Intervensi untuk merawat lymphocytic colitis
Abstract
Background
Lymphocytic colitis is a cause of chronic diarrhea. It is a subtype of microscopic colitis characterized by chronic, watery, non‐bloody diarrhea and normal endoscopic and radiologic findings. The etiology of this disorder is unknown.Therapy is based mainly on case series and uncontrolled trials, or by extrapolation of data for treating collagenous colitis, a related disorder. This review is an update of a previously published Cochrane review.
Objectives
To evaluate the efficacy and safety of treatments for clinically active lymphocytic colitis.
Search methods
The MEDLINE, PUBMED and EMBASE databases were searched from inception to 11 August 2016 to identify relevant papers. Manual searches from the references of included studies and relevant review articles were performed.
Abstracts from major gastroenterological meetings were also searched to identify research submitted in abstract form only. The trial registry web site www.ClinicalTrials.gov was searched to identify registered but unpublished trials. Finally, the Cochrane Central Register of Controlled Trials and the Cochrane Inflammatory Bowel Disease and Functional Bowel Disorders Group Specialized Trials Register were searched for other studies.
Selection criteria
Randomized controlled trials assessing medical therapy for patients with biopsy‐proven lymphocytic colitis were considered for inclusion
Data collection and analysis
Data was independently extracted by at least two authors. Any disagreements were resolved by consensus. Data were analyzed on an intention‐to‐treat (ITT) basis. The primary outcome was clinical response as defined by the included studies. Secondary outcome measures included histological response as defined by the included studies, quality of life as measured by a validated instrument and the occurrence of adverse events. Risk ratios (RR) and 95% confidence intervals (CI) were calculated for dichotomous outcomes. The methodological quality of included studies was evaluated using the Cochrane risk of bias tool. The overall quality of the evidence supporting the primary outcome and selected secondary outcomes was assessed using the GRADE criteria. Data were combined for analysis if they assessed the same treatments. Dichotomous data were combined using a pooled RR along with corresponding 95% CI. A fixed‐effect model was used for the pooled analysis.
Main results
Five RCTs (149 participants) met the inclusion criteria. These studies assessed bismuth subsalicylate versus placebo, budesonide versus placebo, mesalazine versus mesalazine plus cholestyramine and beclometasone dipropionate versus mesalazine. The study which assessed mesalazine versus mesalazine plus cholestyramine and the study which assessed beclometasone dipropionate versus mesalazine were judged to be at high risk of bias due to lack of blinding. The study which compared bismuth subsalicylate versus us placebo was judged as low quality due to a very small sample size and limited data. The other 3 studies were judged to be at low risk of bias. Budesonide (9 mg/day for 6 to 8 weeks) was significantly more effective than placebo for induction of clinical and histological response. Clinical response was noted in 88% of budesonide patients compared to 38% of placebo patients (2 studies; 57 participants; RR 2.03, 95% CI 1.25 to 3.33; GRADE = low). Histological response was noted in 78% of budesonide patients compared to 33% of placebo patients (2 studies; 39 patients; RR 2.44, 95% CI 1.13 to 5.28; GRADE = low). Forty‐one patients were enrolled in the study assessing mesalazine (2.4 g/day) versus mesalazine plus cholestyramine (4 g/day). Clinical response was noted in 85% of patients in the mesalazine group compared to 86% of patients in the mesalazine plus cholestyramine group (RR 0.99, 95% CI 0.77 to 1.28; GRADE = low). Five patients were enrolled in the trial studying bismuth subsalicylate (nine 262 mg tablets daily for 8 weeks versus placebo). There were no differences in clinical (P=0.10) or histological responses (P=0.71) in patients treated with bismuth subsalicylate compared with placebo (GRADE = very low). Forty‐six patients were enrolled in the trial studying beclometasone dipropionate (5 mg/day or 10 mg/day) versus mesalazine (2.4 g/day). There were no differences in clinical remission at 8 weeks (RR 0.97; 95% CI 0.75 to 1.24; GRADE = low) and 12 months of treatment (RR 1.29; 95% CI 0.40 to 4.18; GRADE = very low). Although patients receiving beclometasone dipropionate (84%) and mesalazine (86%) achieved clinical remission at 8 weeks, it was not maintained at 12 months (26% and 20%, respectively). Adverse events reported in the budesonide studies include nausea, vomiting, neck pain, abdominal pain, hyperhidrosis and headache. Nausea and skin rash were reported as adverse events in the mesalazine study. Adverse events in the beclometasone dipropionate trial include nausea, sleepiness and change of mood. No adverse events were reported in the bismuth subsalicylate study.
Authors' conclusions
Low quality evidence suggests that budesonide may be effective for the treatment of active lymphocytic colitis. This benefit needs to be confirmed by a large placebo ‐controlled trial. Low quality evidence also suggests that mesalazine with or without cholestyramine and beclometasone dipropionate may be effective for the treatment of lymphocytic colitis, however this needs to be confirmed by large placebo‐controlled studies. No conclusions can be made regarding bismuth subsalicylate due to the very small number of patients in the study, Further trials studying interventions for lymphocytic colitis are warranted.
PICO
Ringkasan bahasa mudah
Rawatan untuk lymphocytic colitis
Apakah lymphocytic colitis?
Lymphocytic colitis adalah sejenis kolitis mikroskopik, keadaan yang dicirikan oleh cirit‐birit berair kronik yang tidak berdarah. Pesakit dengan lymphocytic colitis mempunyai usus yang kelihatan normal apabila dinilai endoskopi (kamera yang digunakan untuk melihat usus) atau X‐ray; tetapi mempunyai keradangan mikroskopik (histologi) usus apabila dinilai dengan biopsi (sampel tisu yang diambil semasa endoskopi). Punca gangguan ini tidak diketahui. Ulasan ini adalah kemaskini ulasan Cochrane yang telah diterbitkan terdahulu.
Apakah rawatan yang telah diuji untuk lymphocytic colitis?
Budesonide, mesalazine dengan atau tanpa cholestyramine, beclometasone dipropionate dan bismut subsalicylate (iaitu Pepto‐Bismol®) telah diuji sebagai rawatan untuk lymphocytic colitis. Budesonide adalah sejenis ubat steroid imunosupresif yang cepat dimetabolisma oleh hati yang mengakibatkan penurunan kesan sampingan steroid. Ianya diambil melalui mulut. Beclometasone dipropionate juga merupakan ubat steroid. Ubat steroid digunakan untuk merawat keradangan. Mesalazine (juga dikenali sebagai 5‐ASA) adalah ubat anti‐radang yang sering diambil melalui mulut. Cholestyramine adalah ubat yang membantu badan membuang asid hempedu. Pepto‐Bismol®, adalah ubat antasid yang digunakan untuk merawat ketidakselesaan sementara perut dan saluran gastrousus.
