outcomes | Primary outcomes: see notes | |
| Funding | Supported by the Tokos Medical Corporation and the March of Dimes | |
| Notes | Trial discontinued as the similarities between this and a companion trial (Iams 1987, above) were confusing participating physicians. Study end points in this trial were preterm births < 36 weeks, and parenteral tocolysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. "subjects were assigned randomly in a ratio of 1:2 " (control: experimental) No comparison of demographic and risk factors presented in tables |
| Allocation concealment (selection bias) | Unclear risk | No details of implementation of randomisation procedures provided |
| Blinding of participants and personnel (performance bias) | High risk | Authors note multiple physicians and hospitals involved. Women and monitoring centre staff aware of group allocation |
| Blinding of outcome assessment (detection bias) | High risk | Tocolysis outcome could be affected by advice given by monitoring centre staff, who were aware of group allocation |
| Incomplete outcome data (attrition bias) | Unclear risk | Frequent contact permitted assessment of compliance. Withdrawal statistically significantly greater in the control group as 6 of 27 withdrew (22%) compared to 3 of 49 (6%) in experimental group. 67 out of 76 participants completed the study |
| Selective reporting (reporting bias) | High risk | Authors state all births before 37 completed weeks reviewed in detail, but data only provided for births before 36 weeks (defined as preterm) and end point different from companion trial |
| Other bias | High risk | Authors suggest the trial was underpowered |
| Methods | Randomised controlled trial | |
| Participants | Women at risk of preterm birth in a dependent military population, allocated to experimental (n = 31) and control (n = 31) groups | |
| Interventions | Women in the experimental group transmitted 1 hour of uterine activity data twice daily to the diagnostic centre, and women in the control were monitored weekly at the diagnostic centre. Subjective information obtained daily by nurses for the experimental group | |
| Outcomes | Primary outcomes: | |
| Funding | No details provided in conference abstract | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "A randomized prospective study was done....Sixty two patients at risk for preterm delivery were randomly assigned to one of two groups." |
| Allocation concealment (selection bias) | Unclear risk | No details of implementation of randomisation in conference abstract |
| Blinding of participants and personnel (performance bias) | High risk | "Subjective information was obtained by trained nursing personnel on a daily basis" in intervention group, "at each clinic visit" in control group, and "evaluated by the responsible physicians." |
| Blinding of outcome assessment (detection bias) | High risk | Women and nursing staff aware of allocation and prenatal outcome (number of days in hospital antenatally) |
| Incomplete outcome data (attrition bias) | Unclear risk | It appears that all women completed the study, but difficult to state for sure |
| Selective reporting (reporting bias) | Unclear risk | Few details provided in conference abstract |
| Other bias | Unclear risk | Few details provided in conference abstract |
| Methods | Quasi‐randomised controlled trial | |
| Participants | Women (n = 75) supported through Medicaid, and judged at high risk for preterm labour, were identified as eligible from the clinic over a 9‐month period, between 14 and 24 weeks' gestational age. Of the 75 identified, 69 were randomised (n = 35 experimental, n = 34 control) | |
| Interventions | Intervention group used a home uterine activity monitoring device, twice daily for an hour, and data transmitted to a monitoring centre (with daily phone contact). If other symptoms developed the women were told to re‐monitor and to contact the study nurse. Both intervention and control groups received education in signs of preterm labour and were instructed to come to the hospital if symptoms developed. Both groups were examined once every 2 weeks. The control group were contacted by phone twice a week | |
| Outcomes | Primary outcomes: see notes. | |
