مداخلات غیر دارویی در پیشگیری از بروز دلیریوم در بیماران بستری در بخشهای غیر از ICU
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع مطالعات در حال انجام
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Design: Randomised controlled trial of a short‐term occupational therapy intervention in an acute geriatric unit Date of study: November 2002 to June 2003 Inclusion criteria: All patients aged 65 and over consecutively admitted to the acute geriatric unit with an acute medical illness or exacerbation of existing chronic condition | |
| Participants | Number in study: 400 Country: Spain Age: Mean age 83.7 years (SD 6.1) in intervention group, 83.3 years (SD 6.5) in control group Sex: 43.4% male in intervention group, 43.1% male in control group | |
| Interventions | Intervention: Occupational therapy intervention (OTI) schedule consisted of a daily 45‐minute session with patient and relative/caregiver Monday‐Friday for the duration of admission. Activities were carried out according to needs and day of admission. Therapeutic plan included: cognitive stimulation; instruction on preventing complications including immobility, confusion, falls, urinary incontinence, pressure sores; retraining in ADL; assessment of technical aids for home. Control: All participants received medical treatment, nursing care, physical therapy and social assistance. | |
| Outcomes | 1. Incident delirium, measured using CAM 2. Length of admission 3. Activities of daily living (ADL), measured using Barthel index 4. In‐hospital mortality 5. Adverse events | |
| Notes | Funding source: Institute of Health Sciences, Junta de Comunidades de Castilla‐La Mancha. Declarations of interest: "All authors declare that there is not any personal, financial or potential conflict of interest, and therefore have nothing to declare." Delirium excluded at enrolment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Assignment to randomised group by a geriatrician who did not participate in the clinical management of participants |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation system |
| Blinding of participants and personnel (performance bias) | High risk | The geriatricians caring for the patients and providing their routine care were blinded to allocated group. Participants were not blinded due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessor and the individual performing data analysis were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Number with missing data are balanced between groups and there do not appear to be any systematic differences between the groups. |
| Selective reporting (reporting bias) | Low risk | No changes were made to trial outcomes after the trial was initiated |
| Other bias | Low risk | No evidence of other bias |
| Methods | Design: Randomised controlled trial of a delirium‐free protocol administered postoperatively in a general and colorectal surgery unit Date of study: November 1996 to March 1999 Inclusion criteria: Consecutive patients over 70 and under 86 years who underwent resection of gastric or colorectal cancer under general anaesthesia in one hospital department | |
| Participants | Number in study: n = 42 randomised, outcomes reported for n = 40 Country: Japan Age: Mean age 75.9 (SD 4.5) for intervention group; mean age 76.2 (SD 4.1) for control group Sex: 26 males and 14 females (15/20 males in intervention and 11/20 in control group) Ilness severity: APACHE score 8.3 (SD 1.4) for intervention and 7.6 (SD 1.7) in control group | |
| Interventions | Intervention: Delirium‐free protocol (DFP): Post surgery, Diazepam 0.1 mg/kg IM at 20.00, Flunitrazepam 0.04 mg/kg IV and Pethidine 1 mg/kg IV infusions 20.00‐04.00 for 3 nights Control: Treatment as usual. No placebo | |
| Outcomes | 1. Incident delirium in 7 postoperative days by psychiatrist using DSM‐IV criteria 2. Behavioural disturbance in 7 postoperative days 3. Length of admission | |
| Notes | Funding source: Not reported Declarations of interest: Not reported Delirium not excluded at enrolment Intervention used likely to sedate and therefore interfere with assessments for delirium Very specific patient group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Randomisation method unclear thus allocation is unclear |
| Random sequence generation (selection bias) | Unclear risk | Stated random assignment but method not described |
| Blinding of participants and personnel (performance bias) | High risk | All participants and personnel unblinded |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessment made by psychiatrist unaware of original allocation |
| Incomplete outcome data (attrition bias) | Unclear risk | Two dropouts but not clear from which group and no data presented for these |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information presented to make judgment |
| Other bias | High risk | The issue of how delirium was assessed in patients who might be sedated by the DFP is not addressed |
| Methods | Design: Randomised controlled trial of melatonin for 14 days or until discharge in a medical unit in a tertiary care hospital Date of study: October 2007 to February 2008 Inclusion criteria: admissions of 65 years and older to through the emergency department to Internal Medicine inpatient services | |
| Participants | Number in study: 145 Country: Canada Age mean (SD): Intervention: 84.3 (5.9), Control 84.6 (6.2); P = 0.8 Sex: Male Intervention 46%, Control 39%; P= 0.58 | |
| Interventions | Intervention: Melatonin tablets half of 1 mg, rapid dissolving, daily for 14 days or until discharge Control: Lactose tablets 100 mg halved, similar in appearance | |
| Outcomes | 1. Incident delirium measured using CAM 2. Delirium severity, measured using MDAS but included prevalent cases 3. Length of admission 4. Use of psychotropic medication 5. Withdrawal from protocol 6. Mortality | |
| Notes | Funding source: Divison of Geriatric Medicine, University of Western Ontario Declarations of interest: "None of the authors or study team members has had any conflict of interest or any affiliation or relation with any melatonin producing organization" Delirium not excluded at enrolment, but data available for prevalent delirium Four participants not randomised‐ unclear why | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Pharmacy kept randomisation code |
| Random sequence generation (selection bias) | Low risk | Patients were assigned using computer‐generated blocked‐randomisation (block size: 4) |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and clinicians blinded. In case of emergency, an independent physician could request unmasking of the treatment allocation |
| Blinding of outcome assessment (detection bias) | Low risk | All the assessments were carried out by research assistants and clinicians blinded to group assignment. The investigators did not become aware of treatment allocation until several months after study completion |
| Incomplete outcome data (attrition bias) | High risk | Withdrawals and missing data for 11 in intervention group, 12 in control group. Reasons for missing data not separated by group, therefore difficult to tell whether reasons could be due to side effect of study medication, or more delirium episodes in one group. The results are presented as available case analysis rather than intention‐to‐treat. The authors present a sensitivity analysis to consider worst case figures for delirium incidence that all those missing from the intervention group have delirium and that none of those in the control group had delirium. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information presented to make judgment |
| Other bias | Low risk | No evidence of other bias |
| Methods | Design: Randomised controlled trial of oral premedication with diazepam and diphenhydramine versus no premedication in older people undergoing cardiac catheterisation Date of study: Not reported Inclusion criteria: Aged > 70 years; elective cardiac catheterisation | |
| Participants | Number in study: 93 (53% inpatients; demographic data for entire sample) Country: USA Age: Mean age 78 years (SD 4.8) in intervention group; 77 years (SD 3.5) in control group Sex: Males 25 (53%) in intervention; 28 (61%) in control | |
| Interventions | Intervention: Oral premedication with diazepam 5 mg and diphenhydramine 25 mg Control: No premedication prior to procedure | |
| Outcomes | 1. Incident delirium using CAM 2. Cognitive function using MMSE (data not fully reported in paper) 3. Length of stay (data not fully reported in paper) | |
| Notes | Funding source: Not reported Declaration of interest: Not reported Delirium excluded at enrolment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | High risk | No placebo given to the control group |
| Blinding of outcome assessment (detection bias) | High risk | States ‘the catheterization laboratory staff and nursing staff that took care of patients after the procedure and majority of the operators were unaware of the randomisation' |
| Incomplete outcome data (attrition bias) | Low risk | Complete reporting of all included participants |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information presented to make judgment |
| Other bias | Low risk | No evidence of other bias |
| Methods | Design: Randomised controlled trial of intrathecal morphine versus patient‐controlled intravenous morphine for postoperative analgesia and recovery after major colorectal surgery Date of study: July 2001 to December 2003 Inclusion criteria: Cancer of left colon or rectum with surgical indication for resection in patients over 70 years with normal preoperative functional status | |
| Participants | Number in study: 59 Country: France Age: Mean age 78 years (SD 5 years) in intervention group, 77 years (SD 5 years) in control group Sex: 58% male in intervention group, 46% male in control group | |
| Interventions | Intervention: Preoperatively, a dose of 300 mcg of morphine was injected via the L4/L5 interspace. Postoperatively, patients had IV PCA. Control: Preoperatively, a 3 mL dose of saline was injected into the subcutaneous space between L4/L5. Postoperatively, patients had PCA. Postoperative management was identical for all patients. | |
| Outcomes | 1. Incident delirium, measured using CAM 2. Cognitive status, defined as number of days for MMSE to return to preoperative value 3. Length of admission 4. Mortality 5. Withdrawal from protocol | |
| Notes | Funding Source: Institutional grant from the Assistance Publique‐Hopitaux de Paris Declarations of interest: Not reported Delirium not excluded at enrolment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A physician independent from the study group opened a sealed letter that assigned the group of allocation according to the rank of inclusion |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number list |
| Blinding of participants and personnel (performance bias) | Low risk | Participants blinded as already under general anaesthesia. Personnel providing care for the patient blinded to their assignment. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind RCT but no statement of outcome assessor blinding |
| Incomplete outcome data (attrition bias) | Low risk | 7/59 patients not included in final analysis although reasons for exclusion reported |
| Selective reporting (reporting bias) | High risk | Reported outcomes which were not pre‐specified in the methods |
| Other bias | Low risk | No evidence of other bias |
| Methods | Design: Randomised trial of epidural and general anaesthesia in patients operated on for fracture neck of femur Date of study: March 1983 to November 1984 Inclusion criteria: All fully lucid, consenting patients admitted to an orthopaedic unit for fracture neck of femur | |
| Participants | Number in study: 57 Country: Sweden Age mean years (SD): Epidural 78(8), General 77(7) Sex M:F: Epidural 4/24, General 7/22 | |
| Interventions | Intervention: Epidural anaesthesia | |
| Outcomes | 1. Incident delirium measured using a modified version of the Organic Brain Syndrome Scale on postoperative days 1 and 7 2. Length of admission (data not fully reported) 3. Physical morbidity (stroke, urinary tract infection) 4. Psychological morbidity (depression) 5. Pressure ulcers | |
