Fármacos antitusivos sin prescripción como complemento de los antibióticos para la neumonía aguda en niños y adultos
Resumen
Antecedentes
La tos suele ser angustiosa para los pacientes con neumonía. En consecuencia, suelen utilizar fármacos sin prescripción para la tos (mucolíticos o supresores de la tos). Éstos podrían proporcionar alivio para reducir la gravedad de la tos, pero la supresión del mecanismo de la tos podría impedir la limpieza de las vías respiratorias y causar daños.
Objetivos
Evaluar la eficacia de los fármacos sin prescripción para la tos como complemento de los antibióticos en niños y adultos con neumonía.
Métodos de búsqueda
Se hicieron búsquedas en CENTRAL 2013, número 12, MEDLINE (enero 1966 hasta enero, semana 2, 2014), OLDMEDLINE (1950 hasta 1965), EMBASE (1980 hasta enero 2014), CINAHL (2009 hasta enero 2014), LILACS (2009 hasta enero 2014) y Web of Science (2009 hasta enero 2014).
Criterios de selección
Ensayos controlados aleatorizados (ECA) en niños y adultos que comparen cualquier tipo de fármaco sin prescripción para la tos con placebo o fármacos de control, con la tos como resultado y cuando la tos es secundaria a la neumonía aguda.
Obtención y análisis de los datos
Se seleccionaron de forma independiente los ensayos para su inclusión. Se extrajeron los datos de estos estudios, se evaluó su calidad metodológica sin desacuerdos y se analizaron con métodos estándar.
Resultados principales
No hay nuevos ensayos para incluir en esta actualización de la revisión. Anteriormente, se habían incluido cuatro estudios con un total de 224 participantes; uno se realizó exclusivamente en niños y tres en adolescentes o adultos. Uno que usaba un antitusivo no tenía datos específicos de neumonía extraíbles. En los estudios restantes, se utilizaron tres mucolíticos diferentes (bromhexina, ambroxol, neltenexina), de los cuales sólo dos tenían datos extraíbles. No demostraron ninguna diferencia significativa para el resultado primario de "no curado o no mejorado" para los mucolíticos. Se redujo un resultado secundario de "no curado" (odds‐ratio (OR) para los niños 0,36, intervalo de confianza (IC) del 95%: 0,16 a 0,77; número necesario a tratar para obtener un beneficio (NNTB) al día 10 = 5 (IC del 95%: 3 a 16) y OR 0,32 para los adultos (IC del 95%: 0,13 a 0,75); NNTB al día 10 = 5 (IC del 95%: 3 a 19)). En un análisis post hoc que combinó datos de niños y adultos, nuevamente no hubo diferencias en el resultado primario de "no curado o no mejorado" (OR 0,85; IC del 95%: 0,40 a 1,80), aunque los mucolíticos redujeron el resultado secundario "no curado" (OR 0,34; IC del 95%: 0,19 a 0,60; NNTB 4; IC del 95%: 3 a 8). El riesgo de sesgo era bajo o incierto.
Conclusiones de los autores
No hay evidencia suficiente para decidir si los fármacos sin prescripción para la tos asociada a la neumonía aguda son beneficiosos. Los mucolíticos pueden ser beneficiosos, pero no hay evidencia suficiente para recomendarlos como tratamiento adyuvante para la neumonía aguda. Esto deja sólo recomendaciones teóricas de que los fármacos sin prescripción que contienen codeína y antihistamínicos no deben utilizarse en niños pequeños.
PICOs
Resumen en términos sencillos
Fármacos sin prescripción para ayudar a reducir la tos en niños y adultos que toman antibióticos para la neumonía aguda
Hay muchas causas de la tos aguda, una de las cuales es la neumonía. La tos es una carga y perjudica la calidad de vida. Los pacientes suelen comprar y utilizar fármacos sin prescripción y el personal sanitario los recomienda como fármacos adicionales para el tratamiento de la neumonía. Existen muchas clases de fármacos sin prescripción para la tos, como los mucolíticos (fármacos que pueden reducir el grosor de la mucosidad) y los antitusígenos (fármacos que suprimen la tos). La presente revisión tiene por objeto equilibrar los posibles beneficios de esos agentes con sus posibles riesgos.
En esta revisión, se encontraron cuatro estudios con un total de 224 participantes que eran adecuados para su inclusión; uno se realizó exclusivamente en niños y tres en adolescentes o adultos. Sin embargo, sólo se pudieron obtener datos de dos estudios; en ambos, se utilizaron mucolíticos (ambroxol y bromhexina) junto con antibióticos. Combinando estos dos estudios, la tasa de curación o mejora de la tos de los pacientes que recibieron mucolíticos fue similar a la de los que no los recibieron. Sin embargo, en el análisis secundario, los niños que recibieron un mucolítico tenían más probabilidades de curarse de la tos (el número necesario a tratar para beneficiarse (NNTB) al día 10 era de cinco para los niños y cuatro para los adultos). No se informó de un aumento de los eventos adversos en el grupo de tratamiento.
