Inhibidores de la colinesterasa para la enfermedad de Alzheimer
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | 104 week, randomized, double‐blind, parallel group | |
| Participants | Country: Australia, Canada, France, Germany, Italy, Spain, UK | |
| Interventions | 1. donepezil (10mg/day) | |
| Outcomes | SIB | |
| Notes | ||
| Methods | 24‐week, randomized, double‐blind, parallel‐group, placebo‐controlled study ‐ a computer randomization schedule was used | |
| Participants | Country: USA | |
| Interventions | 1. placebo | |
| Outcomes | Primary: | |
| Notes | The group on 10mg/d of donepezil was on a blinded forced titration scheme of 5mg/d for week 1, and 10mg/d for the remainder of the study. | |
| Methods | 24‐week double‐blind, parallel‐group, placebo‐controlled, randomized study ‐ the randomization schedule was computer‐generated | |
| Participants | Country: Europe | |
| Interventions | 1. placebo | |
| Outcomes | ADAS‐Cog | |
| Notes | Patients in the 10mg/day group received 5mg/day for the first week of treatment. 6‐week placebo washout phase followed the double‐blind phase. | |
| Methods | 24‐week double‐blind, parallel group, placebo controlled, randomized study | |
| Participants | Country: USA | |
| Interventions | 1. placebo | |
| Outcomes | NPI‐NH | |
| Notes | The group on donepezil took 5 mg/d for the first 4 weeks, followed by 10 mg/d for 20 weeks. | |
| Methods | 24‐week double‐blind, parallel‐group, placebo‐controlled, randomized study | |
| Participants | Country: USA | |
| Interventions | 1. placebo | |
| Outcomes | mADAS‐Cog | |
| Notes | patients unable to tolerate 10mg/day were dropped from the study | |
| Methods | 24‐week double‐blind, parallel‐group, placebo‐controlled, randomized study ‐ the randomization schedule was computer‐generated | |
| Participants | Country: Canada, Australia, France | |
| Interventions | 1. placebo | |
| Outcomes | CIBIC plus | |
| Notes | The group on donepezil took 5 mg/d for the first 4 weeks, followed by 10 mg/d for 20 weeks. The dose could be reduced to 5mg/day at any point if necessary | |
| Methods | 52‐week, double‐blind, parallel‐group, placebo‐controlled, randomized study | |
| Participants | Country: Northern Europe | |
| Interventions | 1. placebo | |
| Outcomes | GBS | |
| Notes | The group on donepezil received 5mg/d for 28 days initially, and then 10mg/d according to the clinician's judgement for 1 year. | |
| Methods | Randomized | |
| Participants | Country: 8 European | |
| Interventions | Route: oral | |
| Outcomes | ADAS‐cog | |
| Notes | No. excluded after randomization: 128 | |
| Methods | Randomized | |
| Participants | Country: USA | |
| Interventions | Route: oral | |
| Outcomes | ADAS‐cog | |
| Notes | No. excluded after randomization: 198 | |
| Methods | Randomized | |
| Participants | Country: United States | |
| Interventions | Route: oral | |
| Outcomes | ADAS‐cog ADCS‐CGIC | |
| Notes | No. excluded after randomization: 199 | |
| Methods | 26 week | |
| Participants | Country: Europe and North America | |
| Interventions | 1.rivastigmine 1‐4mg/day divided into 2 doses | |
| Outcomes | ADAS‐Cog | |
| Notes | Main hypothesis: to assess the effects of rivastigmine on the core domains of AD | |
| Methods | 26 week | |
| Participants | Country: Australia, Canada, Italy, South Africa, UK | |
| Interventions | 1.rivastigmine 2‐12 mg/day divided into 2 doses | |
| Outcomes | ADAS‐Cog | |
| Notes | Main hypothesis: to evaluate the efficacy and safety of individual highest well tolerated doses (range 2‐12 mg/d) of rivastigmine bid or tid for 26 weeks compared to placebo, in the therapy of patients with probable AD | |
| Methods | 26 week | |
| Participants | Country: USA | |
| Interventions | 1.rivastigmine:3 mg/day divided into 2 doses | |
| Outcomes | ADAS‐Cog | |
| Notes | Main hypothesis: to evaluate the efficacy and safety of 3 fixed doses of rivastigmine (3,6,9 mg/d) and placebo for 26 weeks of treatment, and dose/efficacy and dose/safety relationships in patients with probable mild to moderate AD | |
| Methods | 26 week | |
| Participants | Country: USA | |
| Interventions | 1.rivastigmine 1‐4mg/day divided into 2 doses | |
| Outcomes | ADAS‐Cog | |
| Notes | primary hypothesis:to evaluate efficacy and safety of rivastigmine | |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Randomized, placebo controlled, double blind trial of donepezil. Results for the 5 and 10 mg/day groups were not reported separately. Complex design and high numbers of dropouts made analysis and interpretation difficult. | |
| Single‐blinded study of 12 weeks only. | |
| Single blinded study. | |
| Single‐blinded study of 12 weeks only. | |
| Randomized, placebo controlled, double blind trial of donepezil. Designed to evaluate effect on brain glucose metabolism and no data to contribute to this review. | |
| Randomized, placebo controlled, double blind trial of donepezil. Designed to evaluate effect on brain glucose metabolism and no data to contribute to this review. | |
| Randomized study of donepezil 5 mg/day compared with rivastigmine 6‐9 mg/day for AD. Not blinded. | |
| Randomized, double‐blind, placebo controlled study of galantamine. Dose available could be as high as 24 mg/day, but the mean dose was 17mg/day, which was too low for this review. | |
| Diagnosis of the included patients was not for simply AD. Randomized, placebo controlled, double blind trial of donepezil, for AD with cerebrovascular disease | |
| Randomized, placebo controlled, double blind trial of donepezil. Designed to evaluate effect on N‐acetylasparate concentration and hippocampal volume and no data to contribute to this review. | |
| Non‐randomized study of donepezil, metrifonate or galantamine. Outcome is response to cerebral metabolic activation. | |
| Donepezil compared with rivastigmine in a non‐randomzied study. | |
| Donepezil compared with rivastigmine compared with galantamine. No mention of randomization | |
| Randomized, placebo controlled, double blind trial of donepezil. Designed to evaluate preservation of function. Patients left the trial when function declined to a specified level and no data to contribute to this review. | |
| Donepezil compared with rivastigmine in a non‐randomized study. | |
| Open label, randomized study, comparing rivastigmine with donepezil. | |
| Non‐randomized study of tacrine, donepezil or rivastigmine. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ADAS‐Cog mean changes in score from baseline at 6 months or later (ITT‐LOCF) Show forest plot | 10 | 4236 | Mean Difference (IV, Fixed, 95% CI) | ‐2.37 [‐2.73, ‐2.02] |
| Analysis 1.1  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 1 ADAS‐Cog mean changes in score from baseline at 6 months or later (ITT‐LOCF). | ||||
| 2 MMSE mean change in score from baseline at 6 months or later (ITT‐LOCF) Show forest plot | 9 | 3118 | Mean Difference (IV, Fixed, 95% CI) | 1.37 [1.13, 1.61] |
| Analysis 1.2  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 2 MMSE mean change in score from baseline at 6 months or later (ITT‐LOCF). | ||||
| 3 Activities of daily living (DAD) mean changes in score from baseline at 6 months or later (ITT‐LOCF) Show forest plot | 2 | 669 | Mean Difference (IV, Fixed, 95% CI) | 4.39 [1.96, 6.81] |
| Analysis 1.3  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 3 Activities of daily living (DAD) mean changes in score from baseline at 6 months or later (ITT‐LOCF). | ||||
| 4 Activities of daily living (PDS) mean change in score from baseline at 6 months (ITT) Show forest plot | 5 | 2188 | Mean Difference (IV, Fixed, 95% CI) | 2.46 [1.55, 3.37] |
| Analysis 1.4  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 4 Activities of daily living (PDS) mean change in score from baseline at 6 months (ITT). | ||||
| 5 Behavioural disturbance (NPI) mean changes from score from baseline at 6 months (ITT) Show forest plot | 3 | 1005 | Mean Difference (IV, Fixed, 95% CI) | ‐2.44 [‐4.12, ‐0.76] |
| Analysis 1.5  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 5 Behavioural disturbance (NPI) mean changes from score from baseline at 6 months (ITT). | ||||
| 6 Global assessment with carer input (CIBIC‐Plus) (numbers improved or unchanged) at 6 months (ITT) Show forest plot | 3 | 1306 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.84 [1.47, 2.30] |
| Analysis 1.6  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 6 Global assessment with carer input (CIBIC‐Plus) (numbers improved or unchanged) at 6 months (ITT). | ||||
| 7 Global assessment with carer input (CIBIC‐Plus) (numbers improved) at 6 months (ITT) Show forest plot | 8 | 3402 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.32, 1.85] |
| Analysis 1.7  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 7 Global assessment with carer input (CIBIC‐Plus) (numbers improved) at 6 months (ITT). | ||||
| 8 GBS‐global assessment mean change in score from baseline at 52 weeks (ITT) Show forest plot | 1 | 282 | Mean Difference (IV, Fixed, 95% CI) | ‐3.26 [‐7.38, 0.86] |
| Analysis 1.8  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 8 GBS‐global assessment mean change in score from baseline at 52 weeks (ITT). | ||||
| 9 Time spent by carer assisting in IADL and PSMS (mean changes in score from baseline min/day) at 6 months (ITT) Show forest plot | 1 | 221 | Mean Difference (IV, Fixed, 95% CI) | ‐52.4 [‐118.78, 13.98] |
| Analysis 1.9  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 9 Time spent by carer assisting in IADL and PSMS (mean changes in score from baseline min/day) at 6 months (ITT). | ||||
| 10 Total number of withdrawals before end of treatment at 6 months or later (ITT) Show forest plot | 13 | 5143 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.76 [1.54, 2.02] |
| Analysis 1.10  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 10 Total number of withdrawals before end of treatment at 6 months or later (ITT). | ||||
| 11 Total number of withdrawals due to an adverse event before end of treatment at 6 months or later (ITT) Show forest plot | 13 | 5143 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.32 [1.95, 2.76] |
| Analysis 1.11  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 11 Total number of withdrawals due to an adverse event before end of treatment at 6 months or later (ITT). | ||||
| 12 Number who suffered at least one adverse event before end of treatment at 6 months or later Show forest plot | 12 | 4824 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.51 [2.14, 2.95] |
| Analysis 1.12  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 12 Number who suffered at least one adverse event before end of treatment at 6 months or later. | ||||
| 13 Number who suffered at least one adverse event of abdominal pain before end of treatment at 6 months or later Show forest plot | 7 | 2704 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.95 [1.46, 2.61] |
| Analysis 1.13  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 13 Number who suffered at least one adverse event of abdominal pain before end of treatment at 6 months or later. | ||||
| 14 Number who suffered at least one adverse event of abnormal gait before end of treatment at 6 months or later Show forest plot | 1 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.63, 4.09] |
| Analysis 1.14  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 14 Number who suffered at least one adverse event of abnormal gait before end of treatment at 6 months or later. | ||||
