Intervenciones para la prevención y el tratamiento del pie cavo
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Prospective randomized, single‐blind, sham controlled trial. | |
| Participants | 154 adults with chronic foot pain. Pes cavus was defined by a Foot Posture Index score of –2 or less, which is 2 standard deviations below the reported normal mean of +5 (Redmond 2006). | |
| Interventions | Custom‐made foot orthoses versus sham orthoses. | |
| Outcomes | Foot pain, functional limitation, health‐related quality of life, in‐shoe plantar pressure at 3 months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Using a computer random number generator |
| Allocation concealment? | Low risk | Central allocation by telephone by a third party not involved in the study |
| Blinding? | Low risk | Participants were blinded to treatment allocation for the duration of the study |
| Blinding? | High risk | Blinding of the investigator was not appropriate because of the potential need for ongoing contact with the participants concerning adverse effects |
| Blinding? | Low risk | No blinding, but primary outcome measure was self‐reported and not likely to be influenced by lack of blinding |
| Incomplete outcome data addressed? | Low risk | Only one missing outcome data |
| Free of selective reporting? | Low risk | Protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
| Free of other bias? | Low risk | The study appears to be free of other sources of bias. Explicit inclusion criteria, no baseline differences |
| Methods | Prospective randomized, single‐blind, experimental trial. | |
| Participants | 10 children aged 3 to 14 years with Charcot‐Marie‐Tooth disease type 1A. Pes cavus was defined weight bearing by a Foot Posture Index score between –12 and –1 (Redmond 2006) | |
| Interventions | One leg received intramuscular injections at six‐monthly intervals of botulinum toxin type‐A in the posterior tibialis and peroneus longus muscles, and one leg received no intervention. | |
| Outcomes | Primary outcome was radiographic alignment at 24‐months. Secondary outcomes were objective clinical measures of foot structure, ankle flexibility and strength. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | A simple randomization sequence was determined by coin‐toss |
| Allocation concealment? | Low risk | Central allocation by Paediatric Neurologist not involved in recruitment |
| Blinding? | High risk | Each child/parent was aware of the leg selection for treatment |
| Blinding? | High risk | The injecting physician was aware of the leg selection for treatment |
| Blinding? | Low risk | The principal investigator conducting all outcome measures was blinded to the injected leg |
| Incomplete outcome data addressed? | Low risk | No missing data |
| Free of selective reporting? | Low risk | Protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
| Free of other bias? | Low risk | The study appears to be free of other sources of bias. Explicit inclusion criteria, no baseline differences |
| Methods | Experimental, randomized cross‐over trial. | |
| Participants | 10 healthy young adults. Cavus feet were subjectively categorised as those having a high medial | |
| Interventions | Three off‐the‐shelf orthotic combinations (neutral post, medial post, lateral post) versus no orthotic device. | |
