Отказ от приема стероидов или их отмена у реципиентов почечного трансплантата
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods |
| |
| Participants |
| |
| Interventions | Withdrawal group
Maintenance group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomization was stratified by centre and was done centrally to maintain a 1:1 ratio at each centre' |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind placebo controlled. Stated 'After randomisation, recipients received blister packs containing tablets for their 'prednisone' dose. Neither recipients nor physicians knew whether a randomised patient was in the withdrawal group' |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind placebo controlled. Stated 'After randomisation, recipients received blister packs containing tablets for their 'prednisone' dose. Neither recipients nor physicians knew whether a randomised patient was in the withdrawal group' |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind placebo controlled. Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis performed; all participants were followed for the primary endpoint until study closure on 22 July 1998 |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | High risk | The study was supported by Roche Laboratories |
| Methods |
| |
| Participants |
| |
| Interventions | Avoidance group
Withdrawal group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear whether ITT analysis performed, total number of patients by group analysed not reported, results presented as percentages/rates |
| Selective reporting (reporting bias) | High risk | Acute rejection not reported |
| Other bias | Unclear risk | Funding sources not reported |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Stated 'randomly assigned' but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Unclear risk | Number of patients by group not reported for outcomes; unclear if ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | Funding source not reported Abstract‐only publication |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated 'Randomization was performed with a 1:1 ratio stratified by centre. The randomization list was generated by the Data Operation Department of Fujisawa GmbH. Each centre received a unique sequence of patient numbers and a set of sealed envelopes.' |
| Allocation concealment (selection bias) | Low risk | Stated 'sealed envelopes' |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis performed; all patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review have been reported |
| Other bias | High risk | Sponsored by a grant from Fujisawa GmbH The investigator‐initiated 1‐year follow‐up was supported by an unrestricted grant from Astellas, Munich, Germany |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated 'centrally randomised' but no further information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Stated 'in a placebo controlled double‐blinded fashion' but no further information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Stated 'in a placebo controlled double‐blinded fashion' but no further information provided |
| Blinding of outcome assessment (detection bias) | Low risk | Stated 'in a placebo controlled double‐blinded fashion' but no further information provided. Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Low risk | Total number of patients by group not reported for outcomes; ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review have been reported |
| Other bias | High risk | High drop‐out rate before randomisation (52%) Choice of calcineurin inhibitor was centre specific (TAC or CsA) Support provided by NIH UO1‐A1‐46135 and Wyeth Pharmaceuticals The study was terminated early due to an unanticipated high incidence of PTLD |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomly assigned' but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | High risk | Number of patients in whom the outcome were measured is ambiguous (two reports with different number of patients in each group); 14% failed to comply with follow‐up protocol; unclear if ITT analysis performed |
| Selective reporting (reporting bias) | High risk | Death and graft loss are only reported in one of the two published reports, but number of participants in each group vary between reports |
| Other bias | Unclear risk | Unclear whether informative censoring is present, because the two published reports are different in regard to number of participants and time period of study Funding source not reported |
| Methods |
| |
| Participants |
| |
| Interventions | Avoidance group
Withdrawal group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was performed by opening a closed opaque numbered envelope |
| Allocation concealment (selection bias) | Low risk | Stated 'closed opaque envelopes' |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Low risk | All patients followed up or accounted for; ITT analysis performed ('Analyses were made on an ITT basis.' |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review have been reported |
| Other bias | Unclear risk | Funding source not reported |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis performed; all patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | High risk | This study was supported by a grant from Sandoz, The Netherlands |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'all patients were randomised' but no further information provided. |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind placebo controlled, but partially unblinded for interim analysis |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind placebo controlled, but partially unblinded for interim analysis |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind placebo controlled, outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | High risk | 43% of patients were withdrawn from the study for various reasons; patients who died/lost their graft were excluded from the study; unclear if ITT analysis performed |
| Selective reporting (reporting bias) | High risk | Primary endpoints for this review not reported, primarily surrogate outcomes reported |
| Other bias | Unclear risk | Abstract data only available Funding source not reported |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomly assigned' but no further information provided |
| Allocation concealment (selection bias) | Low risk | Stated 'assigned by sealed envelopes' |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | High risk | ITT analysis not performed; 6 patients in treatment group and 5 patients in control group excluded because of switch to different immunosuppression |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Low risk | Funded by grant of the Consiglio Nazionale delle Richerche |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis performed; all patients followed‐up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | Funding source not reported; abstract data only |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Patients were randomised using a block size of 4 with no stratification by the contract research organization using a validated automated system.' |
| Allocation concealment (selection bias) | Low risk | 'With sealed envelopes distributed to the participating centers...opened after randomization by the investigator.' |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis performed, all patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | High risk | The study was funded by Novartis Pharma SAS, Rueil‐Malmaison, France The manuscript was prepared with editorial support from a freelance medical writer funded by Novartis Pharma SAS |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | '...eligible patients were randomised 1:1 to 1 of the treatment arms. Randomization was stratified according to centre, recipient age at transplantation (<60 and 60 years) and creatinine clearance at month 3 (55 and >55 mL/min), according to a biased coin design.' |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | ITT analysis for primary analysis, but total number of patients by group for outcomes not reported |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | High risk | Difference in CsA levels between groups (higher levels in treatment group) The study was sponsored by Novartis according to ClinicalTrials.gov. 'Editorial assistance was provided by Mary Hines, Springer Healthcare Communications, and funded by Novartis Farma, Italy.' |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but no further information provided. |
| Allocation concealment (selection bias) | Low risk | 'Using sealed envelopes'. |
| Blinding (performance bias and detection bias) | High risk | 'Patients were informed to which arm of the trial they had been allocated.' |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | High risk | "The patients randomised to the withdrawal group were followed with more frequent serum creatinine estimation." A rise in serum creatinine prompted kidney biopsy to detect biopsy proven acute cellular rejection which is the primary endpoint of this study |
