Trening mięśni oddechowych w mukowiscydozie
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Parallel design over 12 weeks. | |
| Participants | Total cohort: n = 27. Age range: 6 ‐ 18 years. Gender split: no information. | |
| Interventions | RMT: no details; plus, cycle ergometer training 3 times per week. Control: cycle ergometer training 3 times per week. | |
| Outcomes | FEV1, FVC, IMS, IME, MEC, perceived breathlessness, antibiotic use and ease or degree of expectoration. | |
| Notes | RME protocol: abstract only, no details given. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no details given. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | High risk | Performance bias: clear difference between the interventions received. Dectection bias: No reference to any blinding. |
| Incomplete outcome data (attrition bias) | Unclear risk | No information provided. Intention‐to‐treat: unclear. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information available to arrive at a conclusion. |
| Other bias | Unclear risk | Insufficient information available to arrive at a conclusion. |
| Methods | Parallel design over 6 weeks. Single‐centre study in Russia. | |
| Participants | Total cohort: n = 20. Treatment group: n = 10; control group: n = 10. Age range was not stated, but all were adults. Gender split: no information. States no significant differences between groups in terms of gender, age, weight, height, pulmonary function. | |
| Interventions | Threshold loading device: Intervention group: 30% of PImax Control group: 7 cm H2O Training regimen: 10 to 15 minutes twice daily for 6 weeks. | |
| Outcomes | FEV1, FVC, PImax, IC, RMS, RME and exercise capacity. | |
| Notes | Abstract only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors only state that the allocation was random without explaining the process involved. |
| Allocation concealment (selection bias) | Unclear risk | No details are provided. |
| Blinding (performance bias and detection bias) | Unclear risk | Performance bias: The comparison group are referred to only as the "control group" with no mention of the intensity of the training used; i.e. if it was at "sham" or sub‐maximal levels. Dectection bias: No reference to any blinding. |
| Incomplete outcome data (attrition bias) | High risk | No statistical data is presented for the control group. 1 participant from the intervention group did not complete the study; it was not stated whether they were included or excluded from the final analysis. |
| Selective reporting (reporting bias) | High risk | 2 outcomes (respiratory muscle strength and dyspnoea) are mentioned as having been analysed, but no data are provided for them. |
| Other bias | Unclear risk | Insufficient information available to arrive at a conclusion. |
| Methods | Consecutive, self‐control design over 8 weeks. Study run in Canada, unclear if single‐ or multicentre. | |
| Participants | Total cohort: n = 11. Age range: 9 ‐ 24 years. Gender split: no information. | |
| Interventions | RMT: inspiratory resistance, 15 minutes twice daily, no dosage. Control: treatment at usual. | |
| Outcomes | IMS, Wmax, VO₂max, VE and heart rate. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no details given. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | High risk | Performance bias: no details of the control training regimen are provided = high risk. Dectection bias: observer blind = low risk. |
| Incomplete outcome data (attrition bias) | High risk | 2 participants were unable to satisfactorily perform the outcome measure PIMax, due to expiration up to residual volume resulting in coughing. The authors do not stipulate whether this occurred during the intervention or control phase of the study. Intention‐to‐treat: unclear. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information available to arrive at a conclusion. |
| Other bias | Unclear risk | Insufficient information available to arrive at a conclusion. |
| Methods | Randomised cross‐over design. Single‐centre study in Switzerland. | |