| Notes | Funding source: Swedish Medical Council; King Gustav V Birthday Foundation; Umea University Research Foundation Declarations of interest: Not reported Delirium not excluded at enrolment No data presented for length of admission but reported as no difference between the two groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method not described |
| Random sequence generation (selection bias) | Unclear risk | Method for random sequence generation not described |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel not blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors did not know allocation of participants at time of testing for delirium |
| Incomplete outcome data (attrition bias) | Low risk | All participants included in outcome reporting |
| Selective reporting (reporting bias) | High risk | Reported outcomes which were not pre‐specified in the methods |
| Other bias | Low risk | No evidence of other bias |
| Methods | Design: Randomised controlled trial of a multi‐component intervention, the Intervention to Prevent Delirium (IPD) in older patients admitted to medical and geriatric wards Date of study: 2005 to 2006 Inclusion criteria: Age > or = to 65 years admitted to medical and geriatric wards in one hospital Exclusion criteria: MMSE score < or =25, at least 1 relative not present, transfer out of ward, pre‐existing dementia, blindness, deafness, aphasia or unable to understand Italian | |
| Participants | Number in study: 60 Country: Italy Age: Not given Sex M:F: Intervention 12/18, Control 12/18 | |
| Interventions | Intervention: Intervention to Prevent Delirium (IPD), a series of structured and standardised welfare actions based on existing guidelines, including support in the following areas: cognitive re‐orientation, sensory and environmental, mobilisation, hydration, and 'socio‐emotional' Control: Usual care, not described further | |
| Outcomes | 1. Incident delirium measured using CAM & DRS‐R‐98 on days 1, 2, 4, 7 of hospital stay 2. Cogntive status using MMSE 3. Functional performance using Barthel Index | |
| Notes | Funding source: Not reported Declarations of interest: Not reported Delirium not excluded at enrolment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Odd and even days of admission used so concealment unlikely |
| Random sequence generation (selection bias) | High risk | Sequence generated using day of admission |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel not blinded, not possible given nature of the intervention |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessment blinding not described |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information presented to make judgment |
| Other bias | Low risk | No evidence of other bias |
| Methods | Design: Randomised controlled trial of a clinical decision support system to improve the care of hospitalised older adults with cognitive impairment Date of study: July 2006 to March 2008 Inclusion criteria: At least 65 years of age, hospitalised on a medical ward, English‐speaking, and cognitive impairment at the time of hospital admission. | |
| Participants | Number in study: 427 Country: USA Age: Mean age 76.8 years (SD 7.9 years) in intervention group, 77.6 years (SD 8.3 years) in control group Sex: 39.7% male in intervention group, 28.9% male in control group | |
| Interventions | Intervention: Electronically delivered clinical decision support system (CDSS) (1) Each time a physician enters an order for a patient randomised to the intervention arm, the physician received non‐interruptive alerts of the presence of CI, Foley catheter, physical restraints, anticholinergic drugs, or the need for ACE services; (2) If the physician orders a urinary catheter, s/he will receive interruptive alerts to recommending discontinuing the catheter; (3) If the physician orders physical restraints, s/he will receive interruptive alerts recommending substituting physical restraints with the use of a professional sitter or low dose trazodone; (4) If the physician orders any of the 18 inappropriate anticholinergics, s/he will receive interruptive alerts recommending stopping the drug, suggesting an alternative, or recommending dose modification. (5) The physician was required to make a decision to accept, reject, or modify any of the interruptive alerts. Control: Patients randomised into usual care did not receive CDSS | |
| Outcomes | 1. Incident delirium, measured using CAM 2. Mortality 3. Length of hospital stay 4. Falls 5. Pressure ulcers | |
| Notes | Funding source: NIA Paul B. Beeson K23 Career Development Award Declarations of interest: "Dr Boustani has work supported by grants from the NIA and AHRQ. He is also a member of the Pfizer speakers' bureau. Dr Buckley has provided expert testimony for local law firms. Mr Perkins owns stock in several pharmaceutical firms" Delirium assessed but not excluded at enrolment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Central process following computer generation |
| Random sequence generation (selection bias) | Low risk | A computer‐generated process was employed for sequence generation in a 1:1 ratio |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind personnel treating the patients in the CDSS group |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of research assistants conducting outcome assessments not known |
| Incomplete outcome data (attrition bias) | Low risk | 427 enrolled into trial, outcome data available for 424 with no account given for missing participants or which group they were assigned to. However, small as proportion of total sample. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information presented to make judgment |
| Other bias | Low risk | No evidence of other bias |
| Methods | Design: Prospective randomised double‐blinded parallel group study assessing BIS‐guided anaesthesia in elective surgical patients Date of study: January 2007‐December 2009 Inclusion criteria: > 60yrs old; scheduled for elective major surgery anticipated to last > 2 hours or longer which has an anticipated hospital stay of at least 4 days | |
| Participants | Number in study: 921 Country: Hong‐Kong Age: Mean age of 68.1 (SD 8.2) in intervention group 67.6 (SD 8.3) in control group Sex: 62.2% of intervention group and 60.4% of control group were male | |
| Interventions | Intervention: BIS‐guided anaesthesia ‐ anaesthetic dosage adjusted to maintain BIS value between 40‐60 from commencement of anaesthesia to the end of surgery; alarm sounded when out of range Control: Routine care, anaesthetic drug administration was titrated according to clinical judgment. BIS monitoring was continued in this group, but the BIS number, its trend, and the EEG waveform were omitted from the display, specifically designed for this trial | |
| Outcomes | 1. Incident delirium, measured using CAM 2. Length of admission 3. Cognitive status (postoperative cognitive dysfunction) at 1 week and 3 months 4. Mortality at 1 week and 3 months 5. Postoperative complications 6. Psychological morbidity, measured using Short‐Form‐36 Mental Score | |
| Notes | Funding source: Research Grants Council of Hong Kong and Health and Health Services Research Fund Declarations of interest: "The authors have no conflicts of interest to disclose" Delirium not excluded at enrolment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | No evidence that allocations know |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random assignment accessed via intranet |
| Blinding of participants and personnel (performance bias) | High risk | Patients, surgeons and all research staff were blinded but, concern re: anaesthetists and theatre team in view of alarm system for intervention group only |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors blinded |
| Incomplete outcome data (attrition bias) | High risk | Outcome data available for n = 783 at one week and n = 835 at 3 months but n = 921 were randomised. Reasons for exclusion reported: n = 80 were excluded in the intervention group and n = 58 in the control group at one week; n = 32 were excluded in the intervention group and n = 25 in the control group at three months. In n = 97 cases participants were not assessed at one week due to being 'unfit for testing', compared with n = 5 at three months |
| Selective reporting (reporting bias) | Unclear risk | Limited protocol available on Centre for Clinical Trials online registry |
| Other bias | Low risk | No evidence of other bias |
| Methods | Design: Multi‐centre randomised controlled trial Date of study: November 2008‐May 2012 Inclusion criteria: Patients 65 years and older admitted for surgical treatment of hip fractures; enrolment within 24 hours of admission; individual willing to participate; medically able to receive study medication according to the protocol for the duration of the study | |
| Participants | Number in study: 452 Country: The Netherlands Age: Mean age 84.1 (SD 8.0) in intervention group, 83.4 (SD 7.5) in control group Sex: 53 (28.5%) male in intervention group, 62 (32.3%) of control group | |
| Interventions | Intervention: 3 mg of melatonin Control: Placebo | |
| Outcomes | 1. Incident delirium during the first eight days after initiation of the study medication using DSM‐IV and DOSS 2. Duration of delirium 3. 'Severe' delirium (defined as percentage of patients who received a total of ≥3mg haloperidol) 4. Length of admission 5. Use of psychotropic medications (reported as total dose rather than frequency of administration) 6. Cognitive outcomes at 3 months, using Charlson Index, IQCODE and MMSE 7. Functional outcomes at 3 months, using Katz ADL Index 8. In‐hospital mortality 9. Mortality at 3 months | |
| Notes | Funding source: Dutch National Program of Innovative Care for vulnerable older persons (a program operated by ZonMw, a Dutch institute that funds health research) Declarations of interest: None declared Delirium excluded at enrolment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Allocation blinded, randomisation list maintained by the trial pharmacist |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was stratified by study centre, with fixed blocks of 10 patients within each stratum. Before the start of the study, an independent statistician generated a randomisation schedule and the trial pharmacist maintained the randomisation list Not described method of sequence generation |
| Blinding of participants and personnel (performance bias) | Low risk | Investigators, other staff members and patients remained blinded until after the last patient had completed the study and the follow‐up and data analyses had been completed |
| Blinding of outcome assessment (detection bias) | Low risk | As above, blinded to allocation |
| Incomplete outcome data (attrition bias) | Low risk | 452 were randomised of which 70 did not complete the study, generally balanced between the groups although rates of prevalent delirium different between groups. Complete reporting of reasons for withdrawals and missing data. |
| Selective reporting (reporting bias) | Low risk | Outcome data presented as per pre‐published protocol |
| Other bias | Low risk | No evidence of other bias |
| Methods | Design: Randomised controlled study of citicoline in hip fracture surgery patients Date of study: Study dates not reported Inclusion criteria: 70 years or over, admitted with hip fracture | |
| Participants | Number in study: 81 Country: Chile Age mean years (SD): Citicoline 79.5 (6.6), Control 80.0 (5.9) P = 0.9 Sex M:F: Citicoline 4/31, Control 10/36; P = 0.2 | |
| Interventions | Intervention: Citicoline 400 mg orally 8 hourly, given between 24 hrs before and 4 days after surgery (n = 35). | |
| Outcomes | 1. Incident delirium immediately, day 1, day 2 and day 3 postoperatively using MMSE, AMT, CAM | |
| Notes | Funding source: Not reported Declarations of interest: Not reported Delirium excluded at enrolment using MMSE, AMT, CAM Study underpowered, as incidence of delirium much lower than the 20% used in power calculation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Carried out and codes kept by hospital pharmacy independently of researchers |
| Random sequence generation (selection bias) | Low risk | 'Lottery drawing' independently of researchers |
| Blinding of participants and personnel (performance bias) | Low risk | Matched placebo used |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors blind to allocation |