| Incomplete outcome data (attrition bias) | Unclear risk | Total number of patients by group for outcomes not reported. Number of patients who were lost to follow up is unclear |
| Selective reporting (reporting bias) | High risk | Patient and graft survival are not reported |
| Other bias | Unclear risk | Time lead bias, because follow up started with date steroids were completely withdrawn in treatment group but with randomisation for control group Funding source not reported |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Eligible patients were assigned to CS or non‐CS treatment at a 1:1 ratio using block randomization with stratification according to the recipient's age and cold ischaemia time.' |
| Allocation concealment (selection bias) | Low risk | 'Treatment codes were provided in sealed envelopes'. |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints. |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis performed; 4 patients in control group excluded from analysis for acute rejection but included for patient and graft survival analysis. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported. |
| Other bias | High risk | TAC, SRL, EVL, AZA could be introduced according to centre practice Steroid dosing after 6 months according to centre practice, unclear whether patients were withdrawn from steroids or maintained on steroids Study was sponsored by the Nantes University Hospital Statistical analysis of study data was supported by Fresenius Biotech GmbH, Germany |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1
Treatment group 2
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated 'Randomization was undertaken in a 1:1:1 ratio using a validated system that automates the random assignment of treatment groups to randomization numbers.' |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis performed; all patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary endpoints for this review reported |
| Other bias | High risk | The study was funded by Novartis Pharma AG |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline Immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated 'randomised blocks of various sizes were generated and used to attain a balanced, restricted randomization according to treatment centre. The order of randomization did not have a repeating sequence' |
| Allocation concealment (selection bias) | Low risk | Stated 'Physicians did not know the randomization number until the patient was enrolled, and the code was not broken until the analysis' |
| Blinding (performance bias and detection bias) | Low risk | Stated '...the code was not broken until the analysis. Patients were randomly assigned at 90 days to receive either a placebo or prednisone by means of a process that prevented prior knowledge of their treatment group' |
| Blinding of participants and personnel (performance bias) | Low risk | Stated 'The study was doubly blinded. The placebo and prednisone were prepared in an indistinguishable form and dispensed as coded therapy' |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blinded placebo controlled, outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Low risk | Stated 'No patients were excluded after entry (as distinct from withdrawals in the survival analysis) or lost to follow‐up.'; ITT analysis performed |
| Selective reporting (reporting bias) | High risk | Acute rejection not reported |
| Other bias | High risk | This work was supported by Sandoz Ltd., Basel, Switzerland, Sandoz Canada Inc., Dorval, Que., Upjohn Ltd., Kalamazoo, Mich., the Richard and Jean Ivey Fund, London, Ont., the Michael Fung Endowment Fund, London, Ont., the Claudine Keown Endowment Fund, London, Ont., the University Hospital Transplant Research Fund, London, Ont., Robarts Research Institute endowment funds and the City of London, Ont |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated 'central randomization by the principal investigator', stated 'block randomization stratified by pubertal status'. |
| Allocation concealment (selection bias) | Unclear risk | Stated 'concealed allocation' but not further information provided |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis performed; all patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | 'Because recruitment of patients for this study was more difficult than anticipated (because some patient's parents and covering physicians had a strong bias pro or con steroid withdrawal, we performed an interim analysis, which revealed a significant difference in growth between both groups. Hence, the study was finished prematurely.' Funding source not reported |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear if ITT analysis conducted |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | High risk | Funding source not reported but authors disclose 'Grant/Research Support, Novartis (Myfortic)', Co‐authors affiliated with Novartis Pharma SAS, Rueil‐Malmaison, France Abstract data only Lack of important information regarding design and conduct of study |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but no further information provided. |
| Allocation concealment (selection bias) | Low risk | Stated 'using the sealed envelope method' |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis performed; all patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Low risk | 'The study was supported by a grant from the Sigrid Juselius Foundation.' AZA dose was increased during and after steroid withdrawal in treatment group while it remained unchanged in maintenance group |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear whether ITT analysis performed; number of patients by group not reported for outcomes |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | Funding source not reported Patients in treatment group were enrolled later after transplantation compared to control group |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'The recipient was entered into the trial by drawing a card to determine immunosuppressive therapy.' |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear if ITT analysis performed; total number of patients by group not reported for outcomes, results presented as rates and percentages |
| Selective reporting (reporting bias) | High risk | Acute rejection not reported |
| Other bias | Unclear risk | Funding sources not reported 465 patients included in first publication (1989), 700 patients included in second publication in (1990). Patients in third arm (AZA + steroids) remained equal in size, while the treatment group (steroid avoidance = CsA monotherapy) gained most of the additional patients, which was the group with the better outcomes in first publication. Immunosuppressive protocol differs between these two publications with lower CsA target levels and more steroids in 2nd publication. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear whether ITT analysis performed; total number of patients by group for outcomes not reported |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | Funding source not reported Abstract‐only publication |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised 1:1 ratio' but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Unclear risk | One patient lost to follow up in withdrawal group (8%), unlikely to affect results; unclear if ITT analysis performed |
| Selective reporting (reporting bias) | High risk | Graft loss not reported |
| Other bias | Unclear risk | Funding source not reported Abstract‐only publication |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomization was completed using the first generator plan from randomization.com.' |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis performed; all patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | High risk | First 17 patients (38%) in withdrawal group received steroids until day 7 and two additional doses of basiliximab, the remaining 28 patients (62%) received steroids until day 2 and no additional basiliximab 'The study was funded internally by clinical revenue. The manuscript was support by an unrestricted educational grant from Novartis Pharm. Corp.' |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Patients were randomised by a blinded nurse coordinator according to random numbers.' |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | 'A single pathologist who was blinded to the treatment arms, evaluated biopsy specimens for severity of rejection and fibrosis.' |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear whether ITT analysis performed. In treatment group 16 of 32 patients and in control group 14 of 28 patients completed 1 year follow‐up |
| Selective reporting (reporting bias) | High risk | Mortality and graft loss are not reported |