La gama de posibles eventos adversos asociados con los fármacos sin prescripción para la tos es amplia e incluye desde eventos adversos mínimos (como con el uso de la miel) hasta eventos adversos graves, como la alteración de los patrones de frecuencia cardíaca, la somnolencia y la muerte en niños pequeños. Los estudios incluidos en esta revisión no informaron de ningún aumento detectable de los eventos adversos. No hubo sesgos obvios en los estudios.
Esta revisión tiene limitaciones sustanciales debido a la falta de disponibilidad de datos de los estudios. Tampoco hay estudios de otros fármacos sin prescripción comunes utilizados para la tos, como los antihistamínicos y los antitusígenos.
Así pues, no hay evidencia suficiente para extraer conclusiones definitivas sobre el papel de los fármacos sin prescripción tomados como tratamiento adicional para la tos asociada con la neumonía aguda. Los mucolíticos pueden ser beneficiosos, pero la falta de evidencia consistente impide recomendar el uso sistemático de los mucolíticos como complemento en el tratamiento de la tos molesta asociada con la neumonía en niños o adultos. La evidencia está actualizada hasta enero 2014.
Authors' conclusions
Summary of findings
| Mucolytics as an adjunct to antibiotics to reduce cough in acute pneumonia in children and adults | ||||||
| Patient or population: children and adults with acute pneumonia Settings: any Comparison: antibiotics | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Mucolytics | |||||
| Number of people who had not improved or had not been cured | 16 per 100 | 14 per 100 | OR 0.85 | 221 | ⊕⊕⊝⊝ | Fewer people represents a benefit |
| Cough score | The mean cough score in the control groups was | The mean cough score in the intervention groups was | 120 | ⊕⊕⊝⊝ | Data for children only | |
| Adverse events | See comment | See comment | Not estimable | 120 | See comment | 1 study in children provided data specific to participants with pneumonia ‐ there were no adverse events |
| Complications (e.g. medication change) | See comment | See comment | Not estimable | 0 | See comment | Complications were not measured in the trials |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1In addition to antibiotics, people with pneumonia often use over‐the‐counter (OTC) cough medications when at home or request OTC cough medications when in hospital to suppress an annoying cough. There is a question as to whether suppressing cough may prolong pneumonia. Over‐the‐counter cough medications can include antitussives, expectorants, antihistamine‐decongestants, antihistamines and mucolytics (such as bromhexine, ambroxol and neltenexine). 2Allocation concealment unclear. 3Scale not validated. 4Sparse data. 5Sparse data; confidence interval does not rule out the potential for 'more people' not improved or cured with mucolytics. | ||||||
Background
Description of the condition
Cough is the most common symptom presenting to general practitioners (Britt 2002; Cherry 2003). Acute cough (duration less than two weeks) (Chang 2006) has multiple causes, including pneumonia. Whatever the cause, attempting to reduce the impact of the symptom of cough is reflected in the billions spent on over‐the‐counter (OTC) cough medications. Cough impairs quality of life (French 2002) and causes significant anxiety to the parents of children (Cornford 1993). Accordingly, patients with pneumonia sometimes self medicate with over‐the‐counter (OTC) cough medications in ambulatory settings, or ask for them in hospital.
Description of the intervention
A Cochrane review showed that antihistamine‐analgesic‐decongestion combinations have some general benefit in adults and older children with the common cold but not in young children (de Sutter 2012). In the management of acute cough, in the ambulatory setting, a Cochrane review found no good evidence for or against the use of OTC medications (Smith 2012). There is no clear benefit of antihistamines (either singly or in combination) in young children for relieving acute cough (de Sutter 2012; Smith 2012). Moreover, they are associated with potentially significant adverse events including altered consciousness, arrhythmia and death (Gunn 2001; Kelly 2004). None of these reviews included patients with pneumonia (Smith 2012). There are also Cochrane reviews on chronic non‐specific cough but this is unrelated to this review, which focuses on acute cough associated with pneumonia.
How the intervention might work
Cough is usually divided into acute or chronic according to its duration and age group. It is defined as chronic if over eight weeks duration in adults, and over three to four weeks in children (Chang 2005). This reflects the different conditions causing chronic cough in different age groups. In contrast, in this review we examined the efficacy of OTC medication for acute cough in acute pneumonia, where the pathophysiological processes (albeit poorly understood) are likely to be the same in children and adults. Methods for determining cough outcomes are similar in adults and children, although these methods remain poorly standardised. Objective measurements of cough include cough frequency and cough sensitivity outcomes, whilst subjective measurements of cough may broadly encompass quality‐of‐life and outcomes based on diaries etc. (Birring 2006; Chang 2003).
