Добавки глютамина для младенцев с тяжелыми желудочно‐кишечными заболеваниями
Abstract
Background
Endogenous glutamine biosynthesis may be insufficient to meet the needs of people with severe gastrointestinal disease. Results from studies using experimental animal models of gastrointestinal disease have suggested that glutamine supplementation improves clinical outcomes. This review examines evidence on the effect of glutamine supplementation in young infants with severe gastrointestinal disease.
Objectives
To assess the effect of supplemental glutamine on mortality and morbidity in young infants with severe gastrointestinal disease.
Search methods
We searcheed the Cochrane Central Register of Controlled Trials (The Cochrane Library, 2014, Issue 8), MEDLINE, EMBASE, and CINAHL (from inception to September 2014), conference proceedings, and reference lists from previous reviews.
Selection criteria
Randomised or quasi‐randomised controlled trials that compared glutamine supplementation versus no glutamine supplementation in infants up to three months old (corrected for preterm birth if necessary) with severe gastrointestinal disease defined as a congenital or acquired gastrointestinal condition that is likely to necessitate providing parenteral nutrition for at least 24 hours.
Data collection and analysis
Two review authors assessed trial eligibility and risk of bias and undertook data extraction independently. We analysed the treatment effects in the individual trials and reported the risk ratio (RR) and risk difference (RD) for dichotomous data and mean difference for continuous data, with 95% confidence intervals (CI). We used a fixed‐effect model in meta‐analyses and explored the potential causes of heterogeneity in sensitivity analyses.
Main results
We found three trials in which a total of 274 infants participated. The trials were of good methodological quality but were too small to detect clinically important effects of glutamine supplementation. Meta‐analyses did not reveal a statistically significant difference in the risk of death before hospital discharge (typical RR 0.79, 95% CI 0.19 to 3.20; typical RD ‐0.01, 95% CI ‐0.05 to 0.03) or in the rate of invasive infection (typical RR 1.37, 95% CI 0.89 to 2.11; typical RD 0.08, 95% CI ‐0.03 to 0.18]).
Authors' conclusions
The available data from randomised controlled trials do not suggest that glutamine supplementation has any important benefits for young infants with severe gastrointestinal disease.
PICO
Резюме на простом языке
Добавки глютамина для младенцев с тяжелыми желудочно‐кишечными заболеваниями
Глютамин ‐ это аминокислота, которая помогает тканям, особенно в желудочно‐кишечном тракте, восстанавливаться после повреждения. Мы провели поиск доказательств, что дополнительное введение глютамина младенцам с тяжелыми проблемами с кишечником помогает более быстрому и полному выздоровлению. В настоящее время имеются только три клинических испытания; результаты позволяют предположить, что добавки глютамина не имеют никакой пользы для этих детей.
Authors' conclusions
Background
Description of the condition
Severe gastrointestinal disease ‐ including acute necrotising enterocolitis (NEC), spontaneous localised intestinal perforation, congenital gut anomalies and atresias, anterior abdominal wall defects, Hirschsprung's disease, and acute malrotation or volvulus ‐ is an important cause of morbidity, mortality and neuro‐disability in newborn and young infants (Kays 1996; Hajivassiliou 2003). Infants with severe gastrointestinal disease experience more episodes of invasive infection, have lower levels of nutrient intake, grow more slowly, and have longer duration of intensive care and hospital stay than gestation‐comparable infants without gastrointestinal disease (Dalla Vecchia 1998; Donnell 2002; Abdullah 2007). Severe NEC, especially if treated surgically, is associated with a higher rate of long‐term neurological disability, which may be a consequence of infection and under‐nutrition during a critical period of brain development (Stoll 2004; Rees 2007).
