رسیده شدن دهانه رحم پیش از جراحی قبل از انجام هیستروسکوپی
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع مطالعات در انتظار ارزیابی
منابع اضافی
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomized, placebo‐controlled clinical trial | |
| Participants | Patients undergoing hysteroscopy, for simultaneous diagnostic and operative indications such as uterine septae, synechiae, submucous myomas, endometrial polyps and lost intrauterine devices, were included into the study. They studied a total of 43 women scheduled for hysteroscopy at Guhane school of medicine Ankara, Turkey | |
| Interventions | 43 patients undergoing hysteroscopy as an operative office procedure were randomised to misoprostol (n = 22) or placebo (n = 21) groups. Indications for hysteroscopy were similar in both groups and included: uterine septae, synechiae, submucous myomas, endometrial polyps and removal of intrauterine devices. In the misoprostol group, the number of patients having undergone previous delivery was nine (41%) and it was eight (38%) in the placebo group. The median age for misoprostol group was 26.2 years with range of(17‐36), while in placebo group was 27.1 years with range of (18‐38). Misoprostol 400 mcg, was administered to the posterior fornix 4 h before hysteroscopic intervention. The same protocol was performed for the placebo (control) group. | |
| Outcomes | Dilatation time, pain score, cervical bleeding and laceration, and uterine perforation. | |
| Notes | The procedure was done as an office hysteroscopy, but Intravenous analgesia was used in addition to cervical block which possible in case of operative procedure. For operative hysteroscopy in this study, the 7 mm operative hysteroscopic sheath was used.The cervix was dilated to 7‐8 mm in all patients even if it was started as diagnostic. Author contacted for: Method of randomisation, Method of treatment allocation, Was blinding used, and if so who was blinded, intention‐to‐treat analysis, Power Calculation, Source of funding, Declaration of interest, mean +/‐ SD ( or cervical width, dilation time, and operation time), and no.of women who had chills as a side effect, with no response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | 43 women were included in the study and all were included in the analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | no other sources of bias can be identified |
| Methods | Prospective randomised controlled trial. | |
| Participants | From June to December 2004, they studied a total of 105 women scheduled for hysteroscopy at the department of obstetrics and gynaecology of Kaunas University hospital of Medicine, Kaunas, Lithuania. The inclusion criteria were being perimenopausal or postmenopausal women, having definite indication for hysteroscopy and being in good health. Allergy to prostaglandin and lesions of the endocervical canal were considered as exclusion criteria. | |
| Interventions | 105 women were assigned to 2 groups using a computer‐generated randomisation table. In the study group (n = 51) 400 micrograms of misoprostol was inserted in the posterior vaginal fornix at least 12 h before hysteroscopy. No patient received any cervical ripening agent prior to surgery in the control group (n = 54). The hysteroscopy was performed under general anaesthesia by 2 investigators. Cervical dilation was performed using successively larger Hegar dilators until resistance was met. Cervical width was reported as 1 size smaller as the final Hegar dilator used. Further, cervix was dilated to Hegar No. 8.5 for diagnostic purposes and minor operative procedures, and to Hegar No. 10 for hysteroscopic resection of fibroids. An 8mm hysteroscope was used and the uterine cavity distended with normal saline solution. The 2 groups were similar with respect to age, gravidity and parity, number of menopausal women, and number of years after menopause. None of the menopausal women were taking hormone therapy. The number of women who had previous cervical dilation and cervical surgery and the indications for hysteroscopy were also similar in the 2 groups. | |
| Outcomes | The primary outcome measure was the number of women who required cervical dilation. The secondary outcomes were the cervical width (measured by the largest size of Hegar dilator that could be inserted without resistance), total operative time, complications, and adverse effects of misoprostol | |
| Notes | Diagnostic procedures were included, but the cervix in these cases was dilated under general anaesthesia to Hegar No. 8.5 mm using 8 mm hysteroscope. Such dilatation use for operative hysteroscopic procedures. Author contacted for: Was blinding used, and if so who was blinded, intention to treat analysis, Source of funding, Declaration of interest, mean +/‐ SD dilation time. The author responded as: the blinding was not used, Intention to treat analysis was used. No source of funding and no conflict of interest. They did not record dilatation time. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated randomization table was used |
| Allocation concealment (selection bias) | Unclear risk | No information supplied |
| Blinding of participants and personnel (performance bias) | High risk | No blinding, based in author's response |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up all 105 included patients were included in the analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | no other sources of bias can be identified |
| Methods | Prospective randomized placebo controlled study. | |
| Participants | Forty nulliparous reproductive age women at University of teaching centre, Royal Victoria Hospital,who required operative hysteroscopy were considered eligible for the study. | |
| Interventions | Forty nulliparous reproductive age women who received injection of leuprolide acetate 3.75 mg, 4 weeks before operative hysteroscopy were randomly allocated by computer generated random table. 20 of them received sublingual misoprostol 100 mug and the other 20 received placebo administered 12 hours before operative hysteroscopy. The hysteroscopy performed under general anaesthesia by the senior author.stop watch was used to evaluate the length of the time required to dilate the cervix to 9 mm, the 2 (difficult), or 3 (very difficult).Indication for hysteroscopy was similar in the two groups. | |
| Outcomes | The time to dilate the cervix to 9 mm, complications, and adverse effects of misoprostol | |
| Notes | The author was contacted for : Method of treatment allocation; Was blinding used, and if so who was blinded, intention to treat analysis, Power Calculation, Source of funding, Declaration of interest, Duration of operation and number of women required cervical dilatation, with no response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly allocated by computer‐generated random table" |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | No mention of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | No mention of blinding |
| Incomplete outcome data (attrition bias) | Low risk | No loss of follow‐up, all randomised women were analysed |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | No other sources of bias can be identified |
| Methods | Randomized controlled trial. The allocation concealment was by means of sealed envelopes. | |
| Participants | The study was carried at the Gynecologic Endoscopy Unit, Assiut University Hospital from February 2002 to July 2003. It comprised 144 patients recruited from the Gynaecology, Infertility, and Family Planning Clinics with different indications for operative hysteroscopy Inclusion criteria included nulliparous or multiparous women with primary or secondary cervical stenosis (defined as difficult or failed cervical sounding in the office) who were scheduled for operative hysteroscopy. Selected cases had a full history, thorough general and pelvic examinations, and transvaginal ultrasonography to determine the nature, site and extent of intrauterine lesions. | |
| Interventions | 144 patients were included in this double blind randomised study. The evaluator included as: Group A (72 cases) received 200 mcg misoprostol in to the posterior fornix 8 h prior to surgery. Patients in group B (72 cases) received a single laminaria (Med Gyn Products, Inc., USA) as fine as 2mm inserted in to the cervical canal. There was no difference between the groups for age and parity, indications and type of surgery. Primary or secondary infertility were the main indications in 38 (52.8%) and 38 (52.71%) patients in both groups respectively due to a suspected intrauterine cause as diagnosed by transvaginal scan (TVS) or hysterosalpingography (HSG). In the operating room, the degree of initial cervical dilatation was assessed by introducing Hegar dilators under general anaesthesia.It was defined as the maximal calibre dilator that passed without resistance in a descending order, starting with the largest size dilator. | |
| Outcomes | The duration of subsequent cervical dilatation until reaching 10mm, and feasibility of the procedure, were recorded. Cervical canal dilatation complications (false passage or perforation) were reported. At the end of the procedure, doctor assessment in the form of feasibility of the hysteroscopic operation was reported, and patient impression in the form of insertion difficulties, convenience and fear of either method. All operations were done by only three members of the Endoscopic Unit with a comparable level of experience. | |
| Notes | Author contacted for: Allocation concealment, intention to treat analysis, Source of funding, Declaration of interest, number of women required cervical dilatation, and side effects of both, with no response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The randomisation was done by means of sealed envelopes" |
| Allocation concealment (selection bias) | Low risk | The allocation concealment was by means of sealed envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | It was a double‐blind randomised study in that the evaluator (first author) masked the key from the researcher (third author) to avoid bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | No loss of follow‐up, all included women were analysed |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | no other sources of bias can be identified |
| Methods | Randomized placebo‐controlled study. | |
| Participants | 48 women of reproductive age who required operative hysteroscopy for submucous myoma or polyps and considered medically fit were included. The exclusion criteria were: contraindication to prostaglandins (asthma, glaucoma, hypertension), history of cervical surgery or of cervical incompetence, menopausal women, and treatment with GnRH agonists. | |
| Interventions | Between January 1 and March 30, 2001, 48 women of reproductive age who required operative hysteroscopy for submucous myoma (n= 36) or polyps (n= 12) were randomly allocated by computer‐generated randomisation table to receive either four placebo tablets or three placebo tablets and 200 mcg misoprostol or two placebo tablets and 400 or 800 mcg misoprostol (Cytotec Laboratories Searle, France), given vaginally 4 h before surgery. The placebo tablets were identical to misoprostol in appearance. Four hours before the procedure, the dry tablets were placed by a nurse in the posterior vaginal fornix. | |
| Outcomes | The primary outcome measure was cervical width, which was assessed by the subjective force required to enter the cervical os without resistance with successive Hegar dilators from 3‐8 mm. Secondary outcome measurements included the subjective ease of cervical dilatation, the time required for dilatation up to Hegar 10, preoperative pain, and the adverse effects and complications of the procedure (cervical injuries, uterine perforation, false passage, bleeding). | |
| Notes | Author contacted for: Method of treatment allocation. Source of funding, Declaration of interest. number of women required cervical dilatation, side effects of both placebo and misoprostol, and duration of surgery for both groups, with no response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Surgeons and operating theatre nurses who removed the tablets which were not totally disintegrated after 4 h were blinded to patient allocation |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information supplied. |
| Incomplete outcome data (attrition bias) | Low risk | One patient had to withdraw from the study because she was found during surgery to be pregnant. The data for this patient were included in the analysis, in compliance with the principle of intention‐to‐treat analysis. Similarly, one patient received a different treatment than that to which she was randomly allocated. Indeed, she received placebo tablets instead of 800 mcg of misoprostol. So, the analysis considered her to be in the group to which she was allocated, that is, group 1. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | no other sources of bias can be identified |
| Methods | Randomized placebo‐controlled study. | |
| Participants | 55 patients of reproductive age, who have undergone a cesarean section at least once, from January 2008 to December 2010 with an indication for diagnostic hysteroscopy for menstrual problems or during investigation for some intra‐uterine lesion. They were classified into two groups by a computer generated randomizations table, group I (misoprostol study group) and group II (control group). Study’s protocol was approved by the ethical committee of our hospital. Age of the patient, number of caesarean sections, time from last cesarean operation and complications during cervical dilatation were also recorded. Patients who delivered vaginally, or had undergone any other transcervical or transabdominal uterine and cervical intervention, such as loop electrosurgical procedures and spontaneous abortions, were not included in the study. Main indications (as far as are concerned intrauterine lesions) for hysteroscopy were endometrial polyps, submucosal myomas and endometrial hyperplasia, mainly diagnosed at a routine visit or during diagnostic process. Hysteroscopy was, by routine, performed on patients during the follicular phase of their cycles. | |
| Interventions | In group I (n = 30), a 200 microgram of misoprostol tablet (Cytotec, Cipla Limited, Athens, Greece) was inserted in the posterior fornix 12 h before hysteroscopy, by a medical doctor of our team. In group II (n = 25), hysteroscopy was performed without misoprostol tablet or other placebo drugs. Both patient and surgeon were unaware of the classification of the groups (study and control). All operations were performed by the same surgeon to avoid possible discrepancies between different surgeons. A 12 mm sheath diameter resectoscope with a 15° oblique lens (Karl Storz) was used. The uterine cavity was expanded with a NaCl 0.9 % solution. Cervical width was measured by a Hegar bougie. After operative procedure, patients were hospitalised for 6 h before being sent home. The groups were similar regarding age, number of previous caesarean section operations and number of months since the last caesarean operation. | |
| Outcomes | The outcome included cervical width detected with Hegar dilators and complication rate. | |
| Notes | Author contacted for: Method of treatment allocation. Intention‐to‐treat analysis, Source of funding, Declaration of interest, number of women required cervical dilatation, time required for cervical dilatation,failure to dilate the cervix, duration for surgery, preoperative pain score and side effects of both. Author's response ‐ The method of treatment a location was a computer‐generated random table. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Not clear |
| Blinding of participants and personnel (performance bias) | Low risk | Both patient and surgeon were unaware of the classification of the groups (study and control) |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information supplied. |
| Incomplete outcome data (attrition bias) | Low risk | All patients were included in the analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | no other sources of bias can be identified |
| Methods | Randomized controlled trial. | |
| Participants | Fifty postmenopausal women, who underwent hysteroscopy, between March 2010 and January 2011, were included in the study. The women requiring hysteroscopy randomised to into two groups: pretreatment with misoprostol group 25 women ( study group) and no pretreatment group 25 women (control group). A full medical, obstetrical, and gynaecological history was taken followed by physical examination. | |
| Interventions | The study group was given 200 micrograms of misoprostol to be inserted in the vagina at least 12 hours before the procedure and the control group did not receive any cervical priming agent. All hysteroscopies were carried out under general anaesthesia. Before hysteroscopy the dilatation of cervix was assessed with the number of Hegar's dilator passed without resistance (pre‐procedural dilatation). If sufficient cervical dilatation did not occur then dilatation was done using successively larger Hegar's dilators. Using a stop watch, the length of time required to dilate the cervix to 8 mm was noted. Indication for hysteroscopy in all women was postmenopausal bleeding. | |
| Outcomes | Preprocedural cervical width, number of women requiring additional dilatation, time required for dilatation and also the intraoperative complications. | |
