止血剂治疗急性自发性脑出血
Abstract
研究背景
自发性(非外伤性)脑出血(ICH)的预后受血肿体积的影响;多达三分之一的ICHs在发病24小时内扩大。早期止血治疗可能通过限制血肿生长来改善预后。这是2006年首次发布的Cochrane系统综述的更新,最近更新于2009年。
研究目的
为检查1)对于急性自发性脑内出血的成人,与安慰剂或开放式对照相比,各类止血疗法的有效性和安全性,以及2) ICH发病前根据抗血栓药物的类型立即采取各类止血疗法的效果(即抗凝血剂,抗血小板药或无)。
检索策略
我们检索了2017年第11期的Cochrane脑卒中小组试验注册库( Cochrane Stroke Trials Register),CENTRAL; 并于2017年11月27日检索了MEDLINE Ovid,Embase Ovid。为了进一步确定已发表、正在进行和未发表的随机对照试验(RCT),我们筛查了相关文章的参考文献,并在2017年11月检索了国际注册的RCT。
标准/纳入排除标准
我们寻找急性自发性ICH的任何止血干预(即促凝治疗,如凝血因子,抗纤维蛋白溶解药物或血小板输注),与安慰剂对照,开放对照或活性对照,并报告相关临床结局指标的随机对照试验(RCTs)。
数据收集与分析
两位作者独立提取数据资料,评估偏倚风险,若已发表的RCT报告中未提供具体数据,则与相应的RCT相关作者联系。
主要结果
我们纳入了12项RCT,涉及1732名受试者。有7项RCTs是凝血因子与安慰剂或开放对照比较,涉及1480名受试者,3项抗纤维蛋白溶解药物与安慰剂或开放对照的RCT,涉及57名受试者,1项血小板输注与开放对照的RCT,涉及190名受试者,以及1项血液凝固因子与新鲜冰冻血浆比较,涉及5名受试者。我们无法纳入两项符合条件的随机对照试验,因为它们提供了成人ICH和其他类型颅内出血的综合数据。我们识别了10篇进行中的试验。在12项纳入随机对照试验的所有7项标准中,偏倚风险分别为不清楚37(44%),高16(19%)和低31(37%)。在所有标准中,只有一项RCT的偏倚风险低。
在一项与抗血小板药物使用相关的急性自发性ICH的血小板输注与开放对照的RCT中,血小板输注组在第90天死亡或依赖(改良Rankin量表评分4‐6)显着增加(70/97对比52/93;风险比(RR)=1.29,95%置信区间(CI)=1.04‐1.61,一项试验,190名受试者,中等质量证据)。对于有或无手术的急性自发性ICH的凝血因子与安慰剂对照或开放式对照(中等质量证据),对于急性自发性ICH抗纤维蛋白溶解药物与安慰剂对照(中等质量证据)或开放对照(中等质量证据),以及对于与抗凝血药物相关的急性自发性ICH的凝血因子与新鲜冷冻血浆对照(无证据),所有发现均无显着性。
作者结论
根据一项试验的中等质量证据,与抗血小板相关的ICH成人的标准治疗相比,血小板输注似乎是危险的。
我们无法得出关于治疗急性自发性ICH(有或没有手术)的凝血因子,治疗急性自发性ICH的抗纤维蛋白溶解药物,以及与新鲜冰冻血浆相比,治疗与抗凝血药物使用相关的急性自发性ICH的的凝血因子的效力和安全性的肯定结论。
有必要进行进一步的随机对照试验,我们有兴趣等待10项正在进行的随机对照试验的结果。
PICO
Plain language summary
有助于血液凝固的治疗,以改善因脑出血引起成人中风的康复。
系统综述问题
有助于血液凝块的治疗可以减少因脑部出血引起的中风成人死亡和残疾的风险吗?
研究背景
超过十分之一的中风是由大脑出血引起的(称为脑出血)。出血越多,致死的可能性越大。大约三分之一的脑出血在最初的24小时内出血范围显著扩大。因此,如果在出血开始后立即给予促进血凝的治疗,则可以通过限制其范围扩大来降低脑出血后死亡或致残的风险。然而,止血药物可能会导致不必要的凝血,导致不必要的副作用,如心脏病和腿静脉血栓。
研究特征
截至2017年11月,我们发现了12项随机对照试验,纳入1732名受试者。
主要结果
我们发现中等质量的证据显示,直到使用抗血小板药物的患者脑出血,血小板输注才是有害的。我们没有发现证据证明其他止血疗法对脑出血患者有任何益处或伤害。
证据质量
总的来说,证据的质量从中到低。
目前正在进行的10项试验将提供更多信息。
Authors' conclusions
Summary of findings
| Blood clotting factors compared with placebo or open control for acute spontaneous intracerebral haemorrhage | ||||
| Patient or population: adults with acute spontaneous intracerebral haemorrhage Settings: secondary care Intervention: recombinant activated factor VII Comparison: placebo or open control | ||||
| Outcomes | Relative effect | No of Participants | Quality of the evidence | Comments |
| Death or dependence (mRS 4 to 6) at day 90 | RR 0.87 (0.70 to 1.07) | 1390 | ⊕⊕⊕⊝ | |
| GRADE Working Group grades of evidence | ||||
| CI: confidence interval; RR: risk ratio; mRS: modified Rankin Scale ¹We downgraded the quality of the evidence once because of imprecision. | ||||
| Antifibrinolytic drugs compared with placebo for acute spontaneous intracerebral haemorrhage | ||||
| Patient or population: adults with acute spontaneous intracerebral haemorrhage Settings: secondary care Intervention: tranexamic acid Comparison: placebo | ||||
| Outcomes | Relative effect | No of Participants | Quality of the evidence | Comments |
| Death or dependence (mRS 4 to 6) at day 90 | RR 1.25 (0.57 to 2.75) | 24 | ⊕⊕⊕⊝ | |
| GRADE Working Group grades of evidence | ||||
| CI: confidence interval; RR: risk ratio; mRS: modified Rankin Scale ¹We downgraded the quality of the evidence once because of imprecision. | ||||
| Platelet transfusion compared with open control for acute spontaneous intracerebral haemorrhage associated with antiplatelet drug use | ||||
| Patient or population: adults with acute spontaneous intracerebral haemorrhage associated with antiplatelet drug use Settings: secondary care Intervention: platelet transfusion Comparison: open control | ||||
| Outcomes | Relative effect | No of Participants | Quality of the evidence | Comments |
| Death or dependence (mRS 4 to 6) at day 90 | RR 1.29 (1.04 to 1.61) | 190 | ⊕⊕⊕⊝ | |
| GRADE Working Group grades of evidence | ||||
| CI: confidence interval; RR: risk ratio; mRS: modified Rankin Scale ¹We downgraded the quality of the evidence once because of imprecision. | ||||
| Blood clotting factors compared with fresh frozen plasma for acute spontaneous intracerebral haemorrhage associated with anticoagulant drug use | ||||
| Patient or population: adults with acute spontaneous intracerebral haemorrhage associated with anticoagulant drug use Settings: secondary care Intervention: fresh frozen plasma (intravenous), vitamin K (subcutaneous), and factor IX complex concentrate Comparison: fresh frozen plasma (intravenous) | ||||
| Outcomes | Relative effect | No of Participants | Quality of the evidence | Comments |
| Death or dependence (mRS 4 to 6) at day 90 | No evidence | No evidence as mRS not measured | ||
| GRADE Working Group grades of evidence | ||||
| CI: confidence interval | ||||
Background
Description of the condition
The burden of haemorrhagic stroke is out of proportion to its frequency as a subtype of stroke. Although haemorrhagic stroke causes 11% to 22% of all new strokes (Feigin 2009), it caused 50% of all stroke deaths, and approximately 42% (47 million) of the disability‐adjusted life years lost due to stroke in the 2013 Global Burden of Disease study (Feigin 2015). Spontaneous (non‐traumatic) intracerebral haemorrhage (ICH) accounts for two‐thirds of haemorrhagic stroke, amounting to more than two million strokes per year (Al‐Shahi Salman 2009a). ICH is due to cerebral small vessel disease, and may be associated with the use of antithrombotic (i.e. anticoagulant or antiplatelet) drugs. The age‐specific incidence of ICH – like the age‐specific incidence of ischaemic stroke and myocardial infarction – rises with age (Feigin 2015). Two‐thirds of people who have an ICH are 75 years or older (Lovelock 2007; Samarasekera 2015). Given that the number of people aged 75 years and older is projected to rise in many parts of the world, the burden due to ICH incidence, recurrence, and prevalence will rise.
Outcome after stroke due to ICH is poor: one‐year survival is 46% (95% confidence interval (CI) 43% to 49%), five‐year survival is 29% (95% CI 26% to 33%), and predictors most consistently associated with death are increasing age, decreasing Glasgow Coma Scale score, increasing ICH volume, presence of intraventricular haemorrhage, and deep or infratentorial ICH location (Poon 2014). Approximately one‐third of acute ICHs (i.e. less than 24 hours after symptom onset or time last seen well) enlarge from three to 24 hours after onset (Brott 1997); ICH growth is independently associated with death and poor outcome (Davis 2006).
Description of the intervention
The three main components of haemostasis (the process that stops bleeding) are vasoconstriction, platelet plug formation, and coagulation.
Vascular smooth muscle cell vasoconstriction is a blood vessel's first response to injury. They constrict the damaged vessels, which reduces the amount of blood flow through the area and limits the amount of blood loss. Collagen is exposed at the site of injury, which causes platelets to adhere to the injury site.
Primary haemostasis is achieved by platelets, which adhere to damaged endothelium to form a platelet plug. Plug formation is activated by the Von Willebrand factor, which is found in plasma. When platelets are activated, they express glycoprotein receptors that interact with other platelets, producing aggregation and adhesion. Platelets release cytoplasmic granules that contain serotonin, adenosine diphosphate (ADP), and thromboxane A2, all of which increase the effect of vasoconstriction. ADP attracts more platelets to the affected area, serotonin is a vasoconstrictor, and thromboxane A2 assists in platelet aggregation, vasoconstriction, and degranulation.
