重度消化管疾患の乳児(乳児期初期)に対するグルタミン補充
Appendices
Appendix 1. Electronic search strategy
Database searches
Cochrane Central Register of Controlled Trials (CENTRAL)
Wiley http://onlinelibrary.wiley.com/
Issue 8 of 12, August 2014
Searched on 22nd September 2014. 70 records were retrieved.
-
MeSH descriptor: [Infant, Newborn] explode all trees 13194
-
MeSH descriptor: [Premature Birth] this term only 397
-
(neonat* or neo next nat*):ti,ab,kw 10126
-
(newborn* or new next born* or newly next born*):ti,ab,kw 16527
-
(preterm or preterms or pre next term or pre next terms):ti,ab,kw 6017
-
(preemie* or premie or premies):ti,ab,kw 14
-
(prematur* near/3 (birth* or born or deliver*)):ti,ab,kw 1126
-
(low near/3 (birthweight* or birth next weight*)):ti,ab,kw 3243
-
(lbw or vlbw or elbw):ti,ab,kw 887
-
infan*:ti,ab,kw 36861
-
(baby or babies):ti,ab,kw 3163
-
MeSH descriptor: [Enterocolitis, Necrotizing] this term only 121
-
enterocolitis:ti,ab,kw 683
-
NEC:ti,ab,kw 173
-
#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 43510
-
MeSH descriptor: [Glutamine] this term only 491
-
glutam*:ti,ab,kw 3181
-
levoglutam*:ti,ab,kw 0
-
#16 or #17 or #18 3181
-
#15 and #19 189
-
#15 and #19 in Trials 186
-
#15 and #19 Publication Year from 2007 to 2014, in Trials 70
Key
MeSH descriptor = indexing term (MeSH heading)
* = truncation
:ti,ab,kw = terms in either title or abstract or keyword fields
near/3 = terms within three words of each other (any order)
next = terms are next to each other
EMBASE
OvidSP http://ovidsp.ovid.com/
1980 to 2014 Week 38
Searched on 22nd September 2014. 87 records were retrieved. A search strategy developed by Lefebvre 2008 to identify randomised trials in EMBASE was used to limit retrieval to clinical trials (lines 22‐36).
-
exp infant/ (841476)
-
prematurity/ (70117)
-
premature labor/ (29157)
-
exp low birth weight/ (39329)
-
(neonat$ or neo nat$).ti,ab. (229859)
-
(newborn$ or new born$ or newly born$).ti,ab. (143427)
-
(preterm or preterms or pre term or pre terms).ti,ab. (60787)
-
(preemie$ or premie or premies).ti,ab. (154)
-
(prematur$ adj3 (birth$ or born or deliver$)).ti,ab. (14127)
-
(low adj3 (birthweight$ or birth weight$)).ti,ab. (29286)
-
(lbw or vlbw or elbw).ti,ab. (6941)
-
infan$.ti,ab. (364930)
-
(baby or babies).ti,ab. (64354)
-
necrotizing enterocolitis/ (6320)
-
enterocolitis.ti,ab. (9685)
-
NEC.ti,ab. (4115)
-
or/1‐16 (1134787)
-
glutamine/ (28305)
-
glutam$.ti,ab. (162556)
-
levoglutam$.ti,ab. (3)
-
18 or 19 or 20 (173526)
-
random$.ti,ab. (900117)
-
factorial$.ti,ab. (23315)
-
crossover$.ti,ab. (49098)
-
cross‐over$.ti,ab. (21608)
-
placebo$.ti,ab. (202020)
-
(doubl$ adj blind$).ti,ab. (143459)
-
(singl$ adj blind$).ti,ab. (14632)
-
assign$.ti,ab. (242053)
-
allocat$.ti,ab. (85229)
-
volunteer$.ti,ab. (177796)
-
Crossover Procedure/ (40222)
-
double blind procedure/ (115438)
-
Randomized Controlled Trial/ (350056)
-
single blind procedure/ (18827)
-
or/22‐35 (1432268)
-
17 and 21 and 36 (364)
-
animal/ (1570185)
-
exp animal experiment/ (1697956)
-
Nonhuman/ (4374620)
-
(rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh. (4568271)
-
38 or 39 or 40 or 41 (6746352)
-
exp human/ (15016996)
-
human experiment/ (329080)
-
43 or 44 (15018382)
-
42 not (42 and 45) (5182957)
-
37 not 46 (225)
-
limit 47 to em=201100‐201438 (87)
Key:
/ = indexing term (EMTREE heading)
$ = truncation
.ti,ab. = terms in either title or abstract fields
adj3 = terms within three words of each other (any order)
.sh.= subject heading field
.em. = entry date – date added to database
Maternity and Infant Care
OvidSP http://ovidsp.ovid.com/
1971 to August 2014
Searched on 22nd September 2014. 59 records were retrieved.