| Participants | Total cohort: n = 22. Age range: 9 ‐ 18 years. Gender split: 10 male, 12 female. | |
| Interventions | Intervention group: 8 weeks voluntary eucapnic hyperventilation, 5 self‐selected days per week, 2x daily, 5 to 10 min per session. Control group: 8 weeks standardised chest physiotherapy. | |
| Outcomes | RME time, exercise duration, FEV1, FVC, FEF25‐75%, CF questionnaire (overall score) and CF clinical score. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no details given. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | High risk | Performance bias: the comparison was made to "standardised chest physiotherapy" and would likely be aware of group allocation. Dectection bias: No reference to any blinding. |
| Incomplete outcome data (attrition bias) | Low risk | 6 participants (27.3%) discontinued the study (4 in the control period, 2 in the intervention period). |
| Selective reporting (reporting bias) | High risk | Some health‐related quality of life domains are unreported. All other outcomes are reported. |
| Other bias | Unclear risk | Insufficient information available to arrive at a conclusion. |
| Methods | Parallel design over 8 weeks. Study run in UK, not clear if single centre or 2 centres. | |
| Participants | Total cohort: n = 18. Treatment group: n = 9; control group: n = 9. All participants were adults, but no specific age details or information on gender split given. | |
| Interventions | Intervention: computer‐generated through range RMT (TIRE) at 80% of individual capacity. Control: threshold loading device at 30% of peak; the measure used is not named. | |
| Outcomes | Chronic Respiratory Disease Questionnaire ('mastery' and 'emotion' elements), RMS and RME. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no details given. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information available to arrive at a conclusion. |
| Blinding (performance bias and detection bias) | Unclear risk | Perfomance bias: the training intensities employed (80% and "threshold" 30% training) could, potentially, have led the participants to know which group they were in. Detection bias: no reference to any blinding. |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information available to arrive at a conclusion; no statistical data is presented for the control group. Intention‐to‐treat: 3 from 18 (17%). |
| Selective reporting (reporting bias) | Unclear risk | As this study (to date) is only published in abstract form it is unclear whether the reported outcomes are all that were analysed. |
| Other bias | Unclear risk | Insufficient information available to arrive at a conclusion. |
| Methods | Parallel design over 6 weeks. Single‐centre study in the Netherlands. | |
| Participants | Total cohort: n = 16. Treatment group: n = 8; control group: n = 8. Age range for total cohort 10 ‐ 25 years. Treatment group: mean (SD) age = 17 (5.2) years; control group: mean (SD) age = 19 (5.5) years. Gender split for total cohort: 8 male, 8 female. Treatment group: 4 male, 4 female; control: 4 male, 4 female. | |
| Interventions | RMT: threshold loading: 20 minutes a day, 5 days per week, at 40% of PImax. Control: threshold loading: 20 minutes a day, 5 days per week. at 10% of PImax. | |
| Outcomes | FEV1, FVC, Wmax, VO2max, VEmax, RME, perceived breathlessness, general fatigue, physical fatigue, reduced activity score, reduced motivation score, mental fatigue and dyspnoea. | |
| Notes | RME protocol: a commercially‐available threshold‐loading device (Threshold, Healthscan Products, Inc. USA) was used during an incremental loading procedure. In order to obtain pressures over 41 cm H2O an additional spring was inserted with a double‐spring constant. Participants started inspiring from a threshold‐loading device set at 30% of PImax for 2 min. The threshold load was then increased every 2 min in increments of 10% of PImax. The maximal load was defined as the highest load which could be reached and maintained for at least 1 min as a percentage of PImax. The breathing pattern was not regulated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned to 1 of 2 groups by 5 factors: gender; age; FEV1; FVC; and BMI using the minimization method. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | High risk | Performance bias: both training intensities were low; however, no attempt was made to ascertain whether the participants knew if the received the training intensity. Detection bias: no reference to any blinding. |