| Notes | Author contacted for: Method of treatment allocation. Intention to treat analysis, Source of funding, Declaration of interest, failure to dilute the cervix, duration for surgery, preoperative pain score and side effects of both, and no answer. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized controlled trial; method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not clear |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not clear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | All study subjects were included in the analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | no other sources of bias can be identified |
| Methods | Randomized, controlled study. Patients were randomly allocated to receive a laminaria tent (n=59) or misoprostol (n=58) using sealed envelopes and a computer‐generated random‐number allocation table. A study nurse assigned the patients to treatment protocols. | |
| Participants | From March 2005 to January 2007. All premenopausal women who were to undergo operative hysteroscopy were recruited for the study. Exclusion criteria were contraindication to prostaglandins including asthma, glaucoma, and hypertension. | |
| Interventions | In the laminaria group (n=59), patients received a single laminaria tent (Mizutani Laminana Inc., Nagoya, Japan) about 3 mm in diameter 12 hours before operative hysteroscopy. In the misoprostol group (n=58), patients were given 400 microgram of misoprostol (Orally Cytotec; Pharmacia Corp, Chicago, Illinois) 12 and 24 hours before operative hysteroscopy. No patients were given any analgesics to compare the pain intensity associated with cervical priming. All operative hysteroscopies were performed by the same surgeon (YR. Lin). Patients received intravenous general anaesthesia with propofol, or in those with submucous leiomyoma, endotracheal general anaesthesia. After the patients were anaesthetized, residents in training prepared the patients, including disinfection and draping, and the surgeon was summoned to perform the hysteroscopy. The hysteroscopist was blinded to the priming method. Hegar dilators (Atom Medical Co., Tokyo, Japan.) were introduced through the cervical os starting with No. I and followed by increasingly larger dilators until resistance was encountered. Postpriming cervical width was defined as the smallest dilator that passed with resistance. Operative hysteroscopies were performed using a 22F resectoscope (Karl Storz GmbH, Tuttlingen, Germany) with a sheath diameter of 7 mm. A 5% glucose solution was used for uterine distention and irrigation. No complications occurred during cervical dilation or hysteroscopic surgeries. | |
| Outcomes | Primary outcomes were post‐priming cervical width and need for cervical dilation, and secondary outcomes were adverse effects of the priming methods. | |
| Notes | A total of 117 patients were included in the study. In 6 patients randomised to the laminaria group (10.2%), laminaria tent insertion failed because of cervical stenosis. All of these patients were nulliparous. In the misoprostol group, 1 patient discontinued the second dosage of misoprostol because of unbearable pain. Therefore, data for analysis were available for 53 patients in the laminaria group and 57 in the misoprostol group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly allocated using sealed envelopes and a computer‐generated random‐number allocation table |
| Allocation concealment (selection bias) | Low risk | A study nurse assigned the patients to treatment protocols |
| Blinding of participants and personnel (performance bias) | Low risk | The hysteroscopist was blinded to the priming method |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | A total of 117 patients were included in the study In 6 patients randomised to the laminaria group (10.2%), laminaria tent insertion failed because of cervical stenosis. All of these patients were nulliparous. In the misoprostol group, 1 patient discontinued the second dosage of misoprostol because of unbearable pain. Therefore, data for analysis were available for 53 patients in the laminaria group and 57 in the misoprostol group |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | no other sources of bias can be identified |
| Methods | Randomised, double‐blind, placebo‐controlled sequential trial (premenopausal study) The randomisation was performed with permuted blocks, using the randomisation plan generator. The randomisation procedure was third party concealed randomisation, performed by the hospital pharmacist. Placebo misoprostol tablets are difficult to make; therefore, gelatine capsules with an identical appearance were manufactured by the hospital pharmacist. The hospital pharmacist prepared numbered, opaque, sealed plastic containers for intervention agents. | |
| Participants | 69 Premenopausal women referred to outpatient operative hysteroscopy at Ullevâl University Hospital between 1 August 2006 and 20 April 2007. Women who had a medical indication for operative hysteroscopy and who had given informed consent were eligible for study recruitment. Exclusion criteria were as follows: women who were unable to communicate in Norwegian, women without an indication for hysteroscopy, women who were medically unfit for surgery and women with a known allergy to misoprostol. | |
| Interventions | 84 women referred to outpatient operative hysteroscopy at Ullevâl University Hospital between 1 August 2006 and 20 April 2007.During the 8 months the study was carried out, 82% of the total number of women referred to operative hysteroscopy participated in the study. 11% declined the offer and 7% excluded based on the exclusion. Two separate studies (Oppegaard a for premenopausal and Oppegaard b for postmenopausal), but identical, studies were conducted in parallel, based on the women's menopausal status. 69 Premphase women were randomised to either misoprostol or placebo (34 in misoprostol group and 35 in placebo group). Each participant received either 1000 micrograms of misoprostol or placebo, which they self‐ inserted vaginally at least 12 hours before operative hysteroscopy. Those involved in administering the intervention and the women were blinded to the treatment received. The procedures were done under general intravenous anaesthetic (propofol/fentanyl/alfentanil). Six experienced senior gynaecologists (with 5‐20 years experience in operative hysteroscopy) performed the operative hysteroscopies during the study period .Before the operative hysteroscopy, the operator measured the preoperative degree of cervical dilatation by passing Hegar dilators through the cervix in ascending order starting with a size of 4 mm. The size of the largest dilator passed into the inner cervical ostium without subjective resistance felt by the operator was recorded as the preoperative degree of dilatation. If there was initial resistance with Hegar dilator of size 4 mm, then dilators of size 3 or 2 mm were tried. If there was resistance with Hegar dilator of size 2 mm, the result was recorded as 0 mm. After the cervical canal was dilated to a Hegar dilator of size 10 or 11 mm, a rigid resectoscope equipped with a Hopkins 12° rigid fibre optic was passed into the uterine cavity. A sodium chloride 9% solution was infused for uterine irrigation. A bipolar diathermal current of 280 watts (pure cut) was routinely used for resection of pathological uterine masses (myomas, polyps, uterine septae, etc.) and endometrium. The two treatment groups were comparable regarding baseline clinical preoperative characteristics, and the indications for operative hysteroscopy and the operative procedure. | |
| Outcomes | Preoperative cervical dilatation (primary outcome). Secondary end points were as follows: the number of women who achieve satisfactory cervical priming (cervical dilatation 5 mm); 5 mm was chosen as satisfactory, as this would permit insertion of a diagnostic hysteroscope without further dilatation. A preoperative cervical dilatation of 5 mm would also make it much easier to further dilate the cervix with Hegar dilators if necessary (for insertion of an operative resectoscope of 10‐11 mm), decreasing the risk of creating a false passage, acceptability of self‐administration of vaginal capsules at home, the number of dilatations judged as easy or difficult by the operator, and the frequency of complications, as registered by the nurses preoperatively and the operators intraoperatively. | |
| Notes | 4 patients were not analysed for the outcomes in the placebo group, the reason for this is either did not attend the surgery, Asherman's syndrome, postponed operation or no indication for hysteroscopy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed with permuted blocks, using the randomisation plan generator |
| Allocation concealment (selection bias) | Low risk | The randomisation procedure was third party concealed randomisation, performed by the hospital pharmacist and the hospital pharmacist prepared numbered, opaque, sealed plastic containers labelled Misoprostol 0.5 mg/Placebo, 2 vaginal capsules |
| Blinding of participants and personnel (performance bias) | Low risk | Those administering the intervention and the women were blinded to the treatment received |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | 4 patients were not analysed for the outcomes in the placebo group |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | no other sources of bias can be identified |
| Methods | Randomized, double‐blind, placebo‐controlled sequential trial (postmenopausal study) The randomisation was performed with permuted blocks, using the randomisation plan generator. The randomisation procedure was third party concealed randomisation, performed by the hospital pharmacist. Placebo misoprostol tablets are difficult to make; therefore, gelatine capsules with an identical appearance were manufactured by the hospital pharmacist. The hospital pharmacist prepared numbered, opaque, sealed plastic containers for intervention agents. | |
| Participants | 24 postmenopausal women referred to outpatient operative hysteroscopy at Ullevâl University Hospital between 1 August 2006 and 20 April 2007. Women who had a medical indication for operative hysteroscopy and who had given informed consent were eligible for study recruitment. Exclusion criteria were as follows: women who were unable to communicate in Norwegian, women without an indication for hysteroscopy, women who were medically unfit for surgery and women with a known allergy to misoprostol. | |
| Interventions | 29 women referred to outpatient operative hysteroscopy at Ullevâl University Hospital between 1 August 2006 and 20 April 2007. Two separate (Oppegaard 2008 afor premenopausal andOppegaard 2008 bfor postmenopausal), but identical, studies were conducted in parallel, based on the women's menopausal status. 24 postmenopausal women were randomised to either misoprostol or placebo. Each participant received either 1000 micrograms of misoprostol or placebo, which they self‐ inserted vaginally at least 12 hours before operative hysteroscopy. Those involved in administering the intervention and the women were blinded to the treatment received. Each study participant opened a numbered container at home, containing either misoprostol or lactosum monohydricum in capsules. The women were instructed to insert the capsules as deep as possible vaginally after voiding urine at approximately 9 pm the evening before the operation.On admission to the operating theatre, nurses recorded symptoms and comments from the women on the case report form. The procedures were done under general intravenous anaesthetic (propofol/fentanyl/alfentanil). Six experienced senior gynaecologists (with 5‐20 years experience in operative hysteroscopy) performed the operative hysteroscopies during the study period .Before the operative hysteroscopy, the operator measured the preoperative degree of cervical dilatation by passing Hegar dilators through the cervix in ascending order starting with a size of 4 mm. The size of the largest dilator passed into the inner cervical ostium without subjective resistance felt by the operator was recorded as the preoperative degree of dilatation. If there was initial resistance with Hegar dilator of size 4 mm, then dilators of size 3 or 2 mm were tried. If there was resistance with Hegar dilator of size 2 mm, the result was recorded as 0 mm. After the cervical canal was dilated to a Hegar dilator of size 10 or 11 mm, a rigid resectoscope equipped with a Hopkins 12° rigid fibre optic was passed into the uterine cavity. A sodium chloride 9% solution was infused for uterine irrigation. A bipolar diathermal current of 280 watts (pure cut) was routinely used for resection of pathological uterine masses (myomas, polyps, uterine septae, etc.) and endometrium. The two treatment groups were comparable regarding basal clinical preoperative characteristics, and the indications for operative hysteroscopy and the operative procedure. | |
| Outcomes | Preoperative cervical dilatation (primary outcome). Secondary end points were as follows: the number of women who achieve satisfactory cervical priming (cervical dilatation 5 mm); 5 mm was chosen as satisfactory, as this would permit insertion of a diagnostic hysteroscope without further dilatation. A preoperative cervical dilatation of 5 mm would also make it much easier to further dilate the cervix with Hegar dilators if necessary (for insertion of an operative resectoscope of 10‐11 mm), decreasing the risk of creating a false passage, acceptability of self‐administration of vaginal capsules at home, the number of dilatations judged as easy or difficult by the operator, and the frequency of complications, as registered by the nurses preoperatively and the operators intraoperatively. | |
| Notes | 2 patients were not analysed for the outcomes one in each group; reason for this is that they did not attend the surgery. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed with permuted blocks, using the randomisation plan generator |
| Allocation concealment (selection bias) | Low risk | The randomisation procedure was third party concealed randomisation, performed by the hospital pharmacist and the hospital pharmacist prepared numbered, opaque, sealed plastic containers labelled Misoprostol 0.5 mg/Placebo, 2 vaginal capsules |
| Blinding of participants and personnel (performance bias) | Low risk | Administering the intervention and the women were blinded to the treatment received |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | 2 patients were not analysed for the outcomes one in each group reason for this is did not attend the surgery; thus 92% of randomised participants were included in analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | no other sources of bias can be identified |
| Methods | Randomised double‐blind placebo‐controlled sequential trial. Participants were randomly assigned at a ratio of 1:1 to 1000 Microgram of vaginal misoprostol or placebo, according to a randomised list of three permuted blocks on a double‐blind basis at the outpatient consultation. The hospital pharmacist manufactured the study drug and placebo capsules, which were delivered in numbered, opaque, sealed plastic containers. Both patient and examiner were blind to randomisation. | |
| Participants | Postmenopausal women referred for day‐care operative hysteroscopy at Norwegian university teaching hospital. The inclusion criteria: All postmenopausal (>1 year since last menstruation) women who are referred to day‐care hysteroscopy with a medical indication for hysteroscopy, and who have given informed consent, were eligible for study recruitment. Exclusion criteria: women who do not wish to participate, women who are medically unfit for hysteroscopy, women who are medically unfit for participation in any clinical trial, women who do not have a medical indication for hysteroscopy, women who have previously had, or currently have breast or gynaecological cancer, women who have a medical contraindication for locally applied estradiol or misoprostol, the current use of hormone therapy or unable to communicate in Norwegian. Between January 2008 and April 2009, 72 women were enrolled and randomly assigned to treatment .The study was stopped on 13 May 2009 as a result of a significant difference between the misoprostol and placebo groups on the primary efficacy outcome. 75 women were excluded (52 on hormone therapy,15 history of breast cancer in mamma, 3 no indication for operative hysteroscopy, 2 previous D&C in the last three months, one suspected uterine cancer , one cervical dysplasia, and one could not speak Norwegain), 79 women were eligible for randomisation, 7 of them did not wish to participate in the study, 72 women were randomised (36 women in each group). 5 women withdrew their consent (2 in the placebo group and 3 in the misoprostol group), so the analysis was done for 33 women in the misoprostol group and 34 in the placebo group. | |
| Interventions | Before a cervical smear and endometrial biopsy were taken as part of the routine outpatient examination, cervical dilatation was measured by passing Hegar dilators through the cervix in ascending order, starting with a Hegar dilator of size 2 mm. Women participating in the study started daily treatment with 25‐Microgram estradiol vaginal tablets for 2 weeks before operative hysteroscopy. The study participants were instructed to insert the misoprostol/placebo capsules vaginally, as deep as possible, after voiding urine at approximately 21.00 hours on the evening before the operation. Those involved in administering the intervention and the women were blinded to the treatment received. Each study participant opened a numbered container at home, containing either misoprostol or lactosum monohydricum in capsules. On admission to the operating theatre, nurses recorded symptoms and comments from the women on the case report form. The procedures were done under general intravenous anaesthetic (propofol/fentanyl/alfentanil). Six experienced senior gynaecologists (with 5‐20 years experience in operative hysteroscopy) performed the operative hysteroscopies during the study period .Before the operative hysteroscopy, the operator measured the preoperative degree of cervical dilatation by passing Hegar dilators through the cervix in ascending order starting with a size of 2 mm. The size of the largest dilator passed into the inner cervical ostium without subjective resistance felt by the operator was recorded as the preoperative degree of dilatation. The size of the largest huger dilator passed through the internal os without resistance was recorded as the preoperative degree of dilatation. A rigid resectoscope equipped with a Hopkins 12° rigid fibre optic was passed into the uterine cavity. A sodium chloride 9% solution was infused for uterine irrigation. A bipolar diathermal current of 280 watts (pure cut) was routinely used for resection of pathological uterine masses (myomas, polyps, uterine septae, etc.) and endometrium. The two treatment groups were comparable regarding basal clinical preoperative characteristics, and the indications for operative hysteroscopy and the operative procedure. | |
