اکسیژن درمانی در عفونتهای دستگاه تنفسی تحتانی در کودکان سنین 3 ماه تا 15 سال
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع مطالعات در انتظار ارزیابی
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | A cross‐sectional study at the Kanti Children's Hospital at Kathmandu Valley (1336 MASL) | |
| Participants | 264 children from 2 months to 5 years of age, presenting with cough or difficult breathing. 14 were excluded because they could not be classified into any category of respiratory illness | |
| Interventions | No interventions assessed | |
| Outcomes | Prediction of hypoxaemia (SpO2 < 90%) was based on clinical signs and symptoms presented at the time of admission before any treatment. Sensitivity and specificity are presented for symptoms and signs | |
| Notes | Results are presented for the global population. Only tachypnoea was presented with age subgroups. No disease severity was associated with signs and symptoms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) | Unclear risk | NA |
| Incomplete outcome data (attrition bias) | Low risk | All children included in the study completed the final outcome assessment |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | Physicians caring for patients were blinded to the SpO2 results. The oximeter readings were used as the gold standard. The definition of hypoxaemia was established in advance |
| Methods | Observational study conducted in the Garoka Hospital at Eastern Highlands of Papua New Guinea (1600 MASL), with the aim of determining the incidence and severity of hypoxaemia in neonates and children requiring admission to hospital with acute respiratory and non‐respiratory illnesses | |
| Participants | 491 neonates and children were enrolled. 245 out of these met the clinical criteria for LRTIs | |
| Interventions | No interventions were assessed | |
| Outcomes | Sensitivity, specificity, and positive and negative predictive values. Presence of hypoxaemia was determined using the oximeter readings as the gold standard. Clinical symptoms or signs such as inability to feed, reduced activity, cyanosis, fast respiratory rate, failure to resist examination, grunting and head nodding were assessed as indicators of hypoxaemia (SpO2 < 86%) | |
| Notes | To establish normal values of oxygen saturation among well neonates and children they studied 67 neonates and 151 children from 1 to 60 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA (observational study ‐ dx accuracy study) |
| Allocation concealment (selection bias) | Unclear risk | NA (observational study) |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Low risk | Physicians caring for patients were blinded to the SpO2 results. The oximeter readings were used as the gold standard |
| Incomplete outcome data (attrition bias) | Low risk | All children included in the study completed the final outcome assessment.SpO2 was measured in every child included in the study |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The definition of hypoxaemia was established in advance. To establish normal values of oxygen saturation among well neonates and children they studied 67 neonates and 151 children from 1 to 60 months |
| Methods | Cohort study in Papua New Guinea Institute of Medical Research | |
| Participants | This study included 91 children between 3 months and 5 years with a clinical diagnosis of pneumonia | |
| Interventions | The oximeter readings were used as the gold standard. To establish the 'adequate' values of oxygen saturation, 100 healthy children from Tari were assessed with oximeter and hypoxaemia was defined as SpO2 equal to or less than 85% | |
| Outcomes | Clinical signs present at the initial evaluation (cyanosis, poor feeding, crepitations, bronchial breathing, grunting, chest indrawing, nasal flaring, drowsiness and hepatomegaly) were recorded. Sensitivity and specificity of each sign were calculated, to indicate whether hypoxaemia was present, taking the oximeter readings as the gold standard. Prediction of hypoxaemia (SpO2 < 85%) was based on clinical signs presenting at the time of admission, before any treatment | |
| Notes | Results for sensitivity and specificity of clinical signs are for global population. Sensitivity and specificity of each sign were calculated for this review: they are not presented by age group, nor by disease severity | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA (observational study ‐ dx accuracy study) |
| Allocation concealment (selection bias) | Unclear risk | NA (observational study ‐ dx accuracy study) |
| Blinding of participants and personnel (performance bias) | Unclear risk | NA (observational study ‐ dx accuracy study) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Physicians caring for patients were blinded to the SpO2 results |
| Incomplete outcome data (attrition bias) | Unclear risk | All children included in the study completed the final outcome assessment SpO2 was measured in every child included in the study |
| Selective reporting (reporting bias) | Unclear risk | All expected outcomes were reported |
| Other bias | Unclear risk | The definition of hypoxaemia was established in advance |
| Methods | Prospective cohort study conducted at Clinica Pediatrica "A" from Centro Hospitalario Pereira Rossell in Montevideo, Uruguay (43 MASL) | |
| Participants | A total of 216 hospitalised children between 1 month and 5 years with LRTI or with asthma were evaluated. Children with chronic respiratory distress and neuromuscular diseases were excluded from the study. Viral LRTI 65%, bacterial pneumonia 24%, asthma attacks 11%. Median age 14 months | |
| Interventions | No interventions were assessed. Oxygen saturation measured by oximeter was taken as the gold standard | |
| Outcomes | Prediction of hypoxaemia (SpO2 < 95%, SpO2 < 93%) based on tachypnoea, tachycardia and chest indrawing presented at the time of admission before any treatment. Sensitivity, specificity and predictive values are reported | |
| Notes | Hypoxaemia was defined as SpO2 < 95%. Results are presented for global population. No type of disease or disease severity subgroups were considered in the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA (observational study ‐ dx accuracy study) |
| Allocation concealment (selection bias) | Unclear risk | NA (observational study ‐ dx accuracy study) |
| Blinding of participants and personnel (performance bias) | Unclear risk | NA (observational study ‐ dx accuracy study) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Physicians caring for patients were blinded to the SpO2 results |
| Incomplete outcome data (attrition bias) | Unclear risk | All children included in the study completed the final outcome assessment SpO2 was measured in every child included in the study |
| Selective reporting (reporting bias) | Unclear risk | All expected outcomes were reported |
| Other bias | Unclear risk | The definition of hypoxaemia was established in advance |
| Methods | Non‐randomised. Sequential assignment | |
| Participants | 80 children with acute respiratory disease including asthma, less than 5 years old | |
| Interventions | Head box at 4 L/min Nasopharyngeal catheter at 1 L/min Twin‐holed prenasal catheter 1 L/min | |
| Outcomes | Achieve PaO2 greater than 60 mmHg | |
