Zinkergänzung zur Prävention von Lungenentzündungen bei Kindern im Alter von 2 bis 59 Monaten
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | RCT in which the children were individually randomised by a computer‐generated simple randomisation scheme in blocks of 8. Zinc or placebo bottles were labelled with a unique child identification number according to the randomisation scheme. Six bottles, one for each month and two extra, for each child were produced and labelled before enrolment commenced. The supplies for each child were kept separately in labelled plastic bags. The zinc and placebo syrups were similar in appearance, taste, and packaging. Blinding was maintained during analyses by coding the groups as A or B. The study took place in the urban slum of Dakshinpuri in New Delhi, India. For episodes to be counted as individual, there had to be at least 14 intervening days. The children in the two groups were comparable for age, anthropometry, child feeding practices, morbidity in the previous 24 hours, socioeconomic characteristics and plasma zinc concentration. | |
| Participants | The study included children aged from 6 to 30 months. There were 1241 children in each group, and after dropouts, the number reduced to 1093 in the zinc and 1133 in the placebo groups. Children were excluded if consent was refused, were likely to move out of the study area within the next four months, needed urgent admission to hospital on the enrolment day or had received a massive dose of vitamin A (100,000 IU for infants and 200,000 IU for older children) within the two months before enrolment. | |
| Interventions | Doses of elemental zinc were 10 mg for infants and 20 mg for older children (twice the recommended daily dosage) as zinc gluconate. Zinc or placebo was taken daily for four months. Both groups received single massive doses of vitamin A (100,000 IU for infants and 200,000 IU for older children) at enrolment. Immunisations and treatment for acute illnesses were provided as per WHO guidelines. Children with acute lower respiratory tract infections received cotrimoxazole. Amoxicillin was substituted if the child did not respond within three days. Children were sent to hospital if they had signs and symptoms that warranted referral according to WHO guidelines. | |
| Outcomes | Incidence of ALRI ALRIs were defined by cough and fast breathing or lower chest indrawing as assessed by the physician; other clinical signs were not taken into account. Fast breathing was defined as 2 counts of > 50 breaths/minute for infants and > 40 breaths/minute for older children. Pneumonia was diagnosed either by a combination of cough with crepitations or bronchial breathing by auscultation or as an episode of ALRI associated with at least one of lower chest indrawing, convulsions, not able to drink or feed, extreme lethargy, restlessness or irritability, nasal flaring, or abnormal sleepiness. | |
| Notes | Funding: European Union (Contract No IC18CT960045), Norwegian Council of Universities' Committee for Development Research and Education (PRO 53/96), Department of Child and Adolescent Health and Development (CAH), WHO. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "children were individually randomised by a simple randomisation scheme in blocks of eight. The randomisation scheme was generated by a statistician at Statens Serum Institut, not otherwise involved with this study, using the SAS software" |
| Allocation concealment (selection bias) | Low risk | Quote: "zinc or placebo syrups were prepared and packaged in unbreakable bottles by GK Pharma ApS Koge, Denmark, who also labelled bottles with a unique child identification number according to the randomisation scheme. The supplies of each child were kept separately in labelled plastic bags. The zinc and placebo were similar in appearances, taste and packaging. Masking was maintained during the analysis by coding the groups as A and B" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "the supplies for each child were kept separately in labelled plastic bags". "Masking was maintained during analyses by coding the groups as A or B" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgment |
| Incomplete outcome data (attrition bias) | Low risk | Exclusion (35%) with their reasons documented. Attrition was 12% in the zinc group and 8.7% in the control group. Loss to follow‐ups were mainly because they refused further participation, moved and died (3 died in the placebo group only) |
| Selective reporting (reporting bias) | Low risk | We could not locate the protocol of this study. We could not find the trial registration number of the study. The outcomes mentioned in the methods were reported in the results |
| Other bias | Unclear risk | Sources of funding: Not mentioned if they had any role in design or results of study |