| Outcomes | Electromyography of 3 muscles (vastus medialis, vastus lateralis and gluteus medius) during 3 activities (squat, step down and vertical jump). | |
| Notes | No follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Latin square design |
| Allocation concealment? | Unclear risk | Insufficient information to permit judgement |
| Blinding? | High risk | No blinding |
| Blinding? | High risk | No blinding |
| Blinding? | High risk | No blinding |
| Incomplete outcome data addressed? | Low risk | No missing data |
| Free of selective reporting? | Unclear risk | Insufficient information to permit judgement |
| Free of other bias? | Low risk | The study appears to be free of other sources of bias. Explicit inclusion criteria, no baseline differences |
| Methods | Experimental, randomized, single‐blind, cross‐over trial. | |
| Participants | 22 healthy athletic adults. Pes cavus was defined weight bearing by a Foot Posture Index | |
| Interventions | Two common off‐the‐shelf running footwear models versus a control footwear. | |
| Outcomes | In‐shoe plantar pressure during running. | |
| Notes | No follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Using a computer random number generator |
| Allocation concealment? | Low risk | Allocation by opaque envelopes |
| Blinding? | Low risk | Participants were blinded to the manufacturer and model of each shoe condition to reduce preference bias |
| Blinding? | High risk | The chief investigator who recruited and assessed the participants was not blinded, to enable footwear fitting and data collection |
| Blinding? | High risk | The chief investigator who recruited and assessed the participants was not blinded, to enable footwear fitting and data collection |
| Incomplete outcome data addressed? | Low risk | No missing data |
| Free of selective reporting? | Low risk | Study protocol is not available but it is clear that the published report (and thesis) include all expected outcomes |
| Free of other bias? | Low risk | The study appears to be free of other sources of bias. Explicit inclusion criteria, no baseline differences |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not a randomized controlled trial. | |
| Not a randomized controlled trial and does not include pes cavus. | |
| Does not include participants with pes cavus | |
| Does not include participants with pes cavus | |
| Does not include participants with pes cavus | |
| Pes cavus subgroup data (N = 7) not published. Author unwilling to provide additional data. | |
| Does not include participants with pes cavus | |
| Does not include participants with pes cavus | |
| Not a randomized controlled trial. | |
| Not a randomized controlled trial. | |
| Excludes participants with pes cavus. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in foot pain at three months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Custom‐made foot orthoses versus sham, Outcome 1 Change in foot pain at three months. | ||||
| 1.1 Foot pain | 1 | 154 | Mean Difference (IV, Fixed, 95% CI) | 10.90 [3.21, 18.59] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change of calcaneal‐first metatarsal angle Show forest plot | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐2.31, 4.31] |