| Funding | Supported in part by the Vicksburg Hospital Medical Foundation | |
| Notes | Some cost analysis figures given. This study is related to a later and larger cost analysis study of 130 women, supported by Medicaid, who were recruited from clinics in Mississippi and Michigan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Tables show demographic and risk characteristics of the groups similar. Randomisation based on last digits of hospital number |
| Allocation concealment (selection bias) | High risk | Randomisation based on last digits of hospital number |
| Blinding of participants and personnel (performance bias) | Unclear risk | Women aware and nursing staff aware of allocation. Authors state that women admitted for observation "were observed by staff unaware of the participants' involvement in the study" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Authors state that women admitted for observation "were observed by staff unaware of the participants' involvement in the study". Extent of nursing contact varied between the groups, and nursing advice could affect prenatal outcomes (number of unscheduled visits, use of tocolysis/admission for preterm labour) |
| Incomplete outcome data (attrition bias) | Low risk | 2 women withdrew from 69 randomised, data obtained from 67 women |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting, several related publications on this trial |
| Other bias | Unclear risk | Authors state tocolysis protocol the same for both groups |
| Methods | Randomised controlled trial | |
| Participants | Women who had been treated successfully for preterm labour, between 20 and 34 weeks' gestation, were recruited from University medical system, and randomised (n = 59) to experimental (n = 28) and control (n = 29) | |
| Interventions | Intervention group used the home uterine monitoring device twice daily and transmitted data to the perinatal monitoring centre. Both intervention and control groups received education in signs of preterm labour and were instructed to call or return to hospital if signs were persistent. All women were seen once weekly in the office, and all women were given prescriptions for terbutaline | |
| Outcomes | Primary outcomes: preterm birth < 34 weeks | |
| Funding | Supported in part by a grant from Tokos Medical Inc (supplier of the device) and by an Interagency project agreement project grant | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Pseudo‐random number generator" used for randomisation Notes that 2 additional participants, initially randomised, were excluded from analysis as they were not found eligible medically |
| Allocation concealment (selection bias) | Low risk | Numbered, sealed opaque envelopes used |
| Blinding of participants and personnel (performance bias) | High risk | Authors report that neither the women nor their caregivers were blinded to the allocation group |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes preterm or term birth |
| Incomplete outcome data (attrition bias) | Unclear risk | In the experimental group, 4 women never left hospital and never received the intervention. 1 control participant lost to follow‐up. 2 participants initially randomised excluded from the analysis for medical reasons |
| Selective reporting (reporting bias) | Unclear risk | Authors comment that analysis on available cases did not change direction of significance (analysis presented as ITT) |
| Other bias | High risk | Power calculations (1‐sided alpha of 0.05. 80% power) based on incidences of preterm delivery of 0.45 in routine care and 0.15 in the monitoring group (based on previous randomised controlled trial, Morrison 1987). Estimated that 28 required in each arm, but authors also suggest that the trial may be underpowered |
| Methods | Randomised controlled trial with 3 arms | |
| Participants | Women "at high risk for preterm birth" allocated to | |
| Interventions | Women doing the monitoring transmitted 2 hours of data daily. For the active analysis group, contraction > 4 per hour referred for evaluation following a protocol. All women appear to have had daily phone contact with the study centre | |
| Outcomes | None of relevance to the review (only reports preterm birth < 36 weeks) | |