| Methods | Randomised, double‐blind, placebo‐controlled trial conducted in Grey's Hospital in Pietermaritburg, South Africa. Baseline measurements of plasma HIV‐1 viral load and the percentage of CD4T lymphocytes were established at two study visits before randomisation, and measurements were repeated 3, 6, and 9 months after the start of supplementation. | |
| Participants | 96 children with HIV‐1 infection between the ages of 6 months and 60 months, being cared for as outpatients at Grey’s Hospital, and not receiving anti‐retroviral therapy were recruited. Pneumonia was diagnosed by history and physical examination, including chest auscultation, and confirmed by chest radiograph. | |
| Interventions | Children either received 10 mg of elemental zinc as sulphate or placebo every day for 6 months. The child’s parent or guardian was given one packet at the first two visits and two packets at each monthly follow‐up visit thereafter, and was instructed on how to give the tablet. | |
| Outcomes | The primary outcome measure was plasma HIV‐1 viral load and incidence of pneumonia. | |
| Notes | Outpatient management of children with HIV‐1 infection is provided by a team of paediatricians, medical officers, and nurses who care for about 20 to 30 children per week. After starting zinc or placebo, children were assessed at Grey’s Hospital every 2 weeks for the first month, monthly for 5 months, and a final visit 9 months after zinc or placebo supplementation started. Pneumonia was diagnosed by history and physical examination, including chest auscultation, and confirmed by chest radiograph. Funding: This study was funded by the Johns Hopkins Family Health and Child Survival Cooperative Agreement with the Office of Health, Infectious Diseases, and Nutrition, Global Health Bureau, US Agency for International Development. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Children were block–randomised in three age strata (6 to 23, 24 to 41, and 42 to 60 months)"; "Randomisation lists were computer generated at the WHO in a fixed block size of eight" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Tablets of zinc sulphate or placebo were produced by the same manufacturer (Nutriset, Bierne, France) and supplied in blister packets of 14 dispersible tablets"; "An investigator at Grey’s Hospital assigned children to the treatment groups. The investigators were unaware of the treatment allocation until follow up was completed" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "An investigator at Grey’s Hospital assigned children to the treatment groups. The investigators were unaware of the treatment allocation until follow up was completed." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | Low risk | 9/105 participants (8.6%) were excluded. 2/46 (4.4%) and 9/50 (18%) participants did not complete the trial in zinc and placebo groups, respectively. The reasons for lost to follow‐up were mainly deaths and refused to participate |
| Selective reporting (reporting bias) | Low risk | We could not locate the protocol of this study. We could not find the trial registration number of the study. The outcomes mentioned in the methods were reported in the results |
| Other bias | Low risk | Sources of funding: funding agencies had no role in views and opinions mentioned in the study |
| Methods | RCT in which random assignment to zinc or placebo was done with permuted blocks of variable length between 2 and 8. Placebo was designed to be identical to the zinc syrup in colour, odour, and taste. The study was conducted at Kamalapur, southeastern Dhaka, Bangladesh. The medical officer diagnosed pneumonia if crepitations were heard on inspiration with a respiratory rate greater than 50 breaths per minute; severe pneumonia was diagnosed if there was also chest indrawing, or at least one other danger sign. | |
| Participants | Children aged 60 days to 12 months at the time of enrolment and excluded those with known or suspected tuberculosis, chronic respiratory or congenital heart disease, or severe malnutrition requiring hospital admission. Pneumonia was diagnosed if crepitations were heard on inspiration with a respiratory rate greater than 50 breaths per minute; severe pneumonia was diagnosed if there was also chest indrawing, or at least one other danger sign. Children with wheezing or rhonchi with crepitations were also diagnosed with pneumonia. 809 children were randomly assigned to zinc and 812 to placebo. There were no significant differences between groups at baseline, except for a slightly higher proportion of boys in the zinc group. There was no difference between the groups in serum zinc values at baseline. | |