-
Infant.de. (7384)
-
Infant ‐ newborn.de. (23849)
-
Infant ‐ premature.de. (7331)
-
infant ‐ very premature.de. (753)
-
Infant ‐ low birth weight.de. (2367)
-
Infant ‐ very low birth weight.de. (2147)
-
Premature birth.de. (1625)
-
Infant ‐ small for gestational age.de. (887)
-
(neonat$ or neo nat$).ti,ab. (32059)
-
(newborn$ or new born$ or newly born$).ti,ab. (14916)
-
(preterm or preterms or pre term or pre terms).ti,ab. (18476)
-
(preemie$ or premie or premies).ti,ab. (41)
-
(prematur$ adj3 (birth$ or born or deliver$)).ti,ab. (3141)
-
(low adj3 (birthweight$ or birth weight$)).ti,ab. (8455)
-
(lbw or vlbw or elbw).ti,ab. (2237)
-
infan$.ti,ab. (48220)
-
(baby or babies).ti,ab. (23514)
-
Enterocolitis.de. (8)
-
enterocolitis.ti,ab. (1349)
-
NEC.ti,ab. (493)
-
or/1‐20 (94518)
-
Glutamine.de. (16)
-
glutam$.ti,ab. (161)
-
levoglutam$.ti,ab. (0)
-
22 or 23 or 24 (162)
-
21 and 25 (108)
-
limit 26 to yr="2007 ‐Current" (59)
Key
.de. = indexing term
$ = truncation
.ti,ab. = terms in either title or abstract fields
adj3 = terms within three words of each other (any order)
yr = year published
MEDLINE
OvidSP http://ovidsp.ovid.com/
1946 to September week 2 2014
Searched on 22nd September 2014. 61 records were retrieved. The Cochrane highly sensitive search strategy for identifying randomized trials in MEDLINE (sensitivity‐maximizing version) was used to limit retrieval to clinical trials (lines 21‐31) (Lefebvre 2011).
-
exp Infant, Newborn/ (504876)
-
Premature Birth/ (6949)
-
(neonat$ or neo nat$).ti,ab. (189942)
-
(newborn$ or new born$ or newly born$).ti,ab. (128353)
-
(preterm or preterms or pre term or pre terms).ti,ab. (45250)
-
(preemie$ or premie or premies).ti,ab. (106)
-
(prematur$ adj3 (birth$ or born or deliver$)).ti,ab. (11353)
-
(low adj3 (birthweight$ or birth weight$)).ti,ab. (24580)
-
(lbw or vlbw or elbw).ti,ab. (5285)
-
infan$.ti,ab. (323868)
-
(baby or babies).ti,ab. (50568)
-
Enterocolitis, Necrotizing/ (2030)
-
enterocolitis.ti,ab. (7862)
-
NEC.ti,ab. (2655)
-
or/1‐14 (818716)
-
Glutamine/ (15076)
-
glutam$.ti,ab. (145071)
-
levoglutam$.ti,ab. (4)
-
16 or 17 or 18 (149488)
-
15 and 19 (5020)
-
randomized controlled trial.pt. (388375)
-
controlled clinical trial.pt. (89809)
-
randomized.ab. (284414)
-
placebo.ab. (150745)
-
drug therapy.fs. (1744932)
-
randomly.ab. (201022)
-
trial.ab. (295504)
-
groups.ab. (1282228)
-
or/21‐28 (3283677)
-
exp animals/ not humans.sh. (4011372)
-
29 not 30 (2797292)
-
15 and 19 and 31 (437)
-
limit 32 to ed=20111101‐20140911 (61)
Key
/ = indexing term (MeSH heading)
exp = exploded MeSH heading
$ = truncation
.ti,ab. = terms in either title or abstract fields
adj3 = terms within three words of each other (any order)
.pt. = publication type
.fs. = floating subheading
.sh.= subject heading
.ed. = entry date ‐ date added to the database
MEDLINE In‐Process & Other Non‐Indexed Citations
OvidSP http://ovidsp.ovid.com/
September 19, 2014
Searched on 22nd September 2014. 60 records were retrieved.