| Other bias | Unclear risk | Funding source not reported Unclear whether groups were similar at baseline, because 'steroid withdrawal patients were at greater risk for rejection, having a higher average number of HLA mismatches and a greater number of African American patients' |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'Patients were randomly assigned to one of two treatment groups in a 1:1 ratio, with stratification by cadaveric/ living related donor transplant recipient and by type of cyclosporine' but random sequence generation unclear |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Stated 'Treatment continued in a blinded fashion for 6 months, after which the study was to be unblinded during a further 6 months, for a total study length of 1 year' |
| Blinding of participants and personnel (performance bias) | High risk | Stated 'Treatment continued in a blinded fashion for 6 months, after which the study was to be unblinded during a further 6 months, for a total study length of 1 year' |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear whether ITT analysis performed; stated 'At 12 months 17% in the control group and 25% in the treatment group were prematurely withdrawn from the study' |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | Funding source not reported |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated' randomised' but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Unclear risk | All patients followed up or accounted for; unclear if ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | Funding source not reported |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients were randomised according to the month of birth |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Low risk | All patients followed up or accounted for; ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Low risk | The study was supported by grant N°3631‐3 awarded by the Internal Grant Agency of the Ministry of Health of the Czech Republic |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated 'central randomization by the principle investigator'. |
| Allocation concealment (selection bias) | Low risk | Stated 'stratified treatment allocation on the basis of block randomization carried out by a statistician who was not participating in this study using numbered containers by a computerized statistical program'. |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear whether ITT analysis performed; outcomes for prepubertal patients only reported. Number of events and per group not reported |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Low risk | This study was supported by Fondecyt 1080166 (National Fund for Scientific and Technological Development) |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised randomisation by a randomisation list, stratified within centres using an interactive voice‐response system |
| Allocation concealment (selection bias) | Low risk | 'The sequence was concealed until interventions were assigned.' |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Low risk | All patients followed up or accounted for; ITT analysis performed ('All the analyses considered all the randomised patients, grouped originally by randomised treatment as per ITT concept.') |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | High risk | Supported by grant from Novartis |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | '...patients were randomised to receive...' but no further information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | Unclear if ITT analysis performed; total number of patients by group for outcomes not reported |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | Funding source not reported |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | '100 similar closed opaque envelopes were made, each containing a slip of opaque paper with the type of maintenance immunosuppression. Therefore, 50 envelopes were with steroid and the rest were without. All envelopes were kept closed until the morning of the transplant day, when one envelope was selected for each patient'. |
| Allocation concealment (selection bias) | Low risk | 'Similar closed opaque envelopes, each containing a slip of opaque paper'. |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear whether ITT analysis performed; number of patients in groups varies slightly between reports |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | Different protocol between groups for steroid dosing before withdrawal Funding source not reported |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was achieved by drawing a card |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Unclear risk | Total number of patients by group not reported for outcomes; ITT analysis performed |
| Selective reporting (reporting bias) | High risk | Acute rejection not reported |
| Other bias | Unclear risk | Immunosuppressive protocol differs between publications No patient characteristics shown, unclear whether the groups were similar at baseline Funding source not reported |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but no further information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Unclear risk | Number of patients in which the outcome was measured are not reported, survival only reported as rates; unclear if ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | Funding source not reported There's a substantial difference between number of participants in first published report (1994) (294) and second report (1998) (68) which is not explained |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but no further information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear if ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | Funding source not reported Steroids have been withdrawn at different time points after transplantation and the time point of steroid withdrawal is unclear Different induction treatments used, 14% of patients did not receive any induction treatment |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but no further information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear whether ITT analysis was performed; number of patients per group and in total vary between reports |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | Funding source not reported Abstract‐only data Lack of important information regarding design and conduct of study |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Random assignments were made according to a randomization list balanced per centre through a telephone call to the coordinating centre.' |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis performed; all patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | Funding source not reported Sandoz Prodotti Farmaceutici SpA provided logistic support for the SIMTRE group meetings |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but no further information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints. |
| Incomplete outcome data (attrition bias) | Low risk | All patients followed up or accounted for; ITT analysis performed ("Unless otherwise stated, data were analysed with groups assigned on the basis of "intention‐to‐treat") |
| Selective reporting (reporting bias) | High risk | Acute rejection is not reported |
| Other bias | Unclear risk | Funding source not reported |
| Methods |
| |
| Participants |
| |
| Interventions | Avoidance group
Withdrawal group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label, primary study endpoint was the percentage of patients who could be successfully withdrawn from steroids at 1 and 4 years after transplantation |
| Incomplete outcome data (attrition bias) | Unclear risk | Stated ' The results were analyzed on an ITT basis' but patients were excluded from analysis due to protocol violation; reasons for loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | Funding source not reported Lack of important information regarding design and conduct of study High percentage of protocol failure (38% in avoidance group and 33% in withdrawal group not withdrawn from steroids at 1 year after transplantation) |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Patients were randomised using a table of random numbers' |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Low risk | All patients followed up or accounted for; ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | Groups at baseline were different regarding gender, race and causes of kidney failure with more females, less African‐Americans, more diabetics in the steroid avoidance group Funding source not reported |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'Patients were randomly assigned to one of the three treatment groups in a 1:1:1 ratio, with stratification for cadaveric/living related transplant, for centre, and for the number of acute rejections during the first 6 mo after transplantation' but random sequence generation not reported |