| Interventions | Zinc was given orally as a syrup (35 mg zinc acetate per 5 mL). The placebo was non‐nutritious and vitamin‐free. Compliance required intake of two teaspoons of syrup (10 mL). Children with pneumonia were treated with co‐trimoxazole (10 mg/kg trimethoprim, twice daily for 5 days) for pneumonia. Children on antibiotics were assessed within 72 hours of starting treatment; those who did not improve (i.e. the respiratory rate did not change by more than 5 breaths/minute from baseline) were switched to treatment with amoxicillin (40 mg/kg, three times daily for 5 days). If oral treatment failed, or if they had severe pneumonia, children were referred to hospital for parenteral treatment (ceftriaxone 75 mg/kg intramuscularly per day). Children with only expiratory wheezes or rhonchi were managed with salbutamol syrup (0.3 mg/kg, 3 times daily), or referred to hospital for danger signs. | |
| Outcomes | Pneumonia incidence. Other outcomes included frequency of other illnesses and mortality. | |
| Notes | Sources of funding: The research was funded by Johns Hopkins Family Health and Child Survival Cooperative Agreement with the US Agency for International Development, the Swiss Development Corporation, and a cooperative agreement between the US Agency for International Development (HRN‐A‐00‐96‐90005‐00) and core donors to the Centre for Health and Population Research. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random assignment to zinc or placebo was done with permuted blocks of variable length between two and eight" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "ACME Laboratories (Dhaka) prepared, labelled and masked the identity of both preparations. Both placebo and treatment were designed to be identical in colour, odour, and taste"; "identity of both the preparations were masked" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Blinding of FRAs was not affected because a proportion of children in both zinc and placebo groups reacted to the taste such that the treatment could not be distinguished" |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up was 9.1%. Withdrawal from both groups was most commonly attributed to the child’s reaction to the taste of the syrup, which sometimes resulted in regurgitation. Most of those who withdrew were young, primarily breast fed infants. The highest proportion (37·1%) of withdrawals for both groups occurred at age 2 months, with 77·1% younger than 6 months |
| Selective reporting (reporting bias) | Low risk | We could not locate the protocol of this study. We could not find the trial registration number of the study. The outcomes mentioned in the methods were reported in the results |
| Other bias | Low risk | Study seems to be free from other biases; the funding sources had no role in the study design, data collection, data analysis, interpretation of results, or decision to publish this research Funding: The research was funded by Johns Hopkins Family Health and Child Survival Cooperative Agreement with the US Agency for International Development, the Swiss Development Corporation, and a cooperative agreement between the US Agency for International Development (HRN‐A‐00‐96‐90005‐00) and core donors to the Centre for Health and Population Research |
| Methods | What was the study design? The study was conducted in northern KwaZulu‐Natal Province, South Africa. Children were enrolled into the study by nurses at five government primary health care clinics. Pneumonia by maternal report was considered to have occurred if there was a history of either fast breathing or chest in‐drawing. Confirmed pneumonia was defined as an elevated respiratory rate at rest measured by the fieldworker using WHO/UNICEF Integrated Management of Childhood Illness guidelines. | |
| Participants | Add number of participants. Children eligible for study were 4 to 6 months old. Children were excluded from the study if they were: less than 60% of median weight‐for‐age using United States National Center for Health Statistics standards; had nutritional oedema; had received vitamin or micronutrient supplements in the previous month; had diarrhoea for more than seven days at the time of study enrolment; or were enrolled in another study of a clinical intervention. Confirmed pneumonia was defined as an elevated respiratory rate at rest measured by the fieldworker using WHO/UNICEF Integrated Management of Childhood Illness guidelines. | |
| Interventions | The 3 treatment arms were: vitamin A alone; vitamin A plus zinc; and vitamin A, zinc and multiple micronutrients. All supplements were given daily at home from entry into the study until 24 months of age. | |
| Outcomes | Diarrhoea, pneumonia Incidence? prevalence? | |
| Notes | Funding: Supported by grants from the US National Institute of Health (1 UO1 AI45508‐01, 1 K24 AI/HDO1671‐01, D43TW05572‐01 to Dr Bennish) and the Wellcome Trust (Wellcome 62925 to Dr Bennish and Wellcome 063009 to Dr Van den Broeck. The sponsor for the study was the host institution, the Africa Centre for Health and Population Studies, which gave discretion in the investigative team in study design, data analysis, manuscript preparation, and decisions on manuscript submission and publication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An allocation list was prepared using computer‐generated random numbers and a block size of six"; assignment to the three treatment arms was done separately for three cohorts of children stratified by HIV status of child and mother: HIV‐infected children and mothers; HIV‐uninfected children of HIV‐infected mothers; and HIV‐uninfected children of HIV‐uninfected mothers |