| Analysis 2.1  Comparison 2 Botulinum toxin type‐A versus control (note number of participants refers to legs not people ‐ see text), Outcome 1 Change of calcaneal‐first metatarsal angle. | ||||
| 2 Change of tibia‐calcaneal angle Show forest plot | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 1.1 [‐1.81, 4.01] |
| Analysis 2.2  Comparison 2 Botulinum toxin type‐A versus control (note number of participants refers to legs not people ‐ see text), Outcome 2 Change of tibia‐calcaneal angle. | ||||
| 3 Change of Foot Posture Index Show forest plot | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐3.65, 0.65] |
| Analysis 2.3  Comparison 2 Botulinum toxin type‐A versus control (note number of participants refers to legs not people ‐ see text), Outcome 3 Change of Foot Posture Index. | ||||
| 4 Change of ankle dorsiflexion range of motion Show forest plot | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐8.22, 3.82] |
| Analysis 2.4  Comparison 2 Botulinum toxin type‐A versus control (note number of participants refers to legs not people ‐ see text), Outcome 4 Change of ankle dorsiflexion range of motion. | ||||
| 5 Change of dorsiflexion foot strength Show forest plot | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐20.44, 21.24] |
| Analysis 2.5  Comparison 2 Botulinum toxin type‐A versus control (note number of participants refers to legs not people ‐ see text), Outcome 5 Change of dorsiflexion foot strength. | ||||
| 6 Change of plantarflexion foot strength Show forest plot | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 10.90 [‐40.82, 62.62] |
| Analysis 2.6  Comparison 2 Botulinum toxin type‐A versus control (note number of participants refers to legs not people ‐ see text), Outcome 6 Change of plantarflexion foot strength. | ||||
| 7 Change of inversion foot strength Show forest plot | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 7.00 [‐26.21, 40.21] |
| Analysis 2.7  Comparison 2 Botulinum toxin type‐A versus control (note number of participants refers to legs not people ‐ see text), Outcome 7 Change of inversion foot strength. | ||||
| 8 Change of eversion foot strength Show forest plot | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐6.20 [‐32.98, 20.58] |
| Analysis 2.8  Comparison 2 Botulinum toxin type‐A versus control (note number of participants refers to legs not people ‐ see text), Outcome 8 Change of eversion foot strength. | ||||
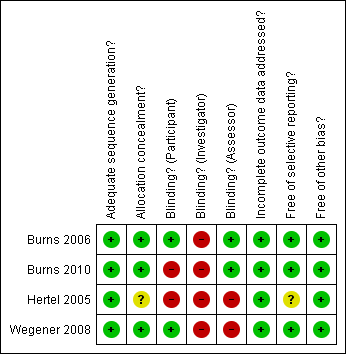
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
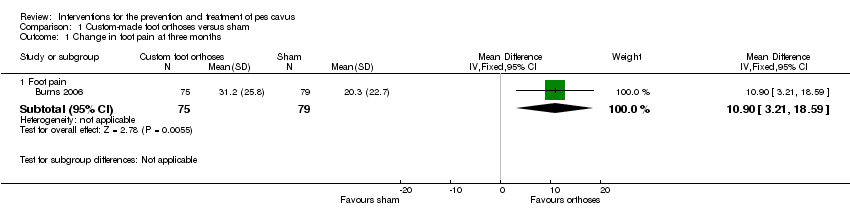
Comparison 1 Custom‐made foot orthoses versus sham, Outcome 1 Change in foot pain at three months.
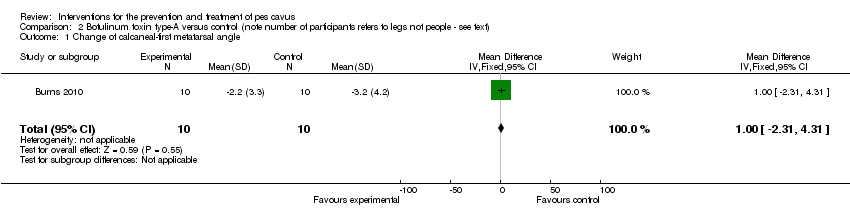
Comparison 2 Botulinum toxin type‐A versus control (note number of participants refers to legs not people ‐ see text), Outcome 1 Change of calcaneal‐first metatarsal angle.
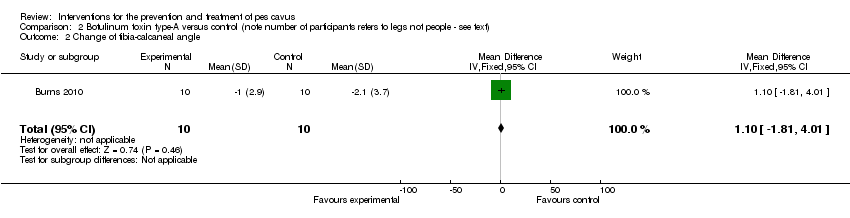
Comparison 2 Botulinum toxin type‐A versus control (note number of participants refers to legs not people ‐ see text), Outcome 2 Change of tibia‐calcaneal angle.
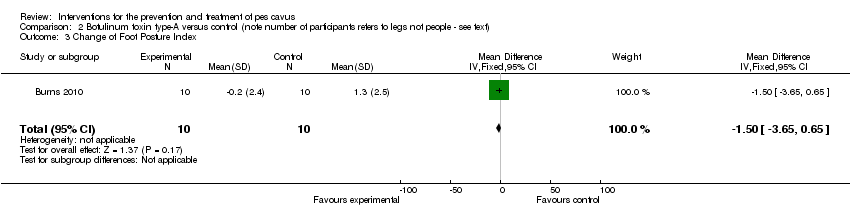
Comparison 2 Botulinum toxin type‐A versus control (note number of participants refers to legs not people ‐ see text), Outcome 3 Change of Foot Posture Index.