Apakah yang dikaji oleh penyelidik?
Para penyelidik menyiasat sama ada ubat‐ubatan ini memperbaiki gejala lymphocytic colitis (contohnya cirit‐birit) atau keradangan mikroskopik dan sama ada sebarang kesan sampingan terhasil daripada rawatan. Para penyelidik mencari kesusasteraan perubatan secara meluas sehingga 11 Ogos 2016.
Apakah yang ditemui penyelidik?
Lima kajian termasuk 149 peserta telah dikenal pasti. Kajian ini menilai budesonide berbanding plasebo (cth pil gula), mesalazine berbanding mesalazine ditambah cholestyramine dan beclometasone dipropionate berbanding mesalazine dan Pepto‐Bismol® berbanding plasebo. Kajian yang membandingkan mesalazine dengan mesalazine ditambah cholestyramine dan kajian yang membandingkan beclometasone dipropionate dengan mesalazine dinilai sebagai kualiti yang rendah. Kajian yang membandingkan Pepto‐Bismol® bismut subsalicylate kepada plasebo dinilai sebagai kualiti rendah kerana saiz sampel yang sangat kecil (5 peserta) dan data yang terhad. Tiga kajian lain dinilai sebagai berkualiti tinggi.
Analisis gabungan dua kajian (57 peserta) menunjukkan bahawa budesonide (9 mg/hari selama 6 hingga 8 minggu) adalah lebih baik daripada plasebo untuk penambahbaikan cirit‐birit dan penambahbaikan keradangan usus mikroskopik. Penambahbaikan dalam cirit‐birit dicatatkan dalam 88% peserta budesonide berbanding 38% peserta plasebo. Peningkatan dalam keradangan mikroskopik dilaporkan dalam 78% peserta budesonide berbanding 33% peserta plasebo. Empat puluh satu peserta telah didaftarkan dalam kajian yang membandingkan mesalazine (2.4. g / hari) kepada mesalazine ditambah cholestyramine (4 g / hari). Peningkatan dalam cirit‐birit dicatatkan dalam 85% peserta dalam kumpulan mesalazine berbanding 86% peserta dalam kumpulan mesalazine plus cholestyramine. Lima pesakit telah didaftarkan dalam kajian yang mengkaji Pepto‐Bismol® (sembilan tablet 262 mg setiap hari selama 8 minggu berbanding plasebo). Tiada perbezaan dalam peningkatan cirit‐birit atau peningkatan keradangan usus mikroskopik. Empat puluh enam peserta telah didaftarkan dalam percubaan yang mengkaji beclometasone dipropionate (5 mg / hari atau 10 mg / hari) berbanding mesalazine (2.4 g / hari). Walaupun peserta yang menerima beclometasone dipropionate (84%) dan mesalazine (86%) telah meningkatkan cirit‐birit selepas 8 minggu, peningkatan ini tidak dikekalkan pada 12 bulan (masing‐masing 26% dan 20%). Kesan sampingan yang dilaporkan dalam kajian budesonide termasuklah loya, muntah, sakit leher, sakit perut, berpeluh berlebihan dan sakit kepala. Kesan sampingan yang dilaporkan dalam kajian melibatkan mesalamine termasuklah loya dan ruam kulit. Kesan sampingan dalam percubaan beclometasone dipropionate termasuk loya, mengantuk dan perubahan mood. Tiada kesan sampingan dilaporkan dalam kajian yang melibatkan Pepto‐Bismol®.
Bukti kualiti rendah menunjukkan bahawa budesonide mungkin merupakan terapi yang berkesan untuk rawatan lymphocytic colitis. Bukti kualiti rendah juga menunjukkan bahawa mesalazine dengan atau tanpa kolestiramin dan beclometasone dipropionate mungkin berkesan untuk rawatan lymphocytic colitis. Tiada kesimpulan boleh dibuat mengenai bismut subsalicylate kerana bilangan peserta yang sangat kecil dalam kajian ini. Pada masa hadapan, penyelidik harus mempertimbangkan lagi kajian terkawal plasebo yang besar bagi budesonide untuk mengesahkan manfaat dan keselamatan yang dicadangkan bagi terapi ini. Bismuth subsalicylate, yang mempunyai potensi kurang ketoksikan daripada budesonide, juga memerlukan kajian lanjut. Keberkesanan dan keselamatan mesalazine dengan atau tanpa cholestyramine, dan beclometasone dipropionate perlu disiasat dalam kajian terkawal plasebo yang besar.
Authors' conclusions
Summary of findings
| Bismuth subsalicylate versus placebo for treating lymphocytic colitis | ||||||
| Patient or population: patients with active lymphocytic colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| control | bismuth vs placebo | |||||
| Clinical response | 0 per 10001 | 0 per 1000 | RR 5.25 | 5 | ⊕⊝⊝⊝ | |
| Histological response | 500 per 10001 | 665 per 1000 (135 to 3300) | RR 1.33 (0.27 to 6.6) | 5 (1 study) | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. | ||||||
| Budesonide versus mesalazine for treating lymphocytic colitis | ||||||
| Patient or population: patients with active lymphocytic colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| control | budesonide versus placebo | |||||
| Clinical response | 440 per 10001 | 893 per 1000 | RR 2.03 | 57 | ⊕⊕⊝⊝ low2,3 | |
| Histological response | 313 per 10001 | 763 per 1000 | RR 2.44 (1.13 to 5.28) | 39 | ⊕⊕⊝⊝ low4 | |
| Adverse events | 143 per 10001 | 96 per 1000 (17 to 513) | RR 0.67 (0.12 to 3.59) | 42 | ⊕⊕⊝⊝ low5 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. | ||||||
| Mesalazine versus mesalazine + cholestyramine for treating lymphocytic colitis | ||||||
| Patient or population: patients with maintenance of remission in lymphocytic colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| mesalazine | mesalazine plus cholestyramine | |||||
| Clinical response | 857 per 1000 | 849 per 1000 (660 to 1000) | RR 0.99 | 41 | ⊕⊕⊝⊝ | |
| Histological response | 857 per 1000 | 849 of 1000 (660 to 1000) | RR 0.99 | 41 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. | ||||||
| Beclometasone dipropionate versus mesalazine for treating lymphocytic colitis | ||||||
| Patient or population: Patients with active or quiescent lymphocytic colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Mesalazine | beclometasone | |||||
| Clinical response at 8 weeks | 867 per 10001 | 841 per 1000 (650 to 1075) | RR 0.97 (0.75 to 1.24) | 46 | ⊕⊕⊝⊝ low2,3 | |
| Maintenance of clinical response at 12 weeks | 200 of 10001 | 258 per 1000 (80 to 836) | RR 1.29 (0.40 to 4.18) | 46 | ⊕⊝⊝⊝ very low2,4 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. | ||||||
Background
Lymphocytic colitis is a cause of chronic watery diarrhea. It is related to collagenous colitis, and together these two disorders are more broadly categorized as "microscopic colitis", with normal radiologic and endoscopic findings, but typical histological features on biopsies. Population‐based studies in Europe have reported an annual incidence of collagenous colitis ranging from 0.6 to 5.2 per 100,000 persons and a prevalence of 10 to 15.7 per 100,000 population, while lymphocytic colitis has a reported annual incidence ranging from 3.1 to 4.4 per 100,000 persons and a prevalence of 14.2 per 100,000 population (Agnarsdottir 2002; Bohr 1995; Fernandez 1999; Olesen 2004a; Raclot 1994). A population based study in the United States reported an annual incidence of collagenous and lymphocytic colitis of 5.1 and 9.8 per 100,000 persons and a prevalence of 36 and 64 per 100,000 population respectively (Pardi 2004a).