Description of the intervention
Glutamine is a key source of energy for rapidly dividing cells such as enterocytes, lymphocytes, macrophages, and neutrophils (Windmueller 1980; Newsholme 1999). Although glutamine can be synthesised in vivo, it is considered a 'conditionally essential' amino acid in catabolic states, where demand outstrips supply (Lacey 1990). Plasma glutamine levels fall during critical illness or following major surgery and glutamine deficiency may limit tissue recovery in these situations (Parry‐Billings 1990; Parry‐Billings 1992; Newsholme 2001). While glutamine is present in human breast milk, infant formula is low in glutamine, and standard parenteral nutrition solutions contain no glutamine because it is not stable in an aqueous environment (Khan 1991; Agostini 2000). Thus, infants who have severe gastrointestinal disease or who are recovering from major gastrointestinal surgery are unlikely to receive sufficient glutamine. Synthetic glutamine‐containing dipeptides that are stable in aqueous solution are available, however (Furst 1997). It is therefore feasible to provide supplemental glutamine to infants with these conditions.
In theory, glutamine supplementation may confer several benefits to young infants with severe gastrointestinal disease. Accelerating anastomosis and wound healing and enhancing gut mucosal integrity might help infants to tolerate enteral feeding earlier and thereby shorten their duration of hospitalisation. In fact, improving gut barrier function and lymphocyte production may reduce the rate of acquired infection, lower mortality, and in the longer term, lower rates of adverse neurodevelopmental outcomes.
How the intervention might work
In experimental animal models of enterocolitis, providing supplemental glutamine aided gut recovery, probably by providing additional energy for enterocyte division and proliferation (Klimberg 1990; Rombeau 1990). Peri‐ or postoperative glutamine supplementation may also accelerate bowel anastomosis healing (McCauley 1991; da Costa 2003). In adult surgical or critically ill patients, some evidence exists that glutamine supplementation preserves gut mucosal integrity and reduces infectious complications and (possibly) mortality (Van der Hulst 1993; Novak 2002; Estivariz 2008). However, concerns about trial quality and publication bias have limited the reliability and applicability of these findings (Avenell 2009; Murray 2009). More recent, larger and better quality trials have failed to demonstrate clinically important benefits of glutamine supplementation in this population (Andrews 2011).
There is a theoretical concern that supplemental glutamine, via its metabolic products glutamate and ammonia, may have adverse neurological effects in high concentrations (Garlick 2001). However, metabolic studies in preterm infants have found that parenteral glutamine supplementation (up to 0.6 g/kg/day) limits whole body proteolysis without increasing plasma amino acid or ammonia levels (Kalhan 2005). Enterally administered glutamine is metabolised entirely in the splanchnic compartment and does not affect whole‐body ammonia or urea nitrogen levels (Parimi 2004). Systematic reviews have not found evidence of adverse effects of glutamine supplementation in adults or in clinically stable preterm infants (Novak 2002; Avenell 2009; Murray 2009; Moe‐Byrne 2012).
Why it is important to do this review
A previous Cochrane review on routine glutamine supplementation for preterm infants concluded that there is no evidence of any benefits or harms (Moe‐Byrne 2012). Although the infants who participated in these trials were all of very low birth weight, most were clinically stable. Any putative benefits of glutamine supplementation might be confined to critically ill infants for whom glutamine availability is rate‐limiting for tissue repair, for example, infants with severe gastrointestinal disease such as NEC or infants who have undergone major gastrointestinal surgery (Pierro 2002).
Objectives
To determine whether supplemental glutamine for infants with severe gastrointestinal disease reduces mortality, decreases the time taken to establish enteral feeding, reduces the incidence of late‐onset invasive infection, shortens the duration of hospitalisation, increases growth rates, and prevents adverse neurodevelopmental outcomes.
Methods
Criteria for considering studies for this review
Types of studies
Controlled trials using either random or quasi‐random patient allocation.
Types of participants
Newborn and young infants (up to and including three months old, corrected for preterm birth) with severe gastrointestinal disease defined as a congenital or acquired gastrointestinal condition that is likely to necessitate parenteral nutrition; for example, NEC, anterior abdominal wall defect, or intestinal obstruction. Infants should have been enrolled to participate in the trial within one week after gastrointestinal surgery or after the onset of NEC (if treated non‐surgically).
NEC should have been diagnosed using Bell's criteria or modifications, that is, the presence of at least two of the following features: pneumatosis coli on abdominal radiograph; abdominal distension or abdominal radiograph with gaseous distension or frothy appearance of bowel lumen (or both); blood in stool; and lethargy, hypotonia, or apnoea, or combination of these (Bell 1978; Walsh 1986).