| Allocation concealment (selection bias) | Low risk | Quote: "The manufacturer prepared numbered packs of tablets corresponding to the allocation list. Children enrolled in the study were assigned by a study physician to one of the three study cohorts after results of the HIV tests became available. The physician then allocated the next pack of tablets from the blocks assigned to that cohort to the participant" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Investigators, study staff and participants were blind to the treatment assignments" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Investigators, study staff and participants were blind to the treatment assignments" |
| Incomplete outcome data (attrition bias) | Low risk | Exclusion (12.7%) and attrition (8.1% in vitamin A + zinc and 8.9% in vitamin A group) data were reported along with their reasons. Thirty‐seven children withdrew and one died before any home visits took place |
| Selective reporting (reporting bias) | Low risk | The study appears to be free of selective reporting. Trial Registration |
| Other bias | Low risk | Sources of funding: The sponsor for the study was the host institution, the Africa Centre for Health and Population Studies, which gave discretion in the investigative team in study design, data analysis, manuscript preparation, and decisions on manuscript submission and publication |
| Methods | This randomised, double‐blind, placebo‐controlled, community‐based trial was carried out in Canto Grande, a shanty town on the outskirts of Lima, Peru. The study was carried out in two phases. During the first phase researchers evaluated the effect of zinc or multiple micronutrient supplementation on the recovery from persistent diarrhoea. During the second phase researchers assessed the effect of continued supplementation on morbidity from new infections during the following 6 months. | |
| Participants | 412 children aged 6 to 36 months with diarrhoea for 14 days were randomly assigned, after being stratified for breastfeeding status, to receive two weeks of daily supplementation with one of three indistinguishable supplements: placebo; 20 mg zinc daily as zinc gluconate (zinc group); or 20 mg zinc daily as zinc gluconate plus a mixture of other micronutrients, i.e. vitamins and minerals (zinc VM group). A subset of children consisting of the first 246 children enrolled who intended to remain in the study area subsequently received the same assigned supplement at one‐half the initial daily dose (10 mg zinc daily) and continued under observation for a total of 6 months. | |
| Interventions | Supplements were supplied as individual doses of a dry micronutrient mixture with added sugar, colouring and flavouring agents, which were dissolved in clean water in participants’ homes and provided as a liquid beverage under the supervision of study personnel on Monday through Friday and by parents or other caregivers during the weekends. There were two intervention arms, zinc plus vitamins and minerals who were given 10 mg of zinc supplementation along with different combinations of mineral and vitamins. Another interventional arm was given zinc 10 mg and the control group was not given any supplementation. | |
| Outcomes | Changes in plasma zinc, haematocrit, haemoglobin, plasma ferritin, pneumonia incidence. | |
| Notes | In this review, groups with zinc and placebo are included for analysis. Examination included assessment of hydration status, measurement of rectal temperature and monitoring of respiratory rate, which was counted for 1 minute and repeated if the rate was greater than age‐specific upper limits (50/minute for children aged 6 to 11 months and 40/minute for children aged 11 months). Children were referred to the study physician for diagnosis and treatment when the fieldworker or caregiver was concerned about the child’s health status or if the child had any one of several predefined signs of illness, including fever, presentation or worsening of cough with elevated respiratory rate (i.e. fieldworker‐defined acute lower respiratory infection), persistent diarrhoea, diarrhoea with signs of dehydration, or vomiting or skin conditions requiring diagnosis. Sources of funding: Supported primarily by the Thrasher Research Fund and the World Health Organization; additional funds were provided by the University of California Pacific Rim Program. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "double‐masked" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "double‐masked" |
| Incomplete outcome data (attrition bias) | High risk | 14/81 (17.3%) in zinc and 13/83 (15.7%) placebo groups lost to follow‐up but no reasons were reported |