| Notes | All children were placed in each of the 4 delivery method groups and changed every 15 minutes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Used a quasi‐random method to assign patients to the treatment groups in a cross‐over design (predetermined sequence). There is no description of the order in which children were placed in the different delivery method groups |
| Allocation concealment (selection bias) | High risk | The allocation sequence was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Due to the intervention under assessment it was not possible to blind participants and personnel, but it is unlikely to affect the final results because assessment of hypoxaemia was done by blood gas analysis and pulse oximetry |
| Blinding of outcome assessment (detection bias) | Low risk | The evaluation of their main outcome was completely objective even though it was not blinded; they used arterial blood gas analysis and pulse oximetry |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the methods section are also reported in the results section |
| Other bias | Low risk | None |
| Methods | Observational study in a population attending at the Pediatric Ward of the Basse Major Health Centre during 6 months, in rural Gambia, Africa | |
| Participants | 420 children aged 2 to 59 months with severe and very severe pneumonia using the WHO criteria. The distribution of age was: 2 to 11 months 168 (40%), 12 to 23 months 137 (32.6%), 24 to 35 months 56 (13.3%), 36 to 47 months 40 (9.5%), 48 to 59 months 19 (4.5%) | |
| Interventions | No interventions were assessed | |
| Outcomes | Signs and symptoms that predict hypoxaemia (SaO2 < 90%) | |
| Notes | Sensitivity and specificity were calculated for this review; they are not presented by age group nor by disease severity. Results for sensitivity and specificity of clinical signs are for the global population | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA (observational study ‐ dx accuracy study) |
| Allocation concealment (selection bias) | Unclear risk | NA (observational study ‐ dx accuracy study) |
| Blinding of participants and personnel (performance bias) | Unclear risk | NA (observational study ‐ dx accuracy study) |
| Blinding of outcome assessment (detection bias) | Low risk | Physicians caring for patients were blinded to the SpO2 results |
| Incomplete outcome data (attrition bias) | Low risk | All children included in the study completed the final outcome assessment SpO2 was measured in every child included in the study |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The definition of hypoxaemia was established in advance |
| Methods | Cross‐sectional study conducted at Port Moresby General Hospital, Papua New Guinea | |
| Participants | 77 children 1 to 60 months of age with clinical diagnosis of moderate or severe pneumonia according to WHO classification. Median age: 8 months (IQR 4 to 12). 9 patients were excluded for not meeting the classification criteria. 48 moderate pneumonia, 15 severe pneumonia | |
| Interventions | No interventions were assessed | |
| Outcomes | Risk ratios, sensitivity, specificity and positive predictive values of clinical signs at 3 levels of hypoxaemia. Prediction of hypoxaemia (SpO2 < 93%; SpO2 < 90%; SpO2 < 85%) based on reasonably objective signs presented at the time of admission before any treatment | |
| Notes | Results are presented for the global population; no age subgroups were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA (observational study ‐ dx accuracy study) |
| Allocation concealment (selection bias) | Unclear risk | NA (observational study ‐ dx accuracy study) |
| Blinding of participants and personnel (performance bias) | Unclear risk | NA (observational study ‐ dx accuracy study) |
| Blinding of outcome assessment (detection bias) | Low risk | Physicians caring for patients were blinded to the SpO2 results |
| Incomplete outcome data (attrition bias) | Low risk | All children included in the study completed the final outcome assessment SpO2 was measured in every child included in the study |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The definition of hypoxaemia was established in advance |
| Methods | Observational cross‐sectional study, conducted at the Emergency Department of India Institute of Medical Sciences, New Delhi, India (239 MASL), with the aim of determining the prevalence of hypoxaemia (SpO2 < 90%) in children with acute LRTI and identifying the clinical signs associated with the presence of hypoxaemia in children with LRTI | |
| Participants | 109 children less than 5 years of age were evaluated. Children with a history of cough and rapid respiration or difficulty in breathing were included. Children with asthma, congenital heart disease, severe anaemia, peripheral circulatory failure, needing ventilatory support and severe dehydration were excluded | |
| Interventions | No interventions were assessed. The oximeter readings were taken as the gold standard | |
| Outcomes | Sensitivity, specificity and likelihood ratios were calculated for each symptom or sign and for various combinations of clinical signs as well. Signs assessed were: appearance, weight, heart rate, respiratory rate, oxygen saturation, cyanosis, chest retraction, grunting, nasal flaring, head nodding, pallor, crepitation or rhonchi and the state of consciousness. Prediction of hypoxaemia (SpO2 < 90%) was based on clinical signs and symptoms presented at the time of admission before any treatment | |
| Notes | Sensitivity and specificity of tachypnoea were reported by age group at 3 cut‐off points in the 3 age groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA (observational study ‐ dx accuracy study) |
| Allocation concealment (selection bias) | Unclear risk | NA (observational study ‐ dx accuracy study) |
| Blinding of participants and personnel (performance bias) | Unclear risk | NA (observational study ‐ dx accuracy study) |
| Blinding of outcome assessment (detection bias) | Low risk | Physicians caring for patients were blinded to the SpO2 results |
| Incomplete outcome data (attrition bias) | Low risk | All children included in the study completed the final outcome assessment SpO2 was measured in every child included in the study |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The definition of hypoxaemia was established in advance. The presence of respiratory symptoms was established by physical examination of the child |
| Methods | Cross‐sectional study conducted in a tertiary care centre in Bogotá, Colombia (2640 MASL) at the emergency room or the outpatient department of the Clinica Infantil Colsubsidio | |
| Participants | 201 children aged from 7 days to 36 months, presenting with cough lasting up to 7 days and whose evaluation included a chest radiograph. Children were excluded if they had cardiovascular, pulmonary, neurological or congenital defects; a chronic disease including asthma, cancer, immunosuppression and metabolic disorders; or previous episodes of wheezing. The age distribution of studied children was: < 12 months 62 (31%), 13 to 24 months 83 (42%) and > 24 months 55 (28%) | |
| Interventions | No interventions were assessed. Oxygen saturation measured by oximeter/the gold standard was a chest radiograph | |
| Outcomes | Sensitivity and specificity for each symptom. Prediction of hypoxaemia (SpO2 < 88%) was based on clinical signs and symptoms presenting at the time of admission before any treatment | |
