Tratamiento con metformina antes y durante la FIV o la ICSI en pacientes con síndrome de ovario poliquístico
Appendices
Appendix 1. Search strategies
Database: Cochrane Menstrual Disorders and Subfertility Group Specialised Register
Search strategy
Keywords CONTAINS "PCOS" or "polycystic ovary syndrome" or "Polycystic Ovary Syndrome" or Title CONTAINS "PCOS" or "polycystic ovary syndrome" or "Polycystic Ovary Syndrome"
AND
Keywords CONTAINS "IVF" or "in‐vitro fertilisation " or "in vitro fertilization" or "ICSI" or "intracytoplasmic morphologically selected sperm injection" or "intracytoplasmic sperm injection" or "Embryo Transfer" or "ovulation" or "ovarian stimulation" or Title CONTAINS "IVF" or "in‐vitro fertilisation " or "in vitro fertilization" or "ICSI" or "intracytoplasmic morphologically selected sperm injection" or "intracytoplasmic sperm injection" or "Embryo Transfer" or "ovulation" or "ovarian stimulation"
AND
Keywords CONTAINS "metformin" or Title CONTAINS "metformin"
Database: MEDLINE via OVID <1980 to 20 October 2014>
Search strategy
1 exp Polycystic Ovary Syndrome/ (10671)
2 Polycystic Ovar$.tw. (11225)
3 (PCOS or PCOD).tw. (6980)
4 (stein‐leventhal or leventhal).tw. (694)
5 (ovar$ adj (scelerocystic or polycystic or degeneration)).tw. (76)
6 or/1‐5 (13885)
7 exp metformin/ (7654)
8 metformin.tw. (10249)
9 (dimethylbiguanidium or dimethylguanylguanidine or glucophage or glucovance).tw. (112)
10 or/7‐9 (11652)
11 exp fertilization in vitro/ (28847)
12 (ivf or icsi).tw. (19896)
13 (in vitro fertil$ or ((intracytoplasmic or intra‐cytoplasmic) adj sperm$)).tw. (21463)
14 exp embryo transfer/ or exp sperm injections, intracytoplasmic/ (16614)
15 or/11‐14 (42331)
16 6 and 10 and 15 (85)
17 randomized controlled trial.pt. (397392)
18 controlled clinical trial.pt. (90499)
19 randomized.ab. (316865)
20 placebo.tw. (167401)
21 clinical trials as topic.sh. (175872)
22 randomly.ab. (227168)
23 trial.ti. (137785)
24 (crossover or cross‐over or cross over).tw. (63575)
25 or/17‐24 (977646)
26 exp animals/ not humans.sh. (4078006)
27 25 not 26 (901386)
28 16 and 27 (32)
Database: EBM Reviews ‐ Cochrane Central Register of Controlled Trials <September 2014>
Search strategy
1 exp Polycystic Ovary Syndrome/ (772)
2 Polycystic Ovar$.tw. (1234)
3 (PCOS or PCOD).tw. (924)
4 (stein‐leventhal or leventhal).tw. (8)
5 (ovar$ adj (scelerocystic or polycystic or degeneration)).tw. (3)