| Selective reporting (reporting bias) | Low risk | We could not locate the protocol of this study. We could not find the trial registration number of the study. The outcomes mentioned in the methods were reported in the results |
| Other bias | Unclear risk | Sources of funding: funding agencies had no role in views and opinions mentioned in the study |
| Methods | Double‐blind RCT in which the loss of follow‐up was less than 2% The study was conducted in a low socioeconomic population of urban India. | |
| Participants | Children, 6 to 35 months of age, presenting to a community‐based clinic for acute diarrhoea and before enrolment, a parent of the child was given an explanation of the study and written informed consent was obtained. The baseline characteristics for the child‐periods included in the analysis were similar between the two groups. The zinc group had 298 participants and the placebo one had 311. | |
| Interventions | Children were randomised to receive either zinc or placebo in a liquid preparation containing vitamins A (800 units), B1 (0.6 mg), B2 (0.5 mg), B6 (0.5 mg), D3 (100 IU), and E (3 mg) and niacinamide (10 mg); the zinc preparation contained zinc gluconate (10 mg elemental zinc). The liquid preparation 5 mL was given daily for 6 months to all enrolled children; during diarrhoeal illness this was increased to 10 mL to provide for excess zinc losses. | |
| Outcomes | Incidence and prevalence of ALRI. ALRI was diagnosed as using WHO criteria for respiratory disease episodes based on fast breathing alone. ALRI was also defined as child having cough and at least one assessment documenting: a) an elevated respiratory rate more than the age‐specific value on both 1‐minute estimations; and b) a recorded temperature of more than 101°F or lower chest indrawing. | |
| Notes | Funding: This work was supported by grants from the WHO Diarrheal Disease Control Program, the Thrasher Research Fund, the Johns Hopkins Family Health and Child Survival Cooperative Agreement with funding from the US Agency for International Development and the US National Institutes of Health (R29 HD34724). The assistance of Ms Usha Dhingra and Mr Dharminder Kashyap in data management and of Sandoz India Ltd for providing the supplements is appreciated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation schedules with permuted blocks of 10 were used for children" |
| Allocation concealment (selection bias) | Low risk | Quote: "Supplements were prepared and coded by Sandoz India Ltd (Mumbai). Both formulation were liquid preparations, similar in colour and taste" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind"; "The Code, which was kept by WHO personnel, was not available to the investigator until the end of the study; "both formulation were liquid preparations, similar in colour and taste" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | High risk | Exclusion and attrition rates with their reasons were not described in the study |
| Selective reporting (reporting bias) | Low risk | We could not locate the protocol of this study. We could not find the trial registration number of the study. The outcomes mentioned in the methods were reported in the results |
| Other bias | Unclear risk | Sandoz India provided the supplements. Not clear of their role and other funding agencies |
ALRI: acute lower respiratory infection
IU: international unit
RCT: randomised controlled trial
WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Wrong participants: included children with recurrent respiratory infections | |
| Wrong duration: zinc supplementation was given for 2 weeks | |
| Wrong diagnosis: ALRI was diagnosed if the child had reported symptoms of cough or difficulty in breathing with rapid breathing with or without chest indrawing | |
| Wrong mode of supplementation: zinc supplement was delivered in a fortified drink | |
| Wrong duration; wrong outcomes: zinc was supplemented for 60 days; did not study effects on diarrhoea or respiratory illnesses | |
| Wrong duration: duration of intervention was 2 weeks | |
| Wrong duration: duration of intervention was 10 days | |
| Wrong diagnosis: did not use this review's specific ARI definition | |
| Wrong diagnosis: considered ‘cough and fever’ as ALRI outcome | |
| Wrong intervention and outcomes: infants were recruited and supplemented from birth; short course supplementation was provided; only cough was reported | |
| Wrong outcome: respiratory tract infection outcomes were defined as the occurrence of cough alone, cough and fever, or cough and rapid respiratory rate as reported by the mother | |
| Wrong diagnosis: considered "cough or cold with or without fever. ALRI was diagnosed if the child had symptoms of cough with difficult and/or rapid breathing or chest indrawing as informed by the caregiver" as ALRI | |
| Wrong diagnosis: considered caregiver’s report of cough or difficulty in breathing along with rapid breathing | |
| Wrong population: infants aged less than 2 months at start of intervention (5 to 7 weeks) | |