| 15 Number who suffered at least one adverse event of abnormal dreams before end of treatment at 6 months or later Show forest plot | 1 | 153 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.38 [1.34, 21.55] |
| Analysis 1.15  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 15 Number who suffered at least one adverse event of abnormal dreams before end of treatment at 6 months or later. | ||||
| 16 Number who suffered at least one adverse event of accidental injury before end of treatment at 6 monthsorlater Show forest plot | 3 | 651 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.86, 2.10] |
| Analysis 1.16  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 16 Number who suffered at least one adverse event of accidental injury before end of treatment at 6 monthsorlater. | ||||
| 17 Number who suffered at least one adverse event of agitation before end of treatment at 6 months or later Show forest plot | 2 | 767 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.57, 1.56] |
| Analysis 1.17  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 17 Number who suffered at least one adverse event of agitation before end of treatment at 6 months or later. | ||||
| 18 Number who suffered at least one adverse event of anorexia before end of treatment at 6 months or later Show forest plot | 10 | 4419 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.75 [2.89, 4.87] |
| Analysis 1.18  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 18 Number who suffered at least one adverse event of anorexia before end of treatment at 6 months or later. | ||||
| 19 Number who suffered at least one adverse event of anxiety before end of treatment at 6 months or later Show forest plot | 1 | 286 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.82, 4.90] |
| Analysis 1.19  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 19 Number who suffered at least one adverse event of anxiety before end of treatment at 6 months or later. | ||||
| 20 Number who suffered at least one adverse event of arthralgia before end of treatment at 6 months or later Show forest plot | 2 | 498 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.62, 2.40] |
| Analysis 1.20  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 20 Number who suffered at least one adverse event of arthralgia before end of treatment at 6 months or later. | ||||
| 21 Number who suffered at least one adverse event of asthenia before end of treatment at 6 months or later Show forest plot | 3 | 729 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.47 [1.27, 4.81] |
| Analysis 1.21  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 21 Number who suffered at least one adverse event of asthenia before end of treatment at 6 months or later. | ||||
| 22 Number who suffered at least one adverse event of back pain before end of treatment at 6 months or later Show forest plot | 1 | 290 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.62, 4.36] |
| Analysis 1.22  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 22 Number who suffered at least one adverse event of back pain before end of treatment at 6 months or later. | ||||
| 23 Number who suffered at least one adverse event of confusion before end of treatment at 6 months or later Show forest plot | 4 | 1331 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.52, 1.32] |
| Analysis 1.23  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 23 Number who suffered at least one adverse event of confusion before end of treatment at 6 months or later. | ||||
| 24 Number who suffered at least one adverse event of conjunctivitis before end of treatment at 6 months or later Show forest plot | 1 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.70, 5.55] |
| Analysis 1.24  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 24 Number who suffered at least one adverse event of conjunctivitis before end of treatment at 6 months or later. | ||||
| 25 Number who suffered at least one adverse event of constipation before end of treatment at 6 months or later Show forest plot | 1 | 286 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.23, 1.91] |
| Analysis 1.25  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 25 Number who suffered at least one adverse event of constipation before end of treatment at 6 months or later. | ||||
| 26 Number who suffered at least one adverse event of depression before end of treatment at 6 months or later Show forest plot | 2 | 576 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.82, 3.04] |
| Analysis 1.26  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 26 Number who suffered at least one adverse event of depression before end of treatment at 6 months or later. | ||||
| 27 Number who suffered at least one adverse event of diarrhoea before end of treatment at 6 months or later Show forest plot | 13 | 5173 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.91 [1.59, 2.30] |
| Analysis 1.27  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 27 Number who suffered at least one adverse event of diarrhoea before end of treatment at 6 months or later. | ||||
| 28 Number who suffered at least one adverse event of dizziness before end of treatment at 6 months or later Show forest plot | 12 | 4583 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.64, 2.42] |
| Analysis 1.28  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 28 Number who suffered at least one adverse event of dizziness before end of treatment at 6 months or later. | ||||
| 29 Number who suffered at least one adverse event of ecchymosis before end of treatment at 6 months or later Show forest plot | 1 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.54, 4.61] |
| Analysis 1.29  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 29 Number who suffered at least one adverse event of ecchymosis before end of treatment at 6 months or later. | ||||
| 30 Number who suffered at least one adverse event of fatigue before end of treatment at 6 months or later Show forest plot | 1 | 319 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.39 [1.21, 15.85] |
| Analysis 1.30  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 30 Number who suffered at least one adverse event of fatigue before end of treatment at 6 months or later. | ||||
| 31 Number who suffered at least one adverse event of fever before end of treatment at 6 months or later Show forest plot | 1 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.39, 1.93] |
| Analysis 1.31  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 31 Number who suffered at least one adverse event of fever before end of treatment at 6 months or later. | ||||
| 32 Number who suffered at least one adverse event of fracture before end of treatment at 6 months or later Show forest plot | 5 | 2269 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.53, 1.74] |
| Analysis 1.32  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 32 Number who suffered at least one adverse event of fracture before end of treatment at 6 months or later. | ||||
| 33 Number who suffered at least one adverse event of haemorrhage before end of treatment at 6 months or later Show forest plot | 1 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.35, 3.02] |
| Analysis 1.33  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 33 Number who suffered at least one adverse event of haemorrhage before end of treatment at 6 months or later. | ||||
| 34 Number who suffered at least one adverse event of headache before end of treatment at 6 months or later Show forest plot | 9 | 3686 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.27, 1.91] |
| Analysis 1.34  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 34 Number who suffered at least one adverse event of headache before end of treatment at 6 months or later. | ||||
| 35 Number who suffered at least one adverse event of hostility before end of treatment at 6 months or later Show forest plot | 2 | 576 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.49, 1.87] |
| Analysis 1.35  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 35 Number who suffered at least one adverse event of hostility before end of treatment at 6 months or later. | ||||
| 36 Number who suffered at least one adverse event of increased cough before end of treatment at 6 months or later Show forest plot | 1 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.56, 2.52] |
| Analysis 1.36  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 36 Number who suffered at least one adverse event of increased cough before end of treatment at 6 months or later. | ||||
| 37 Number who suffered at least one adverse event of infection before end of treatment at 6 months or later Show forest plot | 1 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.51, 2.37] |
| Analysis 1.37  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 37 Number who suffered at least one adverse event of infection before end of treatment at 6 months or later. | ||||
| 38 Number who suffered at least one adverse event of insomnia before end of treatment at 6 months or later Show forest plot | 7 | 2906 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.49 [1.12, 2.00] |
| Analysis 1.38  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 38 Number who suffered at least one adverse event of insomnia before end of treatment at 6 months or later. | ||||
| 39 Number who suffered at least one adverse event of muscle cramp before end of treatment at 6 months or later Show forest plot | 1 | 319 | Odds Ratio (M‐H, Fixed, 95% CI) | 13.32 [1.71, 103.74] |
| Analysis 1.39  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 39 Number who suffered at least one adverse event of muscle cramp before end of treatment at 6 months or later. | ||||
| 40 Number who suffered at least one adverse event of myasthenia before end of treatment at 6 months or later Show forest plot | 1 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.10 [0.51, 8.64] |
| Analysis 1.40  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 40 Number who suffered at least one adverse event of myasthenia before end of treatment at 6 months or later. | ||||
| 41 Number who suffered at least one adverse event of nausea before end of treatment at 6 months or later Show forest plot | 13 | 5089 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.87 [4.13, 5.74] |
| Analysis 1.41  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 41 Number who suffered at least one adverse event of nausea before end of treatment at 6 months or later. | ||||
| 42 Number who suffered at least one adverse event of pain before end of treatment at 6 months or later Show forest plot | 1 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.47, 1.78] |
| Analysis 1.42  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 42 Number who suffered at least one adverse event of pain before end of treatment at 6 months or later. | ||||
| 43 Number who suffered at least one adverse event of peripheral oedema before end of treatment at 6 monthsorlater Show forest plot | 1 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.08 [1.01, 4.28] |
| Analysis 1.43  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 43 Number who suffered at least one adverse event of peripheral oedema before end of treatment at 6 monthsorlater. | ||||
| 44 Number who suffered at least one adverse event of a rash before end of treatment at 6 months or later Show forest plot | 1 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.37, 1.42] |