| Allocation concealment (selection bias) | Low risk | Stated 'Randomization was carried out by opening a sealed envelope with the lowest available study number' |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis performed; all patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | High risk | The study was supported by Roche Pharmaceuticals, Mijdrecht, the Netherlands |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline Immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Unclear risk | Number of events and number of patients analysed not reported; unclear if ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | Funding source not reported |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'A computer‐derived randomised blocks of varying size was generated and noted in a series of opaque envelopes held by the research pharmacist at each participating centre.' |
| Allocation concealment (selection bias) | Low risk | Stated 'opaque envelopes' |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear whether ITT analysis performed and whether all patients have been followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | High risk | The study was supported by Medical Research Council of Canada; Richard and Jean Ivey Fund, London, Ontario; Sandoz Ltd, Basel; the Micheal Fung Endowment Fund, London, Ontario; the University Hospital Transplant Research Fund, London, Ontario |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'Randomization (1:1:1) was stratified by centre and donor type' but random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Stated 'The investigators were blinded with respect to randomization until the month‐3 visit.' which is the time before start of the intervention, but thereafter investigators were unblinded, thus this is an open‐label study |
| Blinding of participants and personnel (performance bias) | High risk | Stated 'The investigators were blinded with respect to randomization until the month‐3 visit.' which is the time before start of the intervention, but thereafter investigators were unblinded, thus this is an open‐label study |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis performed; all patients followed‐up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | High risk | Unclear whether the rather short follow‐up period allows sufficient time for endpoints to occur This study was supported by Fujisawa GmbH, Munich, Germany |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis performed; all patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | High risk | The study was supported by Novartis Pharmaceuticals Corporation, East Hanover, NJ |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated 'Randomization was based on a permuted block design with block sizes of 6 within each clinical site. Randomization was performed using a central randomization service at the EMMES Corporation (Potomac, Md, US). Patients were randomised 1:1 stratified by race and donor type' |
| Allocation concealment (selection bias) | Low risk | Stated 'The EMMES Corporation generated the allocation sequence and maintained the allocation code. The randomization order did not have a repeating sequence, and the randomization code was not broken or revealed to patients/investigators until subjects completed study' |
| Blinding (performance bias and detection bias) | Low risk | Stated 'Patients received a blinded study drug beginning on posttransplant day 8' |
| Blinding of participants and personnel (performance bias) | Low risk | Stated 'Study subjects, investigators, study personnel, and those assessing outcomes remained blinded throughout 5‐year duration of the study, unless medical necessity to unblind occurred' |
| Blinding of outcome assessment (detection bias) | Low risk | Stated 'Study subjects, investigators, study personnel, and those assessing outcomes remained blinded throughout 5‐year duration of the study, unless medical necessity to unblind occurred' |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis performed; all patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | Funding source not reported |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Baseline Immunosuppression
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) | Unclear risk | Number of patients and number of events per group not reported; unclear whether ITT analysis was performed. Number of patients lost to follow up not reported |
| Selective reporting (reporting bias) | High risk | Graft loss not reported |
| Other bias | Unclear risk | Funding source not reported It was not reported how many of the participants were randomised to either group, whether the timing of outcome assessment is similar in all groups, whether the groups were similar at baseline, whether co‐interventions were avoided or similar Important information on design and conduct of study not reported |
ALG ‐ anti‐lymphocyte globulin; ATG ‐ anti‐thymocyte globulin; AZA ‐ azathioprine; CMV ‐ cytomegalovirus; CNI ‐ calcineurin inhibitor; CrCl ‐ creatinine clearance; CsA ‐ cyclosporin; EC‐MPS ‐ enteric‐coated mycophenolate sodium; eGFR ‐ estimated glomerular filtration rate; EVL ‐ everolimus; GFR ‐ glomerular filtration rate; HBsAG ‐ hepatitis B surface antigen; HCT ‐ haematocrit; HIV ‐ human immunodeficiency virus; HLA ‐ human leukocyte antigen; HTLV‐1 ‐ human T‐lymphotropic virus type 1; IL‐2RA ‐ interleukin 2 receptor antagonist; ITT ‐ intention‐to‐treat analysis; IV ‐ intravenous; MMF ‐ mycophenolate mofetil; NODAT ‐ new‐onset diabetes post transplant; PO ‐ oral; PRA ‐ panel reactive antibodies; PTLD ‐ Post‐transplant lymphoproliferative disease; RCT ‐ randomised controlled trial; SCr ‐ serum creatinine; SD ‐ standard deviation; SRL ‐ sirolimus; TAC ‐ tacrolimus; WCC ‐ white cell count
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Wrong co‐intervention | |
| Wrong co‐intervention | |
| Not RCT | |
| Pancreatic islet transplantation | |
| Not RCT | |
| Pancreatic islet transplantation | |
| Wrong co‐intervention PLEASE ADD REASON FOR EXCLUSION | |
| Wrong co‐intervention | |
| Wrong co‐intervention | |
| Wrong co‐intervention | |
| No steroid withdrawal or avoidance | |
| No steroid withdrawal or avoidance | |
| No steroid withdrawal or avoidance | |
| No steroid withdrawal or avoidance | |
| Wrong co‐intervention | |
| Difference in co‐intervention | |
| Wrong co‐intervention | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not steroid withdrawal or avoidance | |
| Wrong co‐intervention | |
| Not RCT | |
| Pancreatic islet transplantation | |
| Not steroid withdrawal or avoidance | |
| Not steroid withdrawal or avoidance | |
| Wrong co‐intervention | |
| Wrong co‐intervention | |
| Wrong co‐intervention | |
| Wrong co‐intervention | |
| Not steroid withdrawal or avoidance | |
| Wrong co‐intervention | |
| Not steroid withdrawal or avoidance | |
| Not RCT | |
| Wrong co‐intervention | |
| Wrong co‐intervention | |
| Wrong co‐intervention | |
| Not RCT | |
| Not steroid withdrawal or avoidance | |
| Wrong co‐intervention | |
| Not RCT | |
| Wrong co‐intervention | |
| Wrong co‐intervention | |
| Wrong co‐intervention | |
| Wrong co‐intervention |
RCT ‐ randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Unclear if this was a RCT |
| Participants | Kidney transplant recipients not further specified, unclear time frame, but before 1989 |
| Interventions | Steroid withdrawal versus steroid maintenance plus CsA |
| Outcomes | Serum creatinine and acute rejection |
| Notes | Abstract‐only data; unable to contact authors |
CsA ‐ cyclosporin; RCT ‐ randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death and graft loss Show forest plot | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Steroid withdrawal versus steroid maintenance, Outcome 1 Death and graft loss. | ||||
| 1.1 Death up to one year | 10 | 1913 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.36, 1.30] |
| 1.2 Death one to five years | 7 | 1118 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.73, 2.17] |
| 1.3 Graft loss including death up to one year | 8 | 1817 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.64, 1.49] |
| 1.4 Graft loss including death one to five years | 7 | 1092 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [1.00, 2.01] |
| 1.5 Graft loss excluding death up to one year | 8 | 1817 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.72, 1.92] |
| 1.6 Graft loss excluding death one to five years | 7 | 1092 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.98, 2.64] |
| 2 Rejection Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.2 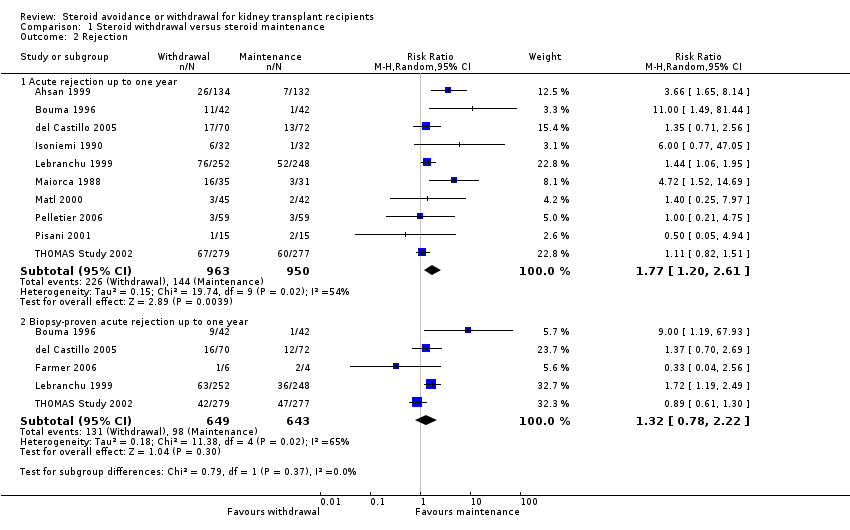 Comparison 1 Steroid withdrawal versus steroid maintenance, Outcome 2 Rejection. | ||||
| 2.1 Acute rejection up to one year | 10 | 1913 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [1.20, 2.61] |
| 2.2 Biopsy‐proven acute rejection up to one year | 5 | 1292 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.78, 2.22] |
| 3 New‐onset diabetes after transplantation and cardiovascular events Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Steroid withdrawal versus steroid maintenance, Outcome 3 New‐onset diabetes after transplantation and cardiovascular events. | ||||
| 3.1 New onset diabetes after transplantation up to five years | 6 | 1439 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.49, 1.21] |
| 3.2 Cardiovascular events up to five years | 2 | 607 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.42, 2.33] |
