Traitement par metformine administré avant et pendant une FIV ou une IICS chez les femmes atteintes du syndrome ovarien polykystique
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Generation of the allocation sequence: not reported Allocation concealment method: not reported Blinding method: not reported Number and reasons for withdrawals: not reported ITT analysis: yes The authors did not provide additional information about allocation concealment and generation of allocation sequence methods Prospective randomised trial | |
| Participants | 40 PCOS participants were randomised (20 in the metformin group and 20 in the placebo group) | |
| Interventions | Group A was pretreated for 2 months with metformin 1.5 g/day until the embryo transfer day Protocol for controlled ovarian hyperstimulation: short‐protocol GnRH‐antagonist (cetrorelix acetate, Cetrotide®) with step up rec‐FSH (Gonal F® ‐ starting dose 150 IU). GnRH‐antagonist, cetrorelix acetate 0.25 mg/day, was started when the leading follicle reached 14 mm diameter on ultrasound scan and stopped on the day of hCG | |
| Outcomes | a) Number of ampoules of rec‐FSH | |
| Notes | Country of the study: Italy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Reasons for withdrawals were not reported |
| Selective reporting (reporting bias) | Unclear risk | Live birth and clinical pregnancy rates were not assessed |
| Other bias | High risk | No power calculation. Neither the causes of infertility nor the baseline characteristics of the 2 groups were reported |
| Methods | Generation of the allocation sequence: table of random numbers Allocation concealment method: sealed, opaque envelopes serially numbered Blinding method: does not apply (open‐label cross‐over trial) Number and reasons for withdrawals: not reported ITT analysis: yes Prospective, open‐label, randomised cross‐over trial. Only data from the pre‐cross‐over phase of this study were considered for meta‐analysis | |
| Participants | 17 PCOS participants were randomised (9 in the metformin group and 8 in the placebo group) | |
| Interventions | Metformin 500 mg tid was started 3 weeks before down‐regulation with GnRH‐agonist began and continued until the day of hCG injection Catheter used for transfer: not reported | |
| Outcomes | Primary outcomes: Secondary outcomes: | |
| Notes | Country of the study: Norway | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate ‐ random numbers table |
| Allocation concealment (selection bias) | Low risk | Adequate ‐ sealed, opaque envelopes serially numbered |
| Blinding (performance bias and detection bias) | High risk | Open‐label cross‐over trial |
| Incomplete outcome data (attrition bias) | Low risk | There were no withdrawals in the phase analysed (pre‐cross‐over phase) |
| Selective reporting (reporting bias) | Unclear risk | Live birth rate was not evaluated |
| Other bias | Unclear risk | No power calculation. The causes of infertility were not reported |
| Methods | Generation of the allocation sequence: computer randomisation system Allocation concealment method: codes were kept by a third party in the pharmacy department Blinding method: yes Number and reasons for withdrawals: reported ITT analysis: yes Prospective, randomised, double‐blind, placebo‐controlled trial | |
| Participants | 73 PCOS participants were randomised (37 in the metformin group and 36 in the placebo group) ITT analysis was performed for primary outcomes | |
| Interventions | Metformin 500 mg bid (gradually increasing the dose during the first 2 weeks) for at least 16 weeks until the day of hCG injection Catheter used for transfer: not reported | |
| Outcomes | Primary outcomes: Secondary outcomes: h) incidence of OHSS | |
| Notes | Country of the study: Norway | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate ‐ computer randomisation system |
| Allocation concealment (selection bias) | Low risk | Adequate ‐ codes kept by a third party in the pharmacy department |
| Blinding (performance bias and detection bias) | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) | Low risk | Number and reasons for withdrawals were reported |
| Selective reporting (reporting bias) | Low risk | All main outcomes were reported |
| Other bias | Low risk | Power calculation was performed. There were no significant differences in the baseline characteristics of the participants between the 2 groups |