Secondary haemostasis starts once a platelet plug has been formed. Blood plasma clotting factors are activated in a sequence of events known as the coagulation cascade. Inactive fibrinogen produces a fibrin mesh around the platelet plug to hold it in place. Red and white blood cells are trapped in the mesh to produce a thrombus, or clot. Tissue factor (also called platelet tissue factor, factor III, or thromboplastin) is a protein that is exposed after endothelial damage, which leads to thrombin formation and activation of the tissue factor pathway, as well as its circulating ligand factor VII.
Therapies that intervene in primary haemostasis (e.g. platelet transfusion) or secondary haemostasis (e.g. administration of clotting factors (fresh frozen plasma (FFP), or prothrombin complex concentrate (PCC), or antifibrinolytic drugs) might promote clot formation, decrease bleeding, and thus improve outcomes by limiting ICH growth.
How the intervention might work
Theoretically, early interventions to reduce acute ICH volume might improve outcomes. Although surgical craniotomy to evacuate spontaneous supratentorial ICH and reduce ICH volume was found to reduce the odds of dying or becoming dependent compared with medical management alone, the result was not very robust, and surgical evacuation is not frequently used (Prasad 2008). Therefore, medical (non‐surgical) interventions to promote haemostasis, and limit haematoma growth, have become the main focus of acute ICH therapeutic research.
Why it is important to do this review
Various haemostatic therapies have been investigated in a variety of spontaneous bleeding conditions with little evidence for their effectiveness in some settings (Johansen 2015: Stanworth 2012; Wikkelsø 2013), but clear benefit in others (Ker 2015). It is unclear whether they are effective for acute ICH. Therefore, we systematically reviewed the literature for randomised controlled trials (RCTs) of all potential haemostatic therapies for the treatment of acute spontaneous ICH.
Objectives
To examine 1) the effectiveness and safety of individual classes of haemostatic therapies, compared against placebo or open control, in adults with acute spontaneous intracerebral haemorrhage (ICH), and 2) the effects of each class of haemostatic therapy according to the type of antithrombotic drug taken immediately before ICH onset (i.e. anticoagulant, antiplatelet, or none).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials, whether published or unpublished, regardless of the language of publication. Pseudo‐randomised studies were not eligible.
Types of participants
People of any sex, aged 16 years or older. We restricted this review to people with radiographically‐confirmed acute spontaneous intracerebral haemorrhage (ICH). Where possible, we grouped RCTs, or participant subgroups, by whether ICH was associated with antiplatelet drug use, anticoagulant drug use, or neither.
Types of interventions
Single or multiple haemostatic therapies (including antifibrinolytic drugs, blood coagulation factors, antidotes to specific antithrombotic drugs, platelet transfusion, or other platelet activation therapies), regardless of dosage or route of administration. Interventions could be compared against placebo, open control, or an active comparator.
Types of outcome measures
We assessed the following outcomes at 90 days after randomization (or at the end of scheduled follow‐up, if not provided at 90 days).
Primary outcomes
-
Death or dependence from any cause (measured on a standard rating scale, such as the modified Rankin Scale)
Secondary outcomes
-
All serious adverse events (if possible, with a separate analysis of arterial and venous thromboembolic events, including deep vein thrombosis symptomatic pulmonary embolism, arterial embolism, myocardial infarction, ischaemic stroke, and disseminated intravascular coagulation)
-
Change in volume of ICH on follow‐up brain imaging
-
Death from any cause (categorised into early (e.g. less than seven days) and late (e.g. between seven days and the end of follow‐up) if possible)
-
Quality of life
-
Mood
-
Cognitive function
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. We searched for trials in all languages, and arranged for the translation of relevant articles when necessary.
Electronic searches
The Cochrane Stroke Group Information Specialist searched the Cochrane Stroke Group Trials Register (last searched 27 November 2017), the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 11) in the Cochrane Library (searched 27 November 2017; Appendix 1); MEDLINE Ovid (1946 to 27 November 2017; Appendix 2); and Embase Ovid (1974 to 27 November 2017; Appendix 3).
One review author (ZL) also searched the following international registers of RCTs on 27 November 2017.
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov) using the search strategy in Appendix 4;
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP; apps.who.int/trialsearch) using the search strategy in Appendix 5.
Searching other resources
In an effort to identify further published, ongoing, and unpublished RCTs, three authors (RA‐SS, NS, and ZL) scanned bibliographies of relevant articles.
Data collection and analysis
Selection of studies
Two authors independently checked the titles and abstracts of studies identified by the search strategy for RCTs meeting the selection criteria for the first version of this review (You 2006). Two authors (ZL or RA‐SS) independently screened the results of the updated searches for potentially eligible studies for this updated review, and obtained the full published articles or trial registry entries for studies likely to be relevant RCTs. Two authors (NS and RA‐SS) independently read these potentially eligible RCTs in full, and confirmed their inclusion according to the inclusion criteria.
Data extraction and management
Two authors (NS and RA‐SS) used a standard data collection form to independently extract data on risk of bias, other RCT characteristics, participants, methods, interventions, outcomes, and results. If necessary, we sought additional data from the principal investigators of RCTs that met, or potentially met the inclusion criteria. We sought unpublished data that were not quantified in the original publications, or not presented as stratified by intracranial haemorrhage type, from the principal investigators and pharmaceutical companies. In the one study for which these data were not forthcoming, RA‐SS measured the numbers in the relevant groups in the stacked bar charts, using Adobe Acrobat Professional measuring tools on the PDF of the published study (Mayer 2008 (FAST)). In one phase II study, we could obtain only limited data from the Novo Nordisk website (F7ICH‐1602 2007).
Assessment of risk of bias in included studies
Two authors (NS and RA‐SS) independently assessed included RCTs' risk of bias, according to the seven criteria of the Cochrane 'Risk of bias' tool (Higgins 2011). The same two authors discussed and agreed on the overall quality of the evidence for each outcome, using the GRADE approach (Higgins 2011).
Measures of treatment effect
We calculated risk ratio (RR) for dichotomous data, and mean difference (MD), or standardized mean difference (SMD) for different measures of the same outcome, for continuous data.
Dealing with missing data
We sought missing data from studies' corresponding authors, and used all the data that were available to us.
Assessment of heterogeneity
We estimated inconsistency between RCTs using the I² statistic.
Assessment of reporting biases
We used funnel plots to assess publication bias where there were sufficient data.
Data synthesis
We used a random‐effects model (because we expected studies of different drugs and doses to estimate different, yet related, treatment effects) to calculate RRs and 95% confidence intervals (CIs), with the inverse variance method.
Subgroup analysis and investigation of heterogeneity
