Suplementos de zinc para la prevención de la neumonía en niños de dos a 59 meses de vida
Información
- DOI:
- https://doi.org/10.1002/14651858.CD005978.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 04 diciembre 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Infecciones respiratorias agudas
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Zohra S Lassi (ZSL) entered data, created the comparisons, carried out the analysis and wrote the text of the review under the guidance of Dr Zulfiqar A Bhutta (ZAB). Anoosh Moin (AM) took part in screening results and updating the review.
Sources of support
Internal sources
-
The Aga Khan University, Pakistan.
External sources
-
No sources of support supplied
Declarations of interest
Zohra S Lassi: none known.
Anoosh Moin: none known.
Zulfiqar A Bhutta: none known.
Acknowledgements
The authors wish to thank Ammad Saeed, who assisted in the development of protocol and Ms Sarah Thorning for her assistance with the literature search. The review authors would like to thank the following people for commenting on the draft review: Abdullah Brooks, Robert Black, Teenah Handiside and Jiaan‐Der Wang, Mark Griffin, and John Holden.The draft protocol was written by Dr Batool A Haider (BAH) who also took part in the initial stages of the 2010 review and assisted in data extraction.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Dec 04 | Zinc supplementation for the prevention of pneumonia in children aged 2 months to 59 months | Review | Zohra S Lassi, Anoosh Moin, Zulfiqar A Bhutta | |
| 2010 Dec 08 | Zinc supplementation for the prevention of pneumonia in children aged 2 months to 59 months | Review | Zohra S Lassi, Batool A Haider, Zulfiqar A Bhutta | |
| 2006 Apr 19 | Zinc supplementation for the prevention of pneumonia in children aged 2 months to 59 months | Protocol | Batool A Haider, Muhammad Ammad Saeed, Zulfiqar A Bhutta | |
Differences between protocol and review
We have added mandatory sections such as subgroup analysis and sensitivity analysis for this update.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Child, Preschool; Humans; Infant;
PICO

Study flow diagram

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies

Methodological quality summary: review authors' judgements about each methodological quality item for each included study

Comparison 1 Zinc supplementation vs placebo, Outcome 1 Pneumonia incidence.
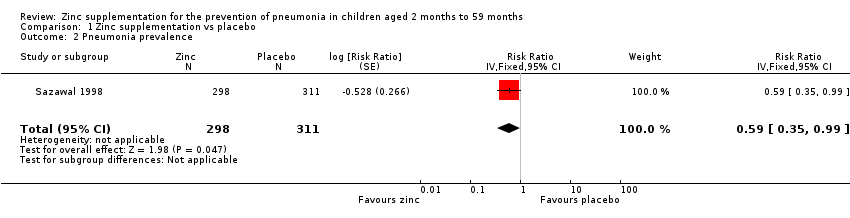
Comparison 1 Zinc supplementation vs placebo, Outcome 2 Pneumonia prevalence.
| Zinc supplementation compared with placebo for the prevention of pneumonia in children aged 2 months to 59 months | ||||||
| Patient or population: children aged 2 months to 59 months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Zinc supplementation | |||||
| Pneumonia incidence | 343 per 1000 | 299 per 1000 | RR 0.87 | 5193 | ⊕⊕⊝⊝ | |
| Pneumonia prevalence | 22 per 1000 | 13 per 1000 | RR 0.59 | 609 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Studies have unclear information on allocation concealment, blinding and reporting biases. | ||||||
| Study | Supplement | Schedule | Duration | Surveillance | |
| Zinc | Control | ||||
| Zinc gluconate 10 mg | Both groups vitamin A | Daily | 4 months | Once weekly | |
| Zinc sulphate 10 mg | Placebo | Daily | 6 months | Every 2 weeks | |
| Zinc acetate 35 mg to infants 70 mg to children aged > 12 months | Placebo | Weekly | 12 months | Once weekly | |
| Zinc gluconate 10 mg | Both groups vitamin A | Daily | (Continued until 24 months of age) | Once weekly | |
| Zinc gluconate 10 mg | Placebo | Daily | 6 months | Once weekly | |
| Zinc gluconate 10 mg | Placebo | Daily | 4 months | Every 5th day | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pneumonia incidence Show forest plot | 6 | 5193 | Risk Ratio (Fixed, 95% CI) | 0.87 [0.81, 0.94] |
| 1.1 Diagnosis based on age‐specific fast breathing with or without lower chest indrawing | 4 | 1932 | Risk Ratio (Fixed, 95% CI) | 0.95 [0.86, 1.06] |
| 1.2 Diagnosis based age‐specific fast breathing and confirmed by chest examination or chest radiograph | 4 | 3261 | Risk Ratio (Fixed, 95% CI) | 0.79 [0.71, 0.88] |
| 2 Pneumonia prevalence Show forest plot | 1 | 609 | Risk Ratio (Fixed, 95% CI) | 0.59 [0.35, 0.99] |