Types of interventions
Glutamine supplementation versus no supplementation or placebo by the parenteral or enteral route. We did not pre‐specify a minimum duration of intervention.
Types of outcome measures
Primary outcomes
-
Death prior to hospital discharge.
-
Neurodevelopmental outcomes assessed beyond infancy (neurological evaluations, developmental scores, and classifications of disability including auditory and visual disability, non‐ambulant cerebral palsy, and developmental delay) and cognitive and educational outcomes (intelligence quotient and/or indices of educational achievement measured using a validated tool, including school examination results).
Secondary outcomes
-
Time from trial entry to establish full enteral feeds independent of parenteral fluids or nutrition.
-
Incidence of invasive infection during hospital admission as determined by culture of bacteria or fungus from blood, cerebrospinal fluid, urine, or from a normally sterile body space (number of participants per group with one or more episodes).
-
Time from trial entry to discharge from hospital.
-
Growth during the trial period: weight gain (g/day or g/kg/day), linear growth (mm/week), head growth (mm/week), and skinfold thickness growth (mm/week).
Search methods for identification of studies
We used the standard search strategy of the Cochrane Neonatal Review Group.
Electronic searches
An update search was carried out in September 2014 to locate randomised controlled trials of glutamine supplementation in surgical infants since the date of the last search in November 2011.
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (Wiley), EMBASE (OvidSP), Maternity and Infant Care (OvidSP), MEDLINE & MEDLINE in process (OvidSP) and PubMed. We limited the searches to references added to the databases since November 2011. We limited retrieval to clinical trials using a search filter where possible. We did not apply language restrictions.
We searched for ongoing and completed trials in ClinicalTrials.gov, metaRegister of Controlled Trials (mRCT), and the World Health Orgainzation International Clinical Trials Registry Platform (ICTRP), limiting where possible to trials added or updated since November 2011.
Full search strategies with results can be found in Appendix 1.
Searching other resources
We examined the reference lists of studies identified as potentially relevant. We searched the abstracts from the annual meetings of the Pediatric Academic Societies (1993 to 2014), the European Society for Paediatric Research (1995 to 2013), the UK Royal College of Paediatrics and Child Health (2000 to 2014), and the Perinatal Society of Australia and New Zealand (2000 to 2014). We considered trials reported only as abstracts to be eligible if sufficient information was available from the report, or from contact with the authors, to fulfil the inclusion criteria.
Data collection and analysis
We used the standard methods of the Cochrane Neonatal Review Group.
Selection of studies
Two review authors screened the title and abstract of all studies identified by the above search strategy. We assessed the full text of any potentially eligible reports and excluded those studies that did not meet all of the inclusion criteria. We discussed any disagreements until consensus was achieved.
Data extraction and management
We used a data collection form to aid extraction of relevant information from each included study. Two review authors extracted the data separately. We discussed any disagreements until consensus was achieved. We asked the investigators for further information if data from the trial reports were insufficient.
Assessment of risk of bias in included studies
We used the criteria and standard methods of the Cochrane Neonatal Review Group to assess the methodological quality of any included trials. Additional information from the trial authors was requested to clarify methodology and results as necessary. We evaluated and reported the following issues in the 'Risk of bias' tables.
-
Sequence generation: we categorised the method used to generate the allocation sequence as:
-
low risk: any random process (e.g. random number table; computer random number generator);
-
high risk: any non‐random process (e.g. odd or even date of birth; patient case‐record number);
-
unclear.
-
-
Allocation concealment: we categorised the method used to conceal the allocation sequence as:
-
low risk: (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
-
high risk: open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth;
-
unclear.
-
-
Blinding: we assessed blinding of participants, clinicians and carers, and outcome assessors separately for different outcomes and categorised the methods as:
-
low risk;
-
high risk;
-
unclear.
-
-
Incomplete outcome data: we described the completeness of data including attrition and exclusions from the analysis for each outcome and any reasons for attrition or exclusion where reported. We assessed whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorised completeness as:
-
low risk: < 20% missing data;
-
high risk: ≥ 20% missing data;
-
unclear.
-
Measures of treatment effect
We calculated risk ratio (RR) and risk difference (RD) for dichotomous data and weighted mean difference (WMD) for continuous data with respective 95% confidence intervals (CI). We determined the number needed to treat for benefit (NNTB) or harm (NNTH) for a statistically significant difference in the RD.