| Incomplete outcome data (attrition bias) | Unclear risk | Sample size reported but unclear how many randomised |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information presented to make judgment |
| Other bias | Low risk | No evidence of other bias |
| Methods | Design: Randomised open‐label trial of postoperative low dose intravenous haloperidol in older patients undergoing abdominal, orthopaedic or other surgery Date of study: January 2007 ‐ December 2012 Inclusion criteria: 75 years or older; elective abdominal surgery under general anaesthesia or elective orthopaedic surgery under general or spinal anaesthesia and who could consent to participate | |
| Participants | Number in study: 121 Country: Japan Age: Mean age 80.5 years (SD 0.5) in intervention group versus 80.2 (SD 0.5) for controls Sex: Males: Intervention 32/59; Control: 32/62 | |
| Interventions | Intervention: 2.5 mg/day of intravenous haloperidol dissolved in 100 mL of saline for first 3 days after surgery. Administered by infusion at 6 pm. Control: Usual care | |
| Outcomes | 1. Delirium incidence using NEECHAM 2. Delirium incidence stratified by low MMSE score (data not fully reported in paper) 3. Delirium severity using NEECHAM (data not fully reported in paper) 4. Delirium duration (data not fully reported in paper) 5. Adverse events (data not fully reported in paper) | |
| Notes | Funding source: Research Grant for Longevity Sciences (17C‐3, 21‐13) from the Ministry of Health, Labour and Welfare and The Research Funding for Longevity Sciences (23‐28) from the National Center for Geriatrics and Gerontology (NCGG), Japan Declaration of interest: The authors declare 'no conflicts of interest' Delirium not fully excluded at enrolment ‐ excluded if NEECHAM < 20 but this may not exclude all delirium Haloperidol given one day postoperatively rather than preoperatively or immediately postoperatively as in other studies, and prevalent delirium not excluded. Inclusion criteria only mention abdominal and orthopaedic surgery but results presented for 5 patients who underwent ‘other’ including vascular surgery. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation, adjusted for age, gender and department |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel unblinded to allocation; control group did not receive any IV medication/placebo |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label study; delirium assessment unblinded to allocation |
| Incomplete outcome data (attrition bias) | Unclear risk | Data reported on 119/121 patients. 2 patients in control group received haloperidol for delirium on day of surgery, therefore withdrawn |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess |
| Other bias | Low risk | No evidence of other bias |
| Methods | Design: Randomised controlled trial of optimisation of intraoperative depth of anaesthesia and cerebral oxygenation Date of study: Study dates not reported Inclusion criteria: Aged over 64 years, undergoing coronary artery bypass graft surgery | |
| Participants | Number in study: 81 Country: Not reported Age: Mean age 71.9 years (whole sample) Sex: 86% male (whole sample) | |
| Interventions | Intervention: Intraoperative monitoring of depth of anaesthesia using bispectral index and cerebral oxygenation monitoring | |
| Outcomes | 1. Incidence of postoperative delirium using CAM | |
| Notes | Funding source: Not reported Declarations of interest: Not reported Delirium excluded at enrolment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information provided ‐ abstract only |
| Random sequence generation (selection bias) | Unclear risk | No information provided ‐ abstract only |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided ‐ abstract only |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided ‐ abstract only |
| Incomplete outcome data (attrition bias) | Unclear risk | No information provided ‐ abstract only |
| Selective reporting (reporting bias) | Unclear risk | No information provided ‐ abstract only |
| Other bias | Unclear risk | No information provided ‐ abstract only |
| Methods | Design: Randomised controlled trial of liberal blood transfusion thresholds compared to restrictive transfusion practice for hip fracture patients Date of study: April 2008‐February 2009 Inclusion criteria: aged 50 and older; undergoing surgical repair of hip fracture; Hb < 10 g/dL within 3 days after surgery; clinical evidence of cardiovascular disease or cardiovascular disease risk factors | |
| Participants | Number in study: 139 Country: USA and Canada Age: Mean age 82.4 (SD 7.4) in intervention group compared to 80.6 (SD 10.4) in control group Sex: 81.8% of intervention group were female compared to 47% of control group | |
| Interventions | Intervention (aka liberal treatment): One unit of packed red blood cells and as much blood as needed to maintain a haemoglobin concentration >10 g/dL Control (aka restrictive treatment): only transfused if symptoms of anaemia developed or at the study physicians discretion or if Hb < 8 g/dL | |
| Outcomes | 1. Incident delirium, using CAM 2. Delirium severity, using MDAS 3. Length of admission 4. Psychoactive medication use 5. Physical morbidity (post‐randomisation adverse events) | |
| Notes | Funding source: Research grant from National Heart Lung and Blood Institute Declarations of interest: "Dr Magaziner received support from Amgen, Eli Lilly, Glaxo SmithKline, Merck, Novartis and Sanofi Aventis to conduct research through his institution, provide academic consultation, or serve on an advisory board. Dr Roffey reports working as a consultant for Palladian Health. Dr Cardson reports receiving grant support to his institution from Amgen. Dr Marcantionio is a recipient of a Mid‐Career Investigator Award in Patient‐Oriented Research from the National Institute on Aging" Delirium assessed at baseline but not excluded >1/3 of the restrictive group received transfusion | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | No evidence to suggest allocations revealed |
| Random sequence generation (selection bias) | Low risk | Automated central telephone randomisation system |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Research staff unblinded to treatment status except at one site |
| Incomplete outcome data (attrition bias) | Low risk | 139 randomised, outcome assessment data available for 138 |
| Selective reporting (reporting bias) | Low risk | Data reported for all participants included in the study |
| Other bias | High risk | Imbalance in dementia prevalence between intervention and control groups (27.3% in intervention versus 36.1% in control) |
| Methods | Design: Randomised controlled trial of ramelteon, a melatonin agonist Date of study: September 2011 to October 2012 Inclusion criteria: aged 65‐89; newly admitted for serious medical problems; able to take oral medications | |
| Participants | Number in study: 43 were admitted to acute medical wards (67 in total study cohort, 24 admitted to ICU) Country: Japan Setting: Acute medical wards in four university hospitals and one general hospital Age: Mean age 78.2 (SD 6.6) in the ramelteon group and 78.3 (SD 6.8) in the placebo group Sex: 48% of the intervention group were male compared with 32% of the placebo group Comorbidity: Charlson Index mean 3.2 (SD 2.4) in intervention group compared with 2.6 (SD 2.2) in placebo group Dementia: Clinical Dementia Rating mean score 0.5 (SD 0.7) in the intervention group compared with 0.6 (SD 0.9) in the placebo group | |
| Interventions | Intervention: Ramelteon tablet 8 mg daily at 9 pm until development of delirium or up to seven days Control: Lactose powder 330 mg daily at 9 pm until development of delirium or up to seven days | |
| Outcomes | 1. Incidence of delirium using DRS‐R‐98, cut‐off 14.5 2. Severity of delirium using DRS‐R‐98 3. Withdrawal from protocol 4. Adverse events 5. Inpatient mortality | |
| Notes | Funding source: Japan Society for the Promotion of Science (Grant‐in‐Aid for Scientific Research) Declaration of interest: Authors declare receiving honoraria from & serving as consultants for Eli Lilly, Janssen, GlaxoSmithKline, Shionogi; Merck Sharp &Dohme; Otsuka; Pfizer; Mochida; Tsumura; Dainippon‐Sumitomo; Daiichi‐Sankyo; Eisai, and Ono Delirium excluded at enrolment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Allocation concealed using envelope method |
| Random sequence generation (selection bias) | Low risk | Random number table, sealed opaque envelope |
| Blinding of participants and personnel (performance bias) | High risk | Participants not blinded, nurses administering medication not blinded; although other personnel blinded. Placebo not similar to active tablet |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessment blinded |
| Incomplete outcome data (attrition bias) | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | Reporting of outcomes as identified in the protocol published on the UMIN‐CTR registry 00005591 |
| Other bias | Low risk | No evidence of other bias |
| Methods | Design: multi‐centre, randomised controlled trial Date of study: June 2007‐June 2010 Inclusion criteria: over 65 yrs; due to undergo elective surgery for a solid tumour, deemed to be frail (using Groningen Frailty Indicator >3) | |
| Participants | Number in study: 297 Country: The Netherlands Age: Mean age 77.45 (SD 6.72) in intervention group; 77.63 (SD 7.69) in usual care group Sex: 62.2% of intervention group were female compared with 65.8% of usual care group | |
| Interventions | Intervention: Multi‐component intervention focused on best supportive care and the prevention of delirium. Preoperative geriatric team assessment with daily monitoring during hospital stay, supported by the use of standardised checklists Usual care: only had access to geriatric care if treating physician requested referral | |
| Outcomes | 1. Incident delirium, using DOSS ‐ if > 3 then had specialist assessment using DSM‐IV. Assessments performed up to 10 days postoperatively 2. Delirium severity, using DRS‐R‐98 3. Length of admission 4. Mortality 5. Return to independent living 6. Postoperative complications 7. Quality of life using Short‐Form‐36 8. Falls | |
| Notes | Funding source: Netherlands Organisation for Health Research and Development Declarations of interest: "The authors declared that no competing interests exist" Delirium not excluded at enrolment No record of how many in usual care group received geriatrician input | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Central allocation system |
| Random sequence generation (selection bias) | Low risk | Interactive voice response telephone system for randomisation provided by university |
| Blinding of participants and personnel (performance bias) | High risk | Participants and research nurses unblinded |
| Blinding of outcome assessment (detection bias) | Low risk | Delirium assessment blinded to allocation |
| Incomplete outcome data (attrition bias) | Unclear risk | 297 participants randomised, outcome assessments available for 260 (n = 127 in intervention group and n = 133 in control group) ‐ no information provided, described as 'lost to follow‐up' |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as per original protocol |
| Other bias | Low risk | No evidence of other bias |
| Methods | Design: Randomised controlled trial Date of study: May 2005‐December 2007 Inclusion criteria: aged 65 years or older; admitted to a medical unit in the study area; in hospital < 48 hours | |
| Participants | Number in study: 649 Country: Australia Age: Mean age of 79.6 (SD 7.5) in intervention group, 79.1 (7.9) in control group Sex: 45% of intervention group were male, compared to 50% of control group | |