Lymphocytic colitis is distinguished from collagenous colitis by the presence of an increased number of intraepithelial lymphocytes (>20 per 100 epithelial cells) and the absence of a markedly thickened subepithelial collagen band. The etiology and pathogenesis of lymphocytic colitis are uncertain, although links to autoimmune disorders and the use of nonsteroidal anti‐inflammatory drugs have been suggested (Loftus 2003; Pardi 2004b; Schiller 2004). Treatment has been mainly based on anecdotal evidence, and includes dietary modification, antidiarrheals, bulking agents, bile acid binding agents, spasmolytics, antibiotics, bismuth subsalicylate, sulfasalazine/other 5‐ASA products, traditional corticosteroids, budesonide, azathioprine/6‐mercaptopurine, cyclosporine, probiotics and surgery (See Table 1). Various therapies have been evaluated in randomized controlled studies in patients with collagenous colitis (Chande 2008a), but it is not known if the results of these studies can be extrapolated to treating patients with lymphocytic colitis. Treatments for lymphocytic colitis that have been investigated in randomized controlled trials include budesonide (Miehlke 2009; NCT00184171; Pardi 2009), mesalazine with or without cholestyramine (Calabrese 2007), bismuth subsalicylate (Fine 1999; NCT00184171) and beclometasone dipropionate (Latella 2010). This systematic review is an update of a previously published Cochrane review (Chande 2007; Chande 2008b).
Objectives
To evaluate the effectiveness of therapies for clinically active lymphocytic colitis studied in randomized trials.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials were considered for inclusion. Studies published as abstracts were only included if the authors could be contacted to obtain further information.
Types of participants
Patients with biopsy‐proven clinically active lymphocytic colitis without other significant radiologic or endoscopic findings were considered for inclusion.
Types of interventions
All types of therapy were eligible for inclusion.
Types of outcome measures
The primary outcome measure was clinical response as defined by the included studies (e.g. decreased stool frequency or stool weight or both). Secondary outcome measures included histological response as defined by the included studies, quality of life as measured by a validated instrument and the occurrence of adverse events.
Search methods for identification of studies
Electronic searches
EMBASE, MEDLINE, the Cochrane Library (CENTRAL) and the Cochrane IBD Group Specialized Register were searched from inception to 11 August 2016. The search strategies are listed in Appendix 1. Conference abstracts from Digestive Disease Week and the United European Gastroenterology Week were also searched to identify abstract publications.
Searching other resources
We searched reference lists from potentially relevant papers to identify additional studies that may have been missed using the computer‐assisted search strategy. The trial database clinicaltrials.gov was also searched to identify studies that may have not been reported.
Data collection and analysis
Study Selection:
At least two authors (NY, TMN or TB) Each author independently reviewed potentially relevant studies to determine eligibility based on the criteria specified above. Studies published in abstract form were only included if the authors could be contacted to clarify details about the protocol and results. Any disagreement was resolved by consensus.
Data Collection:
A data extraction form was developed and used to extract information on results of included studies. A minimum of two authors (NY, TMN or TB) independently extracted the data, any disagreement among authors was resolved by consensus.
Statistical Analysis:
Data was analyzed using Review Manager (RevMan 5.3.5). If cross‐over studies are identified in future updates, only data from the first arm will be included. All data were analyzed on an intention‐to‐treat basis. Data were extracted and converted into two by two tables (treatment versus comparator and clinical response versus no response; treatment versus comparator and histological response versus no response). For therapies assessed in one trial only, P values were derived using the Chi2 test. If in the future, therapies are assessed in more than one trial, summary test statistics will be derived by calculating the risk ratio (RR) and corresponding 95% confidence interval (95% CI). The authors of each paper set the definitions of clinical and histological response and the data were combined for analysis only if these definitions were sufficiently similar (determined by consensus). The presence of heterogeneity among studies was assessed using the Chi2 test (a P value of 0.10 will be regarded as statistically significant). If statistically significant heterogeneity is identified the RR and 95% CI will be calculated using a random‐effects model.
Quality Assessment:
At least two authors (NY, TMN or TB) independently assessed the methodological quality of included studies using the Cochrane risk of bias tool (Higgins 2011). Factors assessed included:
-
Random sequence generation;
-
Allocation concealment;
-
Blinding of participants, outcome assessors and investigators;
-
Incomplete outcome reporting (i.e. there was an acceptable method of dealing with attrition);
-
Selective outcome reporting (i.e. all outcomes described in the methods were included in the analysis);
-
Other potential sources of bias.
A judgement of 'Yes' indicates low risk of bias, 'No' indicates high risk of bias, and 'Unclear' indicates unclear or unknown risk of bias. Disagreements were resolved by consensus. Study authors were contacted when insufficient information was provided to determine risk of bias.
