مداخلات برای پیشگیری یا درمان خونریزی شدید قاعدگی یا درد مرتبط با استفاده از دستگاه داخل‐رحمی
Appendices
Appendix 1. Search strategies, 2020 update
Cochrane Central Register of Controlled Trials (CENTRAL; Ovid EBM Reviews) January 2020
1 (IUB or IUBs or IUC or IUCs or IUD or IUDs or IUCD or IUCDs or IUS or IUSs or CuIUB or Cu‐IUB or CuIUBs or Cu‐IUBs or CuIUD or Cu‐IUD or CuIUDs or Cu‐IUDs or CuIUC or Cu‐IUC or CuIUCs or Cu‐IUCs or CuIUCD or Cu‐IUCD or CuIUDs or Cu‐IUDs or CuIUS or Cu‐IUS or CuIUSs or Cu‐IUSs or LNGIUC or LNGIUCs or LNGIUCD or LNGIUCDs or LNGIUD or LNGIUDs or LNGIUS or LNGIUSs or PPIUC or PPIUCs or PPIUCD or PPIUCDs or PPIUD or PPIUDs or PPIUS or PPIUSs).ti. (488)
2 ((intrauterine or intra‐uterine) adj3 (ball or balls or coil or coils or contraceptive or contraception or device or devices or system or systems)).ti. (878)
3 (Jaydess or Kyleena or Liletta or Mirena or Skyla or Copper‐T or CuSafe or Cu‐Safe or Cu375 or Cu‐375 or CuT380* or Cu‐T380* or FlexiT or Flexi‐T or Gyne or Gynefix or Gyneplus or IUB* or Liberte or "Lippes Loop" or Load‐375 or MLCu* or Mini‐TT or MiniTT or Mona Lisa or Multiload or Multi‐load or MultiSafe or Multi‐Safe or MYCu or NeoSafe or Neo‐Safe or NovaT or Nova‐T or Paragard or TCu or TSafe or T‐Safe or T380* or T‐380* or TT380* or TT‐380* or UT380 or UT‐380).ti. (316)
4 or/1‐3 (1372)
5 (analges* or antagonist* or "pain control" or "pain relief" or "pain reliever" or "pain relievers" or NSAID or NSAIDs or tenaculum or ((nonsteroidal or non‐steroidal) adj1 (antiinflammatory or anti‐inflammatory)) or adapalene or alclofenac or ampyrone or antipyrine or apazone or arylpropionic or aspirin or "acetylsalicylic‐acid" or baofuxin or benzydamine or bufexamac or celecoxib or clofazimine or clonixin or "Cox 2" or curcumin or cyclooxygenase or cyclo‐oxygenase or desmopressin or dapsone or diclofenac or diflunisal or dipyrone or epirizole or etanercept or etodolac or etoricoxib or fenamate* or fenoprofen or feprazone or flurbiprofen or flufenamic or glycyrrhizic or ibuprofen or indomethacin or indoprofen or inhibit* or ketoprofen or ketorolac or lumiracoxib$ or masoprocol or meclofenamic or mefenamic or meloxicam or mesalamine or nabumetone or naprosyn or naproxen or niflumic or norpregnadienes or olopatadine hydrochloride or oxaprozin or oxyphenbutazone or paracetamol or parecoxib$ or pentosan or phenylbutazone or piroxicam or prenazone or prophylaxis or prophylactic or prostaglandin or rofecoxib$ or salicylate* or sulfasalazine or sulphonanilide$ or sulindac or suprofen or thiamine or tolfenamic or tolmetin or tranexam?c or trasylol or valdecoxib$).ti,ab. (241691)
6 and/4‐5 (245)
7 6 not ("animal model" or "animal models" or bovine or canine or capra or cat or cats or cattle or cow or cows or dog or dogs or equine or feline or goat or goats or horse or mice or mouse or ovine or pig or pigs or porcine or rabbit or rabbits or rat or rats or rattus or sheep or sow or sows).ti. (244)
MEDLINE ALL (Ovid) 1946 to 20 February 2020
1 Intrauterine Devices/ or Intrauterine Devices, Medicated/ or Intrauterine Devices, Copper/ (11362)
2 (IUB or IUBs or IUC or IUCs or IUD or IUDs or IUCD or IUCDs or IUS or IUSs or CuIUB or Cu‐IUB or CuIUBs or Cu‐IUBs or CuIUD or Cu‐IUD or CuIUDs or Cu‐IUDs or CuIUC or Cu‐IUC or CuIUCs or Cu‐IUCs or CuIUCD or Cu‐IUCD or CuIUDs or Cu‐IUDs or CuIUS or Cu‐IUS or CuIUSs or Cu‐IUSs or LNGIUC or LNGIUCs or LNGIUCD or LNGIUCDs or LNGIUD or LNGIUDs or LNGIUS or LNGIUSs or PPIUC or PPIUCs or PPIUCD or PPIUCDs or PPIUD or PPIUDs or PPIUS or PPIUSs).ti,ab,kf. (10663)
3 ((intrauterine or intra‐uterine) adj3 (ball or balls or coil or coils or contraceptive or contraception or device or devices or system or systems)).ti,ab,kf. (9192)
4 (Jaydess or Kyleena or Liletta or Mirena or Skyla or Copper‐T or CuSafe or Cu‐Safe or Cu375 or Cu‐375 or CuT380* or Cu‐T380* or FlexiT or Flexi‐T or Gyne or Gynefix or Gyneplus or IUB* or Liberte or "Lippes Loop" or Load‐375 or MLCu* or Mini‐TT or MiniTT or Mona Lisa or Multiload or Multi‐load or MultiSafe or Multi‐Safe or MYCu or NeoSafe or Neo‐Safe or NovaT or Nova‐T or Paragard or TCu or TSafe or T‐Safe or T380* or T‐380* or TT380* or TT‐380* or UT380 or UT‐380).ti,ab,kf. (3496)
5 or/1‐4 (18799)
6 Anti‐Inflammatory Agents, Non‐Steroidal/ or Cyclooxygenase Inhibitors/ or Cyclooxygenase 2 Inhibitors/ or "4,5‐Dihydro‐1‐(3‐(trifluoromethyl)phenyl)‐1H‐pyrazol‐3‐amine"/ or Adapalene/ or Adapalene, Benzoyl Peroxide Drug Combination/ or Ampyrone/ or Antipyrine/ or Apazone/ or Aspirin/ or Bufexamac/ or Celecoxib/ or Clonixin/ or Curcumin/ or Diclofenac/ or Diflunisal/ or Dipyrone/ or Epirizole/ or Etanercept/ or Etodolac/ or Etoricoxib/ or Fenoprofen/ or Feprazone/ or Flurbiprofen/ or Ibuprofen/ or Indomethacin/ or Indoprofen/ or Ketoprofen/ or Ketorolac/ or Ketorolac Tromethamine/ or Masoprocol/ or Meclofenamic Acid/ or Mefenamic Acid/ or Meloxicam/ or Mesalamine/ or Nabumetone/ or Naproxen/ or Niflumic Acid/ or Olopatadine Hydrochloride/ or Oxaprozin/ or Oxyphenbutazone/ or Phenylbutazone/ or Piroxicam/ or Prostaglandin Antagonists/ or Salicylates/ or Sodium Salicylate/ or Sulfasalazine/ or Sulindac/ or Suprofen/ or Thiamine/ or Tolmetin/ or Tranexamic Acid/ (209751)
7 (analges* or antagonist* or "pain control" or "pain relief" or "pain reliever" or "pain relievers" or NSAID or NSAIDs or ((nonsteroidal or non‐steroidal) adj1 (antiinflammatory or anti‐inflammatory)) or adapalene or alclofenac or ampyrone or antipyrine or apazone or arylpropionic or aspirin or "acetylsalicylic‐acid" or baofuxin or benzydamine or bufexamac or celecoxib or clofazimine or clonixin or "Cox 2" or curcumin or cyclooxygenase or cyclo‐oxygenase or desmopressin or dapsone or diclofenac or diflunisal or dipyrone or epirizole or etanercept or etodolac or etoricoxib or fenamate* or fenoprofen or feprazone or flurbiprofen or flufenamic or glycyrrhizic or ibuprofen or indomethacin or indoprofen or inhibit* or ketoprofen or ketorolac or lumiracoxib$ or masoprocol or meclofenamic or mefenamic or meloxicam or mesalamine or nabumetone or naprosyn or naproxen or niflumic or norpregnadienes or olopatadine hydrochloride or oxaprozin or oxyphenbutazone or paracetamol or parecoxib$ or pentosan or phenylbutazone or piroxicam or prenazone or prophylaxis or prophylactic or prostaglandin or rofecoxib$ or salicylate* or sulfasalazine or sulphonanilide$ or sulindac or suprofen or thiamine or tolfenamic or tolmetin or tranexam?c or trasylol or valdecoxib$).ti,ab,kf,rn. (3018163)
8 or/6‐7 (3031564)
9 (controlled clinical trial or randomized controlled trial or comparative study).pt. (2276529)
10 (blind or blinded or control* or groups or placebo or random* or trial).ti,ab,oa. (5763664)
11 (meta‐analysis or systematic review).pt. (182738)
12 (meta‐analy* or metaanaly* or ((evidence or systematic) adj2 (review or synthesis))).ti,kf. (188827)
13 or/9‐12 (7143280)
14 13 not ((exp Animals/ not Humans/) or ("animal model" or "animal models" or bovine or canine or capra or cat or cats or cattle or cow or cows or dog or dogs or equine or feline or goat or goats or horse or mice or mouse or ovine or pig or pigs or porcine or rabbit or rabbits or rat or rats or rattus or sheep or sow or sows).ti.) (5647953)
15 and/5,8,14 (537)
Embase.com
#1 'intrauterine contraceptive device'/de OR 'levonorgestrel releasing intrauterine system'/exp OR 'copper intrauterine device'/exp OR 'multiload copper intrauterine device'/exp (19,888)