6 or/1‐5 (1380)
7 exp metformin/ (1414)
8 metformin.tw. (2235)
9 (dimethylbiguanidium or dimethylguanylguanidine or glucophage or glucovance).tw. (30)
10 or/7‐9 (2314)
11 exp fertilization in vitro/ (1616)
12 (ivf or icsi).tw. (2552)
13 (in vitro fertil$ or ((intracytoplasmic or intra‐cytoplasmic) adj sperm$)).tw. (1822)
14 exp embryo transfer/ or exp sperm injections, intracytoplasmic/ (1055)
15 or/11‐14 (3438)
16 6 and 10 and 15 (38)
Database: EMBASE via Elsevier <1980 to 20 October 2014>
Search strategy
1 exp ovary polycystic disease/ (17456)
2 polycystic ovar$.tw. (14017)
3 (PCOD or PCOS).tw. (9383)
4 (stein‐leventhal or leventhal).tw. (651)
5 (ovar$ adj (scelerocystic or polycystic or degeneration)).tw. (72)
6 or/1‐5 (19952)
7 exp METFORMIN/ (35310)
8 metformin.tw. (16359)
9 (dimethylbiguanidium or dimethylguanylguanidine or glucophage or glucovance).tw. (1531)
10 or/7‐9 (36285)
11 exp fertilization in vitro/ (39133)
12 (ivf or icsi).tw. (29725)
13 in vitro fertil$.tw. (21547)
14 ((intracytoplasmic or intra‐cytoplasmic) adj sperm$).tw. (6773)
15 exp intracytoplasmic sperm injection/ (13023)
16 exp embryo transfer/ (21002)
17 or/11‐16 (61145)
18 6 and 10 and 17 (321)
19 Clinical Trial/ (834564)
20 Randomized Controlled Trial/ (351271)
21 exp randomization/ (63592)
22 Single Blind Procedure/ (18900)
23 Double Blind Procedure/ (115714)
24 Crossover Procedure/ (40361)
25 Placebo/ (246816)
26 Randomi?ed controlled trial$.tw. (104177)
27 Rct.tw. (14914)
28 random allocation.tw. (1341)
29 randomly allocated.tw. (20881)
30 allocated randomly.tw. (1949)
31 (allocated adj2 random).tw. (717)
32 Single blind$.tw. (14712)
33 Double blind$.tw. (143861)
34 ((treble or triple) adj blind$).tw. (395)
35 placebo$.tw. (202854)
36 prospective study/ (263230)
37 or/19‐36 (1388883)
38 case study/ (28194)
39 case report.tw. (265152)
40 abstract report/ or letter/ (903050)
41 or/38‐40 (1190552)
42 37 not 41 (1350817)
43 18 and 42 (155)
Database: LILACS <1982 to 2014>
Search strategy
((MH:C04.182.612.765$) OR (MH:C13.351.500.056.630.580.765$) OR (MH:C19.391.630.580.765$) OR (TW:"Polycystic Ovary Syndrome") OR (TW:"Síndrome del Ovario Poliquístico") OR (TW:"Síndrome do Ovário Policístico") OR (TW:"Stein‐Leventhal Syndrome") OR (TW:"Síndrome de Stein‐Leventhal") OR (TW:stein‐leventhal) OR (TW:leventhal) OR (TW:PCOS) OR (TW:PCOD) OR (TW:Ovar$ AND (Poliquístico OR