We expected to find that the choice of intervention and comparator would be largely determined by the use and type of antithrombotic drug taken prior to a spontaneous acute ICH (e.g. fresh frozen plasma (FFP), or prothrombin complex concentrate (PCC) for anticoagulant‐associated ICH, platelet transfusion for antiplatelet‐associated ICH). However, where interventions were used for ICH, whether it was associated with antithrombotic drug use or not (e.g. the clotting factor recombinant activated factor VII, antifibrinolytic drugs), and where ICH evacuation using craniotomy was performed, we stratified our analyses by pre‐ICH antithrombotic drug use and use of surgery.
Results
Description of studies
Results of the search
Our searches identified 31 potentially eligible randomised controlled trials (RCT); see Figure 1 for the flowchart describing the searches done for this update. We excluded nine studies because: they never started (Ciccone 2007; NCT02429453), they did not report any of our primary or secondary outcomes (Meng 2003; Zhou 2005), they did not report data and outcomes in the intracerebral haemorrhage population (Kerebel 2013; Steiner 2016 (INCH)), they did not quantify outcomes (Madjdinasab 2008), the study was stopped due to poor enrolment but never reported results (NCT00222625), or the study did not relate to a haemostatic therapy (Li 2016). Ten RCTs were still in the process of recruitment (Liu 2017 (TRAIGE); Meretoja 2014 (STOP‐AUST); NCT00699621; NCT02777424; NCT02866838; NCT03044184; NOR‐ICH), follow‐up (Sprigg 2016 (TICH‐2)), or reporting (NCT00810888; NCT01359202) at the time of writing, and will be assessed for inclusion with the next update. This left 12 RCTs that we included in this review, all of which included acute intracerebral haemorrhage (ICH) in adults aged 18 years or older (Arumugam 2015; Baharoglu 2016 (PATCH); Boulis 1999; F7ICH‐1602 2007; Imbert 2012 (PRE‐SICH); Li 2012; Mayer 2005a; Mayer 2005b; Mayer 2006; Mayer 2008 (FAST); Sprigg 2014 (TICH‐1); Zazulia 2001 (ATICH)).
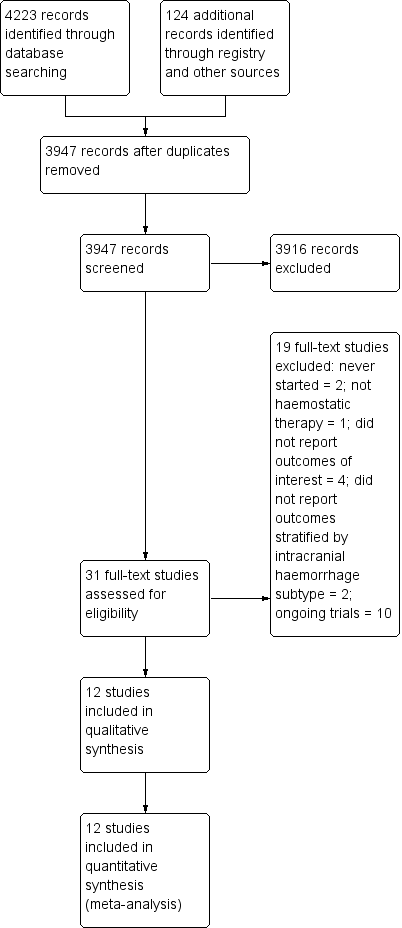
Study flow diagram
We included seven RCTs of blood clotting factors versus placebo or open control involving 1480 participants (F7ICH‐1602 2007; Imbert 2012 (PRE‐SICH); Li 2012; Mayer 2005a; Mayer 2005b; Mayer 2006; Mayer 2008 (FAST)), three RCTs of antifibrinolytic drugs versus placebo or open control involving 57 participants (Arumugam 2015; Sprigg 2014 (TICH‐1); Zazulia 2001 (ATICH)), one RCT of platelet transfusion versus open control involving 190 participants (Baharoglu 2016 (PATCH)), and one RCT of blood clotting factors versus fresh frozen plasma involving five participants (Boulis 1999).
Included studies
We included 12 RCTs. For details, please refer to the 'Characteristics of included studies' table.
Of the seven RCTs of blood clotting factors versus placebo or open control, six examined blood clotting factors in adults with acute spontaneous ICH (F7ICH‐1602 2007; Li 2012; Mayer 2005a; Mayer 2005b; Mayer 2006; Mayer 2008 (FAST)), and one in adults with acute spontaneous ICH undergoing craniotomy (Imbert 2012 (PRE‐SICH)). Five studies were funded and conducted by Novo Nordisk, and compared the use of various doses of intravenous recombinant activated clotting factor VII (rFVIIa; (973 participants)) against placebo (422 participants), started within four hours of ICH onset in adults (F7ICH‐1602 2007; Mayer 2005a; Mayer 2005b; Mayer 2006; Mayer 2008 (FAST)). Novo Nordisk supplied supplementary, unpublished data from three trials (Mayer 2005a; Mayer 2005b; Mayer 2006), but did not respond to several requests to provide further data from two trials (F7ICH‐1602 2007; Mayer 2008 (FAST)). The other study of intravenous rFVIIa was performed independently of Novo Nordisk, as a single centre, phase II study, in adults with acute spontaneous ICH within six hours of ICH onset (Li 2012). We had not pre‐specified the exclusion of RCTs of haemostatic therapies following surgical intervention, so we included Imbert 2012 (PRE‐SICH), which was a phase II study of intravenous rFVIIa in adults with acute spontaneous ICH undergoing craniotomy, administered immediately after the evacuation of the haematoma, within 24 hours of ICH onset.
Of the three RCTs of antifibrinolytic drugs versus placebo or open control, Zazulia 2001 (ATICH) was a phase II RCT of intravenous aminocaproic acid compared against supportive treatment alone, started within three hours of ICH onset in adults. Dr Allyson Zazulia provided unpublished data, because the trial was stopped after the enrolment of three participants because recruitment had been slow, and the investigators decided that the rationale for rFVIIa was better (Zazulia 2005). Sprigg 2014 (TICH‐1) was a phase II RCT of intravenous tranexamic acid compared against supportive treatment alone, started within 24 hours of ICH onset in adults. Arumugam 2015 was a phase II RCT of intravenous tranexamic acid compared against supportive treatment alone, started within eight hours of ICH onset in adults.
We found one RCT of platelet transfusion versus open control involving 190 participants (Baharoglu 2016 (PATCH)).
We found one RCT of blood clotting factors versus fresh frozen plasma involving five participants with acute spontaneous ICH associated with anticoagulant drug use (Boulis 1999).
Excluded studies
We excluded nine studies. For details, please refer to the 'Characteristics of excluded studies' table. We excluded two eligible RCTs because they presented aggregate data for adults with ICH as well as other types of intracranial haemorrhage, and the study authors could not provide data restricted to the ICH group alone by the time this review was submitted (Kerebel 2013; Steiner 2016 (INCH)). One abstract proposed a RCT of tranexamic acid for ICH, but the corresponding author confirmed that funding had not been obtained (Ciccone 2007). We found two studies of aprotinin, but it was unclear whether they included some participants in both studies, and the outcome measures used were unsuitable for meta‐analysis in this review (Meng 2003; Zhou 2005). NCT02429453 was a planned RCT of fresh frozen plasma (FFP) versus prothrombin complex concentrate (PCC), but was terminated before enrolment began. NCT00222625 was a study of coagulation factors, but it was "stopped due to slow recruitment" (Iorio 2012). Dr Iorio has not responded to requests for clarification about whether any data were collected. Madjdinasab 2008 was a study of coagulation factors, but no results were reported, and there was no response from the authors to requests for information for this review. Finally, Li 2016 was excluded as the intervention (TRABC) did not appear to be haemostatic, and had four Traditional Chinese Medicine (TCM) medicinals.
We identified 10 ongoing or recently completed but unreported RCTs. See 'Characteristics of ongoing studies' table for details. Two RCTs examined blood clotting factors versus placebo or open control (NCT00810888; NCT01359202), five examined antifibrinolytic drugs versus placebo or open control in acute spontaneous ICH (Liu 2017 (TRAIGE); Meretoja 2014 (STOP‐AUST); NCT03044184; NOR‐ICH; Sprigg 2016 (TICH‐2)); one RCT examined antifibrinolytic drugs versus placebo or open control in acute spontaneous ICH associated with anticoagulant drug use (NCT02866838); one RCT examined platelet transfusion versus open control (NCT00699621), and one RCT examined blood clotting factors versus fresh frozen plasma in acute spontaneous ICH associated with anticoagulant drug use (NCT02777424).
Risk of bias in included studies
Across all seven domains in the 12 included RCTs, we assessed that the risk of bias, according to the Cochrane 'Risk of bias' tool, and guidance in the Cochrane Handbook for Systematic Reviews of Interventions, was unclear in 37 (44%), high in 16 (19%), and low in 31 (37%; (Figure 2; Figure 3; Higgins 2011)). Only one RCT was at low risk of bias in all domains (Sprigg 2014 (TICH‐1)).