#2 iub:ti,ab,kw OR iubs:ti,ab,kw OR iuc:ti,ab,kw OR iucs:ti,ab,kw OR iud:ti,ab,kw OR iuds:ti,ab,kw OR iucd:ti,ab,kw OR iucds:ti,ab,kw OR ius:ti,ab,kw OR iuss:ti,ab,kw OR cuiub:ti,ab,kw OR 'cu iub':ti,ab,kw OR cuiubs:ti,ab,kw OR 'cu iubs':ti,ab,kw OR cuiud:ti,ab,kw OR 'cu iud':ti,ab,kw OR cuiuc:ti,ab,kw OR 'cu iuc':ti,ab,kw OR cuiucs:ti,ab,kw OR 'cu iucs':ti,ab,kw OR cuiucd:ti,ab,kw OR 'cu iucd':ti,ab,kw OR cuiuds:ti,ab,kw OR 'cu iuds':ti,ab,kw OR cuius:ti,ab,kw OR 'cu ius':ti,ab,kw OR cuiuss:ti,ab,kw OR 'cu iuss':ti,ab,kw OR lngiuc:ti,ab,kw OR lngiucs:ti,ab,kw OR lngiucd:ti,ab,kw OR lngiucds:ti,ab,kw OR lngiud:ti,ab,kw OR lngiuds:ti,ab,kw OR lngius:ti,ab,kw OR lngiuss:ti,ab,kw OR ppiuc:ti,ab,kw OR ppiucs:ti,ab,kw OR ppiucd:ti,ab,kw OR ppiucds:ti,ab,kw OR ppiud:ti,ab,kw OR ppiuds:ti,ab,kw OR ppius:ti,ab,kw OR ppiuss:ti,ab,kw (11,890)
#3 ((intrauterine OR 'intra uterine') NEAR/3 (ball OR balls OR coil OR coils OR contraceptive OR contraception OR device OR devices OR system OR systems)):ti,ab,kw (12,449)
#4 jaydess:ti,ab,kw OR kyleena:ti,ab,kw OR liletta:ti,ab,kw OR mirena:ti,ab,kw OR skyla:ti,ab,kw OR 'copper t':ti,ab,kw OR cusafe:ti,ab,kw OR 'cu safe':ti,ab,kw OR cu375:ti,ab,kw OR 'cu 375':ti,ab,kw OR cut380*:ti,ab,kw OR 'cu t380*':ti,ab,kw OR flexit:ti,ab,kw OR 'flexi t':ti,ab,kw OR gyne:ti,ab,kw OR gynefix:ti,ab,kw OR gyneplus:ti,ab,kw OR iub*:ti,ab,kw OR liberte:ti,ab,kw OR 'lippes loop':ti,ab,kw OR 'load 375':ti,ab,kw OR mlcu*:ti,ab,kw OR 'mini tt':ti,ab,kw OR minitt:ti,ab,kw OR 'mona lisa':ti,ab,kw OR multiload:ti,ab,kw OR 'multi load':ti,ab,kw OR multisafe:ti,ab,kw OR 'multi safe':ti,ab,kw OR mycu:ti,ab,kw OR neosafe:ti,ab,kw OR 'neo safe':ti,ab,kw OR novat:ti,ab,kw OR 'nova t':ti,ab,kw OR paragard:ti,ab,kw OR tcu:ti,ab,kw OR tsafe:ti,ab,kw OR 't safe':ti,ab,kw OR t380*:ti,ab,kw OR 't 380*':ti,ab,kw OR tt380*:ti,ab,kw OR 'tt 380*':ti,ab,kw OR ut380:ti,ab,kw OR 'ut 380':ti,ab,kw (5,404)
#5 #1 OR #2 OR #3 OR #4 (27,836)
#6 'nonsteroid antiinflammatory agent'/exp OR 'prostaglandin receptor blocking agent'/exp OR '3 amino 1 (3 trifluoromethylphenyl) 2 pyrazoline'/de OR 'adapalene'/de OR 'adapalene plus benzoyl peroxide'/de OR '4 aminophenazone'/de OR 'phenazone'/de OR 'azapropazone'/de OR 'curcumin'/de OR 'dipyrone'/de OR 'etanercept'/de OR 'indometacin'/de OR 'ketorolac trometamol'/de OR 'nordihydroguaiaretic acid'/de OR 'mesalazine'/de OR 'olopatadine'/de OR 'salicylic acid derivative'/exp OR 'salazosulfapyridine'/de OR 'tranexamic acid'/de (841,263)
#7 analges*:ti,ab,kw OR antagonist*:ti,ab,kw OR 'pain control':ti,ab,kw OR 'pain relief':ti,ab,kw OR 'pain reliever':ti,ab,kw OR 'pain relievers':ti,ab,kw OR nsaid:ti,ab,kw OR nsaids:ti,ab,kw OR (((nonsteroidal OR 'non steroidal') NEAR/1 (antiinflammatory OR 'anti inflammatory')):ti,ab,kw) OR adapalene:ti,ab,kw OR alclofenac:ti,ab,kw OR ampyrone:ti,ab,kw OR antipyrine:ti,ab,kw OR apazone:ti,ab,kw OR arylpropionic:ti,ab,kw OR aspirin:ti,ab,kw OR 'acetylsalicylic‐acid':ti,ab,kw OR baofuxin:ti,ab,kw OR benzydamine:ti,ab,kw OR bufexamac:ti,ab,kw OR celecoxib:ti,ab,kw OR clofazimine:ti,ab,kw OR clonixin:ti,ab,kw OR 'cox 2':ti,ab,kw OR curcumin:ti,ab,kw OR cyclooxygenase:ti,ab,kw OR 'cyclo oxygenase':ti,ab,kw OR desmopressin:ti,ab,kw OR dapsone:ti,ab,kw OR diclofenac:ti,ab,kw OR diflunisal:ti,ab,kw OR dipyrone:ti,ab,kw OR epirizole:ti,ab,kw OR etanercept:ti,ab,kw OR etodolac:ti,ab,kw OR etoricoxib:ti,ab,kw OR fenamate*:ti,ab,kw OR fenoprofen:ti,ab,kw OR feprazone:ti,ab,kw OR flurbiprofen:ti,ab,kw OR flufenamic:ti,ab,kw OR glycyrrhizic:ti,ab,kw OR ibuprofen:ti,ab,kw OR indomethacin:ti,ab,kw OR indoprofen:ti,ab,kw OR inhibit*:ti,ab,kw OR ketoprofen:ti,ab,kw OR ketorolac:ti,ab,kw OR lumiracoxib?:ti,ab,kw OR masoprocol:ti,ab,kw OR meclofenamic:ti,ab,kw OR mefenamic:ti,ab,kw OR meloxicam:ti,ab,kw OR mesalamine:ti,ab,kw OR nabumetone:ti,ab,kw OR naprosyn:ti,ab,kw OR naproxen:ti,ab,kw OR niflumic:ti,ab,kw OR norpregnadienes:ti,ab,kw OR 'olopatadine hydrochloride':ti,ab,kw OR oxaprozin:ti,ab,kw OR oxyphenbutazone:ti,ab,kw OR paracetamol:ti,ab,kw OR parecoxib?:ti,ab,kw OR pentosan:ti,ab,kw OR phenylbutazone:ti,ab,kw OR piroxicam:ti,ab,kw OR prenazone:ti,ab,kw OR prophylaxis:ti,ab,kw OR prophylactic:ti,ab,kw OR prostaglandin?:ti,ab,kw OR rofecoxib?:ti,ab,kw OR salicylate*:ti,ab,kw OR sulfasalazine:ti,ab,kw OR sulphonanilide*:ti,ab,kw OR sulindac:ti,ab,kw OR suprofen:ti,ab,kw OR thiamine:ti,ab,kw OR tolfenamic:ti,ab,kw OR tolmetin:ti,ab,kw OR tranexam?c:ti,ab,kw OR trasylol:ti,ab,kw OR valdecoxib*:ti,ab,kw (3,769,595)
#8 #6 OR #7 (4,236,328)
#9 'crossover procedure':de OR 'double‐blind procedure':de OR 'randomized controlled trial':de OR 'single‐blind procedure':de OR random*:de,ab,ti OR factorial*:de,ab,ti OR crossover*:de,ab,ti OR ((cross NEXT/1 over*):de,ab,ti) OR placebo*:de,ab,ti OR ((doubl* NEAR/1 blind*):de,ab,ti) OR ((singl* NEAR/1 blind*):de,ab,ti) OR assign*:de,ab,ti OR allocat*:de,ab,ti OR volunteer*:de,ab,ti (2,541,220)
#10 'meta analysis'/de OR 'network meta‐analysis'/de OR 'systematic review'/de (326,469)
#11 'meta analy*':ti OR metaanaly*:ti OR (((evidence OR systematic) NEAR/2 (review OR synthesis)):ti) OR 'meta analy*':kw OR metaanaly*:kw OR (((evidence OR systematic) NEAR/2 (review OR synthesis)):kw) (236,552)
#12 #9 OR #10 OR #11 (2,758,267)
#13 #5 AND #8 AND #12 (574)
#14 #13 NOT ('animal model':ti OR 'animal models':ti OR bovine:ti OR canine:ti OR capra:ti OR cat:ti OR cats:ti OR cattle:ti OR cow:ti OR cows:ti OR dog:ti OR dogs:ti OR equine:ti OR feline:ti OR goat:ti OR goats:ti OR horse:ti OR mice:ti OR mouse:ti OR ovine:ti OR pig:ti OR pigs:ti OR porcine:ti OR rabbit:ti OR rabbits:ti OR rat:ti OR rats:ti OR rattus:ti OR sheep:ti OR sow:ti OR sows:ti) (563)
Scopus
( ( ( TITLE ( iub OR iubs OR iuc OR iucs OR iud OR iuds OR iucd OR iucds OR ius OR iuss OR cuiub OR cu‐iub OR cuiubs OR cu‐iubs OR cuiud OR cu‐iud OR cuiuds OR cu‐iuds OR cuiuc OR cu‐iuc OR cuiucs OR cu‐iucs OR cuiucd OR cu‐iucd OR cuiuds OR cu‐iuds ) OR TITLE ( cuius OR cu‐ius OR cuiuss OR cu‐iuss OR lngiuc OR lngiucs OR lngiucd OR lngiucds OR lngiud OR lngiuds OR lngius OR lngiuss OR ppiuc OR ppiucs OR ppiucd OR ppiucds OR ppiud OR ppiuds OR ppius OR ppiuss ) OR TITLE ( ( ( intrauterine OR intra‐uterine ) PRE/3 ( ball OR balls OR coil OR coils OR contraceptive OR contraception OR device OR devices OR system OR systems ) ) ) OR TITLE ( jaydess OR kyleena OR liletta OR mirena OR skyla OR copper‐t OR cusafe OR cu‐safe OR cu375 OR cu‐375 OR cut380* OR cu‐t380* OR flexit OR flexi‐t OR gyne OR gynefix OR gyneplus OR iub* OR liberte OR "Lippes Loop" OR load‐375 ) OR TITLE ( mlcu* OR mini‐tt OR minitt OR "Mona Lisa" OR multiload OR multi‐load OR multisafe OR multi‐safe OR mycu OR neosafe OR neo‐safe OR novat OR nova‐t OR paragard OR tcu OR tsafe OR t‐safe OR t380* OR t‐380* OR tt380* OR tt‐380* OR ut380 OR ut‐380 ) ) ) AND ( ( TITLE‐ABS‐KEY ( analges* OR antagonist* OR "pain control" OR "pain relief" OR "pain reliever" OR "pain relievers" OR nsaid OR nsaids OR ( ( nonsteroidal OR non‐steroidal ) PRE/1 ( antiinflammatory OR anti‐inflammatory ) ) OR adapalene OR alclofenac OR ampyrone OR antipyrine ) OR TITLE‐ABS‐KEY ( apazone OR arylpropionic OR aspirin OR "acetylsalicylic‐acid" OR baofuxin OR benzydamine OR bufexamac OR celecoxib OR clofazimine OR clonixin OR "Cox 2" OR curcumin OR cyclooxygenase OR cyclo‐oxygenase OR desmopressin OR dapsone OR diclofenac ) OR TITLE‐ABS‐KEY ( diflunisal OR dipyrone OR epirizole OR etanercept OR etodolac OR etoricoxib OR fenamate* OR fenoprofen OR feprazone OR flurbiprofen OR flufenamic OR glycyrrhizic OR ibuprofen OR indomethacin OR indoprofen OR inhibit* OR ketoprofen OR ketorolac ) OR TITLE‐ABS‐KEY ( lumiracoxib* OR masoprocol OR meclofenamic OR mefenamic OR meloxicam OR mesalamine OR nabumetone OR naprosyn OR naproxen OR niflumic OR norpregnadienes OR olopatadine AND hydrochloride OR oxaprozin OR oxyphenbutazone OR paracetamol OR parecoxib* ) OR TITLE‐ABS‐KEY ( pentosan OR phenylbutazone OR piroxicam OR prenazone OR prophylaxis OR prophylactic OR prostaglandin OR rofecoxib* OR salicylate* OR sulfasalazine OR sulphonanilide* OR sulindac OR suprofen OR thiamine OR tolfenamic OR tolmetin OR tranexam?c OR trasylol ) OR TITLE‐ABS‐KEY ( valdecoxib* ) ) ) ) AND ( TITLE‐ABS‐KEY ( blind OR blinded OR control* OR groups OR placebo OR random* OR trial ) )
