Vorbehandlung mit oraler Verhütungspille, Gestagen oder Östrogen bei Protokollen zur Stimulation der Eierstöcke für Frauen, die sich einer künstlichen Befruchtung unterziehen
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Parallel group study. Number of women randomized: 152 (77 in intervention group; 75 in control group). Number of withdrawals: 8 (1 in intervention group due to endometrioma; 7 in control group: 5 due to endometrioma or cysts and 2 chose not to proceed). Number of women analyzed: 144. | |
| Participants | Country of authors: UK. Inclusion criteria: women planning to have an IVF cycle on the Southampton (UK) IVF programme. Exclusion criteria: endometrioma or ovarian cyst seen on vaginal ultrasound scan on day 19 of the menstrual cycle (after recruitment). Mean age ± SD: intervention group: 33.8 ± 4.1 years; control group: 33.5 ± 3.5 years. | |
| Interventions | Intervention: medroxyprogesterone acetate (10 mg/day) on cycle days 19‐25 + GnRH agonist (buserelin acetate, nasal administration 200 μg 3 times daily) from cycle day 21 + hMG 4 ampoules/day (75 IU FSH and 75 IU LH per ampoule) from day 4 of ensuing menses. Control: placebo on cycle days 19‐25 + GnRH agonist (buserelin acetate, 200 μg nasal administration, 3 times daily) from cycle day 21 + hMG 4 ampoules/day (75 IU FSH and 75 IU LH per ampoule) from day 4 of ensuing menses. Both hMG and GnRH agonist continued until hCG injection (10,000 IU, IM), administered when leading 3 follicles reached diameter of ≥ 18 mm and serum oestradiol > 300 pmol/L for every follicle > 14 mm in diameter. | |
| Outcomes |
| |
| Notes | Power calculation performed: yes. ITT analysis performed: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The hospital pharmacy randomized to contain placebo or progestogen." |
| Allocation concealment (selection bias) | Low risk | Quote: "The hospital pharmacy provided a series of consecutively numbered bottles." |
| Blinding (performance bias and detection bias) | Low risk | Double blind. |
| Incomplete outcome data (attrition bias) | High risk | Proportions of withdrawals not balanced between the 2 treatment groups (1 in intervention group; 5 in control group) and data were not analyzed on ITT basis. |
| Selective reporting (reporting bias) | High risk | All planned outcomes not reported. |
| Other bias | Low risk | Quote: "No difference was seen between the study group and control group in the indication of IVF and age." |
| Methods | Academic centre, parallel group study. Number of women randomized: 83 women undergoing 102 cycles (51 cycles in each group; number of women per group not reported). Number of withdrawals: not reported. Number of women analyzed: only number of cycles analyzed reported (102 cycles). | |
| Participants | Country of authors: Canada. Inclusion criteria: women who were receiving a long protocol of pituitary suppression in the early follicular phase as a part of IVF‐ET treatment. Exclusion criteria: not reported. Median age (range): intervention group: 35.2 years (32.5‐39.1); control group: 33.7 years (31.6‐38.3). | |
| Interventions | Intervention: COCP on cycle days 1‐14 + GnRH agonist (buserelin acetate, long protocol 500 μg/day) started on cycle day 14 + hMG (75 IU FSH and 75 IU LH) or pure FSH (75 IU) started after achievement of pituitary suppression. Control: GnRH agonist (buserelin acetate, long protocol 500 μg/day) started on cycle day 2 + hMG (75 IU FSH and 75 IU LH) or pure FSH (75 IU) started after achievement of pituitary suppression. If no pituitary suppression (serum oestradiol < 40 pg/mL) achieved after 14 days of GnRH agonist administration, dosage of buserelin acetate increased to 500 μg twice daily + administration of an IM injection of progesterone 100 mg. Both hMG/FSH and GnRH agonist continued until hCG injection, administered when ≥ 3 follicles reach a mean diameter of ≥ 18 mm. | |
| Outcomes |
| |
| Notes | Power calculation performed: yes. ITT analysis performed: no. No per‐woman data reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "They were randomized in two groups by drawing sealed envelopes that contained randomly generated numbers." |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Numbers and reasons for withdrawals not reported. |
| Selective reporting (reporting bias) | High risk | All planned outcomes not reported. |
| Other bias | Low risk | No significant difference in baseline characteristics between groups with regard to age, cause of infertility, number of previous attempts, and oestradiol, FSH or LH level. |
| Methods | Parallel group study. Number of women randomized: 86 (44 in intervention group; 42 in control group). Number of withdrawals: 14 (9 in intervention group due to cyst development, protocol violation, insufficient ovarian response, did not undergo treatment, did not receive embryo transfer; 5 in control group due to insufficient ovarian response, did not receive embryo transfer). Number of women analyzed (for pregnancy outcome): 72 (35 in intervention group; 37 in control group). | |
| Participants | Country: Belgium. Inclusion criteria: women aged ≤ 36 years, BMI 18‐29 kg/m2, underwent a first or second treatment cycle of IVF with ICSI, serum FSH on day 3 of the menstrual cycle < 12 IU/L, normal ultrasound scan regular ovulatory menstrual cycle of 21 to 35 days. Exclusion criteria: oocyte donors, women with endometriosis ≥ grade 3, endocrine or metabolic abnormalities, PCOS or previous history of poor ovarian response (defined as development of < 4 follicles in previous IVF or ICSI cycle). Mean age ± SD: intervention group: 29.2 ± 3.0 years; control group: 30.2 ± 3.0 years. Setting: assisted reproduction programme in Belgium. | |
| Interventions | Intervention: oestradiol valerate (2 × 2 mg/day) during 6‐10 consecutive days (from cycle day 25 onwards) prior to start of rFSH stimulation so that the first day of stimulation occurred between Friday and Sunday. Control: no pretreatment; standard GnRH antagonist protocol. Both groups received rFSH (150 IU) and on day 6 of stimulation GnRH antagonist protocol (ganirelix 0.25 mg/day). | |
| Outcomes | Primary:
Secondary:
| |
| Notes | Power calculation: not reported. ITT analysis: not reported. Objective of the study was to assess the ability of oestradiol to control the oocyte retrieval of GnRH antagonist cycles prior to COS. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization list. |
| Allocation concealment (selection bias) | Low risk | Consecutive sealed opaque envelopes. |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding not reported. |
| Incomplete outcome data (attrition bias) | Low risk | Reasons for, and proportions of, withdrawals balanced between the 2 treatment groups and data were analyzed on the basis of ITT. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, |
| Other bias | Low risk | Groups comparable at baseline, |
| Methods | Multicentre (6 IVF centres), parallel group study. Number of women randomized: 93 (21 in COCP group; 23 in progestogen group; 25 in oestrogen group; 24 in control group). Number of withdrawals: 3 in oestrogen group (1 did not start any treatment, 1 due to an ovarian cyst and 1 due to major protocol violation). Number of women analyzed: 90. Duration of study: 10 months of recruitment. | |
| Participants | Country of authors: France. Inclusion criteria: regular normo‐ovulatory cycles (28‐35 days), aged < 38 years, BMI 18‐30. Exclusion criteria: high levels of baseline serum FSH or oestradiol, < 5 follicles at the antral follicular count performed on day 3 of a spontaneous cycle, history of high (> 20 oocytes) or low (< 5 oocytes) ovarian response in a previous IVF attempt. Mean age ± SD: COCP group: 30.8 ± 4.6 years; progestogen group: 32.9 ± 2.5 years; oestrogen group: 31.8 ± 3.2 years; control group: 31.2 ± 4.3 years. | |
| Interventions | COCP group: COCP (ethinyl oestradiol 30 μg + desogestrel 150 μg daily), started cycle day 2 or 3 for 15‐21 days (stopped on a Sunday) + rFSH (recombinant follitropin beta 150‐300 IU/day), started post‐treatment day 5 + GnRH antagonist (ganirelix acetate 0.25 mg/day), started when leading follicle reached 14 mm in diameter. Progestogen group: norethisterone 10 mg/day, started cycle day 15 for 10‐15 days (stopped on a Sunday) + rFSH (recombinant follitropin beta 150‐300 IU/day), started post‐treatment day 5 + GnRH antagonist (ganirelix acetate 0.25 mg/day), started when leading follicle reached 14 mm in diameter. Oestrogen group: micronised 17‐βE2 (2 mg twice daily), 10‐15 days, started 10 days before the presumed menses (stopped on a Sunday) + rFSH (recombinant follitropin beta 150‐300 IU/day), started post‐treatment day 5 + GnRH antagonist (ganirelix acetate 0.25 mg/day), started when leading follicle reached 14 mm in diameter. Control group: rFSH (recombinant follitropin beta 150‐300 IU/day), started day 3 after spontaneous menses + GnRH antagonist (ganirelix acetate 0.25 mg/day), started when leading follicle reached 14 mm in diameter. rFSH dose according to age, BMI and previous responses to stimulation; after 5 days of treatment dose adjustment according to ovarian response. Both rFSH and GnRH antagonist were continued until hCG injection (10,000 IU), administered when ≥ 3 mature (of ≥ 17 mm diameter) follicles were obtained. | |
| Outcomes |
| |
| Notes | Power calculation performed: no. ITT analysis performed: no (not for oestrogen group). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random allocation sequence was generated from a table of random numbers... Randomization was stratified by centre..." |
| Allocation concealment (selection bias) | Low risk | Quote: "Random allocation sequence was concealed to each physician who enrolled and randomised patients." Sealed envelopes. |
| Blinding (performance bias and detection bias) | High risk | Quote: "This study was not blind." |
| Incomplete outcome data (attrition bias) | Unclear risk | Reasons for withdrawals and proportions of withdrawals not clearly stated. |
| Selective reporting (reporting bias) | High risk | Some planned outcomes not reported. |
| Other bias | Low risk | No significant baseline imbalance with regard to age and BMI. |
| Methods | Parallel group study. Number of women randomized: 472 (238 in intervention group; 234 in control group). Number of withdrawals: 19 (5 in intervention group due to spontaneous pregnancy, discontinuation and no periods; 14 in control group due to spontaneous pregnancy, discontinuation, ovarian cyst and protocol change). Number of women analyzed: 453 (233 in intervention group; 220 in control group). | |
| Participants | Country France. Inclusion criteria: regular normo‐ovulatory cycles (28‐35 days), aged < 38 years, BMI 18‐30 kg/m2 and first or second IVF/ICSI attempt. Exclusion criteria: high basal levels of serum FSH (> 12 IU/L) or oestradiol (> 80 pg/mL), < 5 follicles at the antral follicular count performed on day 3 of a spontaneous cycle or history of high (> 20 oocytes) or low (< 5 oocytes) ovarian response in earlier IVF attempt. Mean age ± SD: intervention group: 31.1 ± 3.6 years; control group: 31.2 ± 3.7 years. Setting: 10 private or university‐based centres in France. | |
| Interventions | Intervention: oral 17 β‐oestradiol (4 mg; 2 mg x 2/day) started 7 days before the presumed onset of menses and administered up to the next Thursday after the occurrence of menstrual bleeding. Control: no pretreatment. Ovarian stimulation started on Friday in intervention group and on cycle day 2 after spontaneous menses in control group. Followed by GnRH antagonists on day 6, then hCG. | |
| Outcomes | Primary:
Secondary:
| |
| Notes | Power calculation for sample size (225 women per group). ITT analysis not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers. |
| Allocation concealment (selection bias) | Low risk | Authors stated allocation concealed by sealed envelopes. |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding not reported. |
| Incomplete outcome data (attrition bias) | High risk | Reasons for withdrawals and proportions of withdrawals not balanced between groups and data not analyzed on the basis of ITT. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
| Other bias | Low risk | Groups balanced at baseline with respect to demographic characteristics. |
| Methods | Cross‐over study. Number of women randomized: 25 (13 intervention group; 12 in control group). Number of withdrawals: not reported. Number of women analyzed: unclear. | |
| Participants | Country of authors: USA. Inclusion criteria: women, aged < 41 years, who were anticipated to have limited ovarian reserve based on transvaginal ultrasound showing limited follicles on cycle day 2‐3 or hormonal values (inhibin B, FSH, oestradiol). Mean age: not reported. Poor response: yes ("limited ovarian reserve"). | |
| Interventions | Intervention: COCP + GnRH agonist (leuprolide acetate, microdose) + hMG (300 IU FSH + 75 IU LH). Timing of administration of COCP, hMG and GnRH agonist not reported. Control: oestradiol (2 mg) in the luteal phase of the preparation cycle + FSH (300 IU), started cycle day 2 + GnRH antagonist (ganirelix acetate) started in late follicular phase + hMG (375 IU FSH + 150 IU LH), timing not reported. | |
| Outcomes |
| |
| Notes | Power calculation performed: unclear. ITT analysis performed: unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized," method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) | High risk | Unblinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | Only abstract available. Numbers and reasons for withdrawals not reported. |
| Selective reporting (reporting bias) | High risk | Data on all planned outcomes reported. Pre‐cross‐over data on primary outcome reported, but on some secondary outcomes (implantation rate, mature oocytes, good embryos) only post‐cross‐over data reported. |
| Other bias | Unclear risk | No data on baseline characteristics reported. |
| Methods | Parallel group study. Number of women randomized: 105 (47 in intervention group; 58 in control group). Number of withdrawals: 0. Number of women analyzed: 105. Length of follow‐up: until end of treatment cycle. | |
| Participants | Country: USA. Inclusion criteria: day 3 FSH values < 15 mIU/mL. Exclusion criteria: not reported. Mean age ± SD: intervention: 36.7 ± 4.8 years; control: 35.8 ± 4.57 years. | |
| Interventions | Intervention: norethindrone acetate (10 mg/day PO) on cycle days 1‐8 + GnRH agonist (leuprolide acetate, 1 mg/day, SC), started cycle day 1 + hMG (225 IU/day IM), started when serum oestradiol level was < 30 pg/mL. Both hMG and GnRH agonist are continued until hCG injection (10,000 IU, IM), administered when the leading follicles reached a diameter of ≥ 18 mm. | |
| Outcomes |
| |
| Notes | Power calculation performed: no. ITT analysis performed: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned by tossing a coin to one of two groups." |
| Allocation concealment (selection bias) | Low risk | Centralised randomization process. |
| Blinding (performance bias and detection bias) | High risk | No blinding. |
| Incomplete outcome data (attrition bias) | Low risk | No withdrawals. |
| Selective reporting (reporting bias) | High risk | All planned outcomes not reported. |
| Other bias | Low risk | Quote: "The various infertility diagnoses were distributed equally between the control and study groups." |
| Methods | Parallel group study. Number of women recruited: 123. Number of women excluded: 6 (2 due to ovarian cysts ≥ 15 mm, 2 due to raised early follicular phase serum FSH, 2 did not undergo IVF). Number of women randomized: 117 (63 in intervention group; 54 in control group). Number of withdrawals: 1 (in intervention group, due to violation of the study protocol). Number of women analyzed: 116. Duration of study: 1 year of recruitment. Source of funding: Schering Health Care Limited, West Sussex, UK, supplied the norethindrone. | |
| Participants | Country of authors: UK and Canada. Inclusion criteria: aged 18‐44 years at time of screening, duration of infertility ≥ 1 year, early follicular phase serum FSH ≤ 11.0 IU/L, good physical and mental health, suitability for the long‐term buserelin protocol for desensitisation. Exclusion criteria: endometrioma of the ovary, ovarian cysts (≥ 15 mm) in the early follicular phase, known contraindications to the use of progestogen, GnRH agonists or hMG. Mean age ± SD: intervention group: 35.3 ± 4.3 years; control group: 33.8 ± 5.5 years. | |
| Interventions | Intervention: norethindrone (10 mg on cycle day 1 and 5 mg twice daily on cycle day 2‐5) + GnRH agonist (buserelin acetate 500 μg/day, SC, long protocol), started on cycle day 2 (dose adjustment after pituitary suppression to 200 μg/day) + hMG (Normegon, 75 IU FSH 2‐5 ampoules/day) or rFSH, started when serum oestradiol ≤ 150 pmol/L. Control: GnRH agonist (buserelin acetate 500 μg/day, SC, long protocol) started on cycle day 2 (dose adjustment after pituitary suppression to 200 μg/day) + hMG (Normegon, 75 IU FSH 2‐5 ampoules/day) or rFSH, started when serum oestradiol ≤ 150 pmol/L. Pituitary suppression achieved when there was an absence of follicular activity and endometrial thickness < 5 mm. hMG or rFSH dose according to woman's age, previous response, basal serum FSH levels and PCOS. Both hMG/rFSH and GnRH agonist continued until hCG injection (10,000 IU, IM), administered when 2 or 3 leading follicles ≥ 18 mm in diameter. | |
| Outcomes |
| |
| Notes | Power calculation performed: yes. ITT analysis performed: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients were randomly assigned in a ratio of 1:1 by means of computer‐generated random numbers. To ensure similar distributions of age in the two groups, separate randomization schedules were drawn up for women < 40 years old and women ≥ 40 years old by use of stratified randomised blocks." |
| Allocation concealment (selection bias) | Low risk | Quote: "Selection into the groups (and of administration of the appropriate treatment protocol) was performed by the clinic nurses by using a series of consecutively numbered sealed envelopes (one for each age group)." |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "Although the patient could guess her treatment status, treatment allocation was not recorded in the clinical notes, and all clinicians were blinded to the status of study participants until the trial was over." However, no information on outcome assessors. |
| Incomplete outcome data (attrition bias) | Low risk | Proportions of withdrawals fairly balanced between the 2 groups. |
| Selective reporting (reporting bias) | Low risk | Data on all planned outcomes reported. |
| Other bias | Low risk | No significant baseline imbalance between groups with regard to age, duration of infertility, previous attempts, baseline serum FSH, polycystic ovaries and cause of infertility. |
| Methods | Parallel group study. Number of women randomized: 100 (number of women per group not reported). Number of withdrawals: 10 (4 due to personal reasons and 6 due to major protocol violation). Number of women analyzed: 90 (47 in intervention group; 43 in control group). Duration of study: 1 IVF‐ET cycle, from day 20 of the previous cycle to day of hCG administration (information obtained from contact person). | |
| Participants | Country of authors: France. Inclusion criteria: aged ≤ 38 years; regular, ovulatory menstrual cycles every 25‐35 days; both ovaries present; no current or past diseases affecting ovaries or gonadotrophin or sex steroid secretion, clearance or excretion; BMI 18‐27 kg/m2; no hormone therapy during the past 6 weeks; adequate visualisation of both ovaries in transvaginal ultrasound scans. Exclusion criteria: not reported. Median age (range): intervention group: 33 (26‐38) years; control group: 33 (25‐38) years. | |
| Interventions | Intervention: micronised 17β‐E2 (4 mg/day, PO), started cycle day 20 until day 2 of the next cycle + rFSH (225 IU/day, SC) on cycle days 3‐7 + GnRH antagonist (cetrorelix acetate 3 mg single dose, SC) when ≥ 1 follicle > 13 mm in diameter. Control: rFSH (225 IU/day SC) on cycle days 3‐7 + GnRH antagonist (cetrorelix acetate 3 mg single dose, SC) when ≥ 1 follicle > 13 mm in diameter. rFSH dose adjustments according to follicle growth determined by serum oestradiol levels and ultrasound monitoring. | |
| Outcomes |
| |
| Notes | Power calculation performed: yes. ITT analysis performed: no Our data analysis in this review includes 90 women with full follow‐up. We did not include all randomized women because it is unclear how many women were randomized to each group before dropouts. The study publication reports very low measures of variability which can be to be assumed SEs and which we have converted to SDs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Women randomly received...", "...according to a computer‐generated, blocked randomization list." |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment allocation was decided by an independent person." |