We used the GRADE criteria to assess the overall quality of the evidence supporting the primary outcome and selected secondary outcomes (Schünemann 2011). The overall quality of evidence can be graded as:
1. High: Further research is very unlikely to change our confidence in the estimate of effect;
2. Moderate: Further research is likely to have an impact on our confidence in the estimate of effect and may change the estimate;
3. Low: Further research is very likely to have an important impact on our confidence on the estimate of effect and is likely to change the estimate; or
4. Very low: Any estimate of effect is very uncertain.
The overall quality of evidence can be downgraded by one (serious concern) or two levels (very serious concern) for the following reasons: risk of bias, inconsistency (e.g. unexplained heterogeneity), indirectness (e.g. indirect population, intervention, control or outcomes), publication bias, and imprecision (e.g. sparse data, few events). Imprecision was determined principally by a small number of studies for a comparison (two or fewer studies) and fewer than 100 events for that comparison. We used the GRADEproGDT software to produce Summary of Findings Tables.
Results
Description of studies
A literature search conducted on August 11, 2016 identified 333 records. Two additional studies were identified through searching of conference and other sources. After duplicates were removed, a total of 248 trials remained for review of titles and abstracts. Twenty‐seven studies were selected for full text review (see Figure 1). Twenty studies were excluded (See: Characteristics of excluded studies). Seven reports of 5 studies (n = 149 patients) met the pre‐defined inclusion criteria and were included in the review (Fine 1999; Calabrese 2007; Miehlke 2009; Pardi 2009; Latella 2010). A search of clinical trials.gov identified two ongoing randomized trials (NCT00184171; NCT01209208). The NCT00184171 study plans to assess the treatment of lymphocytic colitis with budesonide (9 mg/day), bismuth or fiber. The NCT01209208 plans to assess treatment of lymphocytic colitis with budesonide (9 mg/day), mesalazine (3 g/day) or placebo. The results of these ongoing studies will be included in future updates of this review when the study results become available.
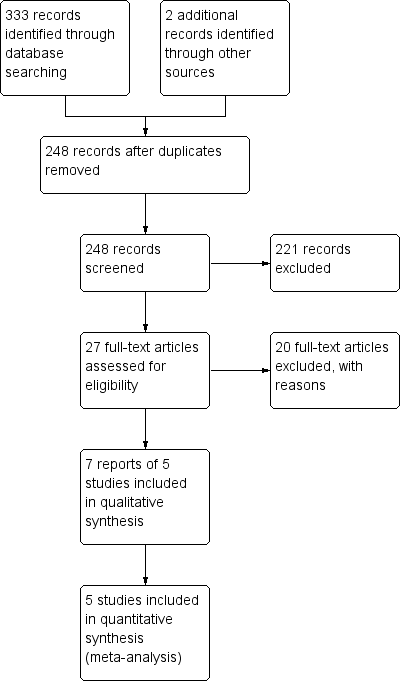
Study flow diagram.
Fine 1999, which was published in abstract form only, was a randomized, double‐blind, placebo‐controlled trial that compared bismuth subsalicylate to placebo in 14 patients (11 females: 3 males; age range 35 to 78 years) with microscopic colitis. Of these, five patients had lymphocytic colitis and nine had collagenous colitis. Patients were treated with bismuth subsalicylate (n = 3; nine 262 mg chewable tablets daily in 3 divided doses) or an identical placebo (n = 2) for 8 weeks. Each patient underwent 48‐hour stool collection prior to treatment and on the last two days of treatment stool weight and consistency were evaluated using standardized criteria. All patients underwent flexible sigmoidoscopy with standardized biopsy prior to entry and after treatment. Patients were not to take antibiotics or anti‐inflammatory agents for a minimum of 6 weeks, and antidiarrheals for minimum of 2 weeks prior to entry. Clinically active lymphocytic colitis was defined as at least eight weeks of non‐bloody watery diarrhea with normal radiologic and endoscopic findings. Lymphocytic colitis was confirmed by histopathology consisting of excess mononuclear inflammatory cells in the lamina propria and surface epithelium without significant neutrophilic or eosinophilic inflammation, numerous crypt abscesses, or granuloma; the absence of a thickened subepithelial collagen band; and no other evidence of Crohn's disease. The histological score ranged from 0 to 10 and was based on the following five parameters: surface epithelium (microulceration, cell flattening and mucin depletion ‐ scored 0 to 2); crypts; lamina propria cellularity; numbers of intraepithelial lymphocytes within the surface epithelium and the number of intra epithelial lymphocytes within the crypt epithelium. A clinical response was defined as the improvement of diarrhea to the passage of two or less formed or semi formed stools per day. A histological response was defined as a reduction in the histopathology score by greater than 50%. Adverse events were also documented.
Calabrese 2007 was a randomized, open‐label trial comparing mesalazine to mesalazine plus cholestyramine for the treatment of patients with active microscopic colitis. The study included 23 patients with collagenous colitis and 41 with lymphocytic colitis. Patients were randomized via a computer generated list to oral mesalazine 800 mg three times daily (n = 20) or oral mesalazine 800 mg three times daily plus cholestyramine 4 g once daily (n = 21) for 6 months of treatment. The diagnosis of lymphocytic colitis was based on clinical and histological criteria. Clinically active lymphocytic colitis was defined as chronic or recurrent non‐bloody diarrhea. Histological criteria included increased chronic inflammatory infiltrate (plasma cells, lymphocytes, eosinophils) in the lamina propria; increased number of intraepithelial lymphocytes, damage to surface epithelium, with flattening of epithelial cells or epithelial loss or both and detachment and minimal crypt architecture distortion. In addition, the number of intraepithelial lymphocytes had to be greater than 20 per 100 epithelial cells in the absence of a thickened subepithelial collagen layer to diagnose lymphocytic colitis. Patients with a clear correlation between symptoms and consumption of drugs (e.g. nonsteroidal anti‐inflammatory drugs (NSAIDS), ticlopidine, or proton pump inhibitors) were excluded. All patients underwent a colonoscopy pre‐treatment and 20 patients also underwent a colonoscopy at the end of 6 months. Outcomes included complete and partial clinical response and histological response. Complete response was defined as complete resolution of diarrhea. Partial clinical response was defined as improvement but not resolution of diarrhea. Histological response was defined as normalization of histologic pattern at the end of six months. Adverse events were also reported.