Sclerocystic OR Polycystic OR Degeneration OR Policístico OR Degeneração))) AND ((MH:D02.078.370.141.450$) OR (TW:Metformin) OR (TW:METFORMINA) OR (TW:Dimethylguanylguanidine) OR (TW:"Dimetil Guanil Guanidina") OR (TW:Dimetilguanilguanidina) OR (TW:Glucophage) OR (TW:Glucovance)) AND ((MH:E02.875.800.500$) OR (MH:E05.820.800.500$) OR (TW:Embryo Transfer) OR (TW:Transferencia de Embrión) OR (TW:Transferência Embrionária) OR (TW:Blastocyst Transfer) OR (TW:Tubal Embryo Transfer) OR (TW:Transferencia de Blastocitos) OR (TW:Transferencia Tubaria del Embrión) OR (TW:Transferência de Blastócitos) OR (TW:Transferência Tubária de Embrião) OR (TW:Transferência de Embrião) OR (MH:E02.875.800.750$) OR (MH:E05.820.800.750$) OR (TW:Fertilization in Vitro) OR (TW:Fertilización In Vitro) OR (TW:Fertilização In Vitro) OR (TW:Test‐Tube Fertilization) OR (TW:Fecundación In Vitro) OR (TW:Fecundación en Probeta) OR (TW:Fertilización en Probeta) OR (TW:Fecundação In Vitro) OR (TW:Fecundação em Tubo de Ensaio) OR (TW:Fertilização em Tubo de Ensaio) OR (MH:E02.875.800.750.700$) OR (MH:E05.820.800.750.700$) OR (TW:Inyecciones de Esperma Intracitoplasmáticas) OR (TW:Injeções de Esperma Intracitoplásmicas) OR (TW:Intracytoplasmic Sperm Injections) OR (TW:ICSI) OR (TW:Inyecciones Intracitoplasmáticas de Esperma) OR (TW:IICE) OR (TW:Injeções Intracitoplásmicas de Esperma) OR (TW:assisted reproductive technique) OR (TW:in vitro fertilization) OR (TW:in vitro fertilisation) OR (TW:FIV) OR (TW:IVF))
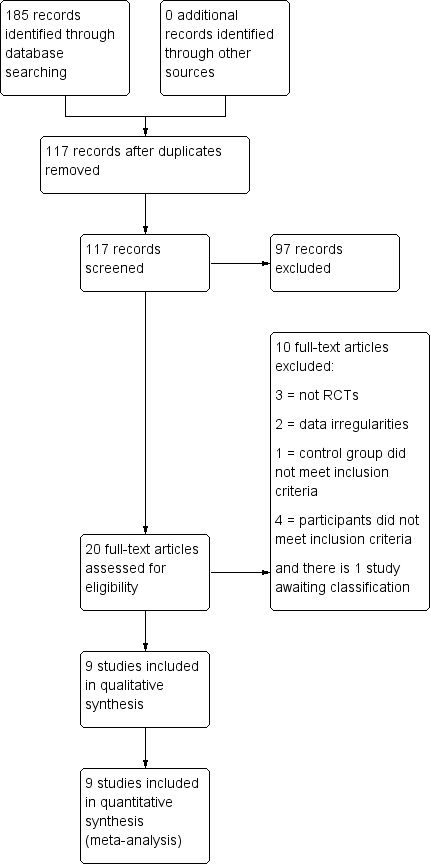
Study flow diagram.

'Risk of bias' graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each methodological quality item for each included study.

Forest plot of comparison: 1 Metformin versus placebo or no treatment, outcome: 1.1 Live birth rate per woman.

Forest plot of comparison: 1 Metformin versus placebo or no treatment, outcome: 1.2 Clinical pregnancy rate per woman.

Forest plot of comparison: 1 Metformin versus placebo or no treatment, outcome: 1.3 Incidence of OHSS per woman.

Comparison 1 Metformin versus placebo or no treatment, Outcome 1 Live birth rate per woman.
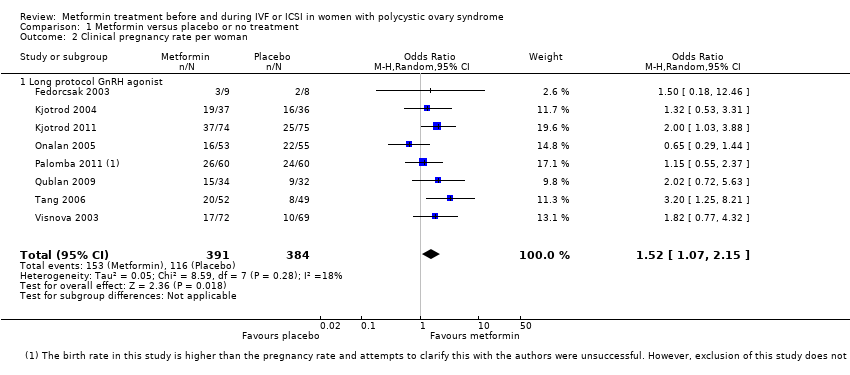
Comparison 1 Metformin versus placebo or no treatment, Outcome 2 Clinical pregnancy rate per woman.
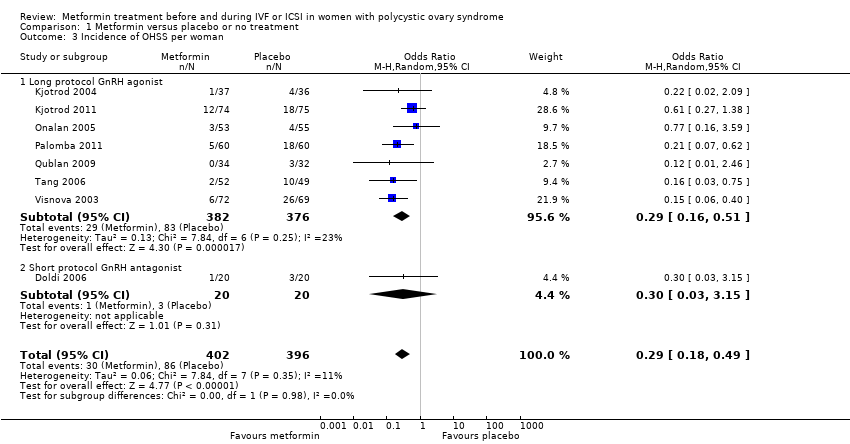
Comparison 1 Metformin versus placebo or no treatment, Outcome 3 Incidence of OHSS per woman.