Why it is important to do this review
Although OTC cough medications might provide some relief by reducing the severity of the cough, they might also be harmful in prolonging pneumonia (by suppressing the cough reflex, which might cause retention of airway debris). Thus, a systematic review of their benefits or harms is useful to help guide clinical practice.
Objectives
To evaluate the efficacy of OTC medications for cough as an adjunct to antibiotics in children and adults with pneumonia.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing any type of OTC cough medication with a placebo (or control group) with cough as an outcome and where cough is secondary to acute pneumonia. We excluded quasi‐randomised trials.
Types of participants
We considered studies of both children and adults with cough of less than four weeks in duration that was related to pneumonia. We specifically excluded studies of cough of more than four weeks in duration and cough related to another underlying cardio‐respiratory condition (for example, suppurative lung disease, chronic obstructive airway disease, asthma). However, we considered studies which included cough of mixed aetiologies if data were available for the subgroup of patients with pneumonia.
Types of interventions
RCT comparisons of any type of OTC cough medication as an adjunct therapy to antibiotics. We did not include trials comparing only two or more medications without a placebo comparison group. We included trials that included the use of other medications or interventions if all participants had equal access to such medications (including antibiotics) or interventions.
Types of outcome measures
We attempted to obtain data on at least one of the following outcome measures.
Primary outcomes
-
Proportion of participants who were not cured or not substantially improved at follow‐up (failure to improve was measured according to the hierarchy listed below in Secondary outcomes).
Secondary outcomes
-
Proportion of participants who were not cured at follow‐up.
-
Change in quantitative differences in cough (cough frequency, cough scores, other quantitative outcomes based on cough diary).
-
Proportion experiencing adverse effects of the intervention (for example, sleepiness, nausea, etc.).
-
Proportion experiencing complications (for example, requirement for medication change, etc.).
We adopted and recorded individual trial definitions.
As it was likely that studies may have differed in their definitions of cure and improvement, we adopted a hierarchical approach that employed the reported outcome measures. For example, if both an objective measure and a subjective measure of cough frequency were reported, we were to adopt the objective measure in assessing the efficacy of treatment. Our hierarchy of outcome measures was as follows.
-
Objective measurements of cough indices (cough frequency, cough receptor sensitivity).
-
Symptomatic (quality of life, Likert scale, visual analogue scale, level of interference of cough, outcomes‐based cough diary): assessed by the patient (adult or child).
-
Symptomatic (quality of life, Likert scale, visual analogue scale, level of interference of cough, outcomes‐based cough diary): assessed by the parents or carers.
-
Symptomatic (Likert scale, visual analogue scale, level of interference of cough, outcomes‐based cough diary): assessed by clinicians.
-
Fever, respiratory rate, oxygen requirement.
-
Non‐clinical outcomes (chest radiology, white cell count, C‐reactive protein, erythrocyte sedimentation rate, lung function test (spirometry)).
-
Eradication of micro‐organism(s) causing the pneumonia.
Search methods for identification of studies
Electronic searches
For this update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2013, Issue 12, part of The Cochrane Library,www.thecochranelibrary.com (accessed 22 January 2014), which contains the Acute Respiratory Infections Group's Specialised Register, MEDLINE (January 1966 to January week 2, 2014), OLDMEDLINE (1950 to 1965), EMBASE (1980 to January 2014), CINAHL (2009 to January 2014), LILACS (2009 to January 2014) and Web of Science (2009 to January 2014).
We used the following search strategy to search MEDLINE and CENTRAL. We adapted the search strategy for EMBASE (see Appendix 2), CINAHL (see Appendix 3), LILACS (see Appendix 4) and Web of Science (see Appendix 5).
MEDLINE (OVID)
1 Cough/
2 cough*.tw.
3 1 or 2
4 exp Pneumonia/
5 (pneumon* or bronchopneumon*).tw.
6 4 or 5
7 3 and 6
8 exp Antitussive Agents/
9 antitussiv*.tw,nm.
10 exp Expectorants/
11 expectorant*.tw,nm.
12 exp Cholinergic Antagonists/
13 (cholinergic adj2 (blocking or antagonist*)).tw,nm.
14 (anticholinergic* or anti‐cholinergic*).tw,nm.
15 exp Histamine H1 Antagonists/
16 histamine h1 antagonist*.tw,nm.
17 (antihistamin* or anti‐histamin*).tw,nm.
18 mucolytic*.tw,nm.