| Notes | Data on symptoms and clinical signs of acute respiratory infection were obtained using a standardised questionnaire and a physical examination performed by a paediatrician. Results for sensitivity and specificity of clinical signs other than tachypnoea are not presented by age group, nor by disease severity. Sensitivity and specificity of tachypnoea was reported at different cut‐off values (from 10 breaths/min to 70 breaths/min) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA (observational study ‐ dx accuracy study) |
| Allocation concealment (selection bias) | Unclear risk | NA (observational study ‐ dx accuracy study) |
| Blinding of participants and personnel (performance bias) | Low risk | A chest radiograph was read by a blinded physician plus the oxygen saturation measured by oximeter |
| Blinding of outcome assessment (detection bias) | Unclear risk | Physicians caring for patients were blinded to the SpO2 results |
| Incomplete outcome data (attrition bias) | Low risk | All children included in the study completed the final outcome assessment SpO2 was measured in every child included in the study |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | None |
| Methods | Multicentre, randomised, open‐label | |
| Participants | 121 children aged 2 weeks to 5 years with LRTI with SaO2 < 89% | |
| Interventions | Nasal prongs (n = 60) 0.25 to 4 L/min | |
| Outcomes | Adequate oxygenation SaO2 > 90% | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Method for randomisation generation was not described. The enrolment of children was limited by the availability of beds and pulse oximeter |
| Allocation concealment (selection bias) | Low risk | Adequate; authors used sealed envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Not blinded due to intervention under assessment. It is unlikely to affect the final results because assessment of hypoxaemia was done using a pulse oximeter |
| Blinding of outcome assessment (detection bias) | Unclear risk | The evaluators of the main outcomes were not blinded but SaO2 was documented by oximetry. Complications and other secondary outcomes were assessed in a non‐blinded way |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All important outcomes were assessed and reported |
| Other bias | Low risk | None |
| Methods | Randomised, open‐label, cross‐over design | |
| Participants | 99 children aged 2 weeks to 5 years with LRTI with hypoxaemia | |
| Interventions | Nasal prongs (n = 50) 0.25 L/min | |
| Outcomes | Time required to achieve adequate oxygenation > 90% for more than 8 hours | |
| Notes | The nasal catheter used was a modification of the traditional nasopharyngeal catheter; it was shorter and was left at half distance from the nostril | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The method of random sequence generation was not described |
| Allocation concealment (selection bias) | Low risk | Adequate; authors used sealed envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not blinded due to the intervention under assessment. It is unlikely to affect the final results because assessment of hypoxaemia was done by using a pulse oximeter |
| Blinding of outcome assessment (detection bias) | Low risk | The evaluators of the main outcomes were not blinded but SaO2 was documented by oximetry. Complications and other secondary outcomes were assessed in a non‐blinded way |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reported results for the same outcomes listed in the methods section of the article |
| Other bias | Low risk | None |
| Methods | Cross‐sectional study conducted in Kenyatta National Hospital (public hospital) in Nairobi (1670 MASL) | |
| Participants | 256 infants and children from the age of 7 days to 36 months with history of cough and other symptoms of acute LRTI for less than 7 days. The distribution by age was: 0 to 2 months 45 infants (17.6%); 3 to 11 months 144 infants (56.25%) and 12 to 36 months 67 children (26.2%) | |
| Interventions | No interventions assessed | |
| Outcomes | Prevalence of hypoxaemia (SpO2 < 90%), sensitivity and specificity of signs and symptoms to determine the presence of hypoxaemia Recorded data included respiratory rates, pulse, central cyanosis, chest retractions, grunting, nasal flaring, wheezing, crepitations or rhonchi on auscultation. SpO2 breathing room air and a chest radiograph read by a blinded physician were taken as the gold standard for diagnosis of hypoxaemia associated with LRTI. Each clinical finding was assessed for its sensitivity and specificity in the diagnosis of hypoxaemia | |
| Notes | To define the SpO2 cut‐off point, oxygen saturation was measured with an oximeter in 87 healthy children attending the child welfare clinics. Results for sensitivity and specificity of clinical signs are presented by age group, but not disaggregated by disease severity | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA (observational study ‐ dx accuracy study) |
| Allocation concealment (selection bias) | Unclear risk | NA (observational study ‐ dx accuracy study) |
| Blinding of participants and personnel (performance bias) | Low risk | NA (observational study ‐ dx accuracy study) |
| Blinding of outcome assessment (detection bias) | Low risk | Physicians caring for patients were blinded to the SpO2 results |
| Incomplete outcome data (attrition bias) | Low risk | All children included in the study completed the final outcome assessment SpO2 was measured in every child included in the study |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The definition of hypoxaemia was established in advance |
| Methods | Cross‐sectional study in high‐altitude population attending the Chulec Hospital and La Oroya Clinic during a 4‐month period in Junin, Peru (1750 MASL) | |
| Participants | 423 children between 2 and 60 months with acute respiratory infection. 188 (44%) with upper respiratory infection (URI). 175 (41%) with acute LRTI non‐pneumonia, 60 (14%) with bronchopneumonia | |
| Interventions | No interventions assessed | |
| Outcomes | Clinical signs and symptoms present at the time of admission were recorded by an expert physician who was blinded to the oximeter reading. SpO2 was also measured at this time. Using 2 clinical categories, upper respiratory tract infection (URTI) and LRTI and balancing by age group, they determined the sensitivity, specificity and likelihood ratios (LR) for several potential indicators of hypoxaemia. The SpO2 cut‐off was determined by studying 153 healthy children from the same population. Hypoxaemia was considered to be present if SpO2 was > 2 standard deviations below the mean value for healthy children (2 to 11 months: SpO2 < 84 and 12 to 60 months: SpO2 < 86) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA (observational study ‐ dx accuracy study) |
| Allocation concealment (selection bias) | Unclear risk | NA (observational study ‐ dx accuracy study) |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Low risk | Physicians caring for patients were blinded to the SpO2 results |
| Incomplete outcome data (attrition bias) | Low risk | SpO2 was measured in every child included in the study |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The definition of hypoxaemia was established in advance |
| Methods | Single‐centre, randomised, open level, parallel trial | |
| Participants | 58 children aged 2 to 59 months presenting with severe pneumonia without hypoxaemia (SpO2 > 90%) | |