(509)
Global Health (Ovid) 1973 to 2020 Week 07
1 (IUB or IUBs or IUC or IUCs or IUD or IUDs or IUCD or IUCDs or IUS or IUSs or CuIUB or Cu‐IUB or CuIUBs or Cu‐IUBs or CuIUD or Cu‐IUD or CuIUDs or Cu‐IUDs or CuIUC or Cu‐IUC or CuIUCs or Cu‐IUCs or CuIUCD or Cu‐IUCD or CuIUDs or Cu‐IUDs or CuIUS or Cu‐IUS or CuIUSs or Cu‐IUSs or LNGIUC or LNGIUCs or LNGIUCD or LNGIUCDs or LNGIUD or LNGIUDs or LNGIUS or LNGIUSs or PPIUC or PPIUCs or PPIUCD or PPIUCDs or PPIUD or PPIUDs or PPIUS or PPIUSs).ti,ab. (1162)
2 ((intrauterine or intra‐uterine) adj3 (ball or balls or coil or coils or contraceptive or contraception or device or devices or system or systems)).ti,ab. (1392)
3 (Jaydess or Kyleena or Liletta or Mirena or Skyla or Copper‐T or CuSafe or Cu‐Safe or Cu375 or Cu‐375 or CuT380* or Cu‐T380* or FlexiT or Flexi‐T or Gyne or Gynefix or Gyneplus or IUB* or Liberte or "Lippes Loop" or Load‐375 or MLCu* or Mini‐TT or MiniTT or Mona Lisa or Multiload or Multi‐load or MultiSafe or Multi‐Safe or MYCu or NeoSafe or Neo‐Safe or NovaT or Nova‐T or Paragard or TCu or TSafe or T‐Safe or T380* or T‐380* or TT380* or TT‐380* or UT380 or UT‐380).ti,ab. (201)
4 or/1‐3 (1985)
5 (analges* or antagonist* or "pain control" or "pain relief" or "pain reliever" or "pain relievers" or NSAID or NSAIDs or tenaculum or ((nonsteroidal or non‐steroidal) adj1 (antiinflammatory or anti‐inflammatory)) or adapalene or alclofenac or ampyrone or antipyrine or apazone or arylpropionic or aspirin or "acetylsalicylic‐acid" or baofuxin or benzydamine or bufexamac or celecoxib or clofazimine or clonixin or "Cox 2" or curcumin or cyclooxygenase or cyclo‐oxygenase or desmopressin or dapsone or diclofenac or diflunisal or dipyrone or epirizole or etanercept or etodolac or etoricoxib or fenamate* or fenoprofen or feprazone or flurbiprofen or flufenamic or glycyrrhizic or ibuprofen or indomethacin or indoprofen or inhibit* or ketoprofen or ketorolac or lumiracoxib$ or masoprocol or meclofenamic or mefenamic or meloxicam or mesalamine or nabumetone or naprosyn or naproxen or niflumic or norpregnadienes or olopatadine hydrochloride or oxaprozin or oxyphenbutazone or paracetamol or parecoxib$ or pentosan or phenylbutazone or piroxicam or prenazone or prophylaxis or prophylactic or prostaglandin or rofecoxib$ or salicylate* or sulfasalazine or sulphonanilide$ or sulindac or suprofen or thiamine or tolfenamic or tolmetin or tranexam?c or trasylol or valdecoxib$).ti,ab. (373116)
6 and/4‐5 (60)
7 6 not ("animal model" or "animal models" or bovine or canine or capra or cat or cats or cattle or cow or cows or dog or dogs or equine or feline or goat or goats or horse or mice or mouse or ovine or pig or pigs or porcine or rabbit or rabbits or rat or rats or rattus or sheep or sow or sows).ti. (57)
8 (blind or blinded or control* or groups or placebo or random* or trial).ti,ab. (1018071)
9 (meta‐analy* or metaanaly* or ((evidence or systematic) adj2 (review or synthesis))).ti. (36292)
10 or/8‐9 (1031128)
11 and/7,10 (25)
LILACS
Words: IUB OR IUBs OR IUC OR IUCs OR IUD OR IUDs OR IUCD OR IUCDs OR IUS OR IUSs OR CuIUB OR Cu‐IUB OR CuIUBs OR Cu‐IUBs OR CuIUD OR Cu‐IUD OR CuIUDs OR Cu‐IUDs OR CuIUC OR Cu‐IUC OR CuIUCs OR Cu‐IUCs OR CuIUCD OR Cu‐IUCD OR CuIUDs OR Cu‐IUDs OR CuIUS OR Cu‐IUS OR CuIUSs OR Cu‐IUSs OR LNGIUC OR LNGIUCs OR LNGIUCD OR LNGIUCDs OR LNGIUD OR LNGIUDs OR LNGIUS OR LNGIUSs OR PPIUC OR PPIUCs OR PPIUCD OR PPIUCDs OR PPIUD OR PPIUDs OR PPIUS OR PPIUSs OR ((intrauterine OR intra‐uterine) AND (ball OR balls OR coil OR coils OR contraceptive OR contraception OR device OR devices OR system OR systems)) OR Jaydess OR Kyleena OR Liletta OR Mirena OR Skyla OR Copper‐T OR CuSafe OR Cu‐Safe OR Cu375 OR Cu‐375 OR CuT380 OR Cu‐T380 OR FlexiT OR Flexi‐T OR Gyne OR Gynefix OR Gyneplus OR IUB OR Liberte OR Lippes OR Load‐375 OR MLCu OR Mini‐TT OR MiniTT OR Mona Lisa OR Multiload OR Multi‐load OR MultiSafe OR Multi‐Safe OR MYCu OR NeoSafe OR Neo‐Safe OR NovaT OR Nova‐T OR Paragard OR TCu OR TSafe OR T‐Safe OR T380 OR T‐380 OR TT380 OR TT‐380 OR UT380 OR UT‐380
AND
Words: analges? OR antagonist? OR "pain control" OR "pain relief" OR "pain reliever" OR "pain relievers" OR NSAID OR NSAIDs OR nonsteroidal OR non‐steroidal OR adapalene OR alclofenac OR ampyrone OR antipyrine OR apazone OR arylpropionic OR aspirin OR "acetylsalicylic‐acid" OR baofuxin OR benzydamine OR bufexamac OR celecoxib OR clofazimine OR clonixin OR "Cox 2" OR curcumin OR cyclooxygenase OR cyclo‐oxygenase OR desmopressin OR dapsone OR diclofenac OR diflunisal OR dipyrone OR epirizole OR etanercept OR etodolac OR etORicoxib OR fenamate? OR fenoprofen OR feprazone OR flurbiprofen OR flufenamic OR glycyrrhizic OR ibuprofen OR indomethacin OR indoprofen OR inhibit? OR ketoprofen OR ketorolac OR lumiracoxib? OR masoprocol OR meclofenamic OR mefenamic OR meloxicam OR mesalamine OR nabumetone OR naprosyn OR naproxen OR niflumic OR norpregnadienes OR olopatadine hydrochloride OR oxaprozin OR oxyphenbutazone OR paracetamol OR parecoxib? OR pentosan OR phenylbutazone OR piroxicam OR prenazone OR prophylaxis OR prophylactic OR prostaglandin OR rofecoxib? OR salicylate? OR sulfasalazine OR sulphonanilide? OR sulindac OR suprofen OR thiamine OR tolfenamic OR tolmetin OR tranexam?c OR trasylol OR valdecoxib?
(49)
Appendix 2. Previous search strategies
2011 Search strategies
MEDLINE via PubMed (2009 to 17 Aug 2011)
(intrauterine devices OR intrauterine system* OR IUD* OR IUC* OR IUS) AND (NSAID* OR anti inflammatory agents, nonsteroidal)
CENTRAL (2009 to 17 Aug 2011)
contracept* in Title, Abstract or Keywords
AND (non‐steroidal anti‐inflammatory OR NSAID*) in Title, Abstract or Keywords
POPLINE (17 Aug 2011 and past five years)
(iud*/iuc*/intrauterine device*) & (NSAID*/nonsteroidal anti inflammatory agent*)
LILACS (17 Aug 2011)
intrauterine devices or intrauterine device or dispositivos intrauterinos or dispositivos Intra‐uterinos OR IUD or iuds OR IUC or IUCD or iucds [Words] and bleeding or pain [Words]
ClinicalTrials.gov (01 Jan 2009 to 18 Aug 2011)
Search terms: non‐steroidal anti‐inflammatory OR NSAID*
Intervention: contraceptive OR contraception
ICTRP
Title or Intervention: non‐steroidal anti‐inflammatory OR NSAID
Condition: contraception OR contraceptive
2006 Search strategies
MEDLINE via PubMed
(intrauterine devices OR intrauterine system* OR IUD* OR IUCD* OR IUS) AND (NSAID* OR anti inflammatory agents, nonsteroidal)
CENTRAL
contracept* AND (non‐steroidal anti‐inflammatory OR NSAID*) in Title, Abstract or Keywords
POPLINE
(iud*/iucd*/intrauterine device*) & (NSAID*/nonsteroidal anti inflammatory agent*)
LILACS
intrauterine devices or intrauterine device or dispositivos intrauterinos or dispositivos Intra‐uterinos OR IUD or iuds OR IUCD or iucds [Words] and bleeding or pain [Words]
Embase
(INTRAUTERINE CONTRACEPTIVE DEVICE or INTRAUTERINE(1N)DEVICE? or IUD? OR IUCD?)
and (NONSTEROID ANTI INFLAMMATORY AGENT or NONSTEROID ANTI INFLAMMATORY DRUG or NONSTEROID ANTIINFLAMMATORY AGENT or NONSTEROIDAL(1N)ANTI(1N)INFLAMMATORY(1N) AGENT or NONSTEROIDAL(1N)ANTI(1N)INFLAMMATORY(1N)DRUG or
NONSTEROIDAL(1N)ANTIINFLAMMATORY(1N)AGENT? or NONSTEROIDAL(1N)ANTIINFLAMMATORY(1N)DRUG? or NSAID?)
CINAHL
(intrauterine device* or IUD* or IUCD*)
and
(non?steroidal anti?inflammatory agent? or non?steroidal anti?inflammatory drug? or non?steroidal anti?inflammatory agent* or non?steroidal anti?inflammatory drug* or NSAID*)
ClinicalTrials.gov
Search terms: non‐steroidal anti‐inflammatory OR NSAID
Condition: contraceptive OR contraception
ICTRP
Title or Intervention: non‐steroidal anti‐inflammatory OR NSAID
Condition: contraception OR contraceptive
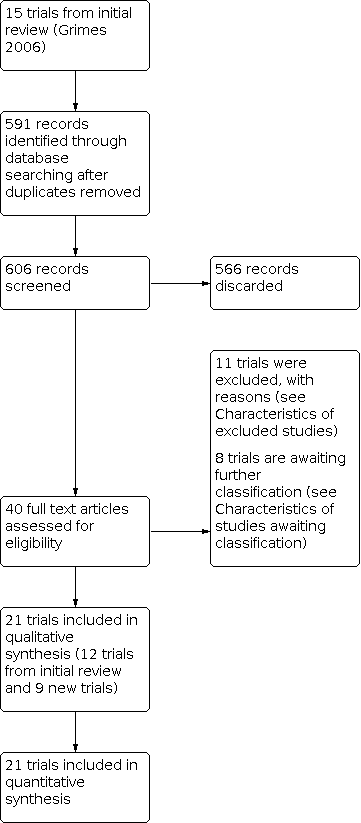
Trial flow diagram