19 exp Drug Combinations/
20 drug combination*.tw.
21 exp Nonprescription Drugs/
22 ((non prescribed or non‐prescribed or nonprescribed or non prescription* or non‐prescription* or nonprescription*) adj3 (drug* or medicin* or pharmaceut* or medicat*)).tw.
23 (over‐the‐counter* or over the counter or otc).tw.
24 (cough* adj3 (mixture* or suppress* or medicin* or remed* or relief* or formula* or syrup*)).tw.
25 or/8‐24
26 7 and 25
There were no language or publication restrictions.
Searching other resources
We searched the trials registries ClinicalTrials.gov and WHO ICTRP (searched 20 January 2014). We also searched lists of references in relevant publications.
Data collection and analysis
Selection of studies
Two review authors (CCC, ABC) independently reviewed the literature searches to identify potentially relevant trials for full review from the title, abstract or descriptors. We conducted searches of bibliographies and texts to identify additional studies. The same two review authors independently selected trials for inclusion from the full text, using specific criteria. There was no disagreement. A third review author (ACC) was the nominated adjudicator in case of any disagreements.
Data extraction and management
We reviewed trials that satisfied the inclusion criteria and extracted the following information: study setting; year of study; source of funding; patient recruitment details (including number of eligible patients); inclusion and exclusion criteria; other symptoms; randomisation and allocation concealment method; numbers of participants randomised; blinding (masking) of participants, care providers and outcome assessors; dose and type of intervention; duration of therapy; co‐interventions; numbers of patients not followed up; reasons for withdrawals from study protocol (clinical, side effects, refusal and other); details on side effects of therapy and whether intention‐to‐treat (ITT) analyses were possible. We extracted data on the outcomes described previously. It was planned that further information would be requested from the trial authors, where required.
Assessment of risk of bias in included studies
Two review authors (CCC, ABC) independently performed a potential bias assessment on studies included in the previous review. We described seven components of potential biases under Assessment of reporting biases in our updated review
Measures of treatment effect
We undertook an initial qualitative comparison of all the individually analyzed studies to determine if pooling of results (meta‐analysis) was reasonable. This took into account differences in study populations, inclusion and exclusion criteria, interventions, outcome assessment and estimated effect size. We included the results from studies that met the inclusion criteria and reported any of the outcomes of interest in the subsequent meta‐analyses.
We calculated individual and pooled statistics for continuous outcomes measured on the same metrics as mean differences (MD) and standard mean differences, as indicated, with 95% confidence intervals (CIs). We combined data for continuous outcomes measured on different metrics, with a standardised mean difference (SMD). We calculated individual and pooled statistics as odds ratio (OR) with 95% CIs for dichotomous variables.
Unit of analysis issues
Had there been any cross‐over studies, we would have calculated mean treatment differences from raw data, extracted or imputed and entered as fixed‐effect generic inverse variance (GIV) outcomes, to provide summary weighted differences and 95% CIs. Only data from the first arm would have been included in a meta‐analysis where data were combined with parallel studies (Elbourne 2002).
Dealing with missing data
We planned to contact trial authors for missing data when the studies were less than 15 years old.
Assessment of heterogeneity
We described any heterogeneity between the study results and tested to see if it reached statistical significance using the I2 statistic (Higgins 2003). Heterogeneity is considered significant when the P value of the Chi2 test is < 0.10 (Higgins 2011). We would have included the 95% CI estimate using a random‐effects model had there been concerns about statistical heterogeneity.
Assessment of reporting biases
In this updated review, in line with the new Cochrane process, we assessed the risk of bias of each study including sequence generation, allocation concealment, blinding and reporting of outcome data.
While there are other possible biases (such as publication bias detected by funnel plot) as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), these were not included.
Data synthesis
We calculated odds ratios (ORs) using a modified ITT analysis for dichotomous outcome variables of each individual study. This analysis assumed that participants not available for outcome assessment had not improved (and probably represented a conservative estimate of effect). We calculated the summary weighted ORs and 95% CI (fixed‐effect model) using the computer program RevMan 2012. We calculated the number needed to treat to benefit (NNTB) from the pooled OR and its 95% CI, applied to a specified baseline risk using an online calculator (Cates 2003).
We assumed the cough indices to be normally distributed continuous variables so that the mean difference in outcomes could be estimated (mean difference). We would have estimated the standardised mean difference if studies had reported outcomes using different measurement scales.
Subgroup analysis and investigation of heterogeneity
We planned an a priori subgroup analysis for:
-
children (14 years and younger) versus adolescents and adults (older than 14 years);
-
hospitalised versus ambulatory settings;
-
classes of OTC cough medications:
-
antitussives (codeine and derivatives);
-
expectorants;
-
mucolytics;
-
antihistamine‐decongestant combinations;
-
antihistamines alone;
-
other drug combinations;
-
males versus females in adults.