| Methods | Generation of the allocation sequence: computer randomisation system Allocation concealment method: codes were kept by a third party in the pharmacy department Blinding method: yes Number and reasons for withdrawals: reported ITT analysis: yes Multi‐centre, prospective, randomised, double‐blind, placebo‐controlled trial | |
| Participants | 150 PCOS participants were randomised (74 in the metformin group and 76 in the placebo group); however 1 participant in the placebo group withdrew her consent just after randomisation The ITT population in the study consisted of 149 women (74 in the metformin group, 75 in the placebo group) b) baseline FSH serum level > 10 IU/l i) alcoholism or drug abuse j) diabetes mellitus | |
| Interventions | The dose of metformin was gradually increased from 500 to 2000 mg per day during the first 2 weeks of treatment for at least 12 weeks prior to controlled ovarian hyperstimulation Catheter used for transfer: not reported | |
| Outcomes | Primary outcomes: Secondary outcomes: a) pregnancy rate b) spontaneous pregnancy rate f) incidence of OHSS g) total dose of FSH given during stimulation h) number of days of gonadotrophins i) miscarriage rate j) incidence of side effects | |
| Notes | Country of the study: Norway | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate ‐ computer randomisation system |
| Allocation concealment (selection bias) | Low risk | Adequate ‐ codes kept by a third party in the pharmacy department |
| Blinding (performance bias and detection bias) | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) | Low risk | Number and reasons for withdrawals were reported |
| Selective reporting (reporting bias) | Low risk | All main outcomes were reported |
| Other bias | Low risk | Power calculation was performed. There were no significant differences in the baseline characteristics of the participants between the 2 groups |
| Methods | Generation of the allocation sequence: computer randomisation system Allocation concealment method: not reported Blinding method: yes Number and reasons for withdrawals: reported ITT analysis: no Prospective, randomised, double‐blind, placebo‐controlled trial | |
| Participants | 110 PCOS participants without concomitant causes of infertility | |
| Interventions | Metformin 850 mg bid or tid (according to BMI) for 8 weeks before their first ICSI cycle, through the luteal phase and until a positive pregnancy test Catheter used for transfer: not reported | |
| Outcomes | a) Number of days of gonadotrophins | |
| Notes | Country of the study: Turkey | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate ‐ computer randomisation system |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) | High risk | Intention‐to‐treat (ITT) was not performed |
| Selective reporting (reporting bias) | Unclear risk | Live birth rate was not reported |
| Other bias | Low risk | Power calculation was not performed. There were no significant differences in the baseline characteristics of the participants between the 2 groups |
| Methods | Generation of the allocation sequence: computer randomisation system Allocation concealment method: random allocation sequence was concealed in the central pharmacy of the University of Catanzaro Blinding method: yes Number and reasons for withdrawals: no participants dropped out ITT analysis: yes Prospective, randomised, double‐blind, placebo‐controlled trial | |
| Participants | Number of eligible cycles: 120 infertile women with PCOS who had a history of 1 previous cancelled cycle due to a high risk of OHSS or history of a moderate or severe case of OHSS during their previous IVF cycle c) BMI > 30 kg/m2 e) hypothyroidism f) hyperprolactinaemia h) Cushing's syndrome i) nonclassic congenital adrenal hyperplasia j) alcohol abuse k) current or previous: a wash‐out period of at least 6 months without use of any antidiabetic, obesity or hormonal drugs except those used during the previous IVF cycle l) male factor infertility | |
| Interventions | Metformin 500 mg tid and placebo tablets were started on the same day that down‐regulation with GnRH‐agonist began and continued until a positive pregnancy test was obtained or menstrual bleeding appeared Catheter used for transfer: not reported | |