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial

Comparison 1: Vitamin B1 vs placebo (Cu IUD, treatment for HMB), Outcome 1: Number of pads/day

Comparison 1: Vitamin B1 vs placebo (Cu IUD, treatment for HMB), Outcome 2: Number of spotting days

Comparison 1: Vitamin B1 vs placebo (Cu IUD, treatment for HMB), Outcome 3: Number of bleeding days

Comparison 2: Naproxen vs placebo (Cu IUD, treatment for HMB), Outcome 1: "More" menstrual flow

Comparison 2: Naproxen vs placebo (Cu IUD, treatment for HMB), Outcome 2: Side effects

Comparison 3: Mefenamic acid vs tranexamic acid (Cu IUD, treatment for HMB), Outcome 1: Volume of blood loss

Comparison 3: Mefenamic acid vs tranexamic acid (Cu IUD, treatment for HMB), Outcome 2: PBAC score

Comparison 3: Mefenamic acid vs tranexamic acid (Cu IUD, treatment for HMB), Outcome 3: Number of bleeding days
| Mean reduction of PBAC score (%) | ||
| Study | desmopressin | mefenamic acid |
|---|---|---|
| Mercorio 2003 | 40.5 | 45.7 |
Comparison 4: Mefenamic acid vs desmopressin (Cu IUD, treatment for HMB), Outcome 1: Mean reduction of PBAC score (%)

Comparison 4: Mefenamic acid vs desmopressin (Cu IUD, treatment for HMB), Outcome 2: Side effects: headache and insomnia

Comparison 5: Tranexamic acid vs sodium diclofenac (Cu IUD, treatment for HMB), Outcome 1: Menstrual blood loss (alkaline hematin)

Comparison 5: Tranexamic acid vs sodium diclofenac (Cu IUD, treatment for HMB), Outcome 2: Duration of menstruation