| Analysis 1.44  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 44 Number who suffered at least one adverse event of a rash before end of treatment at 6 months or later. | ||||
| 45 Number who suffered at least one adverse event of a respiratory tract infection before end of treatment at 6 m Show forest plot | 1 | 290 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.49, 2.12] |
| Analysis 1.45  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 45 Number who suffered at least one adverse event of a respiratory tract infection before end of treatment at 6 m. | ||||
| 46 Number who suffered at least one adverse event of rhinitis before end of treatment at 6 months or later Show forest plot | 2 | 527 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.74, 2.58] |
| Analysis 1.46  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 46 Number who suffered at least one adverse event of rhinitis before end of treatment at 6 months or later. | ||||
| 47 Number who suffered at least one adverse event of skin ulcer before end of treatment at 6 months or later Show forest plot | 1 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.55, 4.12] |
| Analysis 1.47  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 47 Number who suffered at least one adverse event of skin ulcer before end of treatment at 6 months or later. | ||||
| 48 Number who suffered at least one adverse event of syncope before end of treatment at 6 months or later Show forest plot | 5 | 2206 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.90 [1.09, 3.33] |
| Analysis 1.48  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 48 Number who suffered at least one adverse event of syncope before end of treatment at 6 months or later. | ||||
| 49 Number who suffered at least one adverse event of tremor before end of treatment at 6 months or later Show forest plot | 2 | 633 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.82 [1.99, 23.37] |
| Analysis 1.49  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 49 Number who suffered at least one adverse event of tremor before end of treatment at 6 months or later. | ||||
| 50 Number who suffered at least one adverse event of urinary tract infection before end of treatment at 6 month Show forest plot | 3 | 784 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.54, 1.48] |
| Analysis 1.50  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 50 Number who suffered at least one adverse event of urinary tract infection before end of treatment at 6 month. | ||||
| 51 Number who suffered at least one adverse event of vertigo before end of treatment at 6 months or later Show forest plot | 1 | 286 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.95 [1.08, 14.46] |
| Analysis 1.51  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 51 Number who suffered at least one adverse event of vertigo before end of treatment at 6 months or later. | ||||
| 52 Number who suffered at least one adverse event of vomiting before end of treatment at 6 months or later Show forest plot | 11 | 4703 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.82 [3.91, 5.94] |
| Analysis 1.52  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 52 Number who suffered at least one adverse event of vomiting before end of treatment at 6 months or later. | ||||
| 53 Number who suffered at least one adverse event of weight loss before end of treatment at 6 months or later Show forest plot | 4 | 1358 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.99 [1.89, 4.75] |
| Analysis 1.53  Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 53 Number who suffered at least one adverse event of weight loss before end of treatment at 6 months or later. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 MMSE mean change from baseline (ITT‐LOCF) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 1 MMSE mean change from baseline (ITT‐LOCF). | ||||
| 1.1 At 24 months | 1 | 955 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.33, 0.33] |
| 2 Activities of daily living (ADCS‐ADL) (ITT‐LOCF) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 2 Activities of daily living (ADCS‐ADL) (ITT‐LOCF). | ||||
| 2.1 At 24 months | 1 | 929 | Mean Difference (IV, Fixed, 95% CI) | ‐2.08 [‐4.58, 0.42] |
| 3 Behavioural disturbance (NPI‐10) (ITT‐LOCF) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 3 Behavioural disturbance (NPI‐10) (ITT‐LOCF). | ||||
| 3.1 At 24 months | 1 | 955 | Mean Difference (IV, Fixed, 95% CI) | 0.54 [‐1.68, 2.76] |
| 4 Cognitive function (SIB) (ITT‐LOCF) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 4 Cognitive function (SIB) (ITT‐LOCF). | ||||
| 4.1 At 24 months | 1 | 954 | Mean Difference (IV, Fixed, 95% CI) | ‐0.61 [‐3.66, 2.44] |
| 5 Global Deterioration Scale (GDS) (ITT‐LOCF) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.5  Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 5 Global Deterioration Scale (GDS) (ITT‐LOCF). | ||||
| 5.1 At 24 months | 1 | 954 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.00, 0.22] |
| 6 Total number of patients who withdrew before end of treatment Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.6  Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 6 Total number of patients who withdrew before end of treatment. | ||||
| 6.1 At 104 weeks | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.50, 0.83] |
| 7 Total number of patients who withdrew before end of treatment due to an adverse event Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.7  Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 7 Total number of patients who withdrew before end of treatment due to an adverse event. | ||||
| 7.1 At 104 weeks | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.32, 0.68] |
| 8 Total number of patients who suffered an adverse event of nausea Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.8  Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 8 Total number of patients who suffered an adverse event of nausea. | ||||
| 8.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.27, 0.50] |
| 8.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.23, 0.63] |
| 9 Total number of patients who suffered an adverse event of vomiting Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.9  Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 9 Total number of patients who suffered an adverse event of vomiting. | ||||
| 9.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.10, 0.24] |
| 9.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.15, 0.43] |
| 10 Total number of patients who suffered an adverse event of agitation Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.10  Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 10 Total number of patients who suffered an adverse event of agitation. | ||||
| 10.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.93, 2.30] |
| 10.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.79, 2.00] |
| 11 Total number of patients who suffered an adverse event of anorexia Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.11  Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 11 Total number of patients who suffered an adverse event of anorexia. | ||||
| 11.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.24, 0.72] |
| 11.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.24, 0.90] |
| 12 Total number of patients who suffered an adverse event of diarrhoea Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.12  Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 12 Total number of patients who suffered an adverse event of diarrhoea. | ||||
| 12.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.50, 1.30] |
| 12.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.60, 1.77] |
| 13 Total number of patients who suffered an adverse event of weight loss Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.13  Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 13 Total number of patients who suffered an adverse event of weight loss. | ||||
| 13.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.13, 0.61] |
| 13.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.67, 1.71] |
| 14 Total number of patients who suffered an adverse event of headache Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.14  Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 14 Total number of patients who suffered an adverse event of headache. | ||||
| 14.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.47, 1.48] |
| 14.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.37, 1.81] |
| 15 Total number of patients who suffered an adverse event of a fall Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.15  Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 15 Total number of patients who suffered an adverse event of a fall. | ||||
| 15.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.18, 0.81] |
| 15.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.75, 1.94] |
| 16 Total number of patients who suffered an adverse event of hypertension Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.16  Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 16 Total number of patients who suffered an adverse event of hypertension. | ||||
| 16.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.14, 0.81] |
| 16.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.40, 1.44] |
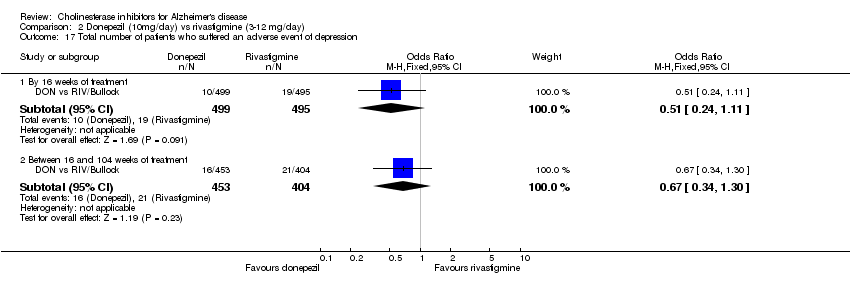
| 17 Total number of patients who suffered an adverse event of depression Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.17  Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 17 Total number of patients who suffered an adverse event of depression. | ||||
| 17.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.24, 1.11] |
| 17.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.34, 1.30] |
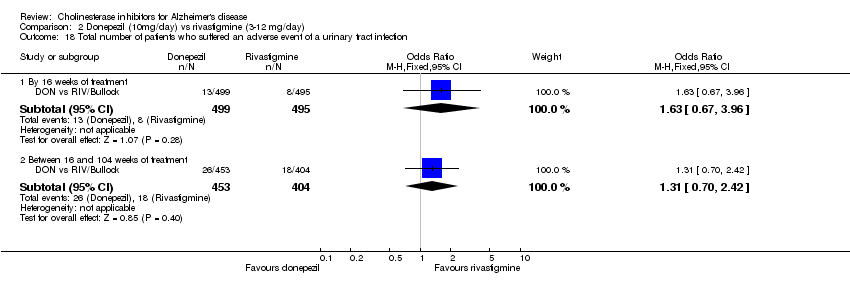
| 18 Total number of patients who suffered an adverse event of a urinary tract infection Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.18  Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 18 Total number of patients who suffered an adverse event of a urinary tract infection. | ||||
| 18.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.67, 3.96] |
| 18.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.70, 2.42] |
| 19 Total number of patients who suffered an adverse event of aggression Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.19  Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 19 Total number of patients who suffered an adverse event of aggression. | ||||
| 19.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.60, 4.09] |
| 19.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.64, 2.18] |
| 20 Total number of patients who suffered a serious adverse event Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.20  Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 20 Total number of patients who suffered a serious adverse event. | ||||
| 20.1 At 104 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.79, 1.35] |
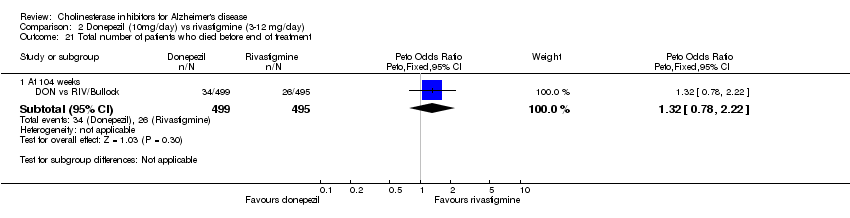
| 21 Total number of patients who died before end of treatment Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.21  Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 21 Total number of patients who died before end of treatment. | ||||
| 21.1 At 104 weeks | 1 | 994 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.32 [0.78, 2.22] |