'Risk of bias' summary: review authors' judgements about each risk of bias criteria for each included study

Risk of bias graph: review authors' judgements about each risk of bias criteria presented as percentages across all included studies
Allocation
The risk of bias in random sequence generation was low in two RCTs, unclear in 10 RCTs, and high in none. The risk of bias in allocation concealment was low in two RCTs, unclear in nine RCTs, and high in one.
Only four RCTs clearly described the method of randomization (Baharoglu 2016 (PATCH); Mayer 2005b; Sprigg 2014 (TICH‐1); Zazulia 2001 (ATICH)), and one RCT simply mentioned 'block randomization according to site' (Mayer 2008 (FAST)).
Allocation was reported as being concealed in the papers for Baharoglu 2016 (PATCH) and Sprigg 2014 (TICH‐1), there was a risk of unblinding in one study (Mayer 2008 (FAST)), and the rest were unclear about allocation concealment.
It became apparent during questioning after the presentation of the Mayer 2008 (FAST) data at the European Stroke Conference in Glasgow, UK, in 2007 (Mayer 2007), that the imbalance in allocation between the three groups in this trial (there were approximately 30 more participants analysed in the 80 mcg/kg dose group than the other two groups) was due to the 80 mcg/kg dose of rFVIIa tending to be packed in the first of the three boxes of study drug for part of the trial (which might have unblinded investigators, in view of the preponderance of thromboembolic adverse events with the higher dose).
Blinding
Five of the 12 RCTs did not blind the intervention and comparator (Arumugam 2015; Baharoglu 2016 (PATCH); Boulis 1999; Imbert 2012 (PRE‐SICH); Zazulia 2001 (ATICH)), four did blind intervention and comparator, and the risk of bias was unclear in three RCTs. Whether participants and personnel were blinded to treatment allocation was only explicitly stated in Baharoglu 2016 (PATCH), but correspondence with the study authors verified that this was the case for Mayer 2006; Zazulia 2001 (ATICH), and Sprigg 2014 (TICH‐1). The risk of bias in the remaining RCTs was unclear in three RCTs, and high in five RCTs.
Risk of bias from blinding of outcome assessment was low in five RCTs, high in one RCT, and unknown in six RCTs. Assessment of radiological outcome was blinded to treatment allocation in nine included RCTs (Arumugam 2015; Baharoglu 2016 (PATCH); Imbert 2012 (PRE‐SICH); Mayer 2005a; Mayer 2005b; Mayer 2006; Mayer 2008 (FAST); Sprigg 2014 (TICH‐1); Zazulia 2001 (ATICH)), but information was not provided in others.
Incomplete outcome data
Overall, the risk of bias from incomplete outcome data was low in six RCTs, high in two RCTs, and unclear in the remaining four RCTs. Only Baharoglu 2016 (PATCH); Mayer 2008 (FAST), and Sprigg 2014 (TICH‐1) provided data about completeness of clinical follow‐up. In Arumugam 2015 and Li 2012, four participants had missing data. Boulis 1999 excluded eight participants after randomization. The last‐observation‐carried‐forward technique was used in Mayer 2005b and Mayer 2008 (FAST), which is likely to be unbiased only if the completeness of follow‐up was high.
Selective reporting
Bias from selective outcome reporting was low in eight RCTs, appeared to be low in two RCTs, and high in two other RCTs.
Other potential sources of bias
There were differences in baseline characteristics between treatment and control arms in the rFVIIa RCTs, especially in Mayer 2008 (FAST): 90‐day case fatality was worse in the placebo group in Mayer 2005b (29%) than in Mayer 2008 (FAST) (19%), which might be one explanation for the difference between the RCTs' findings. There was no visual evidence of funnel plot asymmetry in the largest group of RCTs, which compared blood clotting factors versus placebo or open control for death or dependency (Figure 4).