Comparison 5: Tranexamic acid vs sodium diclofenac (Cu IUD, treatment for HMB), Outcome 3: Side effects

Comparison 6: Tranexamic acid vs flavonoids (Cu IUD, treatment for HMB), Outcome 1: PBAC score

Comparison 6: Tranexamic acid vs flavonoids (Cu IUD, treatment for HMB), Outcome 2: Number of pads/day

Comparison 6: Tranexamic acid vs flavonoids (Cu IUD, treatment for HMB), Outcome 3: Number of bleeding days

Comparison 6: Tranexamic acid vs flavonoids (Cu IUD, treatment for HMB), Outcome 4: Side effects

Comparison 7: Tranexamic acid vs active comparators (mefenamic acid, flavonoids, diclofenac) (Cu IUD, treatment for HMB), Outcome 1: Number of bleeding days

Comparison 8: Ulipristal acetate vs placebo (LNG IUD, treatment of HMB), Outcome 1: Duration of days until bleeding stops

Comparison 8: Ulipristal acetate vs placebo (LNG IUD, treatment of HMB), Outcome 2: Total bleeding in 90 days
| Blood loss reduction (%) | ||
| Study | High dose naproxen | Low dose naproxen |
|---|---|---|
| Davies 1981 | 31.6 | 9.5 |
Comparison 9: High‐dose naproxen vs low‐dose naproxen (unknown IUD, treatment of HMB), Outcome 1: Blood loss reduction (%)

Comparison 10: Mefenamic acid vs Vitex agnus (unknown IUD, treatment for HMB), Outcome 1: PBAC score

Comparison 10: Mefenamic acid vs Vitex agnus (unknown IUD, treatment for HMB), Outcome 2: Side effects

Comparison 11: Tranexamic acid vs sodium diclofenac (Cu IUD, treatment for pain), Outcome 1: Pelvic pain

Comparison 12: Naproxen vs placebo (unknown IUD, treatment for pain), Outcome 1: Overall pain relief score

Comparison 12: Naproxen vs placebo (unknown IUD, treatment for pain), Outcome 2: Daily pain relief score

Comparison 12: Naproxen vs placebo (unknown IUD, treatment for pain), Outcome 3: Pain relief

Comparison 12: Naproxen vs placebo (unknown IUD, treatment for pain), Outcome 4: Need for analgesia

Comparison 13: Ibuprofen vs placebo (Cu IUD, prevention of HMB), Outcome 1: Menstrual blood loss (alkaline hematin)

Comparison 13: Ibuprofen vs placebo (Cu IUD, prevention of HMB), Outcome 2: Duration of bleeding
| Median menstrual blood loss (alkaline hematin) | ||
| Study | Ibuprofen | Placebo |
|---|---|---|
| Fincoid | ||
| Makarainen 1986 | 51 | 49 |
| ML Cu 375 | ||
| Makarainen 1986 | 47 | 62 |
Comparison 13: Ibuprofen vs placebo (Cu IUD, prevention of HMB), Outcome 3: Median menstrual blood loss (alkaline hematin)

Comparison 13: Ibuprofen vs placebo (Cu IUD, prevention of HMB), Outcome 4: Side effects

Comparison 14: Tolfenamic vs placebo (Cu IUD, prevention of HMB), Outcome 1: Number of pads/day

Comparison 14: Tolfenamic vs placebo (Cu IUD, prevention of HMB), Outcome 2: Menstruation "more abundant than normal"

Comparison 14: Tolfenamic vs placebo (Cu IUD, prevention of HMB), Outcome 3: Clots in menstrual blood

Comparison 15: Aspirin vs paracetamol (Cu IUD, prevention of HMB), Outcome 1: Menstrual blood loss (alkaline hematin)
| Occurence of HMB (%) | ||
| Study | 2g tranexamic | 1g tranexamic |
|---|---|---|
| Alkaline hematin | ||
| Lin 2007 | 20 | 11.76 |
| PBAC | ||
| Lin 2007 | 26.79 | 45.83 |
Comparison 16: 2 g tranexamic vs 1 g tranexamic (Cu IUD, prevention HMB), Outcome 1: Occurence of HMB (%)

Comparison 17: Ulipristal acetate (CDB‐2914) vs placebo (LNG IUD, prevention of HMB), Outcome 1: Percentage days bleeding or spotting

Comparison 17: Ulipristal acetate (CDB‐2914) vs placebo (LNG IUD, prevention of HMB), Outcome 2: Longest consecutive run of days without bleeding or spotting
| Median number of bleeding days | |||
| Study | tranexamic acid (n=55) | mefenamic acid (n=57) | placebo (n=56) |
|---|---|---|---|
| Sordal 2013 | 8 | 10 | 11.5 |
Comparison 18: Tranexamic acid vs mefenamic acid (LNG IUD, prevention of HMB), Outcome 1: Median number of bleeding days
| Median number of bleeding and spotting days | |||
| Study | Naproxen (n=35) | Estradiol (n=34) | Placebo (n=37) |
|---|---|---|---|
| Madden 2012 | 27.5 | 44 | 32 |
Comparison 19: Naproxen vs estradiol (LNG IUD, prevention of HMB), Outcome 1: Median number of bleeding and spotting days

Comparison 19: Naproxen vs estradiol (LNG IUD, prevention of HMB), Outcome 2: Satisfied with bleeding pattern at 12 weeks

Comparison 19: Naproxen vs estradiol (LNG IUD, prevention of HMB), Outcome 3: Side effects
| Percentage bleeding and spotting days | ||
| Study | Mifepristone | Vitamin B |
|---|---|---|
| Papaikonomou 2018 | Fewer women reported bleeding or spotting. Less days with normal or heavy intensity bleeding | More bleeding or spotting days. |
Comparison 20: Mifepristone vs vitamin B (LNG IUD, prevention of HMB), Outcome 1: Percentage bleeding and spotting days

Comparison 21: Tolfenamic acid vs placebo (Cu IUD, prevention of pain), Outcome 1: Menstruation "more painful than normal"

Comparison 21: Tolfenamic acid vs placebo (Cu IUD, prevention of pain), Outcome 2: Side effects

Comparison 22: Ibuprofen vs placebo (Cu IUD, prevention of pain), Outcome 1: Reduction in menstrual cramps

Comparison 23: Ibuprofen vs placebo (Cu IUD,prevention of bleed and pain, IUD removal), Outcome 1: IUD removal by 26 weeks due to pain or increased blood loss