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 1 ADAS‐Cog mean changes in score from baseline at 6 months or later (ITT‐LOCF).

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 2 MMSE mean change in score from baseline at 6 months or later (ITT‐LOCF).

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 3 Activities of daily living (DAD) mean changes in score from baseline at 6 months or later (ITT‐LOCF).

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 4 Activities of daily living (PDS) mean change in score from baseline at 6 months (ITT).

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 5 Behavioural disturbance (NPI) mean changes from score from baseline at 6 months (ITT).

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 6 Global assessment with carer input (CIBIC‐Plus) (numbers improved or unchanged) at 6 months (ITT).
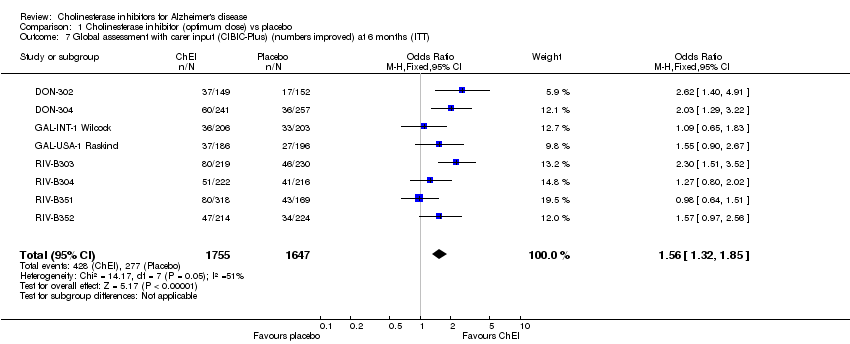
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 7 Global assessment with carer input (CIBIC‐Plus) (numbers improved) at 6 months (ITT).