-
exp Infant, Newborn/ (0)
-
Premature Birth/ (0)
-
(neonat$ or neo nat$).ti,ab. (10428)
-
(newborn$ or new born$ or newly born$).ti,ab. (5627)
-
(preterm or preterms or pre term or pre terms).ti,ab. (3486)
-
(preemie$ or premie or premies).ti,ab. (11)
-
(prematur$ adj3 (birth$ or born or deliver$)).ti,ab. (623)
-
(low adj3 (birthweight$ or birth weight$)).ti,ab. (1523)
-
(lbw or vlbw or elbw).ti,ab. (432)
-
infan$.ti,ab. (16416)
-
(baby or babies).ti,ab. (3393)
-
Enterocolitis, Necrotizing/ (0)
-
enterocolitis.ti,ab. (468)
-
NEC.ti,ab. (298)
-
or/1‐14 (30053)
-
Glutamine/ (0)
-
glutam$.ti,ab. (8117)
-
levoglutam$.ti,ab. (0)
-
16 or 17 or 18 (8117)
-
15 and 19 (176)
-
limit 20 to ed=20111101‐20140918 (60)
Key
/ = indexing term (MeSH heading)
exp = exploded MeSH heading
$ = truncation
.ti,ab. = terms in either title or abstract fields
adj3 = terms within three words of each other (any order)
ed = entry date ‐ date added to the database
PubMed
http://www.ncbi.nlm.nih.gov/pubmed/
Searched on 23rd September 2014. 82 records were retrieved. The Cochrane highly sensitive search strategy for identifying randomized trials in PubMed (sensitivity‐maximizing version) was used to limit retrieval to clinical trials (Lefebvre 2011).
Search (((((((((((((((((((((("Infant, Newborn"[Mesh])) OR ("Premature Birth"[Mesh])) OR (((neonat*[Title/Abstract]) OR neo nat*[Title/Abstract]) OR neo‐nat*[Title/Abstract])) OR (((((newborn*[Title/Abstract]) OR new born*[Title/Abstract]) OR new‐born*[Title/Abstract]) OR newly born*[Title/Abstract]) OR newly‐born*[Title/Abstract])) OR ((((((preterm[Title/Abstract]) OR preterms[Title/Abstract]) OR pre term[Title/Abstract]) OR pre‐term[Title/Abstract]) OR pre terms[Title/Abstract]) OR pre‐terms[Title/Abstract])) OR (((preemie*[Title/Abstract]) OR premie[Title/Abstract]) OR premies[Title/Abstract])) OR ((prematur*[Title/Abstract]) AND birth*[Title/Abstract])) OR ((prematur*[Title/Abstract]) AND born[Title/Abstract])) OR ((prematur*[Title/Abstract]) AND deliver*[Title/Abstract])) OR ((low[Title/Abstract]) AND birthweight*[Title/Abstract])) OR ((low[Title/Abstract]) AND birth weight*[Title/Abstract])) OR ((low[Title/Abstract]) AND birth‐weight*[Title/Abstract])) OR (((lbw[Title/Abstract]) OR vlbw[Title/Abstract]) OR elbw[Title/Abstract])) OR (infan*[Title/Abstract])) OR ((baby[Title/Abstract]) OR babies[Title/Abstract])) OR ("Enterocolitis, Necrotizing"[Mesh:noexp]) OR (enterocolitis[Title/Abstract]) OR (NEC[Title/Abstract]))) AND (((levoglutam*[Title/Abstract]) OR glutam*[Title/Abstract]) OR "Glutamine"[Mesh:noexp]))) AND (((((((((((randomized controlled trial[Publication Type])) OR (controlled clinical trial[Publication Type])) OR (randomized[Title/Abstract])) OR (placebo[Title/Abstract])) OR (drug therapy[MeSH Subheading])) OR (randomly[Title/Abstract])) OR (trial[Title/Abstract])) OR (groups[Title/Abstract]))) NOT (animals[mh] NOT humans[mh])))) AND ("2011/11/01"[Date ‐ Entrez] : "3000"[Date ‐ Entrez])
Key
[Mesh] = exploded Medical Subject heading (MeSH)
[mh] = exploded MeSH
[Mesh:noexp] = MeSH not exploded
* = truncation
[Title/Abstract] = terms in either title or abstract fields
[Date ‐ Entrez] = entry date ‐ date added to the database
Trial register searches
Clinical Trials.gov
Searched on 23rd September 2014. 17 records were retrieved in total using the following search strategies:
4 studies found for: Glutamine AND (infant OR infants OR newborn OR newborns OR premature OR prematurity OR neonate OR neonates OR neonatal OR preterm OR preterms OR preemie OR preemies OR premie OR premies OR birthweight OR baby OR babies) | received on or after 11/01/2011 | updated on or after 11/01/2011
1 study found for: Glutamine AND (NEC OR enterocolitis) | received on or after 11/01/2011 | updated on or after 11/01/2011
12 studies found for: "Glutamine" | Child | received on or after 11/01/2011 | updated on or after 11/01/2011
metaRegister of Controlled Trials (mRCT)
http://www.controlled‐trials.com/mrct/searchform
Searched on 23rd September 2014. 18 trials were retrieved.