Miehlke 2009 was a randomized, double‐blind, placebo‐controlled trial that compared oral budesonide to placebo in patients with lymphocytic colitis. Forty‐two patients (28 females;14 males; median age 61) with lymphocytic colitis were randomized in a 1:1 ratio via a centralized randomization list in blocks of four to receive oral budesonide (9 mg/day, n = 21) or placebo (n = 20) for 6 weeks of treatment. Non‐responders at week six were treated with open‐label budesonide (9 mg/day) for an additional 6 weeks. Clinically active lymphocytic colitis was defined as more than three watery or loose stools per day on average, for at least 4 weeks. The diagnosis of lymphocytic colitis was based on histology and confirmed by the central pathologist. The number of intraepithelial lymphocytes had to be greater than 20/100 epithelial cells, with inflammation of the lamina propria. The primary outcome measure was clinical remission at 6 weeks which was defined as no more than 3 non‐watery stools per day on average, and a reduction in more than stool compared with screening. Complete colonoscopy including histology was assessed at entry and after 6 weeks, and was used to determine rates of histological remission and histological response. Histological remission was defined as a reduction in the number of intraepithelial lymphocytes to less than 20/100 epithelial cells, plus a reduction in lamina propria inflammation. Histological response was defined as a greater than 50% reduction in the number of intraepithelial lymphocytes compared to baseline or a reduction in lamina propria inflammation or both. Other outcome measures included clinical remission from lymphocytic colitis after 3 weeks, quality of life, which was assessed at entry and after 6 weeks with the validated SF‐36 instrument (a 36 item questionnaire measuring both physical and mental components of quality of life), and the occurrence of adverse events.
Pardi 2009 was a double‐blind, randomized controlled trial comparing controlled ileal‐release budesonide to placebo for the treatment of patients with diarrhea and microscopically confirmed lymphocytic colitis (the criteria were not described). Patients with possible drug‐induced lymphocytic colitis were excluded, unless symptoms persisted despite discontinuation of the offending medication. The study included 15 patients who were randomized to receive either 9 mg/day of budesonide (3 x 3 mg; n = 11) or three placebo capsules (n = 4). Symptoms were self‐recorded daily then averaged for each week of the study. The primary outcome was global assessment of improvement in diarrhea as assessed by the patient. This was defined as at least a 50% improvement in the frequency of bowel movements in at least three of the last four weeks of the study, compared to baseline. Clinical histologic features were graded on a four‐point scale which accounted for features such as intraepithelial lymphocyte infiltration and lamina propria cellularity. Colon biopsies were taken before study entry, and follow‐up sigmoidoscopy and biopsy was requested from each subject at the end of the study. No definitions were provided for either complete or partial clinical remission, or histologic remission. As such, all results were described in terms of either improvement or worsening of the patient's condition. In our analysis, we defined clinical response as an improvement in global assessment score and histologic response as an improvement in the histologic score. Secondary outcomes included improvement in number of bowel movements per day, consistency of stool, abdominal discomfort, histology and adverse events.
Latella 2010, which was published in abstract form only, was a randomized, open‐label trial comparing beclometasone dipropionate to mesalazine for the treatment of patients with lymphocytic colitis. The study included 46 patients with lymphocytic colitis. The diagnosis of lymphocytic colitis was based on clinical and histological criteria. Clinically active lymphocytic colitis was defined as greater than 3 watery or loose stools per day on average per week and a history of diarrhea lasting for more than four weeks. Histologic criteria included a complete colonoscopy with colonic biopsies within four weeks of entry, and a histologically confirmed diagnosis of active lymphocytic colitis. Patients were randomized to receive beclometasone dipropionate 5 mg/day (n = 18), beclometasone dipropionate 10 mg/day (n = 13) or mesalazine 2.4 g/day (n = 15) for 8 weeks. Outcomes included clinical remission at 8 weeks, defined as less than or equal to 3 soft or solid stools per day during the last week of treatment, and relapse at 6 and 12 months after treatment, defined as greater than three stools per day on four or more consecutive days.
Risk of bias in included studies
The overall results of the risk of bias analysis are reported in Figure 2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Fine 1999 reported that randomization was achieved by pulling pieces of paper out of a sealed box. Based on this information we decided to rate random sequence generation as low risk. The other four studies reported the use of computer‐generated randomization and these studies were also rated as low risk of bias (Calabrese 2007; Latella 2010; Miehlke 2009; Pardi 2009).
Two studies did not describe methods used for allocation concealment and were rated as unclear for this item (Calabrese 2007; Fine 1999). Two studies employed a centralized randomization scheme and were rated as low risk of bias for allocation concealment (Miehlke 2009; Latella 2010). The Pardi 2009 used opaque sealed envelopes to conceal allocation and therefore this was rated as low risk of bias for this item.
Blinding
Two studies were open‐label and were rated as high risk of bias for blinding (Calabrese 2007; Latella 2010). Both the caregivers and data analysts were blinded for the Latella 2010 study. Two studies were described as double‐blind and utilized an identical placebo and thus were rated as low risk of bias for blinding (Fine 1999; Miehlke 2009). The Pardi 2009 study was described as double‐blind. However, an identical placebo was not available from the sponsor at the time of the study. All research personal including the pathologist were blinded to treatment assignment. Patients did not know treatment assignment unless they discovered what budesonide was supposed to look like. The authors did not formally assess whether patients knew which treatment they received. The Pardi 2009 study was rated as unclear risk of bias for blinding.
Incomplete outcome data
Three studies reported that there were no drop outs (Calabrese 2007; Pardi 2009; Latella 2010). These studies were rated as low risk of bias for incomplete outcome data. One patient dropped out of the Fine 1999 study, which was rated as low risk of bias for incomplete outcome data. Dropouts were balanced across treatment groups in the Miehlke 2009 study and this study was rated as low risk of bias for incomplete outcome data.
Selective reporting
All of the included studies were rated as low risk of bias for selective outcome reporting as all expected outcomes were reported.
Other potential sources of bias
All of the included studies were rated as low risk of bias for other potential sources of bias. These studies appeared to be free of other potential sources of bias.
Effects of interventions
See: Summary of findings for the main comparison Bismuth subsalicylate versus placebo for treating lymphocytic colitis; Summary of findings 2 Budesonide versus mesalazine for treating lymphocytic colitis; Summary of findings 3 Mesalazine versus mesalazine + cholestyramine for treating lymphocytic colitis; Summary of findings 4 Beclometasone dipropionate versus mesalazine for treating lymphocytic colitis
Bismuth subsalicylate versus placebo
Clinical response
In the Fine 1999 study, all 3 patients in the bismuth subsalicylate group achieved a clinical response after 8 weeks of treatment, while those 2 patients assigned to placebo did not (RR 5.25, 95% CI 0.41 to 67.73). A GRADE analysis rated the overall quality of the evidence supporting this outcome as very low due to unclear risk of bias (allocation concealment) and very serious imprecision (3 events; See summary of findings Table for the main comparison).