| Funding | Tradename of device mentioned | |
| Notes | This study is within scope, but not included in data tables or meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "we undertook a randomized prospective study"; "patients were randomly assigned to one of three groups." |
| Allocation concealment (selection bias) | Unclear risk | No details of the method of implementation of randomisation |
| Blinding of participants and personnel (performance bias) | High risk | 1 of the 2 control groups received home uterine monitoring "but uterine activity data was blinded to patient management", other control group did not receive home uterine monitoring. Both control groups had daily participant telephone contact. Monitored participants deemed at risk "were evaluated at the hospital for possible preterm labour by strict protocol" |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome of preterm birth objective |
| Incomplete outcome data (attrition bias) | Unclear risk | Only 1 outcome reported. Authors state that 7 noncompliant women were removed from the analysis |
| Selective reporting (reporting bias) | Unclear risk | Only 1 outcome reported in the abstract |
| Other bias | Unclear risk | Few details provided in conference abstract |
| Methods | Randomised controlled trial | |
| Participants | Women judged at risk for preterm labour were recruited from private and clinic populations, USA sites, and randomly allocated to experimental (home uterine monitoring) (n = 38) and control (self‐palpation) (n = 34) groups. | |
| Interventions | All women monitored contractions for 1 hour daily, all women had daily contact with nurse or physician. Uterine monitoring data used to manage tocolytic dose | |
| Outcomes | Primary outcomes: preterm birth < 34 weeks | |
| Funding | Manufacturer of device mentioned, no further details | |
| Notes | Not included in data tables and meta‐analysis as the results cannot be back‐calculated from the percentages provided (no sensible interpolations possible) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "we performed a randomized clinical trial", authors state that groups comparable for risk factors, preterm delivery, referring physician and mean gestational age on entry |
| Allocation concealment (selection bias) | Unclear risk | No details of implementation of randomisation provided in conference abstract |
| Blinding of participants and personnel (performance bias) | High risk | All women aware of allocation, and all had daily contact with a perinatal nurse or physician, who would therefore be aware of allocation |
| Blinding of outcome assessment (detection bias) | High risk | Prenatal outcome (number of unscheduled visits) would be affected by nature of advice from clinician who was aware of allocation |
| Incomplete outcome data (attrition bias) | High risk | Authors state that 5 participants were removed from analysis for calculation of mean gestational age; unclear whether this covers other outcome data. Raw frequency data impossible to back‐calculate from the percentages provided. |
| Selective reporting (reporting bias) | High risk | Few details provided in conference abstract; authors state that number of "emergency visits were similar" but no figures provided |
| Other bias | Unclear risk | Few details provided in conference abstract |
| Methods | Randomised controlled trial | |
| Participants | Women (24 to 36 weeks' gestation) with a history of preterm delivery were recruited and randomised into monitored group (experimental) (n = 107) and control group (n = 111) | |
| Interventions | Intervention group received routine high‐risk obstetric care and transmitted monitoring data twice daily to the receiving centre. The control group received routine high‐risk obstetric care. Both groups received education in self‐palpation and indications of preterm labour, and instructions on dealing with such indications. All participants were scheduled for routine office visits for evaluation at least once every 4 weeks until 30 weeks' gestation, once every 2 weeks (30 to 35 weeks' gestation) and weekly thereafter | |
| Outcomes | Secondary outcomes: | |
