Maduración preoperatoria del cuello uterino antes de la histeroscopia quirúrgica
Información
- DOI:
- https://doi.org/10.1002/14651858.CD005998.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 23 abril 2015see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Ginecología y fertilidad
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Haya Al‐Fozan: conceptualised and wrote the initial version of the protocol, updated it in the light of the co‐reviewers' changes and comments, and wrote the final version. Updated the protocol, assessment of trials and quality analysis, data collection, analysis, and wrote the initial draft of the review. Checked literature search, wrote the final version of the review.
Belal Firwana: run in analysis and helped in writing the initial draft of the review.
Samar Hassan: updated and commented on the protocol and review.
Hanan Kadri: updated and commented on the protocol and review.
Togas Tulandi: commented on the protocol, evaluated the review and revised the language.
Sources of support
Internal sources
-
IVF and Reproductive Endocrinology Unit , KAMC,Riyadh AND National and Gulf center for Evidence Based Health Practice(NGCEBHP), Saudi Arabia.
Free time with free workshops/courses in relation to systematic review and meta analysis
External sources
-
None, Other.
Declarations of interest
None
Acknowledgements
We thank all the women and investigators who were involved in the clinical trials mentioned in this review. We thank the staff of the Cochrane Menstrual Disorders and Subfertility Group Editorial Team for their contribution and excellent collaboration. We appreciate the support of the National and Gulf Center for Evidence Health Care Practice (NGCEBHP). We acknowledge the advice of statisticians M. Hassan Murad, MD, MPH and Zhen Wang, PhD (Mayo Clinic, Rochester, MN, USA) for advice on combining the intervention groups in Fernandez 2004.
Version history
| Published | Title | Stage | Authors | Version |
| 2015 Apr 23 | Preoperative ripening of the cervix before operative hysteroscopy | Review | Haya Al‐Fozan, Belal Firwana, Hanan Al Kadri, Samar Hassan, Togas Tulandi | |
| 2006 Apr 19 | Preoperative ripening of the cervix before operative hysteroscopy | Protocol | Haya M Al‐Fozan, Hanan Al Kadri, Samar Hassan, Togas Tulandi | |
Differences between protocol and review
Since the protocol was published in 2006 there have been numerous methodological advances. The review now reflects the current methodologies employed by The Cochrane Collaboration.
-
in the primary outcomes the power required to perform procedure was removed.
-
In the full review, we reported the following outcomes in addition to those listed in the protocol.
-
Women requiring cervical dilatation (our primary outcome)
-
Duration of surgery
-
-
In the protocol our primary outcome was cervical width. After peer review, we decided to use need for mechanical dilatation as our primary effectiveness outcome, as this was deemed more clinically relevant.
-
We will consider expanding eligibility to other intervention agents in future updates.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- *Cervical Ripening;
- *Hysteroscopy [adverse effects];
- Cervix Uteri [*drug effects, injuries];
- Dilatation [*methods];
- Dinoprostone [administration & dosage];
- Laminaria;
- Misoprostol [administration & dosage];
- Oxytocics [administration & dosage];
- Preoperative Care [*methods];
- Randomized Controlled Trials as Topic;
Medical Subject Headings Check Words
Female; Humans; Pregnancy;
PICO