| 4 Infection and malignancy Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Steroid withdrawal versus steroid maintenance, Outcome 4 Infection and malignancy. | ||||
| 4.1 Infection (all) up to five years | 5 | 1819 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.84, 1.22] |
| 4.2 CMV infection up to five years | 5 | 1758 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.80, 1.36] |
| 4.3 Malignancy up to five years | 3 | 756 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.41, 1.46] |
| 5 Kidney function Show forest plot | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.5 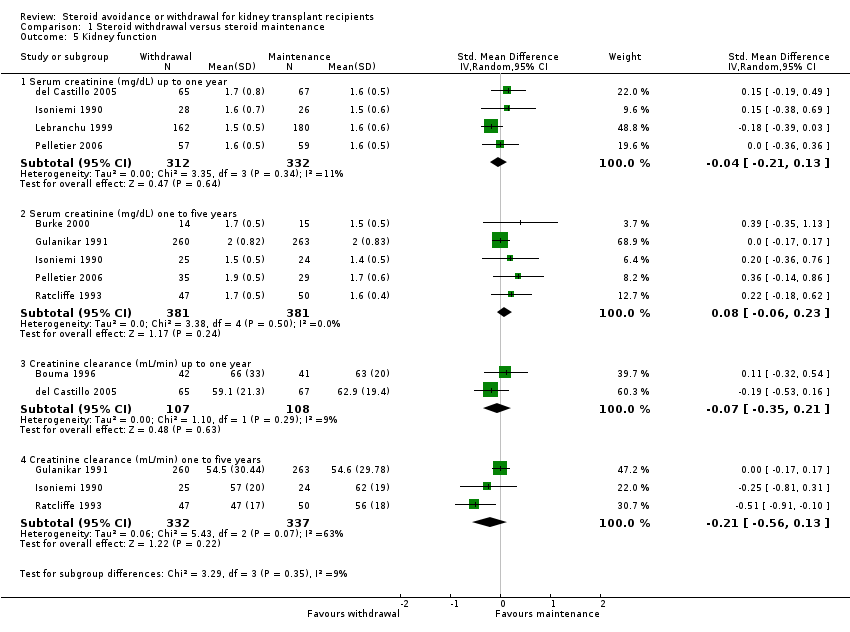 Comparison 1 Steroid withdrawal versus steroid maintenance, Outcome 5 Kidney function. | ||||
| 5.1 Serum creatinine (mg/dL) up to one year | 4 | 644 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.21, 0.13] |
| 5.2 Serum creatinine (mg/dL) one to five years | 5 | 762 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.06, 0.23] |
| 5.3 Creatinine clearance (mL/min) up to one year | 2 | 215 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.35, 0.21] |
| 5.4 Creatinine clearance (mL/min) one to five years | 3 | 669 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.56, 0.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death and graft loss Show forest plot | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Steroid avoidance versus steroid maintenance, Outcome 1 Death and graft loss. | ||||
| 1.1 Death up to one year | 10 | 1462 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.52, 1.80] |
| 1.2 Death one to five years | 7 | 1201 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.32, 1.01] |
| 1.3 Graft loss including death up to one year | 7 | 1211 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.72, 1.62] |
| 1.4 Graft loss including death one to five years | 7 | 1245 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.53, 1.18] |
| 1.5 Graft loss excluding death up to one year | 7 | 1211 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.64, 1.86] |
| 1.6 Graft loss excluding death one to five years | 7 | 1245 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.66, 1.45] |
| 2 Rejection Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.2 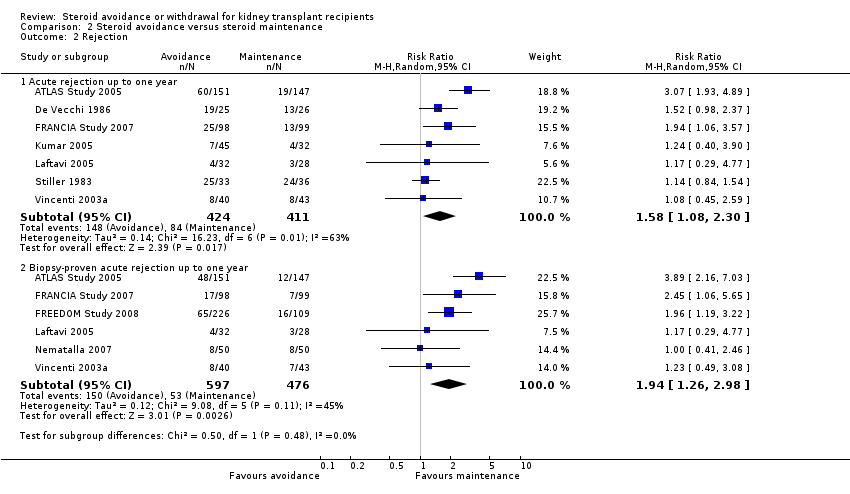 Comparison 2 Steroid avoidance versus steroid maintenance, Outcome 2 Rejection. | ||||
| 2.1 Acute rejection up to one year | 7 | 835 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [1.08, 2.30] |
| 2.2 Biopsy‐proven acute rejection up to one year | 6 | 1073 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [1.26, 2.98] |
| 3 New‐onset diabetes after transplantation and cardiovascular events Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 Steroid avoidance versus steroid maintenance, Outcome 3 New‐onset diabetes after transplantation and cardiovascular events. | ||||
| 3.1 New onset diabetes after transplantation up to five years | 9 | 1618 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.51, 1.10] |
| 3.2 Cardiovascular events up to five years | 4 | 1013 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.30, 1.05] |
| 4 Infection and malignancy Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 Steroid avoidance versus steroid maintenance, Outcome 4 Infection and malignancy. | ||||
| 4.1 Infection (all) up to five years | 9 | 1833 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.84, 1.03] |
| 4.2 CMV Infection up to five years | 6 | 1454 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.70, 1.31] |
| 4.3 Malignancy up to five years | 7 | 1635 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.61, 1.52] |
| 5 Kidney function Show forest plot | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.5  Comparison 2 Steroid avoidance versus steroid maintenance, Outcome 5 Kidney function. | ||||
| 5.1 Serum creatinine (mg/dL) up to one year | 5 | 735 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.12, 0.17] |
| 5.2 Serum creatinine (mg/dL) one to five years | 3 | 688 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.16, 0.14] |
| 5.3 Creatinine clearance (mL/min) up to one year | 6 | 1104 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.23, 0.08] |
| 5.4 Creatinine clearance (mL/min) one to five years | 3 | 563 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.25, 0.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death and graft loss Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Steroid avoidance versus steroid withdrawal, Outcome 1 Death and graft loss. | ||||
| 1.1 Death up to one year | 1 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.08, 1.98] |
| 1.2 Death one to five years | 2 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 2.67 [0.63, 11.32] |
| 1.3 Graft loss including death up to one year | 1 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.32, 2.29] |
| 1.4 Graft loss including death one to five years | 2 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [0.89, 6.70] |
| 1.5 Graft loss excluding death up to one year | 1 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.40, 6.68] |
| 1.6 Graft loss excluding death one to five years | 2 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [0.48, 7.67] |
| 2 Rejection Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 3.2  Comparison 3 Steroid avoidance versus steroid withdrawal, Outcome 2 Rejection. | ||||
| 2.1 Acute rejection up to one year | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Biopsy‐proven acute rejection up to one year | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 New‐onset diabetes after transplantation, infection, malignancy Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.3  Comparison 3 Steroid avoidance versus steroid withdrawal, Outcome 3 New‐onset diabetes after transplantation, infection, malignancy. | ||||
| 3.1 New onset diabetes after transplantation up to five years | 3 | 351 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.36, 1.09] |
| 3.2 Infection (all) up to five years | 3 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.76, 1.50] |
| 3.3 CMV Infection up to five years | 2 | 284 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.30, 0.92] |
| 3.4 Malignancy up to five years | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.28, 8.94] |
| 4 Kidney function Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.4 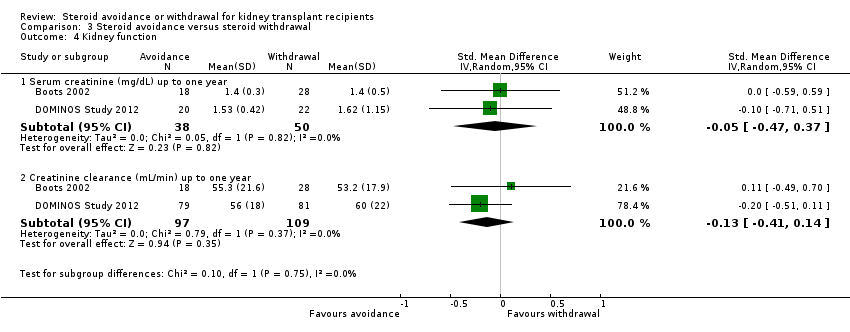 Comparison 3 Steroid avoidance versus steroid withdrawal, Outcome 4 Kidney function. | ||||
| 4.1 Serum creatinine (mg/dL) up to one year | 2 | 88 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.47, 0.37] |
| 4.2 Creatinine clearance (mL/min) up to one year | 2 | 206 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.41, 0.14] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death and graft loss Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.1  Comparison 4 Steroid withdrawal versus maintenance in children, Outcome 1 Death and graft loss. | ||||
| 1.1 Death up to five years | 2 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.02, 1.35] |
| 1.2 Graft loss including death up to five years | 2 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 0.69] |
| 1.3 Graft loss excluding death up to five years | 2 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.00, 1.64] |
| 2 Rejection, malignancy Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.2 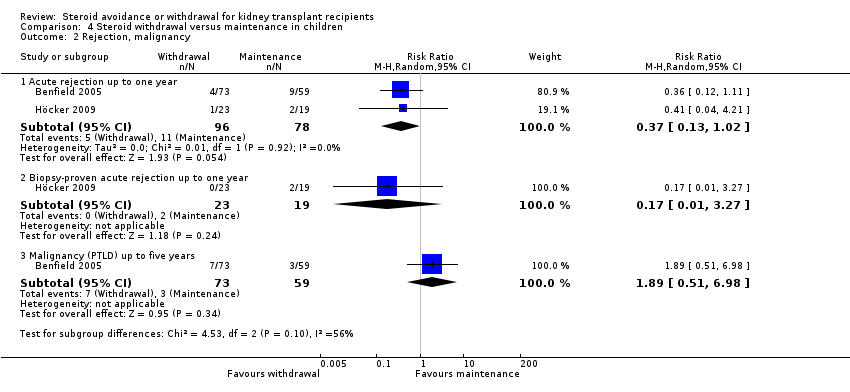 Comparison 4 Steroid withdrawal versus maintenance in children, Outcome 2 Rejection, malignancy. | ||||
| 2.1 Acute rejection up to one year | 2 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.13, 1.02] |
| 2.2 Biopsy‐proven acute rejection up to one year | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.01, 3.27] |
| 2.3 Malignancy (PTLD) up to five years | 1 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [0.51, 6.98] |
| 3 Kidney function Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.3  Comparison 4 Steroid withdrawal versus maintenance in children, Outcome 3 Kidney function. | ||||
| 3.1 Creatinine clearance (mL/min) up to five years | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Funnel plots Show forest plot | 20 | 5288 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [1.15, 1.62] |
| Analysis 5.1  Comparison 5 Publication bias, Outcome 1 Funnel plots. | ||||
| 1.1 Death, steroid withdrawal versus maintenance | 10 | 1913 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.39, 1.29] |
| 1.2 Acute rejection steroid withdrawal versus maintenance | 10 | 1913 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.28, 1.87] |
| 1.3 Death, steroid avoidance versus maintenance | 10 | 1462 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.52, 1.63] |

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
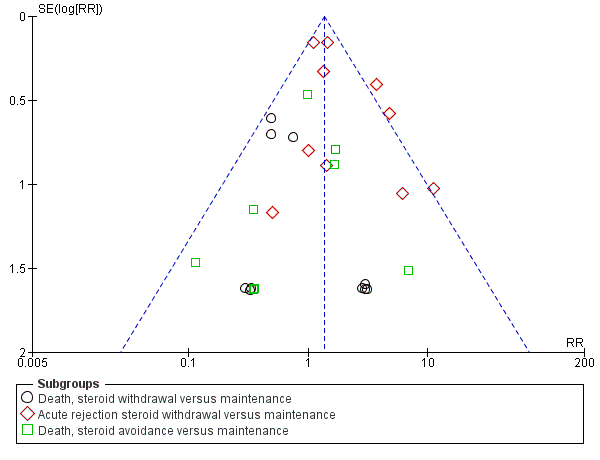
Funnel plot of comparisons that included at least 10 studies in the meta‐analysis

Comparison 1 Steroid withdrawal versus steroid maintenance, Outcome 1 Death and graft loss.
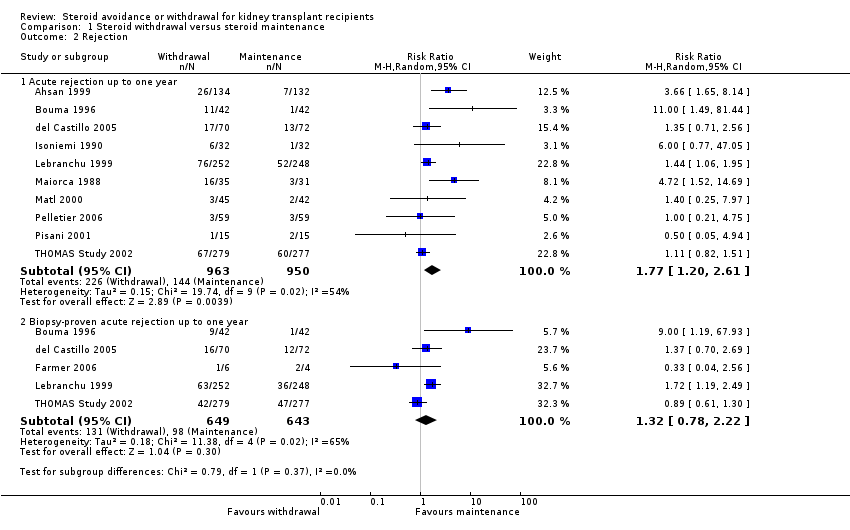
Comparison 1 Steroid withdrawal versus steroid maintenance, Outcome 2 Rejection.