-
Sensitivity analysis
It was planned that sensitivity analyses be carried out to assess the impact of potentially important factors on the overall outcomes:
-
study quality;
-
study size;
-
variation in the inclusion criteria;
-
differences in the medications used in the intervention and comparison groups;
-
differences in outcome measures;
-
analysis using a random‐effects model;
-
analysis by 'treatment received';
-
analysis by 'ITT';
-
analysis by study design, parallel and cross‐over studies.
Results
Description of studies
Results of the search
In the first version of this review (Chang 2007), the search identified 238 potentially relevant titles. After reviewing the abstracts, we obtained 21 full‐text papers, included four studies and excluded 17 papers (details are in the Characteristics of excluded studies table). Most were non‐randomised studies or performed without a placebo. A review article was identified in this search (Ida 1997) which described three studies of dimemorfan (a dextromethorphan analogue) not identified using the original search strategy. One of these three studies was described as a placebo‐controlled trial. Unfortunately we were not able to obtain this and there was insufficient details provided in the review article (Ida 1997). Another review paper (Mancini 1996) described three studies, of which one appeared to include patients with acute lower respiratory tract infection (specified as acute bronchitis or bronchoalveolitis but which may have included patients with pneumonia). We attempted to contact the trial authors but were not able to extract data on the subgroup of patients with pneumonia, and thus we excluded the study from further analysis.
In the 2009 update (Chang 2010), we identified two studies (Balli 2007; Titti 2000) on erdosteine (a mucolytic agent) but these were excluded as this is not readily accessible over the counter (details added to the Characteristics of excluded studies table). In the 2011 and 2013 updates, we identified 32 and 49 potential studies respectively but none fulfilled the inclusion criteria. Of note, a study which examined the role of zinc supplementation (Basnet 2012) was excluded as this is not an OTC drug for cough suppression and cough was not an outcome measure (details added to the Characteristics of excluded studies table, thus totalling to 20 excluded studies in this current update).
Included studies
Four studies were included, as described in the 'Characteristics of included studies' table; all were available in English. However, data specific for pneumonia were only available in two papers (Principi 1986; Roa 1995). Authors of three papers did not respond to our correspondence requesting for further pneumonia‐specific data.
Of the included studies, one study was exclusively in children (Principi 1986), two were exclusively in adults (Aquilina 2001; Azzopardi 1964) and one included adolescents and adults (Roa 1995).
One study utilised an antitussive (Dimyril) (Azzopardi 1964) and three of the studies examined the efficacy of different formulations of mucolytics (bromhexine (Roa 1995), neltenexine (Aquilina 2001) and ambroxol (Principi 1986)). In two of these studies, the concomitant antibiotics used were reported (Principi 1986; Roa 1995).
Two studies were multicentre studies (Principi 1986; Roa 1995), for which the funding was unspecified. Two studies were single‐centre studies (Aquilina 2001; Azzopardi 1964). One study was a controlled non‐placebo study (Roa 1995) and the rest utilised a randomised placebo‐controlled design (Aquilina 2001; Azzopardi 1964; Principi 1986). All but one study (Azzopardi 1964) used a parallel design.
The inclusion and exclusion criteria (that is, including the definition of pneumonia) varied between the studies; only one study was exclusively in patients with pneumonia (Principi 1986). In Roa 1995, bacterial pneumonia was defined as the presence of recent productive phlegm, fever or leucocytosis (> 10,000 mm3) and pulmonary infiltrates on radiographic examination. In Principi 1986, inclusion required either a positive blood culture for a well‐defined bacterium or a chest X‐ray showing lobar or sub lobar involvement together with raised inflammatory markers, erythrocyte sedimentation rate ≥ 30 mm/h and C‐reactive protein ≥ 25 μg/mL. The two smaller papers (Aquilina 2001; Azzopardi 1964) which contributed rather fewer numbers to the analysis did not clearly define pneumonia.
The outcomes of the studies also varied and none utilised a validated scale for cough. The larger trials were performed and published 12 and 21 years ago, respectively, and so were not methodologically as robust as one would expect of current‐day trials (Principi 1986; Roa 1995). The Roa 1995 trial evaluated clinical response, bacteriological response and each clinical symptom using a visual analogue scale. Both clinical and bacteriological responses had clearly defined definitions; they defined cure as complete disappearance of pretreatment signs and symptoms, and improvement as an improvement on the visual analogue scale but less than cure. Principi 1986 evaluated clinical and radiological signs and used absolute numbers and severity scores to evaluate clinical symptoms and signs, including cough. The Aquilina 2001 trial used severity scores on prespecified examination days and at the end of therapy; the investigator expressed an overall assessment of the therapeutic efficacy. The Azzopardi 1964 trial was more obviously subjective in its evaluation.