| Funding | Authors state "supported in part by a grant from Healthdyne Perinatal Services", manufacturers of the tocodynamometer uterine monitoring device | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Separate blocked random number sequences used at each study site. Randomisation carried out by study personnel not responsible for participant care |
| Allocation concealment (selection bias) | Low risk | Group assignments "carried out by study personnel not responsible for patient care, by opening consecutive numbered envelopes" at each site |
| Blinding of participants and personnel (performance bias) | Unclear risk | Women aware of allocation, and authors state that women were instructed "not to inform caretakers of their use or non‐use of the monitor" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Known allocation may have affected 1 outcome (number of antenatal hospital visits) |
| Incomplete outcome data (attrition bias) | Unclear risk | Number of withdrawals = 37 (17.8%). Unclear whether monitored group subject to more withdrawals; authors state that 9 women enrolled into monitoring group, but never received monitoring. Authors state that there were no significant differences in the "enrolled population" between monitored and control groups for mean scheduled and unscheduled office visits |
| Selective reporting (reporting bias) | High risk | Neonatal and pregnancy outcomes only reported for women who experienced preterm labour (n = 43, of which there were 21 monitored, and 22 control) |
| Other bias | Unclear risk | Authors state "neonatal and pregnancy outcomes not chosen as study end points in the design and sample size calculation". Sample size calculated for cervical dilatation at the time of diagnosis of preterm labour, the study endpoint |
ICU: intensive care unit
ITT: intention‐to‐treat
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not in scope, does not mention home uterine monitoring | |
| This paper and the others relating to this study (Monincx 1997; Monincx 2001) are all out of scope, and are therefore excluded. Midwives did the home monitoring and it was fetal heart rate that was transmitted. Uterine activity monitoring is not mentioned | |
| Not in scope, home visiting only | |
| Not in scope, deals with home visiting only, mentioned in discussion | |
| Not in scope, fetal monitoring only | |
| This is a letter, no data provided of relevance | |
| Not in scope, home visiting only | |
| Not in scope, home visiting only | |
| Not in scope, domiciliary care only | |
| Not in scope, domiciliary care only | |
| Not in scope, review discussing contribution of nursing care to monitoring | |
| Not in scope, see Birnie 2000 (above) | |
| Not in scope, see Birnie 2000 (above) | |
| Not in scope, remote fetal monitoring only | |
| Not in scope (personal communication comment) | |
| Notice of trial registration data, and not clear whether trial was ever completed. No evidence found | |
| Not in scope, fetal monitoring | |
| Not in scope, domiciliary care only | |
| Not in scope, domiciliary care only | |
| Not in scope, fetal monitoring | |
| The trial appears as if it should be in scope, but there is no report of any clinical data. The studies only describe the technology |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Perinatal mortality Show forest plot | 2 | 2589 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.86, 1.72] |
| Analysis 1.1  Comparison 1 Home uterine monitoring versus standard care ‐ primary outcomes, Outcome 1 Perinatal mortality. | ||||
| 2 Preterm birth < 34 weeks Show forest plot | 3 | 1596 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.62, 0.99] |
| Analysis 1.2 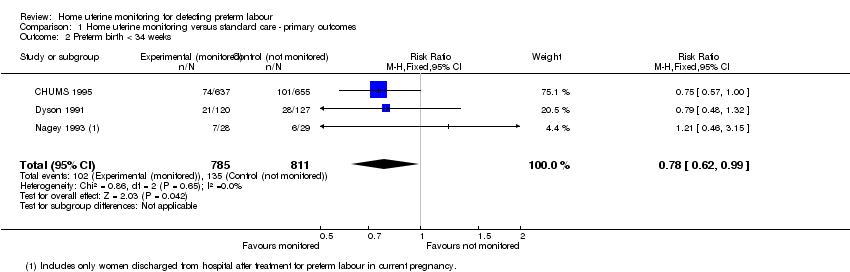 Comparison 1 Home uterine monitoring versus standard care ‐ primary outcomes, Outcome 2 Preterm birth < 34 weeks. | ||||
| 3 Preterm birth < 34 weeks (Subgroup analysis) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Home uterine monitoring versus standard care ‐ primary outcomes, Outcome 3 Preterm birth < 34 weeks (Subgroup analysis). | ||||
| 3.1 Singleton gestations | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.55, 2.27] |
| 3.2 Twin gestations | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.26, 1.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Preterm birth < 37 weeks Show forest plot | 8 | 4834 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.72, 1.01] |