| Outcomes | Primary outcomes: Secondary outcomes: a) live birth rate per woman b) clinical pregnancy rate g) total dose of FSH given during stimulation h) number of days of gonadotrophins | |
| Notes | Country of the study: Italy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate ‐ computer randomisation system |
| Allocation concealment (selection bias) | Low risk | Random allocation sequence was concealed in the central pharmacy |
| Blinding (performance bias and detection bias) | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) | Low risk | There were no withdrawals |
| Selective reporting (reporting bias) | Low risk | All main outcomes were reported |
| Other bias | High risk | There is a discrepancy in the data in the published study: in both the Metformin group and the placebo group the clinical pregnancy rate is lower than the live birth rate (pregnancy 26/60, 24/60; live birth 29/60, 27/60). We have attempted to contact the first author (emailed 8/19/14) but have not received a response. There were no significant differences in the baseline characteristics of the participants between the 2 groups |
| Methods | Generation of allocation sequence: not reported Allocation concealment method: not reported Blinding method: yes Number and reasons for withdrawals: there were no withdrawals ITT analysis: yes Prospective, randomised, double‐blind, placebo‐controlled trial | |
| Participants | 66 clomiphene citrate‐resistant PCOS participants were randomised (34 in the metformin group and 32 in the placebo group) There were no withdrawals All participants were required to have a normal uterine cavity | |
| Interventions | Metformin 850 mg tid for a month before their first ICSI cycle, through the luteal phase and until a positive pregnancy test. If the test was positive metformin was continued until 12 weeks of gestation | |
| Outcomes | a) Number of days of gonadotrophins | |
| Notes | Country of the study: Jordan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) | High risk | Single‐blinded |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat (ITT) was performed |
| Selective reporting (reporting bias) | Unclear risk | Live birth rate was not reported |
| Other bias | Low risk | Power calculation was performed. There were no significant differences in the baseline characteristics of the participants between the 2 groups |
| Methods | Generation of allocation sequence: reported Allocation concealment method: reported Blinding method: yes Number and reasons for withdrawals: reported ITT analysis: yes Prospective, randomised, double‐blind, placebo‐controlled trial | |
| Participants | Diagnosis of PCOS followed the Rotterdam criteria (ESHRE/ASRM) 5 cycles in the metformin group and 2 in the placebo group were abandoned due to poor response | |
| Interventions | Metformin 850 mg bid from the first day of down‐regulation GnRH‐agonist to the day of oocyte retrieval Protocol for controlled ovarian hyperstimulation: long luteal phase pituitary down‐regulation using the GnRH analogue nafarelin 600 μg daily (Synarel®) with step up rec‐FSH (Puregon® starting dose of 100 IU). hCG (Profasi® 10000 IU) was administered when there were more than 3 follicles over 17 mm in diameter Assisted reproductive technology (ART): IVF or ICSI (4 hours after oocyte retrieval) Embryo transfer: maximum of 2 embryos were transferred per participant on day 2 after follicle puncture under abdominal US guidance | |
| Outcomes | Primary outcome: Secondary outcomes: | |
| Notes | Country of the study: United Kingdom | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate ‐ random numbers table |
| Allocation concealment (selection bias) | Low risk | Adequate ‐ codes kept by a third party in the trial office |
| Blinding (performance bias and detection bias) | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) | Low risk | Reasons for withdrawals were reported. ITT analysis was performed |
| Selective reporting (reporting bias) | Low risk | All main outcomes were reported |
| Other bias | Low risk | Power calculation was performed. There were no significant differences in the baseline characteristics of the participants between the 2 groups |
| Methods | Generation of the allocation sequence: not reported Allocation concealment method: not reported Blinding method: no Number and reasons for withdrawals: reported ITT analysis: yes Prospective randomised controlled trial | |