Comparison 23: Ibuprofen vs placebo (Cu IUD,prevention of bleed and pain, IUD removal), Outcome 2: IUD removal due to pain or increased blood loss by 52 weeks
| Interventions versus comparisons | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Certainty of the evidence | |
|---|---|---|---|---|---|
| Risk with comparisons | Risk with interventions | ||||
| Vitamin B1 vs placebo | |||||
| Menstrual blood loss Number of pads (diary record) Follow‐up: 1 month after completion of intervention for 3 months | The mean number of pads was 19 pads | MD 7 pads fewer | ‐ | 110 | ⨁⨁◯◯ |
| Duration of bleeding Number of bleeding days (diary record)
Follow‐up: 1 month after completion of intervention for three months | The mean duration of bleeding was 8 days | MD 2 days fewer | ‐ | 110 | ⨁⨁◯◯ |
| Adverse events or side effects | None reported |
|
|
|
|
| Mefenamic acid vs tranexamic acid | |||||
| Menstrual blood loss Volume blood loss (pictorial chart in mL)
Follow‐up: after 3rd month of intervention | The mean volume of menstrual blood loss was 160 mL | MD 64.26 mL lower | ‐ | 94 | ⨁⨁◯◯ |
| Duration of bleeding Number of bleeding days (menstrual record)
Follow‐up: after 3rd month of intervention Recorded on daily diary | The mean number of bleeding days was 3.5 to 6.4 days | MD 0.08 days more | ‐ | 152 | ⨁⨁◯◯ |
| Adverse events or side effects | No data available |
|
|
|
|
| Mefenamic acid vs desmopressin | |||||
| Menstrual blood loss Mean reduction of PBAC score Follow‐up: after 3rd month of intervention | The mean reduction of PBAC score was 40.5% in desmopressin and 45.7% in mefenamic acid |
| 24 | ⨁◯◯◯ | |
| Tranexamic acid vs sodium diclofenac | |||||
| Menstrual blood loss Volume of blood loss (alkaline hematin method) Follow‐up: each intervention cycle (cross‐over) for 5 menstrual cycles | The mean blood loss was 102mL | MD 42.7mL lower | ‐ | 38 | ⨁◯◯◯ |
| Duration of bleeding
Follow‐up: each intervention cycle (cross‐over) for 5 menstrual cycles | The mean duration of bleeding was 5.1 days | MD 0 days | ‐ | 38 | ⨁◯◯◯ |
| Side effects: gastrointestinal disturbances (diarrhoea, lower abdominal pain), headache and sweating | 316 per 1000 | 684 per 1000 (356 to 895) | OR 4.69 (1.2 to 18.44) | 38 (1 RCT) | ⨁◯◯◯ |
| Tranexamic acid vs flavonoids | |||||
| Menstrual blood loss PBAC score Follow‐up: each cycle for 3 menstrual cycles | The mean PBAC score was 125 | MD 32 lower | ‐ | 100 | ⨁⨁◯◯ |
| Menstrual blood loss Number of pads per day Follow‐up: each cycle for 3 menstrual cycles | The mean number of pads was 3 pads per day | MD 0.5 pads lower | ‐ | 100 | ⨁⨁◯◯ |
| Duration of bleeding Number of bleeding days Follow‐up: each cycle for 3 menstrual cycles | The mean number of bleed days was 6.8 days | MD 1.4 days lower | ‐ | 100 | ⨁⨁◯◯ |
| Side effects: headache Follow‐up: each cycle for 3 menstrual cycles | 160 per 1000 | 99 per 1000 (33 to 268) | OR 0.58 (0.18 to 1.92) | 100 | ⨁⨁◯◯ |
| Side effects: vomiting Follow‐up: each cycle for 3 menstrual cycles | 180 per 1000 | 140 per 1000 (33 to 268) | OR 0.74 (0.25 to 2.18) | 100 | ⨁⨁◯◯ |
| Tranexamic acid vs active comparators (mefenamic acid, flavonoid, sodium diclofenac) | |||||
| Duration of bleeding Number of bleeding days Follow‐up: 2‐5 menstrual cycles
| The mean number of bleeding days was 5.1 to 6.8 days | MD 0.27 days lower | ‐ | 290 | ⨁◯◯◯ |
| CI: confidence interval; MD: mean difference; OR: odds ratio; PBAC: pictorial blood assessment chart; RCT: randomized controlled trial; vs: versus aDowngraded one level due to unclear risk of selection bias. | |||||
| Interventions vs comparisons | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | |
|---|---|---|---|---|---|
| Risk with comparisons | Risk with interventions | ||||
| Ulipristal acetate vs placebo | |||||
| Duration of bleeding Total bleeding in 90 days (bleeding calendar) Follow‐up: 30, 60 and 90 days after treatment initiation | The mean total of bleeding days in 90 days was 29.8 to 39.1 days | MD 9.3 days lower | ‐ | 24 | ⨁◯◯◯ |
| CI: confidence interval; MD: mean difference; OR: odds ratio; RCT: randomized controlled trial; vs: versus aDowngraded one level due to high risk of detection bias. | |||||
| Interventions vs comparisons | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Certainty of the evidence | |||
|---|---|---|---|---|---|---|---|
| Risk with comparisons | Risk with interventions | ||||||
| Mefenamic acid vs Vitex agnus | |||||||
| Menstrual blood loss PBAC score Follow‐up: monthly for 4 months | The mean PBAC score was 89.7 | 2.4 lower | ‐ | 84 | ⨁⨁◯◯ | ||
| Side effects: nausea Follow‐up: monthly for 4 months | 24 per 1000 | 24 per 1000 (1 to 287) | OR 1.00 (0.06 to 16.53) | 84 | ⨁⨁◯◯ | ||
| Side effects: abdominal pain Follow‐up: monthly for 4 months | 24 per 1000 | 8 per 1000 (0 to 167) | OR 0.33 (0.01 to 8.22) | 84 | ⨁⨁◯◯ | ||
| CI: confidence interval; MD: mean difference; OR: odds ratio; PBAC: pictorial blood assessment chart; RCT: randomized controlled trial; vs: versus aDowngraded one level due to unclear reporting bias. | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Certainty of the evidence | |
|---|---|---|---|---|---|
| Risk with comparisons | Risk with interventions | ||||
| Tranexamic acid vs sodium diclofenac | |||||
| Pelvic pain Follow‐up: each intervention cycle (cross over) for 5 menstrual cycles | 53 per 1000 | 53 per 1000 | OR 1.00 | 38 | ⨁◯◯◯ |
| CI: confidence interval; MD: mean difference; OR: odds ratio; RCT: randomized controlled trial; vs: versus aDowngraded two levels due to unclear risk of selection, performance and reporting bias and high risk of detection bias. | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Certainty of the evidence | |
|---|---|---|---|---|---|
| Risk with comparisons | Risk with interventions | ||||
| Naproxen vs placebo | |||||
| Overall pain relief score (4‐point scale) Follow‐up: each cycle for 3 menstrual cycles | The mean overall pain relief score was 7.9 | MD 4.1 higher | ‐ | 33 | ⨁⨁◯◯ |
| Daily pain relief score (6‐point scale) Follow‐up: each cycle for 3 menstrual cycles | The mean daily pain relief score was 10.8 | 3.1 higher | ‐ | 33 | ⨁⨁◯◯ |
| Need for additional analgesia (self‐recorded) Follow‐up: each cycle for 3 menstrual cycles | 313 per 1000 | 116 per 1000 | OR 0.29 | 33 | ⨁⨁◯◯ |
| Side effects: gastrointestinal symptoms Follow‐up: each cycle for 3 menstrual cycles | 48 per 1000 | 95 per 1000 (9 to 557) | OR 2.11 (0.18 to 25.17) | 33 | ⨁⨁◯◯ |
| CI: confidence interval; MD: mean difference; OR: odds ratio; RCT: randomized controlled trial; vs: versus aDowngraded two levels due to unclear risk of selection, performance and reporting bias as well as high risk of detection bias. | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Outcomes |
|---|---|---|---|---|---|
| Ibuprofen vs placebo | |||||
| Menstrual blood loss (alkaline hematin method) Follow‐up: 3 months | The mean menstrual blood loss was 44.7 to 109.2 mL | MD 14.11 mL lower | ‐ | 40 | ⨁◯◯◯ |
| Duration of bleeding (menstrual diary) Follow‐up: 3 months | The mean duration of bleed was 6.4 days | MD 0.2 days lower | ‐ | 28 | ⨁◯◯◯ |
| Side effects: eye and mouth swelling, stomach cramps, fatigue, irritability (diary record) Follow‐up: 3 months | 38 per 1000 | 79 per 1000 (20 to 271) | OR 2.15 (0.5 to 9.31) | 68 (2 RCTs) | ⨁⨁◯◯ |
| Tolfenamic acid vs placebo | |||||
| Menstrual blood loss Menstruation "more abundant than normal" (questionnaire) Follow‐up: 3 months | 658 per 1000 | 510 per 1000 | OR 0.54 | 310 | ⨁◯◯◯ |
| Side effects: dyspepsia, diarrhoea, headache, fatigue (questionnaire) Follow‐up: 3 months | 129 per 1000 | 66 per 1000 | OR 0.48 | ⨁◯◯◯ | |
| Aspirin vs paracetamol | |||||
| Menstrual blood loss (alkaline hematin method) Follow‐up: 3 cycles | The mean menstrual blood loss was 49.5 mL | MD 0.3mL lower | ‐ | 20 | ⨁◯◯◯ |
| Adverse events or side effects | Not reported | ||||
| 2 g tranexamic acid vs 1 g tranexamic acid | |||||
| Menstrual blood loss % occurrence of heavy menstrual bleeding (alkaline hematin method) Follow‐up: each cycle for 3 menstrual cycles | 2 g tranexamic acid: 20 1 g tranexamic acid: 11.76 | 64 | ⨁◯◯◯ | ||
| Menstrual blood loss % occurrence heavy menstrual bleeding (PBAC score) Follow‐up: each cycle for 3 menstrual cycles | 2 g tranexamic acid: 26.79 1 g tranexamic acid: 45.83 | 175 | ⨁◯◯◯ | ||
| Adverse events or side effects | Not reported | ||||
| CI: confidence interval; MD: mean difference; OR: odds ratio; PBAC: pictorial blood assessment chart; RCT: randomized controlled trial; vs: versus aDowngraded one level due to unclear risk of selection and reporting bias. | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Certainty of the evidence | |
|---|---|---|---|---|---|
| Risk with comparisons | Risk with interventions | ||||
| Ulipristal acetate vs placebo | |||||
| Percentage days bleeding or spotting (questionnaire) Follow‐up: 1, 3 and 6 months after IUD insertion | The mean percentage days bleeding or spotting was 20.7 | MD 9.5% higher (1.48 higher to 17.52 higher) | ‐ | 118 | ⨁⨁◯◯ |
| Adverse events or side effects | Not reported | ||||
| Tranexamic acid vs mefenamic acid | |||||
| Menstrual blood loss | No data reported | ||||
| Duration of bleeding Median number of bleed days (diary) Follow‐up: 90 days of study assessment followed by 30 days | Tranexamic acid: 8 Mefenamic acid: 10 | 168 | ⨁⨁◯◯ | ||
| Side effects: gastrointestinal disorders, headache, breast tenderness, musculoskeletal disorders Follow‐up: 90 days of study assessment followed by 30 days | 468 per 1000 | 468 per 1000 | OR 1.00 (0.49 to 2.02) | 168 | ⨁⨁◯◯ |