| Interventions | Intervention: Participants randomised to the intervention arm received a graded physical activity and orientation programme twice daily, which was delivered in addition to usual care. A certified Allied Health Assistant, trained in administering exercise programmes, delivered the intervention after initial assessment of the participant by a physiotherapist. The programme started on the same day as the participant was randomised. Commensurate with ability, participants were prescribed one of four exercise programmes: bed, seated, standing or rails. All programmes were customised to the participant’s ability and were reviewed daily. Exercise programmes were modified to ensure suitable progression for those participants who made significant gains. The orientation programme comprised formal and informal elements. The formal element of the programme comprised a series of seven questions aimed at assessing and improving orientation (day, month, year, date, ward, bed number and name of primary nurse). The participant was asked the questions in sequence and prompted with the correct answer if they were not able to give a correct response. The informal element of the programme related to engaging in the exercise programme and in the social interaction with the Allied Health Assistant and/or Physiotherapist. Control: Usual care included 24‐hour nursing care, daily medical assessment and allied health referral by medical, nursing or other staff. Allied health input was provided on referral only, but daily ward meetings were held to review patient progress and facilitate referrals. Patients with significant functional, cognitive or social issues could be referred to the Aged Care medical consultation service that performed a daily round and could offer advice regarding the recognition, investigation and management of geriatric syndromes including delirium. | |
| Outcomes | 1. Incidence of delirium, using CAM 2. Duration of delirium 3. Severity of delirium, using CAM 4. Length of stay 5. Return to previous residence | |
| Notes | Funding source: HCF Health and Medical Research Foundation Declarations of interest: "No competing interests" Very low rates of delirium in both arms. Authors suggest may be due to 48 hourly assessments or not selecting those at high risk. Delirium excluded at enrolment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes for allocation |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not clear, just states 'randomisation was achieved using sealed opaque envelopes' |
| Blinding of participants and personnel (performance bias) | High risk | Participants not informed of allocation, but unable to fully blind due to nature of intervention |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors blinded to allocation |
| Incomplete outcome data (attrition bias) | Low risk | n = 17 in intervention and n = 18 in control did not receive the intervention, but were assessed on an intention‐to‐treat analysis basis |
| Selective reporting (reporting bias) | Low risk | Trial protocol retrospectively registered with Australian New Zealand Clinical Trials Registry ACTRN 012605000044628; outcomes reported in accordance with protocol |
| Other bias | Low risk | No evidence of other bias |
| Methods | Design: Randomised controlled trial of fast‐track surgery for colorectal cancer compared to usual care Date of study: 2008‐2011 Inclusion criteria: patients aged 70 years and over with colorectal cancers admitted to the Fourth Hospital of Hebei Medical Univerity for open curative resection. | |
| Participants | Number in study: 240 Country: China Age: Mean age of 75.6 (SD 4.2) in intervention group; 74.8 (SD 4) in control group Sex: 65% of intervention group were male, compared to 60% of the control group | |
| Interventions | Fast‐track surgery group: Bowel preparation with oral purgatives instead of a mechanical enema; thoracic epidural anaesthesia and postoperative analgesic maintenance via the epidural catheter maintained for 48h; no nasogastric tube insertion; no drainage tube placement with the exception of the low rectal anastomosis; water was allowed from 6 hours post operation, liquid diet in the morning and semi‐liquid diet at noon and evening of the first and second postoperative day (POD) with regular diet on POD 3; early urine catheter withdrawal; early out‐of‐bed mobilisation Traditional therapy group: usual preoperative and postoperative care | |
| Outcomes | 1. Incidence of delirium, using DRS‐R‐98 2. Length of admission 3. Postoperative complications | |
| Notes | Funding source: Not reported Declarations of interest: "No conflicts of interest" Delirium not clearly excluded at enrolment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Allocation method not clearly described |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel not blinded due to nature of intervention |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear if psychiatrist performing outcome assessment was blinded to allocation or not |
| Incomplete outcome data (attrition bias) | Low risk | n = 240 participants were randomised, outcome assessment available for n = 233. Three in intervention group and four in the control group did not receive their allocated intervention and were excluded from outcome assessment data ‐ these individuals did not meet study inclusion criteria |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information presented to make judgment |
| Other bias | Low risk | No evidence of other bias |
| Methods | Design: Randomised controlled study of haloperidol prophylaxis in patients undergoing hip surgery Date of study: August 2000 to August 2002 Inclusion criteria: Patients aged 70 years or over admitted for acute or elective hip surgery, who were at intermediate or high risk of delirium postoperatively | |
| Participants | Number in study: 430 Country: The Netherlands Age mean (SD): Intervention 78.76.0), Control 79.66.3); P = 0.15 Sex M:F: Intervention 19.9%, Control 21.1% Ilness severity: APACHE scores mean (SD) Intervention 13.4 (3.2), Control 13.3 (3.1) | |
| Interventions | Intervention: Haloperidol 0.5 mg orally three times daily on admission until 3 days postoperatively Control: Placebo tablets identical in appearance Proactive geriatric consultation offered to all patients in both groups | |
| Outcomes | 1. Incident delirium postoperatively using DSM‐IV and CAM 3. Duration of delirium | |
| Notes | Funding source: Medical Center Alkmaar Declarations of interest: "Financial disclosure: none" Delirium at enrolment excluded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Randomisation by hospital pharmacy independent of researchers. Codes held in sealed envelopes. |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation code |
| Blinding of participants and personnel (performance bias) | Low risk | Matched placebos used |
| Blinding of outcome assessment (detection bias) | Low risk | Members of the research team not involved in the clinical care of patients performed all baseline and outcome assessments |
| Incomplete outcome data (attrition bias) | Unclear risk | Complete outcomes data available for n = 395, missing data for n = 35 (24 in control, 11 in intervention) 192/212 in intervention and 190/218 in control treated according to protocol. Outcome data available reported as intention‐to‐treat by study authors. More lost to follow‐up in placebo group than intervention group and lack of information about those who were lost. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information presented to make judgment |
| Other bias | Low risk | No evidence of other bias |
| Methods | Design: Randomised controlled trial of olanzapine to prevent postoperative delirium in elderly joint replacement patients Date of study: 2005 to 2007 Inclusion criteria: All patients aged 65 years and over, patients aged less than 65 years with a history of delirium, impending joint‐replacement surgery, ability to speak English, and ability to provide informed consent | |
| Participants | Number in study: 495 Country: USA Age: Mean age 73.4 years (SD 6.1 years) in intervention group, 74.0 years (SD 6.2 years) in control group Sex: 48% female in intervention group, 60% female in control group | |
| Interventions | Intervention: First dose of olanzapine 5 mg (orally disintegrating tablet (ODT)) administered immediately before surgery in the pre‐anaesthesia care unit by nursing staff. Second dose of olanzapine 5 mg administered in the post‐anaesthesia care unit by nursing staff blind to the intervention arm. Control: Oral dispersible tablet placebo of similar appearance to the olanzapine tablet. | |
| Outcomes | 1. Incident delirium, measured using CAM/DSM‐III‐R 2. Severity of delirium, measured using DRS‐R‐98 3. Duration of delirium 4. Withdrawal from protocol 5. Cognition using MMSE 6. Adverse events | |
| Notes | Funding source: New England Baptist Hospital Research Department Declarations of interest: "Theodore A Stern, has been a consultant to and is on the speaker's bureau of Eli Lilly and Company, and has been a consultant to and shareholder of WiFiMed, the company that designed the Tablet PC data‐management software. No other authors reported conflicts of interest" Delirium not excluded at enrolment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Randomisation sequence held in pharmacy department. Randomisation carried out by pharmacy department. |
| Random sequence generation (selection bias) | Low risk | Statistician provided pharmacy with a computer‐generated random‐number table. |
| Blinding of participants and personnel (performance bias) | Low risk | Hospital pharmacy prepackaged the study drug and placebo in identical packages and blinded investigators and participants. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessments conducted by research assistants and nurses and verified by a clinical psychologist. All were blind to allocation group |
| Incomplete outcome data (attrition bias) | High risk | 95 dropouts not included in final analysis (n = 47 in intervention, n = 48 in control). Reasons stated but imbalance between groups with loss due to anxiety, surgery cancelled and family pressure as significant factors. High rate of delirium (40% in placebo group vs 14.3% in intervention group), concern that some of the exclusions may influence outcome assessment |
| Selective reporting (reporting bias) | Low risk | Study protocol registered on ClinicalTrials.gov NCT000699946; outcomes reported in accordance with protocol |
| Other bias | Low risk | No evidence of other bias |
| Methods | Design: Pilot randomised controlled trial of gabapentin to decrease postoperative delirium in older patients Date of study: 2005 Inclusion criteria: Consecutive patients who were > 45 years of age, undergoing surgery involving the spine, requiring general anaesthesia, and expected to remain in the hospital postoperatively for > 72 hours. | |
| Participants | Number in study: 21 Country: USA Age: Mean age 59.6 years Sex: 48% female | |
| Interventions | Intervention: Gabapentin 900 mg administered by mouth 1 to 2 hours before surgery and anaesthesia. 900 mg dose continued daily for the first 3 postoperative days. Control: Placebo as control. Unclear whether matching placebo used. | |
| Outcomes | 1. Incident delirium, measured using CAM | |
| Notes | Funding source: National Institute of Aging, National Institute of Health Declarations of interest: "Dr Rowbotham consults for, and owns stock in, a company developing an analogue of gabapentin, an investigational agent" Pilot trial Delirium not excluded at enrolment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Random number list given to the research pharmacist who prepared and delivered the designated drug to each study patient according to the randomised allocation. However, not clear how the random number list allocation was concealed from the pharmacist by the co‐investigator who created it. |
| Random sequence generation (selection bias) | Low risk | Computerised random number list generated by co‐investigator |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo‐controlled so participants and personnel blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Trained interviewer blinded to the study drug assignment measured the occurrence of delirium |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for in analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information presented to make judgment |
| Other bias | Low risk | No evidence of other bias |
| Methods | Design: Randomised controlled trial of intravenous parecoxib sodium analgesia for those undergoing femoral head replacement Date of study: January 2011 ‐ May 2012 Inclusion criteria: age >70 years old; weight < 90 kg; diagnosed with femoral neck fracture caused by trauma and required for analgesia; anaesthetic risk ASA II or III; achieved satisfactory intraoperative anaesthesia outcome; sedation only by intravenous midazolam; maintain normal blood pressure and heart rate by ephedrine and atropine. | |