| Analysis 2.1 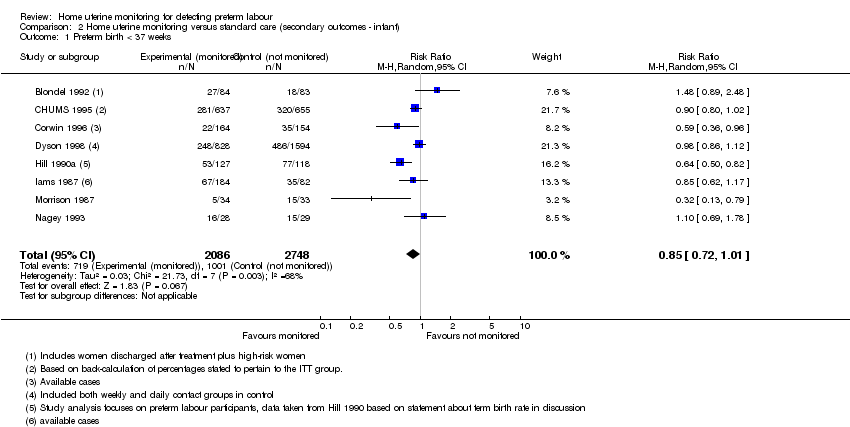 Comparison 2 Home uterine monitoring versus standard care (secondary outcomes ‐ infant), Outcome 1 Preterm birth < 37 weeks. | ||||
| 2 Preterm birth < 37 weeks (Subgroup analysis) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 Home uterine monitoring versus standard care (secondary outcomes ‐ infant), Outcome 2 Preterm birth < 37 weeks (Subgroup analysis). | ||||
| 2.1 Singleton gestations | 1 | 2422 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.62, 1.45] |
| 2.2 Twin gestations | 1 | 844 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.71, 1.30] |
| 3 Preterm birth < 32 weeks Show forest plot | 3 | 2550 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.31, 1.85] |
| Analysis 2.3  Comparison 2 Home uterine monitoring versus standard care (secondary outcomes ‐ infant), Outcome 3 Preterm birth < 32 weeks. | ||||
| 4 Use of antenatal corticosteroids Show forest plot | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.82, 1.25] |
| Analysis 2.4 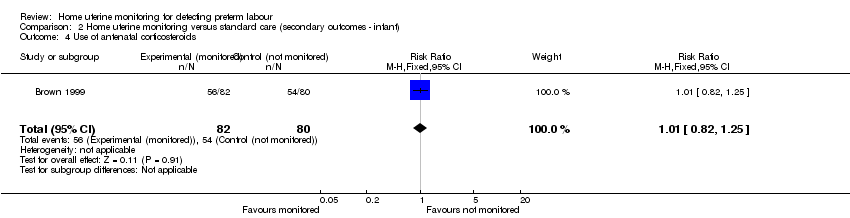 Comparison 2 Home uterine monitoring versus standard care (secondary outcomes ‐ infant), Outcome 4 Use of antenatal corticosteroids. | ||||
| 5 Respiratory distress syndrome Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.5  Comparison 2 Home uterine monitoring versus standard care (secondary outcomes ‐ infant), Outcome 5 Respiratory distress syndrome. | ||||
| 5.1 Singleton gestations | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.40, 3.95] |
| 5.2 Twin gestations | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.13, 1.12] |
| 6 Use of mechanical ventilation Show forest plot | 2 | 539 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.04, 2.38] |
| Analysis 2.6 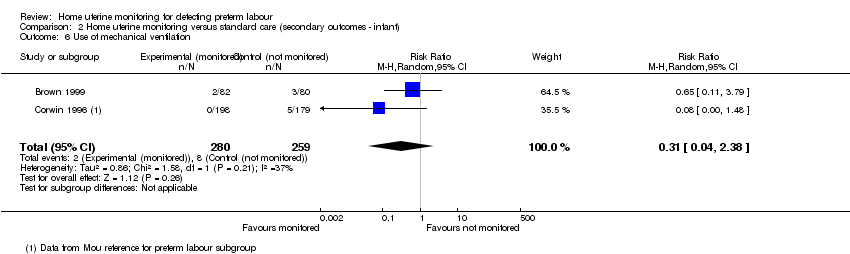 Comparison 2 Home uterine monitoring versus standard care (secondary outcomes ‐ infant), Outcome 6 Use of mechanical ventilation. | ||||
| 7 Admission to neonatal intensive care unit Show forest plot | 5 | 2367 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.62, 0.96] |
| Analysis 2.7 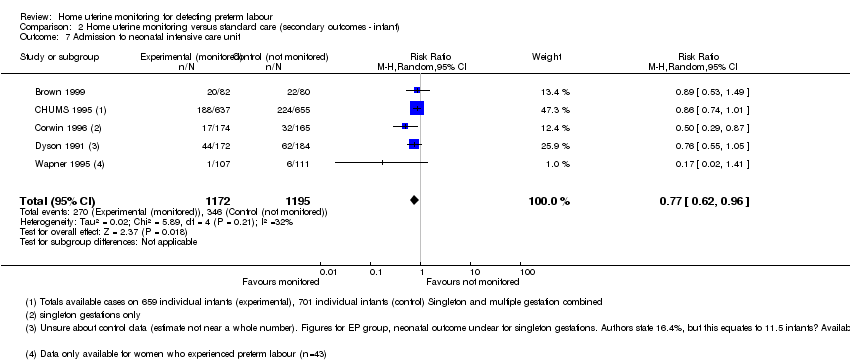 Comparison 2 Home uterine monitoring versus standard care (secondary outcomes ‐ infant), Outcome 7 Admission to neonatal intensive care unit. | ||||
| 8 Mode of delivery Show forest plot | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.36, 2.66] |
| Analysis 2.8 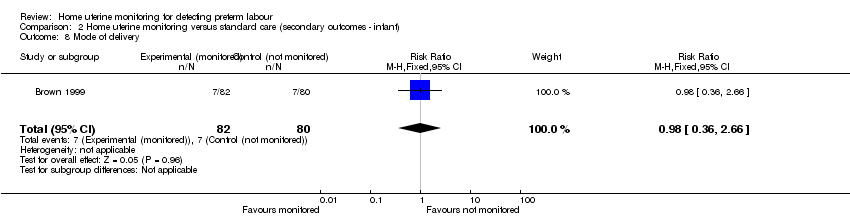 Comparison 2 Home uterine monitoring versus standard care (secondary outcomes ‐ infant), Outcome 8 Mode of delivery. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of antenatal visits (unscheduled) Show forest plot | 2 | 1994 | Mean Difference (IV, Fixed, 95% CI) | 0.48 [0.31, 0.64] |
| Analysis 3.1 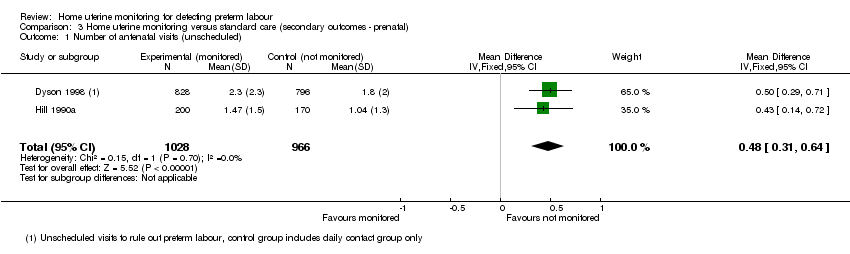 Comparison 3 Home uterine monitoring versus standard care (secondary outcomes ‐ prenatal), Outcome 1 Number of antenatal visits (unscheduled). | ||||
| 2 Number of antenatal hospital admissions Show forest plot | 3 | 1494 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.74, 1.11] |
| Analysis 3.2  Comparison 3 Home uterine monitoring versus standard care (secondary outcomes ‐ prenatal), Outcome 2 Number of antenatal hospital admissions. | ||||
| 3 Number of antenatal visits (unscheduled) (Subgroup analysis) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.3  Comparison 3 Home uterine monitoring versus standard care (secondary outcomes ‐ prenatal), Outcome 3 Number of antenatal visits (unscheduled) (Subgroup analysis). | ||||
| 3.1 Singleton gestations | 1 | 1060 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [0.15, 0.65] |
| 3.2 Twin gestations | 1 | 564 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [0.24, 0.96] |
| 4 Use of tocolysis Show forest plot | 7 | 4316 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.01, 1.45] |
| Analysis 3.4  Comparison 3 Home uterine monitoring versus standard care (secondary outcomes ‐ prenatal), Outcome 4 Use of tocolysis. | ||||

Study flow diagram.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
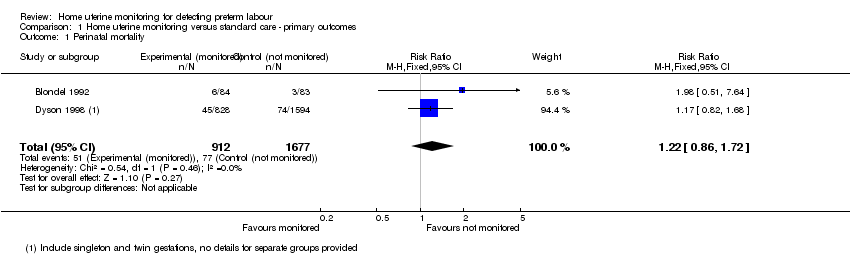
Comparison 1 Home uterine monitoring versus standard care ‐ primary outcomes, Outcome 1 Perinatal mortality.