| Mifepristone vs vitamin B | |||||
| Menstrual blood loss | No data reported | ||||
| Duration of bleeding % spotting and bleeding days Follow‐up: 1, 3 and 6 months after IUD insertion | Mifepristone: fewer women reported bleeding or spotting. Fewer days with normal or heavy intensity bleeding Vitamin B: more bleeding or spotting days | 58 | ⨁◯◯◯ | ||
| Adverse events or side effects | Not reported | ||||
| Naproxen vs estradiol | |||||
| Menstrual blood loss | Not reported | ||||
| Duration of bleeding Median number of bleeding and spotting days (diary) Follow‐up: every 4 weeks for 16 weeks | Naproxen: 27.5 Estradiol: 44 | 106 | ⨁◯◯◯ | ||
| Side effects: gastroesophageal reflux Follow‐up: every 4 weeks for 16 weeks | 0 per 1000 | 0 per 1000 | OR 3.22 (0.13 to 81.19) | 86 | ⨁◯◯◯ |
| CI: confidence interval; MD: mean difference; OR: odds ratio; RCT: randomized controlled trial; vs: versus aDowngraded one level for imprecision as effects were measured from one trial. | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Certainty of the evidence | |
|---|---|---|---|---|---|
| Risk with comparisons | Risk with interventions | ||||
| Ibuprofen vs placebo | |||||
| Reduction in painful menstruation Follow‐up: end of 1st, 2nd and 3rd months | 200 per 1000 | 200 per 1000 | OR 1.00 | 20 | ⨁⨁◯◯ |
| Tolfenamic acid vs placebo | |||||
| Menstruation "more painful than normal" Follow‐up: 3 months | 387 per 1000 | 310 per 1000 | OR 0.71 | 310 | ⨁◯◯◯ |
| CI: confidence interval; MD: mean difference; OR: odds ratio; RCT: randomized controlled trial; vs: versus aDowngraded one level due to unclear risk of selection and reporting bias. | |||||
| Trial | Experimental Intervention | Comparison Intervention | IUD type | Duration of intervention | Follow‐up | Outcome |
|---|---|---|---|---|---|---|
| Effectiveness trials | ||||||
| Bleeding trials | ||||||
| RCT with cross‐over | Oral naproxen 1250 mg/day | Oral naproxen 500 mg on 1st day then 750 mg on subsequent days Placebo | Unknown | 4 cycles Each participant took 2 of the 3 treatments, each for 2 menstrual cycles. Each regimen was begun on the 1st day of menstrual bleeding and continued for a total of 5 days. | After every 2 menstrual cycles | Percentage reduction in menstrual blood loss |
| RCT | Oral ulipristal acetate 5 mg/day for 5 days | Oral placebo | LNG IUD | 5 days, bleeding was assessed at 30 days, 60 days and 70 days | 90 days | Total bleeding days |
| RCT | Oral vitamin B1 100 mg/day during the 2nd, 3rd and 4th months following insertion of the IUD | Oral placebo during the 2nd, 3rd and 4th months following insertion of the IUD | Cu IUD | 3 cycles | 1 month after completion of intervention | Number of pads used Number of spotting days Duration of bleeding |
| Pain trials | ||||||
| RCT | Oral naproxen sodium 550 mg initially, then 275 mg every 6 hours as needed for uterine pain | Placebo (lactose) | Unknown IUD type | 3 cycles | No details | Overall and daily pain relief score (6‐point Likert scale) |
| RCT with cross‐over | Oral naproxen 500 mg followed by 250 mg 2‐4 times a day, with a maximum of 1250 mg, taken at the first sign of menstrual distress. | Oral placebo taken at the first sign of menstrual distress. | Cu IUD | 4 cycles Participant took an intervention for 2 cycles then crossed over to the other intervention for 2 cycles | After 2nd and 4th menstrual cycle | Pain relief (5‐point Likert scale) |
| Comparative effectiveness trials | ||||||
| Bleeding trials | ||||||
| RCT | Oral tranexamic acid 500 mg, 1 tablet 6‐hourly during the 1st 3 days of menstrual bleeding | Oral micronized flavonoid 500 mg, 1 tablet 6‐hourly during the 1st 3 days of menstrual bleeding | Cu IUD | 3 cycles | No details | PBAC score Number of pads used/day Number of bleeding days |
| RCT | Oral mefenamic acid, 250 mg; 1 capsule every 8 hours on the first 3 days of menstruation | Oral tranexamic acid, 250 mg; 1 capsule every 8 hours on the first 3 days of menstruation | Cu IUD | 2 cycles | No details | PBAC score Number of bleeding days |
| RCT | Desmopressin 300 mcg intranasal spray each morning for the first 5 days after start of menstruation | Oral mefenamic acid 1500 mg/day for the first 5 days after the start of menstruation. | Cu IUD | 3 months | Monthly for 3 months | PBAC score |
| RCT | Oral tranexamic acid 500 mg 3 times/day for 3‐5 days based on the duration of bleeding | Oral mefenamic acid 500 mg 3 times/day for 3‐5 days based on the duration of bleeding | Cu IUD | 3 months | Monthly for 3 months | Volume of blood loss Duration of bleeding |
| RCT | Oral mefenamic acid 250 mg 3 times/day from the 1st day of menstruation until day 8 | Oral Vitex agnus 3 times/day from the 1st day of menstruation until day 8 | Unknown IUD type | 4 months | No details | PBAC score |
| RCT with cross‐over | Oral tranexamic acid 4.5 g/day for 5 days | Oral sodium diclofenac 150 mg on day 1 then 75 mg/day on days 2‐4 Placebo | Cu IUD | 5 months Each participant received placebo for 1 month, 2 months of tranexamic acid, and 2 months of sodium diclofenac | No details | Menstrual blood loss (alkaline hematin) Duration of bleeding |
| Cu IUD: copper intrauterine device; HMB: heavy menstrual bleeding; IUD: intrauterine device; LNG IUD: levonorgestrel intrauterine device; PBAC: pictorial blood loss assessment chart | ||||||
| Trial | Experimental intervention | Comparison intervention | IUD type | Duration of intervention | Follow‐up | Outcomes |
|---|---|---|---|---|---|---|
| Effectiveness trials | ||||||
| Bleeding trials | ||||||
| RCT | Oral ibuprofen 1200 mg/day at the beginning of menstruation and to continue to end of bleeding for a maximum of 10 days. | Oral placebo at the beginning of menstruation and to continue to end of bleeding for a maximum of 10 days. | Cu IUD | 3 cycles | No details | Menstrual blood loss (alkaline hematin) Length of menstruation |
| RCT with cross‐over | Oral ibuprofen 1600 mg/day starting with bleeding and continuing until end of bleeding or maximum of 7 days | Placebo starting with bleeding and continuing until end of bleeding or maximum of 7 days | Cu IUD Cu Lippes loop | 3 cycles Each participant had 1 month of observation, 1 month on ibuprofen or placebo, and 1 month crossed over to the alternative treatment. | End of month 1, 2, 3 | Menstrual blood loss (alkaline hematin) |
| RCT | Oral CDB‐2914 50 mg/day | Oral placebo | LNG IUD | Intervention taken for 3 consecutive days with separate treatments starting 21, 49 and 77 days after LNG IUD insertion | 1,3 and 6 months after IUD insertion | Percentage days bleeding/ spotting, removal of LNG IUD within 6 months; the longest run of amenorrhoea in the 64 days after third treatment, side effects |
| Bleeding and pain trials | ||||||
| RCT | Oral ibuprofen 1200 mg/day to be taken with the first 6 menstruations after IUD insertion and for a maximum of 5 days at a time. | Oral placebo to be taken with the first 6 menstruations after IUD insertion and for a maximum of 5 days at a time. | Cu IUD | 6 cycles | 6, 13, 26 and 52 weeks after IUD insertion | IUD removal within 12 months of insertion due to HMB/pain |
| RCT | Oral tolfenamic acid 600 mg/day starting at IUD insertion and continuing for 7 days; this was to be repeated during the next 3 menstruations. | Placebo starting at IUD insertion and continuing for 7 days; this was to be repeated during the next 3 menstruations | Cu IUD | 3 cycles | No details | Number of pads used, menstruation more abundant than normal, clots, menstruation more painful than normal |
| Comparative effectiveness trials | ||||||
| Bleeding trials | ||||||
| RCT with cross‐over | Oral aspirin 1500 mg/day | Oral paracetamol 1500 mg/day Placebo | Cu IUD | 3 cycles Interventions taken on day 1 of menstruation and continued for the duration of bleeding. Each participant received each treatment in alternating order. | No details | Menstrual blood loss (alkaline hematin) |
| RCT | Oral tranexamic acid 2 g/day, taken for the 1st 5 days of 3 consecutive cycles after IUD insertion | Oral tranexamic acid 1 g/day, taken for the 1st 5 days of 3 consecutive cycles after IUD insertion | Cu IUD | 3 cycles | No details | Percentage occurrence of HMB by alkaline hematin and PBAC |
| RCT | Oral naproxen 500 mg twice a day first 5 days of a 4‐week period | Transdermal estradiol 0.1 mg on day after insertion of IUD, changing it weekly Oral placebo twice a day | LNG IUD | First 12 weeks of IUD use | Telephone surveys at 4, 8, and 16 weeks and an in‐person follow‐up visit at 12 weeks | Median bleed days, patient satisfaction, continuation rates of IUD |
| RCT | Oral 50 mg mifepristone every other day starting on the 1st day of the menstrual cycle for the pre‐treatment period | Oral vitamin B every other day starting on the 1st day of the menstrual cycle for the pre‐treatment period | LNG IUD | 2 months (corresponding to 2 menstrual cycles i.e. 2 × 28 days) prior to insertion and until 3 days (± 2 days) following the LNG IUD insertion | Monthly for the first 3 months, 6 months post‐LNG IUD insertion and 12 months end‐of‐trial evaluation | Bleeding and spotting days |