Excluded studies
As described above, we excluded 20 trials (details are provided in the Characteristics of excluded studies table), most on the basis of being non‐randomised, with no placebo.
Risk of bias in included studies
In previous reviews, the agreement between the review authors for the scores was good: the weighted Kappa score for the quality assessment scale was 0.63. In the updated 2011 version we completed a 'Risk of bias' table (Figure 1; Figure 2). Generally, there were no glaring biases in the included studies ‐ the majority of parameters were assessed as low‐risk or unclear risk. There were no high‐risk biases identified. Specifically the method used for sequence generation and whether allocation was concealed were not clear in all the studies. However the blinding of participants and outcomes seemed reasonable.
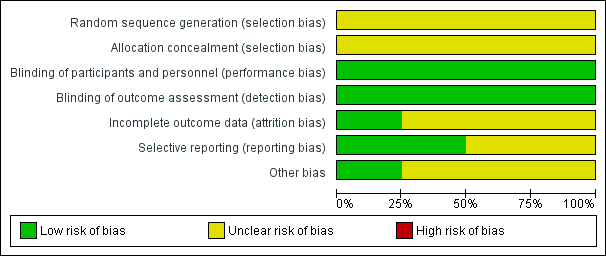
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
See 'Characteristics of included studies' table.
Blinding
See 'Characteristics of included studies' table.
Incomplete outcome data
See 'Characteristics of included studies' table.
Selective reporting
There was limited reporting in the studies. See 'Characteristics of included studies' table.
Other potential sources of bias
Unclear or low‐risk.
Effects of interventions
See: Summary of findings for the main comparison
In one study (Azzopardi 1964) the number of participants with pneumonia was not specified. In the other three included studies (Aquilina 2001; Principi 1986; Roa 1995) the total number of randomised participants was 555, of which 224 had pneumonia. The total number who completed the trials was 518, of which 219 had pneumonia. Given the lack of data, meta‐analysis could not be performed on any outcome when children and adults were considered separately and, thus, sensitivity analysis was irrelevant. Single study results and the data and analysis section are described below.
Paediatrics ‐ Primary outcomes
Mucolytics
Principi reported that cough disappeared more rapidly in children treated with ambroxol than in the placebo group (Principi 1986). However, in the data and analysis section for the primary outcome of 'not cured or not improved' (defined on chest X‐ray), there was no significant difference between groups (odds ratio (OR) 0.40, 95% confidence interval (CI) 0.10 to 1.62) (Analysis 1.1).
Paediatrics ‐ Secondary outcomes
1. Proportion of participants who were not cured at follow‐up
There was also no difference between groups for the secondary outcome of 'no improvement' (OR 0.40, 95% CI 0.10 to 1.62) (Analysis 1.2). However, for the secondary outcome of clinically 'not cured' there was a significant difference between groups (defined on chest X‐ray), as presented in the data and analysis section, favouring the ambroxol group. The OR was 0.36 (95% CI 0.16 to 0.77) (Analysis 1.3) and the number needed to treat to benefit (NNTB) was 5 (95% CI 3 to 16).
2. Change in quantitative differences in cough
Principi reported a significant difference between groups, favouring the ambroxol group from day three onwards (Principi 1986). The data and analysis section for mean cough scores on days 3 and 10 is shown in Analysis 2.1 and Analysis 2.2. For day 3, the mean difference was ‐0.25 (95% CI ‐0.33 to ‐0.17). For day 10, the mean difference was ‐0.15 (95% CI ‐0.17 to ‐0.13).
3. Adverse effects
The trial authors reported no significant adverse events in either group (Principi 1986).
4. Other complications and reported data
Data for other secondary outcomes were not available. There were no studies on any other type of over‐the‐counter (OTC) medication for cough associated with pneumonia in children.
Adults ‐ Primary outcomes
Mucolytics
Two studies (Aquilina 2001; Roa 1995) used a mucolytic but only data from one study (Roa 1995) could be included in this review. In the study using neltenexine (a mucolytic), we could not obtain data specific for those with pneumonia (n = 3) (Aquilina 2001). The trial authors reported no significant adverse events in any of the groups (Aquilina 2001).
Data specifically described for pneumonia were available only for global 'clinical response' and this is presented in the data and analysis section (Analysis 3). For the primary outcome of clinically 'not cured or not improved' there was no significant difference between groups (OR 1.21, 95% CI 0.48 to 3.04) (Analysis 3.1).