| Participants | Number in study: 80 Country: China Age: Mean 76.6 (SD 2.6) Sex: Male sex 29 (36%) | |
| Interventions | Intervention: Intravenous parecoxib sodium (non‐steroidal anti‐inflammatory medication). Dosage based by weight. Given 12 hourly over 3 days (total of 6 injections). Given up to 2 mg IV morphine if pain score elevated despite intervention. Control: Intravenous morphine 2 mg or 4 mg at first injection, thereafter given 5 injections of 2 mL of saline every 12 hours over 3 days (total of 6 injections). Could also be given up to 2 mg IV morphine if pain score elevated. | |
| Outcomes | 1. Incident delirium using DSM‐IV 2. Length of admission 3. Postoperative cognitive dysfunction using APA criteria (3 days, 1 week, 3 months, 6 months) | |
| Notes | Funding source: Science and Technology Development Project of Qingdao Science and Technology Bureau Declaration of interest: Not reported Unclear if delirium excluded at enrolment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Group assignment 'managed by one specific staff’ but not clear if allocation concealment maintained |
| Random sequence generation (selection bias) | Low risk | Random number tables used to generate randomisation sequence |
| Blinding of participants and personnel (performance bias) | Low risk | Participants, personnel administering medications and monitoring patient were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Paper states study was double‐blind, outcome assessment procedure not described in translation |
| Incomplete outcome data (attrition bias) | Low risk | Paper reports complete follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess |
| Other bias | High risk | Potential confounding for unbalanced use of additional morphine doses between group; 7.9 mg in parecoxib group vs. 31.3 mg in morphine and saline group. |
| Methods | Design: Randomised controlled trial of donepezil in patients undergoing elective arthroplasty of the knee or hip Date of study: May 2000 to April 2003 Inclusion criteria: Patients over 50 years, able to give informed consent, admitted for elective knee or hip arthroplasty | |
| Participants | Number in study: 90 Country: USA Age mean(SD) years: Intervention 67.2 (8.7), Control 69.4 (8.9); P = 0.03 Sex M:F: Intervention 43%, Control 35%; P = 0.17 | |
| Interventions | Intervention: Donepezil 5 mg once daily for 14 days before and after surgery, doubled to 10 mg if developed any symptoms of delirium | |
| Outcomes | 1. Incident postoperative delirium, using DSM‐IV criteria from DSI and CAM | |
| Notes | Funding source: Pfizer Corporation Declarations of interest: "This study was supported by an unrestricted research grant from Pfizer Corporation. Dr Liptzin has also been a consultant or speaker for Pfizer, Novartis, Janssen, Forest Labs, and Bristol Myers Squibb" Delirium not excluded at enrolment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information on concealment not provided |
| Random sequence generation (selection bias) | Unclear risk | Randomisation by research pharmacist, method not described |
| Blinding of participants and personnel (performance bias) | Low risk | Identical capsules of active drug and placebo used so participants and personnel blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessment by research assistant blinded to allocation |
| Incomplete outcome data (attrition bias) | High risk | Incomplete follow‐up. Intention‐to‐treat analysis not conducted. Number of dropouts similar in both groups but sufficiently high to potentially affect results |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess |
| Other bias | Low risk | No evidence of other bias |
| Methods | Design: Randomised controlled trial of multi‐component delirium prevention intervention for older hip fracture patients Date of study: May 2000 to December 2002 Inclusion criteria: Patients aged 70 years and older consecutively admitted to the orthopaedic department in Umea hospital, Sweden. | |
| Participants | Number in study: 199 Country: Sweden Age: Mean age 82 years Sex: 74% female | |
| Interventions | Intervention: Multi‐disciplinary team providing comprehensive geriatric assessment, management and rehabilitation on a geriatric ward. Intervention comprising: staff education; teamwork; individual care planning; delirium prevention detection and treatment; prevention and treatment of complications; bowel/bladder function; sleep; decubitus ulcer prevention/treatment; pain management; oxygenation; body temperature measurement; nutrition; rehabilitation; secondary prevention of falls/fractures and osteoporosis prophylaxis. Control: Usual care on orthopaedic ward. | |
| Outcomes | 1. Incident delirium, diagnosed retrospectively using DSM‐IV based on nursing notes (for the duration of the inpatient stay) and OBS (measured once between the 3rd and 5th postoperative day) 2. Duration of delirium, diagnosed retrospectively using DSM‐IV based on nursing notes and OBS 3. Length of admission 4. Cognitive status, measured using MMSE 5. Falls 6. New pressure ulcers 7. Psychological morbidity (Depression) 8. Mortality ‐ inpatient and at 12 months | |
| Notes | Funding source: Swedish Research Council & Vardal Foundation Declarations of interest: Not reported Prevalent delirium not excluded at enrolment (21.8% intervention group, 30.9% control group) and patients with prevalent delirium appear to have been included in outcome data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes to conceal allocation |
| Random sequence generation (selection bias) | Unclear risk | No information given on how randomisation sequence generated |
| Blinding of participants and personnel (performance bias) | High risk | All staff aware of allocation group, patients potentially aware due to nature of intervention |
| Blinding of outcome assessment (detection bias) | High risk | Staff recording outcome measurements not blind to study arm. Blinded specialist made diagnosis of delirium retrospectively based on staff measurements and medical/ nursing records |
| Incomplete outcome data (attrition bias) | Low risk | All randomised patients included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess |
| Other bias | High risk | Imbalance in dementia prevalence between intervention and control groups (27.5% in intervention versus 37.1% in control) |
| Methods | Design: Randomised controlled trial Date of study: February 2006‐October 2010 Inclusion criteria: patients scheduled for surgery under general anaesthesia were eligible if they either had proven coronary artery disease (CAD) and were scheduled for major surgery or had 2 or more risk factors for CAD and were scheduled for major vascular surgery | |
| Participants | Number in study: 385 Country: Switzerland Age: Mean age 78 (SD 8) in sevoflurane group; 73 (SD 8) in propofol group Sex: 75% of sevoflurane group were male compared with 77.6% of propofol group | |
| Interventions | In both groups anaesthesia induction was with etomidate. The protocol did not regulate dosage for the induction or maintenance of anaesthesia or any other aspects of intraoperative management. Sevoflurane: Anaesthesia maintained using sevoflurane Propofol: Anaesthesia maintained using propofol | |
| Outcomes | 1. Incidence of delirium using CAM 2. Mortality at 12 months | |
| Notes | Funding source: University Hospital Basel; Roche Diagnostics; Abbot AG Declarations of interest: "Roche Diagnostics Switzerland provided in‐kind support (assay kits). Abbott AG Switzerland provided some financial support for the conduction of the study. No other potential conflicts of interest are to be disclosed for any of the authors." Delirium not excluded at enrolment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Numbered, sealed opaque envelopes to conceal allocation |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random allocation sequence |
| Blinding of participants and personnel (performance bias) | High risk | Participants blinded to allocation, anaesthesiologists not blinded as able to work‐out allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessment blinded to allocation |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up. Seventeen patients randomised in error, but reasons reported and excluded from analysis |
| Selective reporting (reporting bias) | High risk | Protocol for Trial of the Effect of Anesthetics on Morbidity and Mortality (TEAM‐Project) NCT00286585 but no information about reporting of delirium outcomes in original protocol |
| Other bias | Low risk | No evidence of other bias |
| Methods | Design: Randomised controlled trial of proactive geriatric consultation in patients with hip fracture Date of study: Study dates not reported Inclusion criteria: All patients aged 65 years and older, admitted for primary surgical repair of hip fracture, who were at intermediate or high risk of delirium (presence of 1 or more delirium risk factors) | |
| Participants | Number in study: 126 Country: USA Age mean (SD): Intervention 78 (8), Control 80 (8); P = 0.39 Sex M:F: Intervention 21%, Control 22%; P = 0.9 | |
| Interventions | Intervention: Proactive consultation by Consultant Geriatrician, with daily visits starting preoperatively or within 24 hrs post operatively for duration of admission. Protocol based targeted recommendations over and above what was already being done by team, limited to 5 at initial visit and 3 at follow‐up visits. | |
| Outcomes | 1. Delirium incidence‐ total cumulative during admission, using CAM (performed daily throughout inpatient stay) 2. Delirium incidence in dementia subgroup 6. Withdrawals from protocol | |
| Notes | Funding source: Older Americans Independence Center; Charles Farnworth Trust; Delirium examined but not reported at intake, making interpretation of results for primary outcome of cumulative delirium incidence difficult | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes prepared with allocation |
| Random sequence generation (selection bias) | Low risk | Random number table used to generate sequence |
| Blinding of participants and personnel (performance bias) | High risk | Nature of intervention precluded blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) | Low risk | Independent researchers conducted delirium assessments and timed not to coincide with Geriatrician consultation. States blinding successfully maintained |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess |
| Other bias | High risk | Imbalance in dementia prevalence between intervention and control groups (37% in intervention and 51% in control) |
| Methods | Design: Pilot randomised controlled trial of donepezil for delirium after hip fracture Date of study: January 2007 ‐ August 2008 Inclusion criteria: Admitted to the orthopaedic service for surgical repair of hip fracture and: age 70 and older, English speaking, residence within 40 mile radius of medical centre, life expectancy 6 months or greater, not currently taking cholinesterase inhibitor therapy | |
| Participants | Number in study: 16 Country: USA Age: Mean age 88.0 years (SD 5.2) in intervention group; 87.0 (3.7) in control group Sex: 71% female in intervention group; 44% female in control group | |
| Interventions | Intervention: 5 mg dose of donepezil initiated on the day before or within 24 hours of surgery and continued for a total of 30 days. Control: Matching placebo. All participants received perioperative co‐management from a geriatric team on orthogeriatric ward | |
| Outcomes | 1. Incident delirium, measured using CAM but not included in meta‐analysis as reported as cumulative measures within individuals 2. Delirium severity, measured using MDAS 3. Withdrawal from trial 4. Adverse events | |
| Notes | Funding Source: National Institute of Aging Declarations of interest: "The authors have no financial or any other kind of personal conflicts with this paper" Delirium not excluded at enrolment Only 16 participants in pilot trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate allocation concealment likely: on‐site pharmacy prepared and dispensed active medication and placebo; study team masked to treatment assignment. |
| Random sequence generation (selection bias) | Unclear risk | Permuted block randomisation used but method of sequence generation not described. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel blinded to allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Delirium assessment conducted by trained research interviewer blinded to allocation |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis performed, all randomised participants included in the analysis |