| Blinding (performance bias and detection bias) | Unclear risk | No blinding, information obtained from contact person. |
| Incomplete outcome data (attrition bias) | High risk | Unclear how many withdrew in each group and data not analyzed on the basis of ITT. |
| Selective reporting (reporting bias) | High risk | All planned outcomes not reported. |
| Other bias | Low risk | No difference in baseline characteristics with regard to age, indication for IVF‐ET, duration of infertility, rank of the current IVF‐ET attempt, menstrual cycle length, day 3 serum FSH and oestradiol. |
| Methods | Parallel group study. Number of women recruited: 22. Number of women randomized: 22 (16 in intervention group; 6 in control group). Number of withdrawals: 1 (both in intervention group, due to spontaneous pregnancies). Number of women analyzed: 20. | |
| Participants | Country of authors: Brazil. Inclusion criteria: women without specific ovulatory dysfunction, aged ≤ 37 years, who would be submitted to ovarian stimulation. Exclusion criteria: not reported. Mean age ± SD: intervention group: 32.2 ± 2.1 years; control group: 31.8 ± 1.9 years. | |
| Interventions | Intervention: oestradiol valerate (4 mg/day) for 14 days, started cycle day 21 + rFSH (150‐300 IU) (fixed dose for 5 days), started post‐treatment day 1 + GnRH antagonist (ganirelix acetate 0.25 mg/day), started when follicular diameter ≥ 15 mm. Control: GnRH agonist (nafarelin acetate 200 μg twice daily, nasal), started cycle day 21 + rFSH (150‐300 IU) (fixed dose for 5 days), started stimulation day 14. Both rFSH and GnRH analogues continued until hCG injection (5000‐10,000 IU), administered when ≥ 2 follicles were ≥ 17 mm in diameter. | |
| Outcomes |
| |
| Notes | Power calculation performed: no. ITT analysis performed: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by drawing lots after constructing a table of distribution. |
| Allocation concealment (selection bias) | High risk | After drawing lots, clinicians and participants could see in the table to which treatment they were assigned to. |
| Blinding (performance bias and detection bias) | Unclear risk | Insufficient information to make a conclusive judgement. |
| Incomplete outcome data (attrition bias) | High risk | There was imbalance in the proportions of withdrawals between the 2 groups and data analyzed on the basis of ITT. |
| Selective reporting (reporting bias) | High risk | All planned outcomes not reported. |
| Other bias | Low risk | No significant difference in baseline characteristics with regard to age. |
| Methods | 2‐arm parallel RCT. Number of women randomized: 228 (115 in intervention; 113 in control). Withdrawals: not reported. Number of women analyzed: not reported. | |
| Participants | Country: Spain. Inclusion criteria: regular cycle women under 39 years, < 3 previous IVF attempts. Exclusion criteria: previous low response to COH, ovarian surgery or PCOS. Mean age: intervention group: 34.1 years; control group: 33.7 years. Setting: not reported. | |
| Interventions | Intervention: COCP (ethinyl oestradiol 30 μg + desogestrel 150 μg) for 12‐16 days and COH started on day 5 post COCP using GnRH antagonist. Control: GnRH agonist long protocol from days 20‐22 of the previous cycle. | |
| Outcomes |
| |
| Notes | Duration of stimulation, FSH used and number of oocyte retrieved were continuous variables. Power calculation was not reported. ITT analysis: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list with consecutive inclusion. |
| Allocation concealment (selection bias) | Low risk | Opaque consecutively numbered envelopes with assignment under nurse control. |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Proportions of withdrawals and reasons for withdrawals not reported. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
| Other bias | Low risk | Groups comparable at baseline with regard to demographic characteristics. |
| Methods | Parallel group study. Number of women randomized: 100 (50 in each group). Number of withdrawals: 16 (7 in intervention group; 9 in control group). Number of women analyzed: 100 (ITT analysis). | |
| Participants | Country: Spain. Inclusion criteria: aged 18‐38 years, regular normo‐ovulatory menstrual cycles (26‐35 days), BMI < 30 kg/m2, normal cycle day 3 basal serum hormone levels (FSH < 10 IU/L and oestradiol < 60 pg/mL) and < 3 previous IVF/ICSI attempts. Exclusion criteria: previous ovarian surgery, low ovarian response (cancellation of cycle due to poor follicular development after ≥ 7 days of gonadotropin stimulation or < 5 oocytes retrieved) in previous IVF/ICSI cycle and PCOS. Mean age ± SD: intervention group: 33.9 ± 3.4 years; control group: 34.5 ± 3.1 years. Setting: single hospital clinic in Madrid, Spain. | |
| Interventions | Intervention: COCP: (ethinyl oestradiol 30 μg + levonorgestrel 150 μg) on day 1 or 2 of cycle prior to IVF/ICSI and continued for 12‐16 days, with stimulation starting 5 days after stopping pretreatment. Control: (oestradiol valerate 4 mg/day (2 mg × 2)) from day 20 of menstrual cycle for 5‐12 days until the day before starting stimulation. In both groups, GnRH antagonist (ganirelix 0.25 mg/day) started when the leading follicle reached 13 mm in diameter and ovarian triggering performed with rhCG (250 μg) (when 2 leading follicles reached ≥ 17 mm mean diameter). | |
| Outcomes | Primary:
Secondary:
| |
| Notes | Power calculation for sample size: yes but not achieved because this was a single‐centre study. ITT analysis: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number list. |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered opaque envelopes. |
| Blinding (performance bias and detection bias) | Unclear risk | Although personnel involved in data collection and data analysis blinded it was not reported whether other personnel and participants were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Reasons for withdrawals and proportions of withdrawals were fairly balanced between groups and data were analyzed on the basis of ITT. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
| Other bias | Low risk | Groups balanced at baseline with respect to demographic characteristics. |
| Methods | Parallel group study. Number of women randomized: 45 (20 in intervention group; 25 in control group). Number of withdrawals: not reported. Number of women analyzed: not reported. | |
| Participants | Country of authors: France. Inclusion criteria: not reported. Exclusion criteria: not reported. Mean age: not reported. | |
| Interventions | Intervention: norethisterone (10 mg/day) for 10‐15 days + GnRH agonist (DTRP6‐LHRH 100 μg/day). Control: GnRH agonist (DTRP6‐LHRH 100 μg/day). Timing of treatments not reported. | |
| Outcomes |
| |
| Notes | Power calculation performed: unclear. ITT analysis performed: unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised," method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Numbers and reasons of withdrawals not reported. |
| Selective reporting (reporting bias) | High risk | All planned outcomes not reported. No data on pregnancy rates. |
| Other bias | Unclear risk | Baseline demographic characteristics not reported. |
| Methods | Academic, multicentre, parallel group study. Number of women randomized: 64 (32 in each group). Number of withdrawals: 1 (in intervention group, due to unwillingness to take OCP). Number of women analyzed: 63. Source of funding: Serono Geneva supplied the antide. | |
| Participants | Country: the Netherlands and Belgium. Inclusion criteria: regular IVF or ICSI indication (i.e. idiopathic infertility after 6 unsuccessful IUIs, infertility based on a male or tubal factor); spontaneous, regular ovulatory menstrual cycle; 2 ovaries and a normal uterine cavity; aged 18‐38 years. Exclusion criteria: FSH ≥ 12 IU/L on cycle day 2‐4; BMI > 30 kg/m2; abnormal gynaecological bleeding; extrauterine pregnancy within the last 3 months; previous ART cycles with < 3 oocytes or severe OHSS; any contraindication to receive gonadotrophins or OCP; PCOS. Mean age ± SD: intervention group: 32.3 ± 4.0 years; control group: 33.3 ± 3.8 years. | |
| Interventions | Intervention: COCP (ethinyl oestradiol 30 μg + levonorgestrel 150 μg) for 14‐28 days, started cycle day 2 or 3 + rFSH (150‐300 IU), started post‐treatment day 2 or 3 (= stimulation day 1) + GnRH antagonist (antide 0.5 mg/mL daily), started stimulation day 6. Control: rFSH (150‐300 IU), started on cycle days 2 or 3 (= stimulation day 1) + GnRH antagonist (antide 0.5 mg/mL daily), started on stimulation day 6. rFSH dose adjustments after 5 days of stimulation (up to a maximum of 450 IU), according to number and size of oocytes and risk for OHSS. Both rFSH and GnRH antagonist were continued until hCG injection (6500 IU), administered when ≥ 1 follicle reached a diameter ≥ 18 mm + ≥ 2 follicles reached a diameter ≥ 16 mm. | |
| Outcomes |
| |
| Notes | Power calculation performed: yes. ITT analysis performed: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "64 patients were randomly allocated according to a computer‐generated, blocked randomization list. The randomization was stratified by centre." |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment allocation was decided by an independent person from an independent monitoring company..." |
| Blinding (performance bias and detection bias) | High risk | Open‐label study. |
| Incomplete outcome data (attrition bias) | Low risk | Proportions of withdrawals were fairly balanced between groups (3% in intervention group vs 0% in control group). |
| Selective reporting (reporting bias) | Low risk | Data on all planned outcomes reported. |
| Other bias | Low risk | No significant differences in baseline characteristics with regard to age, BMI, cycle length, primary infertility, smoking habits, duration of infertility, type of infertility and antral follicle count. |
| Methods | Multicentre (8 IVF centres), parallel group study. Number of women recruited: 216. Number of women excluded: 34 (reasons not reported). Number of women randomized: 182 (91 in each group). Number of withdrawals: 22 (10 in intervention group: 1 due to hepatitis B, 1 due to non‐compliance, 1 due to personal reasons, 2 due to insufficient follicular response, 1 due to conversion to IUI, 1 due to absence of mature oocytes, 3 due to absence of viable embryos; 12 in control group: 2 due to spontaneous pregnancy, 3 due to failure of desensitisation, 1 due to personal reasons, 1 due to stimulation failure, 3 due to absence of 'mature' oocytes, 2 due to failure of fertilisation). Number of women analyzed: 182. | |
| Participants | Country of authors: the Netherlands, Belgium, France and Austria. Inclusion criteria: regular IVF/ICSI indication, male partner with viable sperm in the ejaculate, aged 18‐39 years. Exclusion criteria: any previous ART cycles with < 3 oocytes or ≥ 3 consecutive ART cycles without a clinical pregnancy, any contraindication to ART, gonadotrophins or OCPs, significant systemic disease. Mean age ± SD: intervention group: 32.8 ± 3.8 years; control group: 32.2 ± 4.2 years. | |
| Interventions | Intervention: COCP (ethinyl oestradiol 30 μg + levonorgestrel 150 μg daily), started within 5 days of onset of menses for 21‐28 days (stop on a Sunday) + r‐hFSH (150‐225 IU/day), started post‐treatment day 5 (= stimulation day 1) + GnRH antagonist (cetrorelix acetate 0.25 mg/day, SC), started stimulation day 6. Control: GnRH agonist (buserelin acetate 500 μg/day, SC), started cycle day 18‐22 (reducing dose to 200 μg/day when downregulation was achieved) + r‐hFSH (150‐225 IU/day), started when downregulation was achieved. After 5 days of r‐hFSH treatment, the dose could be adjusted by steps of 75 IU (maximal dose 450 IU/day), according to the ovarian response. Both rhFSH and GnRH analogues were continued until hCG injection, administered when the largest follicle reached a mean diameter ≥ 18 mm and ≥ 2 other follicles had a mean diameter ≥ 16 mm. | |
| Outcomes |
| |
| Notes | Power calculation performed: yes. ITT analysis performed: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "182 were randomly allocated to...", "The treatment assigned to each patient was determined according to a computer‐generated concealed randomization list. Randomization was performed by centre." |
| Allocation concealment (selection bias) | Unclear risk | "Concealed randomization list," method not reported. |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Low risk | All women randomized were included in final analysis. |
| Selective reporting (reporting bias) | Low risk | Data on all planned outcomes reported. |
| Other bias | Low risk | No significant difference in baseline characteristics with regard to age, race, duration of infertility, cause of infertility, smoking habits, primary infertility, number of previous ART attempts, number of follicles, endometrial thickness, FSH levels and oestradiol levels. P value of BMI was 0.04. |
| Methods | Single‐centre, parallel group study. Number of women recruited: 60. Number of women excluded: 4 (2 refused to participate, 2 did not meet inclusion criteria). Number of women randomized: 56 (27 in intervention group; 29 in control group). Number of withdrawals: 7 (2 in intervention group: 1 due to poor ovarian response, 1 due to personal reasons; 5 in control group: 2 due to inadequate ovarian response, 3 due to risk of severe OHSS). Number of women analyzed: 49. | |
| Participants | Country: Taiwan. Inclusion criteria: PCOS. Exclusion criteria: diagnosis of congenital adrenal hyperplasia, Cushing's syndrome, androgen‐producing tumours, hyperprolactinaemia or thyroid dysfunction; aged > 38 years; serum FSH levels > 12 mIU/mL. Mean age ± SD: intervention group: 31.4 ± 3.5 years; control group: 31.7 ± 3.7 years | |
| Interventions | Intervention: COCP (Diane‐35, oral) on cycle days 5‐25 for 3 consecutive cycles + GnRH antagonist (cetrorelix acetate 0.25 mg single dose, SC on post‐treatment day 3; 0.125 mg/day on post‐treatment days 4‐9; and 0.25 mg/day started post‐treatment day 10 + hMG 150 IU/day), started post‐treatment day 4. Control: GnRH agonist (buserelin acetate 500 μg/day, long protocol) started day 3 of induced or spontaneous menstruation, and 250 μg/day started day of ensuing pituitary downregulation + hMG (150 IU/day) for 6 days started when pituitary downregulation was achieved. hMG dose can be adjusted according to woman's follicular response. Pituitary downregulation achieved when serum oestradiol levels < 50 pg/mL and there was an absence of ovarian cysts > 10 mm in diameter. Both GnRH analogues and hMG were continued until hCG injection (10,000 IU, IM), administered when ≥ 2 follicles reached 18 mm in diameter with adequate oestradiol response. | |
| Outcomes |
| |
| Notes | Power calculation performed: yes. ITT analysis performed: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done by opening sealed envelopes containing computer‐generated block randomization numbers with a block size of 10." |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "The laboratory staff were blinded to the stimulation protocol." Unclear if treating physicians were blinded. |
| Incomplete outcome data (attrition bias) | High risk | Reasons for withdrawals and proportions of withdrawals were not balanced between groups and data were not analyzed on the basis of ITT. |
| Selective reporting (reporting bias) | Low risk | Data on all planned outcomes were reported. |
| Other bias | Low risk | No significant difference in baseline characteristics with regard to age, duration of infertility, BMI and hormonal levels. |
| Methods | Parallel group study. Number of women randomized: 120. Number of withdrawals: 0. Number of women analyzed: 120. Duration of study: 1 cycle. | |
| Participants | Country of authors: South Korea. 120 poor responders (repeated day 3 levels of FSH > 8.5 mIU/mL or antral follicle count ≤ 5, or both). 40 in each group. Inclusion criteria: not clearly stated. Exclusion criteria: PCOS (Rotterdam criteria). Mean age ± SD: group 1: 36.7 ± 3.1 years; group 2: 35.9 ± 2.8 years; group 3: 36.4 ± 3.3 years. Setting: university‐based infertility clinic, Seoul. Korea. | |
| Interventions | Pretreatment was ethinyl oestradiol 0.03 mg and levonorgestrel 0,15 mg for 21 days in the cycle preceding COS. Group 1: GnRH antagonist multiple dose protocol after OCP pretreatment. Ovarian stimulation started 5 days after OCP discontinued using rFSH (225 IU/day, dose adjusted every 3‐4 days). Cerotide (0.25 mg) started when lead follicle was 14 mm diameter and continued until day of hCG injection. Group 2 GnRH antagonist multiple dose protocol without OCP pretreatment. Ovarian stimulation started on cycle day 3 using rFSH (225 IU/day, dose adjusted every 3‐4 days). Cerotide (0.25 mg) started when lead follicle was 14 mm diameter and continued until day of hCG injection. Group 3: GnRH agonist luteal low‐dose long protocol without OCP pretreatment. Daily injection of decapeptyl (0.1 mg) started from mid‐luteal phase and continued until menses followed by a dose reduction to 0.05 mg daily and continued until day of hCG injection. | |
| Outcomes | Primary:
Secondary:
| |
| Notes | Power calculation: yes. ITT analysis: yes. Earlier publications were Kim 2005 and Kim 2009. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer generated lists." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The sequence of allocation to the three groups was provided to the investigating physicians." |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Low risk | In groups 1 and 3 there were no losses, withdrawals or cancellations. In group 2, 1 cycle was cancelled before embryo transfer; all women randomized were included in data analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed were reported although multiple pregnancy was reported it is not listed a priori. |
| Other bias | Low risk | Groups similar at baseline with respect to demographic characteristics. |
| Methods | Academic, single centre, parallel group study. Number of women randomized: 504 (250 in intervention group; 254 in control group). Number of withdrawals: 79 (36 in intervention group: 28 due to personal reasons, 6 due to abnormal steroid levels, 2 due to spontaneous pregnancy; 43 in control group: 31 due to personal reasons, 10 due to abnormal steroid levels, 2 due to spontaneous pregnancy). Number of women analyzed: 425. Duration of study: 3 years of recruitment. Source of funding: the Fund for Scientific Research Flanders. | |
| Participants | Country: Belgium. Inclusion criteria: aged < 39 years, ≤ 3 previous ART attempts, BMI 18‐29 kg/m2, FSH < 10 IU/L, LH < 10 IU/L. Exclusion criteria: polycystic ovaries, endometriosis > stage II, poor response to ovarian stimulation Mean age ± SD: intervention group: 31.2 ± 0.3 years; control group: 31.5 ± 0.3 years. | |
| Interventions | Intervention: COCP (ethinyl oestradiol 30 μg + desogestrel 150 μg) for 14 days, started cycle day 1 + rFSH (200 IU/day) (fixed dose), started post‐treatment day 5 + GnRH antagonist (ganirelix acetate). Control: rFSH (200 IU/day) (fixed dose), started cycle day 2 + GnRH antagonist (ganirelix acetate). Timing of GnRH antagonist not reported. Both rFSH and GnRH antagonist continued until hCG injection (10,000 IU), administered when ≥ 3 follicles ≥ 17 mm in diameter. | |
| Outcomes |
| |
| Notes | Power calculation performed: yes. ITT analysis performed: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomized on the basis of a computer‐generated list." |
| Allocation concealment (selection bias) | High risk | Quote: "... randomized at the outpatient clinic by the treating physician." "The sequence of allocation was not concealed and thus it was possible for the treating physician to be aware of the next treatment to be allocated." |
| Blinding (performance bias and detection bias) | High risk | Treating physician not blinded as this was the person to allocate the participants. |
| Incomplete outcome data (attrition bias) | Low risk | Reasons for withdrawals and proportions of withdrawals fairly balanced between groups. |
| Selective reporting (reporting bias) | Low risk | Data on all planned outcomes reported. |
| Other bias | Low risk | No significant differences in baseline characteristics with regard to age, BMI, primary/secondary infertility, duration of infertility, number of previous IVF trials, indication for treatment. |
| Methods | 2‐arm parallel multicentre RCT. Number of women randomized: 298 (154 in intervention group; 144 in control group). Withdrawals: not reported. Number of women analyzed: not reported. | |
| Participants | Country: Poland. Inclusion criteria: women aged < 40 years, AMH > 0.6 ng/mL, BMI 18‐29 kg/m2, and undergoing a first or second treatment cycle of IVF with ICSI. Exclusion criteria: presence of endometriosis and pre‐implantation diagnosis cycles. Mean age ± SD: intervention group: 32.5 ± 3.97; control group: 32.5 ± 2.96 years. BMI (mean ± SD): intervention group: 22.2 ± 1.3 kg/m2; control group: 22.4 ± 1.1 kg/m2. Setting: infertility clinics, 2 centres. | |
