La supplémentation en glutamine chez les nourrissons atteints d'une grave maladie gastro‐intestinale
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial | |
| Participants | 80 children of gestational age > 30 weeks and chronological age < 2 years Exclusion criteria: renal or hepatic dysfunction, an inborn error of metabolism, immunodeficiency, use of corticosteroids, pre‐existent life expectancy < 6 months, and simultaneous participation in another trial | |
| Interventions | Intervention (n = 41): on the second postoperative day infants in the intervention group received a standard parenteral nutrition solution supplemented with L‐glutamine sufficient to provide 0.4 g/kg/day. Control (n = 39): infants received parenteral nutrition with an iso‐nitrogenous amino acid solution. | |
| Outcomes | 1. Intestinal permeability (sugar absorption test) | |
| Notes | This trial did not strictly fulfil our a priori population criterion of including only infants < 3 months old. However, we made a consensus decision to include the trial because the report stated that most (69 of the 80) participants were < 6 months old at enrolment. We have contacted the trial investigator to determine whether outcome data are available for infants less than 3 months old. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated, blocks of 4 |
| Allocation concealment (selection bias) | Low risk | Randomisation schedule available only to pharmacist |
| Blinding of participants and personnel (performance bias) | Low risk | Physicians and nurses blinded |
| Incomplete outcome data (attrition bias) | Low risk | Data were analysed by intention‐to‐treat |
| Methods | Randomised controlled trial | |
| Participants | 20 infants with significant gastrointestinal illness including NEC requiring laparotomy and resection, intestinal atresia, omphalocoele, gastroschisis, intestinal volvulus, or malrotation Exclusion criteria: significant hepatic, renal, or metabolic disease necessitating protein restriction < 1.0 g/kg/day; significant extra‐intestinal disease (cystic fibrosis, severe hypoxic ischaemic encephalopathy); or extreme short bowel syndrome (defined as < 25 cm of residual bowel length) | |
| Interventions | Intervention (n = 9): glutamine‐supplemented (up to 0.31 g/kg/day) expressed human milk or hydrolysed formula milk | |
| Outcomes | 1. Time taken to establish full enteral feeding | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers list in blocks of 4 |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes, opened by hospital pharmacists |
| Blinding of participants and personnel (performance bias) | Low risk | Investigators, parents, physicians, nurses blinded |
| Incomplete outcome data (attrition bias) | Low risk | > 80% followed up |
| Methods | Randomised controlled trial | |
| Participants | 174 infants with significant gastrointestinal illness, including abdominal wall defect, congenital bowel obstruction, and NEC Exclusion criteria: renal failure, inborn errors of metabolism or immune deficiency Setting: 14 paediatric surgical units in the UK | |
| Interventions | Intervention (n = 87): parenteral nutrition supplemented with glutamine dipeptide (0.6 g/kg/d) Control (n = 87): isonitrogenous, isocaloric parenteral nutrition solution | |
| Outcomes | 1. Time on parenteral nutrition 2. Time to full enteral feeding 3. Incidence of sepsis and septicaemia Adverse events and mortality also reported | |
| Notes | This trial was stopped when funding came to an end before the target of 250 participants was achieved. It was judged that there was a less than 5% chance of identifying the specified differences in the primary outcome measure (duration of parenteral nutrition), even if recruitment had continued as planned. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods used to generate sequence in pharmacy not stated explicitly |