Comparison 1 Steroid withdrawal versus steroid maintenance, Outcome 3 New‐onset diabetes after transplantation and cardiovascular events.
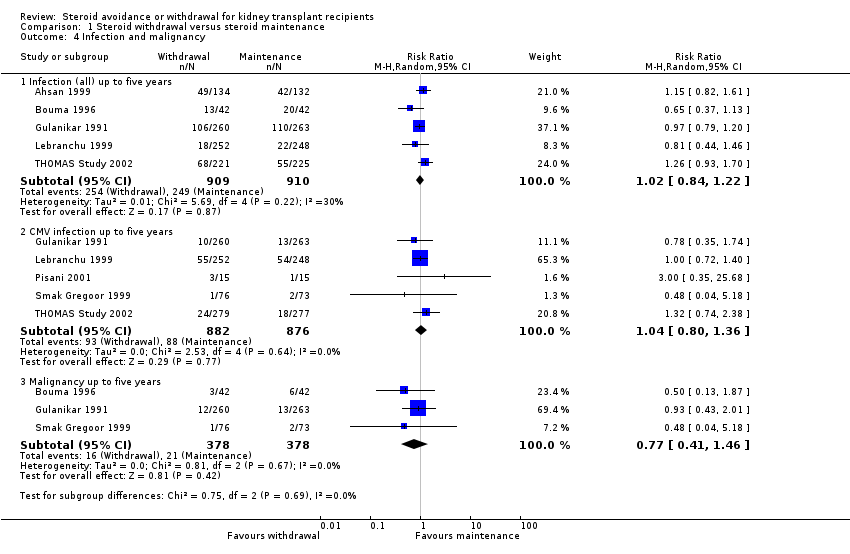
Comparison 1 Steroid withdrawal versus steroid maintenance, Outcome 4 Infection and malignancy.
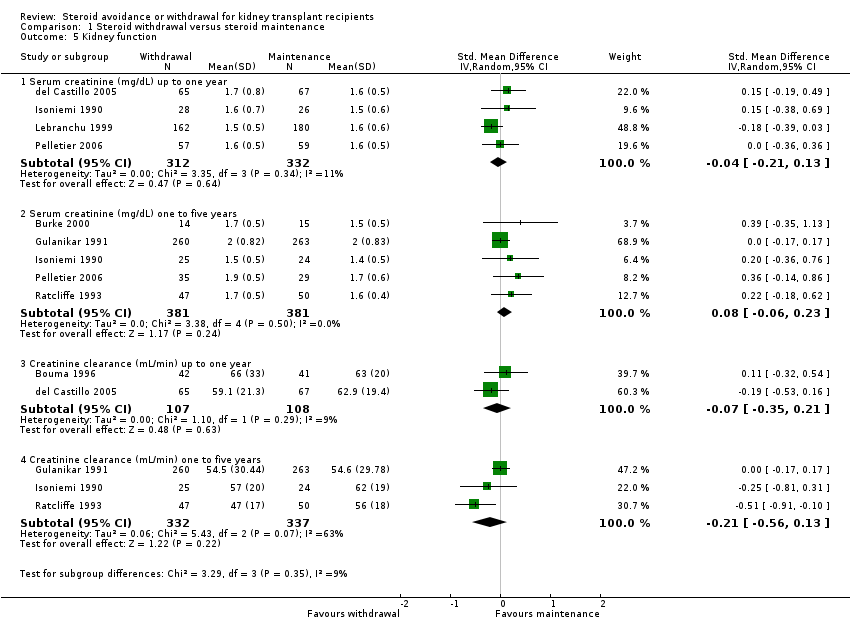
Comparison 1 Steroid withdrawal versus steroid maintenance, Outcome 5 Kidney function.

Comparison 2 Steroid avoidance versus steroid maintenance, Outcome 1 Death and graft loss.

Comparison 2 Steroid avoidance versus steroid maintenance, Outcome 2 Rejection.
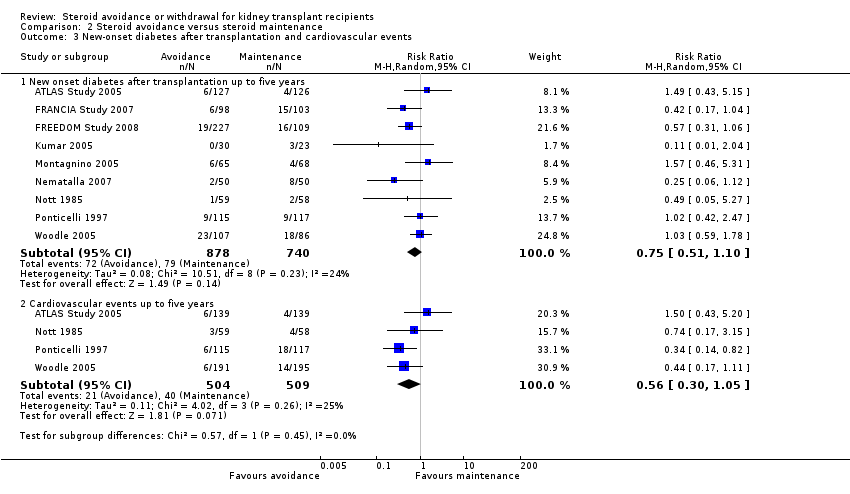
Comparison 2 Steroid avoidance versus steroid maintenance, Outcome 3 New‐onset diabetes after transplantation and cardiovascular events.

Comparison 2 Steroid avoidance versus steroid maintenance, Outcome 4 Infection and malignancy.

Comparison 2 Steroid avoidance versus steroid maintenance, Outcome 5 Kidney function.

Comparison 3 Steroid avoidance versus steroid withdrawal, Outcome 1 Death and graft loss.

Comparison 3 Steroid avoidance versus steroid withdrawal, Outcome 2 Rejection.

Comparison 3 Steroid avoidance versus steroid withdrawal, Outcome 3 New‐onset diabetes after transplantation, infection, malignancy.

Comparison 3 Steroid avoidance versus steroid withdrawal, Outcome 4 Kidney function.
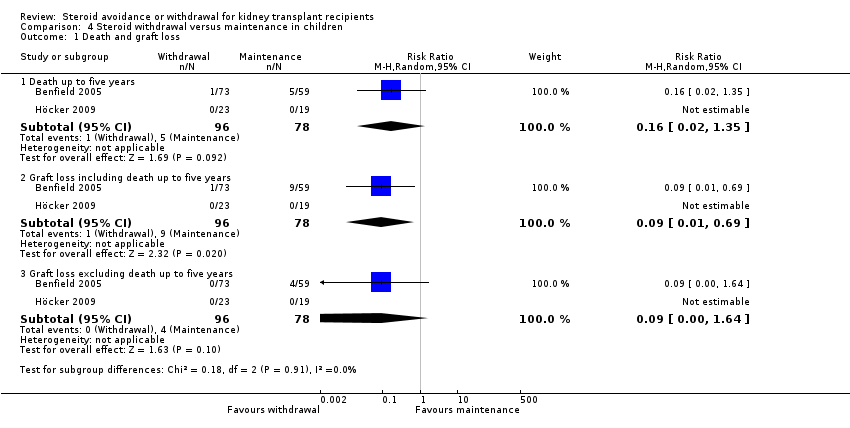
Comparison 4 Steroid withdrawal versus maintenance in children, Outcome 1 Death and graft loss.