Unit of analysis issues
The unit of analysis is the participating infant in individually randomised trials and the neonatal unit for cluster randomised trials.
Dealing with missing data
We requested missing study data from the trial investigators.
Assessment of heterogeneity
If more than one trial was included in a meta‐analysis, we examined the treatment effects of individual trials and heterogeneity between trial results by inspecting the forest plots. We calculated the I² statistic for each analysis to quantify inconsistency across studies and describe the percentage of variability in effect estimates that may be due to heterogeneity rather than sampling error. If substantial (I² > 50%) heterogeneity was detected, we explored the possible causes (for example, differences in study design, participants, interventions, or completeness of outcome assessments) in sensitivity analyses.
Data synthesis
We used a fixed‐effect model for meta‐analyses.
Subgroup analysis and investigation of heterogeneity
We pre‐specified the following subgroup analyses:
-
trials where participants were predominantly (> 80%) term infants versus trials where participants were predominantly preterm infants;
-
trials where participants were infants with NEC versus trials where participants were infants who had undergone gastrointestinal surgery for other indications;
-
trials where infants received enteral glutamine supplementation versus trials where supplemental glutamine was given parenterally;
-
trials where the aim was to give at least 0.2 g/kg/day of glutamine versus trials where less glutamine supplementation was given.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies.
Results of the search
We have included a trial previously identified as ongoing in this review update (Ong 2012). The status of NCT00647036, which was also identified as ongoing in 2011, is not known.
Three trials, in which a total of 274 infants participated, fulfilled eligibility criteria (Duggan 2004; Albers 2005, Ong 2012). The abstract of another small trial (n = 13) was found by our searches in 2011 (Nolin 2001). Further searching in September 2014 identified the full‐text of a master's thesis (Nolin 2000). As this study did not focus on infants undergoing gastrointestinal surgery we have now excluded it from this review.
Included studies
Two trials were single‐centre studies undertaken in surgical neonatal and paediatric intensive care centres in Europe (Albers 2005) and North America (Duggan 2004). The third trial was conducted in 14 paediatric surgical units across the UK (Ong 2012). All participants were recruited between 2000 and 2005. The smallest trial was a pilot study that aimed to assess feasibility and safety (Duggan 2004).
Participants
Duggan 2004 enrolled 20 young infants with severe gastrointestinal diseases (NEC, intestinal atresia, or anterior abdominal wall defects). Most participants were preterm (average gestational age at birth 33 weeks). Their median postnatal age at enrolment was 15 days.
Albers 2005 enrolled 80 children of gestational age at birth > 30 weeks and chronological age less than two years. This trial, therefore, did not strictly fulfil our a priori population criterion (infants < 3 months old). However, we decided to include the trial because the report stated that most (69 of the 80) participants were less than six months old at enrolment. The report did not provide subgroup data for infants less than three months old. The most common gastrointestinal conditions affecting participants were NEC, congenital bowel atresia or obstruction, anterior abdominal wall defects, and Hirschsprung's disease. Infants were not expected to be able to tolerate enteral nutrition for at least four days following gastrointestinal tract surgery. The median postnatal age at enrolment was 11 days.
Ong 2012 enrolled 174 young infants with severe gastrointestinal diseases (abdominal wall defect, congenital bowel obstruction, or NEC). Most participants were born at term (median gestational age at birth 37 weeks). Their median postnatal age at enrolment was five days.
Interventions
Duggan 2004: When judged by the attending clinicians to be ready to tolerate the introduction of enteral feeds, participating infants were randomly allocated to either glutamine‐supplemented expressed human milk or hydrolysed formula milk versus human milk or formula without added glutamine (but with an iso‐osmolar mix of non‐essential amino acids). The glutamine‐supplemented and non‐supplemented milks were indistinguishable to the parents, carers, and assessors. The intervention group received 0.08 g/kg/day of glutamine at the start of the study. This increased to 0.31 g/kg/day by two weeks post enrolment. Glutamine supplementation was stopped on day 30. All infants received the same level of parenteral nutrition (without added glutamine) while enteral feeds were being advanced.