| Interventions | Intervention: OCP pretreatment: pretreated with COCP (Ovulastan, Adamed, Pabianice, Poland) from day 2‐4 of the cycle. Beginning from the day 14 of cycle, pituitary was suppressed by administering triptorelin (0.1 mg) every 2 days; treatment continued for 2 weeks. Ovarian stimulation started with gonadotropin injections (150‐225 IU/day) starting from days 2‐4 of cycle and continued with a daily dose of triptorelin (0.1 mg) until hCG injection was administered 36 hours before retrieval. Control: oestradiol pretreatment: pretreated with oral oestradiol (2 mg twice daily) from day 20 of natural cycle until the day 1‐4 of the new cycle. Ovarian stimulation started with hMG injections (150‐225 IU/day) starting from days 2‐4 of cycle, 2 days after discontinuation of oestradiol administration, and continued with a daily dose of triptorelin (0.1 mg) until the hCG injection 5000 IU was administered 36 hours before retrieval. | |
| Outcomes |
| |
| Notes | All outcomes were measured in denominators other than "per woman randomized." Power calculation was performed. No, outcome measured in denominators other than "per woman randomized." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using "block randomization software." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) | Unclear risk | Only doctors who retrieved oocytes and embryologists were blinded to treatment groups. |
| Incomplete outcome data (attrition bias) | Unclear risk | No information reported on reasons for withdrawals and proportion of withdrawals. |
| Selective reporting (reporting bias) | Low risk | Important outcome measures were prespecified in the methods section and data were reported. |
| Other bias | Low risk | Demographic and infertility characteristics similar between the two groups. |
| Methods | Parallel group multicentre study. Number of women randomized: 442 (223 in intervention group; 219 in control group). Number of withdrawals: 34 (14 in intervention group; 20 in the control group due to adverse events, withdrawal of consent, spontaneous pregnancy and "other" reasons). Number of women analyzed: 408 (209 in intervention group; 199 in control group). | |
| Participants | Country: multicentre (USA, Europe). Inclusion criteria: aged 18‐39 years, BMI ≤ 32 kg/m2, menstrual cycle length 24‐35 days, access to ejaculatory sperm, indication for COS and IVF or ICSI or IVF plus ICSI and scheduled for first COS cycle. Exclusion criteria: history of endocrine abnormality, < 2 ovaries or any other ovarian abnormality, presence of unilateral or bilateral hydrosalpinx, clinically relevant pathology affecting the uterine cavity, fibroids ≥ 5 cm, history of recurrent miscarriage (≥ 3), with or without FSH or LH levels > 12 IU/L in the early follicular phase. Mean age ± SD: intervention group: 31.8 ± 3.7 years; control group: 31.6 ± 4.1 years. Setting: 8 centres in USA, 6 centres in Europe (Denmark, Germany, Spain and Turkey). | |
| Interventions | Intervention: COCP pretreatment: Marvelon (ethinyl oestradiol 30 μg + desogestrel 150 μg) for 14‐21 days. Women started daily rFSH 5 days after stopping COCP pretreatment provided a withdrawal bleed occurred. Control: no pretreatment: daily rFSH on day 2 or 3 of the next menstrual cycle. In both groups, a single SC injection of rFSH (200 IU) started on stimulation day 1 and continued daily up to and including day of triggering of final oocyte maturation by urinary hCG. Maximum total duration of stimulation 19 days. Starting on day 5 of stimulation, all women received ganirelix (0.25 mg/day). | |
| Outcomes | Primary:
Secondary:
Other:
| |
| Notes | Power calculation for sample size: yes (200 women per group with an additional 20 to compensate for discontinuation). ITT analysis: modified: all randomized women who received at least 1 dose of rFSH. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) | High risk | Study reported as open label. |
| Incomplete outcome data (attrition bias) | Low risk | Minimal (< 10%) discontinuations and balanced between groups with similar reasons given. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
| Other bias | Low risk | Groups appeared balanced at baseline with respect to age, BMI, age at menarche, duration of infertility, cycle length, alcohol use and smoking. |
| Methods | Parallel group study. Number of women randomized: 150 (75 in each group). Number of withdrawals: not reported. Number of women analyzed: unclear. | |
| Participants | Country of authors: Austria. Inclusion criteria: women undergoing COS and IVF. Exclusion criteria: not reported. Mean age: not reported. | |
| Interventions | Intervention: COCP (ethinyl oestradiol 30 μg + desogestrel 150 μg daily), started cycle day 1 for 18‐28 days (stopped on a Sunday) + rFSH (150 IU/day), started post‐treatment day 5 (= stimulation day 1) + GnRH antagonist (cetrorelix acetate 0.25 mg/day), started stimulation day 6. Control: rFSH 150 IU/day, started cycle day 3 (= stimulation day 1) + GnRH antagonist (cetrorelix acetate 0.25 mg/day), started stimulation day 6. Both rFSH and GnRH antagonist continued until final follicular maturation. | |
| Outcomes |
| |
| Notes | Power calculation performed: unclear. ITT analysis performed: unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized," method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reasons for withdrawals not reported. |
| Selective reporting (reporting bias) | High risk | All planned outcomes not reported. |
| Other bias | Unclear risk | No data on baseline characteristics reported. |
| Methods | 2‐arm parallel group study. Number of women randomized: 150 women (75 in each group). Number of withdrawals: 4 women in intervention group; 3 in control group did not have embryo transfer. Number of women analyzed: 71 in intervention group; 72 in control group. Duration of study: 1 year of recruitment. Source of funding: Yazd IVF Centre, Yazd, Iran. | |
| Participants | Country of authors: Argentina. Inclusion criteria: aged < 39 years, first IVF attempt, baseline FSH < 12 mlU/mL. Exclusion criteria: PCOS, prior ovarian surgery and TESE needing. Mean age: not reported. | |
| Interventions | Intervention: COCP group: April® (ethinyl oestradiol 20 μg + levonorgestrel 100 μg) for 21 days in preceding cycle and follicular development induced using rFSH (200 IU/day) from menstrual cycle day 2‐3. Control: rFSH (200 IU/day) from menstrual cycle day 2‐3. Both groups received GnRH antagonist (cetrorelix 0.25 mg, SC) in flexible protocol starting when the leading follicle reached 14 mm continuing daily until the day of hCG administration. | |
| Outcomes |
| |
| Notes | Power calculation not reported. ITT: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Low risk | Proportions of exclusion and reason for exclusion similar between groups. |
| Selective reporting (reporting bias) | Low risk | All reported outcomes pre‐specified in the methods section. |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement. |
| Methods | Academic, single‐centre, parallel group study. Number of women randomized: 54 women (number of women per group not reported). Number of withdrawals: 3 women excluded due to incomplete data. Number of women analyzed: 51. Duration of study: 1 year of recruitment. Source of funding: Yazd IVF Centre, Yazd, Iran. | |
| Participants | Country: Iran. Inclusion criteria: women undergoing IVF and ICSI. Exclusion criteria: not reported. Mean age ± SD: intervention: 31.48 ± 5.82 years; control: 35.27 ± 4.13 years. | |
| Interventions | Intervention: COCP (ethinyl oestradiol 30 μg + desogestrel 150 μg), on cycle days 1‐14 + GnRH agonist (triptorelin acetate depot 3.75 mg, IM) single dose on post‐treatment day 1 + hMG (FSH 75 IU + LH 75 IU), started post‐treatment day 2. Control: GnRH agonist (triptorelin acetate depot 3.75 mg, IM) single dose on cycle day 1 + hMG (FSH 75 IU + LH 75 IU), started cycle day 1. | |
| Outcomes |
| |
| Notes | Power calculation performed: no. ITT analysis performed: unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized allocation method," method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | No information on reasons for withdrawals and proportions of withdrawals per treatment group. |
| Selective reporting (reporting bias) | Unclear risk | All planned outcomes not reported. |
| Other bias | Unclear risk | Number of women per group not reported. Quote: "The etiology and duration of infertility were equally distributed among the groups." No table of demographic characteristics available. |
| Methods | Multicentre (10 IVF centres), parallel group study. Number of women randomized: 351 (117 in each group). Number of withdrawals: 19 (5 due to spontaneous pregnancy: 2 in COCP group; 3 in GnRH antagonist group). Other reasons not reported. Number of women analyzed: 332 (111 in COCP group; 110 in GnRH antagonist group; 111 in GnRH agonist group). | |
| Participants | Country: Australia, Denmark, Jordan and Norway. Exclusion criteria: contraindications for the use of gonadotrophins, endocrine abnormalities (e.g. PCOS), > 3 unsuccessful COS cycles, history of low or no ovarian response during FSH/hMG treatment, clinically relevant abnormal laboratory values (including hormones) or medical examination findings. Mean age ± SD: COCP group: 32.7 ± 3.9 years; GnRH antagonist group: 32.1 ± 3.7 years; GnRH agonist group: 32.2 ± 4.0 years. | |
| Interventions | COCP group: COCP (ethinyl oestradiol 30 μg + desogestrel 150 μg daily), started cycle day 1 for 14‐28 days (depending on the planned started of rFSH treatment) + rFSH (follitropin beta 200 IU/day, SC), started post‐treatment day 2 (= stimulation day 1) + GnRH antagonist (ganirelix acetate 0.25 mg/day, SC), started stimulation day 5 or 6. GnRH antagonist: rFSH (follitropin beta 200 IU/day, SC), started cycle day 2 or 3 (= stimulation day 1) + GnRH antagonist (ganirelix acetate 0.25 mg/day, SC), started stimulation day 5 or 6. GnRH agonist: nafarelin acetate (0.8 mg/day, intranasal), started cycle day 21‐24 + rFSH (follitropin beta 200 IU/day, SC), started when downregulation (i.e. serum oestradiol ≤ 50 pg/mL) achieved after 2‐4 weeks of GnRH agonist treatment). After 5‐6 days of rFSH treatment, dose could be adjusted depending on the ovarian response as assessed by ultrasound. rFSH and GnRH analogues are both continued until hCG injection (10,000 IU, SC or IM), administered when ≥ 3 follicles ≥ 17 mm in diameter, or ≥ 1 follicle ≥ 20 mm in diameter. | |
| Outcomes |
| |
| Notes | Power calculation performed: yes. ITT analysis performed: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The subjects were randomly assigned..." "To improve balance, the randomization of subjects to treatment was stratified for type of infertility (primary or secondary), IVF or ICSI, centre, and age." Method not reported. |
| Allocation concealment (selection bias) | Low risk | Quote: "...by central remote allocation." |
| Blinding (performance bias and detection bias) | High risk | Open‐label study. |
| Incomplete outcome data (attrition bias) | Low risk | Proportions of withdrawals fairly balanced between groups although reasons for withdrawals were not completely reported. |
| Selective reporting (reporting bias) | Low risk | Data on all planned outcomes reported. |
| Other bias | Low risk | No differences in baseline characteristics with regard to age, BMI, height and weight. |
| Methods | Parallel group study. 4 study arms (A1+A2 and B1+B2), of which we can only include 2 study arms (A2 and B2). Number of women randomized: 42 (21 in intervention group (A2); 21 in control group (B2)). Number of withdrawals: 13 (8 in intervention group (A2): 3 due to poorly followed treatment, 1 due to inadequate response, 2 due to spontaneous ovulation, 2 due to other reasons; 5 in control group (B2): 1 due to ovarian cyst, 4 due to inadequate response). Number of women analyzed: 29. Duration of study: 7 months of recruitment. | |
| Participants | Country of authors: France. Inclusion criteria: infertile women scheduled for IVF treatment, aged < 38 years. Exclusion criteria: not reported. Mean age ± SD: intervention group (A1+A2): 32.8 ± 0.7 years; control group (B1+B2): 31.7 ± 0.5 years. | |
| Interventions | Intervention (A2): progestogen (ethynodiol acetate 2 mg twice daily) for 11‐17 days, started cycle day 15 + pure FSH 4 ampoules on post‐treatment days 6‐7 and 2 ampoules on post‐treatment days 8‐9 + hMG (FSH 75 IU + LH 75 IU) 2 ampoules on post‐treatment days 10‐11. Control (B2): pure FSH 4 ampoules on cycle days 2‐3 and 2 ampoules on cycle days 4‐5 + hMG (FSH 75 IU + LH 75 IU) when needed. FSH and GnRH agonist both continued until hCG injection (10,000 IU), administration depending on follicular maturity. | |
| Outcomes |
| |
| Notes | Power calculation performed: no. ITT analysis performed: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized," method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | High risk | Proportions of, and reasons for, withdrawals not balanced between groups and data not analyzed on the basis of ITT. |
| Selective reporting (reporting bias) | High risk | All planned outcomes not reported. |
| Other bias | Low risk | Baseline demographic characteristics balanced between groups. |
| Methods | Parallel group study. Number of women randomized: 49 (number per group not reported; 22 cycles in intervention group; 29 cycles in control group) but total number of cycles not the same as total number of women randomized. Number of withdrawals: 11 cycles (3 in intervention group: 2 due to poor response, 1 due to failure of embryo cleavage; 8 in control group: 3 due to conversion to IUI, 1 due to poor response, 2 due to failed fertilisation, 2 due to risk of OHSS). Number of women analyzed: unclear. Duration of study: 8 months of recruitment. | |
| Participants | Country of authors: UK. Inclusion criteria: women who underwent IVF treatment cycles and had an ovarian cyst > 15 mm in diameter or an endometrial thickness > 5 mm and serum oestradiol concentration > 100 pmol/L after 14 days of GnRH agonist (buserelin acetate) treatment Exclusion criteria: relevant uterine or ovarian pathology. Mean age ± SEM: intervention group: 36.0 ± 0.86 years; control group: 35.72 ± 0.69 years. | |
| Interventions | Intervention: GnRH agonist (buserelin acetate 500 μg/day) started cycle day 2 or 3 + progestogen (100 mg IM single dose) on cycle day 16 or 17 + hMG, started when serum oestradiol concentration ≤ 100 pmol/L. Control: GnRH agonist (buserelin acetate 500 μg/day), started cycle day 2 or 3 + hMG, started when serum oestradiol concentration ≤ 100 pmol/L. hMG start dose according to women's age, baseline serum FSH level, response to stimulation in previous treatment cycles. hMG and GnRH agonist both continued until hCG injection (10,000 IU), administered when 3 follicles ≥ 18 mm in diameter. | |
| Outcomes |
| |
| Notes | Power calculation performed: no. ITT analysis performed: unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done by drawing sequentially labelled sealed envelops, each containing a number obtained from a table of random numbers." |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Reasons for withdrawals and proportions of participants who withdrew not reported per treatment group. |
| Selective reporting (reporting bias) | Low risk | Data on all planned outcomes reported. |
| Other bias | Low risk | No significant differences in baseline characteristics between groups with regard to age, length of infertility, number of previous IVF cycles and cause of infertility. |
| Methods | Parallel group study. Number of women randomized: 117 (number per group not reported). Number of withdrawals: not reported. Number of women analyzed: unclear. | |
| Participants | Country of authors: Canada. Inclusion criteria: not reported. Exclusion criteria: not reported. Mean age: not reported. | |
| Interventions | Intervention: progestogen (norethindrone) for 5 days, started cycle day 1 + GnRH agonist, start cycle day 2. Control: GnRH agonist. Timing of treatment not reported. | |
| Outcomes |
| |
| Notes | Power calculation performed: unclear. ITT analysis used: unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | No data reported. |
| Selective reporting (reporting bias) | High risk | All prespecified outcomes not reported. |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement. |
| Methods | Parallel group study. Number of women randomized: 210 (105 to each group). Number of withdrawals: 5 (4 in intervention group; 1 in control group). Number of women analyzed: not clear; assumed 205. | |
| Participants | Country: Argentina. Inclusion criteria: aged ≤ 39 years, first IVF attempt, basal FSH ≤ 12 mIU/mL. Exclusion criteria: PCOS, prior ovarian surgery, "TESE needing." Mean age: not reported. Setting: assume single fertility centre in Buenos Aires, Argentina. | |
| Interventions | Intervention: COCP (ethinyl oestradiol 0.02 mg + levonorgestrel 0.1 mg) for 14/25 days in the preceding cycle. Control: no pretreatment. All women stimulated with rFSH (200 IU/day) from menstrual cycle day 2 or 3, hMG (225 UI/day) from day 4 and GnRH antagonist (cetrorelix 0.25 mg) in a flexible protocol starting with 14 mm leading follicle continuing both daily until the day of hCG. Oocyte maturation was triggered by rhCG (250 μg). Embryo transfer performed 3 days later. | |
| Outcomes | Primary:
Other:
| |
| Notes | Abstract with minimal data reported. Power calculation for sample size: not reported. ITT analysis: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Low risk | Proportions of withdrawals fairly balanced between groups although reasons for withdrawals not reported. |
| Selective reporting (reporting bias) | Unclear risk | Outcome data not clearly reported. |
| Other bias | Unclear risk | Not clear whether groups were comparable at baseline with respect to demographic characteristics. |
| Methods | Parallel group study. Number of women randomized: 220 (109 in intervention group; 111 in control group). Number of withdrawals: none reported. Number of women analyzed: 208 cycles (103 in intervention group; 105 in control group) and numbers of cycles were equivalent to numbers of participants. | |
| Participants | Country: China. Inclusion criteria: aged 25‐35 years; BMI 18‐25 kg/m2; number of previous IVF cycles < 3, and no previous poor response to ovarian stimulation (poor ovarian response characterised by cancellation of the cycle due to either poor follicular development or ≤ 4 cumulus‐oocyte‐complexes collected at oocyte retrieval); normal ovulatory cycles (25‐35 days); both ovaries present and normal uterus; no hormone therapy within the past 3 months; no current or past diseases affecting ovaries, gonadotrophin, sex steroid secretion, clearance or excretion. Exclusion criteria: not explicitly reported. Age range: 25‐35 years. Setting: IVF centre, China. | |
| Interventions | Intervention: oral oestradiol valerate (4 mg/day) preceding the IVF cycle from day 21 until day 2 of next cycle before GnRH antagonist protocol. Control: standard long GnRH agonist protocol. | |
| Outcomes |
| |
| Notes | Outcomes measured as per 'embryo transfer cycle' but numbers of cycles transferred were equivalent to numbers of women randomized. Power calculation for sample size: not reported. ITT analysis: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization allocation sequence was generated from a table of computer‐generated random numbers." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) | High risk | "This study was not blind." |
| Incomplete outcome data (attrition bias) | Low risk | No withdrawals reported, cycle cancellation (number of cycles were equivalent to numbers of women randomized) similar between groups. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
| Other bias | Low risk | Groups comparable at baseline with respect to demographic characteristics. |
17‐βE2: 17‐beta oestradiol; AMH: anti‐mullerian hormone; BMI: body mass index; COCP: combined oral contraceptive pill; COH: controlled ovarian hyperstimulation; COS: controlled ovarian stimulation; FSH: follicle‐stimulating hormone; rFSH: recombinant follicle‐stimulating hormone; GnRH: gonadotrophin‐releasing hormone; GnRHa: gonadotrophin‐releasing hormone analogue; hMG: human menopausal gonadotrophin; IM: intramuscular; ITT: intention to treat; IU: international unit; IUI: intrauterine insemination; IVF: in vitro fertilisation; IVF‐ET: in vitro fertilisation with embryo transfer; LH: luteinising hormone; OCP: oral contraceptive pill; OHSS: ovarian hyperstimulation syndrome; PCOS: polycystic ovary syndrome; PO: per os (oral); rhCG: recombinant human chorionic gonadotropin; SC: subcutaneous; SD: standard deviation; SEM: standardized mean difference; TESE: testicular epididymal sperm extraction; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Randomisation status unknown. | |