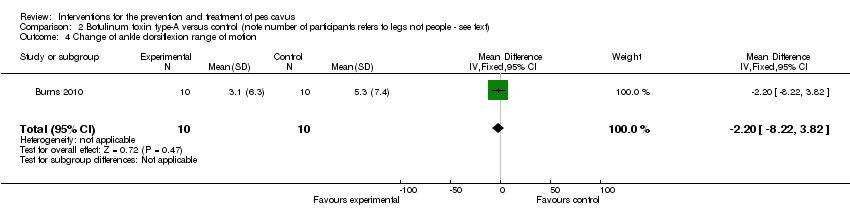
Comparison 2 Botulinum toxin type‐A versus control (note number of participants refers to legs not people ‐ see text), Outcome 4 Change of ankle dorsiflexion range of motion.
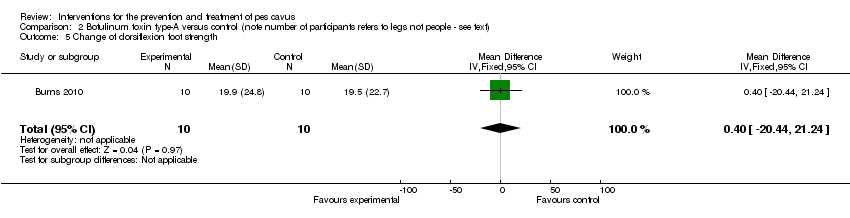
Comparison 2 Botulinum toxin type‐A versus control (note number of participants refers to legs not people ‐ see text), Outcome 5 Change of dorsiflexion foot strength.

Comparison 2 Botulinum toxin type‐A versus control (note number of participants refers to legs not people ‐ see text), Outcome 6 Change of plantarflexion foot strength.
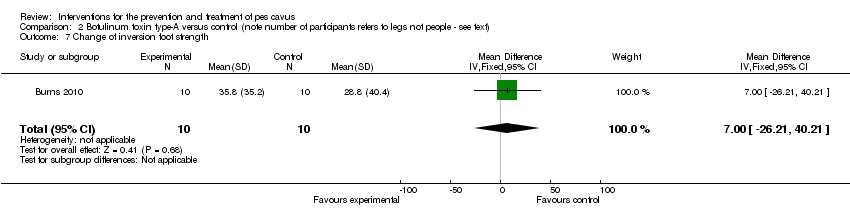
Comparison 2 Botulinum toxin type‐A versus control (note number of participants refers to legs not people ‐ see text), Outcome 7 Change of inversion foot strength.
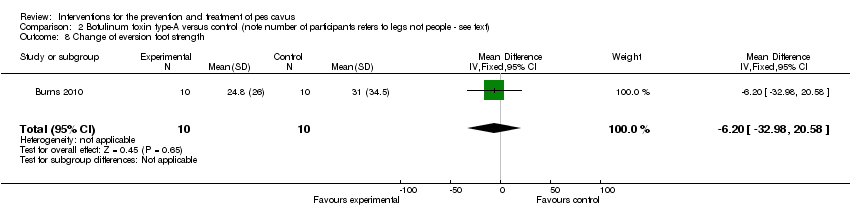
Comparison 2 Botulinum toxin type‐A versus control (note number of participants refers to legs not people ‐ see text), Outcome 8 Change of eversion foot strength.