| Participants | 172 participants were selected but only 141 were randomised because they met the inclusion criteria for PCOS according to the Rotterdam criteria (ESHRE/ASRM) | |
| Interventions | Metformin 500 mg bid from the first day of ovulation induction to the day of oocyte retrieval | |
| Outcomes | a) Incidence of OHSS | |
| Notes | Country of the study: Czech Republic | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) | High risk | Not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Reasons for withdrawals were reported. However, 4 participants were excluded due to poor response to ovulation induction after randomisation and were not included in the ITT by study |
| Selective reporting (reporting bias) | Unclear risk | Live birth rate was not assessed |
| Other bias | Unclear risk | No power calculation was performed. Infertility factors were not reported |
AMH: anti‐Müllerian hormone
ART: assisted reproductive technology
bid: twice a day
BMI: body mass index
CI: confidence interval
E2: oestradiol
FSH: follicle‐stimulating hormone
GnRH: gonadotrophin‐releasing hormone
hCG: human chorionic gonadotrophin
ICSI: intracytoplasmic sperm injection
ITT: intention‐to‐treat
IU: international units
IVF: in vitro fertilisation
OCP: oral contraceptive pill
OHSS: ovarian hyperstimulation syndrome
OR: odds ratio
PCOS: polycystic ovarian syndrome
RCT: randomised controlled trial
SC: subcutaneous
SD: standard deviation
SE: standard error
SHBG: sex hormone‐binding globulin
tid: three times a day
US: ultrasonography
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not a randomised controlled trial | |
| Data were not properly reported. The study did not state how many participants withdrew from each group (1 and 2). Moreover, the author does not present the means and standard deviations (SD) nor the standard errors (SE) | |
| The study was excluded because of data irregularities (summarised reported data were not consistent with table data). We were not successful in contacting the authors to clarify the queries | |
| Control group was treated with oral contraceptives instead of placebo or no treatment | |
| Participants were poor responder women | |
| Participants specifically undergoing ICSI were not randomised separately | |
| It is not a randomised controlled trial | |
| It is not a randomised controlled trial | |
| The study is not a randomised controlled trial | |
| Participants undergoing ovulation induction cycles; not IVF or ICSI cycles |
ICSI: intracytoplasmic sperm injection
IVF: in vitro fertilisation
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised controlled trial |
| Participants | 69 PCOS participants were randomised (39 in the metformin group and 38 in the placebo group) |
| Interventions | Metformin versus placebo |
| Outcomes | Live birth rate; fertilisation rate; number of follicles; number of oocytes; number of embryos transferred; clinical pregnancy rate per woman; incidence of OHSS |
| Notes | Information retrieved from abstract of congress. Authors were contacted to give more information. We are awaiting more details to classify the study |
PCOS: polycystic ovarian syndrome
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||
| 1 Live birth rate per woman Show forest plot | 5 | 551 | Odds Ratio (M‐H, Random, 95% CI) | 1.39 [0.81, 2.40] | ||||||||||||||||
| Analysis 1.1 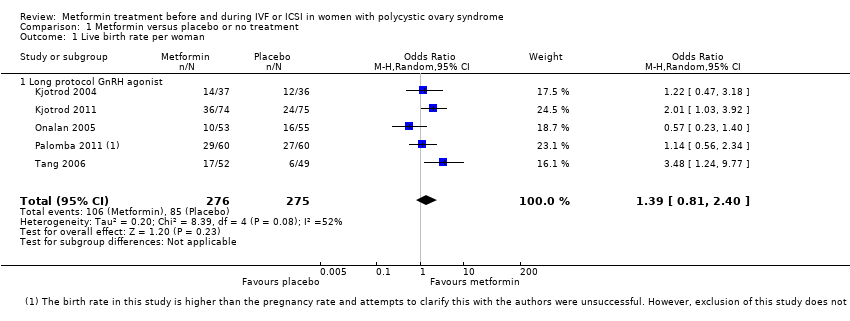 Comparison 1 Metformin versus placebo or no treatment, Outcome 1 Live birth rate per woman. | ||||||||||||||||||||
| 1.1 Long protocol GnRH agonist | 5 | 551 | Odds Ratio (M‐H, Random, 95% CI) | 1.39 [0.81, 2.40] | ||||||||||||||||
| 2 Clinical pregnancy rate per woman Show forest plot | 8 | 775 | Odds Ratio (M‐H, Random, 95% CI) | 1.52 [1.07, 2.15] | ||||||||||||||||
| Analysis 1.2  Comparison 1 Metformin versus placebo or no treatment, Outcome 2 Clinical pregnancy rate per woman. | ||||||||||||||||||||
| 2.1 Long protocol GnRH agonist | 8 | 775 | Odds Ratio (M‐H, Random, 95% CI) | 1.52 [1.07, 2.15] | ||||||||||||||||
| 3 Incidence of OHSS per woman Show forest plot | 8 | 798 | Odds Ratio (M‐H, Random, 95% CI) | 0.29 [0.18, 0.49] | ||||||||||||||||
| Analysis 1.3  Comparison 1 Metformin versus placebo or no treatment, Outcome 3 Incidence of OHSS per woman. | ||||||||||||||||||||
| 3.1 Long protocol GnRH agonist | 7 | 758 | Odds Ratio (M‐H, Random, 95% CI) | 0.29 [0.16, 0.51] | ||||||||||||||||