| Interventions | Supplemental oxygen by nasal prongs at flow of 1 to 2 L/min versus no oxygen supplementation (room air) | |
| Outcomes | Development of subsequent hypoxaemia (SpO2 < 90% or PaO2 < 60%) Duration of tachypnoea (respiratory rate > 50 breaths/min in children from 2 to 12 months; > 40 breaths/min in children from 13 to 59 months) Duration of chest indrawing Duration of fever after enrolment | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised study assignments were prepared beforehand |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed using a serially numbered, opaque, sealed envelopes, which contain study assignments |
| Blinding of participants and personnel (performance bias) | High risk | According to the authors the nature of the intervention prevented blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Although the trial was not blinded, the outcome 'hypoxaemia' was measured by using a pulse oximeter every 6 hours |
| Incomplete outcome data (attrition bias) | Unclear risk | Authors did not report loss to follow‐up. However, it is not clear for how long the patients were followed up |
| Selective reporting (reporting bias) | Low risk | Clinically important outcomes are reported. There is no reason to suspect reporting bias |
| Other bias | Low risk | None |
| Methods | Observational diagnostic accuracy study performed in Saint Francis Hospital, Katete Zambia (1150 MASL), to investigate the clinical signs (respiratory rate, chest indrawing, grunting, crepitations/bronchial breathing, cyanosis, failure to drink) that predict hypoxaemia | |
| Participants | The study included 158 rural children between 4 weeks and 5 years with severe or very severe pneumonia according to the WHO classification. 4 children out of 167 were excluded because of widespread wheezing and 5 left the hospital before completing treatment | |
| Interventions | No interventions were assessed. The SpO2 measure was taken as the gold standard | |
| Outcomes | Sensitivity and specificity of each sign and symptom. Prediction of hypoxaemia (SpO2 < 92%) was based on clinical signs and symptoms presenting at the time of admission before any treatment | |
| Notes | In a pilot study with 85 healthy infants they established a cut‐off point of normal oxygen saturation at Zambia altitude (SpO2 > 92%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA (observational study ‐ dx accuracy study) |
| Allocation concealment (selection bias) | Unclear risk | NA (observational study ‐ dx accuracy study) |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Low risk | Physicians caring for patients were blinded to the SpO2 results |
| Incomplete outcome data (attrition bias) | Low risk | SpO2 was measured in every child included in the study. The definition of hypoxaemia was established in advance |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | None |
| Methods | An observational study undertaken in 2 hospitals in Banjul, Gambia at sea level | |
| Participants | The study included 1072 children aged between 2 and 33 months in the trial cohort who were admitted with pneumonia or any other form of acute LRTI. Any child who had signs of structural heart disease, Down's syndrome or those who had been included in a previous case‐control study of hypoxaemia were excluded | |
| Interventions | No interventions were assessed. The oximeter readings were taken as the gold standard | |
| Outcomes | The sensitivity and specificity of symptoms and clinical signs reported by the patients' mothers, as well as multi‐regression models. Prediction of hypoxaemia (SpO2 < 90%) based on clinical signs presenting at the time of admission before any treatment | |
| Notes | Presence of hypoxaemia was defined as SpO2 < 90%. Results are presented for the global population. No age or disease severity subgroups were considered in the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA (observational study ‐ dx accuracy study) |
| Allocation concealment (selection bias) | Unclear risk | NA (observational study ‐ dx accuracy study) |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Physicians caring for patients were blinded to the SpO2 results |
| Incomplete outcome data (attrition bias) | Unclear risk | SpO2 was measured in every child included in the study |
| Selective reporting (reporting bias) | Unclear risk | All expected outcomes were reported |
| Other bias | Low risk | The definition of hypoxaemia was established in advance |
| Methods | Multicentre, randomised, open‐label, cross‐over design | |
| Participants | 118 children aged 7 days to 5 years with LRTI with Sa02 < 90% | |
| Interventions | Nasal prongs 0.2 to 4 L/min (n = 62) | |
| Outcomes | Adequate oxygenation SaO2 > 95% | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The method of random sequence generation was not described. A maximum of 3 children could be included in the study at any time |
| Allocation concealment (selection bias) | Low risk | Authors used sequentially numbered envelopes and after stabilisation with the first delivery method children were changed to the other delivery method |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not blinded due to the intervention under assessment. It is unlikely to affect the final results because assessment of hypoxaemia was done using a pulse oximeter |
| Blinding of outcome assessment (detection bias) | Low risk | Therapies were not masked and the evaluation assessment was not blinded, but the measurement of the main outcome was objective (pulse oximeter readings) |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up was complete in both arms |
| Selective reporting (reporting bias) | Low risk | Reported results on the same outcomes listed in the methods section of the article. All important outcomes were assessed and reported |
| Other bias | Low risk | None |
| Methods | A case‐control study conducted in the Royal Victoria Hospital in Banjul, Gambia at sea level, with the aim of studying the signs and symptoms indicating hypoxaemia in children with pneumonia | |
| Participants | 69 children between 2 months and 5 years admitted to hospital with acute LRTI and oxygen saturation (SpO2 < 90%) were compared with 67 children matched for age and diagnosis from the same referral hospital (control group 1) and 80 from another hospital (control group 2). All controls had SpO2 of 90% or above | |
| Interventions | Clinical signs/gold standard chest radiographic | |
| Outcomes | The sensitivity and specificity of each single model was calculated | |
| Notes | Results are presented as the distribution of frequencies of presenting signs by categories of severity | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA (observational study ‐ dx accuracy study) |
| Allocation concealment (selection bias) | Unclear risk | NA (observational study ‐ dx accuracy study) |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Low risk | Chest radiographic findings were evaluated by a blinded physician |
| Incomplete outcome data (attrition bias) | Low risk | All children included in the study completed the final outcome assessment SpO2 and chest radiographs were measured in every child included in the study |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | None |
dx: diagnostic
IQR: interquartile range
L/min: litres per minute of oxygen delivered by each method
LRTI: lower respiratory tract infection
MASL: metres above sea level
mmHg: millimetres of mercury (Hg)