| Outcomes | The primary outcome is the preoperative baseline cervical dilatation in the two treatment groups. | |
| Notes | Funded by research grants. No pharmaceutical funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomly assigned at a ratio of 1:1 to 1000 lg of vaginal misoprostol or placebo, according to a randomised list of three permuted blocks" |
| Allocation concealment (selection bias) | Low risk | "The hospital pharmacist manufactured the study drug and placebo capsules, which were delivered in numbered containers". |
| Blinding of participants and personnel (performance bias) | Low risk | "Those involved in administering the intervention and the women will be blinded to the treatment received" |
| Blinding of outcome assessment (detection bias) | Low risk | Primary outcome assessed by operator, who was blinded |
| Incomplete outcome data (attrition bias) | Low risk | 72 women were randomised (36 women in each group). 5 women withdrew their consent (2 in the placebo group and 3 in the misoprostol group), so the analysis was done for 33 women in the misoprostol group and 34 in the placebo group: thus 93% of women randomised were analysed. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | no other sources of bias can be identified |
| Methods | Prospective randomised double‐blind placebo‐controlled trial. | |
| Participants | All women scheduled for planned operative hysteroscopy at their University based Obstetrics and Gynecology residency training program were included for study. | |
| Interventions | Forty‐six women were enrolled in the study and randomised to two groups (active medication and placebo) containing 23 subjects each. Patients were randomised to receive an opaque coded envelope that contained capsules of either misoprostol (400 micrograms) or placebo. Group selection was determined by random permuted blocks. Patients were instructed to ingest the capsules 12 hours prior to the planned procedure. The groups were similar in age, number of prior vaginal deliveries, and menopausal status. | |
| Outcomes | Time required for cervical dilation and hysteroscopy, first dilator used that encountered resistance, subjective ease of the procedure, patient pain, side‐effects, and the incidence of complications. | |
| Notes | The result was recorded as P value only ( see below please). The author was contacted but no response. The groups did not differ for time of dilation (P = 0.830), time of hysteroscopy (P = 0.243), dilator with first resistance (P = 0.402), ease of the procedure (P = 0.302) or pain rating after surgery (P = 0.880). Discomfort and side effects were similar in both groups. One cervical laceration and one false track were found in the misoprostol group. There were no uterine perforations. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not fully described: "Patients were randomized to receive an opaque coded envelope that contained capsules of either misoprostol(400 micrograms) or placebo. Group selection was determined by random permuted blocks" |
| Allocation concealment (selection bias) | Unclear risk | Method not fully described: "Patients were randomized to receive an opaque coded envelope that contained capsules of either misoprostol(400 micrograms) or placebo." |
| Blinding of participants and personnel (performance bias) | Low risk | Described as double blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Described as double blinded but does not clearly state whether outcomes assessment was blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Does not state how many women were inlcuded in analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Unclear risk | Abstract available only, no statistical data reported, other than p values |
| Methods | Randomized controlled double blind trial. Randomization done by resident in training who picked random numbers to assign the subjects to receive either 200 mcg of misoprostol (n=46) or an identical looking placebo (lactose filler, n=45). | |
| Participants | From October 1997 through September 1998, 91 women who underwent routine investigation for infertility were recruited for a randomized study at Ramathibodi hospital. These patients were suspected of having intrauterine abnormalities that needed further definitive diagnosis and treatment by hysteroscopy. Women who were suspected of being in early pregnancy or of having genital tract infections were excluded. | |
| Interventions | All subjects were outpatients. The drug or placebo was placed in the posterior vaginal fornix 9‐10 hours before the procedure. In the operating room, with the women in lithotomy position, a number 1 Hegar dilator was inserted through the internal os, followed by successively larger Hegar dilator until resistance was met ( cervical width). For diagnostic purposes if the endocervical canal was tight, the cervix was dilated to Hegar dilator number 6. If operative sheath was required for targeted biopsy or another minor procedure, the cervix was dilated to Hegar dilator number 8. For hysteroscopic resection the cervix was dilated for Hegar dilator number 9. All women were nulliparous , most of them suffer from primary infertility.The mean age of the women in misoprostol group was 29.6±5.3 and31.2±5 years. The indications for hysteroscopy were similar in the two groups. The hysteroscopy was usually performed in the follicular phase of the menstrual cycle.They used rigid 4mm hysteroscope with 30 degree forward oblique lens ans a 5.5 mm diagnostic sheath. The women were placed under general anesthesia.In most cases the uterine cavity was distended with CO2, when the visualization was inadequate because of excessive bleeding or when an operative procedure was needed, the distention media changed to 1.5% glycine solution. | |
| Outcomes | Cervical width, number of women who required cervical dilatation, duration of hysteroscopy form the insertion of the hysteroscope till the completion of hysteroscopic examination, and side effects. | |
| Notes | Diagnostic and operative procedures are included because 90.1% of cases the cervix was dilated till 8mm or 9mm Hegar dilator, such dilatation use for operative hysteroscopic procedures. 9.9% (5 patients in misoprostol and 4 patients in placebo group) did not required operative procedure nor dilatation beyond 6 mm Hegar dilator, the % is fairly equal in both groups. Author was contacted for: Method of treatment allocation, Was blinding used, and if so who was blinded, intention‐to‐treat analysis. Power Calculation, Source of funding, Declaration of interest. mean +/‐ duration to dilate the cervix and duration of surgery for both groups, with no response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was done by a resident‐in‐training who picked random numbers to assign the subjects to receive either |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Described as double‐blinded. "Subjects received either 200 mcg of misoprostol or an identical looking placebo". |
| Blinding of outcome assessment (detection bias) | Unclear risk | Described as double‐blinded but does not clearly state whether outcomes assessment was blinded. |
| Incomplete outcome data (attrition bias) | Low risk | No loss of follow‐up, all included women were analysed |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | no other sources of bias can be identified |
| Methods | A randomised controlled double‐blind trial. Randomization was done by random numbers to assign the subjects to receive either 200 mcg of misoprostol or an identical looking placebo. | |
| Participants | From October 1998 through December 1999, 174 women who underwent routine investigation for infertility were recruited for a randomised study at Ramathibodi hospital, Bangkok, Thailand. These patients were suspected of having intrauterine abnormalities that needed further definitive diagnosis and treatment by hysteroscopy. Patients with intrauterine pathology were treated with operative hysteroscopy after the diagnostic procedure. Women who were suspected of being in early pregnancy or of having genital tract infections and normal hysterosalpingogram were excluded. | |
| Interventions | The study was performed on an outpatients basis. The drug was placed in the posterior vaginal fornix 9‐10 hours before the procedure. In the operating room, with the women in lithotomy position, a number 1 Hegar dilator was inserted through the internal os, followed by successively larger Hegar dilator until resistance was met (cervical width). For diagnostic purposes ,if the endocervical canal was tight , the cervix was dilated to Hegar dilator number 6.The time taken to this stage was noted. After the completion of the diagnostic procedure, women with intrauterine pathology proceeded for operative hysteroscopy. For the operative procedure, if the endocervical canal was tight, the cervix was dilated to Hegar number 9 and the time taken was noted. The hysteroscopy was mostly performed in the follicular phase of the menstrual cycle under general anaesthesia. Diagnostic procedure was done using 5.5 mm diagnostic sheath, while operative procedures was done using either 7 mm operative sheath or a resectoscope with an outer sheath 9mm in diameter. Operation was done by the same operator.The patients's age, body weight and number of women with previous cervical dilatation were similar in the two groups. | |
| Outcomes | Cervical width, number of women who required cervical dilatation, duration of cervical dilatation, duration of operative hysteroscopy, intraoperative complications and side effects | |
| Notes | 174 patients were recruited and randomised for the study, 22 of them were excluded, 3 were in early pregnancy, 2 had genital tract infections and 13 had normal hysteroscopic findings. All 152 nulliparous patients had operative procedures ( Based on author response) where either 7 mm operative sheath or resectoscope with an outer sheath 9 mm in diameter were used. Author contacted for : Method of treatment allocation, Was blinding used, and if so who was blinded, intention to treat analysis, Power Calculation, Source of funding, Declaration of interest. and duration to dilate the cervix and duration of surgery for both groups, with no response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was done using a random number |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | The study was performed in double‐blind manner |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | They excluded 22 patients after randomisation and not included in the analysis (13%) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | no other sources of bias can be identified |
| Methods | A prospective randomised controlled double‐blind trial. Randomization was performed by a resident in training by picking a random number to assign the patients to receive either 200 mcg of misoprostol or 3 mg of dinoprostone. | |
| Participants | The study was done between January 2000 through December 2004; 329 women who had completed routine investigation for infertility were recruited for a prospective randomised study at Ramathibodi Hospital, Bangkok, Thailand. These patients were those with suspected intrauterine abnormalities that needed further definite diagnosis and treatment by hysteroscopy. Patients with suspected early pregnancy or having genital tract infection were excluded from this study. | |
| Interventions | The study was conducted as an outpatient protocol. Patients to receive either 200 mcg of misoprostol or 3 mg of dinoprostone placed digitally in the posterior fornix of the vagina 9‐10 hours before operative hysteroscopy. In the theatre room, with the patient in lithotomy position, Hegar's dilator number 1 was first introduced through the internal os followed by subsequent larger Hegar dilators until resistance was met. The cervical width was assessed by the size of the Hegar's dilator. For diagnostic purposes, if the endocervical canal was believed to be tight, the cervix was dilated to Hegar number 6. If operative hysteroscopy was required, the cervix needed to be dilated to Hegar number 8. For hysteroscopic resection, the cervix was then dilated to Hegar number 9. Hysteroscopy was timed mostly in the proliferative phase of the menstrual cycle under general anaesthesia, using propofol as total IV anaesthesia. Diagnostic hysteroscopy was performed with a standard rigid hysteroscope with a 5.5‐mm diagnostic sheath using carbon dioxide running on an electronic Hamou hysteroflator as a distending medium. Operative procedures were performed using either an operative hysteroscope with a 7‐mm operative sheath or a resectoscope with an outer sheath of 9 mm diameter. Sterile 1.5% glycine solution was used for uterine distention and irrigation. All of the recruited patients were nulliparous. Most of them suffered from primary infertility. | |
| Outcomes | The outcome assessed included cervical width at hysteroscopy, number of patients requiring cervical dilatation, the combined time of cervical dilatation up to Hegar numbers 6 and 8‐9 before inserting the operative instrument, the duration of the operative hysteroscopy, the cervicouterine complication related to cervical dilatation and hysteroscopic surgery, and the associated side effects. Any side effects such as nausea, vomiting, diarrhoea, headache, feeling an increase in body temperature, lower abdominal pain, and vaginal bleeding were also recorded. | |
| Notes | 329 patients were recruited for the study, 19 of them were excluded, 8 were in early pregnancy, 6 had genital tract infections and 5 had normal hysteroscopic findings. All 310(94%) nulliparous patients had operative procedures (Based on author response) Author contacted for: Method of treatment allocation, Was blinding used, and if so who was blinded, intention‐to‐treat analysis, Power Calculation, Source of funding, Declaration of interest. , and duration to dilate the cervix and duration of surgery for both groups, with no response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was done using a random number table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind manner |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | They excluded 19 patients after randomisation and not included in the analysis (6%) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | no other sources of bias can be identified |
| Methods | Randomized controlled trial | |
| Participants | Between April 2013 and February 2014 , women who were suspected as having an intrauterine pathology were included in the study. Inclusion criteria were as follows: women who were of reproductive age and were not pregnant at the time of presentation. Exclusion criteria included any evidence of a contraindication to prostaglandins. | |
| Interventions | Subjects were randomly assigned to the oral, sublingual, vaginal, or control group at a 1:1:1: ratio .160 subjects underwent randomization.Groups of 40 subjects each were randomly assigned to the oral group,sublingual group,vaginal group (in each case 400 micrograms of misoprostol),or control group.The four groups were comparable in age,body mass index, marital status, gravidity, parity, history of vaginal or cesarean section delivery,and indication for operative hysteroscopy. | |
| Outcomes | The primary outcome measure was the preoperative cervical width at the time of surgery and patient preference for the administration route of misoprostol. The cervical width was assessed by performing cervical dilation,Secondary outcome measurements included the time required for dilation,the subjective ease of cervical dilation recorded by a surgeon on a 5‐point Likert scale,patient, acceptability of the self‐administration of medications preoperatively at home on a 5‐point Likert scale,self‐reported misoprostol‐associated adverse effects before the procedure and complications arising within 4 weeks after surgery. | |
| Notes | Intervention group data combined, as described in Methods section. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were randomly assigned to the oral, sublingual, vaginal, or control group at a 1:1:1:1 ratio using a random |
| Allocation concealment (selection bias) | Low risk | "Sequentially numbered, sealed, opaque envelopes were prepared by the study nurse (not directly involved in the study), and each contained |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded, not placebo‐controlled |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded, not placebo‐controlled |
| Incomplete outcome data (attrition bias) | Low risk | All randomised women included in analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | No other potential bias identifed. |
| Methods | Prospective randomised placebo‐controlled trial. | |