Adults ‐ Secondary outcomes
1. Proportion of participants who were not cured at follow‐up
There was also no significant difference between groups for the secondary outcome 'not improved' (OR 1.21, 95% CI 0.48 to 3.04) (Analysis 3.2). However, like the results for children treated with a mucolytic, there was a significant difference between groups for the secondary outcome 'not cured' (OR 0.32, 95% CI 0.13 to 0.75), NNTB 5 (95% CI 3 to 19), favouring those on bromhexine (Analysis 3.3).
2. Change in quantitative differences in cough
The Roa study reported that for the total group (that is, adults with pneumonia and bronchitis) the differences between cough frequency on days three, five and seven and baseline were significantly larger in the bromhexine group compared to the control group (Roa 1995). Data could not be extracted.
3. Adverse effects
The authors reported a total of 11 adverse events, six in the active treatment group and five in the control group (Roa 1995).
4. Other complications and reported data
There was also a difference between groups (favouring bromhexine) for cough discomfort and ease of expectoration on days three and five, but not on day seven, as well as sputum volume on day three, but not on days five or seven. There was no difference between the groups for difficulty in breathing or chest pain on any day. At final evaluation significantly more participants were 'cured' (46%) in the bromhexine group compared to the control group (34%) (Roa 1995). There were no studies on any other type of OTC medication for cough.
Antitussives
The Azzopardi study on 34 adults (total number assumed based on study design (see the Characteristics of included studies table) included adults with pneumonia (number unknown) in addition to other lower respiratory tract infection aetiologies (Azzopardi 1964). Data on those with pneumonia alone were not available and are not described here.
Combined data for children and adults ‐ Primary outcomes
Mucolytics
In a post hoc analysis, we combined data on children and adults. There was no significant statistical heterogeneity in any of the outcomes (Analysis 4.1 to Analysis 4.3).
In the combined data, meta‐analysis showed no significant difference between groups for the primary outcome of 'not cured or not improved' (OR 0.85, 95% CI 0.40 to 1.80) (Analysis 4.1).
Combined data for children and adults ‐ Secondary outcomes
1. Proportion of participants who were not cured at follow‐up
There was also no significant difference between groups for the secondary outcome 'not improved' (OR 0.80, 95% CI 0.38 to 1.67) (Analysis 4.2). However, Analysis 4.3 showed a significant difference between groups for the outcome 'not cured' (OR 0.34, 95% CI 0.19 to 0.60), NNTB 4 (95% CI 3 to 8), favouring those on a mucolytic.
2. Change in quantitative differences in cough
No data could be combined for this outcome.
3. Adverse effects
There was no significant difference between the groups in the number of people who had an adverse event (OR 1.20, 95% CI 0.34 to 4.22) (Analysis 4.4).
4. Other complications and reported data
Data for other secondary outcomes could not be combined.
Sensitivity analyses
The only appropriate sensitivity analysis that could be performed was that for Analysis 4 (combined children and adults). Statistical heterogeneity was absent but given the clinical heterogeneity we used a random‐effects model to re‐examine the results. This revealed that there was still no significant difference between groups for Analysis 4.1 ('not cured or not improved') but the OR was altered, with a wider confidence interval (OR 0.79, 95% CI to 0.27 to 2.29). For Analysis 4.2 ('not improved'), the non‐significant difference was also unaltered but the OR changed to 0.72 (95% CI 0.21 to 2.29). For Analysis 4.3 ('not cured'), the significant difference between groups was also preserved and there was no difference in the OR or 95% CI (OR 0.34, 95% CI 0.19 to 0.60), NNTB 4 (95% CI 3 to 8), favouring those on a mucolytic.
Discussion
Only a few studies have examined over‐the‐counter (OTC) medications for cough related to pneumonia.
Summary of main results
Although four studies were included in this review, only data from two studies could be used (Principi 1986; Roa 1995). Both of these studies examined the efficacy of a mucolytic as an adjunct in the management of pneumonia and used cough as the principal outcome. For the primary outcome of 'not cured or not improved', there was no difference between groups when we considered children and adults separately, or when we combined data in a post hoc analysis. However, for one of the secondary outcomes ('not cured') the use of a mucolytic increased the cure rate similarly in both children and adults (number needed to treat to benefit (NNTB) = 5). Therefore, we cannot be confident of its efficacy. Nevertheless, based on Analysis 2.1, if a mucolytic is tried then the time to response (that is, the "expected timeframe to which a significant improvement is seen" (Chang 2006)), is three days. However, these data come from only a single study.