Histological response
In Fine 1999, 2 of 3 patients in the bismuth subsalicylate group achieved a histological response after 8 weeks of treatment, compared to 1 of the 2 patients treated with placebo (RR 1.33, 95% CI 0.27 to 6.61). A GRADE analysis rated the overall quality of the evidence supporting this outcome as very low due to unclear risk of bias (allocation concealment) and very serious imprecision (3 events; See summary of findings Table for the main comparison).
Quality of life
Fine 1999 did not report on quality of life as an outcome measure.
Adverse events
Fine 1999 reported no adverse events in either the bismuth subsalicylate or placebo groups.
Budesonide versus placebo
Clinical response
In Miehlke 2009, 18 of 21 patients in the budesonide group achieved a clinical response after 6 weeks of treatment, compared to 10 of 21 patients in the placebo group (RR 1.80, 95% CI 1.11 to 2.91). The number needed to treat to achieve a clinical response with budesonide was 3 patients.
In Pardi 2009, 10 of 11 patients in the budesonide group achieved a clinical response after 8 weeks of treatment, compared to 1 of 4 patients in the placebo group (RR 3.64, 95% CI 0.66 to 20.06).
The pooled analysis for clinical response showed a statistically significant benefit for budesonide over placebo. Clinical response was noted in 88% of budesonide patients compared to 38% of placebo patients (2 studies; 56 participants; RR 2.03, 95% CI 1.25 to 3.33; I2 = 0%). The number needed to treat to achieve a clinical response with budesonide was two. A GRADE analysis rated the overall quality of the evidence supporting this outcome as low due to unclear risk of bias for blinding and sparse data (39 events; See summary of findings Table 2).
Histological response
In Miehlke 2009, 11 of 15 patients in the budesonide group who underwent a follow‐up colonoscopy achieved a histological response after 6 weeks of treatment, compared to 4 of 13 patients in the placebo group who underwent a follow‐up colonoscopy (RR 2.81, 95% CI 1.00 to 5.69). The number needed to treat to achieve a histological response with budesonide was 3 patients.
In Pardi 2009, 7 of 8 patients in the budesonide group who underwent follow‐up sigmoidoscopy achieved a histological response after 8 weeks of treatment, compared to 1 of 3 patients ) in the placebo group (RR 2.63 95% CI 0.52 to 13.29).
The pooled analysis for histological response showed a statistically significant benefit for budesonide over placebo. Histological response was noted in 78% of budesonide patients compared to 33% of placebo patients (2 studies; 39 participants; RR 2.44, 95% CI 1.13 to 5.28; I2 = 0%). The number needed to treat to achieve a histological response with budesonide was three. A GRADE analysis rated the overall quality of the evidence supporting this outcome as low due to very sparse data (23 events; See summary of findings Table 2).
Quality of life
SF‐36 scores at baseline were reduced compared to normal values for both the physical and mental domains. In the budesonide group, the mean physical sum score increased from 42.0 at baseline to 49.7 after 6 weeks of treatment, while the mean mental sum score was unchanged, with a value of 46.5 at baseline and 46.9 after 6 weeks (Miehlke 2009). In the placebo group, the mean physical sum score increased from 44.1 at baseline to 48.0 after 6 weeks of treatment, while the mean mental sum score was unchanged, with a value of 49.0 at baseline and 49.1 after 6 weeks (Miehlke 2009).
Adverse events
In Miehlke 2009, 6 adverse events occurred in 2 patients (10%) in the budesonide group, compared to 9 adverse events in 3 patients (15%) in the placebo group (RR 0.63, 95% CI 0.12 to 3.41). A GRADE analysis rated the overall quality of the evidence supporting this outcome as low due to very sparse data (5 events; See summary of findings Table 2). All adverse events were graded as mild or moderate in intensity. The most common adverse event were headaches. One patient in the budesonide group withdrew due to adverse events (nausea and headache) and one patient in the placebo group withdrew due to adverse events. Serious adverse events were reported in two placebo patients (nausea, abdominal pain, and hyperhidrosis in one patient; and headache, neck pain, and abdominal discomfort in the other patient).
Pardi 2009 reported no adverse events in either budesonide or placebo treatment groups.
Mesalazine versus mesalazine + cholestyramine
Clinical response
In Calabrese 2007, 17 of 20 patients in the mesalazine alone group achieved a clinical response after 6 months of treatment, compared to 18 of 21 patients in the mesalazine + cholestyramine group (RR 0.99; 95% CI 0.77 to 1.28). A GRADE analysis rated the overall quality of the evidence supporting this outcome as low due to high risk of bias (blinding), unclear allocation concealment and sparse data (35 events; See summary of findings Table 3).
Histological response
In Calabrese 2007, all patients with lymphocytic colitis who had a clinical response showed a normalization of histological features, specifically normalization of intraepithelial lymphocytes. Seventeen of 20 patients in the mesalazine group had a histological response compared to 18 of 21 patients in the combined mesalazine + cholestyramine group (RR 0.99; 95% CI 0.77 to 1.28). A GRADE analysis rated the overall quality of the evidence supporting this outcome as low due to high risk of bias (blinding), unclear allocation concealment and sparse data (35 events; See summary of findings Table 3).
Quality of life
In Calabrese 2007, quality of life was not reported as an outcome measure.
Adverse events
In Calabrese 2007, 2 of 21 patients (10%) in the mesalazine + cholestyramine group reported nausea as an adverse event. This was considered a mild event and did not lead to study withdrawal. No events were reported in the mesalazine alone group.
Beclometasone dipropionate versus mesalazine
Clinical response
In Latella 2010, 15 of 18 patients in the 5 mg/day beclometasone dipropionate group achieved a clinical remission after 8 weeks of treatment, compared to 11 of 13 in the 10 mg/day beclometasone dipropionate group, and 13 of 15 in the mesalazine group. There was no statistically significant difference in the proportion of patients who achieved clinical remission. Eighty‐four per cent (26/31) of patients in the beclometasone dipropionate group achieved remission compared to 86% (13/15) of mesalazine patients (RR 0.97, 95% CI 0.75 to 1.29). A GRADE analysis rated the overall quality of the evidence supporting this outcome as low due to high risk of bias (blinding) and sparse data (39 events; See summary of findings Table 4).