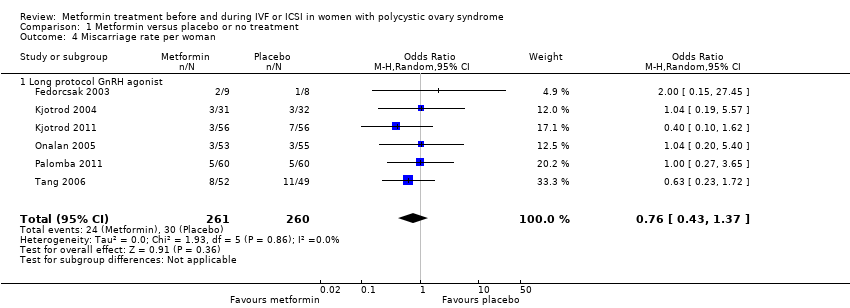
Comparison 1 Metformin versus placebo or no treatment, Outcome 4 Miscarriage rate per woman.
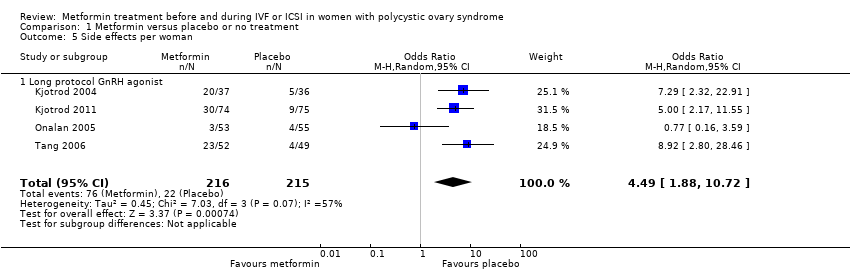
Comparison 1 Metformin versus placebo or no treatment, Outcome 5 Side effects per woman.
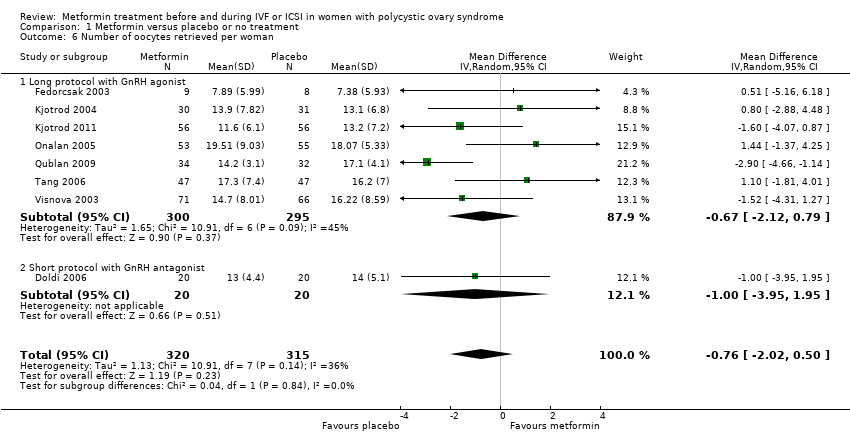
Comparison 1 Metformin versus placebo or no treatment, Outcome 6 Number of oocytes retrieved per woman.

Comparison 1 Metformin versus placebo or no treatment, Outcome 7 Mean total dose of FSH (IU) per woman.

Comparison 1 Metformin versus placebo or no treatment, Outcome 8 Mean days of gonadotrophin per woman.