| Selective reporting (reporting bias) | Low risk | Protocol for Supporting the Health of Adults Undergoing Orthopedic Surgery During the Recovery Period (SHARP) NCT00586196; reporting in accordance with protocol |
| Other bias | Low risk | No evidence of other bias |
| Methods | Design: Randomised controlled trial of a multi‐component delirium prevention intervention provided by family members Date of study: September 2009‐June 2010 Inclusion criteria: All patients at risk for delirium (> 70 years, cognitive impairment (MMSE < 24 prior to admission) alcoholism or metabolic imbalance at admission) | |
| Participants | Number in study: 287 Country: Chile Age: Mean age 78.1 years (SD 6.3) in intervention group; 78.3 years (6.1) in control group Sex: 42% female in intervention group; 33% female in control group | |
| Interventions | Intervention: Multi‐component non‐pharmacological intervention provided by family members, including education regarding confusional syndromes; provision of a clock and calendar; avoidance of sensory deprivation (glasses, denture and hearing aids available as needed); presence of familiar objects in the room; re‐orientation of patient provided by family members; extended visiting times (5 hours daily). Control: Usual care from the attending physician | |
| Outcomes | 1. Incident delirium, measured using CAM performed daily, throughout admission 2. Duration of delirium 3. Length of admission 4. Falls | |
| Notes | Funding source: Not reported Declarations of interest: "No conflicts of interest declared" Delirium excluded at enrolment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Randomisation performed by a statistician who was not involved in data collection |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel unblinded due to the nature of the intervention |
| Blinding of outcome assessment (detection bias) | High risk | Outcome assessors unblinded |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐1 treat analysis performed, 5% loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess |
| Other bias | Low risk | No evidence of other forms of bias |
| Methods | Design: Randomised placebo‐controlled trial of fascia iliaca compartment block (FICB) prophylaxis for hip fracture patients at risk for delirium. Date of study: July 2004‐March 2008 Inclusion criteria: Men and women aged 70 years and older admitted for hip fracture surgery | |
| Participants | Number in study: 219 Country: Greece Age: Mean age 72.7 years Sex: 74% female | |
| Interventions | Intervention: Fascia iliaca compartment block (FICB) using a 0.25 mg dose of 0.3 mL/kg bupivacaine at admission and repeated daily until either delirium developed or hip fracture surgery was performed. 24 hours after surgery, the same dose of FICB was administered and repeated every 24 hours until either delirium occurred or discharge. Control: Matching placebo using water for injection following same regimen. | |
| Outcomes | 1. Incident delirium measured using DSM‐IV/CAM 2. Delirium severity, measured using DRS‐R‐98 3. Duration of delirium 4. Mortality | |
| Notes | Funding source: Not reported Declarations of interest: "The authors declare that they have no conflict of interest related to the publication of this manuscript" Delirium excluded at enrolment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Allocation concealed by central allocation method |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence |
| Blinding of participants and personnel (performance bias) | High risk | Single (participant) blinding. Orthopaedic surgeons performing the local anaesthetic injection do not appear to be blind. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear who performed outcome assessments and if blinded or not |
| Incomplete outcome data (attrition bias) | High risk | Nine patients not included in outcome assessment and lack of information about those lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess |
| Other bias | Low risk | No evidence of other forms of bias |
| Methods | Design: Randomised controlled trial of donepezil in preventing delirium and postoperative cognitive decline following orthopaedic surgery. Date of study: Study dates not reported Inclusion criteria: Aged 65 years and over, no prior donepezil use and scheduled for hip fracture repair or elective hip or knee replacement surgery. | |
| Participants | Number in study: 15 Country: USA Age: Mean age 74.1 years Sex: 66% female | |
| Interventions | Elective patients: donepezil 5 mg starting 7 days prior to surgery and tapering off during the third week following surgery Hip fracture patients: donepezil 5 mg starting on the day of surgery ending 5 days postoperatively Control: placebo | |
| Outcomes | 1) Incident delirium, but reported using mean CAM rather than dichotomous data 2) Length of admission 3) Cognitive status using MMSE | |
| Notes | Funding source: Clarian Values Fund, Pfizer Inc Declarations of interest: Not reported Pilot study, 15 participants. Mean CAM reported as opposed to numbers of people with delirium so limitations regarding interpretation of data. Although MMSE measured daily, frequency of CAM, MDAS not reported. Four time points were reported in the results table but not stated when these were. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information provided ‐ abstract data only |
| Random sequence generation (selection bias) | Unclear risk | No information provided ‐ abstract data only |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided ‐ abstract data only |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided ‐ abstract data only |
| Incomplete outcome data (attrition bias) | Unclear risk | No information provided ‐ abstract data only |
| Selective reporting (reporting bias) | Unclear risk | No information provided ‐ abstract data only |
| Other bias | Unclear risk | No information provided ‐ abstract data only |
| Methods | Design: Randomised trial of regional and general anaesthesia in elective surgery patients Date of study: Study dates not reported Inclusion criteria: Patients aged 60 years or over scheduled for elective surgery that could be performed under regional or general anaesthesia and who had agreed to be randomly allocated to receive either type of anaesthesia | |
| Participants | Number in study: 50 Country: Greece Age 60‐69/70 and over: Regional 14/5, General 15/13 Sex M/F: Regional 12/7, General 18/10 ASA score: ASA I‐II/II‐IV: Regional 16/3, General 27/1 | |
| Interventions | Intervention: Regional anaesthesia (epidural or spinal) Control: General anaesthesia via propofol infusion or inhaled anaesthetic Both given to achieve a Ramsay sedation score of ≤2. Benzodiazepines not administered for premedication or intraoperative sedation. | |
| Outcomes | 1. Incident delirium using DSM‐III criteria with informant history from attending relatives and nurses. Unclear whether patients interviewed 2. Length of admission 3. Cognitive status using MMSE 4. Postoperative complications | |
| Notes | Funding source: European Commission BIOMED2 program BMH4‐98‐3335 and Greek Ministry of Health Declarations of interest: Not reported Delirium diagnosed using informant history from attending relatives and nurses. Unclear whether patients interviewed. Delirium not excluded at enrolment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Allocation concealed by central |
| Random sequence generation (selection bias) | Low risk | Computer programme used |
| Blinding of participants and personnel (performance bias) | High risk | Unable to blind due to nature of intervention |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of outcome assessment is unclear, "incidence of delirium was evaluated by asking the attending nurses and relatives for features fulfilling the DSM III criteria" |
| Incomplete outcome data (attrition bias) | High risk | 50 patients randomised, 4 randomised to intervention crossed‐over to general anaesthesia. Delirium incidence results presented are per protocol, intention‐to‐treat not reported in original paper |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess |
| Other bias | High risk | Potential confounding from unbalanced neuraxial analgesia use 18 in regional anaesthesia, 3 in general anaesthesia group |
| Methods | Design: Randomised controlled trial of pregabalin as an opioid‐sparing agent in elderly patients after cardiac surgery. Date of study: April 2008‐September 2009 Inclusion criteria: Aged 75 years and over and undergoing primary elective coronary artery bypass grafting with cardiopulmonary bypass (CPB) or single valve repair or replacement with CPB | |
| Participants | Number in study: 70 Country: Finland Age: Median age 79.5 years (IQR 75‐89) in intervention group, 79.6 years (IQR 75‐91) in control group Sex: 40% female in intervention group, 54% female in control group | |
| Interventions | Intervention: Patients were premedicated orally 1 hour before surgery with lorazepam (0.02‐0.03 mg/kg) and the study drug, pregabalin 150 mg (Lyrica 75 mg capsule, Pfizer GmbH, Freiburg, Germany) or placebo. Beginning on the first postoperative morning, patients received 75 mg pregabalin or placebo twice daily until the fifth postoperative day. Control: Patients received matching placebo | |
| Outcomes | 1. Delirium, measured using CAM‐ICU (continuous score) ‐ not included in meta‐analysis 2. Length of admission 3. Cognition, mean CAM‐ICU score on day 5 4. Psychotropic medication use 5. Withdrawal from protocol | |
| Notes | Funding source: Helsinki University Hospital Research Fund and Finska Lakaresallskapet (Finnish Medical Association). Declarations of interest: "No conflicts of interest declared" Continuous score of CAM‐ICU reported as opposed to delirium present/absent so unable to use data in outcome table. Continuous delirium score slightly higher on postoperative day 1 in intervention group (median 24 versus 21, P = 0.04), but no differences on days 2, 3, 4 or 5. Delirium not excluded at admission | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Pharmacy conducted randomisation |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Blinding of participants and personnel (performance bias) | Low risk | Identical placebo used |
| Blinding of outcome assessment (detection bias) | Low risk | Identical placebo used |
| Incomplete outcome data (attrition bias) | High risk | 10/70 patients randomised excluded from analysis; 6 from intervention, and 4 from control group. |
| Selective reporting (reporting bias) | Unclear risk | Insuffiecient information to assess |
| Other bias | Low risk | No evidence of other bias |
| Methods | Design: parallel group randomised controlled trial Date of study: March 2009‐May 2010 Inclusion criteria: aged 60 years or older; planned for elective surgery lasting at least 60 minutes | |
| Participants | Number in study: 1277 Country: Berlin Age: Mean age 69.7 (SD 6.3) in intervention group, 70.1 (SD 6.5) in control group Sex: 44.7% of intervention group were female with 47.6% in the control group | |
| Interventions | Intervention: BIS data were allowed to be included in the management of anaesthesia Control: Anaesthesia was provided with blinded BIS monitoring; unblinding of monitoring was allowed if it was deemed necessary for the patient's benefit | |
| Outcomes | 1. Incident delirium, using DSM‐IV 2. Mortality, at 3 months 3. Length of admission 4. Cognitive status (Postoperative cognitive dysfunction) | |
| Notes | Funding source: Charite‐Universitatsmedizin Berlin and Aspect Medical Systems (now Covidien) Declarations of interest: "None declared" Delirium not excluded at enrolment Stopped early due to lack of funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Method for allocation concealment unclear |
| Random sequence generation (selection bias) | Unclear risk | Not clearly described ‐ "patients were consecutively recruited and after stratification electronically randomised into two study groups" |
| Blinding of participants and personnel (performance bias) | High risk | Allocation of anaesthetist dependent on whether for intervention or control so blinding not possible and unblinding of group in ˜10% of cases |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessment performed by trained medical personnel under Psychiatrist supervision, blinded to allocation |