n: number of participants
NA: not applicable
PaO2: arterial oxygen tension
SaO2: arterial oxygen saturation
SpO2: arterial oxygen saturation read by pulse oximeter
WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Event reported was presented in a 2‐month old infant | |
| Event reported was presented in a 2‐month old infant | |
| Study population was malnourished children | |
| Study population included children that required oxygen therapy for any reason and did not report desegregated data for children with LRTIs | |
| Evaluated impact cost of introducing oxygen concentrators and pulse oximeters in hospitals, but did not evaluate the cost or relative cost‐effectiveness of oxygen delivery systems | |
| The population studied included infants of less than 2 months of age (range 0.3 to 11.3 months) | |
| The study population included children with all‐cause respiratory distress. 38% of the participants had a diagnosis of asthma and the results are presented for the whole population studied | |
| Presented the results in different categories of combined clinical signs and it was not possible to contact the author to obtain disaggregated data | |
| Evaluated the prediction of hypoxaemia based on clinical signs in a cohort of children and did not report disaggregated data for children with LRTI | |
| All patients in the cohort studied used nasal prongs, so comparative evaluation of outcomes was not possible. Did not assess the indicators for oxygen therapy | |
| Authors compared the change in PCO2 between the groups after 12 h and 24 h. Secondary outcomes were change in capillary pH, respiratory rate, pulse rate. The study did not address any clinical outcome described in the criteria for selecting studies for this review |
h: hours
LRTI: lower respiratory tract infection
PCO2: carbon dioxide partial pressure
pH: measurement of the acidity of the blood
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Cross‐sectional study conducted in a Pediatric Emergency Unit of University College Hospital (Tertiary Health Facility) in Ibadan, South Western Nigeria. (237 MASL). During a period in April 2010 to March 2011 |
| Participants | 1726 children with age distribution between 0 months and more than 60 months admitted with medical emergencies were recruited. A total of 313 were diagnosed with ALRI |
| Interventions | No interventions assessed (descriptive study) |
| Outcomes | The main outcome measures were hypoxaemia and outcome of illness (died or survived). Recognised signs and symptoms, including very fast breathing (> 60 breaths/min), cyanosis, grunting, nasal flaring, chest retractions, head nodding and auscultatory signs and signs of general depression in the child, were compared between ALRI and non‐ALRI cases. From all the patients, 494/1726 (28.6%) had hypoxaemia (SpO2 < 90%) (268 were female and 208 male) and from this, only (49.2%) presented hypoxaemia among those having diagnosis of ALRI (154/313). A total of 141 children died, 60 (42.1%) female, and hypoxaemia was documented in 56 (39.6%) of the deaths, mortality was reported in 33 of the ALRI patients. Nasal flaring (OR 3.86, 95% CI 1.70 to 8.74) and chest retraction (OR 4.77, 95% CI 1.91 to 11.92) predicted hypoxaemia in ALRI but not in non‐ALRI |
| Notes | Results are presented as several distributions: stratification into 5 age groups between (< 2, 2 to 12, 13 to 24, 25 to 60, > 60 months), distribution according to main primary diagnoses, distribution according to prevalence of hypoxaemia by primary diagnosis including ALRI and others. Type of disease subgroups were considered in the analysis for hypoxaemic on arrival and non‐hypoxaemic after 10 min of oxygen therapy. Only ALRI was presented with different signs (fast breathing, cyanosis, grunting, nasal flaring, chest retractions, head nodding and inability to feed or lethargy) and compared between hypoxaemic and non hypoxaemic. Sensitivity and specificity were calculated for this review; they are not presented by age group nor by disease severity. Results for sensitivity and specificity of clinical signs are for the global population |
ALRI: acute lower respiratory infections
MASL: metres above sea level
OR: odds ratio
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of subsequent hypoxaemia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1 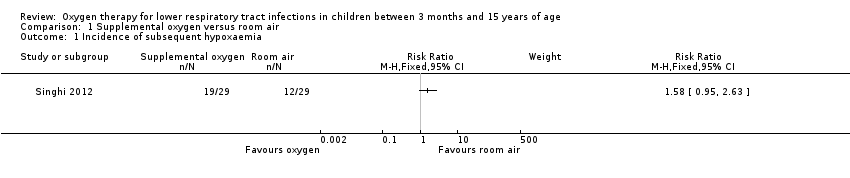 Comparison 1 Supplemental oxygen versus room air, Outcome 1 Incidence of subsequent hypoxaemia. | ||||
| 2 Duration of tachypnoea Show forest plot | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | 4.49 [‐16.30, 25.28] |
| Analysis 1.2  Comparison 1 Supplemental oxygen versus room air, Outcome 2 Duration of tachypnoea. | ||||
| 2.1 Normoxaemic children | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 6.00 [‐18.02, 30.02] |
| 2.2 Hypoxaemic children | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐41.48, 41.48] |
| 3 Duration of chest indrawing Show forest plot | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | 6.64 [‐10.77, 24.06] |
| Analysis 1.3  Comparison 1 Supplemental oxygen versus room air, Outcome 3 Duration of chest indrawing. | ||||
| 3.1 Normoxaemic children | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [‐13.65, 25.65] |
| 3.2 Hypoxaemic children | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 9.0 [‐28.58, 46.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Treatment failure to achieve adequate oxygenation Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Nasal prongs versus nasopharyngeal catheter, Outcome 1 Treatment failure to achieve adequate oxygenation. | ||||
| 1.1 Randomised clinical trials | 2 | 239 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.36, 2.38] |
| 1.2 Non‐randomised studies | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.44, 2.27] |
| 2 Oxygen required in the first 24 hours (litres per minute (L/min)) Show forest plot | 3 | 338 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.14, 0.29] |
| Analysis 2.2  Comparison 2 Nasal prongs versus nasopharyngeal catheter, Outcome 2 Oxygen required in the first 24 hours (litres per minute (L/min)). | ||||
| 3 Nasal obstruction/severe mucus production Show forest plot | 3 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.09, 0.44] |
| Analysis 2.3 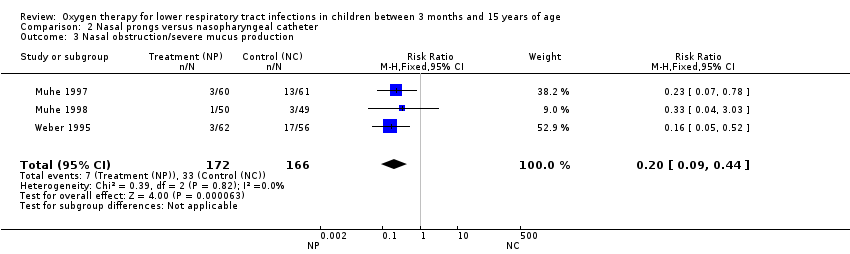 Comparison 2 Nasal prongs versus nasopharyngeal catheter, Outcome 3 Nasal obstruction/severe mucus production. | ||||
| 4 Nose ulceration or bleeding Show forest plot | 3 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.18, 1.02] |
| Analysis 2.4  Comparison 2 Nasal prongs versus nasopharyngeal catheter, Outcome 4 Nose ulceration or bleeding. | ||||
| 5 Fighting/discomfort in the first 24 hours Show forest plot | 2 | 239 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.46, 1.28] |
| Analysis 2.5  Comparison 2 Nasal prongs versus nasopharyngeal catheter, Outcome 5 Fighting/discomfort in the first 24 hours. | ||||
| 6 Death during treatment Show forest plot | 3 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.35, 1.15] |
| Analysis 2.6 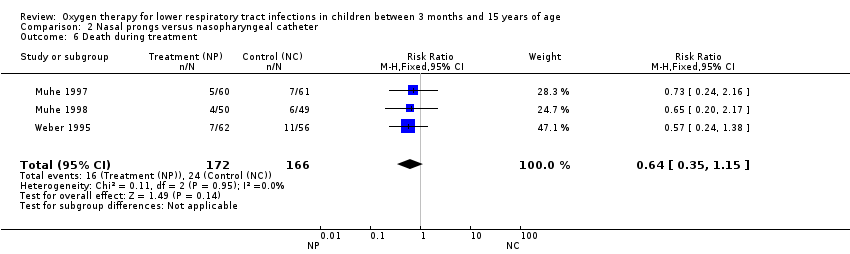 Comparison 2 Nasal prongs versus nasopharyngeal catheter, Outcome 6 Death during treatment. | ||||

Forest plot of comparison: 1 Supplemental oxygen versus room air, outcome: 1.1 Incidence of subsequent hypoxaemia.

Forest plot of comparison: 1 Supplemental oxygen versus room air, outcome: 1.2 Duration of tachypnoea.