| Not an RCT. Retrospective study. | |
| Not an RCT. Women only received controlled ovarian stimulation, but no embryo transfer as part of an ART cycle. | |
| Did not state that allocation randomised. | |
| Not an RCT. Retrospective study. | |
| Not an RCT. | |
| Not an RCT. Retrospective study. | |
| Not an RCT. Retrospective study. Each woman served as her own control. | |
| Not an RCT. Each woman served as her own control. | |
| Not an RCT. Single arm study. | |
| Not an RCT. Retrospective study. Monophasic OCP vs triphasic OCP. | |
| Not an RCT. | |
| Not an RCT. | |
| Not an RCT. Naltrexone used in treatment protocol. | |
| Not an RCT. | |
| Cross‐over design with no pre‐cross‐over data. | |
| Not an RCT. Retrospective study. | |
| RCT status unclear. Number of women randomised at baseline not stated. | |
| Compared different durations of COCP pretreatment. | |
| Not an RCT. Open single‐arm study. | |
| Not an RCT. Retrospective study. | |
| Not an RCT. | |
| Not an RCT. Women were oocyte donors. Compared different durations of COCP pretreatment. | |
| Not an RCT. Retrospective study. | |
| Number of women randomised or analysed in each treatment group not reported. | |
| Cross‐over design with no pre‐cross‐over data. | |
| Not an RCT. Each woman served as her own control. Women only received controlled ovarian stimulation, but no embryo transfer as part of an ART cycle. | |
| Randomised comparison of different types of COCP. | |
| Not an RCT. Each woman served as her own control. | |
| Not an RCT. | |
| Not an RCT. Retrospective study. | |
| Not an RCT. | |
| Not an RCT. | |
| Not an RCT. Single‐arm study. | |
| Intervention not relevant: luteal phase support. | |
| Not an RCT. | |
| Compared 2 different ways of administration of oestrogen. | |
| Not an RCT. Clomiphene citrate used in treatment protocol. | |
| Not an RCT. Retrospective study. | |
| Intervention not relevant: OCP used for endometrial preparation before embryo transfer and used as luteal phase support. | |
| Quasi‐randomised | |
| Both groups received COCP pretreatment, no control group. | |
| Not an RCT. Single‐arm study. | |
| Oestrogen pretreatment not stopped before oocyte retrieval, but also used as luteal phase support. | |
| Compared 2 different ways of administration of a combined contraceptive (Nuvaring vs oral Desogen). | |
| Not an RCT. Retrospective study. | |
| Not an RCT. Retrospective study. | |
| Interventions not relevant (OCP vs OCP). | |
| Not an RCT. Retrospective study. | |
| Women only received oestrogen plus progestogen, but no gonadotrophins or GnRH analogues as part of an ART cycle. Compared 2 different timings of administration. | |
| Compared 2 different doses of oestrogen treatment for endometrial preparation. | |
| Not an RCT. | |
| Interventions not relevant (OCP vs Nuvaring with similar contents as OCP). | |
| Not an RCT. Retrospective study. | |
| Women were oocyte donors. | |
| Compared different durations of COCP pretreatment. | |
| Not an RCT. Open‐label single‐arm study. | |
| Not an RCT. Open‐label single‐arm study. | |
| Alternate randomisation was used. | |
| Not an RCT. Retrospective study. | |
| Not an RCT. Retrospective study. | |
| Not an RCT. Each woman served as her own control. | |
| Not an RCT. | |
| Not an RCT. Retrospective study. Prednisolone used in treatment protocol. | |
| Not an RCT. | |
| Not an RCT. Retrospective study. | |
| Not an RCT. Retrospective study. | |
| Intervention not relevant: luteal phase support. | |
| Compared different doses and timings of oestrogen pretreatments. | |
| Not an RCT. Retrospective study. Single‐arm study. | |
| Not an RCT. | |
| Women only received ovulation induction, no embryo transfer as part of an ART cycle. | |
| Not an RCT. | |
| Not an RCT. Each woman served as her own control. | |
| Not an RCT. Retrospective study. | |
| Not an RCT. Retrospective study. | |
| Not an RCT. | |
| Women had premature ovarian failure. | |
| Interventions not relevant (OCP vs OCP) | |
| Cross‐over design with no pre‐cross‐over data. | |
| Not a true RCT: randomisation according to alternate number allocation; intervention not relevant: co‐administration of medroxyprogesterone acetate with the stimulation agent. | |
| Design not relevant: retrospective cohort study. | |
| Not an RCT. | |
| Not an RCT. | |
| Not an RCT. Retrospective study. | |
| Not relevant: compared mild vs standard stimulation (with pretreatment in the mild group). | |
| Not an RCT. Retrospective study. |
ART: assisted reproductive technique; COCP: combined oral contraceptive pill; OCP: oral contraceptive pill; GnRH: gonadotrophin‐releasing hormone; RCT: randomised controlled trial.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1 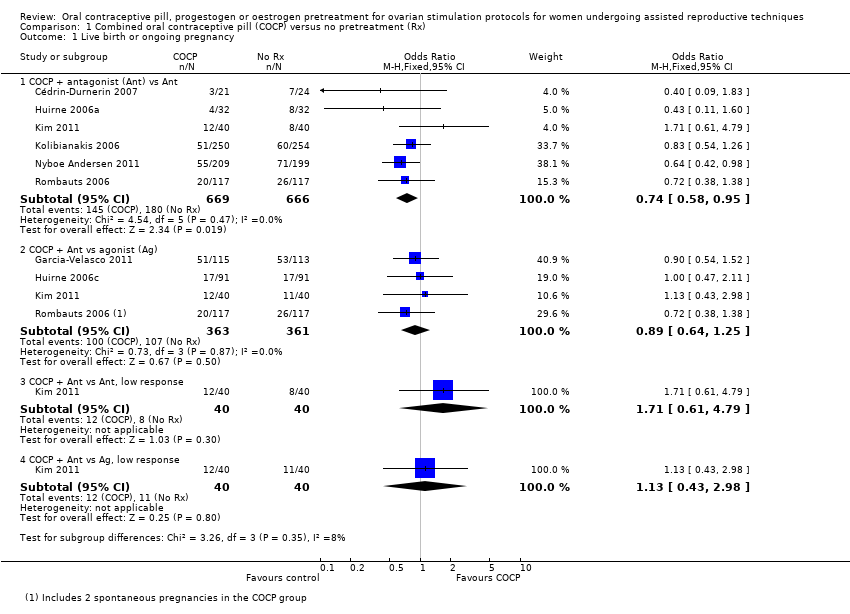 Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 1 Live birth or ongoing pregnancy. | ||||
| 1.1 COCP + antagonist (Ant) vs Ant | 6 | 1335 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.58, 0.95] |
| 1.2 COCP + Ant vs agonist (Ag) | 4 | 724 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.64, 1.25] |
| 1.3 COCP + Ant vs Ant, low response | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.61, 4.79] |
| 1.4 COCP + Ant vs Ag, low response | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.43, 2.98] |
| 2 Pregnancy loss Show forest plot | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 2 Pregnancy loss. | ||||
| 2.1 COCP + Ant vs Ant | 5 | 868 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.82, 2.26] |
| 2.2 COCP + Ant vs Ag | 5 | 780 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.22, 0.72] |
| 2.3 COCP + Ant vs Ant, low response | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.18, 23.59] |
| 2.4 COCP + Ant vs Ag, low response | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.13, 7.47] |
| 3 Clinical pregnancy rate Show forest plot | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 3 Clinical pregnancy rate. | ||||
| 3.1 COCP + Ant vs Ant | 5 | 740 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.63, 1.15] |
| 3.2 COCP + Ant vs Ag | 4 | 546 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.59, 1.20] |
| 3.3 COCP + Ant vs Ant, low response | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.69, 4.97] |
| 3.4 COCP + Ant vs Ag, low response | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.44, 2.83] |
| 4 Multiple pregnancy rate Show forest plot | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4 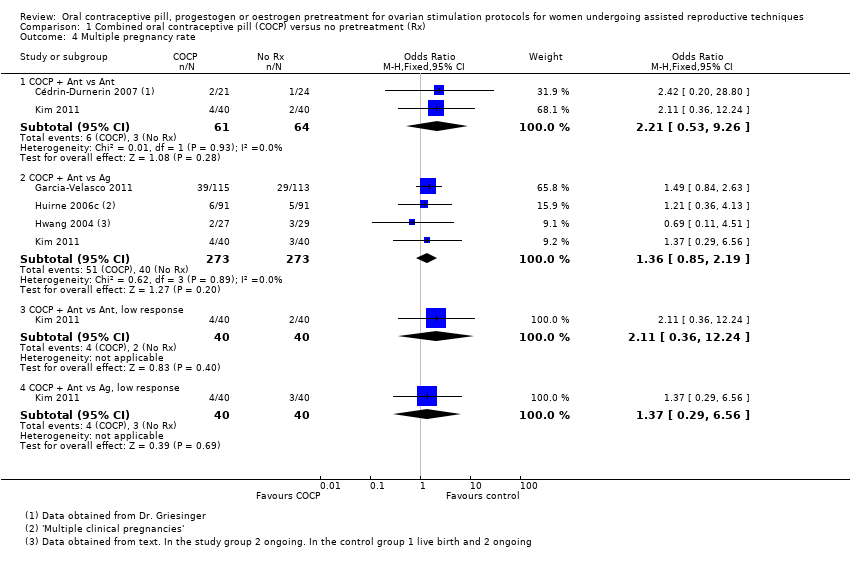 Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 4 Multiple pregnancy rate. | ||||
| 4.1 COCP + Ant vs Ant | 2 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.21 [0.53, 9.26] |
| 4.2 COCP + Ant vs Ag | 4 | 546 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.85, 2.19] |
| 4.3 COCP + Ant vs Ant, low response | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.11 [0.36, 12.24] |
| 4.4 COCP + Ant vs Ag, low response | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.29, 6.56] |
| 5 Ovarian hyperstimulation syndrome rate Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 5 Ovarian hyperstimulation syndrome rate. | ||||
| 5.1 COCP + Ant vs Ant | 2 | 642 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.28, 3.40] |
| 5.2 COCP + Ant vs Ag | 2 | 290 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.20, 1.96] |
| 6 Number of oocytes retrieved Show forest plot | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6 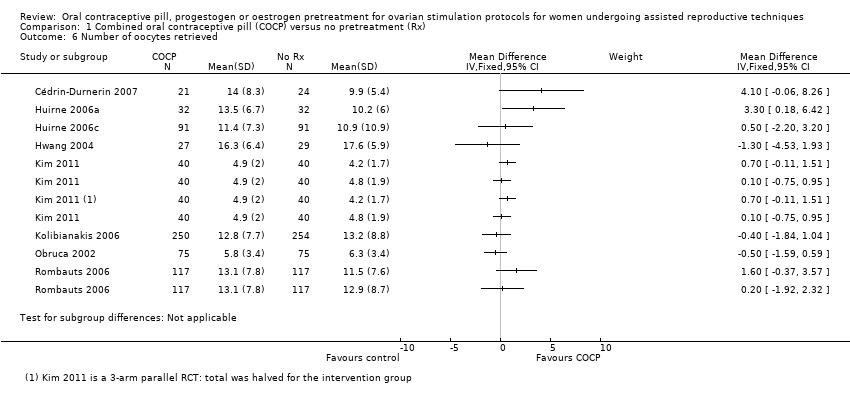 Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 6 Number of oocytes retrieved. | ||||
| 7 Days of gonadotrophin treatment Show forest plot | 8 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.7 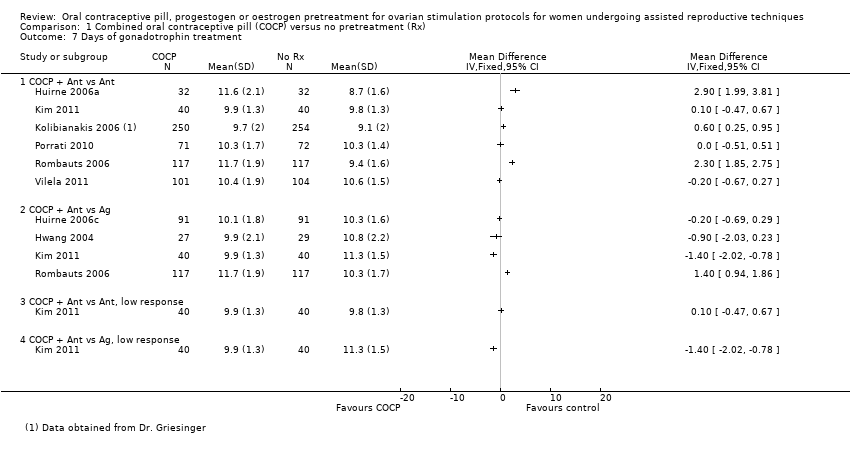 Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 7 Days of gonadotrophin treatment. | ||||
| 7.1 COCP + Ant vs Ant | 6 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 COCP + Ant vs Ag | 4 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 COCP + Ant vs Ant, low response | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 COCP + Ant vs Ag, low response | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Amount of gonadotrophins administered Show forest plot | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.8  Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 8 Amount of gonadotrophins administered. | ||||
| 8.1 COCP + Ant vs Ant | 7 | 1275 | Mean Difference (IV, Fixed, 95% CI) | 190.10 [134.91, 245.28] |
| 8.2 COCP + Ant vs Ag | 3 | 496 | Mean Difference (IV, Fixed, 95% CI) | 9.96 [‐104.09, 124.02] |
| 8.3 COCP + Ant vs Ant, low response | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 20.0 [‐165.39, 205.39] |
| 8.4 COCP + Ant vs Ag, low response | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐349.0 [‐537.92, ‐160.08] |
| 9 Ovarian cyst formation rate Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.9  Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 9 Ovarian cyst formation rate. | ||||
| 9.1 COCP + Ant vs Ant | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.08, 2.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1 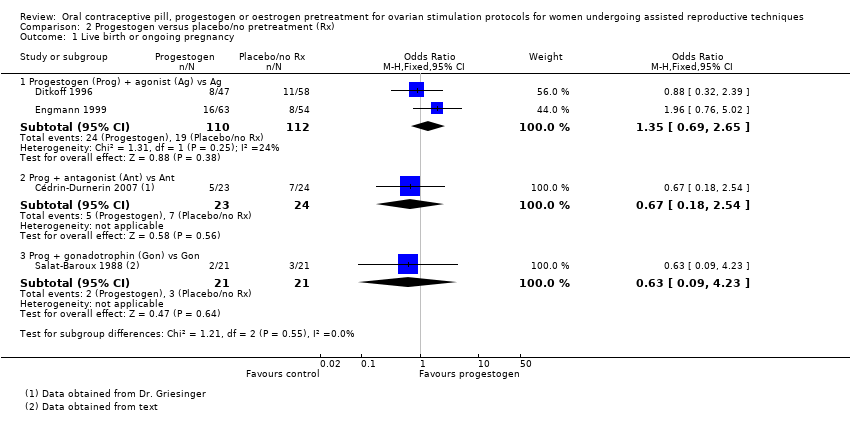 Comparison 2 Progestogen versus placebo/no pretreatment (Rx), Outcome 1 Live birth or ongoing pregnancy. | ||||
| 1.1 Progestogen (Prog) + agonist (Ag) vs Ag | 2 | 222 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.69, 2.65] |
| 1.2 Prog + antagonist (Ant) vs Ant | 1 | 47 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.18, 2.54] |
| 1.3 Prog + gonadotrophin (Gon) vs Gon | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.09, 4.23] |
| 2 Pregnancy loss Show forest plot | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2 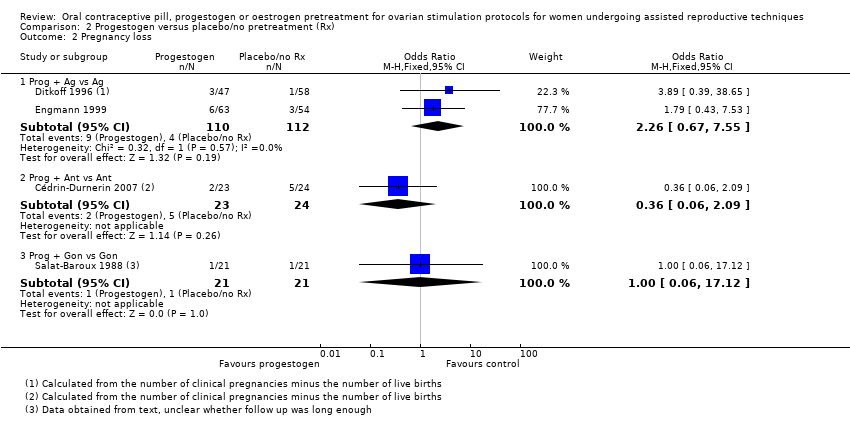 Comparison 2 Progestogen versus placebo/no pretreatment (Rx), Outcome 2 Pregnancy loss. | ||||
| 2.1 Prog + Ag vs Ag | 2 | 222 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.26 [0.67, 7.55] |
| 2.2 Prog + Ant vs Ant | 1 | 47 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.06, 2.09] |
| 2.3 Prog + Gon vs Gon | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 17.12] |
| 3 Clinical pregnancy rate Show forest plot | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3 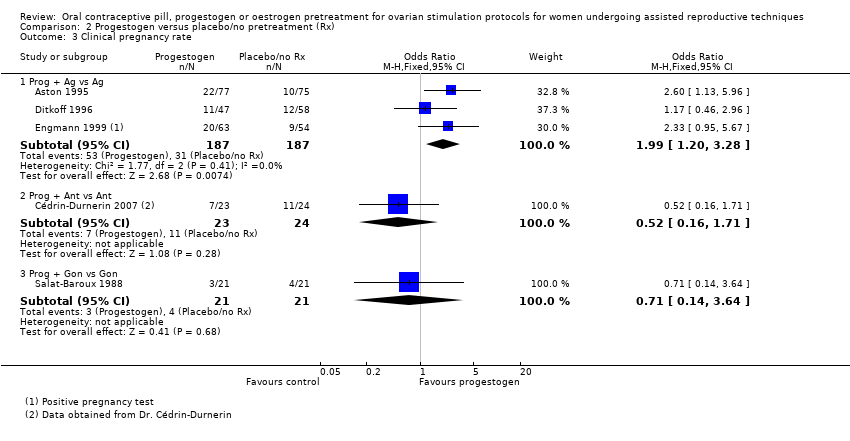 Comparison 2 Progestogen versus placebo/no pretreatment (Rx), Outcome 3 Clinical pregnancy rate. | ||||
| 3.1 Prog + Ag vs Ag | 3 | 374 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.20, 3.28] |
| 3.2 Prog + Ant vs Ant | 1 | 47 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.16, 1.71] |
| 3.3 Prog + Gon vs Gon | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.14, 3.64] |
| 4 Multiple pregnancy rate Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.4 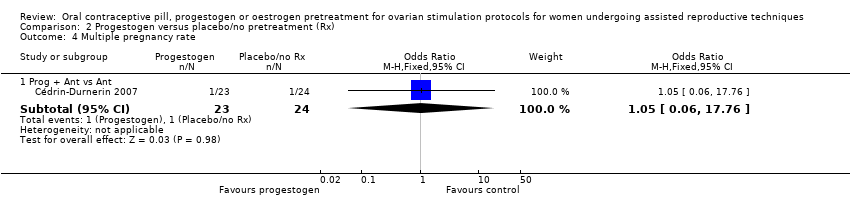 Comparison 2 Progestogen versus placebo/no pretreatment (Rx), Outcome 4 Multiple pregnancy rate. | ||||
| 4.1 Prog + Ant vs Ant | 1 | 47 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.06, 17.76] |
| 5 Number of oocytes retrieved Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.5 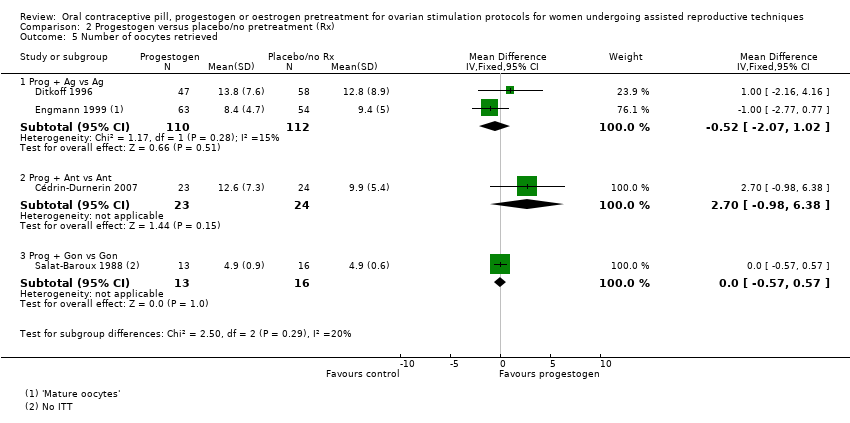 Comparison 2 Progestogen versus placebo/no pretreatment (Rx), Outcome 5 Number of oocytes retrieved. | ||||
| 5.1 Prog + Ag vs Ag | 2 | 222 | Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐2.07, 1.02] |
| 5.2 Prog + Ant vs Ant | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | 2.70 [‐0.98, 6.38] |
| 5.3 Prog + Gon vs Gon | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.57, 0.57] |
| 6 Days of gonadotrophin treatment Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.6 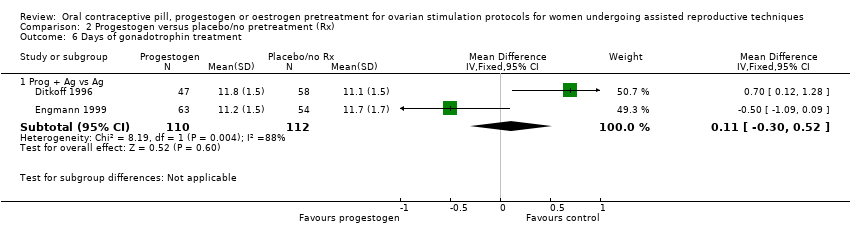 Comparison 2 Progestogen versus placebo/no pretreatment (Rx), Outcome 6 Days of gonadotrophin treatment. | ||||
| 6.1 Prog + Ag vs Ag | 2 | 222 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.30, 0.52] |
| 7 Amount of gonadotrophins administered Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.7 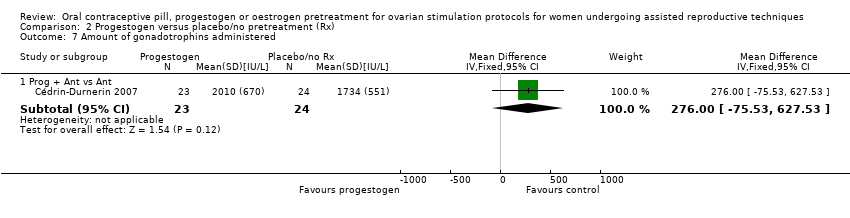 Comparison 2 Progestogen versus placebo/no pretreatment (Rx), Outcome 7 Amount of gonadotrophins administered. | ||||
| 7.1 Prog + Ant vs Ant | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | 276.0 [‐75.53, 627.53] |
| 8 Ovarian cyst formation rate Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.8  Comparison 2 Progestogen versus placebo/no pretreatment (Rx), Outcome 8 Ovarian cyst formation rate. | ||||
| 8.1 Prog + Ag vs Ag | 3 | 374 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.08, 0.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Oestrogen versus no pretreatment (Rx), Outcome 1 Live birth or ongoing pregnancy. | ||||
| 1.1 Oestrogen (Oestr) + antagonist (Ant) vs Ant | 2 | 502 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.53, 1.17] |
| 1.2 Oestr + Ant vs agonist (Ag) | 2 | 242 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.51, 1.50] |
| 2 Pregnancy loss Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.2 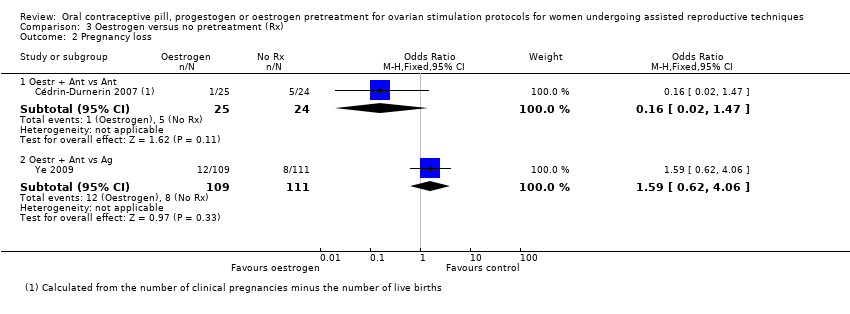 Comparison 3 Oestrogen versus no pretreatment (Rx), Outcome 2 Pregnancy loss. | ||||
| 2.1 Oestr + Ant vs Ant | 1 | 49 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.47] |
| 2.2 Oestr + Ant vs Ag | 1 | 220 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.62, 4.06] |
| 3 Clinical pregnancy rate Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.3  Comparison 3 Oestrogen versus no pretreatment (Rx), Outcome 3 Clinical pregnancy rate. | ||||
| 3.1 Oestr + Ant vs Ant | 4 | 688 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.66, 1.24] |
| 3.2 Oestr + Ant vs Ag | 2 | 242 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.45, 1.27] |
| 4 Multiple pregnancies Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.4  Comparison 3 Oestrogen versus no pretreatment (Rx), Outcome 4 Multiple pregnancies. | ||||
| 4.1 Oestr + Ant vs Ag | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.24 [0.09, 53.59] |
| 5 Ovarian hyperstimulation syndrome rate Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.5  Comparison 3 Oestrogen versus no pretreatment (Rx), Outcome 5 Ovarian hyperstimulation syndrome rate. | ||||
| 5.1 Oestr + Ant vs Ag | 1 | 220 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.25, 9.42] |
| 6 Number of oocytes retrieved Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.6  Comparison 3 Oestrogen versus no pretreatment (Rx), Outcome 6 Number of oocytes retrieved. | ||||
| 6.1 Oestr + Ant vs Ant | 2 | 139 | Mean Difference (IV, Fixed, 95% CI) | 2.23 [0.71, 3.75] |
| 6.2 Oestr + Ant vs Ag | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐4.47, 5.27] |
| 7 Days of gonadotrophin treatment Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.7  Comparison 3 Oestrogen versus no pretreatment (Rx), Outcome 7 Days of gonadotrophin treatment. | ||||
| 7.1 Oestr + Ant vs Ant | 2 | 529 | Mean Difference (IV, Fixed, 95% CI) | 0.83 [0.58, 1.08] |
| 7.2 Oestr + Ant vs Ag | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐4.07, ‐0.93] |
| 8 Amount of gonadotrophins administered Show forest plot | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.8  Comparison 3 Oestrogen versus no pretreatment (Rx), Outcome 8 Amount of gonadotrophins administered. | ||||
| 8.1 Oestr + Ant vs Ant | 4 | 668 | Mean Difference (IV, Fixed, 95% CI) | 168.35 [111.53, 225.17] |
| 8.2 Oestr + Ant vs Ag | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐16.0 [‐470.12, 438.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.1 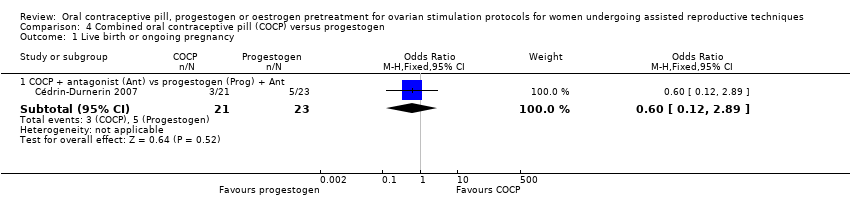 Comparison 4 Combined oral contraceptive pill (COCP) versus progestogen, Outcome 1 Live birth or ongoing pregnancy. | ||||
| 1.1 COCP + antagonist (Ant) vs progestogen (Prog) + Ant | 1 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.12, 2.89] |
| 2 Pregnancy loss Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.2  Comparison 4 Combined oral contraceptive pill (COCP) versus progestogen, Outcome 2 Pregnancy loss. | ||||
| 2.1 COCP + Ant vs Prog + Ant | 1 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.14, 8.64] |
| 3 Clinical pregnancy rate Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.3  Comparison 4 Combined oral contraceptive pill (COCP) versus progestogen, Outcome 3 Clinical pregnancy rate. | ||||
| 3.1 COCP + Ant vs Prog + Ant | 1 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.19, 2.73] |
| 4 Multiple pregnancy rate Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.4 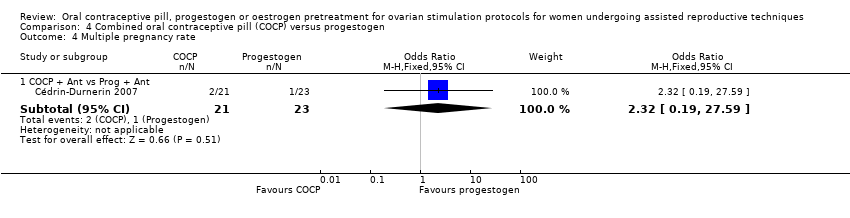 Comparison 4 Combined oral contraceptive pill (COCP) versus progestogen, Outcome 4 Multiple pregnancy rate. | ||||
| 4.1 COCP + Ant vs Prog + Ant | 1 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.32 [0.19, 27.59] |
| 5 Number of oocytes retrieved Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.5  Comparison 4 Combined oral contraceptive pill (COCP) versus progestogen, Outcome 5 Number of oocytes retrieved. | ||||
| 5.1 COCP + Ant vs Prog + Ant | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [‐3.24, 6.04] |
| 6 Amount of gonadotrophins administered Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.6  Comparison 4 Combined oral contraceptive pill (COCP) versus progestogen, Outcome 6 Amount of gonadotrophins administered. | ||||
| 6.1 COCP + Ant vs Prog + Ant | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 164.0 [‐249.03, 577.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.1 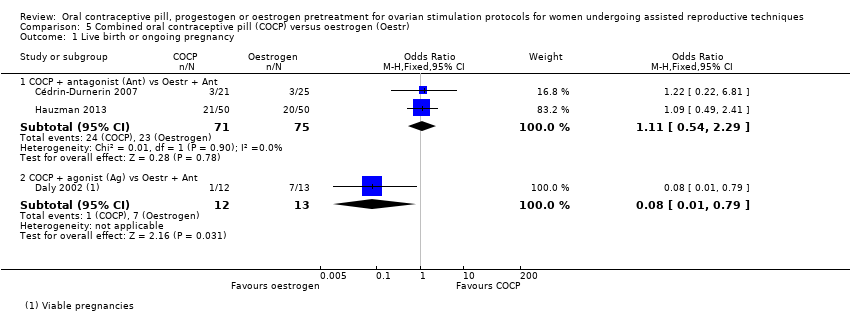 Comparison 5 Combined oral contraceptive pill (COCP) versus oestrogen (Oestr), Outcome 1 Live birth or ongoing pregnancy. | ||||
| 1.1 COCP + antagonist (Ant) vs Oestr + Ant | 2 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.54, 2.29] |
| 1.2 COCP + agonist (Ag) vs Oestr + Ant | 1 | 25 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.01, 0.79] |
| 2 Pregnancy loss Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.2  Comparison 5 Combined oral contraceptive pill (COCP) versus oestrogen (Oestr), Outcome 2 Pregnancy loss. | ||||
| 2.1 COCP + Ag vs Oestr + Ant | 1 | 25 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.06, 19.63] |
| 3 Clinical pregnancy rate Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.3 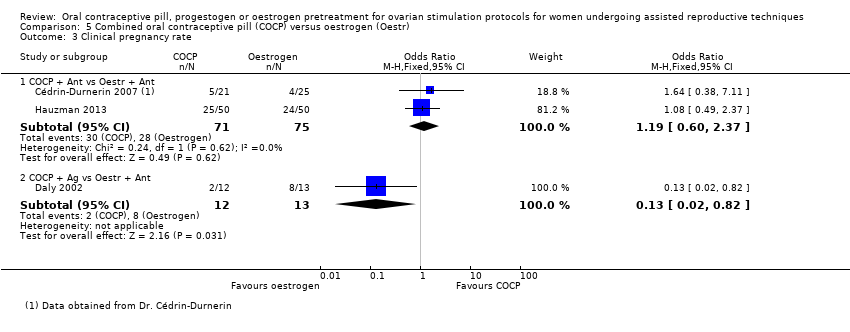 Comparison 5 Combined oral contraceptive pill (COCP) versus oestrogen (Oestr), Outcome 3 Clinical pregnancy rate. | ||||
| 3.1 COCP + Ant vs Oestr + Ant | 2 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.60, 2.37] |
| 3.2 COCP + Ag vs Oestr + Ant | 1 | 25 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.02, 0.82] |
| 4 Number of oocytes retrieved Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.4  Comparison 5 Combined oral contraceptive pill (COCP) versus oestrogen (Oestr), Outcome 4 Number of oocytes retrieved. | ||||
| 4.1 COCP + Ant vs Oestr + Ant | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐3.59, 5.39] |
| 5 Days of gonadotropin treatment Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.5  Comparison 5 Combined oral contraceptive pill (COCP) versus oestrogen (Oestr), Outcome 5 Days of gonadotropin treatment. | ||||
| 5.1 COCP + Ant vs Oestr + Ant | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.23, 0.03] |
| 6 Amount of gonadotrophins administered Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 5.6 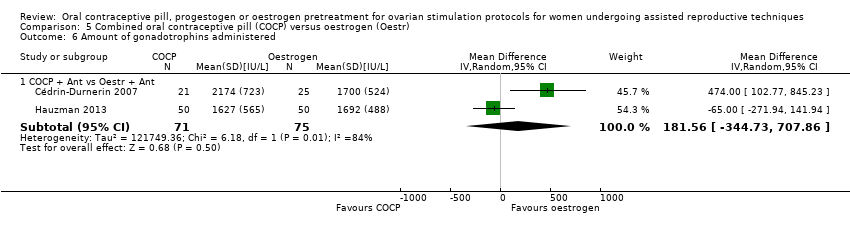 Comparison 5 Combined oral contraceptive pill (COCP) versus oestrogen (Oestr), Outcome 6 Amount of gonadotrophins administered. | ||||
| 6.1 COCP + Ant vs Oestr + Ant | 2 | 146 | Mean Difference (IV, Random, 95% CI) | 181.56 [‐344.73, 707.86] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.1 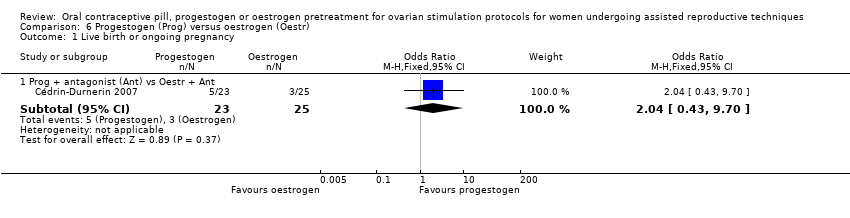 Comparison 6 Progestogen (Prog) versus oestrogen (Oestr), Outcome 1 Live birth or ongoing pregnancy. | ||||
| 1.1 Prog + antagonist (Ant) vs Oestr + Ant | 1 | 48 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.43, 9.70] |
| 2 Clinical pregnancy rate Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.2 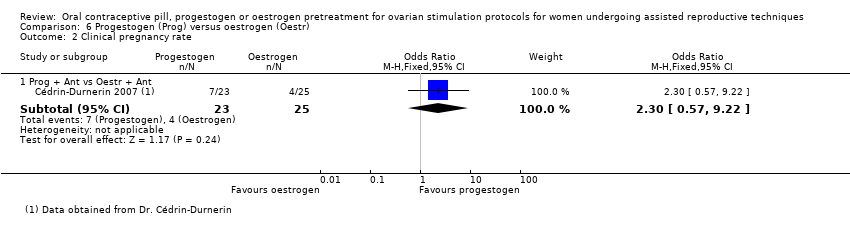 Comparison 6 Progestogen (Prog) versus oestrogen (Oestr), Outcome 2 Clinical pregnancy rate. | ||||
| 2.1 Prog + Ant vs Oestr + Ant | 1 | 48 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.30 [0.57, 9.22] |
| 3 Number of oocytes retrieved Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.3  Comparison 6 Progestogen (Prog) versus oestrogen (Oestr), Outcome 3 Number of oocytes retrieved. | ||||
| 3.1 Prog + Ant vs Oestr + Ant | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐4.55, 3.55] |
| 4 Amount of gonadotrophins administered Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.4  Comparison 6 Progestogen (Prog) versus oestrogen (Oestr), Outcome 4 Amount of gonadotrophins administered. | ||||
| 4.1 Prog + Ant vs Oestr + Ant | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 310.0 [‐32.30, 652.30] |
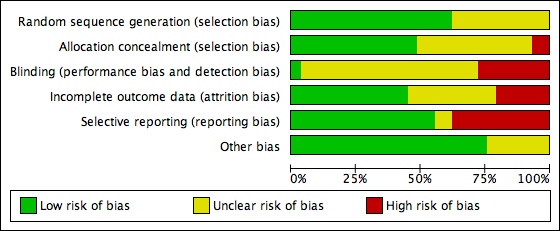
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
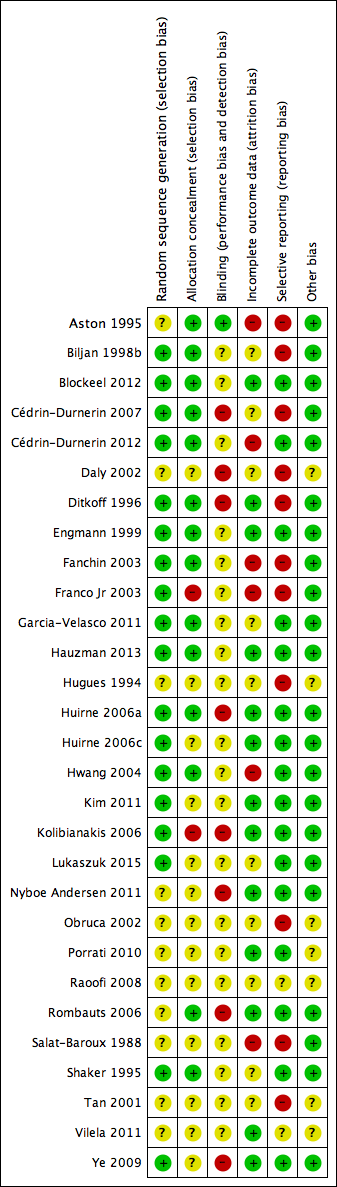
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Study PRISMA flow chart