| Incomplete outcome data (attrition bias) | High risk | 1 participant in the intervention group was withdrawn due to earache experienced whilst training at 40% of PImax. Intention‐to‐treat: 1 from 15 (6%). |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information available to arrive at a conclusion. |
| Other bias | Unclear risk | Insufficient information available to arrive at a conclusion. |
| Methods | Parallel design over 8 weeks. Single centre in UK. | |
| Participants | Total cohort: n = 29. Treatment group 1: n = 9; treatment group 2: n = 10; control group: n = 10. Age Total cohort (all adults): mean (SD) age = 22 (4.2) years. Treatment group 1: mean (SD) age = 24.8 (5.5) years. Treatment group 2: mean (SD) age = 20 (4.7) years; control group: mean (SD) age = 21.3 (2.7) years. Gender split Total cohort: 16 male, 14 female. Treatment group 1: 4 male, 6 female (according to table in paper). Treatment group 2: 6 male, 4 female; control group: 6 male, 4 female. All had similar age, height, weight and lung function at baseline. | |
| Interventions | Intervention 1: RMT at 80% of "maximal inspiratory effort". Control: "No Training" RMT is incremental maximal effort with progressively shorter rest periods, 3 times a week. | |
| Outcomes | FEV1 (% predicted), FVC (% predicted), PImax, SPImax, heart rate, perceived exertion, dyspnoea and Chronic Respiratory Disease Questionnaire. | |
| Notes | Sample size calculation undertaken such that study needed at least 9 participants in each group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) | High risk | Performance bias: the comparison was "no training" making it clear to the participants which arm they were in. Dectection bias: outcome assessors at the final data collection session, although they did not state whether this was the case at the initial assessment or even if the same assessors carried out all the assessments. |
| Incomplete outcome data (attrition bias) | Unclear risk | No mention is made of whether all participants completed the study or not. Nor are there any statistical indications. Intention‐to‐treat: unclear. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information available to arrive at a conclusion. |
| Other bias | Unclear risk | Insufficient information available to arrive at a conclusion. |
| Methods | Parallel design over 8 weeks. Not clear if single or multicentre, authors from UK and USA. | |
| Participants | Total cohort: n = 39 (19 with CF and 20 matched healthy controls). Treatment group CF: n = 9; control group CF: n = 10. Age of CF adults: mean (SD) age = 22.5 (3.5) years; age of healthy adults: mean (SD) age = 21.5 (3.5) years. Gender matched groups. | |
| Interventions | Treatment: RMT at 80% of "maximal effort", no dosage stated. Control: no training. | |
| Outcomes | VC, TLC. | |
| Notes | Only CF participants eligible for this review, 10 healthy adults in another RMT group and another control group. Abstracts only available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no details given. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | High risk | Performance bias: the comparison was "no training" making it clear to the participants which arm they were in. Detection bias: no reference to any blinding. |
| Incomplete outcome data (attrition bias) | Unclear risk | No information provided. Intention‐to‐treat: unclear. |
| Selective reporting (reporting bias) | High risk | The post‐training pulmonary function results were not presented. |
| Other bias | Unclear risk | Insufficient information available to arrive at a conclusion. |