| Participants | From January 2000 to March 2001, they studied a total of 204 women scheduled for hysteroscopy at the Mt Sinai Hospital and women's College Hospital, both of which are tertiary hospitals in Toronto, Ontario, Canada. Any patient who was considered medically fit and scheduled for an operative hysteroscopy were considered eligible for the study. Exclusion criteria included any difficulty with oral administration of misoprostol. | |
| Interventions | A total of 204 women were assigned to 2 groups using a computer‐generated randomizations table. In the study group (n = 102) 400 micrograms of misoprostol was given orally 12 and 24 hours before the surgery .The placebo group (n=102), the capsules were identical to misoprostol in appearance and dosing schedule. Seven surgeons, all experienced in the use of the hysteroscope, performed all the procedures. GnRH was used in 24 patients in misoprostol group and in 26 in placebo group. The age, parity, number of women premenopausal and postmenopausal and previous cervical surgery were similar in the two groups. | |
| Outcomes | The primary outcome measure was the cervical width (measured by the largest size of Hegar dilator that could be inserted without resistance). The secondary outcome measurements were time required for dilatation, subjective assessment of the ease of dilatation on a 5 points like scale, operative complication and adverse effects. | |
| Notes | 23 patients did not complete the protocol, 13 in the treatment group and 10 in placebo group. The reasons for not completing the protocol included surgery was cancelled in 6, adverse effects in 7, forgot to take medication 8 and others 2. Author contacted for: Method of treatment allocation, Was blinding used, and if so who was blinded, intention‐to‐treat analysis, Power Calculation, Source of funding, Declaration of interest. mean +/‐ SD for cervical width, duration of surgery, number of women required cervical dilatation and number of women who had side effects in both groups, with no response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated number table |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Surgeons, patients, and the outcome assessors were blinded to patient allocation.The placebo capsules were identical to misoprostol in appearance and dosing schedule." |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Surgeons, patients, and the outcome assessors were blinded to patient allocation. The placebo capsules were identical to misoprostol in appearance and dosing schedule." |
| Incomplete outcome data (attrition bias) | High risk | Numbers randomised to each group unclear, numbers reported in each group also unclear |
| Selective reporting (reporting bias) | Unclear risk | All prespecified outcomes reported, including complications |
| Other bias | Unclear risk | Data unsuitable for analysis due to poor reporting of study conduct |
| Methods | A randomised placebo‐controlled trial. Eligible patients were assigned to 2 groups with a computer‐generated randomization table. | |
| Participants | Patients of reproductive age referred to the Konya Investigation and Practice Center of Baskent University from September 2003 through September 2007, who had undergone a cesarean section at least once and were scheduled for operative hysteroscopy for various medical conditions were included in the study. Patients who had delivered vaginally, those who had undergone any transcervical or trans‐ abdominal uterine and cervical intervention other than cesarean section, such as hysteroscopy, cervical cryotherapy, cervical biopsies, loop electrosurgical excision procedures, spontaneous abortions, previous dilation, and previous elective abortions were excluded. The patients with a contraindication to prostaglandins were omitted. | |
| Interventions | A total of 60 patients eligible were assigned to 2 groups. In the study group (n = 32), misoprostol 400 mcg was inserted in the posterior vaginal fornix twice, 6 and 12 hours before the procedure. In the control group (n =28) a hexetidine vaginal pill as placebo was administered in the posterior vaginal fornix twice, 6 and 12 hours before the hysteroscopy. Indications for hysteroscopy were endometrial polyp, submucous myoma, uterus septum, and uterine synechia. All patients were hospitalised 1 day before surgery. The written treatment scheme followed a randomised allocation that was prepared by an independent statistician, with computer‐generated random numbers. Misoprostol and placebo were given by the attending physician, according to the route indicated in the randomisation envelope. The patient and surgeon performing the surgery were unaware of which group (study/control) these patients were from. To decrease individual differences, the operation was performed by 2 surgeons. Each surgeon was given an equal number of patients. Spinal anaesthetic was administered to patients during the operation. Hysteroscopy was performed on patients during the follicular phase of their cycles. The groups were similar with regard to age, number of previous cesarean section operations, and number of months since the last caesarean operation. The indications for hysteroscopy and intraoperative findings were similar in both groups. | |
| Outcomes | The primary end point was the highest cervical canal width detected with the largest Hegar bougie that could be passed without resistance. Secondary end points were the rates of surgical complications, including failure of cervical dilation, cervical tear, uterine perforation, the creation of a false tract, bleeding, and adverse drug effects. | |
| Notes | Author contacted for: Method of treatment allocation, Was blinding used, and if so who was blinded, intention‐to‐treat analysis, Power Calculation, Source of funding, Declaration of interest, mean +/‐ SD ( dilation time, and operation time), and no. of women who required cervical dilatation, with no response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | The patient and surgeon performing the surgery were unaware of which group (study/control) these patients were from |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | no other sources of bias can be identified |
| Methods | A prospective, randomised controlled trial. All patients were assigned to one of two groups by a computer‐generated series of random numbers. | |
| Participants | From August 2001 to Jan 2002, 100 patients from Fuxing Hospital, Beijing, China,scheduled for hysteroscopic operations (with a 9‐mm hysteroscope) were recruited into the study. | |
| Interventions | In group 1 (the misoprostol group n=53), 400 mcg misoprostol was inserted into the posterior fornix of the vagina 12 h prior to the operation. In group 2 (the osmotic dilator group n=47), a cervical osmotic dilator (disposable, synthetic hygroscopic polyacrylonitrile rod‐shaped dilators, with a diameter of 6 mm and a sectional expanding rate of 100% ) was inserted into the cervical canal until it passed the internal os. 12 h before the surgical procedure. The osmotic dilator was removed immediately before the patient was transferred to theatre. The surgeons therefore had no knowledge of what the patient received (misoprostol or osmotic dilator) prior to the surgery. All the hysteroscopic surgeries were performed in the early proliferative phase of the menstrual cycle with an Olympus 9‐mm hysteroscope under epidural anaesthesia). There were no differences between the two groups with regard to age. number of previous miscarriages, parity and types of hysteroscopic procedures performed. | |
| Outcomes | The primary outcome measure in this study was cervical dilatation, which was assessed by the size of the Hegar dilator entering the cervix without resistance, up to a maximum of 11 mm. The largest number of Hegar dilators that could be inserted into the cervix without resistance, called spontaneous dilatation, was recorded. The secondary outcome measure was subjective assessments of the ease of dilatation recorded by the surgeon when inserting a 9‐mm Hegar dilator into the cervix. Adverse effects experienced, including preoperative pain, preoperative vaginal bleeding and any other complications, were recorded for each group. | |
| Notes | Author was contacted for: Method of treatment allocation, Was blinding used, and if so who was blinded, intention‐to‐treat analysis, Power Calculation, Source of funding, Declaration of interest , mean +/‐ SD ( for cervical width, dilation time, the cervix and operation time), and no.of women who had chills as a side effect, with no response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated series of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Surgeons were blind, as the osmotic dilator was removed immediately before the patient was transferred to theatre |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | No loss of follow‐up, all included women were analysed |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, including complications |
| Other bias | Low risk | no other sources of bias can be identified |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| No eligible control intervention: in this RCT, sublingual misoprostol is compared with lidocaine spray (which is not a cervical ripening agent) | |
| It is diagnostic hysteroscopy not operative hysteroscopy | |
| It is for outpatient hysteroscopy | |
| Surgery is diagnostic not operative hysteroscopy | |
| It is for mixed diagnostic and operative hysteroscopy, cases of each not distinguished | |
| It is for diagnostic not operative hysteroscopy | |
| It is diagnostic not operative hysteroscopy | |
| Surgery is diagnostic not operative hysteroscopy | |
| This RCT compares different routes of misoprostol (sublingual and vaginal). There is no placebo or no‐intervention control group |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised double blinded trial |
| Participants | Women scheduled for operative hysteroscopy |
| Interventions | Intravaginal isosorbide mononitrate versus placebo |
| Outcomes | Cervical width, operative complications, adverse effects |
| Notes | Intervention is inhaled. Review authors to assess whether intervention is eligible for next review update |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ease of dilatation: need for mechanical dilatation Show forest plot | 5 | 441 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.04, 0.16] |
| Analysis 1.1 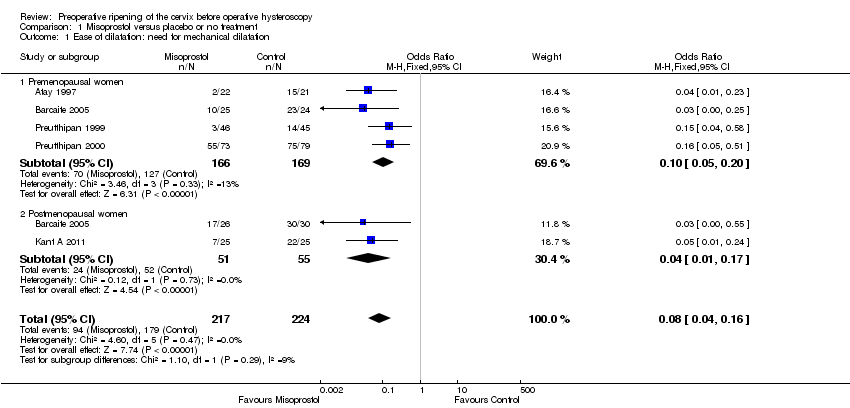 Comparison 1 Misoprostol versus placebo or no treatment, Outcome 1 Ease of dilatation: need for mechanical dilatation. | ||||
| 1.1 Premenopausal women | 4 | 335 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.05, 0.20] |
| 1.2 Postmenopausal women | 2 | 106 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.01, 0.17] |
| 2 Intraoperative complications Show forest plot | 12 | 901 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.18, 0.77] |
| Analysis 1.2  Comparison 1 Misoprostol versus placebo or no treatment, Outcome 2 Intraoperative complications. | ||||
| 2.1 Premenopausal women | 8 | 667 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.11, 0.65] |
| 2.2 Postmenopausal women and women pretreated with GnRHa | 5 | 234 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.21, 4.47] |
| 3 Specific intraoperative complications Show forest plot | 11 | Odds Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3 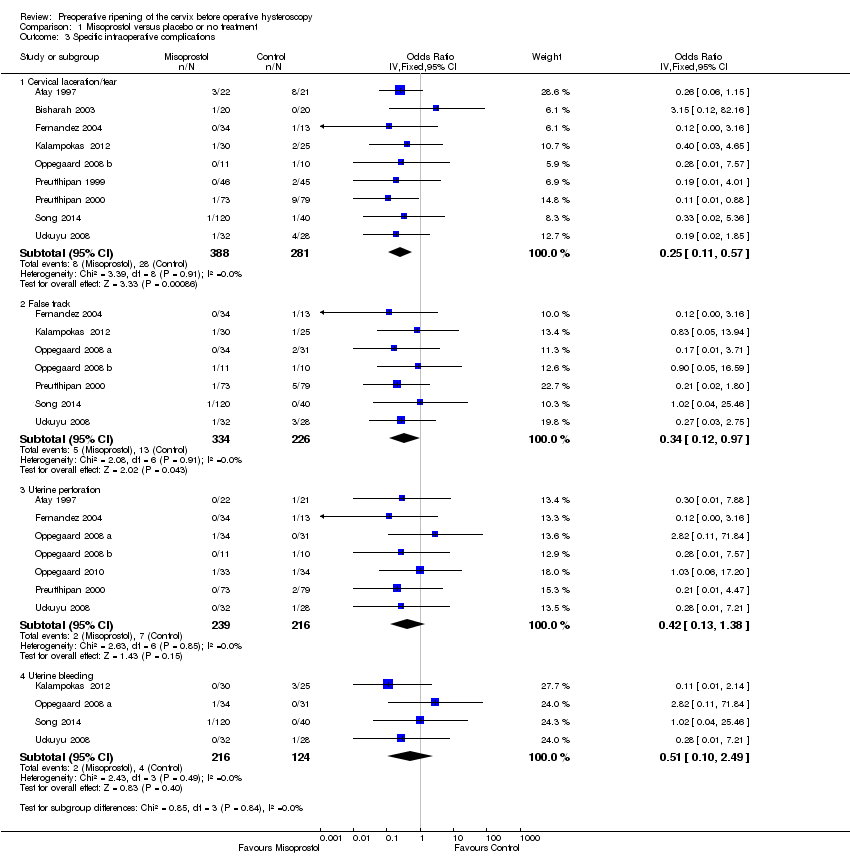 Comparison 1 Misoprostol versus placebo or no treatment, Outcome 3 Specific intraoperative complications. | ||||
| 3.1 Cervical laceration/tear | 9 | 669 | Odds Ratio (IV, Fixed, 95% CI) | 0.25 [0.11, 0.57] |
| 3.2 False track | 7 | 560 | Odds Ratio (IV, Fixed, 95% CI) | 0.34 [0.12, 0.97] |
| 3.3 Uterine perforation | 7 | 455 | Odds Ratio (IV, Fixed, 95% CI) | 0.42 [0.13, 1.38] |
| 3.4 Uterine bleeding | 4 | 340 | Odds Ratio (IV, Fixed, 95% CI) | 0.51 [0.10, 2.49] |
| 4 Time required to dilate the cervix (sec.) Show forest plot | 7 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Misoprostol versus placebo or no treatment, Outcome 4 Time required to dilate the cervix (sec.). | ||||
| 5 Preoperative pain score Show forest plot | 4 | 200 | Mean Difference (IV, Fixed, 95% CI) | 1.25 [0.77, 1.72] |
| Analysis 1.5 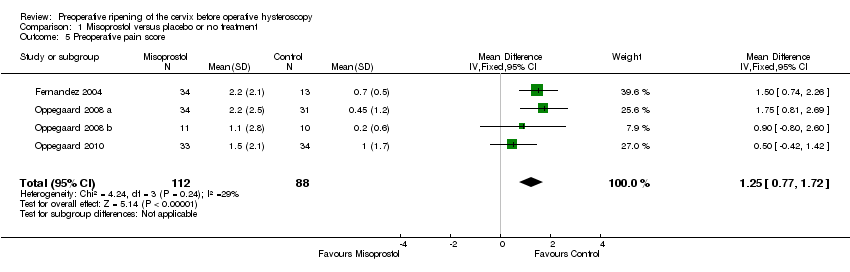 Comparison 1 Misoprostol versus placebo or no treatment, Outcome 5 Preoperative pain score. | ||||
| 6 Cervical width (dilator size in mm) Show forest plot | 12 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.6  Comparison 1 Misoprostol versus placebo or no treatment, Outcome 6 Cervical width (dilator size in mm). | ||||
| 7 Failure to dilate the cervix Show forest plot | 10 | 690 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.82] |
| Analysis 1.7 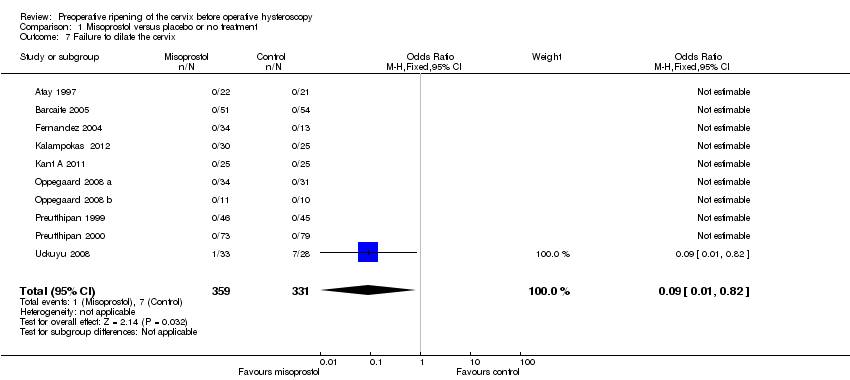 Comparison 1 Misoprostol versus placebo or no treatment, Outcome 7 Failure to dilate the cervix. | ||||
| 8 Side effects Show forest plot | 4 | 272 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.59 [1.15, 5.79] |
| Analysis 1.8  Comparison 1 Misoprostol versus placebo or no treatment, Outcome 8 Side effects. | ||||
| 9 Types of side effects Show forest plot | 7 | Odds Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.9 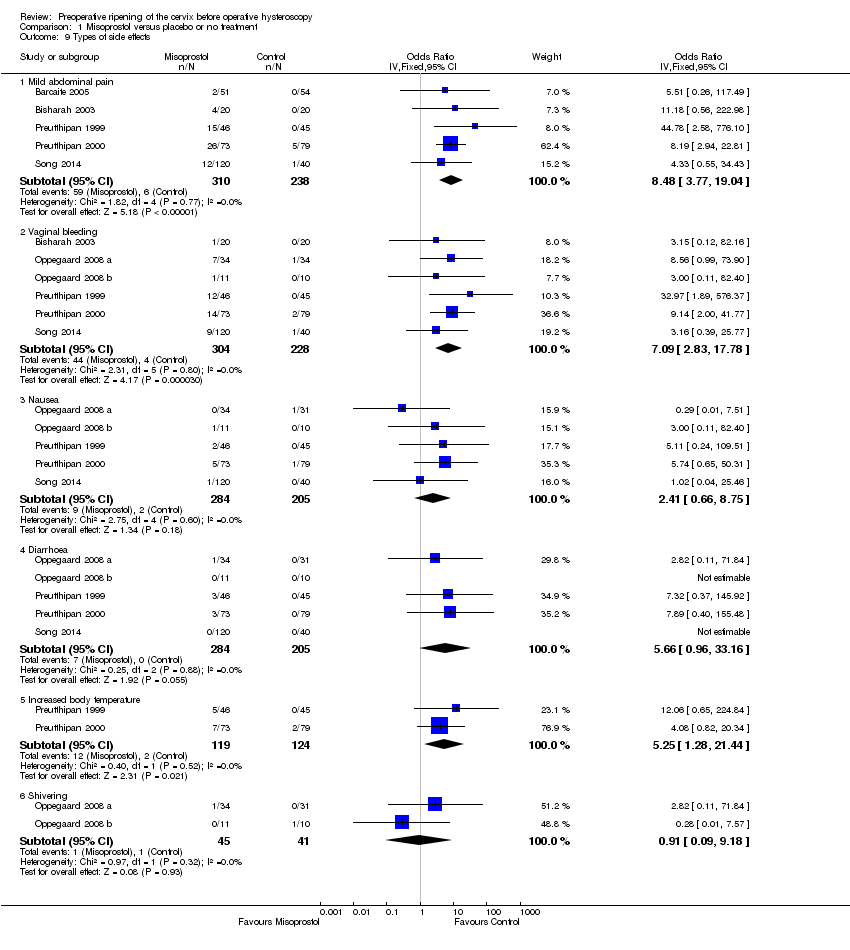 Comparison 1 Misoprostol versus placebo or no treatment, Outcome 9 Types of side effects. | ||||
| 9.1 Mild abdominal pain | 5 | 548 | Odds Ratio (IV, Fixed, 95% CI) | 8.48 [3.77, 19.04] |
| 9.2 Vaginal bleeding | 6 | 532 | Odds Ratio (IV, Fixed, 95% CI) | 7.09 [2.83, 17.78] |
| 9.3 Nausea | 5 | 489 | Odds Ratio (IV, Fixed, 95% CI) | 2.41 [0.66, 8.75] |
| 9.4 Diarrhoea | 5 | 489 | Odds Ratio (IV, Fixed, 95% CI) | 5.66 [0.96, 33.16] |
| 9.5 Increased body temperature | 2 | 243 | Odds Ratio (IV, Fixed, 95% CI) | 5.25 [1.28, 21.44] |
| 9.6 Shivering | 2 | 86 | Odds Ratio (IV, Fixed, 95% CI) | 0.91 [0.09, 9.18] |
| 10 Duration of operation( min..) Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.10  Comparison 1 Misoprostol versus placebo or no treatment, Outcome 10 Duration of operation( min..). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ease of dilatation: need for mechanical dilatation Show forest plot | 1 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.34, 0.98] |
| Analysis 2.1 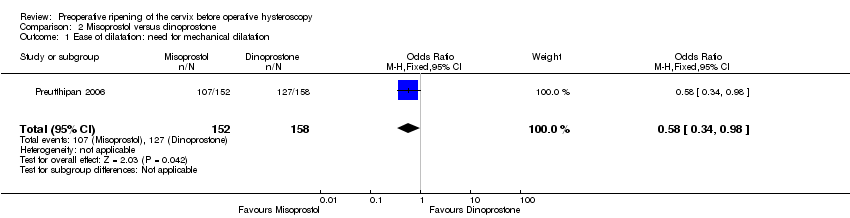 Comparison 2 Misoprostol versus dinoprostone, Outcome 1 Ease of dilatation: need for mechanical dilatation. | ||||
| 2 Intraoperative complications Show forest plot | 1 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.12, 0.83] |
| Analysis 2.2 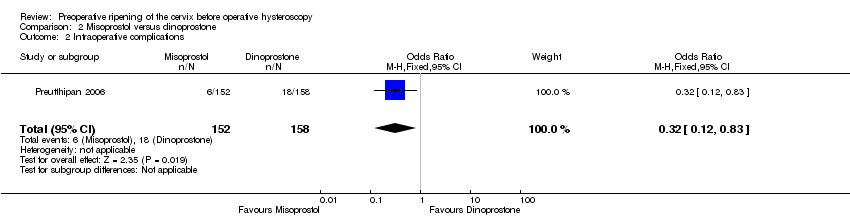 Comparison 2 Misoprostol versus dinoprostone, Outcome 2 Intraoperative complications. | ||||
| 3 Types of side effects Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3 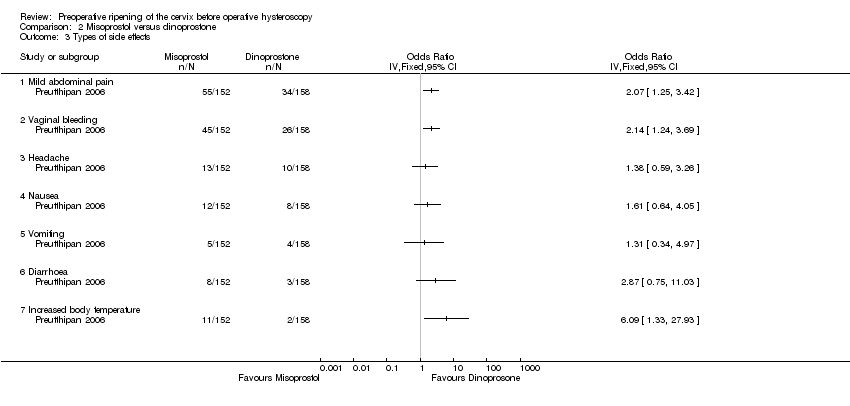 Comparison 2 Misoprostol versus dinoprostone, Outcome 3 Types of side effects. | ||||
| 3.1 Mild abdominal pain | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Vaginal bleeding | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Headache | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Nausea | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Vomiting | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Diarrhoea | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Increased body temperature | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Time required to dilate the cervix (sec.) Show forest plot | 1 | 310 | Mean Difference (IV, Fixed, 95% CI) | ‐4.60 [‐8.61, ‐0.59] |
| Analysis 2.4  Comparison 2 Misoprostol versus dinoprostone, Outcome 4 Time required to dilate the cervix (sec.). | ||||
| 5 Cervical width (dilator size in mm) Show forest plot | 1 | 310 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [0.21, 0.59] |
| Analysis 2.5  Comparison 2 Misoprostol versus dinoprostone, Outcome 5 Cervical width (dilator size in mm). | ||||
| 6 Duration of operative hysteroscopy (min.) Show forest plot | 1 | 310 | Mean Difference (IV, Fixed, 95% CI) | 3.20 [1.16, 5.24] |
| Analysis 2.6 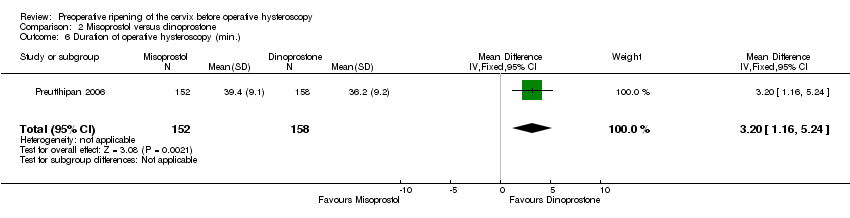 Comparison 2 Misoprostol versus dinoprostone, Outcome 6 Duration of operative hysteroscopy (min.). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ease of dilatation: need for mechanical dilatation Show forest plot | 1 | 110 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.96 [2.61, 13.59] |
| Analysis 3.1  Comparison 3 Misoprostol versus osmotic dilator, Outcome 1 Ease of dilatation: need for mechanical dilatation. | ||||
| 1.1 Misoprostol vs natural osmotic dilator | 1 | 110 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.96 [2.61, 13.59] |
| 2 Intraoperative complications Show forest plot | 3 | 354 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.14 [0.24, 109.01] |
| Analysis 3.2  Comparison 3 Misoprostol versus osmotic dilator, Outcome 2 Intraoperative complications. | ||||
| 2.1 Misoprostol vs natural osmotic dilator | 2 | 254 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.14 [0.24, 109.01] |
| 2.2 Misoprostol vs synthetic osmotic dilator | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Time required to dilate the cervix (sec.) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.3  Comparison 3 Misoprostol versus osmotic dilator, Outcome 3 Time required to dilate the cervix (sec.). | ||||
| 3.1 Misoprostol versus natural osmotic dilator | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Cervical width (dilator size in mm) Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.4 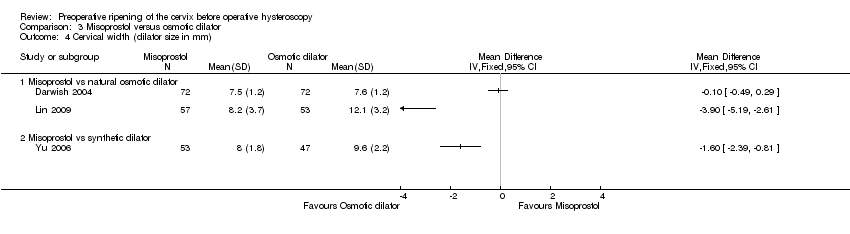 Comparison 3 Misoprostol versus osmotic dilator, Outcome 4 Cervical width (dilator size in mm). | ||||
| 4.1 Misoprostol vs natural osmotic dilator | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Misoprostol vs synthetic dilator | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