| 3.2 Short protocol GnRH antagonist | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.30 [0.03, 3.15] | ||||||||||||||||
| 4 Miscarriage rate per woman Show forest plot | 6 | 521 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.43, 1.37] | ||||||||||||||||
| Analysis 1.4  Comparison 1 Metformin versus placebo or no treatment, Outcome 4 Miscarriage rate per woman. | ||||||||||||||||||||
| 4.1 Long protocol GnRH agonist | 6 | 521 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.43, 1.37] | ||||||||||||||||
| 5 Side effects per woman Show forest plot | 4 | 431 | Odds Ratio (M‐H, Random, 95% CI) | 4.49 [1.88, 10.72] | ||||||||||||||||
| Analysis 1.5 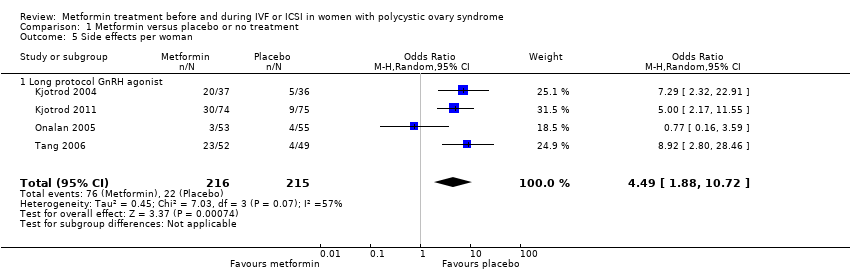 Comparison 1 Metformin versus placebo or no treatment, Outcome 5 Side effects per woman. | ||||||||||||||||||||
| 5.1 Long protocol GnRH agonist | 4 | 431 | Odds Ratio (M‐H, Random, 95% CI) | 4.49 [1.88, 10.72] | ||||||||||||||||
| 6 Number of oocytes retrieved per woman Show forest plot | 8 | 635 | Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐2.02, 0.50] | ||||||||||||||||
| Analysis 1.6  Comparison 1 Metformin versus placebo or no treatment, Outcome 6 Number of oocytes retrieved per woman. | ||||||||||||||||||||
| 6.1 Long protocol with GnRH agonist | 7 | 595 | Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐2.12, 0.79] | ||||||||||||||||
| 6.2 Short protocol with GnRH antagonist | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐3.95, 1.95] | ||||||||||||||||
| 7 Mean total dose of FSH (IU) per woman Show forest plot | 8 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |||||||||||||||||
| Analysis 1.7 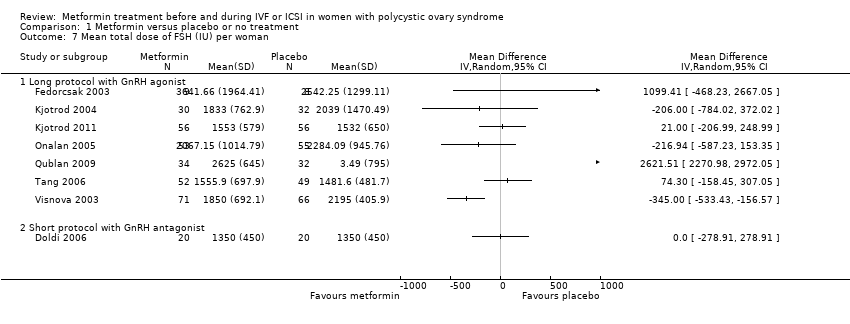 Comparison 1 Metformin versus placebo or no treatment, Outcome 7 Mean total dose of FSH (IU) per woman. | ||||||||||||||||||||
| 7.1 Long protocol with GnRH agonist | 7 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||
| 7.2 Short protocol with GnRH antagonist | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||
| 8 Mean days of gonadotrophin per woman Show forest plot | 8 | 643 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.77, 0.40] | ||||||||||||||||
| Analysis 1.8 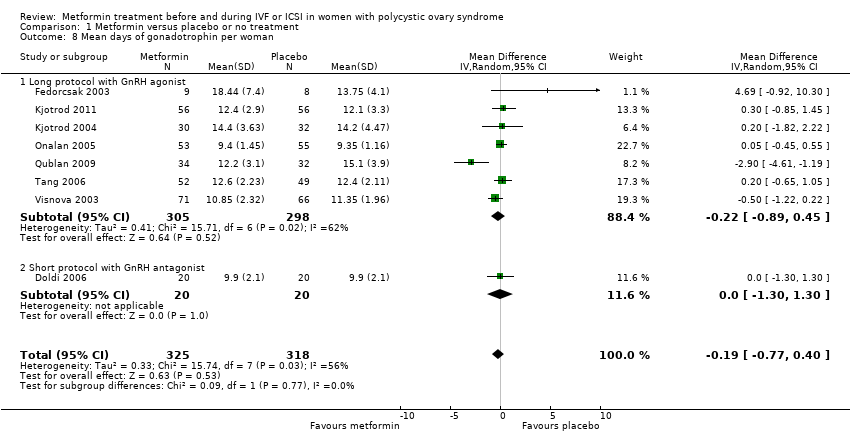 Comparison 1 Metformin versus placebo or no treatment, Outcome 8 Mean days of gonadotrophin per woman. | ||||||||||||||||||||
| 8.1 Long protocol with GnRH agonist | 7 | 603 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.89, 0.45] | ||||||||||||||||
| 8.2 Short protocol with GnRH antagonist | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐1.30, 1.30] | ||||||||||||||||
| 9 Cycle cancellation rate (after ovulation induction) Show forest plot | 6 | 624 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.32, 1.28] | ||||||||||||||||
| Analysis 1.9 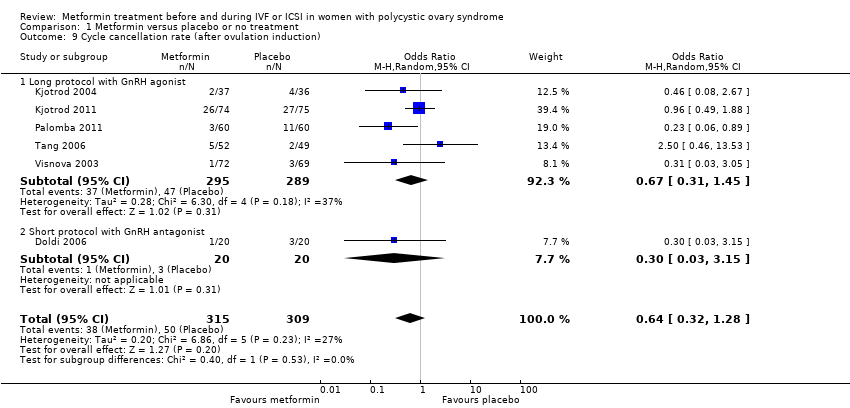 Comparison 1 Metformin versus placebo or no treatment, Outcome 9 Cycle cancellation rate (after ovulation induction). | ||||||||||||||||||||
| 9.1 Long protocol with GnRH agonist | 5 | 584 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.31, 1.45] | ||||||||||||||||
| 9.2 Short protocol with GnRH antagonist | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.30 [0.03, 3.15] | ||||||||||||||||
| 10 Serum oestradiol level (nmol/ l) per woman Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |||||||||||||||||
| Analysis 1.10 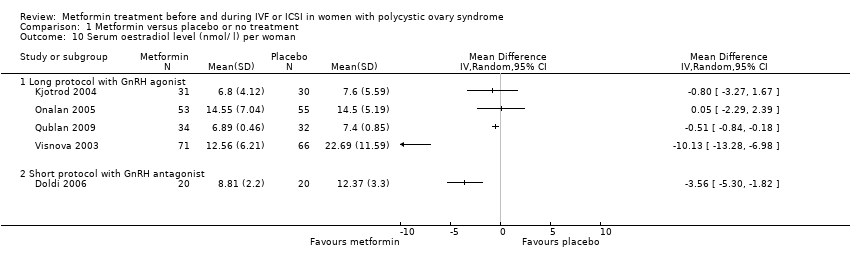 Comparison 1 Metformin versus placebo or no treatment, Outcome 10 Serum oestradiol level (nmol/ l) per woman. | ||||||||||||||||||||
| 10.1 Long protocol with GnRH agonist | 4 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||
| 10.2 Short protocol with GnRH antagonist | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||
| 11 Mean or median serum androgen levels per woman Show forest plot | Other data | No numeric data | ||||||||||||||||||
| Analysis 1.11
Comparison 1 Metformin versus placebo or no treatment, Outcome 11 Mean or median serum androgen levels per woman. | ||||||||||||||||||||
| 12 Mean or median fasting insulin and glucose levels per woman Show forest plot | Other data | No numeric data | ||||||||||||||||||
| Analysis 1.12
Comparison 1 Metformin versus placebo or no treatment, Outcome 12 Mean or median fasting insulin and glucose levels per woman. | ||||||||||||||||||||
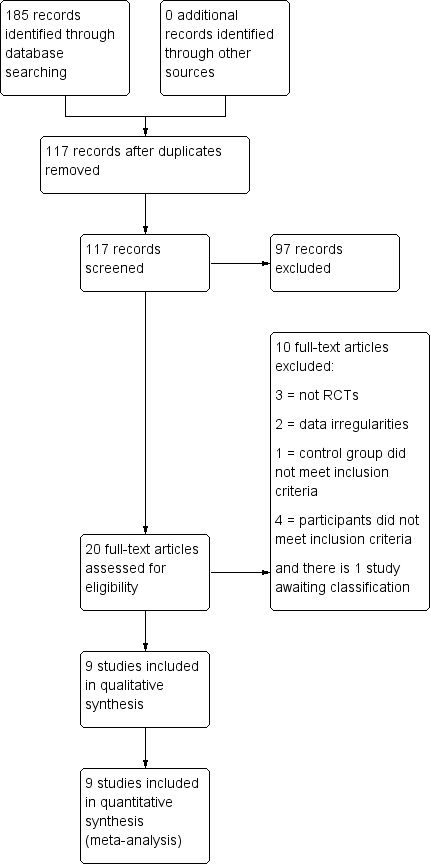
Study flow diagram.