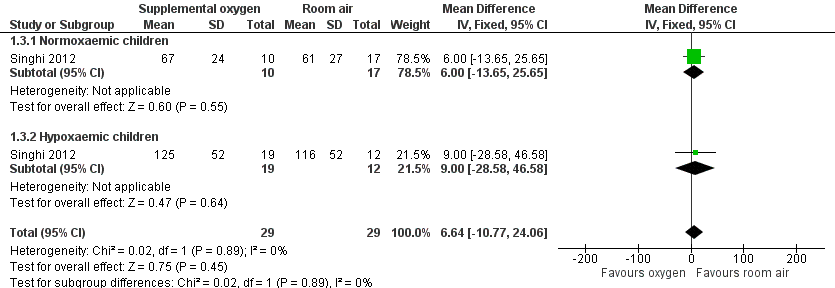
Forest plot of comparison: 1 Supplemental oxygen versus room air, outcome: 1.3 Duration of chest indrawing.

Forest plot of comparison: 2 Nasal prongs versus nasopharyngeal catheter, outcome: 2.1 Treatment failure to achieve adequate oxygenation.

Forest plot of comparison: 2 Nasal prongs versus nasopharyngeal catheter, outcome: 2.4 Nose ulceration or bleeding.

Forest plot of comparison: 2 Nasal prongs versus nasopharyngeal catheter, outcome: 2.6 Death during treatment.

Comparison 1 Supplemental oxygen versus room air, Outcome 1 Incidence of subsequent hypoxaemia.

Comparison 1 Supplemental oxygen versus room air, Outcome 2 Duration of tachypnoea.

Comparison 1 Supplemental oxygen versus room air, Outcome 3 Duration of chest indrawing.

Comparison 2 Nasal prongs versus nasopharyngeal catheter, Outcome 1 Treatment failure to achieve adequate oxygenation.
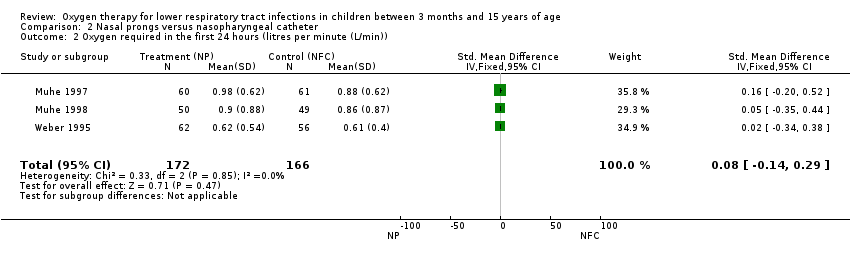
Comparison 2 Nasal prongs versus nasopharyngeal catheter, Outcome 2 Oxygen required in the first 24 hours (litres per minute (L/min)).

Comparison 2 Nasal prongs versus nasopharyngeal catheter, Outcome 3 Nasal obstruction/severe mucus production.

Comparison 2 Nasal prongs versus nasopharyngeal catheter, Outcome 4 Nose ulceration or bleeding.

Comparison 2 Nasal prongs versus nasopharyngeal catheter, Outcome 5 Fighting/discomfort in the first 24 hours.

Comparison 2 Nasal prongs versus nasopharyngeal catheter, Outcome 6 Death during treatment.
| Nasal prongs versus nasopharyngeal catheter for lower respiratory tract infections | ||||||
| Patient or population: children with acute lower respiratory tract infections | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Nasopharyngeal catheter | Nasal prongs | |||||
| Treatment failure | Study population | RR 0.97 | 399 | ⊕⊝⊝⊝ | ||
| 91 per 1000 | 89 per 1000 | |||||
| Moderate | ||||||
| 107 per 1000 | 104 per 1000 | |||||
| Oxygen required in the first 24 hours | The mean oxygen required in the first 24 hours in the intervention groups was | 338 | ⊕⊕⊝⊝ | SMD 0.08 (‐0.14 to 0.29) | ||
| Nasal obstruction/severe mucus production | Study population | RR 0.2 | 338 | ⊕⊕⊝⊝ | ||
| 199 per 1000 | 40 per 1000 | |||||
| Moderate | ||||||
| 213 per 1000 | 43 per 1000 | |||||
| Nose ulceration or bleeding | Study population | RR 0.43 | 338 | ⊕⊕⊝⊝ | ||
| 96 per 1000 | 41 per 1000 | |||||
| Moderate | ||||||
| 61 per 1000 | 26 per 1000 | |||||
| Fighting/discomfort in the first 24 hours | Study population | RR 0.77 | 239 | ⊕⊕⊝⊝ | ||
| 205 per 1000 | 158 per 1000 | |||||
| Moderate | ||||||
| 210 per 1000 | 162 per 1000 | |||||
| Death during treatment | Study population | RR 0.64 | 338 | ⊕⊕⊝⊝ | ||
| 145 per 1000 | 93 per 1000 | |||||
| Moderate | ||||||
| 122 per 1000 | 78 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1One trial used quasi‐randomised methods for assignment of interventions. Evaluation of the main outcome was not blinded in all studies. | ||||||
| Face mask compared to nasopharyngeal catheter for severe acute LRTIs in children | ||||||
| Patient or population: children with severe acute LRTIs | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Nasopharyngeal catheter | Face mask | |||||
| Treatment failure | Moderate | OR 0.20 | 80 | ⊕⊕⊝⊝ | ||
| 107 per 1000 | 23 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Non‐randomised. Used sequential assignment methods. | ||||||
| Head box compared to nasopharyngeal catheter for severe acute LRTIs in children | ||||||
| Patient or population: children with severe acute LRTIs | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Nasopharyngeal catheter | Head box | |||||
| Treatment failure | Moderate | OR 0.40 | 80 | ⊕⊝⊝⊝ | ||
| 107 per 1000 | 46 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Non‐randomised. Used sequential assignment methods. | ||||||
| Study | Adverse event | Oxygen delivery method |
| Pneumocephalus in an 8‐month old girl with severe staphylococcal pneumonia | Nasopharyngeal catheter | |
| Pneumocephalus and right side severe exophthalmos in a 11‐month old boy with bacterial pneumonia and sinusitis | Nasopharyngeal catheter |
| Study | Altitude | Hypoxaemia | Age | Sensitivity | Specificity | LR+ |
| 3750 MASL | SpO2 < 82% | 2 to 11 months | 13 | 99 | 13 | |
| 3750 MASL | SpO2 < 85% | > 11 months | 13 | 99 | 13 | |
| 1670 MASL | SpO2 < 91% | 3 to 11 months | 9 | 96 | 2.3 | |
| 1600 MASL | SpO2 < 86% | 1 month to 5 years | 42 | 84 | 2.6 | |
| Sea level | SpO2 < 90% | 2 months to 5 years | 39 | 100 | ||
| Sea level | SpO2 < 90% | 2 to 36 months | 25 | 95 | 5.0 | |
| 1600 MASL | SpO2 < 88% | 1 month to 5 years | 38 | 98 | 19.9 | |