At 12 months, 5 of 18 patients (28%; 95% CI 12% to 51%) in the beclometasone dipropionate group maintained clinical remission compared to 3 of 13 patients (23%; 95% CI 8% to 50%) in the 10 mg/day beclometasone dipropionate group and 3 of 15 patients (20%; 95% CI 7% ‐ 45%) in the mesalazine group. There was no statistically significant difference in the proportion of patients who maintained clinical remission. Twenty‐six per cent (8/31) of patients in the beclometasone dipropionate group maintained remission at 12 months compared to 20% (3/15) of mesalazine patients (RR 1.29, 95% CI 0.40 to 4.18). A GRADE analysis rated the overall quality of the evidence supporting this outcome as very low due to high risk of bias (blinding) and very sparse data (11 events; See summary of findings Table 4).
Histological response
Latella 2010 did not report on histological response as an outcome measure.
Quality of life
Latella 2010 did not report on quality of life as an outcome measure.
Adverse events
In the Latella 2010 study, 3 of 46 patients discontinued treatment prematurely because of adverse events; one from each of the three groups. Adverse events reported in the beclometasone dipropionate group were nausea,sleepiness and change of mood in the 5 mg/day and 10 mg/day groups, respectively. In the mesalazine group, one patient discontinued treatment due to a skin rash.
Discussion
Lymphocytic colitis is a cause of chronic diarrhea. It is related to collagenous colitis, and together these two disorders fall under the more general term 'microscopic colitis'. In the past, most research has focused on the therapy of collagenous colitis, and as a result, clinicians treating patients with lymphocytic colitis have relied on the results of uncontrolled trials, case series, and the extrapolation of data for treating collagenous colitis. However, there are currently five RCTs that exist for the treatment of lymphocytic colitis.
The results of Miehlke 2009 and Pardi 2009 provide low quality evidence suggesting that budesonide may be effective at inducing both clinical and histological responses in patients with active lymphocytic colitis. Budesonide also appears to be well‐tolerated in these patients. However, both trials were small in size (Miehlke 2009 with 42 patients, Pardi 2009 with 15 patients) and the results should be interpreted with caution. In comparison, three studies examined the use of budesonide as therapy for active collagenous colitis, demonstrating both a clinical and histological benefit after six to eight weeks of treatment with 9 mg daily or in a tapering schedule (Baert 2002; Bonderup 2003; Miehlke 2002). Additionally, two studies demonstrated that budesonide is effective at maintaining clinical and histological responses in collagenous colitis over a period of six months (Bonderup 2007; Miehlke 2007b). It is conceivable that budesonide would be similarly effective in patients with lymphocytic colitis, but this needs to be proven in larger randomized placebo‐controlled trials.
Calabrese 2007 included 41 patients with lymphocytic colitis in a trial of mesalazine versus mesalazine plus cholestyramine. Clinical response was achieved in 85% of patients who received mesalazine compared to 86% of patients who received mesalazine plus cholestyramine (P = 0.95). The addition of cholestyramine to the treatment regimen did not provide any additional benefit for patients with lymphocytic colitis. Both treatments appeared to be well‐tolerated. The results of this study provide low quality evidence suggesting that mesalazine (800 mg three times daily) with or without cholestyramine may be effective for treatment of lymphocytic colitis. Additionally, 23 patients with collagenous colitis enrolled in the same trial responded similarly to these treatments. However, these results should be interpreted with caution due to the small number of patients enrolled, the lack of blinding and no placebo control. Some of the measured effect in both groups may have been due to spontaneous improvement of the disease. The potential benefits and harms of mesalazine with or without cholestyramine needs to be confirmed by a large placebo‐controlled study.
Latella 2010 assessed the 12‐month clinical outcomes of 46 patients with lymphocytic colitis who were randomized to beclometasone dipropionate at doses of 5 mg/day or 10 mg/day or mesalazine at a dose of 2.4 g/day. Clinical remission was achieved in 84% of patients in the beclometasone dipropionate group and 86% of the mesalazine group at 8 weeks (P = 0.80). This study provides low quality evidence suggesting that beclometasone dipropionate and mesalazine may be effective in achieving clinical remission. However, at 12 months only 26% of patients in the beclometasone dipropionate group and 20% in the mesalazine group maintained clinical remission (P = 0.67). This study provides very low quality evidence suggesting that these interventions may not be effective in maintaining remission. Moreover, this trial should be interpreted with caution as there was a small number of patients enrolled, and there was a lack of blinding and no placebo control. Both treatments appear to be well tolerated. The efficacy and safety of beclometasone dipropionate and mesalazine in achieving and maintaining remission needs to be assessed in a large placebo‐controlled trial.
Fine 1999, which studied bismuth subsalicylate therapy for lymphocytic colitis included only five patients with this diagnosis. Although all three patients receiving active drug experienced clinical improvement compared to none of the placebo patients there were no statistically significant differences in clinical or histological response. While these findings are of interest and provide very low quality evidence suggesting that this medication may be effective for treating lymphocytic colitis, no conclusions can be made from such a small number of patients. Additionally, nine patients with collagenous colitis enrolled in this study demonstrated a clinical and histological benefit with bismuth subsalicylate over placebo.
Although lymphocytic colitis and collagenous colitis are similar disorders, it has not been proven that therapies effective for one disorder will also be effective for the other. Epidemiologically, these two disorders are quite similar. Although most early reports of patients with microscopic colitis described collagenous colitis rather than lymphocytic colitis (Zins 1995), recent population‐based studies suggest similar frequencies for these disorders (Fernandez 1999; Olesen 2004a). Both disorders are more common in women than in men, although the relative frequency of collagenous colitis in women is greater than that for lymphocytic colitis (Chande 2005; Fernandez 1999; Olesen 2004a). The mean age at diagnosis is in the sixth or seventh decade (Agnarsdottir 2002; Fernandez 2003; Goff 1997; Olesen 2004a). An association with autoimmune diseases (Bohr 1996; Fernandez 2003; Kitchen 2002), and the use of NSAIDs is also common in both lymphocytic colitis and collagenous colitis (Bohr 1996; Chande 2005; Goff 1997; Kitchen 2002; Pardi 2002; Riddell 1992; Wang 1999).