Study flow diagram.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
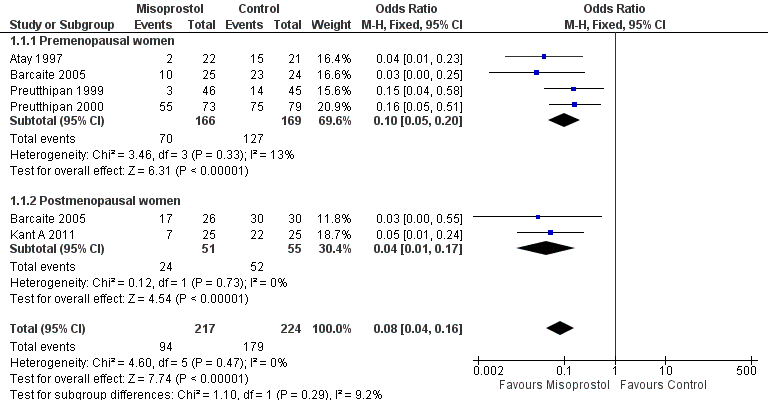
Forest plot of comparison: 1 Misoprostol versus placebo or no treatment, outcome: 1.1 Ease of dilatation: need for mechanical dilatation.
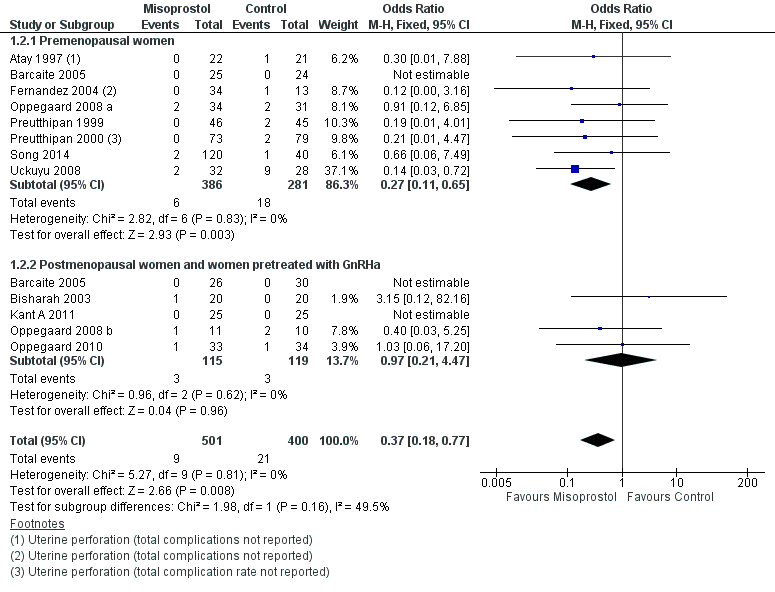
Forest plot of comparison: 1 Misoprostol versus placebo or no treatment, outcome: 1.2 Intraoperative complications.