| Methods | Parallel design over 10 weeks. Single‐centre study in USA. | |
| Participants | Total cohort: n = 20; treatment group: n = 10; control group: n = 10. Treatment group: mean (SD) age = 11.46 (2.45) years; control group: mean (SD) age = 9.76 (2.57) years. No information on gender split. | |
| Interventions | Treatment: RMT at 60% PImax. Control: Sham IMT at 10% PImax. | |
| Outcomes | FEV1, VC, FRC, IC, RV, TLC, RV/TLC, FEV1/FVC, MVV, exercise time. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no details given. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | High risk | Performance bias: there was a clear difference in the intensity of training although no attempt was made to ascertain whether the participants in the training groups knew if they received the training intensity. Dectection bias: outcome assessors at the final data collection session, although they did not state whether this was the case at the initial assessment or even if the same assessors carried out all the assessments. |
| Incomplete outcome data (attrition bias) | High risk | 2 participants removed from analysis and the reasons for this were explained; however, it is unclear which group(s) they were in. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information available to arrive at a conclusion. |
| Other bias | Unclear risk | Insufficient information available to arrive at a conclusion. |
% predicted: the volume of air exhaled expressed as a percentage of the expected volume based on the physical attributes of the individual
BMI: body mass index
FEV1: volume of air exhaled over the first second of a forced exhalation
FEV1/FVC = the ratio of FEV1 to FVC
FRC: functional residual capacity
FVC: total volume of air forcibly exhaled
FEF25‐75%: forced expiratory flow 25‐75%
IC: inspiratory capacity
MEC: maximal exercise capacity
MIV: maximal inspiratory pressure
MVV: maximum voluntary ventilation
RME: respiratory muscle endurance
RMF: inspiratory muscle function
RMS: inspiratory muscle strength
RMT: inspiratory muscle training
n: number of participants
PImax: maximal inspiratory pressure
RV: residual volume; i.e. the volume of air retained in the lungs following a maximal, voluntary exhalation (FVC)
RV/TLC: the ratio of residual volume to total lung capacity
SD: standard deviation
SPImax: sustained maximal inspiratory pressure
TLC: total lung capacity; i.e. the calculated maximum potential volume of an individual's lungs
VC: the total volume of air that can be exhaled in any one breath
VE(max): maximal expired ventilation
VO₂max: maximal oxygen consumption
Wmax: maximum work load
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Inappropriate intervention ‐ ACBT versus TIRE not RMT. | |
| Inappropriate intervention ‐ singing training and not RMT. | |
| Study excluded as allocation not randomised. | |
| Observational study, no randomisation. | |
| Although a form of IMT was used as an intervention it was used in combination with another exercise based intervention; therefore, it was impossible to attribute any observed changes to IMT alone. | |
| Observational study, no randomisation. | |
| Inappropriate intervention ‐ neuromuscular electrical stimulation prior to endurance training not IMT. |
ACBT: active cycle of breathing technique
RMT: respiratory muscle training
TIRE: test of incremental respiratory endurance
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Parallel design over 8 weeks. Unclear if single‐ or multicentre study. |
| Participants | Total cohort: n = 10. Unclear how may were allocated to intervention or control group. Age range: 21 to 40 years. Gender split: males, n = 6; females, n = 4. |
| Interventions | Treatment: RME training at 70% of 12s MVV for 15 minutes daily. Control: standard chest physiotherapy. |
| Outcomes | RME, 6MWT distance, CFQ‐R score, FVC, FEV1, MIP, MEP. |
| Notes |
| Methods | Parallel design over 8 weeks. Unclear if single‐ or multicentre study. |
