تاثیر مکمل گلوتامین در کودکان خردسال مبتلا به بیماری شدید گوارشی
Información
- DOI:
- https://doi.org/10.1002/14651858.CD005947.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 15 diciembre 2014see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Neonatología
- Copyright:
-
- Copyright © 2014 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
In discussion with WM, JVEB searched and screened the studies for inclusion, assessed the methodological quality of the trials, and extracted and entered the relevant information and data from each included study. WM and JVEB completed the final review.
Sources of support
Internal sources
-
University of York, UK.
External sources
-
National Institute for Health Research (NIHR), UK.
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C
Declarations of interest
None.
Acknowledgements
We thank Mellisa Harden (Centre for Reviews and Dissemination, University of York) for revising and running the electronic search.
We thank Professor Christopher Duggan for supporting a previous version of this review by providing further details about his trial (Duggan 2004), Professor Satish Kalhan for sharing data and for advising on glutamine metabolic studies in young infants, and Drs. Tubman and Zubin Grover for contributing to a previous iteration (Grover 2007).
Version history
| Published | Title | Stage | Authors | Version |
| 2014 Dec 15 | Glutamine supplementation for young infants with severe gastrointestinal disease | Review | Jennifer VE Brown, Thirimon Moe‐Byrne, William McGuire | |
| 2012 Jul 11 | Glutamine supplementation for young infants with severe gastrointestinal disease | Review | Jennifer VE Wagner, Thirimon Moe‐Byrne, Zubin Grover, William McGuire | |
| 2007 Jan 24 | Glutamine supplementation for young infants with severe gastrointestinal disease | Review | Zubin Grover, Richard Tubman, William McGuire | |
| 2006 Apr 19 | Glutamine supplementation for young infants with severe gastrointestinal disease | Protocol | Zubin Grover, Richard TRJ Tubman, William McGuire | |
Differences between protocol and review
Albers 2005 enrolled 80 children of gestational age at birth > 30 weeks and chronological age less than two years. This trial therefore did not strictly fulfil our a priori population criterion (infants up to three months old). However, we decided to include the trial because the report stated that most (69 of the 80) participants were less than six months old at enrolment. The report did not provide subgroup data for infants up to three months old.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans; Infant; Infant, Newborn;
PICO
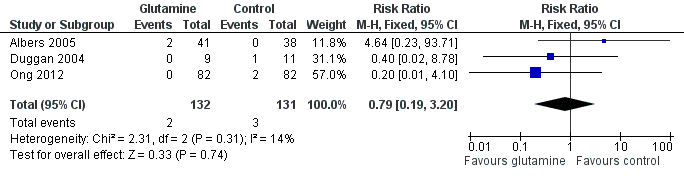
Forest plot of comparison 1: glutamine supplementation (parenteral or enteral) versus no supplementation, outcome 1.1: death prior to hospital discharge.

Forest plot of comparison 1: glutamine supplementation (parenteral or enteral) versus no supplementation, outcome 1.2: incidence of invasive infection.

Forest plot of comparison 1: glutamine supplementation (parenteral or enteral) versus no supplementation, outcome 1.3: weight gain during trial period (g/day).
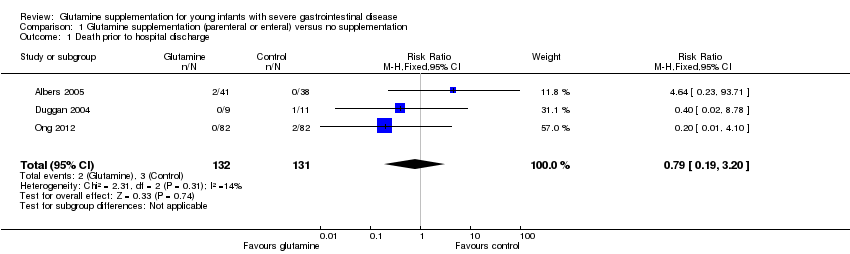
Comparison 1 Glutamine supplementation (parenteral or enteral) versus no supplementation, Outcome 1 Death prior to hospital discharge.
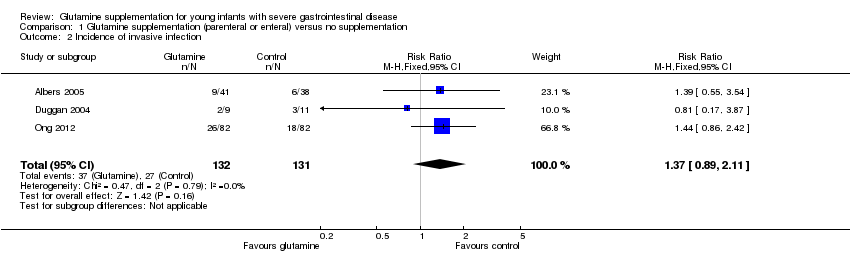
Comparison 1 Glutamine supplementation (parenteral or enteral) versus no supplementation, Outcome 2 Incidence of invasive infection.
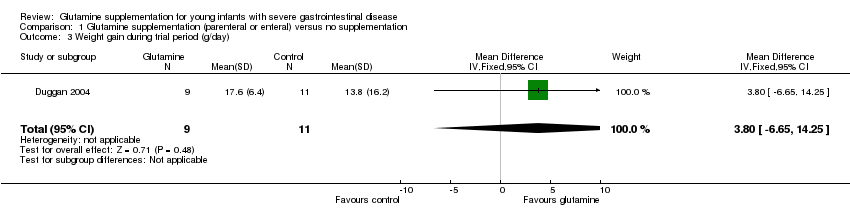
Comparison 1 Glutamine supplementation (parenteral or enteral) versus no supplementation, Outcome 3 Weight gain during trial period (g/day).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death prior to hospital discharge Show forest plot | 3 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.19, 3.20] |
| 2 Incidence of invasive infection Show forest plot | 3 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.89, 2.11] |
| 3 Weight gain during trial period (g/day) Show forest plot | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 3.80 [‐6.65, 14.25] |