Funnel plot of comparison: 1 Misoprostol versus placebo or no treatment, outcome: 1.2 Intraoperative complications.
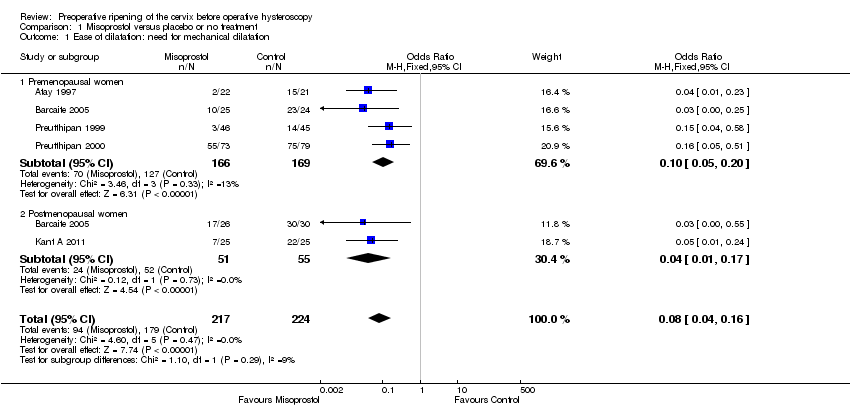
Comparison 1 Misoprostol versus placebo or no treatment, Outcome 1 Ease of dilatation: need for mechanical dilatation.
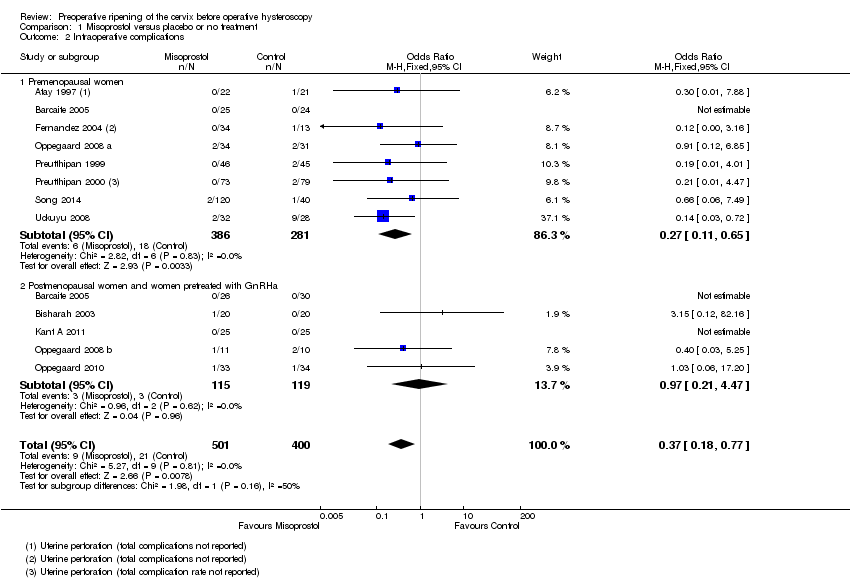
Comparison 1 Misoprostol versus placebo or no treatment, Outcome 2 Intraoperative complications.