Despite sharing certain epidemiologic features, lymphocytic colitis and collagenous colitis have important pathologic differences. The presence of a thickened subepithelial collagen band is the key diagnostic feature found in collagenous colitis. A marked increase in the number of intraepithelial lymphocytes is required to diagnose lymphocytic colitis. Treatments aimed at decreasing the thickness of the collagen band may not necessarily influence the number of intraepithelial lymphocytes. However, most medications used to treat collagenous colitis have anti‐inflammatory activities and consequently might be effective at reducing the number of mucosal inflammatory cells in patients with lymphocytic colitis. In fact, some studies of collagenous colitis treatment with follow‐up histology have shown a decrease in the number of inflammatory cells, findings which could potentially be translated to patients with lymphocytic colitis (Baert 2002; Bonderup 2003; Fine 1999; Miehlke 2002).

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
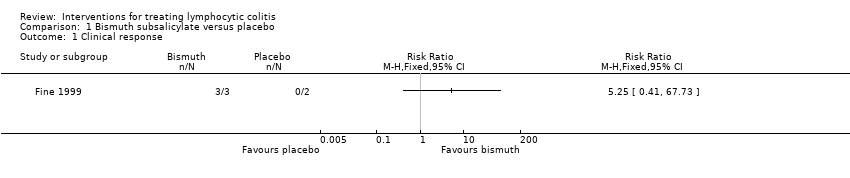
Comparison 1 Bismuth subsalicylate versus placebo, Outcome 1 Clinical response.

Comparison 1 Bismuth subsalicylate versus placebo, Outcome 2 Histological response.

Comparison 2 Budesonide versus placebo, Outcome 1 Clinical response.

Comparison 2 Budesonide versus placebo, Outcome 2 Histological response.
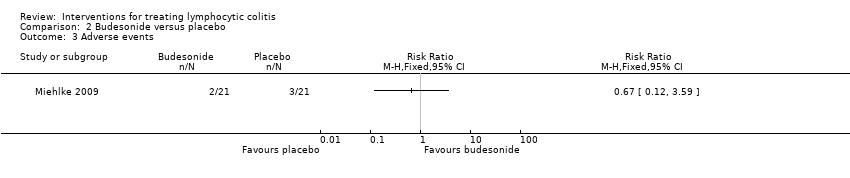
Comparison 2 Budesonide versus placebo, Outcome 3 Adverse events.

Comparison 3 Mesalazine versus mesalazine + cholestyramine, Outcome 1 Clinical response.
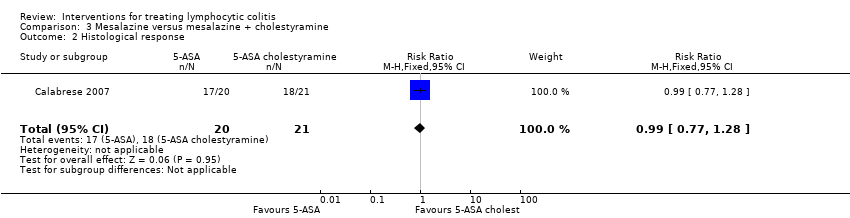
Comparison 3 Mesalazine versus mesalazine + cholestyramine, Outcome 2 Histological response.
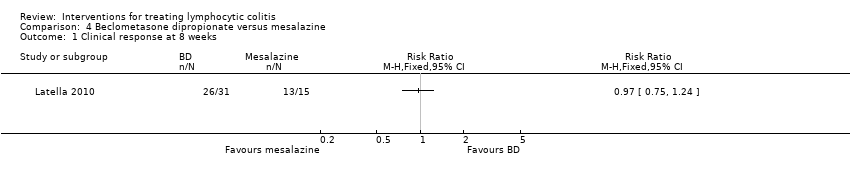
Comparison 4 Beclometasone dipropionate versus mesalazine, Outcome 1 Clinical response at 8 weeks.

Comparison 4 Beclometasone dipropionate versus mesalazine, Outcome 2 Maintenance of clinical response at 12 months.
| Bismuth subsalicylate versus placebo for treating lymphocytic colitis | ||||||
| Patient or population: patients with active lymphocytic colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| control | bismuth vs placebo | |||||
| Clinical response | 0 per 10001 | 0 per 1000 | RR 5.25 | 5 | ⊕⊝⊝⊝ | |
| Histological response | 500 per 10001 | 665 per 1000 (135 to 3300) | RR 1.33 (0.27 to 6.6) | 5 (1 study) | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. | ||||||
| Budesonide versus mesalazine for treating lymphocytic colitis | ||||||
| Patient or population: patients with active lymphocytic colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| control | budesonide versus placebo | |||||
| Clinical response | 440 per 10001 | 893 per 1000 | RR 2.03 | 57 | ⊕⊕⊝⊝ low2,3 | |
| Histological response | 313 per 10001 | 763 per 1000 | RR 2.44 (1.13 to 5.28) | 39 | ⊕⊕⊝⊝ low4 | |
| Adverse events | 143 per 10001 | 96 per 1000 (17 to 513) | RR 0.67 (0.12 to 3.59) | 42 | ⊕⊕⊝⊝ low5 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. | ||||||
| Mesalazine versus mesalazine + cholestyramine for treating lymphocytic colitis | ||||||
| Patient or population: patients with maintenance of remission in lymphocytic colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| mesalazine | mesalazine plus cholestyramine | |||||
| Clinical response | 857 per 1000 | 849 per 1000 (660 to 1000) | RR 0.99 | 41 | ⊕⊕⊝⊝ | |
| Histological response | 857 per 1000 | 849 of 1000 (660 to 1000) | RR 0.99 | 41 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. | ||||||
| Beclometasone dipropionate versus mesalazine for treating lymphocytic colitis | ||||||
| Patient or population: Patients with active or quiescent lymphocytic colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Mesalazine | beclometasone | |||||
| Clinical response at 8 weeks | 867 per 10001 | 841 per 1000 (650 to 1075) | RR 0.97 (0.75 to 1.24) | 46 | ⊕⊕⊝⊝ low2,3 | |
| Maintenance of clinical response at 12 weeks | 200 of 10001 | 258 per 1000 (80 to 836) | RR 1.29 (0.40 to 4.18) | 46 | ⊕⊝⊝⊝ very low2,4 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical response Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Histological response Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical response Show forest plot | 2 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [1.25, 3.33] |
| 2 Histological response Show forest plot | 2 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [1.13, 5.28] |
| 3 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical response Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Histological response Show forest plot | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.77, 1.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical response at 8 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Maintenance of clinical response at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