Forest plot of comparison: 1 Combined oral contraceptive pill (OCP) versus no pretreatment (Rx), outcome: 1.1 Live birth or ongoing pregnancy.

Forest plot of comparison: 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), outcome: 1.2 Pregnancy loss.

Forest plot of comparison: 3 Oestrogen versus no pretreatment (Rx), outcome: 3.1 Live birth or ongoing pregnancy.
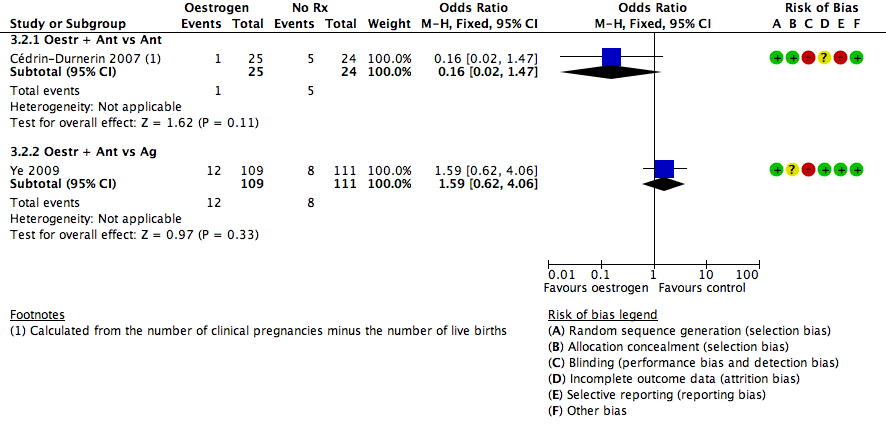
Forest plot of comparison: 3 Oestrogen versus no pretreatment (Rx), outcome: 3.2 Pregnancy loss.

Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 1 Live birth or ongoing pregnancy.
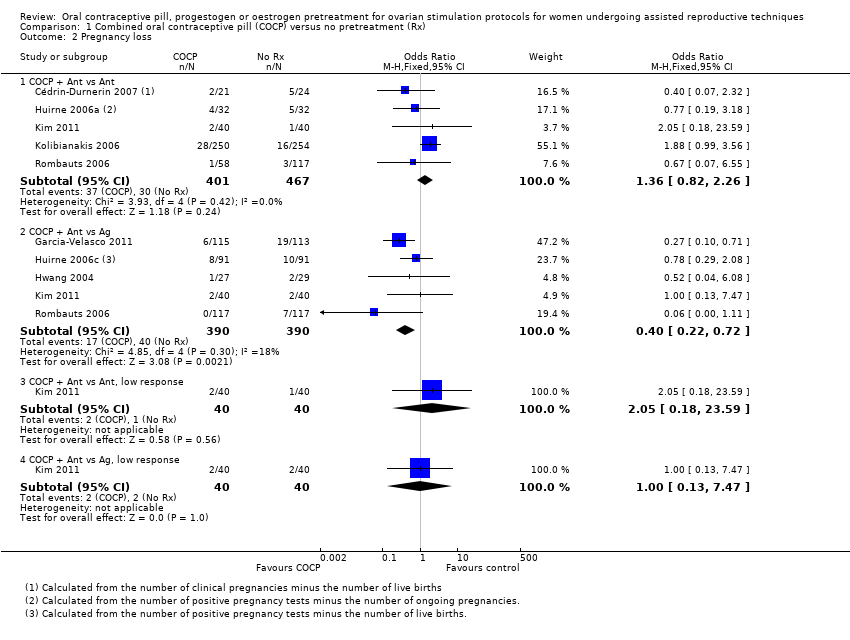
Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 2 Pregnancy loss.

Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 3 Clinical pregnancy rate.

Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 4 Multiple pregnancy rate.
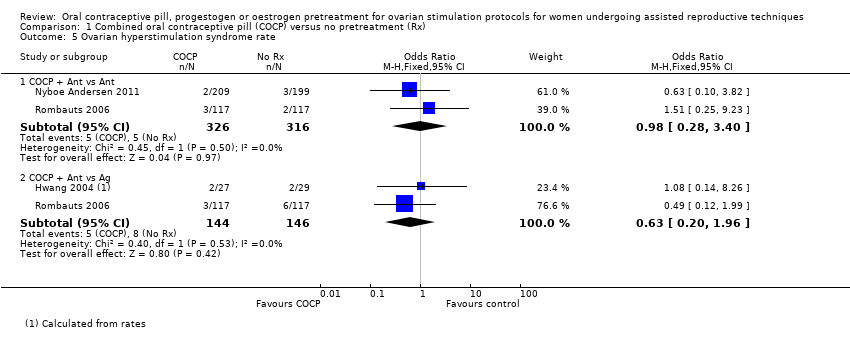
Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 5 Ovarian hyperstimulation syndrome rate.

Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 6 Number of oocytes retrieved.
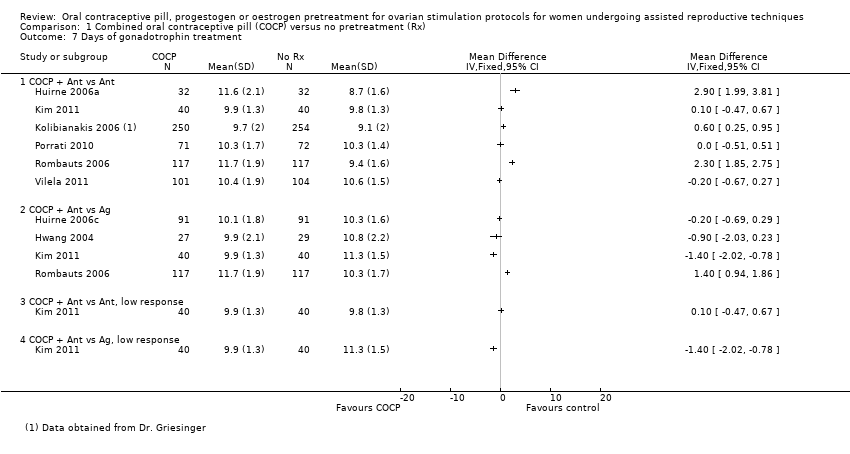
Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 7 Days of gonadotrophin treatment.
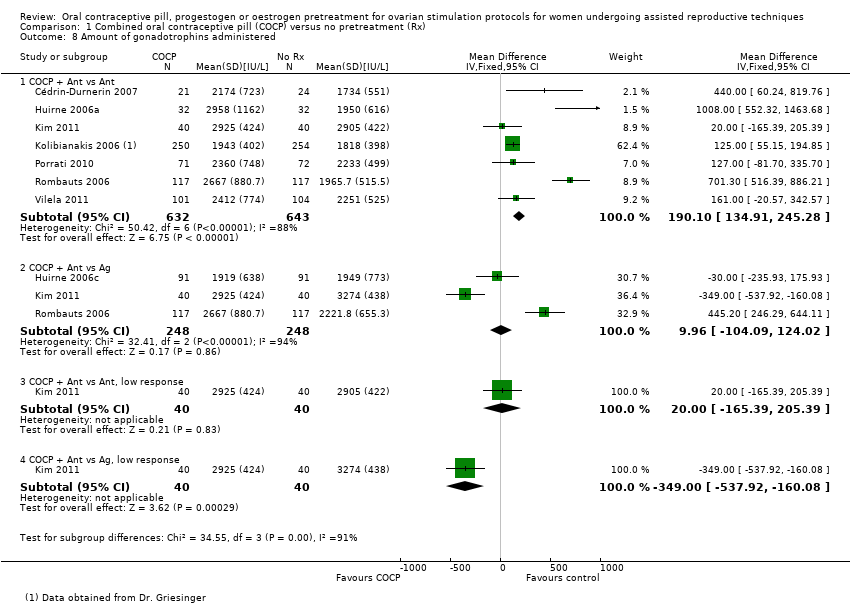
Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 8 Amount of gonadotrophins administered.
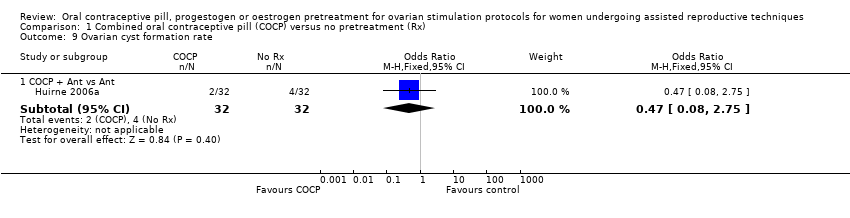
Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 9 Ovarian cyst formation rate.
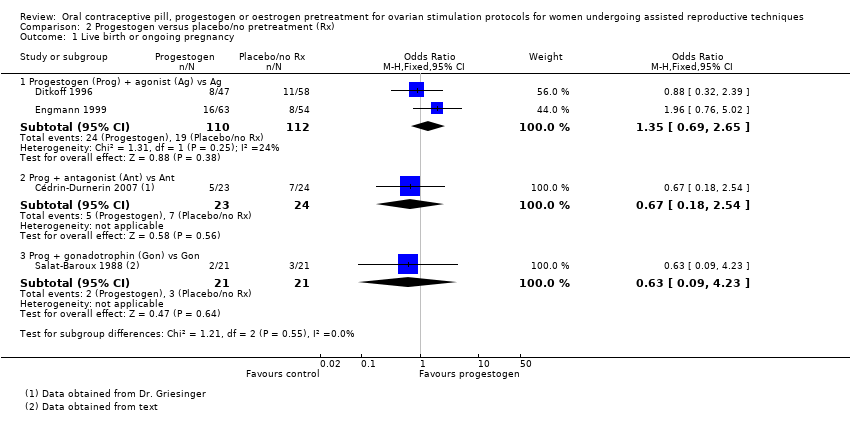
Comparison 2 Progestogen versus placebo/no pretreatment (Rx), Outcome 1 Live birth or ongoing pregnancy.

Comparison 2 Progestogen versus placebo/no pretreatment (Rx), Outcome 2 Pregnancy loss.

Comparison 2 Progestogen versus placebo/no pretreatment (Rx), Outcome 3 Clinical pregnancy rate.
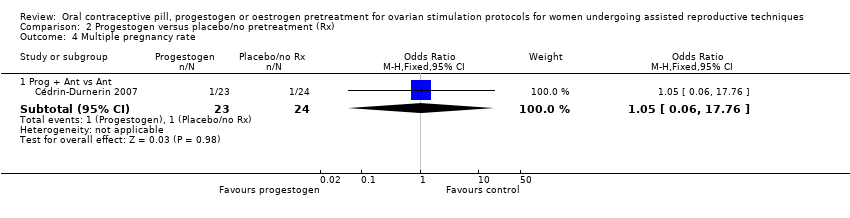
Comparison 2 Progestogen versus placebo/no pretreatment (Rx), Outcome 4 Multiple pregnancy rate.

Comparison 2 Progestogen versus placebo/no pretreatment (Rx), Outcome 5 Number of oocytes retrieved.

Comparison 2 Progestogen versus placebo/no pretreatment (Rx), Outcome 6 Days of gonadotrophin treatment.
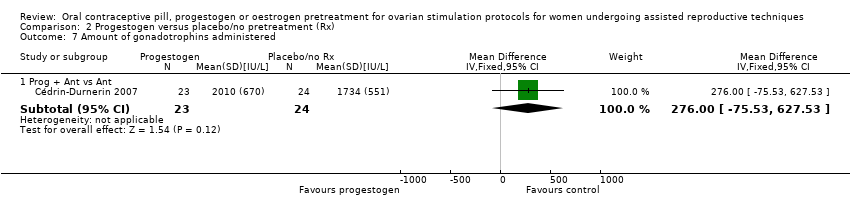
Comparison 2 Progestogen versus placebo/no pretreatment (Rx), Outcome 7 Amount of gonadotrophins administered.

Comparison 2 Progestogen versus placebo/no pretreatment (Rx), Outcome 8 Ovarian cyst formation rate.

Comparison 3 Oestrogen versus no pretreatment (Rx), Outcome 1 Live birth or ongoing pregnancy.
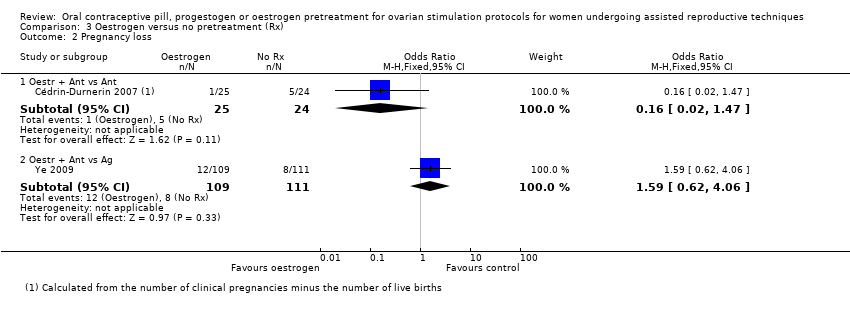
Comparison 3 Oestrogen versus no pretreatment (Rx), Outcome 2 Pregnancy loss.
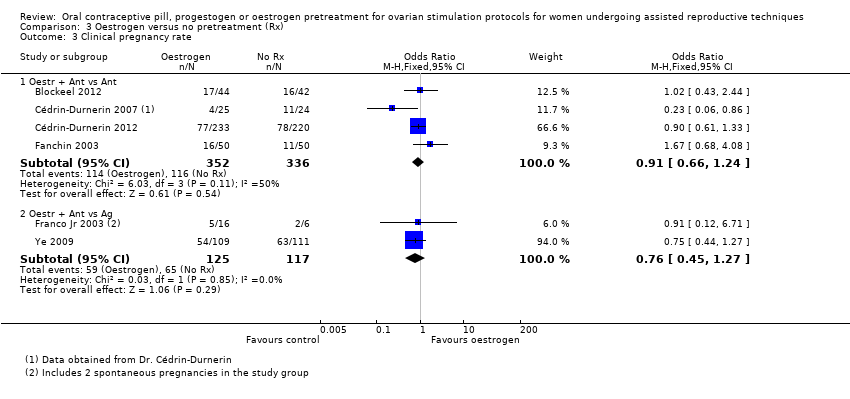
Comparison 3 Oestrogen versus no pretreatment (Rx), Outcome 3 Clinical pregnancy rate.
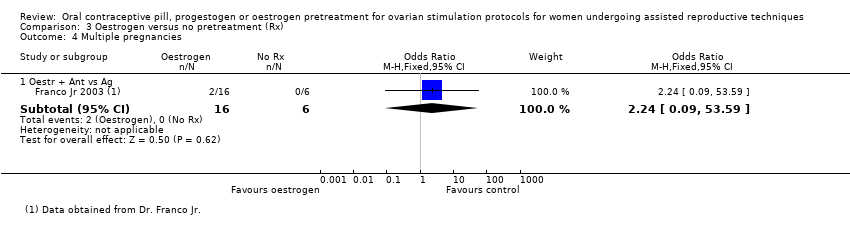
Comparison 3 Oestrogen versus no pretreatment (Rx), Outcome 4 Multiple pregnancies.

Comparison 3 Oestrogen versus no pretreatment (Rx), Outcome 5 Ovarian hyperstimulation syndrome rate.

Comparison 3 Oestrogen versus no pretreatment (Rx), Outcome 6 Number of oocytes retrieved.

Comparison 3 Oestrogen versus no pretreatment (Rx), Outcome 7 Days of gonadotrophin treatment.

Comparison 3 Oestrogen versus no pretreatment (Rx), Outcome 8 Amount of gonadotrophins administered.

Comparison 4 Combined oral contraceptive pill (COCP) versus progestogen, Outcome 1 Live birth or ongoing pregnancy.

Comparison 4 Combined oral contraceptive pill (COCP) versus progestogen, Outcome 2 Pregnancy loss.
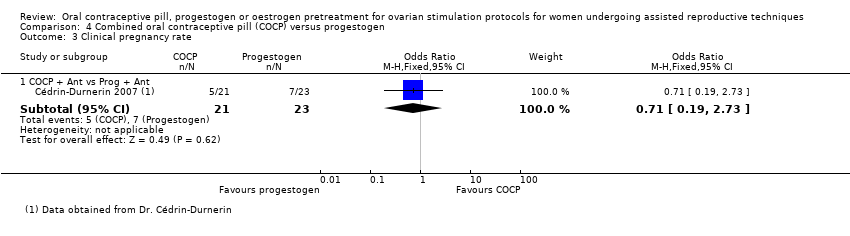
Comparison 4 Combined oral contraceptive pill (COCP) versus progestogen, Outcome 3 Clinical pregnancy rate.
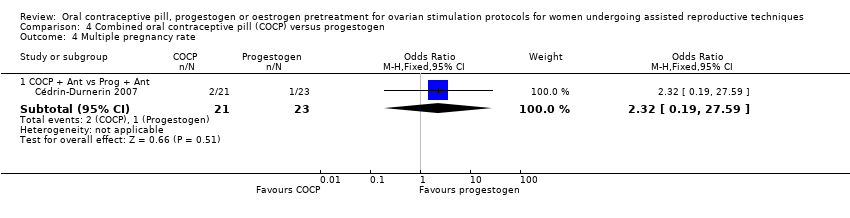
Comparison 4 Combined oral contraceptive pill (COCP) versus progestogen, Outcome 4 Multiple pregnancy rate.
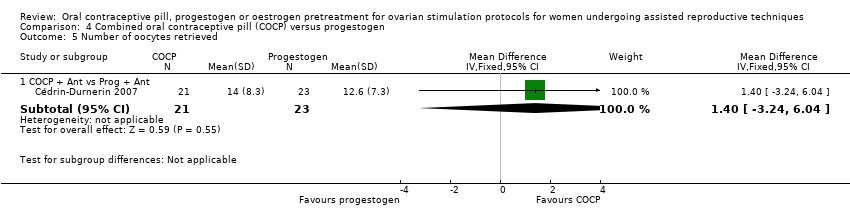
Comparison 4 Combined oral contraceptive pill (COCP) versus progestogen, Outcome 5 Number of oocytes retrieved.
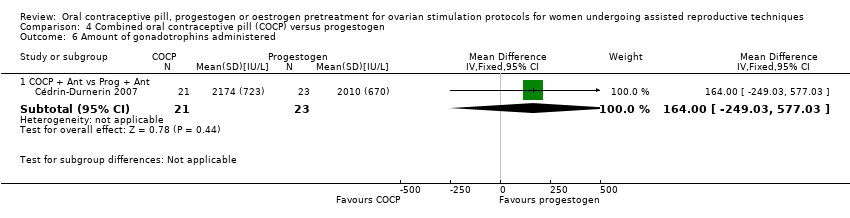
Comparison 4 Combined oral contraceptive pill (COCP) versus progestogen, Outcome 6 Amount of gonadotrophins administered.
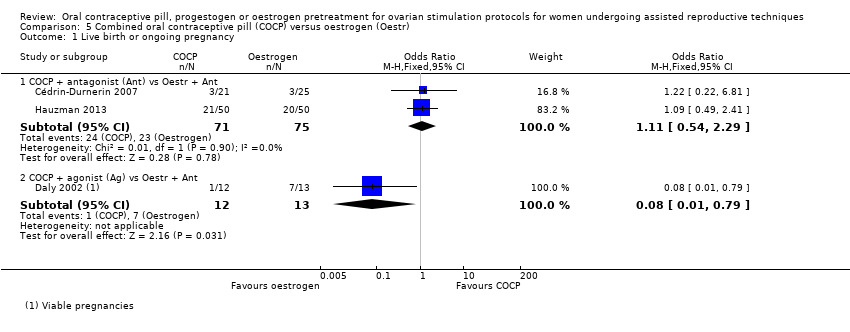
Comparison 5 Combined oral contraceptive pill (COCP) versus oestrogen (Oestr), Outcome 1 Live birth or ongoing pregnancy.
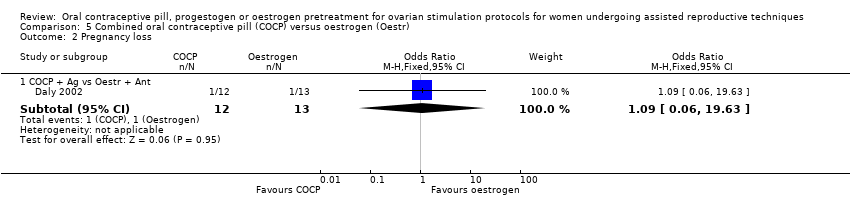
Comparison 5 Combined oral contraceptive pill (COCP) versus oestrogen (Oestr), Outcome 2 Pregnancy loss.
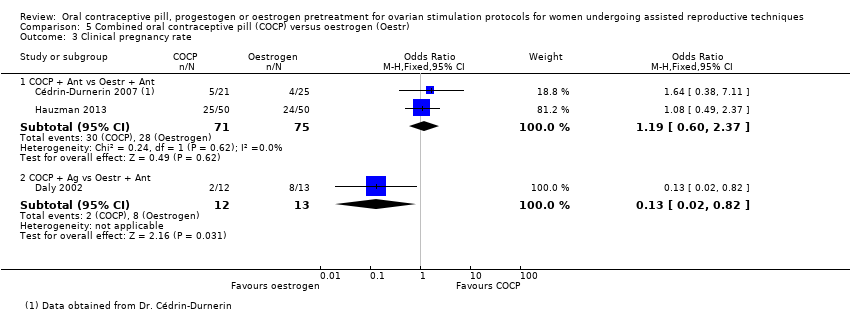
Comparison 5 Combined oral contraceptive pill (COCP) versus oestrogen (Oestr), Outcome 3 Clinical pregnancy rate.