Comparison 1 Misoprostol versus placebo or no treatment, Outcome 3 Specific intraoperative complications.

Comparison 1 Misoprostol versus placebo or no treatment, Outcome 4 Time required to dilate the cervix (sec.).

Comparison 1 Misoprostol versus placebo or no treatment, Outcome 5 Preoperative pain score.

Comparison 1 Misoprostol versus placebo or no treatment, Outcome 6 Cervical width (dilator size in mm).
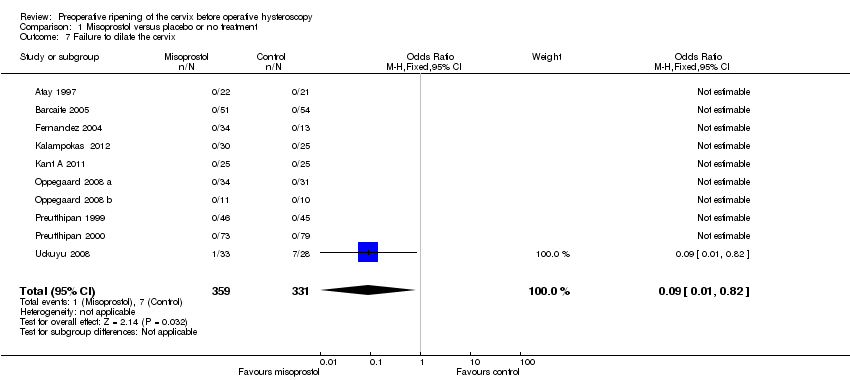
Comparison 1 Misoprostol versus placebo or no treatment, Outcome 7 Failure to dilate the cervix.

Comparison 1 Misoprostol versus placebo or no treatment, Outcome 8 Side effects.

Comparison 1 Misoprostol versus placebo or no treatment, Outcome 9 Types of side effects.