Funnel plot of comparison: 1 Blood clotting factors vs placebo or open control, outcome: 1.1 Death or dependence (mRS 4 to 6) at day 90
Effects of interventions
See: Summary of findings for the main comparison Blood clotting factors versus placebo or open control; Summary of findings 2 Antifibrinolytic drugs versus placebo; Summary of findings 3 Platelet transfusion versus open control; Summary of findings 4 Blood clotting factors versus fresh frozen plasma
We analysed data on intervention effects In 12 RCTs involving 1732 participants (1150 allocated to intervention and 582 allocated to control or active comparator), split by type of intervention as follows.
Blood clotting factors versus placebo or open control
In RCTs of blood clotting factors (N = 1018) versus placebo or open control (N = 462) for acute spontaneous ICH with or without surgery, use of blood clotting factors led to non‐significant reductions in death or dependence (risk ratio (RR) 0.87, 95% confidence interval (CI) 0.70 to 1.07; six trials, 1390 participants; moderate‐quality evidence; Analysis 1.1); in death (RR 0.75, 95% CI 0.51 to 1.09; seven trials, 1480 participants; moderate‐quality evidence; Analysis 1.3); in all serious adverse events (RR 0.81, 95% CI 0.30 to 2.22; two trials, 87 participants; moderate‐quality evidence; Analysis 1.4); and in ICH growth (RR 0.74, 95% CI 0.36 to 1.48; three trials, 151 participants; moderate‐quality evidence; Analysis 1.6). Blood clotting factors resulted in a non‐significant increase in all serious thromboembolic serious adverse events (RR 1.24, 95% CI 0.80 to 1.91; five trials, 1398 participants; moderate‐quality evidence; Analysis 1.5). In these analyses, the I² varied from 0% to 53%. We downgraded the quality of the evidence by one level because of concerns with design and risk of bias.
Antifibrinolytic drugs versus placebo or open control
In RCTs of antifibrinolytic drugs (N = 33) versus placebo or open control (N = 24) for acute spontaneous ICH, use of antifibrinolytic drugs led to non‐significant increases in death or dependence (RR 1.25, 95% CI 0.57 to 2.75; one trial, 24 participants; moderate‐quality evidence; Analysis 2.1), in death (RR 1.16, 95% CI 0.31 to 4.39; three trials, 57 participants; moderate‐quality evidence; Analysis 2.2), in all serious adverse events (RR 1.50, 95% CI 0.39 to 5.83; one trial, 24 participants; moderate‐quality evidence; Analysis 2.3), and in thromboembolic serious adverse events (RR 1.59, 95% CI 0.07 to 35.15; one trial, 24 participants; moderate‐quality evidence; Analysis 2.4). Antifibrinolytic drugs led to a non‐significant reduction in ICH growth (RR 0.76, 95% CI 0.56 to 1.05; three trials, 57 participants; moderate‐quality evidence; Analysis 2.7). In these analyses, the I² was 0% to 1%. We downgraded the quality of the evidence to moderate because of imprecision.
Platelet transfusion versus open control
In one RCT of platelet transfusion (N = 97) versus open control (N = 93) for acute spontaneous ICH associated with antiplatelet drug use, platelet transfusion led to a significant increase in death or dependence (RR 1.29, 95% CI 1.04 to 1.61; one trial, 190 participants; moderate‐quality evidence; Analysis 3.1), and non‐significant increases in death (RR 1.42, 95% CI 0.88 to 2.28; one trial, 190 participants; moderate‐quality evidence; Analysis 3.2), in all serious adverse events (RR 1.46, 95% CI 0.98 to 2.16; one trial, 190 participants; moderate‐quality evidence; Analysis 3.3), in thromboembolic serious adverse events (RR 3.84, 95% CI 0.44 to 33.68; one trial, 190 participants; moderate‐quality evidence; Analysis 3.4), and in ICH growth (RR 1.11, 95% CI 0.56 to 2.20; one trial, 190 participants; moderate‐quality evidence; Analysis 3.5). We downgraded the quality of the evidence by one level because of imprecision.
Clotting factors versus fresh frozen plasma
No conclusions could be drawn from one small RCT of clotting factors (N = 2) versus fresh frozen plasma (N = 3) for acute spontaneous ICH associated with anticoagulant drug use, which did not report the primary outcome of this review.
Discussion
Summary of main results
There was moderate‐quality evidence, based on the findings of one RCT (190 participants), that platelet transfusion appeared hazardous in comparison to standard care for adults with antiplatelet‐associated intracerebral haemorrhage (ICH). Based on moderate‐quality evidence, we were unable to draw firm conclusions about the efficacy and safety of blood clotting factors versus placebo or open control for acute spontaneous ICH with or without surgery (seven trials, 1480 participants), or antifibrinolytic drugs versus placebo or open control for acute spontaneous ICH (three trials, 57 participants). The one very small trial (five participants) we found on clotting factors versus fresh frozen plasma for acute spontaneous ICH associated with anticoagulant drug use did not measure our primary outcome, and provided limited data on the secondary outcomes.
Overall completeness and applicability of evidence
Alongside the 12 included RCTs, we were unable to include two eligible RCTs (N = 113) comparing clotting factors versus fresh frozen plasma for acute spontaneous ICH associated with anticoagulant drug use because data on participants with intracerebral haemorrhage could not be separated from participants with subdural intracranial haemorrhage (Kerebel 2013; Steiner 2016 (INCH)). This resulted in a shortage of data comparing clotting factors versus fresh frozen plasma for acute spontaneous ICH associated with anticoagulant drug use.
The results of 10 ongoing or recently completed unreported RCTs are awaiting completion and publication (Characteristics of ongoing studies). Two of these were recently completed RCTs (N = 142) investigating clotting factors in participants with radiological criteria associated with increased risk of haematoma expansion, so the RCTs in the review only include all patients with ICH and not the subgroup most at risk of ICH growth, i.e. those with the radiological 'spot sign' (tiny, enhancing foci within haematomas) (NCT00810888; NCT01359202).
There were few data available on antifibrinolytic drugs versus placebo after spontaneous acute ICH, but five ongoing RCTs (N > 2500) are investigating the antifibrinolytic drug tranexamic acid versus placebo in acute spontaneous ICH without anticoagulant use (Liu 2017 (TRAIGE); Meretoja 2014 (STOP‐AUST); NCT03044184; NOR‐ICH; Sprigg 2016 (TICH‐2)), and with anticoagulant drug use (NCT02866838). Publication of the largest RCT is expected in 2018 (Sprigg 2016 (TICH‐2)).
It was unclear whether recruitment was complete for one RCT of platelet transfusion (N = 100; NCT00699621). Further RCTs of platelet transfusion seem unlikely, so we await the publication of this RCT to see if the findings of Baharoglu 2016 (PATCH) are externally valid.
Quality of the evidence
We included 12 RCTs with 1732 participants (1150 allocated to intervention, 582 allocated to control or active comparator). One double‐blind RCT (Sprigg 2014 (TICH‐1)), and one open RCT (Baharoglu 2016 (PATCH)), were at low risk of bias, but the risk of bias of most other RCTs was moderate to high (Figure 2; Figure 3). The RCT finding harm from platelet transfusion has not been replicated in another RCT (Baharoglu 2016 (PATCH)).
The largest quantity of data was from RCTs of recombinant activated clotting factor VII (rFVIIa), which were at moderate risk of bias, and their results were moderately inconsistent, showing non‐significant benefits of rFVIIa. These findings have not changed practice (Hemphill 2015 (AHA ICH); RCP 2016; Steiner 2014 (ESO ICH)), and have led to explorations of the use of rFVIIa in ICH sub‐groups in as‐yet‐unpublished RCTs (NCT00810888; NCT01359202).
We rated the overall quality of all of the available evidence as moderate for three comparisons on the grounds of imprecision (Analysis 1.1; Analysis 2.1; Analysis 3.1), and poor for one comparison on the grounds of imprecision and limitations in study design (Analysis 4.1).
Potential biases in the review process
We followed a dual review process: two authors (NS and RA‐SS) independently extracted data that reduced identification bias and improved risk of bias assessment in comparison to the previous update of this review.
We were unable to include data from two eligible studies that separately showed benefits of clotting factors versus FFP on intermediate outcomes (speed of international normalised ratio (INR) reduction) after intracranial haemorrhage as data were not available for the ICH population; we hope to include these RCTs in the next update of this review to also examine the effect of clotting factors versus FFP on clinical outcomes (Steiner 2016 (INCH); Kerebel 2013).
Agreements and disagreements with other studies or reviews
This review was completed after the most recent updates of the European and North American ICH guidelines (Hemphill 2015 (AHA ICH); Steiner 2014 (ESO ICH)). These guidelines predated the results of Baharoglu 2016 (PATCH), and simply found that "the usefulness of platelet transfusions in ICH patients with a history of antiplatelet use is uncertain". Guidance on platelet transfusion has changed to reflect the results of Baharoglu 2016 (PATCH), where platelet transfusion was associated with a worse outcome, hence platelet transfusion is not recommended (Dastur 2017). Consistent with our findings, guidelines have not recommended the use of clotting factors, such as rFVIIa for acute spontaneous ICH not associated with anticoagulant use (Hemphill 2015 (AHA ICH); RCP 2016; Steiner 2014 (ESO ICH)). The AHA and ESO guidelines preceded the publication of Steiner 2016 (INCH), but the national stroke guidelines in the UK recommend using prothrombin complex concentrate for anticoagulant‐associated ICH, reflecting the findings of Steiner 2016 (INCH) on the intermediate outcome of speed of INR reduction (RCP 2016).
Study flow diagram
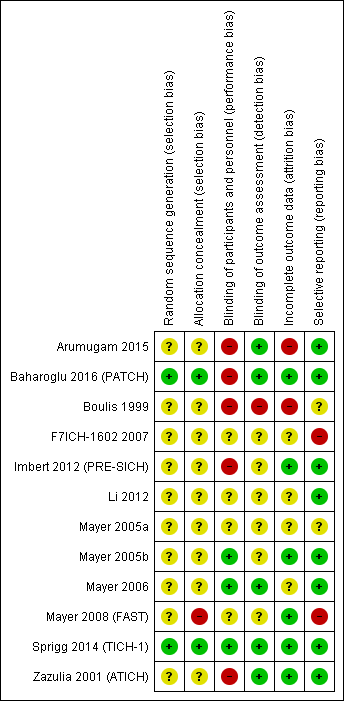
'Risk of bias' summary: review authors' judgements about each risk of bias criteria for each included study