| Incomplete outcome data (attrition bias) | High risk | n = 1277 participants randomised, outcome assessment available for n = 1155. n = 45 in intervention group and n = 39 in control group did not receive their allocated intervention and were excluded from the analysis. Of n = 593 assigned to intervention n = 18 were lost to follow‐up (n = 575 analysed). Of n = 600 assigned to control n = 20 were lost to follow‐up (n = 580 analysed). 9.6% of randomised participants do not have outcome data |
| Selective reporting (reporting bias) | Low risk | ISRCTN registration with protocol, outcomes reported in accordance with protocol |
| Other bias | Low risk | No evidence of other bias |
| Methods | Design: Randomised double‐blind controlled trial of donepezil in patients undergoing elective total hip replacement surgery Date of study: October 2003 to January 2004 Inclusion criteria: All consenting patients undergoing elective hip replacement and attending pre‐admission assessment clinic | |
| Participants | Number in study: 50 Country: UK Age mean (SD) Intervention 69.7 (8.4), Placebo 65.1 (11.1) P = 0.1 Sex % male: Intervention 57.9, Placebo 42.9 P = 0.39 | |
| Interventions | Intervention: Donepezil 5 mg starting postoperatively on returning to orthopaedic ward, every 24 hours for 3 days Control: Identical placebo | |
| Outcomes | 1. Incident delirium measured using Delirium Symptom Interview 2. Length of hospital admission 3. Adverse events | |
| Notes | Funding source: Unrestricted educational grant from Pfizer Esai, UK Declarations of interest: Not reported Delirium not excluded at enrolment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Allocation concealment managed centrally by pharmacy |
| Random sequence generation (selection bias) | Unclear risk | Block randomisation method but sequence generation not described |
| Blinding of participants and personnel (performance bias) | Low risk | Matched placebo used so participants and personnel blinded to allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors not aware of allocation |
| Incomplete outcome data (attrition bias) | High risk | 50 participants randomised, outcome assessment available for 33 (n = 19 in intervention group, n = 14 in control group). Surgery cancelled for 7 participants, 10 withdrew consent |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess |
| Other bias | Low risk | No evidence of other bias |
| Methods | Design: Randomised controlled trial of light sedation during spinal anaesthesia for reducing postoperative delirium in elderly hip fracture patients Date of study: April 2005‐October 2008 Inclusion criteria: Aged 65 years and over undergoing hip fracture repair with spinal anaesthesia and propofol sedation | |
| Participants | Number in study: 114 Country: USA Age: Mean age 81.2 years (SD 7.6) in intervention group, 81.8 years (SD 6.7) in control group Sex: 70% female in intervention group, 75% female in control group | |
| Interventions | Intervention: Sedation was provided during surgery by a propofol infusion targeted to a bispectral index (BIS) of 80 or higher in the light sedation group Control: Sedation was provided during surgery by a propofol infusion targeted to a bispectral index (BIS) of approximately 50 in the deep sedation group. In general, these targets render the light sedation group responsive to voice and the heavy sedation group unresponsive to noxious stimuli. | |
| Outcomes | 1. Incident delirium, measured using CAM 2. Duration of delirium 3. Length of admission 4. Mortality (in hospital, at 1‐year and overall) 5. Cognition using MMSE on postoperative day 2 6. Postoperative complications (Patients with >=1 complications) | |
| Notes | Funding source: Not reported Declarations of interest: Not reported Light sedation group received significantly more midazolam (5.5 mg/kg vs 1.3 mg/kg, P = 0.02). Mean BIS in light sedation group 85.7 (11.3) vs 49.9 (13.5) control P < 0.001 Exclusion of patients with MMSE<15 limits generalisability of findings. Delirium excluded at enrolment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of concealing allocation not clearly described |
| Random sequence generation (selection bias) | Unclear risk | Method of generating sequence not clearly described: "randomised block design with random length blocks.....incorporated a stratification scheme for age and cognitive impairment" |
| Blinding of participants and personnel (performance bias) | Low risk | All study team members, patient and physician blinded to allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Delirium assessments conducted by trained research nurse blinded to allocation |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis performed. No withdrawals. |
| Selective reporting (reporting bias) | Unclear risk | Protocol for the study approved by John Hopkins Medicine Institutional Review Board but this is not publicly available |
| Other bias | Low risk | No evidence of other bias |
| Methods | Design: Randomised controlled trial Date of study: Study dates not reported Inclusion criteria: undergoing elective isolated coronary artery bypass grafting (CABG) with the use of cardiopulmonary by‐pass (CPB); age > 50 years; ASA physical status II‐IV; preserved cardiac function (left ventricular ejection fraction > 50%) and EuroSCORE < or equal to 8 | |
| Participants | Number in study: 30 Country: Germany Age: Mean age 66 (48‐81) in xenon group; 68 (51‐79) in sevoflurane group Sex: 80% of both groups were male | |
| Interventions | Both groups received induction of anaesthesia with propofol and sufentanil. Muscle relaxation was obtained with rocuronium. Anaesthetic depth was adjusted by titration of end‐expiratory xenon or sevoflurane concentrations according to changes in physiological parameters and BIS values. During CPB, patients received a propofol infusion instead of xenon or sevoflurane. Xenon: Maintenance of anaesthesia was achieved by continuous infusion of sufentanil and xenon (end‐expiratory concentrations of 45‐50 vol%) Sevoflurane: Maintenance of anaesthesia was achieved by continuous infusion of sufentanil and sevoflurane (end‐expiratory concentrations of 1‐1.4 vol%) | |
| Outcomes | 1. Incidence of delirium, using CAM‐ICU 2. Mortality 3. Length of stay 4. Adverse events | |
| Notes | Funding source: Deutsche Forschungsgemeinschaft (DFG) grants Declarations of interest: "MC and RR received lecture and consultant fees from Air Liquide Sante International, a company interested in developing clinical applications for medical gases, including xenon" Delirium not clearly excluded at enrolment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Random sequence generation (selection bias) | Unclear risk | Method not described, states patients "randomly assigned to receive...." |
| Blinding of participants and personnel (performance bias) | High risk | Participants and staff not clearly blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessments conducted by trained study scientists blinded to allocation |
| Incomplete outcome data (attrition bias) | Low risk | All randomised patients were included in the analysis |
| Selective reporting (reporting bias) | Low risk | Protocol registered on ClinicalTrials.gov and trial reported in accordance with published protocol |
| Other bias | Low risk | No evidence of other bias |
| Methods | Design: Randomised controlled trial of ketamine as an adjunct to postoperative pain management after spinal fusion Date of study: Study dates not reported Inclusion criteria: Patients scheduled for elective lumbar spinal fusions who were taking opioids on a daily basis | |
| Participants | Number in study: 26 Country: USA Age: Mean age 53 years (SD 12) in intervention group, 48 years (SD 9) in control group Sex: Not reported | |
| Interventions | Intervention: Patients in the ketamine group received 0.2 mg/kg on induction of general anaesthesia and then 2 mcg/kg/hr until discharge from the post‐anaesthesia care unit. Control: All patients received a general anaesthetic with midazolam 5 mg, 70% nitrous oxide, 0.4% isoflurane, fentanyl at 1‐2 mcg/kg/hr and propofol at 70‐100 mg/hr. Spinal morphine (10 mcg/kg) was administered at instrumentation. | |
| Outcomes | 1) Incident delirium, measured using CAM on postoperative day 1 | |
| Notes | Funding source: Department of Anesthesia, Hospital for Special Surgery, New York Declarations of interest: Not reported Delirium not excluded at enrolment Study author conclusion: use of ketamine as an adjunct to postoperative pain management in opioid tolerant patients after spinal fusion reduced postoperative pain. There was no effect on delirium. Small trial (n = 24). Only reported delirium on postoperative day 1. Concern about the integrity of the intervention 3 in control failed their initial pain management and were converted to IV ketamine. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation sequence |
| Blinding of participants and personnel (performance bias) | High risk | Patients blinded but the physicians and nurses were cognitive of the groups |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors (physical therapists) blinded to allocation |
| Incomplete outcome data (attrition bias) | High risk | Intention‐to‐treat analysis performed as there was cross‐over between intervention and control groups. However, two patients excluded after randomised so no outcome assessment data included Any patients who remained at a numerical rating scale of 10 after 2 hours were excluded |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess |
| Other bias | Low risk | No evidence of other bias |
| Methods | Design: Randomised controlled trial comparing care in an acute geriatric ward or standard orthopaedic ward following hip fracture Date of study: September 2009 ‐ January 2012 Inclusion criteria: All acute admissions to Oslo University Hospital with a hip fracture | |
| Participants | Number in study: 332 randomised; 329 included in analyses Country: Norway Age: Mean age 84 years (range: 55 to 99) for intervention group and 85 years (range: 46 to 101) Sex: Male sex 42 (26%) for intervention group; 38 (23%) for controls | |
| Interventions | Intervention: Acute geriatric ward – 20 bed ward mainly admitting patients suffering from acute medical disorder superimposed upon frailty, co‐morbidities and polypharmacy. Comprehensive Geriatric Assessment was the basis for treatment planning. Assessment by geriatrician, nurse, physiotherapist and occupational therapists was expected during their first day on the ward and this team had daily meetings to plan discharge. Checklists and clinical routines based on published literature and previous experience. These included medication reviews, optimal pain control, correction of physiological disturbances preoperatively and postoperatively (hypoxaemia, anaemia, electrolyte disturbances, acid–base disturbances, dehydration, hypotension, blood sugar etc), early and intensive mobilisation, optimising pre and postoperative nutrition and early discharge planning. Outpatient orthopaedic clinic at 4 months. Control: Usual care in orthopaedic ward setting. Staffing levels were similar but there was no multidisciplinary meetings and no geriatric assessments. Early mobilisation was emphasised and patients were seen by a physiotherapist soon after surgery. Outpatient orthopaedic clinic at 4 months. | |
| Outcomes | 1. Incident delirium using CAM 2. Delirium duration (days) 3. Delirium severity using MDAS 4. Length of stay 5. In‐hospital mortality 6. New care home residence at four and 12 months 7. Cognitive function at four months using composite outcome 8. Incident dementia at 12 months 9. ADL function using Barthel Index at four months 10. Falls 11. Pressure ulcers 13. Postoperative complications | |
| Notes | Funding source: Research Council of Norway through the program ‘Improving mental health of older people through multidisciplinary efforts’ (Grant No: 187980/H10) plus Oslo University Hospital, The Sophies Minde Foundation, The Norweigan Association for Public Health and Civitan’s Research Foundation Declaration of interest: The authors declare ‘they have no competing interests’ Delirium not excluded at enrolment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Allocation by sealed opaque numbered envelopes |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers (blocks of variable and unknown size) carried‐out by statistician not involved in clinical service |