Comparison 1 Misoprostol versus placebo or no treatment, Outcome 10 Duration of operation( min..).

Comparison 2 Misoprostol versus dinoprostone, Outcome 1 Ease of dilatation: need for mechanical dilatation.
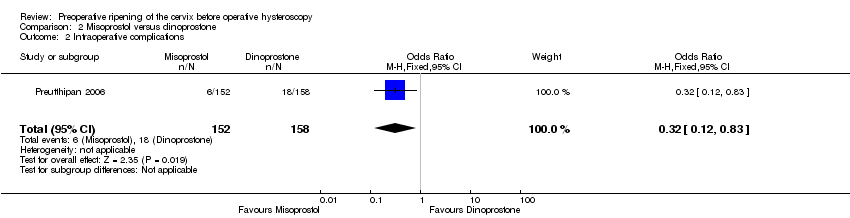
Comparison 2 Misoprostol versus dinoprostone, Outcome 2 Intraoperative complications.

Comparison 2 Misoprostol versus dinoprostone, Outcome 3 Types of side effects.
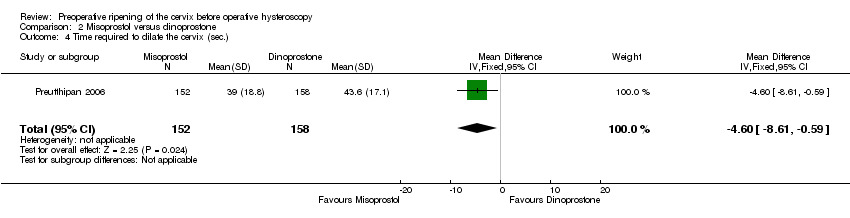
Comparison 2 Misoprostol versus dinoprostone, Outcome 4 Time required to dilate the cervix (sec.).

Comparison 2 Misoprostol versus dinoprostone, Outcome 5 Cervical width (dilator size in mm).
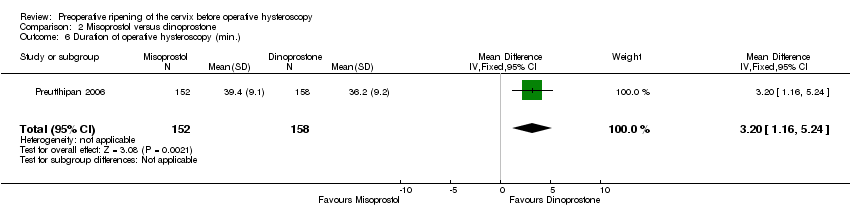
Comparison 2 Misoprostol versus dinoprostone, Outcome 6 Duration of operative hysteroscopy (min.).

Comparison 3 Misoprostol versus osmotic dilator, Outcome 1 Ease of dilatation: need for mechanical dilatation.

Comparison 3 Misoprostol versus osmotic dilator, Outcome 2 Intraoperative complications.
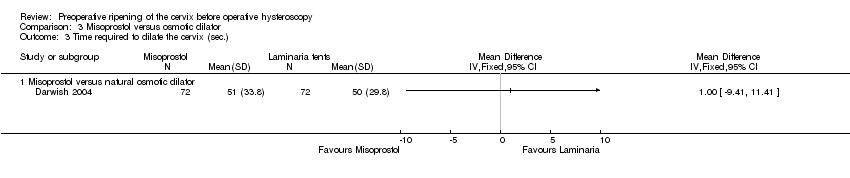
Comparison 3 Misoprostol versus osmotic dilator, Outcome 3 Time required to dilate the cervix (sec.).

Comparison 3 Misoprostol versus osmotic dilator, Outcome 4 Cervical width (dilator size in mm).
| Misoprostol compared to placebo or no treatment for women undergoing hysteroscopy | ||||||
| Population: Pre and post menopausal women | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no treatment | Misoprostol | |||||
| Ease of dilatation: need for mechanical dilatation | 797 per 1000 | 239 per 1000 | OR 0.08 | 441 | ⊕⊕⊕⊝ | |
| Intraoperative complications | 29 per 1000 | 11 per 1000 | OR 0.37 | 901 | ⊕⊕⊕⊝ | |
| Specific intraoperative complications ‐ Cervical laceration/tear | 25 per 1000 | 6 per 1000 | OR 0.25 | 669 | ⊕⊕⊕⊝ | |
| Specific intraoperative complications ‐ False track | 40 per 1000 | 14 per 1000 | OR 0.34 | 560 | ⊕⊕⊕⊝ | |
| Specific intraoperative complications ‐ Uterine perforation | 29 per 1000 | 13 per 1000 | OR 0.42 | 455 | ⊕⊕⊝⊝ | |
| Specific intraoperative complications ‐ Uterine bleeding | 60 per 1000 | 32 per 1000 | OR 0.51 | 340 | ⊕⊕⊝⊝ | |
| Side effects | 18 per 1000 | 112 per 1000 | OR 2.59 | 272 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Inadequate reporting of methodology by all or most of the studies 2Imprecision: low event rates and wide confidence intervals compatible with no effect or with meaningful benefit from misoprostol | ||||||
| Misoprostol compared to dinoprostone for health problem or population | ||||||
| Population: Pre and post menopausal women | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Dinoprostone | Misoprostol | |||||
| Ease of dilatation: need for mechanical dilatation | 804 per 1000 | 704 per 1000 | OR 0.58 (0.34 to 0.98) | 310 | ⊕⊕⊝⊝ | |
| Intraoperative complications | 114 per 1000 | 40 per 1000 | OR 0.32 | 310 | ⊕⊕⊝⊝ | |
| Side effects | Total side effects not reported. Some specific side effects (abdominal pain, vaginal bleeding, diarrhoea and perception of raised temperature) were more common in the misoprostol group. All side effects were mild. | ⊕⊕⊝⊝ | ||||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Inadequate description of study methods, high risk of attrition bias 2Imprecision: wide confidence intervals compatible with meaningful benefit from misoprostol or with little or no meaningful benefit 3Imprecision: wide confidence intervals compatible with harm from misoprostol or no clinically meaningful effect | ||||||
| Misoprostol compared to osmotic dilator for health problem or population | ||||||
| Population: Pre and post menopausal women | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Osmotic dilator | Misoprostol | |||||
| Ease of dilatation: need for mechanical dilatation | 283 per 1000 | 702 per 1000 | OR 5.96 (2.61 to 13.59) | 110 | ⊕⊕⊝⊝ | |
| Intraoperative complications | 0 per 1000 | 0 per 1000 | OR 5.14 | 354 | ⊕⊕⊝⊝ | Only two events altogether. No events in two RCTs |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of attrition bias unclear; unclear whether outcome assessment was blinded 2Imprecision: single small study with total of 55 events 3One of studies does not adequately describe methods, unclear whether outcome assessment blinded 4Imprecision: only 2 events | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ease of dilatation: need for mechanical dilatation Show forest plot | 5 | 441 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.04, 0.16] |
| 1.1 Premenopausal women | 4 | 335 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.05, 0.20] |
| 1.2 Postmenopausal women | 2 | 106 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.01, 0.17] |
| 2 Intraoperative complications Show forest plot | 12 | 901 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.18, 0.77] |
| 2.1 Premenopausal women | 8 | 667 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.11, 0.65] |
| 2.2 Postmenopausal women and women pretreated with GnRHa | 5 | 234 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.21, 4.47] |
| 3 Specific intraoperative complications Show forest plot | 11 | Odds Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Cervical laceration/tear | 9 | 669 | Odds Ratio (IV, Fixed, 95% CI) | 0.25 [0.11, 0.57] |
| 3.2 False track | 7 | 560 | Odds Ratio (IV, Fixed, 95% CI) | 0.34 [0.12, 0.97] |
| 3.3 Uterine perforation | 7 | 455 | Odds Ratio (IV, Fixed, 95% CI) | 0.42 [0.13, 1.38] |
| 3.4 Uterine bleeding | 4 | 340 | Odds Ratio (IV, Fixed, 95% CI) | 0.51 [0.10, 2.49] |
| 4 Time required to dilate the cervix (sec.) Show forest plot | 7 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Preoperative pain score Show forest plot | 4 | 200 | Mean Difference (IV, Fixed, 95% CI) | 1.25 [0.77, 1.72] |
| 6 Cervical width (dilator size in mm) Show forest plot | 12 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Failure to dilate the cervix Show forest plot | 10 | 690 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.82] |
| 8 Side effects Show forest plot | 4 | 272 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.59 [1.15, 5.79] |
| 9 Types of side effects Show forest plot | 7 | Odds Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Mild abdominal pain | 5 | 548 | Odds Ratio (IV, Fixed, 95% CI) | 8.48 [3.77, 19.04] |
| 9.2 Vaginal bleeding | 6 | 532 | Odds Ratio (IV, Fixed, 95% CI) | 7.09 [2.83, 17.78] |
| 9.3 Nausea | 5 | 489 | Odds Ratio (IV, Fixed, 95% CI) | 2.41 [0.66, 8.75] |
| 9.4 Diarrhoea | 5 | 489 | Odds Ratio (IV, Fixed, 95% CI) | 5.66 [0.96, 33.16] |
| 9.5 Increased body temperature | 2 | 243 | Odds Ratio (IV, Fixed, 95% CI) | 5.25 [1.28, 21.44] |
| 9.6 Shivering | 2 | 86 | Odds Ratio (IV, Fixed, 95% CI) | 0.91 [0.09, 9.18] |
| 10 Duration of operation( min..) Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ease of dilatation: need for mechanical dilatation Show forest plot | 1 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.34, 0.98] |
| 2 Intraoperative complications Show forest plot | 1 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.12, 0.83] |
| 3 Types of side effects Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Mild abdominal pain | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Vaginal bleeding | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Headache | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Nausea | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Vomiting | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Diarrhoea | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Increased body temperature | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Time required to dilate the cervix (sec.) Show forest plot | 1 | 310 | Mean Difference (IV, Fixed, 95% CI) | ‐4.60 [‐8.61, ‐0.59] |
| 5 Cervical width (dilator size in mm) Show forest plot | 1 | 310 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [0.21, 0.59] |
| 6 Duration of operative hysteroscopy (min.) Show forest plot | 1 | 310 | Mean Difference (IV, Fixed, 95% CI) | 3.20 [1.16, 5.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ease of dilatation: need for mechanical dilatation Show forest plot | 1 | 110 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.96 [2.61, 13.59] |
| 1.1 Misoprostol vs natural osmotic dilator | 1 | 110 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.96 [2.61, 13.59] |
| 2 Intraoperative complications Show forest plot | 3 | 354 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.14 [0.24, 109.01] |
| 2.1 Misoprostol vs natural osmotic dilator | 2 | 254 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.14 [0.24, 109.01] |
| 2.2 Misoprostol vs synthetic osmotic dilator | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Time required to dilate the cervix (sec.) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Misoprostol versus natural osmotic dilator | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Cervical width (dilator size in mm) Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Misoprostol vs natural osmotic dilator | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Misoprostol vs synthetic dilator | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