| Participants | Total cohort: n = 28; treatment group: n= 14; control group: n= 14. Age, mean (SD): 13.18 (3.65) years. FEV1 % predicted, mean (SD): 89.51 (19.47) % predicted. No information on gender split. |
| Interventions | Treatment: RMT at 30% to 80% of MIV for 20 minutes on 5 days per week‐1. Control: sham RMT at 10% of MIV for 20 minutes on 5 days per week‐1. |
| Outcomes | Pulmonary function, 6MWT distance and peripheral muscle strength (hand grip, shoulder abductors, elbow flexors). |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Forced expiratory volume at one second (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 RMT (80% of maximal effort) versus control, Outcome 1 Forced expiratory volume at one second (L). | ||||
| 1.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Forced vital capacity (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 RMT (80% of maximal effort) versus control, Outcome 2 Forced vital capacity (L). | ||||
| 2.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Chronic Respiratory Disease Questionnaire (mastery) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 RMT (80% of maximal effort) versus control, Outcome 3 Chronic Respiratory Disease Questionnaire (mastery). | ||||
| 4 Chronic Respiratory Disease Questionnaire (emotion) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4 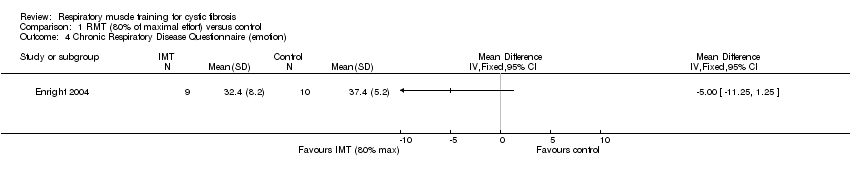 Comparison 1 RMT (80% of maximal effort) versus control, Outcome 4 Chronic Respiratory Disease Questionnaire (emotion). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Forced expiratory volume at one second (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 RMT (60% of maximal effort) versus control, Outcome 1 Forced expiratory volume at one second (L). | ||||
| 1.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 PImax (cm H₂O) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 RMT (60% of maximal effort) versus control, Outcome 2 PImax (cm H₂O). | ||||
| 2.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Forced expiratory volume at one second (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.1 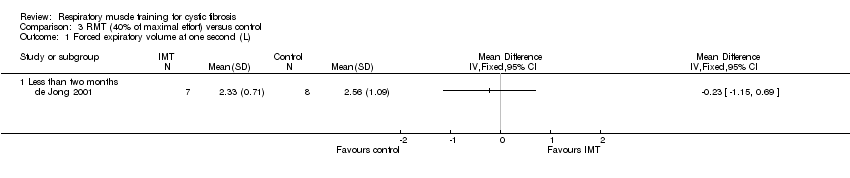 Comparison 3 RMT (40% of maximal effort) versus control, Outcome 1 Forced expiratory volume at one second (L). | ||||
| 1.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Forced expiratory volume at one second (% predicted) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.2  Comparison 3 RMT (40% of maximal effort) versus control, Outcome 2 Forced expiratory volume at one second (% predicted). | ||||
| 2.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Forced vital capacity (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.3 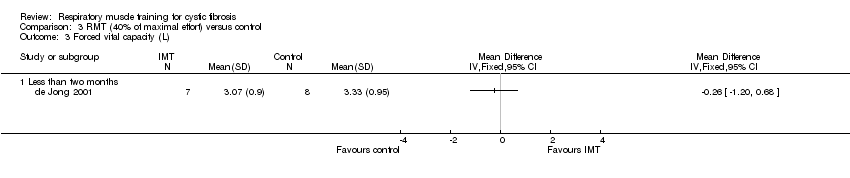 Comparison 3 RMT (40% of maximal effort) versus control, Outcome 3 Forced vital capacity (L). | ||||
| 3.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Forced vital capacity (% predicted) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.4  Comparison 3 RMT (40% of maximal effort) versus control, Outcome 4 Forced vital capacity (% predicted). | ||||
| 4.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Inspiratory muscle endurance (% PImax) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.5  Comparison 3 RMT (40% of maximal effort) versus control, Outcome 5 Inspiratory muscle endurance (% PImax). | ||||
| 5.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Forced expiratory volume at one second (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.1 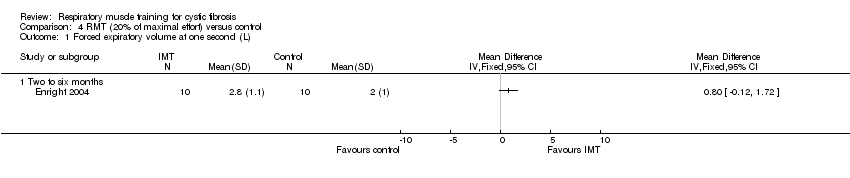 Comparison 4 RMT (20% of maximal effort) versus control, Outcome 1 Forced expiratory volume at one second (L). | ||||
| 1.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Forced vital capacity (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.2 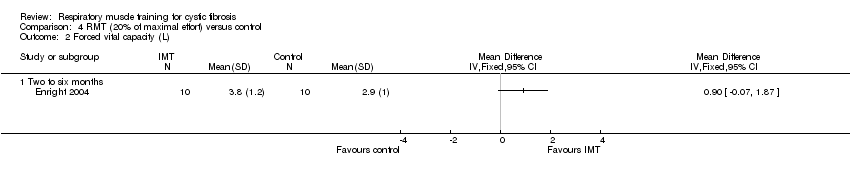 Comparison 4 RMT (20% of maximal effort) versus control, Outcome 2 Forced vital capacity (L). | ||||
| 2.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

Comparison 1 RMT (80% of maximal effort) versus control, Outcome 1 Forced expiratory volume at one second (L).