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 8 GBS‐global assessment mean change in score from baseline at 52 weeks (ITT).

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 9 Time spent by carer assisting in IADL and PSMS (mean changes in score from baseline min/day) at 6 months (ITT).

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 10 Total number of withdrawals before end of treatment at 6 months or later (ITT).

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 11 Total number of withdrawals due to an adverse event before end of treatment at 6 months or later (ITT).
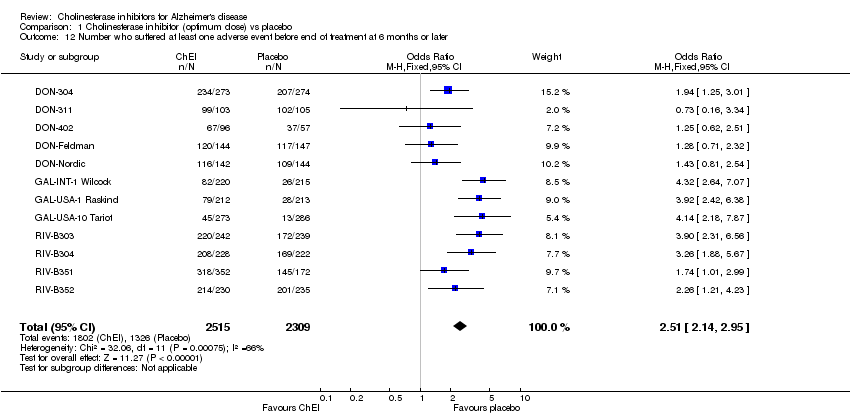
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 12 Number who suffered at least one adverse event before end of treatment at 6 months or later.

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 13 Number who suffered at least one adverse event of abdominal pain before end of treatment at 6 months or later.

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 14 Number who suffered at least one adverse event of abnormal gait before end of treatment at 6 months or later.
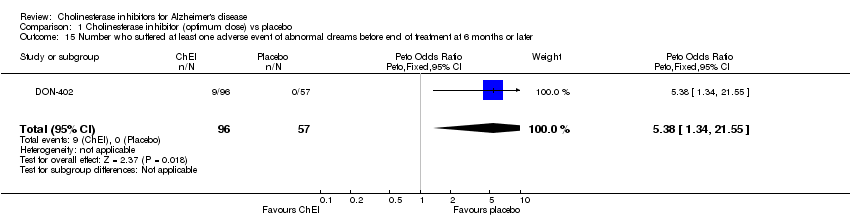
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 15 Number who suffered at least one adverse event of abnormal dreams before end of treatment at 6 months or later.

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 16 Number who suffered at least one adverse event of accidental injury before end of treatment at 6 monthsorlater.

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 17 Number who suffered at least one adverse event of agitation before end of treatment at 6 months or later.

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 18 Number who suffered at least one adverse event of anorexia before end of treatment at 6 months or later.
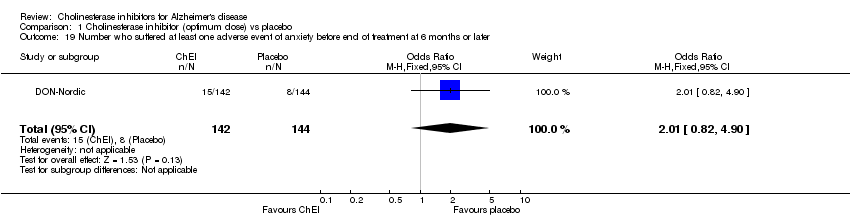
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 19 Number who suffered at least one adverse event of anxiety before end of treatment at 6 months or later.

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 20 Number who suffered at least one adverse event of arthralgia before end of treatment at 6 months or later.
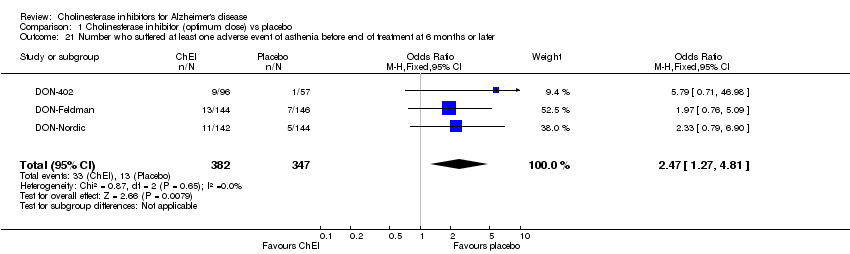
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 21 Number who suffered at least one adverse event of asthenia before end of treatment at 6 months or later.

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 22 Number who suffered at least one adverse event of back pain before end of treatment at 6 months or later.
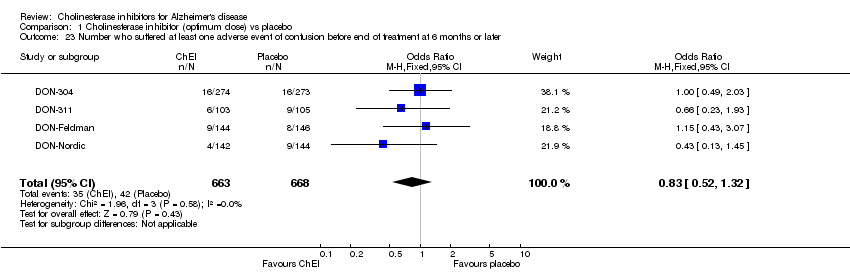
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 23 Number who suffered at least one adverse event of confusion before end of treatment at 6 months or later.

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 24 Number who suffered at least one adverse event of conjunctivitis before end of treatment at 6 months or later.

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 25 Number who suffered at least one adverse event of constipation before end of treatment at 6 months or later.
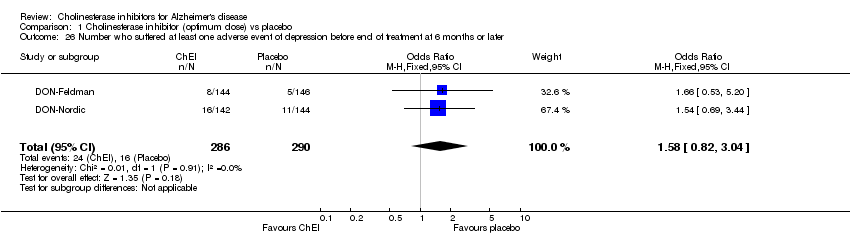
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 26 Number who suffered at least one adverse event of depression before end of treatment at 6 months or later.

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 27 Number who suffered at least one adverse event of diarrhoea before end of treatment at 6 months or later.

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 28 Number who suffered at least one adverse event of dizziness before end of treatment at 6 months or later.

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 29 Number who suffered at least one adverse event of ecchymosis before end of treatment at 6 months or later.

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 30 Number who suffered at least one adverse event of fatigue before end of treatment at 6 months or later.
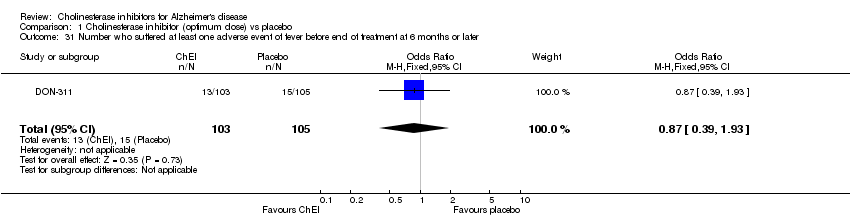
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 31 Number who suffered at least one adverse event of fever before end of treatment at 6 months or later.