Risk of bias graph: review authors' judgements about each risk of bias criteria presented as percentages across all included studies
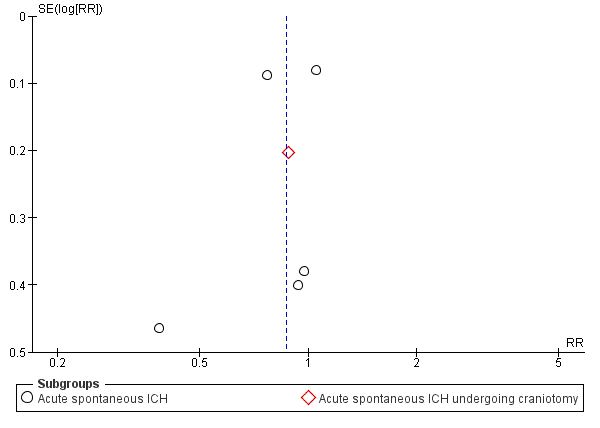
Funnel plot of comparison: 1 Blood clotting factors vs placebo or open control, outcome: 1.1 Death or dependence (mRS 4 to 6) at day 90

Comparison 1 Blood clotting factors vs placebo or open control, Outcome 1 Death or dependence (mRS 4 to 6) at day 90.
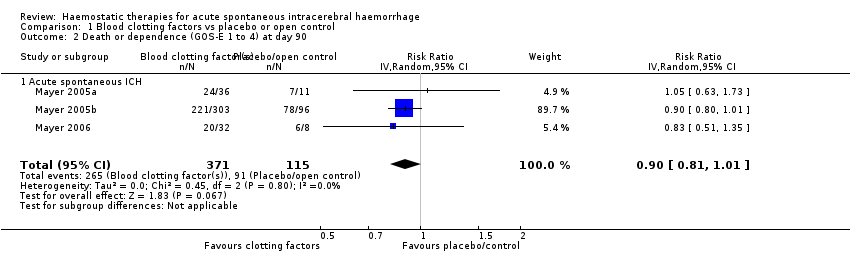
Comparison 1 Blood clotting factors vs placebo or open control, Outcome 2 Death or dependence (GOS‐E 1 to 4) at day 90.

Comparison 1 Blood clotting factors vs placebo or open control, Outcome 3 Death by day 90.