Comparison 1 RMT (80% of maximal effort) versus control, Outcome 2 Forced vital capacity (L).
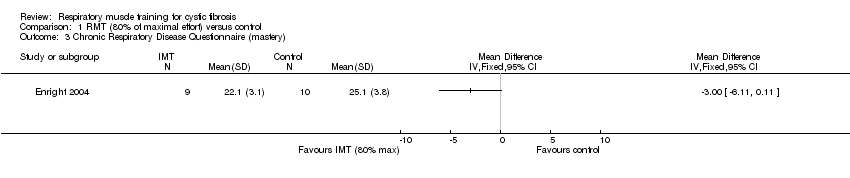
Comparison 1 RMT (80% of maximal effort) versus control, Outcome 3 Chronic Respiratory Disease Questionnaire (mastery).
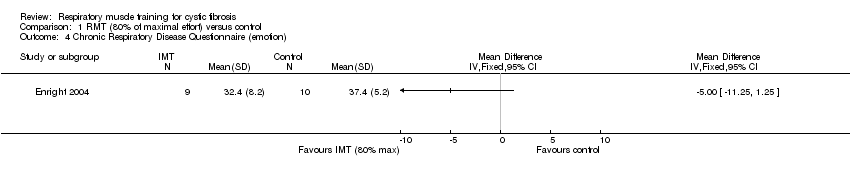
Comparison 1 RMT (80% of maximal effort) versus control, Outcome 4 Chronic Respiratory Disease Questionnaire (emotion).

Comparison 2 RMT (60% of maximal effort) versus control, Outcome 1 Forced expiratory volume at one second (L).

Comparison 2 RMT (60% of maximal effort) versus control, Outcome 2 PImax (cm H₂O).
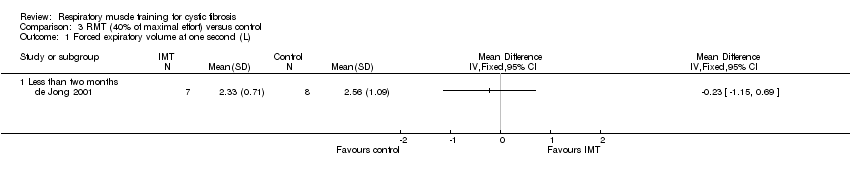
Comparison 3 RMT (40% of maximal effort) versus control, Outcome 1 Forced expiratory volume at one second (L).

Comparison 3 RMT (40% of maximal effort) versus control, Outcome 2 Forced expiratory volume at one second (% predicted).

Comparison 3 RMT (40% of maximal effort) versus control, Outcome 3 Forced vital capacity (L).
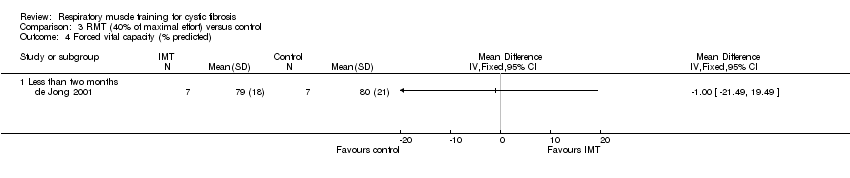
Comparison 3 RMT (40% of maximal effort) versus control, Outcome 4 Forced vital capacity (% predicted).
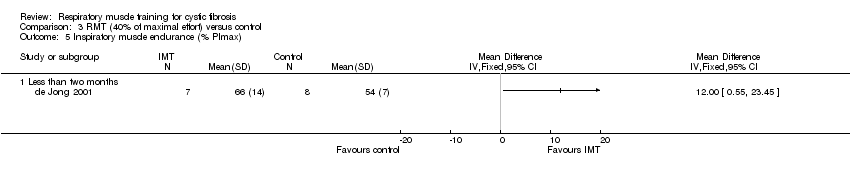
Comparison 3 RMT (40% of maximal effort) versus control, Outcome 5 Inspiratory muscle endurance (% PImax).