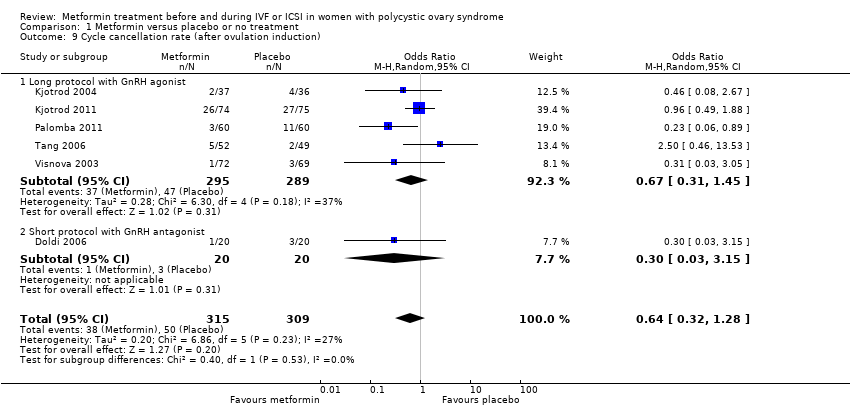
Comparison 1 Metformin versus placebo or no treatment, Outcome 9 Cycle cancellation rate (after ovulation induction).
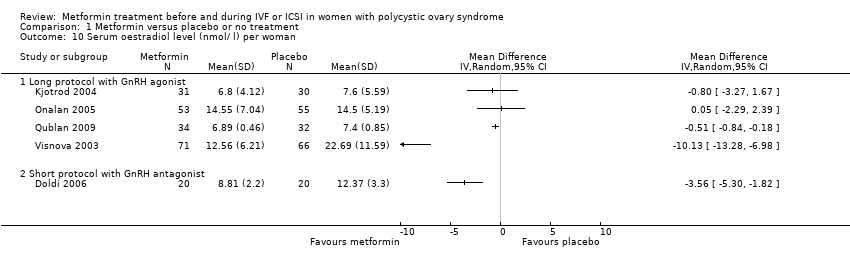
Comparison 1 Metformin versus placebo or no treatment, Outcome 10 Serum oestradiol level (nmol/ l) per woman.
| Study | Results | Metformin | Placebo |
| Onalan 2005 | No significant differences in total testosterone measures from women treated with placebo (P = 0.646) | Median 3.1; range 2.5 to 3.9 | Median 3.1; range 2.4 to 3 |
| Tang 2006 | Testosterone levels did not change significantly in the group taking metformin (P = 0.892); however, participants in the placebo group had a significant increase in testosterone levels (P = 0.040). In the metformin group, on the day of hCG administration, there was a significant decrease in testosterone concentration (P = 0.029) and in the free‐androgen index (P = 0.004) | Baseline geometric mean: 2.03 nmol/l, geometric mean on the day of hCG administration: 1.97 nmol/l. Testosterone concentration (geometric mean: 1.96 nmol/l). Free‐androgen index (geometric mean: 2.43) | Baseline geometric mean: 2.06 nmol/l, geometric mean on the day of hCG administration: 2.52 nmol/l. Testosterone concentration (geometric mean: 2.52 nmol/l). Free‐androgen index (geometric mean: 3.34) |
Comparison 1 Metformin versus placebo or no treatment, Outcome 11 Mean or median serum androgen levels per woman.
| Study | Results | Metformin | Placebo |
| Onalan 2005 | There were no significant changes in the glucose/insulin ratio between groups (P = 0.81) | Median 6; range 2.4 to 8.8 | Median 6; range 3 to 10 |
| Tang 2006 | There were no significant changes in the insulin sensitivity test (QUICKI) between baseline and the day of oocyte retrieval in the metformin group (P = 0.200) and the placebo group (P = 0.572). | Baseline: 0.377 At the day of oocyte retrieval: 0.417 | Baseline: 0.386 At the day of oocyte retrieval: 0.400 |
Comparison 1 Metformin versus placebo or no treatment, Outcome 12 Mean or median fasting insulin and glucose levels per woman.