Albers 2005: On the second postoperative day, infants in the intervention group received a standard parenteral nutrition solution supplemented with L‐glutamine sufficient to provide 0.4 g/kg/day. Control infants received parenteral nutrition with an iso‐nitrogenous amino acid solution. The carers were not aware whether participating infants received glutamine‐supplemented or non‐supplemented parenteral nutrition. The protocol specified that participating infants should continue to receive full parenteral nutrition until at least the sixth postoperative day when enteral feeding was gradually re‐introduced.
Ong 2012: From day three of parenteral nutrition, infants in the intervention group received a glutamine dipeptide supplement providing 0.4 g/kg/day of glutamine. This was continued while parenteral nutrition was administered. The control group received an isonitrogenous, isocaloric parenteral nutrition solution. The solutions were indistinguishable to parents, carers, and assessors, and nutritional information on labels did not allow identification of the solution. All study participants received only parenteral nutrition at the start of the trial and were gradually progressed to full enteral feeds. While exclusively receiving parenteral nutrition the control group did not receive any glutamine. With the introduction of enteral feeds, the control group received some naturally occurring glutamine in breast milk or formula.
Outcomes
Duggan 2004 assessed the effect of enteral glutamine supplementation on the time taken to establish full enteral feeding, rates of nosocomial infection, and growth during the study period.
Albers 2005 assessed the effect of parenteral glutamine supplementation on intestinal permeability (urinary excretion ratios of lactulose and rhamnose) and nitrogen balance. Mortality, duration of intensive care stay, and rates of nosocomial infection were reported as secondary outcomes.
Ong 2012 assessed the effect of parenteral glutamine supplementation on time required to achieve full enteral nutrition and the incidence of "sepsis" and "septicaemia". Adverse events and mortality were also reported.
Excluded studies
Three trials that appeared potentially eligible were excluded after further evaluation of the full report (Barbosa 1999; Nolin 2000, Ehrenkranz 2011). Most participants in these trials were infants with non‐gastrointestinal disease.
Risk of bias in included studies
The overall methodological quality of all studies was good.
Allocation
All trials used methods to conceal allocation.
Blinding
All trials blinded carers and assessors to the intervention.
Incomplete outcome data
All trials achieved complete or near‐complete follow‐up.
Effects of interventions
Glutamine supplementation (parenteral or enteral) versus no supplementation
Mortality prior to hospital discharge (Outcome 1.1):
None of the trials nor a meta‐analysis of data from all trials showed a statistically significant difference in mortality (typical RR 0.79, 95% CI 0.19 to 3.20; typical RD ‐0.01, 95% CI ‐0.05 to 0.03). (Figure 1; Analysis 1.1).
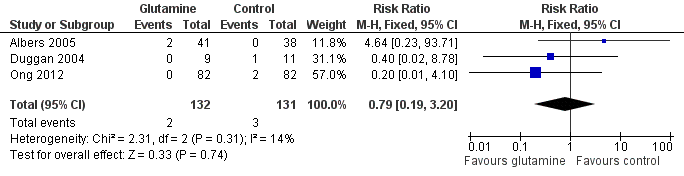
Forest plot of comparison 1: glutamine supplementation (parenteral or enteral) versus no supplementation, outcome 1.1: death prior to hospital discharge.
Neurodevelopmental outcomes
This outcome was not assessed in any trial.
Incidence of invasive infection (Outcome 1.2)
None of the trials nor a meta‐analysis of data from both trials showed a statistically significant difference in infection rates (typical RR 1.37, 95% CI 0.89 to 2.11; typical RD 0.08, 95% CI ‐0.03 to 0.18). (Figure 2; Analysis 1.2)

Forest plot of comparison 1: glutamine supplementation (parenteral or enteral) versus no supplementation, outcome 1.2: incidence of invasive infection.
Time to establish full enteral feeds
Duggan 2004 reported no statistically significant difference in the time taken to establish full enteral feeding in the intervention group (median 39 days; interquartile range 12 to 99) versus the control group (median 21 days; interquartile range 6 to 59). Similarly, Ong 2012 reported that there was no statistically significant difference in the time taken to establish full enteral feeds between the intervention group (median: 19 days; 5th to 95th centile 14.6 to 23.4) and the control group (median: 16 days; 5th to 95th centile 13.6 to 18.4). Albers 2005 did not report on this outcome.