'Risk of bias' graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each methodological quality item for each included study.

Forest plot of comparison: 1 Metformin versus placebo or no treatment, outcome: 1.1 Live birth rate per woman.

Forest plot of comparison: 1 Metformin versus placebo or no treatment, outcome: 1.2 Clinical pregnancy rate per woman.

Forest plot of comparison: 1 Metformin versus placebo or no treatment, outcome: 1.3 Incidence of OHSS per woman.

Comparison 1 Metformin versus placebo or no treatment, Outcome 1 Live birth rate per woman.
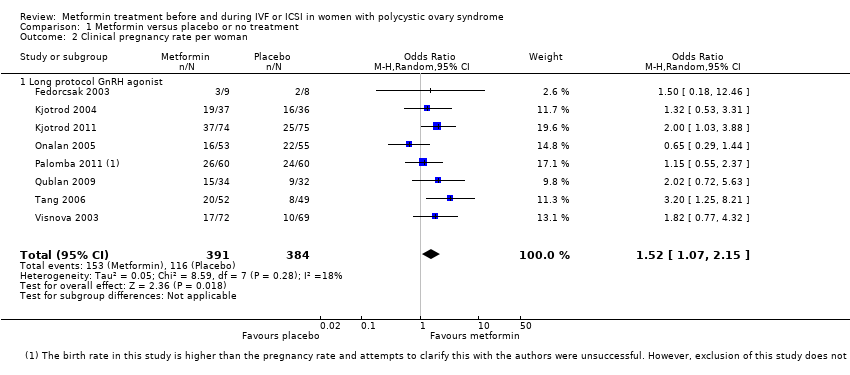
Comparison 1 Metformin versus placebo or no treatment, Outcome 2 Clinical pregnancy rate per woman.
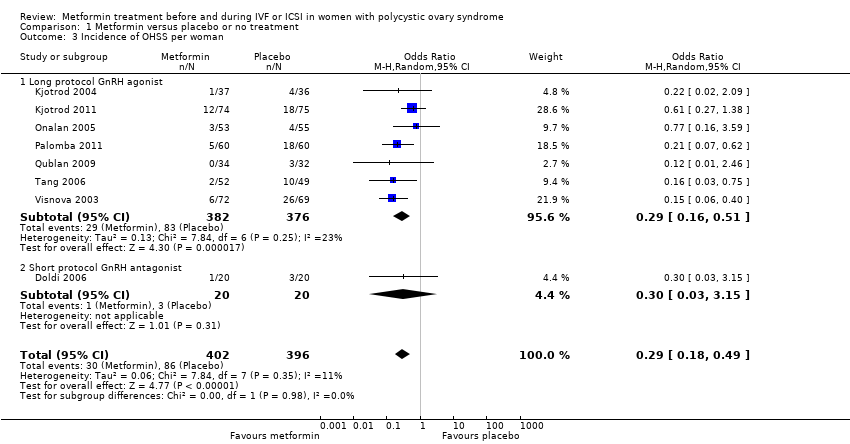
Comparison 1 Metformin versus placebo or no treatment, Outcome 3 Incidence of OHSS per woman.
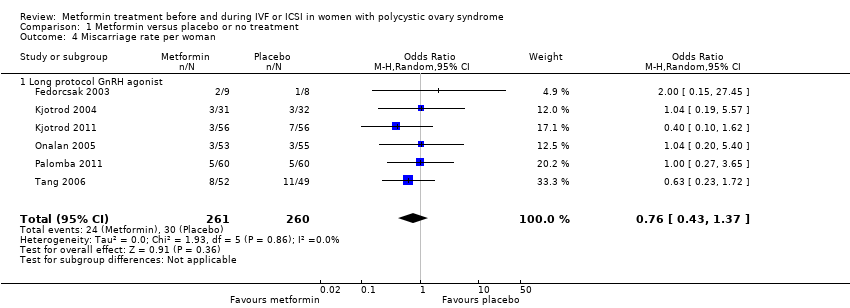
Comparison 1 Metformin versus placebo or no treatment, Outcome 4 Miscarriage rate per woman.
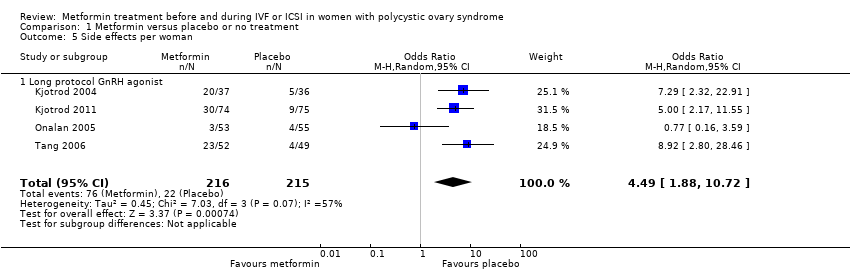
Comparison 1 Metformin versus placebo or no treatment, Outcome 5 Side effects per woman.