Comparison 1 Home uterine monitoring versus standard care ‐ primary outcomes, Outcome 2 Preterm birth < 34 weeks.
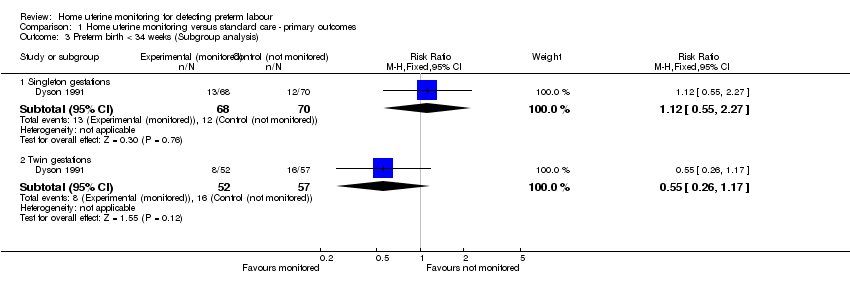
Comparison 1 Home uterine monitoring versus standard care ‐ primary outcomes, Outcome 3 Preterm birth < 34 weeks (Subgroup analysis).

Comparison 2 Home uterine monitoring versus standard care (secondary outcomes ‐ infant), Outcome 1 Preterm birth < 37 weeks.

Comparison 2 Home uterine monitoring versus standard care (secondary outcomes ‐ infant), Outcome 2 Preterm birth < 37 weeks (Subgroup analysis).
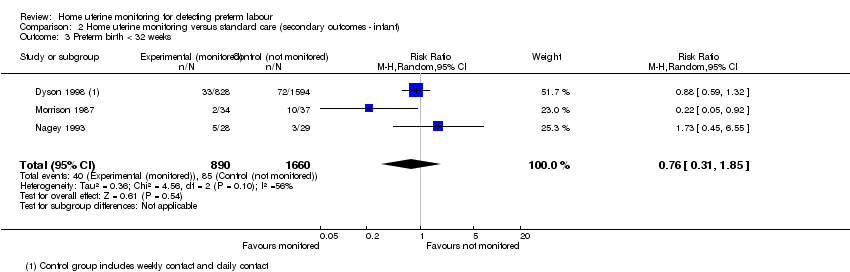
Comparison 2 Home uterine monitoring versus standard care (secondary outcomes ‐ infant), Outcome 3 Preterm birth < 32 weeks.
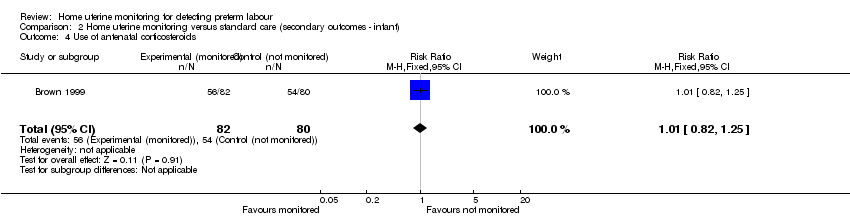
Comparison 2 Home uterine monitoring versus standard care (secondary outcomes ‐ infant), Outcome 4 Use of antenatal corticosteroids.
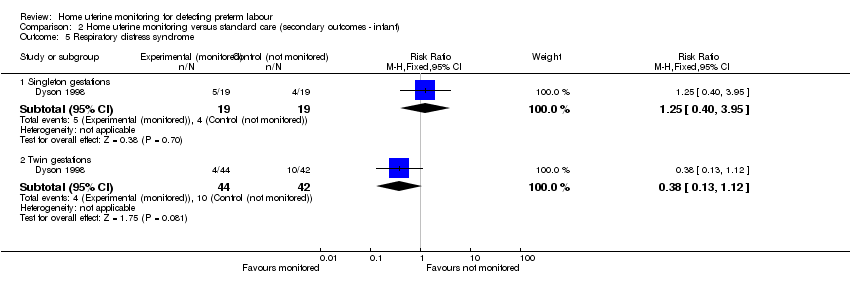
Comparison 2 Home uterine monitoring versus standard care (secondary outcomes ‐ infant), Outcome 5 Respiratory distress syndrome.