Study flow diagram.
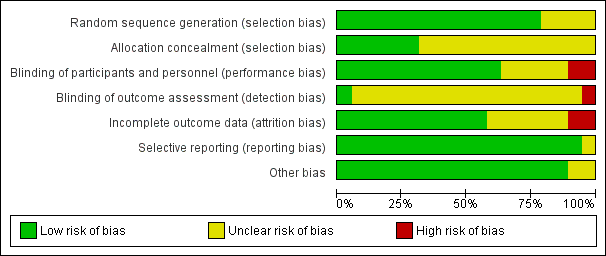
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
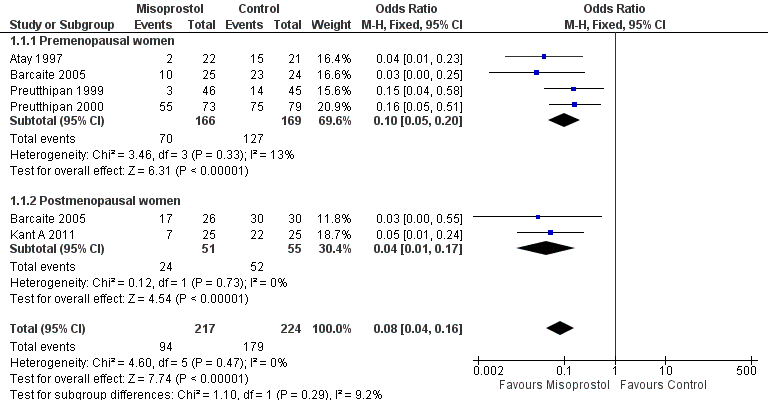
Forest plot of comparison: 1 Misoprostol versus placebo or no treatment, outcome: 1.1 Ease of dilatation: need for mechanical dilatation.
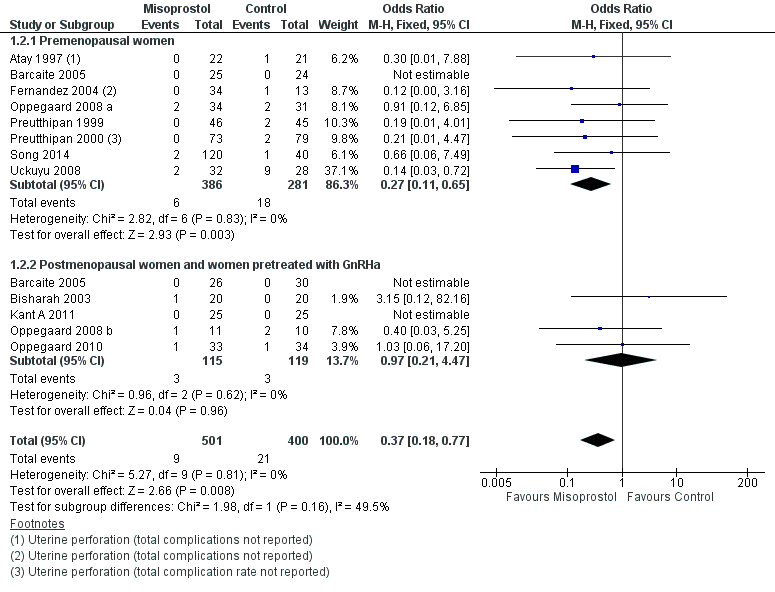
Forest plot of comparison: 1 Misoprostol versus placebo or no treatment, outcome: 1.2 Intraoperative complications.

Funnel plot of comparison: 1 Misoprostol versus placebo or no treatment, outcome: 1.2 Intraoperative complications.
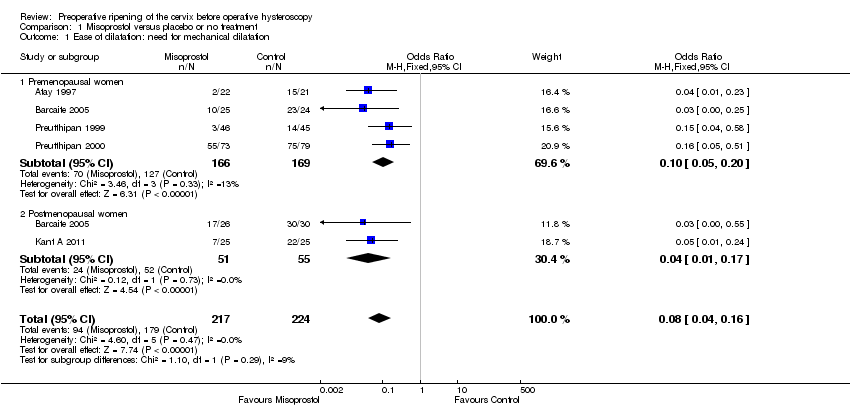
Comparison 1 Misoprostol versus placebo or no treatment, Outcome 1 Ease of dilatation: need for mechanical dilatation.
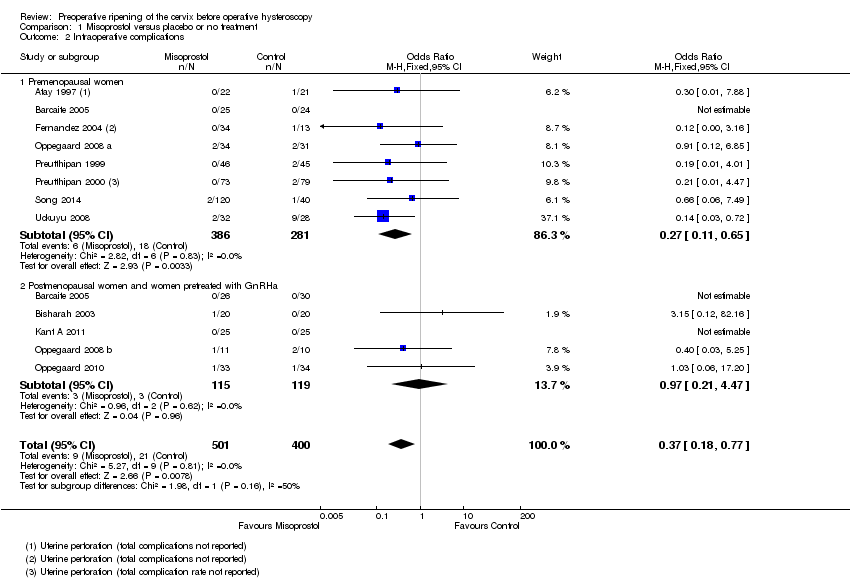
Comparison 1 Misoprostol versus placebo or no treatment, Outcome 2 Intraoperative complications.