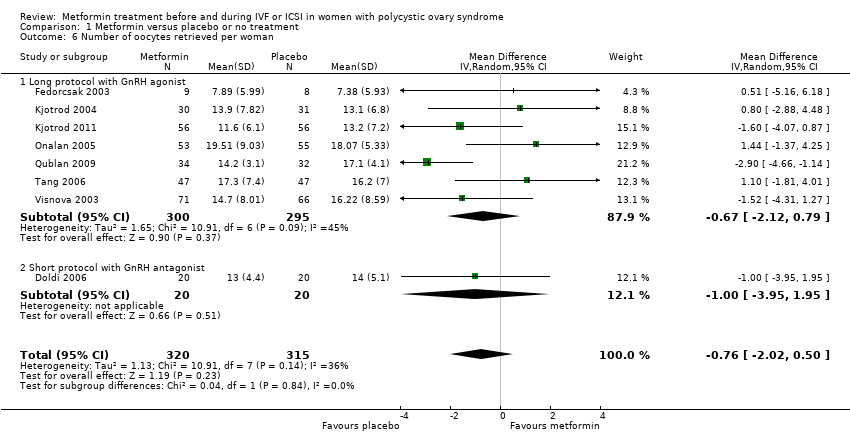
Comparison 1 Metformin versus placebo or no treatment, Outcome 6 Number of oocytes retrieved per woman.

Comparison 1 Metformin versus placebo or no treatment, Outcome 7 Mean total dose of FSH (IU) per woman.

Comparison 1 Metformin versus placebo or no treatment, Outcome 8 Mean days of gonadotrophin per woman.
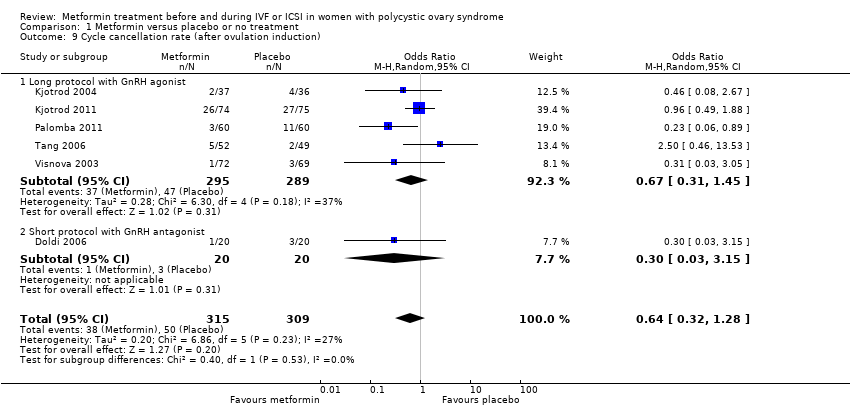
Comparison 1 Metformin versus placebo or no treatment, Outcome 9 Cycle cancellation rate (after ovulation induction).
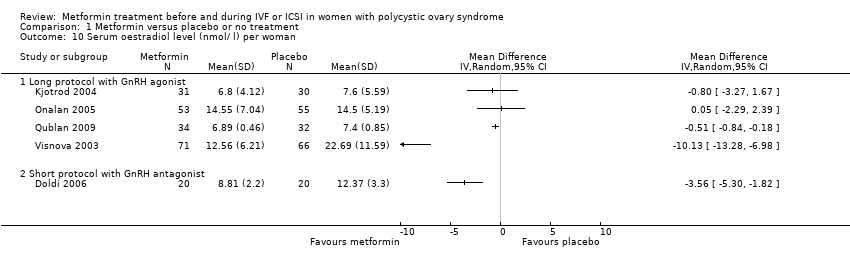
Comparison 1 Metformin versus placebo or no treatment, Outcome 10 Serum oestradiol level (nmol/ l) per woman.
| Study | Results | Metformin | Placebo |
| Onalan 2005 | No significant differences in total testosterone measures from women treated with placebo (P = 0.646) | Median 3.1; range 2.5 to 3.9 | Median 3.1; range 2.4 to 3 |
| Tang 2006 | Testosterone levels did not change significantly in the group taking metformin (P = 0.892); however, participants in the placebo group had a significant increase in testosterone levels (P = 0.040). In the metformin group, on the day of hCG administration, there was a significant decrease in testosterone concentration (P = 0.029) and in the free‐androgen index (P = 0.004) | Baseline geometric mean: 2.03 nmol/l, geometric mean on the day of hCG administration: 1.97 nmol/l. Testosterone concentration (geometric mean: 1.96 nmol/l). Free‐androgen index (geometric mean: 2.43) | Baseline geometric mean: 2.06 nmol/l, geometric mean on the day of hCG administration: 2.52 nmol/l. Testosterone concentration (geometric mean: 2.52 nmol/l). Free‐androgen index (geometric mean: 3.34) |
Comparison 1 Metformin versus placebo or no treatment, Outcome 11 Mean or median serum androgen levels per woman.