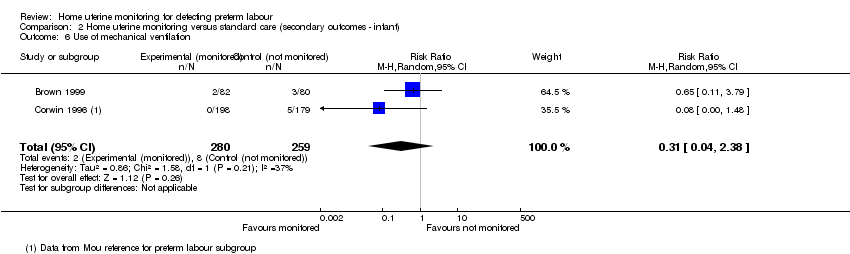
Comparison 2 Home uterine monitoring versus standard care (secondary outcomes ‐ infant), Outcome 6 Use of mechanical ventilation.

Comparison 2 Home uterine monitoring versus standard care (secondary outcomes ‐ infant), Outcome 7 Admission to neonatal intensive care unit.

Comparison 2 Home uterine monitoring versus standard care (secondary outcomes ‐ infant), Outcome 8 Mode of delivery.

Comparison 3 Home uterine monitoring versus standard care (secondary outcomes ‐ prenatal), Outcome 1 Number of antenatal visits (unscheduled).
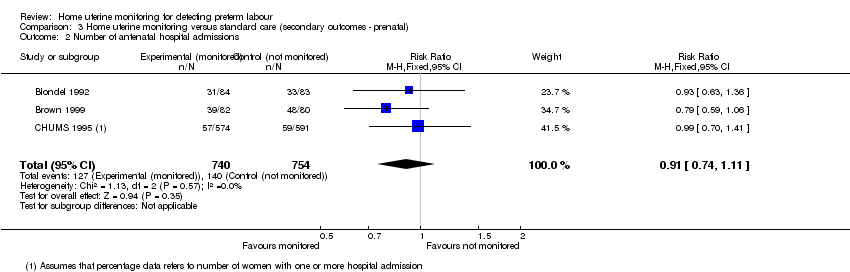
Comparison 3 Home uterine monitoring versus standard care (secondary outcomes ‐ prenatal), Outcome 2 Number of antenatal hospital admissions.
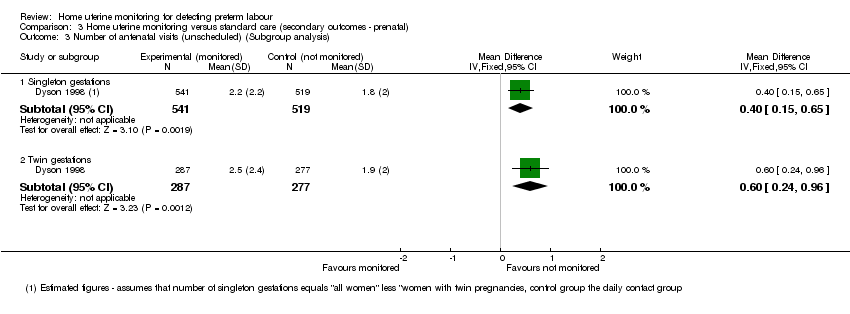
Comparison 3 Home uterine monitoring versus standard care (secondary outcomes ‐ prenatal), Outcome 3 Number of antenatal visits (unscheduled) (Subgroup analysis).
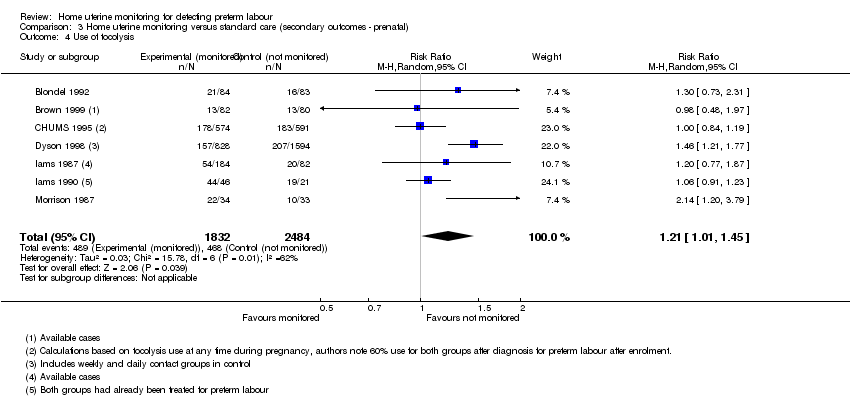
Comparison 3 Home uterine monitoring versus standard care (secondary outcomes ‐ prenatal), Outcome 4 Use of tocolysis.