Comparison 1 Blood clotting factors vs placebo or open control, Outcome 4 All serious adverse events.

Comparison 1 Blood clotting factors vs placebo or open control, Outcome 5 Thromboembolic serious adverse events.

Comparison 1 Blood clotting factors vs placebo or open control, Outcome 6 Intracerebral haemorrhage growth by 24 hours.

Comparison 2 Antifibrinolytic drugs vs placebo or open control, Outcome 1 Death or dependence (mRS 4 to 6) at day 90.

Comparison 2 Antifibrinolytic drugs vs placebo or open control, Outcome 2 Death by day 90.
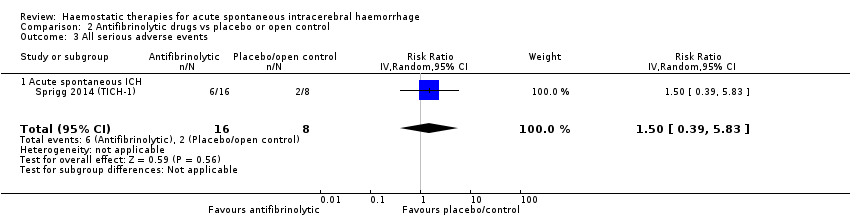
Comparison 2 Antifibrinolytic drugs vs placebo or open control, Outcome 3 All serious adverse events.

Comparison 2 Antifibrinolytic drugs vs placebo or open control, Outcome 4 Thromboembolic serious adverse events.

Comparison 2 Antifibrinolytic drugs vs placebo or open control, Outcome 5 Barthel Index.
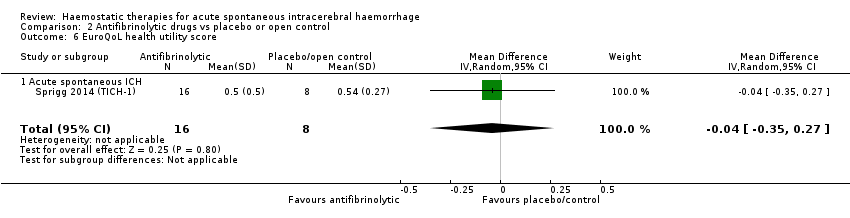
Comparison 2 Antifibrinolytic drugs vs placebo or open control, Outcome 6 EuroQoL health utility score.

Comparison 2 Antifibrinolytic drugs vs placebo or open control, Outcome 7 Intracerebral haemorrhage growth by 24 hours.

Comparison 3 Platelet transfusion vs open control, Outcome 1 Death or dependence (mRS 4 to 6) at day 90.
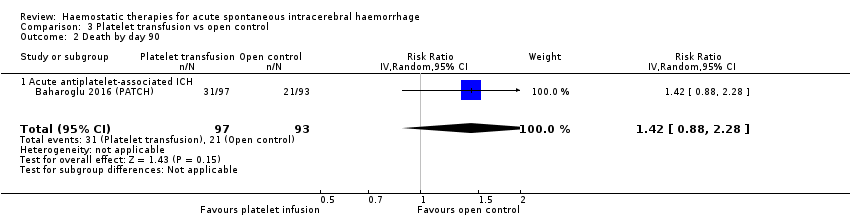
Comparison 3 Platelet transfusion vs open control, Outcome 2 Death by day 90.

Comparison 3 Platelet transfusion vs open control, Outcome 3 All serious adverse events.

Comparison 3 Platelet transfusion vs open control, Outcome 4 Thromboembolic serious adverse events.