| Wrong diagnosis: respiratory outcome was cough and fever | |
| Wrong population: Infants were recruited and supplemented from 4 weeks of age | |
| Wrong duration: supplementation was given for 2 weeks only | |
| Wrong diagnosis: respiratory infections were defined as the presence of at least two of the following symptoms: runny nose, cough, wheezing, difficulty breathing, or fever | |
| Wrong diagnosis: ALRI was reported by parent as presence of cough and rapid respiration | |
| Wrong diagnosis: respiratory illness was presence of runny nose, common cold, sore throat or cough | |
| Wrong duration: zinc supplementation period was 2 weeks | |
| Wrong diagnosis: study used Brazilian Ministry of Health Criteria as ARI definition | |
| Wrong diagnosis: presence of two or more of the following symptoms as ARI: "Cough, runny nose, shortness of breath and sore throat two or more days duration" | |
| Wrong duration: zinc supplementation period was 60 days | |
| Wrong diagnosis: definition of ARI: " Signs (fast breathing, chest indrawing) of acute respiratory illness were recorded as reported by the mother. An acute respiratory illness episode was defined as a minimum of 2 days with signs followed by a significant interval of at least 7 days" | |
| Wrong population: Infants were recruited and supplemented from within 7 days of birth | |
| Wrong population: zinc supplementation was given to infants between 2 to 4 weeks and 12 months of age | |
| Wrong diagnosis and population: trial was on children aged 1 to 35 months with data not stratified by age, ARTI: episodes of acute respiratory illness were defined as one or more consecutive days of fever, cough, or difficulty breathing, with all three symptoms on at least 1 day during the episode and at least 7 days between episodes | |
| Wrong outcomes: cough was only reported respiratory outcome | |
| Wrong population: children included older than review's specified cut off (78 to 120 months) |
ALRI: acute lower respiratory infection
ARI: acute respiratory infection
ARTI: acute respiratory tract infection
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pneumonia incidence Show forest plot | 6 | 5193 | Risk Ratio (Fixed, 95% CI) | 0.87 [0.81, 0.94] |
| Analysis 1.1  Comparison 1 Zinc supplementation vs placebo, Outcome 1 Pneumonia incidence. | ||||
| 1.1 Diagnosis based on age‐specific fast breathing with or without lower chest indrawing | 4 | 1932 | Risk Ratio (Fixed, 95% CI) | 0.95 [0.86, 1.06] |
| 1.2 Diagnosis based age‐specific fast breathing and confirmed by chest examination or chest radiograph | 4 | 3261 | Risk Ratio (Fixed, 95% CI) | 0.79 [0.71, 0.88] |
| 2 Pneumonia prevalence Show forest plot | 1 | 609 | Risk Ratio (Fixed, 95% CI) | 0.59 [0.35, 0.99] |
| Analysis 1.2  Comparison 1 Zinc supplementation vs placebo, Outcome 2 Pneumonia prevalence. | ||||

Study flow diagram

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies

Methodological quality summary: review authors' judgements about each methodological quality item for each included study

Comparison 1 Zinc supplementation vs placebo, Outcome 1 Pneumonia incidence.
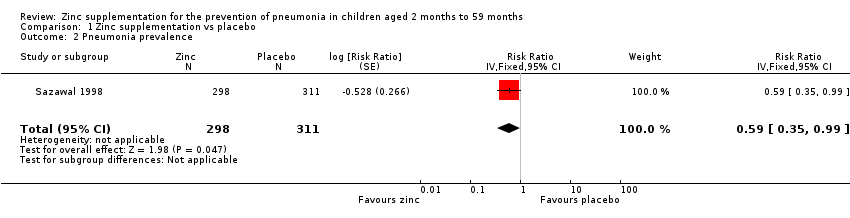
Comparison 1 Zinc supplementation vs placebo, Outcome 2 Pneumonia prevalence.
| Zinc supplementation compared with placebo for the prevention of pneumonia in children aged 2 months to 59 months | ||||||
| Patient or population: children aged 2 months to 59 months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Zinc supplementation | |||||
| Pneumonia incidence | 343 per 1000 | 299 per 1000 | RR 0.87 | 5193 | ⊕⊕⊝⊝ | |
| Pneumonia prevalence | 22 per 1000 | 13 per 1000 | RR 0.59 | 609 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Studies have unclear information on allocation concealment, blinding and reporting biases. | ||||||
| Study | Supplement | Schedule | Duration | Surveillance | |
| Zinc | Control | ||||
| Zinc gluconate 10 mg | Both groups vitamin A | Daily | 4 months | Once weekly | |
| Zinc sulphate 10 mg | Placebo | Daily | 6 months | Every 2 weeks | |
| Zinc acetate 35 mg to infants 70 mg to children aged > 12 months | Placebo | Weekly | 12 months | Once weekly | |
| Zinc gluconate 10 mg | Both groups vitamin A | Daily | (Continued until 24 months of age) | Once weekly | |
| Zinc gluconate 10 mg | Placebo | Daily | 6 months | Once weekly | |
| Zinc gluconate 10 mg | Placebo | Daily | 4 months | Every 5th day | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pneumonia incidence Show forest plot | 6 | 5193 | Risk Ratio (Fixed, 95% CI) | 0.87 [0.81, 0.94] |
| 1.1 Diagnosis based on age‐specific fast breathing with or without lower chest indrawing | 4 | 1932 | Risk Ratio (Fixed, 95% CI) | 0.95 [0.86, 1.06] |
| 1.2 Diagnosis based age‐specific fast breathing and confirmed by chest examination or chest radiograph | 4 | 3261 | Risk Ratio (Fixed, 95% CI) | 0.79 [0.71, 0.88] |
| 2 Pneumonia prevalence Show forest plot | 1 | 609 | Risk Ratio (Fixed, 95% CI) | 0.59 [0.35, 0.99] |