| Home uterine monitoring for preventing preterm birth | ||||||
| Patient or population: women undergoing home monitoring for preventing preterm birth versus women receiving standard care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Home uterine monitoring | |||||
| Perinatal mortality | Study population | RR 1.22 | 2589 | ⊕⊕⊝⊝ | ||
| 46 per 1000 | 56 per 1000 | |||||
| Preterm birth less than 34 weeks' gestation | Study population | RR 0.78 | 1596 | ⊕⊕⊕⊕ | Sensitivity analysis included 1 study at low risk of bias (1292 women) and did not show any difference in results | |
| 166 per 1000 | 130 per 1000 | |||||
| Antenatal hospital admissions | Study population | RR 0.91 | 1494 | ⊕⊕⊝⊝ | ||
| 186 per 1000 | 169 per 1000 | |||||
| Preterm birth less than 37 weeks' gestation | Study population | RR 0.85 | 4834 | ⊕⊝⊝⊝ | ||
| 364 per 1000 | 310 per 1000 | |||||
| Admission to NICU | Study population | RR 0.77 | 2367 | ⊕⊕⊕⊝ | Evidence not downgraded for moderate heterogeneity (I² = 32%) | |
| 290 per 1000 | 223 per 1000 | |||||
| Number of unscheduled antenatal visits | The mean number of days ranged across control groups from approximately 1 to 2 days | The mean number of days in the monitored group was approximately half a day higher MD 0.48 (0.31 to 0.64) | 1994 | ⊕⊕⊕⊝ | Variation in protocol and healthcare delivery structures make it difficult to generalise from 1 large study contributing 65% of the weight for this outcome | |
| Use of tocolysis | Study population | RR 1.21 | 4316 | ⊕⊕⊝⊝ | This outcome may no longer be useful, due to changes in clinical practice. Sensitivity analysis including only 3 studies at low risk of bias (3749 women) did not show any clear difference in results. | |
| 188 per 1000 | 228 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1All studies contributing data with design limitations (‐1). | ||||||
| Methodological item | Adequate | Inadequate |
| Generation of random sequence | Computer‐generated sequence, random‐number tables, lot drawing, coin‐tossing, shuffling cards, throwing dice | Case number, date of birth, date of admission, alternation |
| Concealment of allocation | Central randomisation, coded drug boxes, sequentially‐sealed opaque envelopes | Open allocation sequence, any procedure based on inadequate generation |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Perinatal mortality Show forest plot | 2 | 2589 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.86, 1.72] |
| 2 Preterm birth < 34 weeks Show forest plot | 3 | 1596 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.62, 0.99] |
| 3 Preterm birth < 34 weeks (Subgroup analysis) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Singleton gestations | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.55, 2.27] |
| 3.2 Twin gestations | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.26, 1.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Preterm birth < 37 weeks Show forest plot | 8 | 4834 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.72, 1.01] |
| 2 Preterm birth < 37 weeks (Subgroup analysis) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Singleton gestations | 1 | 2422 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.62, 1.45] |
| 2.2 Twin gestations | 1 | 844 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.71, 1.30] |
| 3 Preterm birth < 32 weeks Show forest plot | 3 | 2550 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.31, 1.85] |
| 4 Use of antenatal corticosteroids Show forest plot | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.82, 1.25] |
| 5 Respiratory distress syndrome Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Singleton gestations | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.40, 3.95] |
| 5.2 Twin gestations | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.13, 1.12] |
| 6 Use of mechanical ventilation Show forest plot | 2 | 539 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.04, 2.38] |
| 7 Admission to neonatal intensive care unit Show forest plot | 5 | 2367 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.62, 0.96] |
| 8 Mode of delivery Show forest plot | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.36, 2.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of antenatal visits (unscheduled) Show forest plot | 2 | 1994 | Mean Difference (IV, Fixed, 95% CI) | 0.48 [0.31, 0.64] |
| 2 Number of antenatal hospital admissions Show forest plot | 3 | 1494 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.74, 1.11] |
| 3 Number of antenatal visits (unscheduled) (Subgroup analysis) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Singleton gestations | 1 | 1060 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [0.15, 0.65] |
| 3.2 Twin gestations | 1 | 564 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [0.24, 0.96] |
| 4 Use of tocolysis Show forest plot | 7 | 4316 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.01, 1.45] |