| RCT | Oral tranexamic acid 1500 mg/day, taken on the 1st day of a bleeding or spotting episode until bleeding or spotting stopped | Oral mefenamic acid 1500 mg/day, taken on the 1st day of a bleeding or spotting episode until bleeding or spotting stopped Oral placebo (lactose and magnesium stearate), taken on the 1st day of a bleeding or spotting episode until bleeding or spotting stopped | LNG IUD | 90 days | No details | Median reduction of bleed days and length of bleed days, satisfaction with drug/placebo, occurrence of pain, number of days pain medication used to alleviate pain, adverse effects |
| Cu IUD: copper intrauterine device; HMB: heavy menstrual bleeding; IUD: intrauterine device; LNG IUD: levonorgestrel intrauterine device; PBAC: pictorial blood loss assessment chart | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Number of pads/day Show forest plot | 1 | 110 | Mean Difference (IV, Random, 95% CI) | ‐7.00 [‐8.50, ‐5.50] |
| 1.2 Number of spotting days Show forest plot | 1 | 110 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐1.94, ‐1.26] |
| 1.3 Number of bleeding days Show forest plot | 1 | 110 | Mean Difference (IV, Random, 95% CI) | ‐2.00 [‐2.38, ‐1.62] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 "More" menstrual flow Show forest plot | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.09 [0.00, 1.78] |
| 2.2 Side effects Show forest plot | 1 | 336 | Odds Ratio (M‐H, Random, 95% CI) | 1.91 [0.72, 5.06] |
| 2.2.1 Irregular flow | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 2.24 [0.36, 13.78] |
| 2.2.2 Fluor | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 3.15 [0.12, 81.74] |
| 2.2.3 Tender vagina | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 3.15 [0.12, 81.74] |
| 2.2.4 Gastrointestinal symptoms | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 2.11 [0.18, 25.17] |
| 2.2.5 Dizziness | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 3.15 [0.12, 81.74] |
| 2.2.6 Trembling legs | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 3.15 [0.12, 81.74] |
| 2.2.7 Blurry vision | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.06, 17.12] |
| 2.2.8 Sore lips | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 8.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Volume of blood loss Show forest plot | 1 | 94 | Mean Difference (IV, Random, 95% CI) | ‐64.26 [‐105.65, ‐22.87] |
| 3.2 PBAC score Show forest plot | 1 | 58 | Mean Difference (IV, Random, 95% CI) | ‐8.19 [‐25.24, 8.86] |
| 3.3 Number of bleeding days Show forest plot | 2 | 152 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.27, 0.42] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Mean reduction of PBAC score (%) Show forest plot | 1 | Other data | No numeric data | |
| 4.2 Side effects: headache and insomnia Show forest plot | 1 | 24 | Odds Ratio (M‐H, Random, 95% CI) | 0.11 [0.00, 2.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Menstrual blood loss (alkaline hematin) Show forest plot | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐42.70 [‐73.33, ‐12.07] |
| 5.2 Duration of menstruation Show forest plot | 1 | 38 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.62, 0.62] |
| 5.3 Side effects Show forest plot | 1 | 38 | Odds Ratio (M‐H, Random, 95% CI) | 4.69 [1.20, 18.44] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 PBAC score Show forest plot | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐32.00 [‐39.84, ‐24.16] |
| 6.2 Number of pads/day Show forest plot | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.91, ‐0.09] |
| 6.3 Number of bleeding days Show forest plot | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐1.93, ‐0.87] |
| 6.4 Side effects Show forest plot | 1 | 200 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.30, 1.48] |
| 6.4.1 Headache | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 0.58 [0.18, 1.92] |
| 6.4.2 Vomiting | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.25, 2.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 7.1 Number of bleeding days Show forest plot | 4 | 290 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐1.14, 0.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 8.1 Duration of days until bleeding stops Show forest plot | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐3.30 [‐9.07, 2.47] |
| 8.2 Total bleeding in 90 days Show forest plot | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐9.30 [‐26.76, 8.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 9.1 Blood loss reduction (%) Show forest plot | 1 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 10.1 PBAC score Show forest plot | 1 | 84 | Mean Difference (IV, Random, 95% CI) | ‐2.40 [‐13.77, 8.97] |
| 10.2 Side effects Show forest plot | 1 | 168 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.07, 5.13] |
| 10.2.1 Nausea | 1 | 84 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.06, 16.53] |
| 10.2.2 Abdominal pain | 1 | 84 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 11.1 Pelvic pain Show forest plot | 1 | 38 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.06, 17.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 12.1 Overall pain relief score Show forest plot | 1 | 33 | Mean Difference (IV, Random, 95% CI) | 4.10 [0.91, 7.29] |
| 12.2 Daily pain relief score Show forest plot | 1 | 33 | Mean Difference (IV, Random, 95% CI) | 3.10 [0.27, 5.93] |
| 12.3 Pain relief Show forest plot | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 2.79 [0.77, 10.04] |
| 12.4 Need for analgesia Show forest plot | 1 | 33 | Odds Ratio (M‐H, Random, 95% CI) | 0.29 [0.05, 1.80] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 13.1 Menstrual blood loss (alkaline hematin) Show forest plot | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐14.11 [‐36.04, 7.82] |
| 13.1.1 Cu IUD | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐9.47 [‐34.45, 15.51] |
| 13.1.2 Lippes Loop | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐29.68 [‐75.44, 16.08] |
| 13.2 Duration of bleeding Show forest plot | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.40, 1.00] |
| 13.3 Median menstrual blood loss (alkaline hematin) Show forest plot | 1 | Other data | No numeric data | |
| 13.3.1 Fincoid | 1 | Other data | No numeric data | |
| 13.3.2 ML Cu 375 | 1 | Other data | No numeric data | |
| 13.4 Side effects Show forest plot | 2 | 108 | Odds Ratio (M‐H, Random, 95% CI) | 2.15 [0.50, 9.31] |
| 13.4.1 Eye and mouth swelling | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 3.15 [0.12, 82.16] |
| 13.4.2 Stomach cramps | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 3.15 [0.12, 82.16] |
| 13.4.3 Tired, irritable | 1 | 28 | Odds Ratio (M‐H, Random, 95% CI) | 1.67 [0.25, 11.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 14.1 Number of pads/day Show forest plot | 1 | 122 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐1.57, ‐0.43] |
| 14.2 Menstruation "more abundant than normal" Show forest plot | 1 | 310 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.34, 0.85] |
| 14.3 Clots in menstrual blood Show forest plot | 1 | 310 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.27, 0.68] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 15.1 Menstrual blood loss (alkaline hematin) Show forest plot | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐26.16, 25.56] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 16.1 Occurence of HMB (%) Show forest plot | 1 | Other data | No numeric data | |
| 16.1.1 Alkaline hematin | 1 | Other data | No numeric data | |
| 16.1.2 PBAC | 1 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 17.1 Percentage days bleeding or spotting Show forest plot | 1 | 118 | Mean Difference (IV, Random, 95% CI) | 9.50 [1.48, 17.52] |
| 17.2 Longest consecutive run of days without bleeding or spotting Show forest plot | 1 | 118 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐6.93, 3.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 18.1 Median number of bleeding days Show forest plot | 1 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 19.1 Median number of bleeding and spotting days Show forest plot | 1 | Other data | No numeric data | |
| 19.2 Satisfied with bleeding pattern at 12 weeks Show forest plot | 1 | 73 | Odds Ratio (M‐H, Random, 95% CI) | 1.10 [0.40, 3.03] |
| 19.3 Side effects Show forest plot | 1 | 86 | Odds Ratio (M‐H, Random, 95% CI) | 3.22 [0.13, 81.19] |
| 19.3.1 Gastroesophageal reflux disease (GERD) | 1 | 86 | Odds Ratio (M‐H, Random, 95% CI) | 3.22 [0.13, 81.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 20.1 Percentage bleeding and spotting days Show forest plot | 1 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 21.1 Menstruation "more painful than normal" Show forest plot | 1 | 310 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.44, 1.14] |
| 21.2 Side effects Show forest plot | 1 | 366 | Odds Ratio (M‐H, Random, 95% CI) | 0.42 [0.02, 9.29] |
| 21.2.1 Dyspepsia and diarrhoea | 1 | 122 | Odds Ratio (M‐H, Random, 95% CI) | 9.96 [0.52, 189.04] |
| 21.2.2 Headache | 1 | 122 | Odds Ratio (M‐H, Random, 95% CI) | 0.09 [0.00, 1.60] |
| 21.2.3 Depression | 1 | 122 | Odds Ratio (M‐H, Random, 95% CI) | 0.09 [0.00, 1.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 22.1 Reduction in menstrual cramps Show forest plot | 1 | 20 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.11, 8.95] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 23.1 IUD removal by 26 weeks due to pain or increased blood loss Show forest plot | 1 | 1962 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.66, 1.38] |
| 23.2 IUD removal due to pain or increased blood loss by 52 weeks Show forest plot | 1 | 1962 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.93, 1.69] |