| Outcome measures | No. of participants | Sham orthoses | Custom orthoses | Statistical method | Effect size |
| Change in foot pain at three months | 154 | 20.30 (22.70) | 31.20 (25.80) | WMD (fixed), 95% CI | 10.90 (3.21to 18.59) |
| Change in foot function at three months | 154 | 14.60 (20.60) | 25.60 (27.20) | WMD (fixed), 95% CI | 11.0 (3.35 to 18.65) |
| Change in physical function at three months | 154 | 2.60 (14.60) | 12.10 (19.30) | WMD (fixed), 95% CI | 9.50 (4.07 to 14.93) |
| Change in general health at three months | 154 | 3.00 (20.80) | 3.50 (18.40) | WMD (fixed), 95% CI | 0.50 (‐5.70 to 6.70) |
| Change in vitality at three months | 154 | 3.00 (15.20) | 8.50 (17.80) | WMD (fixed), 95% CI | 5.50 (0.26 to 10.74) |
| Change in social function at three months | 154 | 6.20 (16.20) | 8.70 (20.10) | WMD (fixed), 95% CI | 2.50 (‐3.28 to 8.28) |
| Change in pressure‐time integral (N.s/cm2, whole foot) at baseline | 154 | ‐1.60 (1.70) | ‐4.50 (2.70) | WMD (fixed), 95% CI | ‐2.90 (‐3.62 to ‐2.18) |
| Change in pressure‐time integral (N.s/cm2, rearfoot) at baseline | 154 | ‐0.70 (0.80) | ‐1.90 (1.40) | WMD (fixed), 95% CI | ‐1.20 (‐1.56 to ‐0.84) |
| Change in pressure‐time integral (N.s/cm2, midfoot) at baseline | 154 | ‐0.20 (0.60) | 0.30 (2.20) | WMD (fixed), 95% CI | 0.50 (‐0.02 to 1.02) |
| Change in pressure‐time integral (N.s/cm2, forefoot) at baseline | 154 | ‐1.40 (2.00) | ‐3.20 (2.90) | WMD (fixed), 95% CI | ‐1.80 (‐2.59to ‐1.01) |
| Adverse events at three months | 154 | 12/79 | 7/75 | RR (fixed), 95% CI | 0.61 (0.26 to 1.48) |
| Outcome measure | No. of participants | 1. Control | 2. Asics Nimbus | 3. Brooks Glycerin | Statistical method | Effect size (1 versus 2) | Effect size (1 versus 3) | Effect size (2 versus 3) |
| Peak pressure (kPa, whole foot) | 22 | 513.4 (78.9) | 399.4 (88.6) | 361.2 (82.2) | WMD (Fixed), 95% CI | ‐114.00 (‐163.58 to ‐64.42) | ‐152.20 (‐199.81 to ‐104.59) | ‐38.20 (‐88.70 to 12.30) |
| Peak pressure (kPa, rearfoot) | 22 | 358.1 (173.8) | 240.9 (91.9) | 264.4 (90.5) | WMD (Fixed), 95% CI | ‐117.20 (‐199.35 to ‐35.05) | ‐93.70 (‐175.58 to ‐11.82) | 23.50 (‐30.40 to 77.40) |
| Peak pressure (kPa, midfoot) | 22 | 168.6 (68.1) | 126.3 (31.0) | 131.4 (34.4) | WMD (Fixed), 95% CI | ‐42.30 (‐73.57to ‐11.03) | ‐37.20 (‐69.08 to ‐5.32) | 5.10 (‐14.25to 24.45) |
| Peak pressure (kPa, forefoot) | 22 | 464.2 (106.4) | 386.1 (100.0) | 340.8 (89.4) | WMD (Fixed), 95% CI | ‐78.10 (‐139.12to ‐17.08) | ‐123.40 (‐181.47to ‐65.33) | ‐45.30 (‐101.35to 10.75) |
| Pressure time integral (kPa.s, whole foot) | 22 | 69.9 (12.4) | 55.6 (12.2) | 51.7 (9.7) | WMD (Fixed), 95% CI | ‐14.30 (‐21.57to ‐7.03) | ‐18.20 (‐24.78to ‐11.62) | ‐3.90 (‐10.41to 2.61) |