| Study | Results | Metformin | Placebo |
| Onalan 2005 | There were no significant changes in the glucose/insulin ratio between groups (P = 0.81) | Median 6; range 2.4 to 8.8 | Median 6; range 3 to 10 |
| Tang 2006 | There were no significant changes in the insulin sensitivity test (QUICKI) between baseline and the day of oocyte retrieval in the metformin group (P = 0.200) and the placebo group (P = 0.572). | Baseline: 0.377 At the day of oocyte retrieval: 0.417 | Baseline: 0.386 At the day of oocyte retrieval: 0.400 |
Comparison 1 Metformin versus placebo or no treatment, Outcome 12 Mean or median fasting insulin and glucose levels per woman.
| Metformin treatment before or during IVF or ICSI for women with polycystic ovary syndrome | ||||||
| Population: Women with polycystic ovary syndrome Control: Placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no treatment | Metformin treatment | |||||
| Live birth rate (per woman) ‐ ITT | 320 per 1000 | 395 per 1000 (276 to 530) | OR 1.39 | 551 | ⊕⊕⊝⊝ | |
| Clinical pregnancy rate (per woman) ‐ ITT | 307 per 1000 | 403 per 1000 | OR 1.52 | 775 | ⊕⊕⊕⊝ | |
| Incidence of OHSS | 270 per 1000 | 97 per 1000 | OR 0.29 | 798 | ⊕⊕⊕⊝ | |
| Miscarriage rate (per woman) | 139 per 1000 | 110 per 1000 | OR 0.76 | 521 | ⊕⊕⊕⊝ | |
| Side effects | 106 per 1000 | 347 per 1000 | OR 4.49 | 431 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Inconsistency: unexplained heterogeneity (I2 = 52%) 2 Imprecision: total number of events is fewer than 300 3Inconsistency: unexplained heterogeneity (I2 = 57%) 4There was a data discrepancy in one of these studies (Palombo 2011). According to the study publication, in both the metformin group and the placebo group the clinical pregnancy rate was lower than the live birth rate. Sensitivity analyses excluding this study yielded an OR of 1.48 (95% CI 0.72 to 3.02) for live birth and 1.61 (95% CI 1.08 to 2.40) for pregnancy, which did not substantially change our findings. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth rate per woman Show forest plot | 5 | 551 | Odds Ratio (M‐H, Random, 95% CI) | 1.39 [0.81, 2.40] |
| 1.1 Long protocol GnRH agonist | 5 | 551 | Odds Ratio (M‐H, Random, 95% CI) | 1.39 [0.81, 2.40] |
| 2 Clinical pregnancy rate per woman Show forest plot | 8 | 775 | Odds Ratio (M‐H, Random, 95% CI) | 1.52 [1.07, 2.15] |
| 2.1 Long protocol GnRH agonist | 8 | 775 | Odds Ratio (M‐H, Random, 95% CI) | 1.52 [1.07, 2.15] |
| 3 Incidence of OHSS per woman Show forest plot | 8 | 798 | Odds Ratio (M‐H, Random, 95% CI) | 0.29 [0.18, 0.49] |
| 3.1 Long protocol GnRH agonist | 7 | 758 | Odds Ratio (M‐H, Random, 95% CI) | 0.29 [0.16, 0.51] |
| 3.2 Short protocol GnRH antagonist | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.30 [0.03, 3.15] |
| 4 Miscarriage rate per woman Show forest plot | 6 | 521 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.43, 1.37] |
| 4.1 Long protocol GnRH agonist | 6 | 521 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.43, 1.37] |
| 5 Side effects per woman Show forest plot | 4 | 431 | Odds Ratio (M‐H, Random, 95% CI) | 4.49 [1.88, 10.72] |
| 5.1 Long protocol GnRH agonist | 4 | 431 | Odds Ratio (M‐H, Random, 95% CI) | 4.49 [1.88, 10.72] |
| 6 Number of oocytes retrieved per woman Show forest plot | 8 | 635 | Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐2.02, 0.50] |
| 6.1 Long protocol with GnRH agonist | 7 | 595 | Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐2.12, 0.79] |
| 6.2 Short protocol with GnRH antagonist | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐3.95, 1.95] |
| 7 Mean total dose of FSH (IU) per woman Show forest plot | 8 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 Long protocol with GnRH agonist | 7 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Short protocol with GnRH antagonist | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Mean days of gonadotrophin per woman Show forest plot | 8 | 643 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.77, 0.40] |
| 8.1 Long protocol with GnRH agonist | 7 | 603 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.89, 0.45] |
| 8.2 Short protocol with GnRH antagonist | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐1.30, 1.30] |
| 9 Cycle cancellation rate (after ovulation induction) Show forest plot | 6 | 624 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.32, 1.28] |
| 9.1 Long protocol with GnRH agonist | 5 | 584 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.31, 1.45] |
| 9.2 Short protocol with GnRH antagonist | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.30 [0.03, 3.15] |
| 10 Serum oestradiol level (nmol/ l) per woman Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.1 Long protocol with GnRH agonist | 4 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Short protocol with GnRH antagonist | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Mean or median serum androgen levels per woman Show forest plot | Other data | No numeric data | ||
| 12 Mean or median fasting insulin and glucose levels per woman Show forest plot | Other data | No numeric data | ||