| Metformin treatment before or during IVF or ICSI for women with polycystic ovary syndrome | ||||||
| Population: Women with polycystic ovary syndrome Control: Placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no treatment | Metformin treatment | |||||
| Live birth rate (per woman) ‐ ITT | 320 per 1000 | 395 per 1000 (276 to 530) | OR 1.39 | 551 | ⊕⊕⊝⊝ | |
| Clinical pregnancy rate (per woman) ‐ ITT | 307 per 1000 | 403 per 1000 | OR 1.52 | 775 | ⊕⊕⊕⊝ | |
| Incidence of OHSS | 270 per 1000 | 97 per 1000 | OR 0.29 | 798 | ⊕⊕⊕⊝ | |
| Miscarriage rate (per woman) | 139 per 1000 | 110 per 1000 | OR 0.76 | 521 | ⊕⊕⊕⊝ | |
| Side effects | 106 per 1000 | 347 per 1000 | OR 4.49 | 431 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Inconsistency: unexplained heterogeneity (I2 = 52%) 2 Imprecision: total number of events is fewer than 300 3Inconsistency: unexplained heterogeneity (I2 = 57%) 4There was a data discrepancy in one of these studies (Palombo 2011). According to the study publication, in both the metformin group and the placebo group the clinical pregnancy rate was lower than the live birth rate. Sensitivity analyses excluding this study yielded an OR of 1.48 (95% CI 0.72 to 3.02) for live birth and 1.61 (95% CI 1.08 to 2.40) for pregnancy, which did not substantially change our findings. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth rate per woman Show forest plot | 5 | 551 | Odds Ratio (M‐H, Random, 95% CI) | 1.39 [0.81, 2.40] |
| 1.1 Long protocol GnRH agonist | 5 | 551 | Odds Ratio (M‐H, Random, 95% CI) | 1.39 [0.81, 2.40] |
| 2 Clinical pregnancy rate per woman Show forest plot | 8 | 775 | Odds Ratio (M‐H, Random, 95% CI) | 1.52 [1.07, 2.15] |
| 2.1 Long protocol GnRH agonist | 8 | 775 | Odds Ratio (M‐H, Random, 95% CI) | 1.52 [1.07, 2.15] |
| 3 Incidence of OHSS per woman Show forest plot | 8 | 798 | Odds Ratio (M‐H, Random, 95% CI) | 0.29 [0.18, 0.49] |
| 3.1 Long protocol GnRH agonist | 7 | 758 | Odds Ratio (M‐H, Random, 95% CI) | 0.29 [0.16, 0.51] |
| 3.2 Short protocol GnRH antagonist | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.30 [0.03, 3.15] |
| 4 Miscarriage rate per woman Show forest plot | 6 | 521 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.43, 1.37] |
| 4.1 Long protocol GnRH agonist | 6 | 521 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.43, 1.37] |
| 5 Side effects per woman Show forest plot | 4 | 431 | Odds Ratio (M‐H, Random, 95% CI) | 4.49 [1.88, 10.72] |
| 5.1 Long protocol GnRH agonist | 4 | 431 | Odds Ratio (M‐H, Random, 95% CI) | 4.49 [1.88, 10.72] |
| 6 Number of oocytes retrieved per woman Show forest plot | 8 | 635 | Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐2.02, 0.50] |
| 6.1 Long protocol with GnRH agonist | 7 | 595 | Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐2.12, 0.79] |
| 6.2 Short protocol with GnRH antagonist | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐3.95, 1.95] |
| 7 Mean total dose of FSH (IU) per woman Show forest plot | 8 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 Long protocol with GnRH agonist | 7 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Short protocol with GnRH antagonist | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Mean days of gonadotrophin per woman Show forest plot | 8 | 643 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.77, 0.40] |
| 8.1 Long protocol with GnRH agonist | 7 | 603 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.89, 0.45] |
| 8.2 Short protocol with GnRH antagonist | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐1.30, 1.30] |
| 9 Cycle cancellation rate (after ovulation induction) Show forest plot | 6 | 624 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.32, 1.28] |
| 9.1 Long protocol with GnRH agonist | 5 | 584 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.31, 1.45] |
| 9.2 Short protocol with GnRH antagonist | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.30 [0.03, 3.15] |
| 10 Serum oestradiol level (nmol/ l) per woman Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.1 Long protocol with GnRH agonist | 4 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Short protocol with GnRH antagonist | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Mean or median serum androgen levels per woman Show forest plot | Other data | No numeric data | ||
| 12 Mean or median fasting insulin and glucose levels per woman Show forest plot | Other data | No numeric data | ||