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 32 Number who suffered at least one adverse event of fracture before end of treatment at 6 months or later.

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 33 Number who suffered at least one adverse event of haemorrhage before end of treatment at 6 months or later.

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 34 Number who suffered at least one adverse event of headache before end of treatment at 6 months or later.

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 35 Number who suffered at least one adverse event of hostility before end of treatment at 6 months or later.

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 36 Number who suffered at least one adverse event of increased cough before end of treatment at 6 months or later.
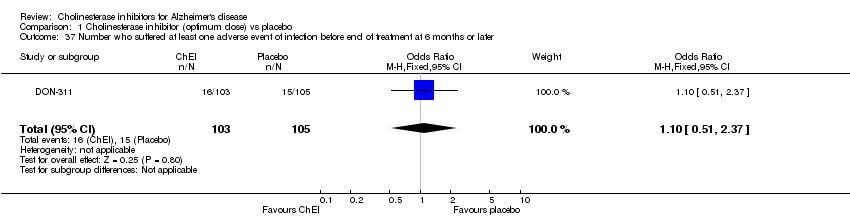
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 37 Number who suffered at least one adverse event of infection before end of treatment at 6 months or later.

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 38 Number who suffered at least one adverse event of insomnia before end of treatment at 6 months or later.
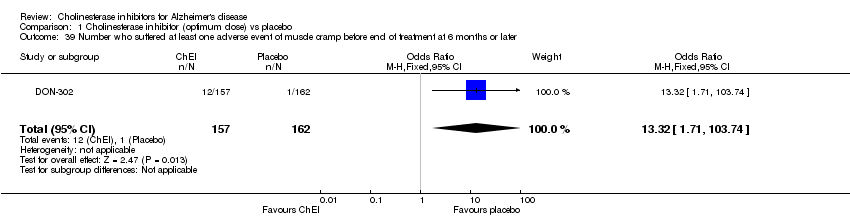
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 39 Number who suffered at least one adverse event of muscle cramp before end of treatment at 6 months or later.

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 40 Number who suffered at least one adverse event of myasthenia before end of treatment at 6 months or later.

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 41 Number who suffered at least one adverse event of nausea before end of treatment at 6 months or later.
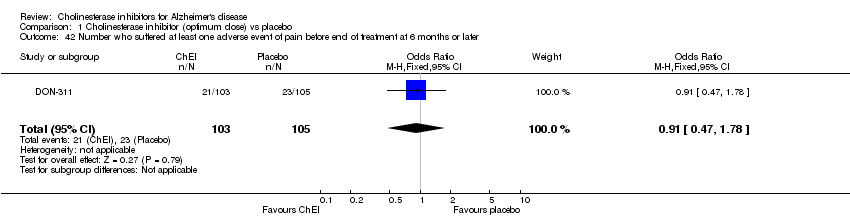
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 42 Number who suffered at least one adverse event of pain before end of treatment at 6 months or later.

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 43 Number who suffered at least one adverse event of peripheral oedema before end of treatment at 6 monthsorlater.

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 44 Number who suffered at least one adverse event of a rash before end of treatment at 6 months or later.

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 45 Number who suffered at least one adverse event of a respiratory tract infection before end of treatment at 6 m.

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 46 Number who suffered at least one adverse event of rhinitis before end of treatment at 6 months or later.
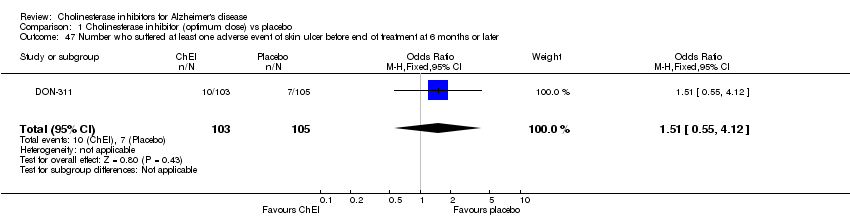
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 47 Number who suffered at least one adverse event of skin ulcer before end of treatment at 6 months or later.

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 48 Number who suffered at least one adverse event of syncope before end of treatment at 6 months or later.

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 49 Number who suffered at least one adverse event of tremor before end of treatment at 6 months or later.

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 50 Number who suffered at least one adverse event of urinary tract infection before end of treatment at 6 month.

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 51 Number who suffered at least one adverse event of vertigo before end of treatment at 6 months or later.
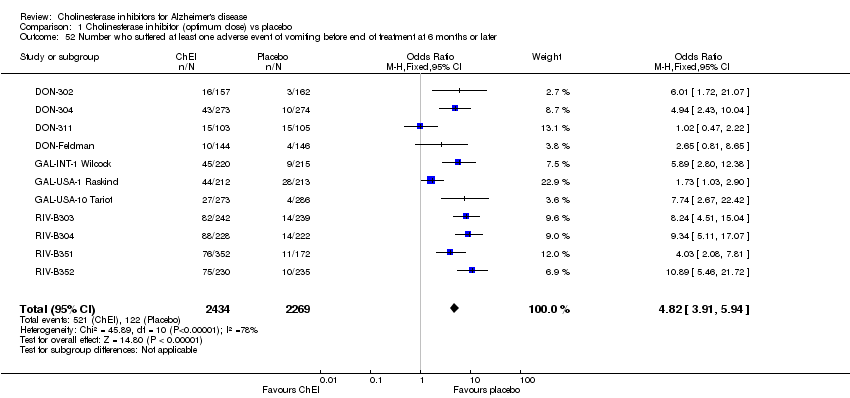
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 52 Number who suffered at least one adverse event of vomiting before end of treatment at 6 months or later.

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 53 Number who suffered at least one adverse event of weight loss before end of treatment at 6 months or later.

Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 1 MMSE mean change from baseline (ITT‐LOCF).

Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 2 Activities of daily living (ADCS‐ADL) (ITT‐LOCF).

Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 3 Behavioural disturbance (NPI‐10) (ITT‐LOCF).

Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 4 Cognitive function (SIB) (ITT‐LOCF).
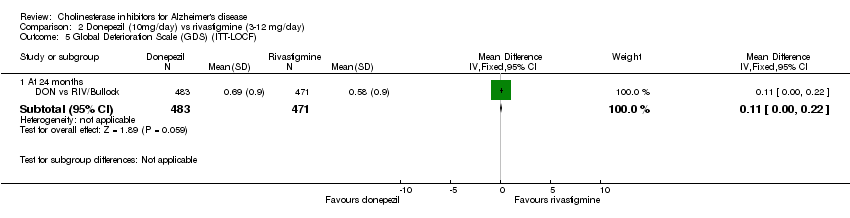
Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 5 Global Deterioration Scale (GDS) (ITT‐LOCF).
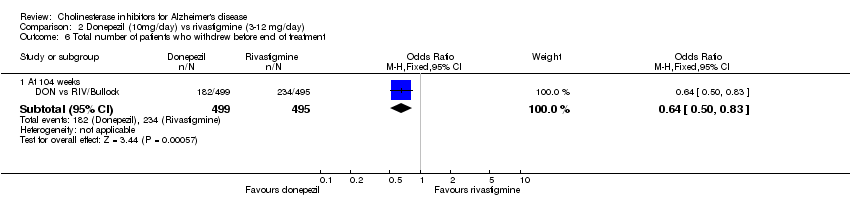
Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 6 Total number of patients who withdrew before end of treatment.

Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 7 Total number of patients who withdrew before end of treatment due to an adverse event.

Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 8 Total number of patients who suffered an adverse event of nausea.

Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 9 Total number of patients who suffered an adverse event of vomiting.

Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 10 Total number of patients who suffered an adverse event of agitation.

Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 11 Total number of patients who suffered an adverse event of anorexia.

Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 12 Total number of patients who suffered an adverse event of diarrhoea.

Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 13 Total number of patients who suffered an adverse event of weight loss.

Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 14 Total number of patients who suffered an adverse event of headache.

Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 15 Total number of patients who suffered an adverse event of a fall.
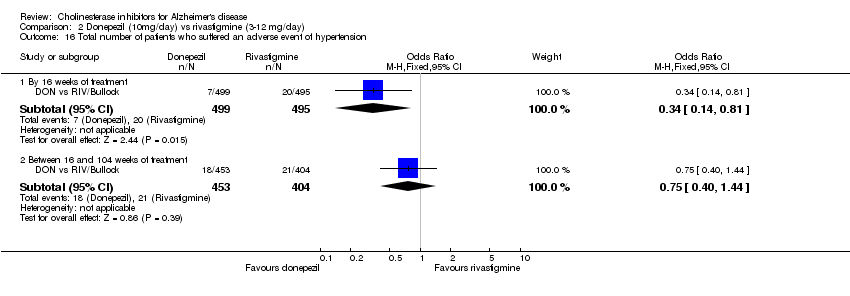
Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 16 Total number of patients who suffered an adverse event of hypertension.
Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 17 Total number of patients who suffered an adverse event of depression.
Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 18 Total number of patients who suffered an adverse event of a urinary tract infection.

Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 19 Total number of patients who suffered an adverse event of aggression.

Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 20 Total number of patients who suffered a serious adverse event.
Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 21 Total number of patients who died before end of treatment.
| Study | duration weeks | number of patients | mean age | % female | mean MMSE | dose mg/day donep. | phase | country | funded by |
| DON vs RIV/Bullock | 104 | 988 | 75.9 | 69 | 15.1 | 10 donepezil, maximum 12 (in 2 doses) rivastigmine | ‐ | Australia, Canada, France, Germany, Italy, Spain, UK | Novartis |
| Donepezil‐302 | 24 | 473 | 73.4 | 62 | 19.0 | 5, 10 | III | USA | Eisai |
| Donepezil‐304 | 24 | 818 | 71.7 | 57 | 20.0 | 5, 10 | III | EUROPE | Eisai |
| Donepezil‐311 | 24 | 208 | 85.7 | 82 | 14.4 | 10 | III | USA | Eisai |
| Donepezil‐402 | 24 | 153 | 74.0 | 53.6 | 24.1 | 10 | USA | Eisai/Pfizer | |
| Donepezil‐Feldman | 24 | 290 | 73.6 | 61 | 11.8 | 10 | CANADA, AUSTRALIA, FRANCE | Eisai/Pfizer | |
| DON‐Nordic | 52 | 286 | 72.5 | 64 | 19.3 | 10 | EUROPE | Pfizer | |
| GAL‐INT‐1 | 26 | 653 | 72.2 | 63 | 19.3 | 24, 32 | EUROPE | UK NHS R&D health technology assessment programme | |
| GAL‐USA‐1 | 26 | 636 | 70.7 | 62 | 19.3 | 24, 32 | USA | Janssen | |
| GAL‐USA‐10 | 22 | 978 | 76.9 | 64 | 17.8 | 8, 16, 24 | USA | Janssen | |
| Rivastigmine‐B303 | 26 | 725 | 72.0 | 59 | 20.0 | 6‐12 | III | EUROPE, CANADA, USA | Novartis |
| Rivastigmine‐B304 | 26 | 677 | 71.4 | 59 | 18.5 | 2‐12 | III | UK, IRELAND, AUSTRALIA, CANADA, RSA, ITALY | Novartis |
| Rivastigmine‐B351 | 26 | 702 | 74.1 | 56 | 20.0 | 6,9 | III | USA | Novartis |
| Rivastigmine‐B352 | 26 | 699 | 74.5 | 61 | 19.7 | 6‐12 | III | USA | Novartis |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ADAS‐Cog mean changes in score from baseline at 6 months or later (ITT‐LOCF) Show forest plot | 10 | 4236 | Mean Difference (IV, Fixed, 95% CI) | ‐2.37 [‐2.73, ‐2.02] |
| 2 MMSE mean change in score from baseline at 6 months or later (ITT‐LOCF) Show forest plot | 9 | 3118 | Mean Difference (IV, Fixed, 95% CI) | 1.37 [1.13, 1.61] |
| 3 Activities of daily living (DAD) mean changes in score from baseline at 6 months or later (ITT‐LOCF) Show forest plot | 2 | 669 | Mean Difference (IV, Fixed, 95% CI) | 4.39 [1.96, 6.81] |
| 4 Activities of daily living (PDS) mean change in score from baseline at 6 months (ITT) Show forest plot | 5 | 2188 | Mean Difference (IV, Fixed, 95% CI) | 2.46 [1.55, 3.37] |
| 5 Behavioural disturbance (NPI) mean changes from score from baseline at 6 months (ITT) Show forest plot | 3 | 1005 | Mean Difference (IV, Fixed, 95% CI) | ‐2.44 [‐4.12, ‐0.76] |
| 6 Global assessment with carer input (CIBIC‐Plus) (numbers improved or unchanged) at 6 months (ITT) Show forest plot | 3 | 1306 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.84 [1.47, 2.30] |
| 7 Global assessment with carer input (CIBIC‐Plus) (numbers improved) at 6 months (ITT) Show forest plot | 8 | 3402 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.32, 1.85] |
| 8 GBS‐global assessment mean change in score from baseline at 52 weeks (ITT) Show forest plot | 1 | 282 | Mean Difference (IV, Fixed, 95% CI) | ‐3.26 [‐7.38, 0.86] |
| 9 Time spent by carer assisting in IADL and PSMS (mean changes in score from baseline min/day) at 6 months (ITT) Show forest plot | 1 | 221 | Mean Difference (IV, Fixed, 95% CI) | ‐52.4 [‐118.78, 13.98] |
| 10 Total number of withdrawals before end of treatment at 6 months or later (ITT) Show forest plot | 13 | 5143 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.76 [1.54, 2.02] |
| 11 Total number of withdrawals due to an adverse event before end of treatment at 6 months or later (ITT) Show forest plot | 13 | 5143 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.32 [1.95, 2.76] |
| 12 Number who suffered at least one adverse event before end of treatment at 6 months or later Show forest plot | 12 | 4824 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.51 [2.14, 2.95] |
| 13 Number who suffered at least one adverse event of abdominal pain before end of treatment at 6 months or later Show forest plot | 7 | 2704 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.95 [1.46, 2.61] |
| 14 Number who suffered at least one adverse event of abnormal gait before end of treatment at 6 months or later Show forest plot | 1 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.63, 4.09] |
| 15 Number who suffered at least one adverse event of abnormal dreams before end of treatment at 6 months or later Show forest plot | 1 | 153 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.38 [1.34, 21.55] |
| 16 Number who suffered at least one adverse event of accidental injury before end of treatment at 6 monthsorlater Show forest plot | 3 | 651 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.86, 2.10] |
| 17 Number who suffered at least one adverse event of agitation before end of treatment at 6 months or later Show forest plot | 2 | 767 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.57, 1.56] |
| 18 Number who suffered at least one adverse event of anorexia before end of treatment at 6 months or later Show forest plot | 10 | 4419 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.75 [2.89, 4.87] |
| 19 Number who suffered at least one adverse event of anxiety before end of treatment at 6 months or later Show forest plot | 1 | 286 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.82, 4.90] |
| 20 Number who suffered at least one adverse event of arthralgia before end of treatment at 6 months or later Show forest plot | 2 | 498 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.62, 2.40] |
| 21 Number who suffered at least one adverse event of asthenia before end of treatment at 6 months or later Show forest plot | 3 | 729 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.47 [1.27, 4.81] |
| 22 Number who suffered at least one adverse event of back pain before end of treatment at 6 months or later Show forest plot | 1 | 290 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.62, 4.36] |
| 23 Number who suffered at least one adverse event of confusion before end of treatment at 6 months or later Show forest plot | 4 | 1331 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.52, 1.32] |
| 24 Number who suffered at least one adverse event of conjunctivitis before end of treatment at 6 months or later Show forest plot | 1 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.70, 5.55] |
| 25 Number who suffered at least one adverse event of constipation before end of treatment at 6 months or later Show forest plot | 1 | 286 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.23, 1.91] |
| 26 Number who suffered at least one adverse event of depression before end of treatment at 6 months or later Show forest plot | 2 | 576 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.82, 3.04] |
| 27 Number who suffered at least one adverse event of diarrhoea before end of treatment at 6 months or later Show forest plot | 13 | 5173 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.91 [1.59, 2.30] |
| 28 Number who suffered at least one adverse event of dizziness before end of treatment at 6 months or later Show forest plot | 12 | 4583 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.64, 2.42] |
| 29 Number who suffered at least one adverse event of ecchymosis before end of treatment at 6 months or later Show forest plot | 1 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.54, 4.61] |
| 30 Number who suffered at least one adverse event of fatigue before end of treatment at 6 months or later Show forest plot | 1 | 319 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.39 [1.21, 15.85] |
| 31 Number who suffered at least one adverse event of fever before end of treatment at 6 months or later Show forest plot | 1 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.39, 1.93] |
| 32 Number who suffered at least one adverse event of fracture before end of treatment at 6 months or later Show forest plot | 5 | 2269 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.53, 1.74] |
| 33 Number who suffered at least one adverse event of haemorrhage before end of treatment at 6 months or later Show forest plot | 1 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.35, 3.02] |
| 34 Number who suffered at least one adverse event of headache before end of treatment at 6 months or later Show forest plot | 9 | 3686 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.27, 1.91] |
| 35 Number who suffered at least one adverse event of hostility before end of treatment at 6 months or later Show forest plot | 2 | 576 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.49, 1.87] |
| 36 Number who suffered at least one adverse event of increased cough before end of treatment at 6 months or later Show forest plot | 1 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.56, 2.52] |
| 37 Number who suffered at least one adverse event of infection before end of treatment at 6 months or later Show forest plot | 1 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.51, 2.37] |
| 38 Number who suffered at least one adverse event of insomnia before end of treatment at 6 months or later Show forest plot | 7 | 2906 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.49 [1.12, 2.00] |
| 39 Number who suffered at least one adverse event of muscle cramp before end of treatment at 6 months or later Show forest plot | 1 | 319 | Odds Ratio (M‐H, Fixed, 95% CI) | 13.32 [1.71, 103.74] |
| 40 Number who suffered at least one adverse event of myasthenia before end of treatment at 6 months or later Show forest plot | 1 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.10 [0.51, 8.64] |
| 41 Number who suffered at least one adverse event of nausea before end of treatment at 6 months or later Show forest plot | 13 | 5089 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.87 [4.13, 5.74] |
| 42 Number who suffered at least one adverse event of pain before end of treatment at 6 months or later Show forest plot | 1 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.47, 1.78] |
| 43 Number who suffered at least one adverse event of peripheral oedema before end of treatment at 6 monthsorlater Show forest plot | 1 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.08 [1.01, 4.28] |
| 44 Number who suffered at least one adverse event of a rash before end of treatment at 6 months or later Show forest plot | 1 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.37, 1.42] |
| 45 Number who suffered at least one adverse event of a respiratory tract infection before end of treatment at 6 m Show forest plot | 1 | 290 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.49, 2.12] |
| 46 Number who suffered at least one adverse event of rhinitis before end of treatment at 6 months or later Show forest plot | 2 | 527 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.74, 2.58] |
| 47 Number who suffered at least one adverse event of skin ulcer before end of treatment at 6 months or later Show forest plot | 1 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.55, 4.12] |
| 48 Number who suffered at least one adverse event of syncope before end of treatment at 6 months or later Show forest plot | 5 | 2206 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.90 [1.09, 3.33] |
| 49 Number who suffered at least one adverse event of tremor before end of treatment at 6 months or later Show forest plot | 2 | 633 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.82 [1.99, 23.37] |
| 50 Number who suffered at least one adverse event of urinary tract infection before end of treatment at 6 month Show forest plot | 3 | 784 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.54, 1.48] |
| 51 Number who suffered at least one adverse event of vertigo before end of treatment at 6 months or later Show forest plot | 1 | 286 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.95 [1.08, 14.46] |
| 52 Number who suffered at least one adverse event of vomiting before end of treatment at 6 months or later Show forest plot | 11 | 4703 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.82 [3.91, 5.94] |
| 53 Number who suffered at least one adverse event of weight loss before end of treatment at 6 months or later Show forest plot | 4 | 1358 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.99 [1.89, 4.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 MMSE mean change from baseline (ITT‐LOCF) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 At 24 months | 1 | 955 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.33, 0.33] |
| 2 Activities of daily living (ADCS‐ADL) (ITT‐LOCF) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At 24 months | 1 | 929 | Mean Difference (IV, Fixed, 95% CI) | ‐2.08 [‐4.58, 0.42] |
| 3 Behavioural disturbance (NPI‐10) (ITT‐LOCF) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 At 24 months | 1 | 955 | Mean Difference (IV, Fixed, 95% CI) | 0.54 [‐1.68, 2.76] |
| 4 Cognitive function (SIB) (ITT‐LOCF) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 At 24 months | 1 | 954 | Mean Difference (IV, Fixed, 95% CI) | ‐0.61 [‐3.66, 2.44] |
| 5 Global Deterioration Scale (GDS) (ITT‐LOCF) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 At 24 months | 1 | 954 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.00, 0.22] |
| 6 Total number of patients who withdrew before end of treatment Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 At 104 weeks | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.50, 0.83] |
| 7 Total number of patients who withdrew before end of treatment due to an adverse event Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 At 104 weeks | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.32, 0.68] |
| 8 Total number of patients who suffered an adverse event of nausea Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.27, 0.50] |
| 8.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.23, 0.63] |
| 9 Total number of patients who suffered an adverse event of vomiting Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.10, 0.24] |
| 9.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.15, 0.43] |
| 10 Total number of patients who suffered an adverse event of agitation Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.93, 2.30] |
| 10.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.79, 2.00] |
| 11 Total number of patients who suffered an adverse event of anorexia Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.24, 0.72] |
| 11.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.24, 0.90] |
| 12 Total number of patients who suffered an adverse event of diarrhoea Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.50, 1.30] |
| 12.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.60, 1.77] |
| 13 Total number of patients who suffered an adverse event of weight loss Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.13, 0.61] |
| 13.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.67, 1.71] |
| 14 Total number of patients who suffered an adverse event of headache Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.47, 1.48] |
| 14.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.37, 1.81] |
| 15 Total number of patients who suffered an adverse event of a fall Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.18, 0.81] |
| 15.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.75, 1.94] |
| 16 Total number of patients who suffered an adverse event of hypertension Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.14, 0.81] |
| 16.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.40, 1.44] |
| 17 Total number of patients who suffered an adverse event of depression Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.24, 1.11] |
| 17.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.34, 1.30] |
| 18 Total number of patients who suffered an adverse event of a urinary tract infection Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.67, 3.96] |
| 18.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.70, 2.42] |
| 19 Total number of patients who suffered an adverse event of aggression Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.60, 4.09] |
| 19.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.64, 2.18] |
| 20 Total number of patients who suffered a serious adverse event Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 At 104 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.79, 1.35] |
| 21 Total number of patients who died before end of treatment Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 21.1 At 104 weeks | 1 | 994 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.32 [0.78, 2.22] |