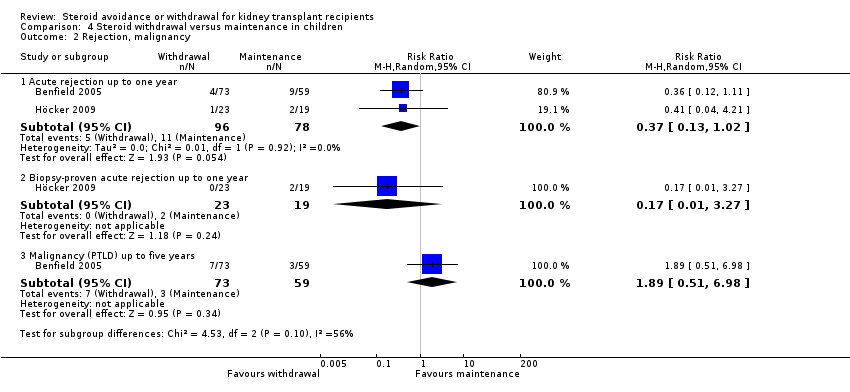
Comparison 4 Steroid withdrawal versus maintenance in children, Outcome 2 Rejection, malignancy.

Comparison 4 Steroid withdrawal versus maintenance in children, Outcome 3 Kidney function.

Comparison 5 Publication bias, Outcome 1 Funnel plots.
| Steroid withdrawal versus steroid maintenance for kidney transplant recipients | |||||
| Patient or population: kidney transplant recipients | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Steroid maintenance | Steroid withdrawal | ||||
| Mortality | 22 per 1000 | 15 per 1000 | RR 0.68 | 1913 (10) | ⊕⊕⊝⊝ |
| Graft loss (excluding death) | 32 per 1000 | 38 per 1000 | RR 1.17 | 1817 (8) | ⊕⊕⊝⊝ |
| Acute rejection | 152 per 1000 | 268 per 1000 | RR 1.77 | 1913 (10) | ⊕⊕⊕⊝ |
| NODAT | 57 per 1000 | 44 per 1000 | RR 0.77 | 1439 (6) | ⊕⊕⊝⊝ |
| CMV infection | 100 per 1000 | 104 per 1000 | RR 1.04 | 1758 (5) | ⊕⊕⊝⊝ |
| *The assumed risk is the baseline risk in the control group treated with steroid maintenance. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Most studies were unblinded (9 studies) and did not report details about random sequence generation or allocation concealment or both (8 studies). One study had inappropriate random sequence generation. Four studies were industry sponsored. ITT analysis was unclear in four. | |||||
| Steroid avoidance versus steroid maintenance for kidney transplant recipients | ||||||
| Patient or population: kidney transplant recipients | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Steroid avoidance versus steroid maintenance | |||||
| Mortality | 31 per 1000 | 30 per 1000 | RR 0.96 | 1462 (10) | ⊕⊕⊝⊝ | |
| Graft loss (excluding death) | 42 per 1000 | 46 per 1000 | RR 1.09 | 1211 (7) | ⊕⊕⊝⊝ | |
| Acute rejection | 204 per 1000 | 323 per 1000 | RR 1.58 | 835 (7) | ⊕⊕⊕⊝ | |
| NODAT | 107 per 1000 | 80 per 1000 | RR 0.75 | 1618 (9) | ⊕⊕⊝⊝ | |
| CMV Infection | 106 per 1000 | 101 per 1000 | RR 0.96 | 1454 (6) | ⊕⊕⊝⊝ | |
| *The assumed risk is the baseline risk in the control group treated with steroid maintenance. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 All studies were unblinded. Six studies were industry sponsored. In six studies random sequence generation or allocation concealment or both was unclear. In two studies ITT was either not performed or unclear. One study had selective outcome reporting | ||||||
| Death | Graft loss | Acute rejection | Biopsy‐proven acute rejection | |||||||||
| Studies | RR | 95% CI | Studies | RR | 95% CI | Studies | RR | 95% CI | Studies | RR | 95% CI | |
| Publication status | ||||||||||||
| Peer reviewed journal | 8 | 0.60 | 0.31 to 1.17 | 7 | 1.25 | 0.73 to 2.13 | 8 | 2.02 | 1.23 to 3.23 | 4 | 1.32 | 0.66 to 2.66 |
| Abstract only | 2 | 3.04 | 0.33 to 28.29 | 1 | 0.82 | 0.23 to 2.94 | 2 | 1.25 | 0.67 to 2.32 | 1 | 1.37 | 0.70 to 2.69 |
| ITT analysis | ||||||||||||
| ITT analysis used | 6 | 0.69 | 0.30 to 1.61 | 6 | 1.31 | 0.69 to 2.46 | 6 | 2.07 | 1.10 to 3.91 | 3 | 1.37 | 0.64 to 2.94 |
| ITT analysis not used/unclear | 4 | 0.67 | 0.25 to 1.81 | 2 | 1.00 | 0.46 to 2.17 | 4 | 1.65 | 0.81 to 3.36 | 2 | 1.04 | 0.24 to 4.59 |
| Calcineurin inhibitor | ||||||||||||
| CsA | 9 | 0.75 | 0.36 to 1.54 | 7 | 0.90 | 0.50 to 5.14 | 9 | 2.08 | 1.29 to 3.35 | 4 | 1.60 | 0.87 to 2.92 |
| TAC | 1 | 0.50 | 0.13 to 1.97 | 1 | 2.13 | 0.88 to 5.14 | 1 | 1.11 | 0.82 to 1.51 | 1 | 0.89 | 0.61 to 1.30 |
| Antimetabolite | ||||||||||||
| MMF or EC‐MPS | 6 | 0.67 | 0.31 to 1.47 | 5 | 1.25 | 0.75 to 2.08 | 6 | 1.41 | 1.02 to 1.94 | 3 | 1.27 | 0.81 to 2.00 |
| AZA | 2 | 0.93 | 0.26 to 3.40 | 2 | 0.25 | 0.03 to 2.18 | 2 | 2.61 | 0.62 to 10.91 | 1 | 0.33 | 0.04 to 2.56 |
| MMF or EC‐MPS or AZA | 8 | 0.73 | 0.38 to 1.43 | 7 | 1.15 | 0.70 to 1.89 | 8 | 1.46 | 1.07 to 1.98 | 4 | 1.19 | 0.75 to 1.90 |
| none | 2 | 0.31 | 0.03 to 2.95 | 1 | 3.00 | 0.13 to 71.61 | 2 | 5.80 | 2.16 to 15.57 | 1 | 9.00 | 1.19 to 67.93 |
| Induction treatment | ||||||||||||
| Induction (yes) | 2 | 3.00 | 0.32 to 27.87 | 1 | 0.33 | 0.01 to 8.02 | 2 | 0.80 | 0.22 to 2.91 | NA | ‐‐ | ‐‐ |
| Induction (no) | 8 | 0.60 | 0.31 to 1.17 | 7 | 1.21 | 0.74 to 1.99 | 8 | 1.93 | 1.26 to 2.94 | NA | ‐‐ | ‐‐ |
| AZA ‐ azathioprine; CI ‐ confidence interval; CsA ‐ cyclosporin A; EC‐MPS ‐ enteric‐coated mycophenolate sodium; ITT ‐ intention to treat; MMF ‐ mycophenolate mofetil; NA ‐ not available; RR ‐ risk ratio; TAC ‐ tacrolimus | ||||||||||||
| Death | Graft loss | Acute rejection | Biopsy‐proven acute rejection | |||||||||
| Studies | RR | 95% CI | Studies | RR | 95% CI | Studies | RR | 95% CI | Studies | RR | 95% CI | |
| ITT analysis | ||||||||||||
| ITT analysis used | 7 | 1.16 | 0.48 to 2.83 | 5 | 1.09 | 0.56 to 2.11 | 4 | 1.92 | 1.18 to 3.14 | 4 | 2.31 | 1.47 to 3.63 |
| ITT analysis not used/unclear | 3 | 0.51 | 0.07 to 3.83 | 2 | 1.11 | 0.46 to 2.67 | 3 | 1.24 | 0.97 to 1.59 | 2 | 1.05 | 0.49 to 2.23 |
| Calcineurin inhibitor | ||||||||||||
| CsA | 8 | 0.88 | 0.47 to 1.66 | 5 | 1.08 | 0.59 to 1.99 | 5 | 1.31 | 1.05 to 1.63 | 3 | 1.89 | 1.29 to 2.79 |
| TAC | 2 | 6.82 | 0.36 to 130.81 | 2 | 1.14 | 0.39 to 3.3 | 2 | 2.40 | 1.05 to 5.49 | 3 | 1.81 | 0.66 to 4.99 |
| Antimetabolite | ||||||||||||
| MMF or EC‐MPS | 6 | 1.15 | 0.36 to 3.69 | 6 | 1.09 | 0.56 to 2.11 | 5 | 1.87 | 1.20 to 2.91 | 6 | 1.94 | 1.26 to 2.98 |
| AZA | 1 | 1.64 | 0.29 to 9.2 | NA | ‐‐ | ‐‐ | NA | ‐‐ | ‐‐ | NA | ‐‐ | ‐‐ |
| MMF or EC‐MPS or AZA | 8 | 1.16 | 0.48 to 2.83 | NA | ‐‐ | ‐‐ | NA | ‐‐ | ‐‐ | NA | ‐‐ | ‐‐ |
| None | 2 | 0.51 | 0.07 to 3.83 | 1 | 1.11 | 0.46 to 2.67 | 2 | 1.26 | 0.95 to 1.65 | NA | ‐‐ | ‐‐ |
| Induction treatment | ||||||||||||
| Induction (yes) | 7 | 0.97 | 0.38 to 2.48 | 5 | 1.06 | 0.45 to 2.46 | 4 | 1.50 | 0.97 to 2.32 | 5 | 1.67 | 1.19 to 2.36 |
| Induction (no) | 3 | 0.92 | 0.17 to 5.01 | 2 | 1.12 | 0.57 to 2.2 | 3 | 1.72 | 0.89 to 3.32 | 1 | 3.89 | 2.16 to 7.03 |
| AZA ‐ azathioprine; CI ‐ confidence interval; CsA ‐ cyclosporin A; EC‐MPS ‐ enteric‐coated mycophenolate sodium; ITT ‐ intention to treat; MMF ‐ mycophenolate mofetil; NA ‐ not available; RR ‐ risk ratio; TAC ‐ tacrolimus | ||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death and graft loss Show forest plot | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Death up to one year | 10 | 1913 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.36, 1.30] |
| 1.2 Death one to five years | 7 | 1118 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.73, 2.17] |
| 1.3 Graft loss including death up to one year | 8 | 1817 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.64, 1.49] |
| 1.4 Graft loss including death one to five years | 7 | 1092 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [1.00, 2.01] |
| 1.5 Graft loss excluding death up to one year | 8 | 1817 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.72, 1.92] |
| 1.6 Graft loss excluding death one to five years | 7 | 1092 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.98, 2.64] |
| 2 Rejection Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Acute rejection up to one year | 10 | 1913 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [1.20, 2.61] |
| 2.2 Biopsy‐proven acute rejection up to one year | 5 | 1292 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.78, 2.22] |