Comparison 3 Platelet transfusion vs open control, Outcome 5 Intracerebral haemorrhage growth by 24 hours.
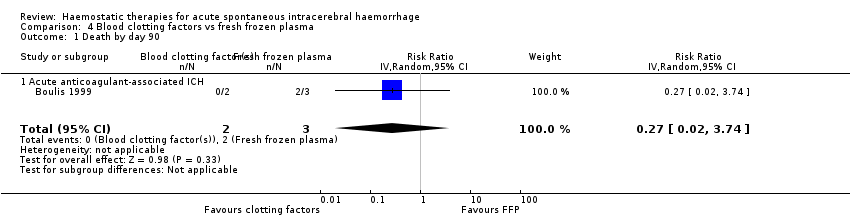
Comparison 4 Blood clotting factors vs fresh frozen plasma, Outcome 1 Death by day 90.
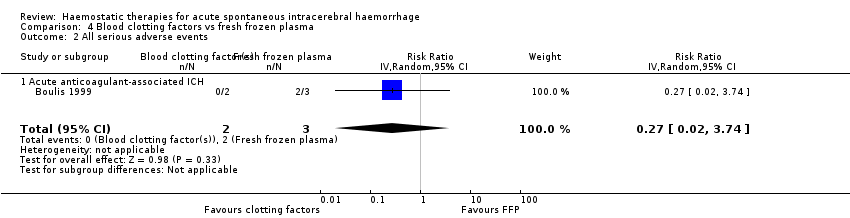
Comparison 4 Blood clotting factors vs fresh frozen plasma, Outcome 2 All serious adverse events.
| Blood clotting factors compared with placebo or open control for acute spontaneous intracerebral haemorrhage | ||||
| Patient or population: adults with acute spontaneous intracerebral haemorrhage Settings: secondary care Intervention: recombinant activated factor VII Comparison: placebo or open control | ||||
| Outcomes | Relative effect | No of Participants | Quality of the evidence | Comments |
| Death or dependence (mRS 4 to 6) at day 90 | RR 0.87 (0.70 to 1.07) | 1390 | ⊕⊕⊕⊝ | |
| GRADE Working Group grades of evidence | ||||
| CI: confidence interval; RR: risk ratio; mRS: modified Rankin Scale ¹We downgraded the quality of the evidence once because of imprecision. | ||||
| Antifibrinolytic drugs compared with placebo for acute spontaneous intracerebral haemorrhage | ||||
| Patient or population: adults with acute spontaneous intracerebral haemorrhage Settings: secondary care Intervention: tranexamic acid Comparison: placebo | ||||
| Outcomes | Relative effect | No of Participants | Quality of the evidence | Comments |
| Death or dependence (mRS 4 to 6) at day 90 | RR 1.25 (0.57 to 2.75) | 24 | ⊕⊕⊕⊝ | |
| GRADE Working Group grades of evidence | ||||
| CI: confidence interval; RR: risk ratio; mRS: modified Rankin Scale ¹We downgraded the quality of the evidence once because of imprecision. | ||||
| Platelet transfusion compared with open control for acute spontaneous intracerebral haemorrhage associated with antiplatelet drug use | ||||
| Patient or population: adults with acute spontaneous intracerebral haemorrhage associated with antiplatelet drug use Settings: secondary care Intervention: platelet transfusion Comparison: open control | ||||
| Outcomes | Relative effect | No of Participants | Quality of the evidence | Comments |
| Death or dependence (mRS 4 to 6) at day 90 | RR 1.29 (1.04 to 1.61) | 190 | ⊕⊕⊕⊝ | |
| GRADE Working Group grades of evidence | ||||
| CI: confidence interval; RR: risk ratio; mRS: modified Rankin Scale ¹We downgraded the quality of the evidence once because of imprecision. | ||||
| Blood clotting factors compared with fresh frozen plasma for acute spontaneous intracerebral haemorrhage associated with anticoagulant drug use | ||||
| Patient or population: adults with acute spontaneous intracerebral haemorrhage associated with anticoagulant drug use Settings: secondary care Intervention: fresh frozen plasma (intravenous), vitamin K (subcutaneous), and factor IX complex concentrate Comparison: fresh frozen plasma (intravenous) | ||||
| Outcomes | Relative effect | No of Participants | Quality of the evidence | Comments |
| Death or dependence (mRS 4 to 6) at day 90 | No evidence | No evidence as mRS not measured | ||
| GRADE Working Group grades of evidence | ||||
| CI: confidence interval | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or dependence (mRS 4 to 6) at day 90 Show forest plot | 6 | 1390 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.70, 1.07] |
| 1.1 Acute spontaneous ICH | 5 | 1369 | Risk Ratio (IV, Random, 95% CI) | 0.86 [0.66, 1.11] |
| 1.2 Acute spontaneous ICH undergoing craniotomy | 1 | 21 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.59, 1.31] |
| 2 Death or dependence (GOS‐E 1 to 4) at day 90 Show forest plot | 3 | 486 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.81, 1.01] |
| 2.1 Acute spontaneous ICH | 3 | 486 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.81, 1.01] |
| 3 Death by day 90 Show forest plot | 7 | 1480 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.51, 1.09] |
| 3.1 Acute spontaneous ICH | 6 | 1459 | Risk Ratio (IV, Random, 95% CI) | 0.71 [0.44, 1.14] |
| 3.2 Acute spontaneous ICH undergoing craniotomy | 1 | 21 | Risk Ratio (IV, Random, 95% CI) | 0.86 [0.41, 1.80] |
| 4 All serious adverse events Show forest plot | 2 | 87 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.30, 2.22] |
| 4.1 Acute spontaneous ICH | 2 | 87 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.30, 2.22] |
| 5 Thromboembolic serious adverse events Show forest plot | 5 | 1398 | Risk Ratio (IV, Random, 95% CI) | 1.24 [0.80, 1.91] |
| 5.1 Acute spontaneous ICH | 5 | 1398 | Risk Ratio (IV, Random, 95% CI) | 1.24 [0.80, 1.91] |
| 6 Intracerebral haemorrhage growth by 24 hours Show forest plot | 3 | 151 | Risk Ratio (IV, Random, 95% CI) | 0.74 [0.36, 1.48] |
| 6.1 Acute spontaneous ICH | 3 | 151 | Risk Ratio (IV, Random, 95% CI) | 0.74 [0.36, 1.48] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or dependence (mRS 4 to 6) at day 90 Show forest plot | 1 | 24 | Risk Ratio (IV, Random, 95% CI) | 1.25 [0.57, 2.75] |
| 1.1 Acute spontaneous ICH | 1 | 24 | Risk Ratio (IV, Random, 95% CI) | 1.25 [0.57, 2.75] |
| 2 Death by day 90 Show forest plot | 3 | 57 | Risk Ratio (IV, Random, 95% CI) | 1.16 [0.31, 4.39] |
| 2.1 Acute spontaneous ICH | 3 | 57 | Risk Ratio (IV, Random, 95% CI) | 1.16 [0.31, 4.39] |
| 3 All serious adverse events Show forest plot | 1 | 24 | Risk Ratio (IV, Random, 95% CI) | 1.5 [0.39, 5.83] |
| 3.1 Acute spontaneous ICH | 1 | 24 | Risk Ratio (IV, Random, 95% CI) | 1.5 [0.39, 5.83] |
| 4 Thromboembolic serious adverse events Show forest plot | 1 | 24 | Risk Ratio (IV, Random, 95% CI) | 1.59 [0.07, 35.15] |
| 4.1 Acute spontaneous ICH | 1 | 24 | Risk Ratio (IV, Random, 95% CI) | 1.59 [0.07, 35.15] |
| 5 Barthel Index Show forest plot | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐22.50 [‐45.65, 0.65] |
| 5.1 Acute spontaneous ICH | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐22.50 [‐45.65, 0.65] |
| 6 EuroQoL health utility score Show forest plot | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.35, 0.27] |
| 6.1 Acute spontaneous ICH | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.35, 0.27] |
| 7 Intracerebral haemorrhage growth by 24 hours Show forest plot | 3 | 57 | Risk Ratio (IV, Random, 95% CI) | 0.76 [0.56, 1.05] |
| 7.1 Acute spontaneous ICH | 3 | 57 | Risk Ratio (IV, Random, 95% CI) | 0.76 [0.56, 1.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or dependence (mRS 4 to 6) at day 90 Show forest plot | 1 | 190 | Risk Ratio (IV, Random, 95% CI) | 1.29 [1.04, 1.61] |
| 1.1 Acute antiplatelet‐associated ICH | 1 | 190 | Risk Ratio (IV, Random, 95% CI) | 1.29 [1.04, 1.61] |
| 2 Death by day 90 Show forest plot | 1 | 190 | Risk Ratio (IV, Random, 95% CI) | 1.42 [0.88, 2.28] |
| 2.1 Acute antiplatelet‐associated ICH | 1 | 190 | Risk Ratio (IV, Random, 95% CI) | 1.42 [0.88, 2.28] |
| 3 All serious adverse events Show forest plot | 1 | 190 | Risk Ratio (IV, Random, 95% CI) | 1.46 [0.98, 2.16] |
| 3.1 Acute antiplatelet‐associated ICH | 1 | 190 | Risk Ratio (IV, Random, 95% CI) | 1.46 [0.98, 2.16] |
| 4 Thromboembolic serious adverse events Show forest plot | 1 | 190 | Risk Ratio (IV, Random, 95% CI) | 3.84 [0.44, 33.68] |
| 4.1 Acute antiplatelet‐associated ICH | 1 | 190 | Risk Ratio (IV, Random, 95% CI) | 3.84 [0.44, 33.68] |
| 5 Intracerebral haemorrhage growth by 24 hours Show forest plot | 1 | 190 | Risk Ratio (IV, Random, 95% CI) | 1.11 [0.56, 2.20] |
| 5.1 Acute antiplatelet‐associated ICH | 1 | 190 | Risk Ratio (IV, Random, 95% CI) | 1.11 [0.56, 2.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death by day 90 Show forest plot | 1 | 5 | Risk Ratio (IV, Random, 95% CI) | 0.27 [0.02, 3.74] |
| 1.1 Acute anticoagulant‐associated ICH | 1 | 5 | Risk Ratio (IV, Random, 95% CI) | 0.27 [0.02, 3.74] |
| 2 All serious adverse events Show forest plot | 1 | 5 | Risk Ratio (IV, Random, 95% CI) | 0.27 [0.02, 3.74] |
| 2.1 Acute anticoagulant‐associated ICH | 1 | 5 | Risk Ratio (IV, Random, 95% CI) | 0.27 [0.02, 3.74] |

