داروهای بدون نیاز به نسخه (OTC) برای کاهش سرفه به عنوان مکمل آنتیبیوتیک در درمان پنومونی حاد در کودکان و بزرگسالان
Información
- DOI:
- https://doi.org/10.1002/14651858.CD006088.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 10 marzo 2014see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Infecciones respiratorias agudas
- Copyright:
-
- Copyright © 2014 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
The protocol was written by Christina C Chang (CCC), Anne B Chang (ABC) and Allen C Cheng (ACC) based on previous protocols on cough in children.
For the review: CCC and ABC selected articles from the search, performed the data extraction and data analysis, and wrote the review.
ACC was the adjudicator if disagreement occurred and contributed to writing the review.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
NHMRC, Australia.
Practitioner fellowship (ABC) grant numbers 545216 and 1058213.
Centre for Research Excellence in Lung Health (grant 1040830).
-
Queensland Health Smart State Funds, Australia.
Salary support for ABC
-
Queensland Children's Medical Research Institute, Australia.
Program Grant
Declarations of interest
ABC: none known. CCC: none known. ACC: none known.
Acknowledgements
We thank Liz Dooley, Managing Editor, and the Cochrane Acute Respiratory Infections (ARI) Group for their advice and support in preparing the protocol and review. We thank Sarah Thorning for the 2011 searches. We also thank Concetto Tartaglia, Thomas Kraemer, Helen Petsky and Margaret McElrea for translation of non‐English articles. Finally, we wish to thank the following people for commenting on the draft review: Chanpen Choprapawon, John Widdicombe, Brandon Carr, Nelcy Rodriguez and Abigail Fraser.
Version history
| Published | Title | Stage | Authors | Version |
| 2014 Mar 10 | Over‐the‐counter (OTC) medications to reduce cough as an adjunct to antibiotics for acute pneumonia in children and adults | Review | Christina C Chang, Allen C Cheng, Anne B Chang | |
| 2012 Feb 15 | Over‐the‐counter (OTC) medications to reduce cough as an adjunct to antibiotics for acute pneumonia in children and adults | Review | Christina C Chang, Allen C Cheng, Anne B Chang | |
| 2007 Oct 17 | Over‐the‐counter (OTC) medications to reduce cough as an adjunct to antibiotics for acute pneumonia in children and adults | Review | Christina C Chang, Allen C Cheng, Anne B Chang | |
| 2006 Jul 19 | Over the counter (OTC) medications to reduce cough as an adjunct to antibiotics for acute pneumonia in children and adults | Protocol | Christina C Chang, Allen C Cheng, Anne B Chang | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Acute Disease;
- Anti‐Bacterial Agents [*therapeutic use];
- Antitussive Agents [*therapeutic use];
- Chemotherapy, Adjuvant [methods];
- Cough [*drug therapy, etiology];
- Drug Therapy, Combination [methods];
- Expectorants [therapeutic use];
- Nonprescription Drugs [*therapeutic use];
- Pneumonia [complications, *drug therapy];
- Randomized Controlled Trials as Topic;
- Treatment Outcome;
Medical Subject Headings Check Words
Adolescent; Adult; Child; Humans;
PICO

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Children ‐ global assessment, Outcome 1 Not cured or not improved.
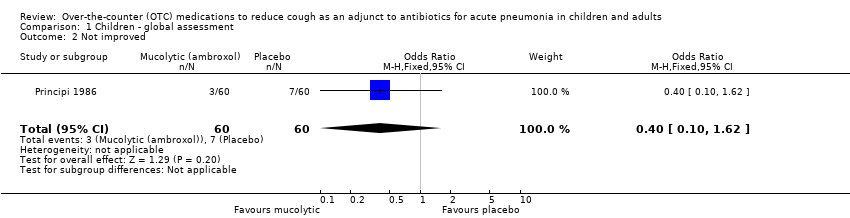
Comparison 1 Children ‐ global assessment, Outcome 2 Not improved.

Comparison 1 Children ‐ global assessment, Outcome 3 Not cured.

Comparison 2 Children ‐ secondary outcomes, Outcome 1 Mean cough score at day 3.
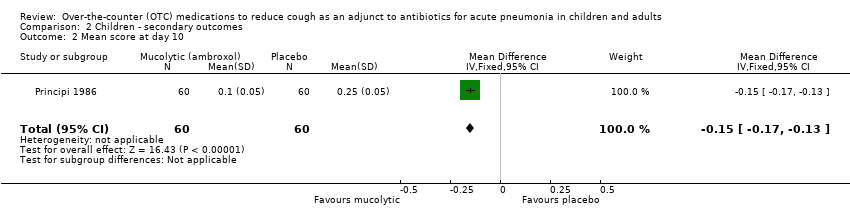
Comparison 2 Children ‐ secondary outcomes, Outcome 2 Mean score at day 10.
Comparison 2 Children ‐ secondary outcomes, Outcome 3 Adverse events (no. of people).