| Pressure time integral (kPa.s, rearfoot) | 22 | 19.8 (10.9) | 17.2 (6.9) | 18.8 (7.6) | WMD (Fixed), 95% CI | ‐2.60 (‐7.99 to 2.79) | ‐1.00 (‐6.55to 4.55) | 1.60 (‐2.69 to 5.89) |
| Pressure time integral (kPa.s, midfoot) | 22 | 15.3 (7.7) | 14.4 (3.9) | 14.8 (4.4) | WMD (Fixed), 95% CI | ‐0.90 (‐4.51to 2.71) | ‐0.50 (‐4.21to 3.21) | 0.40 (‐2.06 to 2.86) |
| Pressure time integral (kPa.s, forefoot) | 22 | 63.9 (13.2) | 50.3 (12.3) | 46.0 (9.6) | WMD (Fixed), 95% CI | ‐13.60 (‐21.14 to ‐6.06) | ‐17.90 (‐24.72 to ‐11.08) | ‐4.30 (‐10.82 to 2.22) |
| Force (%Body Weight, whole foot) | 22 | 226.2 (23.1) | 217.1 (20.4) | 219.4 (17.2) | WMD (Fixed), 95% CI | ‐9.10 (‐21.98 to 3.78) | ‐6.80 (‐18.83 to 5.23) | 2.30 (‐8.85to 13.45) |
| Force (%Body Weight, rearfoot) | 22 | 97.4 (43.3) | 90.3 (34.9) | 95.9 (30.3) | WMD (Fixed), 95% CI | ‐7.10 (‐30.34 to 16.14) | ‐1.50 (‐23.58 to 20.58) | 5.60 (‐13.71 to 24.91) |
| Force (%Body Weight, midfoot) | 22 | 25.6 (12.3) | 30.0 (7.0) | 28.6 (8.3) | WMD (Fixed), 95% CI | 4.40 (‐1.51 to 10.31) | 3.0 (‐3.20 to 9.20) | ‐1.40 (‐5.94 to 3.14) |
| Force (%Body Weight, forefoot) | 22 | 188.0 (21.5) | 176.4 (24.3) | 175.9 (20.6) | WMD (Fixed), 95% CI | ‐11.60 (‐25.16 to 1.96) | ‐12.10 (‐24.54 to 0.34) | ‐0.50 (‐13.81 to 12.81) |
| Outcome measure | No. of participants | 1. No orthoses | 2. Medial orthoses | 3. Neutral orthoses | 4. Lateral orthoses | Statistical method | Effect size (1 versus 2) | Effect size (1 versus 3) | Effect size (1 versus 4) |
| Vastus Medialis EMG during squat | 10 | 1.14 (0.98) | 1.19 (0.94) | 1.24 (1.15) | 1.22 (1.00) | WMD (fixed), 95% CI | 0.05 ‐0.79 to 0.89) | 0.10 (‐0.84 to 1.04) | 0.08 (‐0.79 to 0.95 |
| Vastus Medialis EMG during stepdown | 10 | 0.99 (0.67) | 1.33 (1.36) | 1.27 (1.23) | 1.42 (1.49) | WMD (fixed), 95% CI | 0.34 (‐0.60 to 1.28) | 0.28 (‐0.59 to 1.15) | 0.43 (‐0.58to 1.44) |
| Vastus Medialis EMG during vertical jump | 10 | 1.15 (0.54) | 1.32 (0.88) | 1.26 (0.70) | 1.28 (0.73) | WMD (fixed), 95% CI | 0.17 (‐0.47to 0.81) | 0.11 (‐0.44to 0.66) | 0.13 (‐0.43to 0.69) |
| Vastus Lateralis EMG during squat | 10 | 1.07 (0.63) | 0.95 (0.42) | 0.99 (0.47) | 0.97 (0.47) | WMD (fixed), 95% CI | ‐0.12 (‐0.59to 0.35) | ‐0.08 (‐0.57to 0.41) | ‐0.10 (‐0.59to 0.39) |
| Vastus Lateralis EMG during stepdown | 10 | 0.98 (0.56) | 1.08 (0.60) | 1.09 (0.65) | 1.13 (0.70) | WMD (fixed), 95% CI | 0.10 (‐0.41to 0.61) | 0.11 (‐0.42to 0.64) | 0.15 (‐0.41to 0.71) |
| Vastus Lateralis EMG during vertical jump | 10 | 1.31 (1.31) | 1.25 (0.62) | 1.27 (0.62) | 1.28 (0.54) | WMD (fixed), 95% CI | ‐0.06 (‐0.96to 0.84) | ‐0.04 (‐0.94 to 0.86) | ‐0.03 (‐0.91to 0.85) |
| Gluteus Medius EMG during squat | 10 | 0.66 (0.26) | 0.67 (0.24) | 0.69 (0.26) | 0.70 (0.30) | WMD (fixed), 95% CI | 0.01 (‐0.21to 0.23) | 0.03 (‐0.20 to 0.26) | 0.04 (‐0.21to 0.29) |