Comparison 4 RMT (20% of maximal effort) versus control, Outcome 1 Forced expiratory volume at one second (L).

Comparison 4 RMT (20% of maximal effort) versus control, Outcome 2 Forced vital capacity (L).
| Respiratory muscle training compared with control for cystic fibrosis | ||||||
| Patient or population: adults and children with cystic fibrosis Settings: outpatients Intervention: respiratory muscle trainingₑ Comparison: controlₑ | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Controlₑ | Respiratory muscle trainingₑ | |||||
| FEV1: % predicted Follow‐up: 6‐12 weeks | No significant differences between the respiratory muscle training group and the control group were reported in any study. | NA | 145 (7 studies including 2 cross‐over studies) | ⊕⊝⊝⊝ | Studies reported FEV1 as % predicted, litres or z score. One study with respiratory muscle training level 30% of maximal effort reported a significant improvement within the training group. | |
| FVC: % predicted Follow‐up: 6‐12 weeks | No significant differences between the respiratory muscle training group and the control group were reported in any study. | NA | 114 (5 studies including 1 cross‐over study) | ⊕⊝⊝⊝ | Studies reported FVC as % predicted, litres or z score. One study with respiratory muscle training level 30% of maximal effort reported a significant improvement within the training group. | |
| Exercise capacity: VO2max (mL/kg/min) Follow‐up: 6‐12 weeks | No significant differences between the respiratory muscle training group and the control group were reported in any study. | NA | 54 (3 studies including 1 cross‐over study) | ⊕⊝⊝⊝ | One study with an unspecified level of resistance reported a significant improvement within the respiratory muscle training group. | |
| HRQoL: total score Follow‐up: 8 weeks | Two studies reported no significant differences between the respiratory muscle training group and the control group. One study reported significant improvements in the parameters of mastery and emotion in the respiratory muscle training group compared to the control group. | NA | 69 (3 studies including 1 cross‐over study) | ⊕⊝⊝⊝ | Two studies used the Chronic Respiratory Disease Questionnaire (CRDQ) and one study used the cystic fibrosis questionnaire (CFQ). | |
| Respiratory muscle function: maximal inspiratory pressure (PImax) Follow‐up: 6‐10 weeks | Significant improvements were observed in all respiratory muscle training groups. Two studies reported no significant differences between the respiratory muscle training group and the control group. | NA | 51 (3 studies including 1 cross‐over study) | ⊕⊕⊝⊝ | ||
| Respiratory muscle function: inspiratory capacity Follow‐up: NA | NA | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. The resistance level of the respiratory muscle training intervention was variable; three studies used 80% of maximal effort, one study used 60% of maximal effort, one study used 40% of maximal effort, one study used 30% of maximal effort and three studies did not specify the level of resistance. Control groups were also variable; cycle ergometer, H20, treatment as usual, standard chest physiotherapy, low resistance threshold loading device, no training or sham training. 2. Downgraded twice due to serious risk of bias: the included studies lacked methodological detail relating to methods of randomisation, allocation concealment and blinding. Most of the studies were at high risk of bias due to lack of blinding, incomplete outcome data or selective reporting, or both. 3. Downgraded due to imprecision: studies included a small number of participants and numerical results were not available for some of the studies. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Forced expiratory volume at one second (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Forced vital capacity (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Chronic Respiratory Disease Questionnaire (mastery) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Chronic Respiratory Disease Questionnaire (emotion) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Forced expiratory volume at one second (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 PImax (cm H₂O) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Forced expiratory volume at one second (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Forced expiratory volume at one second (% predicted) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Forced vital capacity (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Forced vital capacity (% predicted) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Inspiratory muscle endurance (% PImax) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Forced expiratory volume at one second (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Forced vital capacity (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