| 239 MASL | SpO2 < 90% | < 5 years | 14 | 96 | 3.7 | |
| 35 MASL | SpO2 < 93% | 1 month to 5 years | 74 | 93 | 10.5 | |
| 35 MASL | SpO2 < 90% | 1 month to 5 years | 70 | 75 | 2.8 | |
| 1336 MASL | SpO2 < 90% | 2 month to 5 years | 5 | 100 | ||
| Sea level | SpO2 < 90% | 2 months to 5 years | 20 | 100 | 66.9 | |
| MASL: metres above sea level LR: likelihood ratio | ||||||
| Study | Altitude | Hypoxaemia | Age | Sensitivity | Specificity | LR+ |
| 1670 MASL | SpO2 < 91% | 3 to 11 months | 64 | 73 | 2.4 | |
| 1670 MASL | SpO2 < 91% | 12 to 36 months | 56 | 76 | 2.3 | |
| 2640 MASL | SpO2 < 88% | 7 days to 36 months | 45 | 72 | 1.6 | |
| 1600 MASL | SpO2 < 86% | 3 months to 5 years | 42 | 89 | 3.8 | |
| Sea level | SpO2 < 90% | 2 months to 5 years | 48 | 61 | 1.2 | |
| Sea level | SpO2 < 90% | 2 to 36 months | 46 | 86 | 3.3 | |
| 1600 MASL | SpO2 < 88% | 1 month to 5 years | 22 | 87 | 1.6 | |
| 239 MASL | SpO2 < 90% | < 5 years | 14 | 93 | 1.9 | |
| 35 MASL | SpO2 < 93% | 1 month to 5 years | 82 | 72 | 3.0 | |
| 35 MASL | SpO2 < 90% | 1 month to 5 years | 90 | 61 | 2.3 | |
| 1336 MASL | SpO2 < 90% | 2 months to 5 years | 36 | 99 | 32.9 | |
| Sea level | SpO2 < 90% | 2 months to 5 years | 60 | 77 | 2.66 | |
| MASL: metres above sea level LR: likelihood ratio | ||||||
| Study | Altitude | Hypoxaemia | Age | Sensitivity | Specificity | LR+ |
| 2640 MASL | SpO2 < 88% | 7 days to 36 months | 63 | 65 | 1.8 | |
| 1600 MASL | SpO2 < 86% | 3 months to 5 years | 56 | 84 | 3.5 | |
| Sea level | SpO2 < 90% | 2 months to 5 years | 71 | 54 | 1.5 | |
| Sea level | SpO2 < 90% | 2 to 36 months | 98 | 17 | 1.2 | |
| 35 MASL | SpO2 < 93% | 1 month to 5 years | 71 | 58 | 1.7 | |
| 1336 MASL | SpO2 < 90% | 2 months to 5 years | 48 | 98 | 22.0 | |
| MASL: metres above sea level LR: likelihood ratio | ||||||
| Study | Altitude | Hypoxaemia | Type of indrawing | Age | Sensitivity | Specificity | LR+ |
| 3750 MASL | SpO2 < 82% | Any chest retractions | 2 to 11 months | 35 | 94 | 5.8 | |
| 3750 MASL | SpO2 < 85% | Any chest retractions | > 11 months | 35 | 94 | ||
| 1670 MASL | SpO2 < 91% | Any retractions | 3 to 11 months | 97 | 29 | 1.4 | |
| 1670 MASL | SpO2 < 91% | Any retractions | > 11 months | 88 | 30 | 1.3 | |
| 2640 MASL | SpO2 < 88% | Intercostal | 7 days to 36 months | 79 | 55 | 1.8 | |
| 2640 MASL | SpO2 < 88% | Subcostal | 7 days to 36 months | 76 | 43 | 1.3 | |
| 2640 MASL | SpO2 < 88% | Any chest retractions | 7 days to 36 months | 83 | 40 | 1.4 | |
| 1600 MASL | SpO2 < 86% | Indrawing | 1 week to 5 years | 98 | 7 | 1.1 | |
| Sea level | SpO2 < 90% | Intercostal indrawing | 2 months to 5 years | 65 | 69 | 2.1 | |
| Sea level | SpO2 < 90% | Lower chest indrawing | 2 months to 5 years | 74 | 37 | 1.2 | |
| 43 MASL | SpO2 < 95% | Any chest retractions | 1 month to 5 years | 59 | 63 | 1.6 | |
| 239 MASL | SpO2 < 90% | Intercostal indrawing | < 5 years | 32 | 88 | 2.6 | |
| 239 MASL | SpO2 < 90% | Lower chest indrawing | < 5 years | 36 | 86 | 2.6 | |
| 1336 MASL | SpO2 < 90% | Chest indrawing | 2 months to 5 years | 69 | 83 | 4.0 | |
| MASL: metres above sea level LR: likelihood ratio | |||||||
| Study | Altitude | Hypoxaemia | Definition | Age | Sensitivity | Specificity | LR+ | LR‐ |
| 1670 MASL | SpO2 < 91% | Unresponsive | 3 to 11 months | 63 | 67 | 1.9 | 0.6 | |
| 1670 MASL | SpO2 < 91% | Unresponsive | > 11 months | 56 | 78 | 2.5 | 0.6 | |
| 2640 MASL | SpO2 < 88% | Difficult to awake/abnormal sleepiness | 7 days to 36 months | 12 | 89 | 1.1 | 1.0 | |
| 1600 MASL | SpO2 < 86% | Decrease of consciousness/restlessness | 3 months to 5 years | 36 | 91 | 4.0 | 0.7 | |
| Sea level | SpO2 < 91% | Arousal | 2 months to 5 years | 70 | 78 | 3.2 | 0.4 | |
| Sea level | SpO2 < 91% | Irritability | 2 months to 5 years | 41 | 43 | 0.7 | 1.4 | |
| Sea level | SpO2 < 91% | Difficult to awake/abnormal sleepiness | 2 months to 5 years | 42 | 78 | 1.9 | 0.7 | |
| Sea level | SpO2 < 90% | No spontaneous movement | 2 to 36 months | 46 | 84 | 2.9 | 0.6 | |
| 1600 MASL | SpO2 < 88% | Reduced activity | 1 month to 5 years | 44 | 69 | 1.4 | 0.8 | |
| 35 MASL | SpO2 < 93% | Drowsy | 1 month to 5 years | 85 | 83 | 7.3 | 0.4 | |
| 35 MASL | SpO2 < 90% | Drowsy | 1 month to 5 years | 68 | 91 | 5.0 | 0.2 | |
| 1336 MASL | SpO2 < 90% | Lethargy | 2 months to 5 years | 40 | 100 | 0.6 | ||
| MASL: metres above sea level LR: likelihood ratio | ||||||||
| Study | Altitude | Hypoxaemia | Age | Sensitivity | Specificity | LR+ |
| 1670 MASL | SpO2 < 91% | 3 to 11 months | 50 | 75 | 2.0 | |
| 1670 MASL | SpO2 < 91% | > 12 months | 40 | 71 | 1.4 | |
| 2640 MASL | SpO2 < 88% | 7 days to 36 months | 35 | 60 | 0.9 | |
| Sea level | SpO2 < 90% | 2 months to 5 years | 71 | 67 | 2.2 | |