Comparison 5 Combined oral contraceptive pill (COCP) versus oestrogen (Oestr), Outcome 4 Number of oocytes retrieved.

Comparison 5 Combined oral contraceptive pill (COCP) versus oestrogen (Oestr), Outcome 5 Days of gonadotropin treatment.
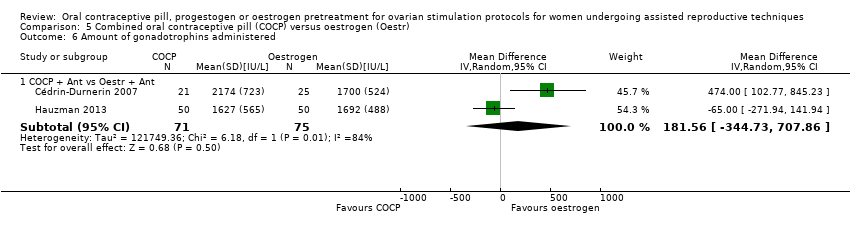
Comparison 5 Combined oral contraceptive pill (COCP) versus oestrogen (Oestr), Outcome 6 Amount of gonadotrophins administered.

Comparison 6 Progestogen (Prog) versus oestrogen (Oestr), Outcome 1 Live birth or ongoing pregnancy.

Comparison 6 Progestogen (Prog) versus oestrogen (Oestr), Outcome 2 Clinical pregnancy rate.

Comparison 6 Progestogen (Prog) versus oestrogen (Oestr), Outcome 3 Number of oocytes retrieved.
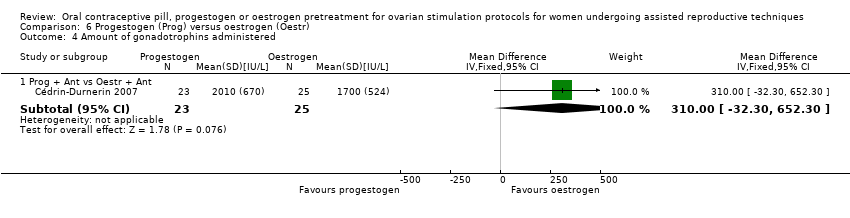
Comparison 6 Progestogen (Prog) versus oestrogen (Oestr), Outcome 4 Amount of gonadotrophins administered.
| Combined oral contraceptive pill compared to no pretreatment for ovarian stimulation protocols for women undergoing assisted reproductive techniques | ||||||
| Population: women undergoing ART Settings: ART clinic Intervention: COCP Comparison: no pretreatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk1 | Corresponding risk | |||||
| No pretreatment | COCP | |||||
| Live birth or ongoing pregnancy (COCP + Ant vs Ant) | 270 per 1000 | 215 per 1000 | OR 0.74 | 1335 | ⊕⊕⊕⊝ | ‐ |
| Live birth or ongoing pregnancy (COCP + Ant vs Ag) | 296 per 1000 | 273 per 1000 | OR 0.89 | 724 | ⊕⊕⊕⊝ | ‐ |
| Pregnancy loss (COCP + Ant vs Ant) | 64 per 1000 | 85 per 1000 | OR 1.36 | 868 | ⊕⊕⊕⊝ | ‐ |
| Pregnancy loss (COCP + Ant vs Ag) | 103 per 1000 | 44 per 1000 | OR 0.40 | 780 | ⊕⊕⊕⊝ | ‐ |
| Multiple pregnancy rate (COCP + Ant vs Ant) | 47 per 1000 | 98 per 1000 | OR 2.21 | 125 | ⊕⊕⊝⊝ | ‐ |
| Multiple pregnancy rate (COCP + Ant vs Ag) | 147 per 1000 | 189 per 1000 | OR 1.36 | 546 | ⊕⊕⊕⊝ | ‐ |
| OHSS rate (COCP + Ant vs Ant) | 16 per 1000 | 16 per 1000 (4 to 52) | OR 0.98 (0.28 to 3.40) | 642 (2 studies) | ⊕⊕⊝⊝ | ‐ |
| OHSS rate (COCP + Ant vs Ag) | 55 per 1000 | 35 per 1000 (11 to 102) | OR 0.63 (0.20 to 1.96) | 290 (2 studies) | ⊕⊕⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Mean baseline risk of control group. | ||||||
| Progestogen compared to placebo or no pretreatment for ovarian stimulation protocols for women undergoing assisted reproductive techniques | ||||||
| Patient or population: ovarian stimulation protocols for women undergoing ART Settings: Intervention: progestogen Comparison: placebo or no pretreatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk1 | Corresponding risk | |||||
| Placebo or no pretreatment | Prog | |||||
| Live birth or ongoing pregnancy (Prog + Ag vs Ag) | 170 per 1000 | 217 per 1000 | OR 1.35 | 222 | ⊕⊕⊝⊝ | ‐ |
| Live birth or ongoing pregnancy (Prog + Ant vs Ant) | 292 per 1000 | 217 per 1000 | OR 0.67 | 47 | ⊕⊕⊝⊝ | ‐ |
| Pregnancy loss (Prog + Ag vs Ag) | 36 per 1000 | 78 per 1000 | OR 2.26 | 222 | ⊕⊕⊝⊝ | ‐ |
| Pregnancy loss (Prog + Ant vs Ant) | 208 per 1000 | 86 per 1000 | OR 0.36 | 47 | ⊕⊕⊝⊝ | ‐ |
| Multiple pregnancy rate (Prog + Ag vs Ag) | No data available | ‐ | ‐ | |||
| Multiple pregnancy rate (Prog + Ant vs Ant) | 42 per 1000 | 44 per 1000 | OR 1.05 | 47 | ⊕⊕⊝⊝ | ‐ |
| OHSS rate (Prog + Ag vs Ag) | No data available | ‐ | ‐ | |||
| OHSS rate (Prog + Ant vs Ant) | No data available | ‐ | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Mean baseline risk of control group. | ||||||
| Oestrogencompared to no pretreatment for ovarian stimulation protocols for women undergoing assisted reproductive techniques | ||||||
| Patient or population: ovarian stimulation protocols for women undergoing ART Settings: Intervention: oestrogen Comparison: no pretreatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk1 | Corresponding risk | |||||
| No pretreatment | Oestr | |||||
| Live birth or ongoing pregnancy (Oestr + Ant vs Ant) | 299 per 1000 | 252 per 1000 | OR 0.79 | 502 | ⊕⊕⊕⊝ | ‐ |
| Live birth or ongoing pregnancy (Oestr + Ant vs Ag) | 350 per 1000 | 322 per 1000 | OR 0.88 | 242 | ⊕⊝⊝⊝ | ‐ |
| Pregnancy loss (Oestr + Ant vs Ant) | 208 per 1000 | 40 per 1000 | OR 0.16 | 49 | ⊕⊝⊝⊝ | ‐ |
| Pregnancy loss (Oestr + Ant vs Ag) | 72 per 1000 | 110 per 1000 | OR 1.59 | 220 | ⊕⊝⊝⊝ | ‐ |
| Multiple pregnancy rate (Oestr + Ant vs Ant) | No data available | ‐ | ‐ | |||
| Multiple pregnancy rate (Oestr + Ant vs Ag) | Not calculable ‐ see comment | OR 2.24 | 22 | ⊕⊝⊝⊝ | Only 2 events (both in oestrogen group) | |
| OHSS rate (Oestr + Ant vs Ant) | No data available | ‐ | ‐ | |||
| OHSS rate (Oestr + Ant vs Ag) | 18 per 1000 | 27 per 1000 (5 to 147) | OR 1.54 (0.25 to 9.42) | 220 (1 study) | ⊕⊝⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Mean baseline risk of control group. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 COCP + antagonist (Ant) vs Ant | 6 | 1335 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.58, 0.95] |
| 1.2 COCP + Ant vs agonist (Ag) | 4 | 724 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.64, 1.25] |
| 1.3 COCP + Ant vs Ant, low response | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.61, 4.79] |
| 1.4 COCP + Ant vs Ag, low response | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.43, 2.98] |
| 2 Pregnancy loss Show forest plot | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 COCP + Ant vs Ant | 5 | 868 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.82, 2.26] |
| 2.2 COCP + Ant vs Ag | 5 | 780 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.22, 0.72] |
| 2.3 COCP + Ant vs Ant, low response | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.18, 23.59] |
| 2.4 COCP + Ant vs Ag, low response | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.13, 7.47] |
| 3 Clinical pregnancy rate Show forest plot | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 COCP + Ant vs Ant | 5 | 740 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.63, 1.15] |
| 3.2 COCP + Ant vs Ag | 4 | 546 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.59, 1.20] |
| 3.3 COCP + Ant vs Ant, low response | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.69, 4.97] |
| 3.4 COCP + Ant vs Ag, low response | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.44, 2.83] |
| 4 Multiple pregnancy rate Show forest plot | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 COCP + Ant vs Ant | 2 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.21 [0.53, 9.26] |
| 4.2 COCP + Ant vs Ag | 4 | 546 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.85, 2.19] |
| 4.3 COCP + Ant vs Ant, low response | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.11 [0.36, 12.24] |
| 4.4 COCP + Ant vs Ag, low response | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.29, 6.56] |
| 5 Ovarian hyperstimulation syndrome rate Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 COCP + Ant vs Ant | 2 | 642 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.28, 3.40] |
| 5.2 COCP + Ant vs Ag | 2 | 290 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.20, 1.96] |
| 6 Number of oocytes retrieved Show forest plot | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7 Days of gonadotrophin treatment Show forest plot | 8 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 COCP + Ant vs Ant | 6 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 COCP + Ant vs Ag | 4 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 COCP + Ant vs Ant, low response | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 COCP + Ant vs Ag, low response | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Amount of gonadotrophins administered Show forest plot | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 COCP + Ant vs Ant | 7 | 1275 | Mean Difference (IV, Fixed, 95% CI) | 190.10 [134.91, 245.28] |
| 8.2 COCP + Ant vs Ag | 3 | 496 | Mean Difference (IV, Fixed, 95% CI) | 9.96 [‐104.09, 124.02] |
| 8.3 COCP + Ant vs Ant, low response | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 20.0 [‐165.39, 205.39] |
| 8.4 COCP + Ant vs Ag, low response | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐349.0 [‐537.92, ‐160.08] |
| 9 Ovarian cyst formation rate Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 COCP + Ant vs Ant | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.08, 2.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Progestogen (Prog) + agonist (Ag) vs Ag | 2 | 222 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.69, 2.65] |
| 1.2 Prog + antagonist (Ant) vs Ant | 1 | 47 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.18, 2.54] |
| 1.3 Prog + gonadotrophin (Gon) vs Gon | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.09, 4.23] |
| 2 Pregnancy loss Show forest plot | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Prog + Ag vs Ag | 2 | 222 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.26 [0.67, 7.55] |
| 2.2 Prog + Ant vs Ant | 1 | 47 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.06, 2.09] |
| 2.3 Prog + Gon vs Gon | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 17.12] |
| 3 Clinical pregnancy rate Show forest plot | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Prog + Ag vs Ag | 3 | 374 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.20, 3.28] |
| 3.2 Prog + Ant vs Ant | 1 | 47 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.16, 1.71] |
| 3.3 Prog + Gon vs Gon | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.14, 3.64] |
| 4 Multiple pregnancy rate Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Prog + Ant vs Ant | 1 | 47 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.06, 17.76] |
| 5 Number of oocytes retrieved Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Prog + Ag vs Ag | 2 | 222 | Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐2.07, 1.02] |
| 5.2 Prog + Ant vs Ant | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | 2.70 [‐0.98, 6.38] |
| 5.3 Prog + Gon vs Gon | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.57, 0.57] |
| 6 Days of gonadotrophin treatment Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Prog + Ag vs Ag | 2 | 222 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.30, 0.52] |
| 7 Amount of gonadotrophins administered Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Prog + Ant vs Ant | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | 276.0 [‐75.53, 627.53] |
| 8 Ovarian cyst formation rate Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Prog + Ag vs Ag | 3 | 374 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.08, 0.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Oestrogen (Oestr) + antagonist (Ant) vs Ant | 2 | 502 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.53, 1.17] |
| 1.2 Oestr + Ant vs agonist (Ag) | 2 | 242 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.51, 1.50] |
| 2 Pregnancy loss Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Oestr + Ant vs Ant | 1 | 49 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.47] |
| 2.2 Oestr + Ant vs Ag | 1 | 220 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.62, 4.06] |
| 3 Clinical pregnancy rate Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Oestr + Ant vs Ant | 4 | 688 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.66, 1.24] |
| 3.2 Oestr + Ant vs Ag | 2 | 242 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.45, 1.27] |
| 4 Multiple pregnancies Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Oestr + Ant vs Ag | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.24 [0.09, 53.59] |
| 5 Ovarian hyperstimulation syndrome rate Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Oestr + Ant vs Ag | 1 | 220 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.25, 9.42] |
| 6 Number of oocytes retrieved Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Oestr + Ant vs Ant | 2 | 139 | Mean Difference (IV, Fixed, 95% CI) | 2.23 [0.71, 3.75] |
| 6.2 Oestr + Ant vs Ag | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐4.47, 5.27] |
| 7 Days of gonadotrophin treatment Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Oestr + Ant vs Ant | 2 | 529 | Mean Difference (IV, Fixed, 95% CI) | 0.83 [0.58, 1.08] |
| 7.2 Oestr + Ant vs Ag | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐4.07, ‐0.93] |
| 8 Amount of gonadotrophins administered Show forest plot | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Oestr + Ant vs Ant | 4 | 668 | Mean Difference (IV, Fixed, 95% CI) | 168.35 [111.53, 225.17] |
| 8.2 Oestr + Ant vs Ag | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐16.0 [‐470.12, 438.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 COCP + antagonist (Ant) vs progestogen (Prog) + Ant | 1 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.12, 2.89] |
| 2 Pregnancy loss Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 COCP + Ant vs Prog + Ant | 1 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.14, 8.64] |
| 3 Clinical pregnancy rate Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 COCP + Ant vs Prog + Ant | 1 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.19, 2.73] |
| 4 Multiple pregnancy rate Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 COCP + Ant vs Prog + Ant | 1 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.32 [0.19, 27.59] |
| 5 Number of oocytes retrieved Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 COCP + Ant vs Prog + Ant | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [‐3.24, 6.04] |
| 6 Amount of gonadotrophins administered Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 COCP + Ant vs Prog + Ant | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 164.0 [‐249.03, 577.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 COCP + antagonist (Ant) vs Oestr + Ant | 2 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.54, 2.29] |
| 1.2 COCP + agonist (Ag) vs Oestr + Ant | 1 | 25 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.01, 0.79] |
| 2 Pregnancy loss Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 COCP + Ag vs Oestr + Ant | 1 | 25 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.06, 19.63] |
| 3 Clinical pregnancy rate Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 COCP + Ant vs Oestr + Ant | 2 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.60, 2.37] |
| 3.2 COCP + Ag vs Oestr + Ant | 1 | 25 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.02, 0.82] |
| 4 Number of oocytes retrieved Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 COCP + Ant vs Oestr + Ant | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐3.59, 5.39] |
| 5 Days of gonadotropin treatment Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 COCP + Ant vs Oestr + Ant | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.23, 0.03] |
| 6 Amount of gonadotrophins administered Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 COCP + Ant vs Oestr + Ant | 2 | 146 | Mean Difference (IV, Random, 95% CI) | 181.56 [‐344.73, 707.86] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Prog + antagonist (Ant) vs Oestr + Ant | 1 | 48 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.43, 9.70] |
| 2 Clinical pregnancy rate Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Prog + Ant vs Oestr + Ant | 1 | 48 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.30 [0.57, 9.22] |
| 3 Number of oocytes retrieved Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Prog + Ant vs Oestr + Ant | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐4.55, 3.55] |
| 4 Amount of gonadotrophins administered Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Prog + Ant vs Oestr + Ant | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 310.0 [‐32.30, 652.30] |