Time to discharge from hospital
Albers 2005 did not find a statistically significant difference between the intervention group (median 32 days; interquartile range 16.8 to 44.8) and the control group (median 31.5 days; interquartile range 14 to 64). Duggan 2004 did not assess time to hospital discharge (according to personal communication from trial authors). Time to discharge was not reported by Ong 2012.
Growth during the trial period
Duggan 2004 did not find a statistically significant difference in the rate of weight gain during the study period (MD 3.80 g/day; 95% CI ‐6.65 to 14.25) (Figure 3; Analysis 1.3). The report also stated that there was not a statistically significant difference in the rate of change in length or 'arm anthropometrics' but data were not presented. Ong 2012 reported no significant difference in weight centile change between groups and maintenance of head circumference centiles in each group. Data were not presented. Albers 2005 did not report growth during the trial period.

Forest plot of comparison 1: glutamine supplementation (parenteral or enteral) versus no supplementation, outcome 1.3: weight gain during trial period (g/day).
Subgroup analyses
Trials where participants were predominantly term infants versus trials where participants were predominantly preterm infants
Duggan 2004 recruited preterm infants predominantly ‐ see above. For Albers 2005 and Ong 2012, subgroup data were not available.
Trials where participants were infants with NEC versus trials where participants were infants who had undergone gastrointestinal surgery for other indications
In Albers 2005, 19 of 80 participating infants had NEC. In Duggan 2004, eight of 20 participating infants had NEC. In Ong 2012, seven of 174 participating infants had NEC. Subgroup data for infants with NEC versus other surgical gastrointestinal conditions were not presented in any of the reports.
Trials where infants received enteral glutamine supplementation versus no supplementation and trials where supplemental glutamine was given parenterally versus no supplementation
Albers 2005 and Ong 2012 studied the effect of parenteral glutamine supplementation ‐ see above. Duggan 2004 studied the effect of enteral glutamine supplementation ‐ see above.
Trials where the aim was to give at least 0.2 g/kg/day of glutamine versus trials where less glutamine supplementation was given
All trials aimed to provide at least 0.2 g/kg/day on average of glutamine.
Discussion
Summary of main results
Three good quality randomised controlled trials in which 274 infants with severe gastrointestinal disease participated assessed the effect of glutamine supplementation versus no glutamine. The available data do not provide evidence that supplemental glutamine affects mortality, rates of invasive infection, or other morbidity.
Overall completeness and applicability of evidence
Although methodologically sound, the included trials were too small to detect moderate but potentially important effects on the specified outcomes of this review. Based on a control event rate for invasive infection (consistent with the included trials), a single trial would need to enrol about 2500 participants (1250 in each group) to be able to detect a 25% risk reduction with 5% significance and 90% power. This optimum study size is an order of magnitude larger than the total number of participants in the trials identified in this review.
Furthermore, the included trials recruited a heterogeneous group of participants with a range of severe gastrointestinal diseases. This heterogeneity may limit meaningful evaluation of the effect of glutamine supplementation. For example, the effect of glutamine supplementation may be different for sick, very preterm infants recovering from severe NEC compared with term infants who have had reduction of an anterior abdominal wall defect but are otherwise metabolically and physiologically stable. If further data become available from ongoing trials, subgroup analyses by gestational age at birth (term versus preterm) or by type of gastrointestinal disease may be needed to define which groups of infants, if any, benefit from glutamine supplementation.
Variation in the route of administration of supplemental glutamine (enteral versus parenteral) is also a potentially important source of trial heterogeneity that may affect outcomes. Systematic review of trials in adults suggests that parenteral supplementation confers more clinical benefits than enteral supplementation (Novak 2002). However, laboratory studies have suggested that using the enteral route results in a much higher concentration of free glutamine being delivered to the intestinal mucosa and that this reduces bacterial translocation and invasive infection (Panigrahi 1997). The theoretical disadvantage to enteral administration is that glutamine is metabolised entirely in the splanchnic compartment with no net increase in free glutamine delivery to other organs (Parimi 2004). The most appropriate route of delivery may also be affected by the underlying gastrointestinal condition. For example, it may not be possible to deliver enteral glutamine to very preterm infants with severe NEC, but this may be the most appropriate route for clinically stable infants recovering from a less physiologically destabilising gastrointestinal condition.