Overall completeness and applicability of evidence
OTC medications for cough consist of a variety of drugs used as sole agents or in combination. These drugs include antitussives (such as codeine derivatives), antihistamines and non‐pharmaceutical medications (for example, menthol) (Eccles 2002). However, it is also possible that non‐pharmaceutical additives used (such as sugar, alcohol) may have a therapeutic effect, such that the placebo effect of medications for cough has been reported to be as high as 85% (Eccles 2002). Thus, it is not surprising that although the total sample size for the combined studies was not small (N = 224), there was no effect seen for the primary outcome. Given that there was a significant difference between groups, further evaluation of mucolytics using more robust outcomes (as outlined in the 'Implications for research') is certainly warranted.
Although adverse events were uncommon in the clinical trials identified in this study, there are case reports of severe adverse events, including severe morbidity and even death (Kelly 2004).
Quality of the evidence
The quality of the evidence is low, as shown in the 'summary of findings Table for the main comparison'.
Potential biases in the review process
This systematic review is limited to four studies (with only two with extractable data) and in these studies only a single type of OTC medication for cough was examined. Thus, there is a clear lack of studies in this area. Also, the inclusion criteria and outcomes varied among trials.
Agreements and disagreements with other studies or reviews
A systematic review on adjunctive therapies for community‐acquired pneumonia (CAP) reported "We found no clinical trials assessing the effectiveness of over‐the‐counter preparations for cough as an adjunct to antimicrobial treatment in patients with CAP" (Siempos 2008).

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Children ‐ global assessment, Outcome 1 Not cured or not improved.

Comparison 1 Children ‐ global assessment, Outcome 2 Not improved.

Comparison 1 Children ‐ global assessment, Outcome 3 Not cured.

Comparison 2 Children ‐ secondary outcomes, Outcome 1 Mean cough score at day 3.

Comparison 2 Children ‐ secondary outcomes, Outcome 2 Mean score at day 10.

Comparison 2 Children ‐ secondary outcomes, Outcome 3 Adverse events (no. of people).

Comparison 3 Adults ‐ global assessment, Outcome 1 Not cured or not improved.

Comparison 3 Adults ‐ global assessment, Outcome 2 Not improved.

Comparison 3 Adults ‐ global assessment, Outcome 3 Not cured.

Comparison 4 Combined children and adults, Outcome 1 Not cured or not improved.

Comparison 4 Combined children and adults, Outcome 2 Not improved.

Comparison 4 Combined children and adults, Outcome 3 Not cured.

Comparison 4 Combined children and adults, Outcome 4 Adverse events (no. of people).
| Mucolytics as an adjunct to antibiotics to reduce cough in acute pneumonia in children and adults | ||||||
| Patient or population: children and adults with acute pneumonia Settings: any Comparison: antibiotics | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Mucolytics | |||||
| Number of people who had not improved or had not been cured | 16 per 100 | 14 per 100 | OR 0.85 | 221 | ⊕⊕⊝⊝ | Fewer people represents a benefit |
| Cough score | The mean cough score in the control groups was | The mean cough score in the intervention groups was | 120 | ⊕⊕⊝⊝ | Data for children only | |
| Adverse events | See comment | See comment | Not estimable | 120 | See comment | 1 study in children provided data specific to participants with pneumonia ‐ there were no adverse events |
| Complications (e.g. medication change) | See comment | See comment | Not estimable | 0 | See comment | Complications were not measured in the trials |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1In addition to antibiotics, people with pneumonia often use over‐the‐counter (OTC) cough medications when at home or request OTC cough medications when in hospital to suppress an annoying cough. There is a question as to whether suppressing cough may prolong pneumonia. Over‐the‐counter cough medications can include antitussives, expectorants, antihistamine‐decongestants, antihistamines and mucolytics (such as bromhexine, ambroxol and neltenexine). 2Allocation concealment unclear. 3Scale not validated. 4Sparse data. 5Sparse data; confidence interval does not rule out the potential for 'more people' not improved or cured with mucolytics. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Not cured or not improved Show forest plot | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.10, 1.62] |
| 2 Not improved Show forest plot | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.10, 1.62] |
| 3 Not cured Show forest plot | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.16, 0.77] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean cough score at day 3 Show forest plot | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.33, ‐0.17] |
| 2 Mean score at day 10 Show forest plot | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.17, ‐0.13] |
| 3 Adverse events (no. of people) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Not cured or not improved Show forest plot | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.48, 3.04] |
| 2 Not improved Show forest plot | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.48, 3.04] |
| 3 Not cured Show forest plot | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.13, 0.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Not cured or not improved Show forest plot | 2 | 221 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.40, 1.80] |
| 2 Not improved Show forest plot | 2 | 221 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.38, 1.67] |
| 3 Not cured Show forest plot | 2 | 221 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.19, 0.60] |
| 4 Adverse events (no. of people) Show forest plot | 2 | 221 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.34, 4.22] |