Comparison 1 Misoprostol versus placebo or no treatment, Outcome 3 Specific intraoperative complications.

Comparison 1 Misoprostol versus placebo or no treatment, Outcome 4 Time required to dilate the cervix (sec.).

Comparison 1 Misoprostol versus placebo or no treatment, Outcome 5 Preoperative pain score.

Comparison 1 Misoprostol versus placebo or no treatment, Outcome 6 Cervical width (dilator size in mm).
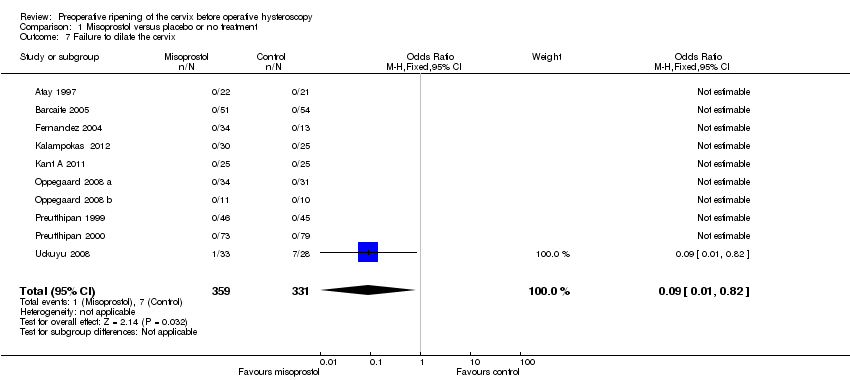
Comparison 1 Misoprostol versus placebo or no treatment, Outcome 7 Failure to dilate the cervix.

Comparison 1 Misoprostol versus placebo or no treatment, Outcome 8 Side effects.

Comparison 1 Misoprostol versus placebo or no treatment, Outcome 9 Types of side effects.

Comparison 1 Misoprostol versus placebo or no treatment, Outcome 10 Duration of operation( min..).

Comparison 2 Misoprostol versus dinoprostone, Outcome 1 Ease of dilatation: need for mechanical dilatation.
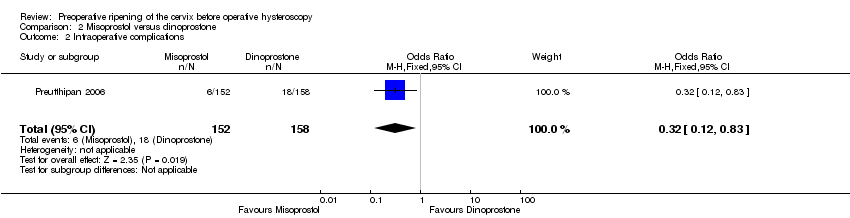
Comparison 2 Misoprostol versus dinoprostone, Outcome 2 Intraoperative complications.

Comparison 2 Misoprostol versus dinoprostone, Outcome 3 Types of side effects.
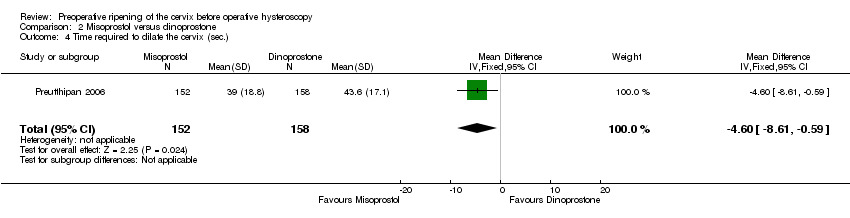
Comparison 2 Misoprostol versus dinoprostone, Outcome 4 Time required to dilate the cervix (sec.).