Comparison 3 Adults ‐ global assessment, Outcome 1 Not cured or not improved.

Comparison 3 Adults ‐ global assessment, Outcome 2 Not improved.

Comparison 3 Adults ‐ global assessment, Outcome 3 Not cured.

Comparison 4 Combined children and adults, Outcome 1 Not cured or not improved.

Comparison 4 Combined children and adults, Outcome 2 Not improved.
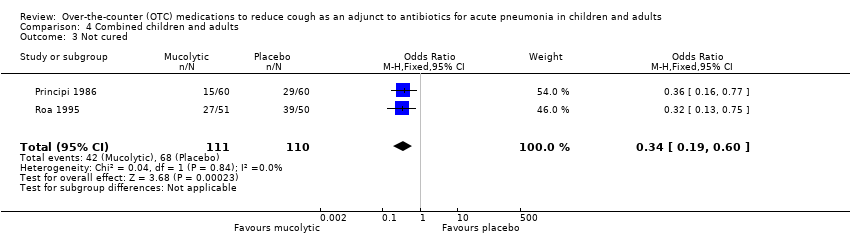
Comparison 4 Combined children and adults, Outcome 3 Not cured.
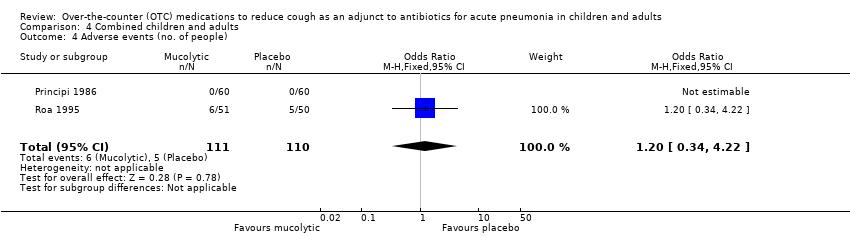
Comparison 4 Combined children and adults, Outcome 4 Adverse events (no. of people).
| Mucolytics as an adjunct to antibiotics to reduce cough in acute pneumonia in children and adults | ||||||
| Patient or population: children and adults with acute pneumonia Settings: any Comparison: antibiotics | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Mucolytics | |||||
| Number of people who had not improved or had not been cured | 16 per 100 | 14 per 100 | OR 0.85 | 221 | ⊕⊕⊝⊝ | Fewer people represents a benefit |
| Cough score | The mean cough score in the control groups was | The mean cough score in the intervention groups was | 120 | ⊕⊕⊝⊝ | Data for children only | |
| Adverse events | See comment | See comment | Not estimable | 120 | See comment | 1 study in children provided data specific to participants with pneumonia ‐ there were no adverse events |
| Complications (e.g. medication change) | See comment | See comment | Not estimable | 0 | See comment | Complications were not measured in the trials |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1In addition to antibiotics, people with pneumonia often use over‐the‐counter (OTC) cough medications when at home or request OTC cough medications when in hospital to suppress an annoying cough. There is a question as to whether suppressing cough may prolong pneumonia. Over‐the‐counter cough medications can include antitussives, expectorants, antihistamine‐decongestants, antihistamines and mucolytics (such as bromhexine, ambroxol and neltenexine). 2Allocation concealment unclear. 3Scale not validated. 4Sparse data. 5Sparse data; confidence interval does not rule out the potential for 'more people' not improved or cured with mucolytics. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Not cured or not improved Show forest plot | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.10, 1.62] |
| 2 Not improved Show forest plot | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.10, 1.62] |
| 3 Not cured Show forest plot | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.16, 0.77] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean cough score at day 3 Show forest plot | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.33, ‐0.17] |
| 2 Mean score at day 10 Show forest plot | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.17, ‐0.13] |
| 3 Adverse events (no. of people) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Not cured or not improved Show forest plot | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.48, 3.04] |
| 2 Not improved Show forest plot | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.48, 3.04] |
| 3 Not cured Show forest plot | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.13, 0.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Not cured or not improved Show forest plot | 2 | 221 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.40, 1.80] |
| 2 Not improved Show forest plot | 2 | 221 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.38, 1.67] |
| 3 Not cured Show forest plot | 2 | 221 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.19, 0.60] |
| 4 Adverse events (no. of people) Show forest plot | 2 | 221 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.34, 4.22] |