| Gluteus Medius EMG during stepdown | 10 | 0.62 (0.23) | 0.74 (0.39) | 0.72 (0.33) | 0.74 (0.44) | WMD (fixed), 95% CI | 0.12 (‐0.16 to 0.40) | 0.10 (‐0.15 to 0.35) | 0.12 (‐0.19to 0.43) |
| Gluteus Medius EMG during vertical jump | 10 | 0.90 (0.34) | 1.02 (0.41) | 0.96 (0.35) | 1.05 (0.45) | WMD (fixed), 95% CI | 0.12 (‐0.21to 0.45) | 0.06 (‐0.24to 0.36) | 0.15 (‐0.20to 0.50) |
| Outcome Measure | No. of participants | Control Leg | BoNT‐A Leg | Statistical Method | Effect Size |
| Change of calcaneal‐first metatarsal angle | 10 | ‐3.2 (4.2) | ‐2.2 (3.3) | WMD (fixed), 95% CI | 1.00 (‐2.31 to 4.31) |
| Change of tibia‐calcaneal angle | 10 | ‐2.1 (3.7) | ‐1.0 (2.9) | WMD (fixed), 95% CI | 1.10 (‐1.81 to 4.01) |
| Change of Foot Posture Index | 10 | 1.3 (2.5) | ‐0.2 (2.4) | WMD (fixed), 95% CI | ‐1.50 ‐3.65 to 0.65) |
| Change of ankle dorsiflexion range of motion | 10 | 5.3 (7.4) | 3.1 (6.3) | WMD (fixed), 95% CI | ‐2.20 ‐8.22 to 3.82) |
| Change of dorsiflexion foot strength | 10 | 19.5 (22.7) | 19.9 (24.8) | WMD (fixed), 95% CI | 0.40 (‐20.44 to 21.24) |
| Change of plantarflexion foot strength | 10 | 50.6 (58.1) | 61.5 (59.9) | WMD (fixed), 95% CI | 10.90 (‐40.82 to 62.62) |
| Change of inversion foot strength | 10 | 28.8 (40.4) | 35.8 (35.2) | WMD (fixed), 95% CI | 7.00 (‐26.21 to 40.21) |
| Change of eversion foot strength | 10 | 31.0 (34.5) | 24.8 (26.0) | WMD (fixed), 95% CI | ‐6.20 (‐32.98 to 20.58) |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in foot pain at three months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Foot pain | 1 | 154 | Mean Difference (IV, Fixed, 95% CI) | 10.90 [3.21, 18.59] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change of calcaneal‐first metatarsal angle Show forest plot | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐2.31, 4.31] |
| 2 Change of tibia‐calcaneal angle Show forest plot | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 1.1 [‐1.81, 4.01] |
| 3 Change of Foot Posture Index Show forest plot | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐3.65, 0.65] |
| 4 Change of ankle dorsiflexion range of motion Show forest plot | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐8.22, 3.82] |
| 5 Change of dorsiflexion foot strength Show forest plot | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐20.44, 21.24] |
| 6 Change of plantarflexion foot strength Show forest plot | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 10.90 [‐40.82, 62.62] |
| 7 Change of inversion foot strength Show forest plot | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 7.00 [‐26.21, 40.21] |
| 8 Change of eversion foot strength Show forest plot | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐6.20 [‐32.98, 20.58] |