Comparison 2 Misoprostol versus dinoprostone, Outcome 5 Cervical width (dilator size in mm).
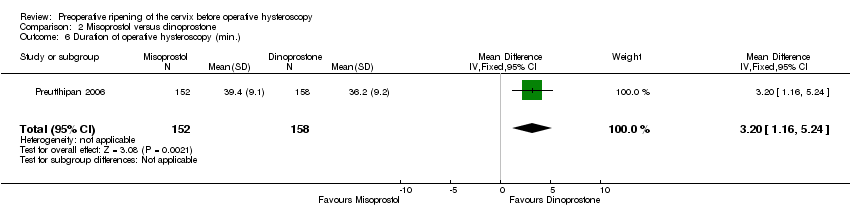
Comparison 2 Misoprostol versus dinoprostone, Outcome 6 Duration of operative hysteroscopy (min.).

Comparison 3 Misoprostol versus osmotic dilator, Outcome 1 Ease of dilatation: need for mechanical dilatation.

Comparison 3 Misoprostol versus osmotic dilator, Outcome 2 Intraoperative complications.
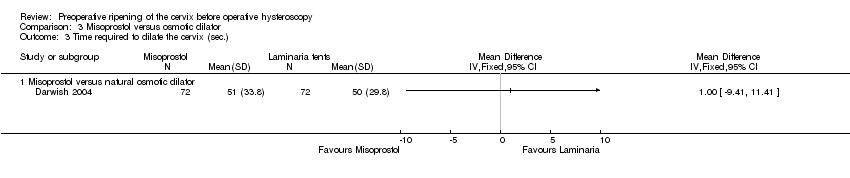
Comparison 3 Misoprostol versus osmotic dilator, Outcome 3 Time required to dilate the cervix (sec.).