Quality of the evidence
All three trials were of good methodological quality but were too small to detect moderate but potentially important effects (Duggan 2004; Albers 2005, Ong 2012).
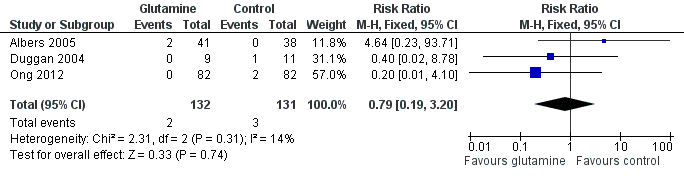
Forest plot of comparison 1: glutamine supplementation (parenteral or enteral) versus no supplementation, outcome 1.1: death prior to hospital discharge.

Forest plot of comparison 1: glutamine supplementation (parenteral or enteral) versus no supplementation, outcome 1.2: incidence of invasive infection.

Forest plot of comparison 1: glutamine supplementation (parenteral or enteral) versus no supplementation, outcome 1.3: weight gain during trial period (g/day).
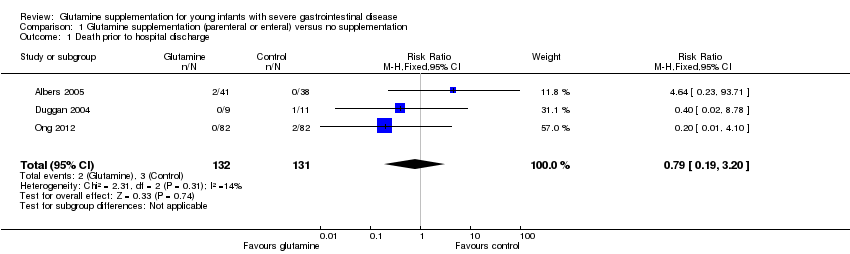
Comparison 1 Glutamine supplementation (parenteral or enteral) versus no supplementation, Outcome 1 Death prior to hospital discharge.
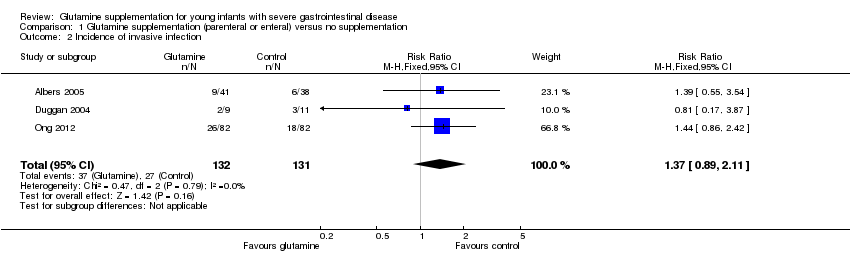
Comparison 1 Glutamine supplementation (parenteral or enteral) versus no supplementation, Outcome 2 Incidence of invasive infection.
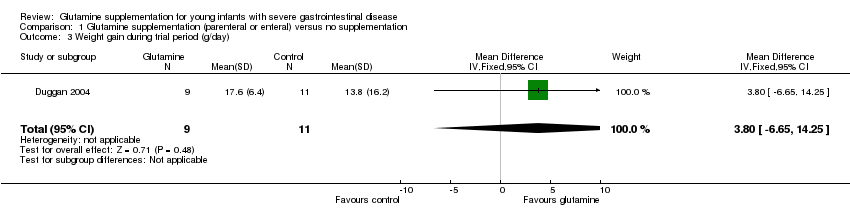
Comparison 1 Glutamine supplementation (parenteral or enteral) versus no supplementation, Outcome 3 Weight gain during trial period (g/day).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death prior to hospital discharge Show forest plot | 3 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.19, 3.20] |
| 2 Incidence of invasive infection Show forest plot | 3 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.89, 2.11] |
| 3 Weight gain during trial period (g/day) Show forest plot | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 3.80 [‐6.65, 14.25] |