| 3 New‐onset diabetes after transplantation and cardiovascular events Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 New onset diabetes after transplantation up to five years | 6 | 1439 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.49, 1.21] |
| 3.2 Cardiovascular events up to five years | 2 | 607 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.42, 2.33] |
| 4 Infection and malignancy Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Infection (all) up to five years | 5 | 1819 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.84, 1.22] |
| 4.2 CMV infection up to five years | 5 | 1758 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.80, 1.36] |
| 4.3 Malignancy up to five years | 3 | 756 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.41, 1.46] |
| 5 Kidney function Show forest plot | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Serum creatinine (mg/dL) up to one year | 4 | 644 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.21, 0.13] |
| 5.2 Serum creatinine (mg/dL) one to five years | 5 | 762 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.06, 0.23] |
| 5.3 Creatinine clearance (mL/min) up to one year | 2 | 215 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.35, 0.21] |
| 5.4 Creatinine clearance (mL/min) one to five years | 3 | 669 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.56, 0.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death and graft loss Show forest plot | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Death up to one year | 10 | 1462 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.52, 1.80] |
| 1.2 Death one to five years | 7 | 1201 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.32, 1.01] |
| 1.3 Graft loss including death up to one year | 7 | 1211 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.72, 1.62] |
| 1.4 Graft loss including death one to five years | 7 | 1245 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.53, 1.18] |
| 1.5 Graft loss excluding death up to one year | 7 | 1211 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.64, 1.86] |
| 1.6 Graft loss excluding death one to five years | 7 | 1245 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.66, 1.45] |
| 2 Rejection Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Acute rejection up to one year | 7 | 835 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [1.08, 2.30] |
| 2.2 Biopsy‐proven acute rejection up to one year | 6 | 1073 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [1.26, 2.98] |
| 3 New‐onset diabetes after transplantation and cardiovascular events Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 New onset diabetes after transplantation up to five years | 9 | 1618 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.51, 1.10] |
| 3.2 Cardiovascular events up to five years | 4 | 1013 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.30, 1.05] |
| 4 Infection and malignancy Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Infection (all) up to five years | 9 | 1833 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.84, 1.03] |
| 4.2 CMV Infection up to five years | 6 | 1454 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.70, 1.31] |
| 4.3 Malignancy up to five years | 7 | 1635 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.61, 1.52] |
| 5 Kidney function Show forest plot | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Serum creatinine (mg/dL) up to one year | 5 | 735 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.12, 0.17] |
| 5.2 Serum creatinine (mg/dL) one to five years | 3 | 688 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.16, 0.14] |
| 5.3 Creatinine clearance (mL/min) up to one year | 6 | 1104 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.23, 0.08] |
| 5.4 Creatinine clearance (mL/min) one to five years | 3 | 563 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.25, 0.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death and graft loss Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Death up to one year | 1 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.08, 1.98] |
| 1.2 Death one to five years | 2 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 2.67 [0.63, 11.32] |
| 1.3 Graft loss including death up to one year | 1 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.32, 2.29] |
| 1.4 Graft loss including death one to five years | 2 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [0.89, 6.70] |
| 1.5 Graft loss excluding death up to one year | 1 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.40, 6.68] |
| 1.6 Graft loss excluding death one to five years | 2 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [0.48, 7.67] |
| 2 Rejection Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Acute rejection up to one year | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Biopsy‐proven acute rejection up to one year | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 New‐onset diabetes after transplantation, infection, malignancy Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 New onset diabetes after transplantation up to five years | 3 | 351 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.36, 1.09] |
| 3.2 Infection (all) up to five years | 3 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.76, 1.50] |
| 3.3 CMV Infection up to five years | 2 | 284 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.30, 0.92] |
| 3.4 Malignancy up to five years | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.28, 8.94] |
| 4 Kidney function Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Serum creatinine (mg/dL) up to one year | 2 | 88 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.47, 0.37] |
| 4.2 Creatinine clearance (mL/min) up to one year | 2 | 206 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.41, 0.14] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death and graft loss Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Death up to five years | 2 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.02, 1.35] |
| 1.2 Graft loss including death up to five years | 2 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 0.69] |
| 1.3 Graft loss excluding death up to five years | 2 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.00, 1.64] |
| 2 Rejection, malignancy Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Acute rejection up to one year | 2 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.13, 1.02] |
| 2.2 Biopsy‐proven acute rejection up to one year | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.01, 3.27] |
| 2.3 Malignancy (PTLD) up to five years | 1 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [0.51, 6.98] |
| 3 Kidney function Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Creatinine clearance (mL/min) up to five years | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Funnel plots Show forest plot | 20 | 5288 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [1.15, 1.62] |
| 1.1 Death, steroid withdrawal versus maintenance | 10 | 1913 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.39, 1.29] |
| 1.2 Acute rejection steroid withdrawal versus maintenance | 10 | 1913 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.28, 1.87] |
| 1.3 Death, steroid avoidance versus maintenance | 10 | 1462 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.52, 1.63] |