Comparison 3 Misoprostol versus osmotic dilator, Outcome 4 Cervical width (dilator size in mm).
| Misoprostol compared to placebo or no treatment for women undergoing hysteroscopy | ||||||
| Population: Pre and post menopausal women | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no treatment | Misoprostol | |||||
| Ease of dilatation: need for mechanical dilatation | 797 per 1000 | 239 per 1000 | OR 0.08 | 441 | ⊕⊕⊕⊝ | |
| Intraoperative complications | 29 per 1000 | 11 per 1000 | OR 0.37 | 901 | ⊕⊕⊕⊝ | |
| Specific intraoperative complications ‐ Cervical laceration/tear | 25 per 1000 | 6 per 1000 | OR 0.25 | 669 | ⊕⊕⊕⊝ | |
| Specific intraoperative complications ‐ False track | 40 per 1000 | 14 per 1000 | OR 0.34 | 560 | ⊕⊕⊕⊝ | |
| Specific intraoperative complications ‐ Uterine perforation | 29 per 1000 | 13 per 1000 | OR 0.42 | 455 | ⊕⊕⊝⊝ | |
| Specific intraoperative complications ‐ Uterine bleeding | 60 per 1000 | 32 per 1000 | OR 0.51 | 340 | ⊕⊕⊝⊝ | |
| Side effects | 18 per 1000 | 112 per 1000 | OR 2.59 | 272 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Inadequate reporting of methodology by all or most of the studies 2Imprecision: low event rates and wide confidence intervals compatible with no effect or with meaningful benefit from misoprostol | ||||||
| Misoprostol compared to dinoprostone for health problem or population | ||||||
| Population: Pre and post menopausal women | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Dinoprostone | Misoprostol | |||||
| Ease of dilatation: need for mechanical dilatation | 804 per 1000 | 704 per 1000 | OR 0.58 (0.34 to 0.98) | 310 | ⊕⊕⊝⊝ | |
| Intraoperative complications | 114 per 1000 | 40 per 1000 | OR 0.32 | 310 | ⊕⊕⊝⊝ | |
| Side effects | Total side effects not reported. Some specific side effects (abdominal pain, vaginal bleeding, diarrhoea and perception of raised temperature) were more common in the misoprostol group. All side effects were mild. | ⊕⊕⊝⊝ | ||||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Inadequate description of study methods, high risk of attrition bias 2Imprecision: wide confidence intervals compatible with meaningful benefit from misoprostol or with little or no meaningful benefit 3Imprecision: wide confidence intervals compatible with harm from misoprostol or no clinically meaningful effect | ||||||
| Misoprostol compared to osmotic dilator for health problem or population | ||||||
| Population: Pre and post menopausal women | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Osmotic dilator | Misoprostol | |||||
| Ease of dilatation: need for mechanical dilatation | 283 per 1000 | 702 per 1000 | OR 5.96 (2.61 to 13.59) | 110 | ⊕⊕⊝⊝ | |
| Intraoperative complications | 0 per 1000 | 0 per 1000 | OR 5.14 | 354 | ⊕⊕⊝⊝ | Only two events altogether. No events in two RCTs |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of attrition bias unclear; unclear whether outcome assessment was blinded 2Imprecision: single small study with total of 55 events 3One of studies does not adequately describe methods, unclear whether outcome assessment blinded 4Imprecision: only 2 events | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ease of dilatation: need for mechanical dilatation Show forest plot | 5 | 441 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.04, 0.16] |
| 1.1 Premenopausal women | 4 | 335 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.05, 0.20] |
| 1.2 Postmenopausal women | 2 | 106 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.01, 0.17] |
| 2 Intraoperative complications Show forest plot | 12 | 901 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.18, 0.77] |
| 2.1 Premenopausal women | 8 | 667 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.11, 0.65] |
| 2.2 Postmenopausal women and women pretreated with GnRHa | 5 | 234 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.21, 4.47] |
| 3 Specific intraoperative complications Show forest plot | 11 | Odds Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Cervical laceration/tear | 9 | 669 | Odds Ratio (IV, Fixed, 95% CI) | 0.25 [0.11, 0.57] |
| 3.2 False track | 7 | 560 | Odds Ratio (IV, Fixed, 95% CI) | 0.34 [0.12, 0.97] |
| 3.3 Uterine perforation | 7 | 455 | Odds Ratio (IV, Fixed, 95% CI) | 0.42 [0.13, 1.38] |
| 3.4 Uterine bleeding | 4 | 340 | Odds Ratio (IV, Fixed, 95% CI) | 0.51 [0.10, 2.49] |
| 4 Time required to dilate the cervix (sec.) Show forest plot | 7 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Preoperative pain score Show forest plot | 4 | 200 | Mean Difference (IV, Fixed, 95% CI) | 1.25 [0.77, 1.72] |
| 6 Cervical width (dilator size in mm) Show forest plot | 12 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Failure to dilate the cervix Show forest plot | 10 | 690 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.82] |
| 8 Side effects Show forest plot | 4 | 272 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.59 [1.15, 5.79] |
| 9 Types of side effects Show forest plot | 7 | Odds Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Mild abdominal pain | 5 | 548 | Odds Ratio (IV, Fixed, 95% CI) | 8.48 [3.77, 19.04] |
| 9.2 Vaginal bleeding | 6 | 532 | Odds Ratio (IV, Fixed, 95% CI) | 7.09 [2.83, 17.78] |
| 9.3 Nausea | 5 | 489 | Odds Ratio (IV, Fixed, 95% CI) | 2.41 [0.66, 8.75] |
| 9.4 Diarrhoea | 5 | 489 | Odds Ratio (IV, Fixed, 95% CI) | 5.66 [0.96, 33.16] |
| 9.5 Increased body temperature | 2 | 243 | Odds Ratio (IV, Fixed, 95% CI) | 5.25 [1.28, 21.44] |
| 9.6 Shivering | 2 | 86 | Odds Ratio (IV, Fixed, 95% CI) | 0.91 [0.09, 9.18] |
| 10 Duration of operation( min..) Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ease of dilatation: need for mechanical dilatation Show forest plot | 1 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.34, 0.98] |
| 2 Intraoperative complications Show forest plot | 1 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.12, 0.83] |
| 3 Types of side effects Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Mild abdominal pain | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Vaginal bleeding | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Headache | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Nausea | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Vomiting | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Diarrhoea | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Increased body temperature | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Time required to dilate the cervix (sec.) Show forest plot | 1 | 310 | Mean Difference (IV, Fixed, 95% CI) | ‐4.60 [‐8.61, ‐0.59] |
| 5 Cervical width (dilator size in mm) Show forest plot | 1 | 310 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [0.21, 0.59] |
| 6 Duration of operative hysteroscopy (min.) Show forest plot | 1 | 310 | Mean Difference (IV, Fixed, 95% CI) | 3.20 [1.16, 5.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ease of dilatation: need for mechanical dilatation Show forest plot | 1 | 110 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.96 [2.61, 13.59] |
| 1.1 Misoprostol vs natural osmotic dilator | 1 | 110 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.96 [2.61, 13.59] |
| 2 Intraoperative complications Show forest plot | 3 | 354 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.14 [0.24, 109.01] |
| 2.1 Misoprostol vs natural osmotic dilator | 2 | 254 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.14 [0.24, 109.01] |
| 2.2 Misoprostol vs synthetic osmotic dilator | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Time required to dilate the cervix (sec.) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Misoprostol versus natural osmotic dilator | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Cervical width (dilator size in mm) Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Misoprostol vs natural osmotic dilator | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Misoprostol vs synthetic dilator | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