(Glutamine AND (infant OR infants OR newborn OR newborns OR premature OR prematurity OR neonate OR neonates or neonatal OR preterm OR preterms OR preemie OR preemies OR premie OR premies OR birthweight OR baby OR babies OR enterocolitis or NEC))
WHO International Clinical Trials Registry Platform
http://apps.who.int/trialsearch/AdvSearch.aspx
Searched on 23rd September 2014. 21 trials were retrieved in total using the following search strategies:
-
glutam* in title, clinical trials in children – 11 trials.
-
glutam* in intervention field, clinical trials in children – 10 trials.
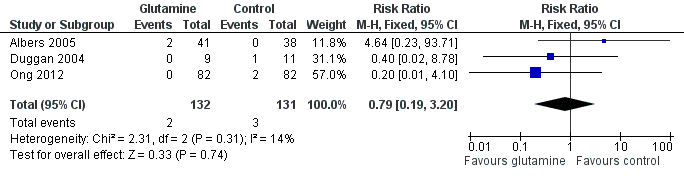
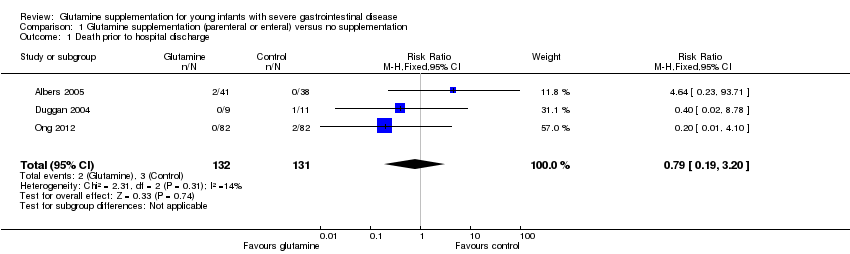
Forest plot of comparison 1: glutamine supplementation (parenteral or enteral) versus no supplementation, outcome 1.1: death prior to hospital discharge.

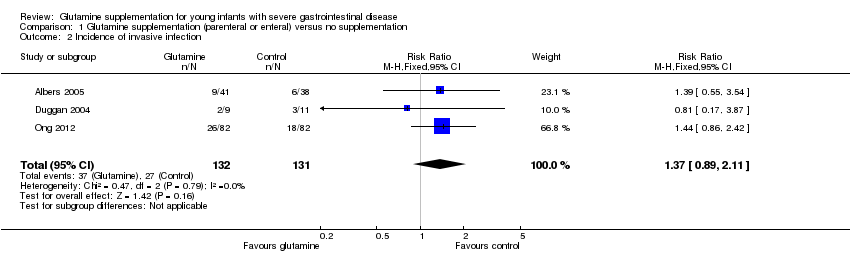
Forest plot of comparison 1: glutamine supplementation (parenteral or enteral) versus no supplementation, outcome 1.2: incidence of invasive infection.

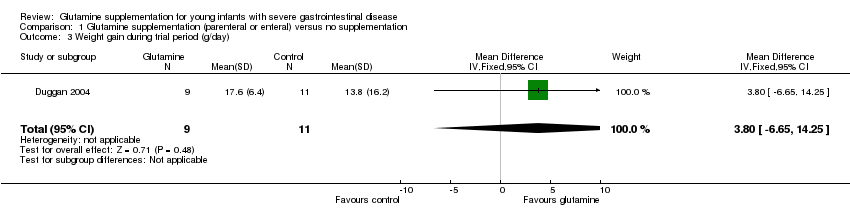
Forest plot of comparison 1: glutamine supplementation (parenteral or enteral) versus no supplementation, outcome 1.3: weight gain during trial period (g/day).
Comparison 1 Glutamine supplementation (parenteral or enteral) versus no supplementation, Outcome 1 Death prior to hospital discharge.
Comparison 1 Glutamine supplementation (parenteral or enteral) versus no supplementation, Outcome 2 Incidence of invasive infection.
Comparison 1 Glutamine supplementation (parenteral or enteral) versus no supplementation, Outcome 3 Weight gain during trial period (g/day).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death prior to hospital discharge Show forest plot | 3 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.19, 3.20] |
| 2 Incidence of invasive infection Show forest plot | 3 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.89, 2.11] |
| 3 Weight gain during trial period (g/day) Show forest plot | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 3.80 [‐6.65, 14.25] |