| Allocation concealment (selection bias) | Low risk | Infants allocated to groups by central pharmacy, allocation communicated to pharmacy of recruiting centre and not shared outside of pharmacy |
| Blinding of participants and personnel (performance bias) | Low risk | Parents, nurses, physicians, surgeons, and trial coordinator blinded Allocation was revealed in one case (control group) where clinically necessary |
| Incomplete outcome data (attrition bias) | Low risk | > 80% followed up, any loss to follow‐up explained and balanced across groups 4 infants in each group who had "intervention discontinued" were not included in final analyses by intention to treat |
NEC: necrotising enterocolitis
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Most participants in this small trial (n = 9) were infants with non‐gastrointestinal disease | |
| Did not focus on infants with gastrointestinal disease | |
| Did not focus on infants with gastrointestinal disease |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised controlled trial |
| Participants | Surgical infants requiring total parenteral nutrition |
| Interventions | |
| Outcomes | |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
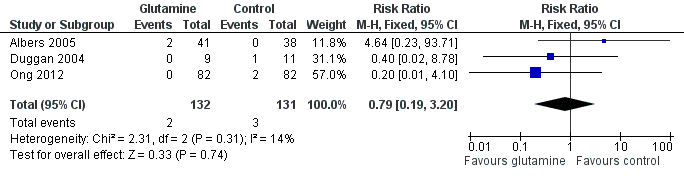
| 1 Death prior to hospital discharge Show forest plot | 3 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.19, 3.20] |
| Analysis 1.1 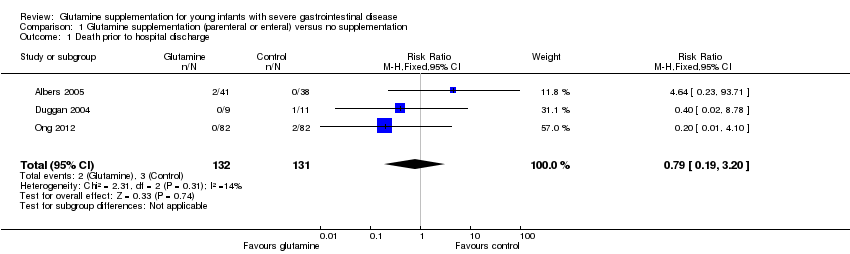 Comparison 1 Glutamine supplementation (parenteral or enteral) versus no supplementation, Outcome 1 Death prior to hospital discharge. | ||||
| 2 Incidence of invasive infection Show forest plot | 3 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.89, 2.11] |
| Analysis 1.2  Comparison 1 Glutamine supplementation (parenteral or enteral) versus no supplementation, Outcome 2 Incidence of invasive infection. | ||||
| 3 Weight gain during trial period (g/day) Show forest plot | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 3.80 [‐6.65, 14.25] |
| Analysis 1.3 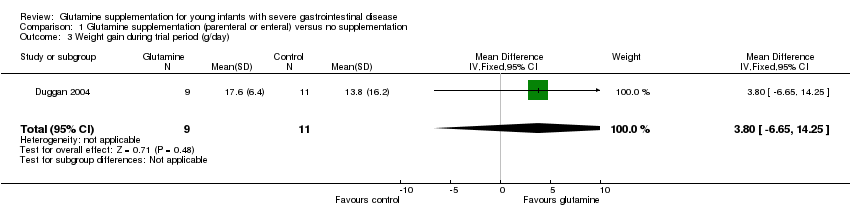 Comparison 1 Glutamine supplementation (parenteral or enteral) versus no supplementation, Outcome 3 Weight gain during trial period (g/day). | ||||
Forest plot of comparison 1: glutamine supplementation (parenteral or enteral) versus no supplementation, outcome 1.1: death prior to hospital discharge.

Forest plot of comparison 1: glutamine supplementation (parenteral or enteral) versus no supplementation, outcome 1.2: incidence of invasive infection.

Forest plot of comparison 1: glutamine supplementation (parenteral or enteral) versus no supplementation, outcome 1.3: weight gain during trial period (g/day).
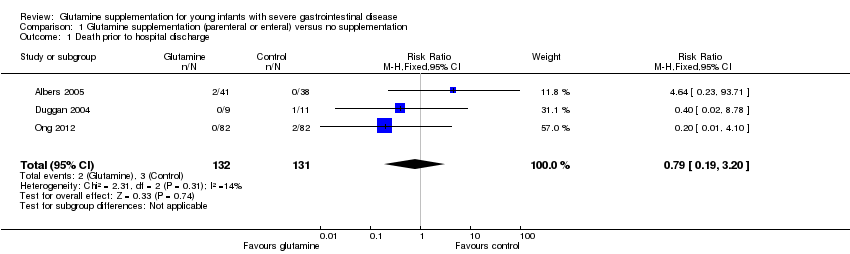
Comparison 1 Glutamine supplementation (parenteral or enteral) versus no supplementation, Outcome 1 Death prior to hospital discharge.
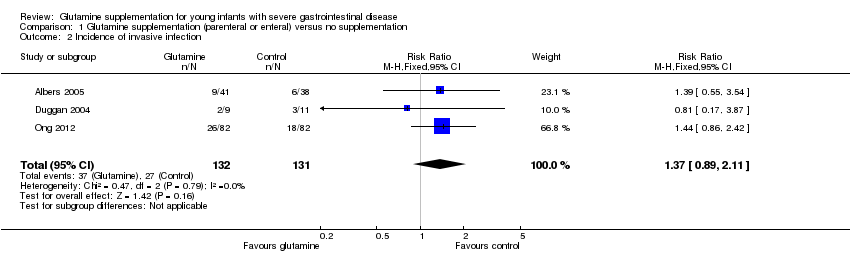
Comparison 1 Glutamine supplementation (parenteral or enteral) versus no supplementation, Outcome 2 Incidence of invasive infection.
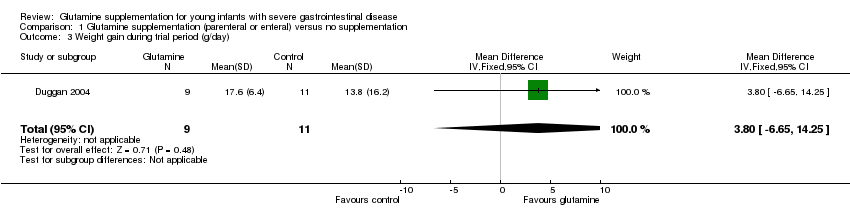
Comparison 1 Glutamine supplementation (parenteral or enteral) versus no supplementation, Outcome 3 Weight gain during trial period (g/day).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death prior to hospital discharge Show forest plot | 3 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.19, 3.20] |
| 2 Incidence of invasive infection Show forest plot | 3 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.89, 2.11] |
| 3 Weight gain during trial period (g/day) Show forest plot | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 3.80 [‐6.65, 14.25] |