| Sea level | SpO2 < 90% | 2 to 36 months | 33 | 91 | 3.7 | |
| 1600 MASL | SpO2 < 88% | 1 month to 5 years | 42 | 76 | 1.8 | |
| 1336 MASL | SpO2 < 90% | 2 months to 5 years | 28 | 99 | 28 | |
| Sea level | SpO2 < 90% | 2 months to 5 years | 9 | 94 | 1.39 | |
| MASL: metres above sea level LR: likelihood ratio | ||||||
| Study | Altitude | Hypoxaemia | Tachypnoea | Age | Sensitivity | Specificity | LR+ |
| 1670 MASL | SpO2 < 91% | > 60 r/min | 3 to 11 months | 86 | 56 | 2.0 | |
| 1670 MASL | SpO2 < 91% | > 70 r/min | 3 to 11 months | 51 | 83 | 3.0 | |
| 2640 MASL | SpO2 < 88% | > 50 r/min | 0 to 11 months | 76 | 71 | 2.6 | |
| 2640 MASL | SpO2 < 88% | > 60 r/min | 0 to 11 months | 40 | 86 | 2.9 | |
| 2640 MASL | SpO2 < 88% | > 70 r/min | 0 to 11 months | 16 | 100 | ||
| 43 MASL | SpO2 < 95% | > 50 r/min | 2 to 11 months | 64 | 56 | 1.5 | |
| 239 MASL | SpO2 < 90% | > 50 r/min | 4 to 12 months | 89 | 24 | 1.2 | |
| 239 MASL | SpO2 < 90% | > 60 r/min | 4 to 12 months | 82 | 52 | 1.7 | |
| 239 MASL | SpO2 < 90% | > 70 r/min | 4 to 12 months | 54 | 78 | 2.5 | |
| 1336 MASL | SpO2 < 90% | > 50 r/min | 2 to 12 months | 90 | 44 | 1.6 | |
| MASL: metres above sea level LR: likelihood ratio | |||||||
| Study | Altitude | Hypoxaemia | Tachypnoea | Age | Sensitivity | Specificity | LR+ |
| 1670 MASL | SpO2 < 91% | > 60 r/min | 12 to 36 months | 65 | 76 | 2.7 | |
| 1670 MASL | SpO2 < 91% | > 70 r/min | 12 to 36 months | 32 | 90 | 3.2 | |
| 2640 MASL | SpO2 < 88% | > 50 r/min | 12 to 36 months | 39 | 71 | 1.3 | |
| 2640 MASL | SpO2 < 88% | > 60 r/min | 12 to 36 months | 12 | 100 | ||
| 2640 MASL | SpO2 < 88% | > 70 r/min | 12 to 36 months | 4 | 100 | ||
| 43 MASL | SpO2 < 95% | > 40 r/min | 12 months to 5 years | 64 | 56 | 1.4 | |
| 239 MASL | SpO2 < 90% | > 40 r/min | 12 months to 5 years | 89 | 24 | 1.2 | |
| 239 MASL | SpO2 < 90% | > 50 r/min | 12 months to 5 years | 82 | 52 | 1.7 | |
| 239 MASL | SpO2 < 90% | > 60 r/min | 12 months to 5 years | 54 | 78 | 2.5 | |
| 1336 MASL | SpO2 < 90% | > 40 r/min | 13 months to 5 years | 100 | 43 | 1.8 | |
| MASL: metres above sea level LR: likelihood ratio | |||||||
| Study | Altitude | Hypoxaemia | Age | Sensitivity | Specificity | LR+ | LR‐ |
| 3750 MASL | SpO2 < 82% | 2 to 11 months | 50 | 92 | 6.3 | 0.5 | |
| 1670 MASL | SpO2 < 91% | 3 to 11 months | 77 | 40 | 1.3 | 0.6 | |
| 1670 MASL | SpO2 < 91% | 12 to 36 months | 91 | 36 | 1.4 | 0.3 | |
| 2640 MASL | SpO2 < 88% | 7 days to 36 moths | 79 | 53 | 1.7 | 0.4 | |
| 1600 MASL | SpO2 < 86% | 3 months to 5 years | 90 | 16 | 1.1 | 0.6 | |
| Sea level | SpO2 < 90% | 2 months to 5 years | 93 | 12 | 1.1 | 0.6 | |
| Sea level | SpO2 < 90% | 2 to 36 months | 86 | 30 | 1.2 | 0.5 | |
| 239 MASL | SpO2 < 90% | < 5 years | 68 | 68 | 2.1 | 0.5 | |
| 1336 MASL | SpO2 < 90% | 2 months to 5 years | 93 | 22 | 1.2 | 0.3 | |
| MASL: metres above sea level LR: likelihood ratio | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of subsequent hypoxaemia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Duration of tachypnoea Show forest plot | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | 4.49 [‐16.30, 25.28] |
| 2.1 Normoxaemic children | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 6.00 [‐18.02, 30.02] |
| 2.2 Hypoxaemic children | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐41.48, 41.48] |
| 3 Duration of chest indrawing Show forest plot | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | 6.64 [‐10.77, 24.06] |
| 3.1 Normoxaemic children | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [‐13.65, 25.65] |
| 3.2 Hypoxaemic children | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 9.0 [‐28.58, 46.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Treatment failure to achieve adequate oxygenation Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Randomised clinical trials | 2 | 239 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.36, 2.38] |
| 1.2 Non‐randomised studies | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.44, 2.27] |
| 2 Oxygen required in the first 24 hours (litres per minute (L/min)) Show forest plot | 3 | 338 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.14, 0.29] |
| 3 Nasal obstruction/severe mucus production Show forest plot | 3 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.09, 0.44] |
| 4 Nose ulceration or bleeding Show forest plot | 3 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.18, 1.02] |
| 5 Fighting/discomfort in the first 24 hours Show forest plot | 2 | 239 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.46, 1.28] |
| 6 Death during treatment Show forest plot | 3 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.35, 1.15] |