| Blinding of participants and personnel (performance bias) | High risk | Blinding not possible due to nature of intervention |
| Blinding of outcome assessment (detection bias) | High risk | Delirium assessments; performed by study nurse/geriatrician aware of allocation |
| Incomplete outcome data (attrition bias) | Low risk | 3 moribund patients erroneously randomised were excluded from the analysis (2 from intervention and 1 from control arm) |
| Selective reporting (reporting bias) | Low risk | Study reported in accordance with published protocol |
| Other bias | High risk | Where a bed was not available in the specialist geriatric unit, care was received in the corridor. As a result there are concerns about the fidelity of the intervention as a delirium prevention intervention as not all participants had the entire length of stay in either unit |
| Methods | Design: Randomised double‐blind controlled trial of methylprednisolone in patients at high risk of morbidity and mortality undergoing cardiac surgery with the use of cardiopulmonary bypass Date of study: June 2007 ‐ December 2013 Inclusion criteria: Patients aged 18 years or older with European System for Cardiac Operative Risk Evaluation (EuroSCORE) of at least 6 (or from 2011, at least 4 if from India or China) and providing written informed consent | |
| Participants | Number in study: 7507 Country: Multinational, 18 countries Age: Mean age 67.5 years (SD 13.6) in intervention group; 67.3 years (SD 13.8) for controls Sex: Male sex 2257 (60%) in intervention group; 2280 (61%) in controls | |
| Interventions | Intervention: Intravenous methylprednisolone (250 mg at anaesthetic induction and 250 mg at initiation of cardiopulmonary bypass) Control: Matched placebo | |
| Outcomes | 1. Incident delirium on postoperative day 3 using CAM 2. Length of hospital stay 3. Mortality at 30 days 4. Physical morbidity (myocardial injury; stroke; respiratory failure; infection) | |
| Notes | Funding source: Canadian Institutes of Health Research Declaration of interest: Authors report ‘no conflicts to declare’ Delirium not excluded at enrolment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Centralised computerised system with drug prepared by local pharmacy |
| Random sequence generation (selection bias) | Low risk | Block randomisation with random block sizes of 2, 4 or 6 stratified by centre |
| Blinding of participants and personnel (performance bias) | Low risk | All participants received intraoperative medication; healthcare providers blinded to medication administered |
| Blinding of outcome assessment (detection bias) | Low risk | Data collection and outcome assessment blinded to allocation |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis presented |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as per published protocol |
| Other bias | Low risk | No evidence of other bias |
ADL: activities of daily living; BIS: Bispectral index; BMI: body mass index; CAM: Confusion Assessment Method; CNS: central nervous system; COPD: chronic obstructive pulmonary disease; DRS‐R‐98: Delirium Rating Scale Revised 98; DSI: Delirium Symptom Interview; DSM: Diagnostic and Statistical Manual; FICB: fascia iliaca compartment block; Hb: haemoglobin; IM: intramuscular; INR: International Normalised Ratio; IQR: interquartile range; IV: intravascular; mcg: micrograms; MDAS: Memorial Delirium Assessment Scale; MMSE: Mini Mental State Examination; OBS: organic brain syndrome; PCA: patient controlled analgesia; SD: standard deviation; RCT: randomised controlled trial; TIA: transient ischaemic attack
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| ICU study. | |
| Not a randomised controlled trial. | |
| The intervention was not designed to prevent delirium. Cognitive impairment rather than delirium was used as an outcome measure. | |
| Not a randomised controlled trial. | |
| A validated method for diagnosis of delirium was not used. | |
| A validated method for diagnosis of delirium was not used. | |
| A validated method for diagnosis of delirium was not used. | |
| Study not in hospitalised patients ‐ active intervention in community setting. | |
| Not a delirium prevention study. | |
| A validated method for diagnosis of delirium was not used. | |
| Not a delirium prevention study. | |
| Randomisation not used and participants were long‐term care residents. | |
| Not a randomised controlled trial. | |
| Not a randomised controlled trial. | |
| PACU study. | |
| PACU study. | |
| ICU study. | |
| ICU study. | |
| ICU study. | |
| ICU study. | |
| ICU study. | |
| Treatment study. | |
| ICU study. | |
| ICU study. | |
| ICU study. | |
| Not original research‐ review article. | |
| Randomisation not used. | |
| A validated method for delirium diagnosis was not used. Although DSM‐IIIR diagnostic criteria used, data obtained from retrospective chart review. | |
| Not a randomised controlled trial. | |
| Nursing home setting. | |
| Outcomes examined did not include delirium. | |
| Not delirium prevention. | |
| Randomisation not used. | |
| Not a randomised controlled trial. | |
| Post‐acute care, not hospital setting. | |
| ICU study. | |
| A validated method for diagnosis of delirium was not used. | |
| Randomisation not used. | |
| ICU study. | |
| Not a randomised controlled trial. Before and after study. | |
| Not a randomised controlled trial. | |
| Delirium not used as an outcome measure. | |
| Randomisation not used. | |
| Not in hospitalised patients. | |
| Treatment study. | |
| Treatment study. | |
| ICU study. | |
| A validated method for diagnosis of delirium was not used. | |
| ICU study. | |
| Commentary. | |
| Treatment study. | |
| ICU study. | |
| A validated method for diagnosis of delirium was not used. | |
| No recruitment, trial stopped. | |
| ICU study. | |
| ICU study. | |
| Not a delirium prevention study. | |
| ICU study and method of delirium diagnosis not validated. | |
| Not a randomised controlled trial. Retrospective observational study. | |
| Randomisation not used. | |
| Treatment study and not randomised controlled trial. | |
| A validated method for diagnosis of delirium was not used. Delirium diagnosis relied on retrospective records review. | |
| A validated method for diagnosis of delirium was not used. | |
| Primary outcome is screening for incidence of delirium; unable to report incidence of delirium as first date of delirium diagnosis is not recorded. | |
| ICU study. | |
| Not a delirium prevention study. | |
| Not a randomised controlled study. Before and after study. | |
| ICU study. | |
| ICU study. |
DSM‐IIR: Diagnostic and Statistical Manual
ICU: Intensive Care Unit
PACU: post‐anaesthesia care unit
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Xenon for the prevention of post‐operative delirium in cardiac surgery: study protocol for a randomised controlled clinical trial |
| Methods | Randomised controlled trial |
| Participants | 190 patients, older than 65 years, and scheduled for elective cardiac surgery with use of cardiopulmonary bypass |
| Interventions | Group 1: General anaesthesia with xenon Group 2: General anaesthesia with sevoflurane |
| Outcomes | Primary outcome: Incidence of postoperative delirium during the first 5 postoperative days measured using 3D‐CAM or CAM‐ICU Secondary outcomes: Duration of postoperative delirium (total number of days and percentage of patients with duration of longer than 2 days; delirium severity; use of physical restraints; postoperative cognitive function; ADL; use of anti delirium medication; duration of sedation; duration of ICU and hospital stay; adverse events. |
| Starting date | May 2013 |
| Contact information | 1 Department of Anesthesiology, KU Leuven – University of Leuven, University |
| Notes | EudraCT Identifier: 2014‐005370‐11. Will need to differentiate between ICU and non‐ICU delirium in results. |
| Trial name or title | BAG‐RECALL Study: BIS or anesthesia gas to reduce explicit recall |
| Methods | Phase IV double‐blind multi‐centre randomised controlled trial |
| Participants | Patients aged over 18 undergoing surgery assessed as high risk for awareness requiring general anaesthesia |
| Interventions | Group 1: Bispectral index‐guided anaesthesia (target range 40‐60) Group 2: End‐tidal anaesthetic gas‐guided anaesthesia (target range 0.7‐1.3 age‐adjusted minimum alveolar concentration) |
| Outcomes | Primary outcome: Awareness with explicit recall during surgical and anaesthetic periods Secondary outcomes: postoperative delirium, postoperative mortality, psychological symptoms, postoperative pain |
| Starting date | March 2008 |
| Contact information | Michael Avidan |
| Notes | ClinicalTrials.gov identifier: NCT00682825 Completed December 2010. Published N Engl J Med 2011 Aug 18;365(7):591‐601 but delirium outcome not reported yet. |
| Trial name or title | The prevention of delirium and complications associated with surgical treatments multi‐centre clinical |
| Methods | Phase 3 double‐blind randomised controlled trial |
| Participants | Patients 60 and over undergoing major surgery and able to provide informed consent |
| Interventions | Intervention: Drug: Low‐dose (sub‐anaesthetic) ketamine (0.5 mg/kg) following induction of anaesthesia or administration of sedative medications |
| Outcomes | Primary outcomes: Incidence of postoperative delirium within three days of surgery (assessed by the CAM or CAM‐ICU) Secondary outcomes: Postoperative acute pain within three postoperative days (assessed by visual analogue pain scale) |
| Starting date | November 2013 |
| Contact information | Michael Avidan |
| Notes | ClinicalTrials.gov identifier: NCT01690988 Estimated primary completion date June 2015 |
| Trial name or title | The effect of physostigmine on cognitive functioning in the immediate period after sedation for colonoscopy |
| Methods | Double‐blind randomised controlled trial |
| Participants | Patients over18 years old, ASA I‐III, fluency in Hebrew, Russian, or Arabic, without serious hearing or visual impairment |
| Interventions | Intervention: Physostigmine Intravenous bolus of physostigmine 1 mg, 3‐5 minutes before completion of colonoscopy Comparator: no physostigmine |
| Outcomes | Primary outcome: Cognitive functioning at time of hospital discharge |
| Starting date | July 2010 |
| Contact information | Bezion Beilin, Hasharon Hospital, Rabin Medical Center |
| Notes | ClinicalTrials.gov identifier: NCT01121497 Estimated Primary Completion Date: July 2011 |
| Trial name or title | Rivastigmine prophylaxis in elderly patients at risk for delirium: a randomised, double‐blind placebo‐controlled pilot study |
| Methods | Phase IV double‐blind randomised controlled trial |
| Participants | 65 years and older undergoing major elective surgery greater than 2 hours duration with any of preoperative cognitive impairment, age >70, use of psychotropic medications, previous history of delirium, severe illness/comorbidity. |
| Interventions | Intervention: Rivastigmine patch delivering 4.6 mg/24hrs applied to upper back preoperatively for 24 hrs. Control: A gauze and Tegaderm dressing applied to upper back within 3 hrs of surgery for 24 hrs |
| Outcomes | Primary outcome: postoperative delirium within 72 hours of surgery (CAM‐ICU) Secondary outcomes: delirium episodes, delirium severity (MDAS), length of hospital stay, cognitive function at 1 and 3 months postoperatively |
| Starting date | December 2008 |
| Contact information | Alex Bekker, NYU School of Medicine, New York |
| Notes | ClinicalTrials.gov identifier: NCT00835159 Data not available to us; manuscript in preparation. New York study, sponsored by Novartis. Study closed prematurely because of emerging safety concerns with this group of drugs, encouraged by Novartis |
| Trial name or title | Effect of prophylactic, perioperative propranolol on peri‐ and postoperative complications in patients With Post Traumatic Stress Disorder |
| Methods | Randomised double‐blind placebo‐controlled trial |
| Participants | Patients over 40 with full or subthreshold PTSD of three months duration admitted for any surgical procedure (except open‐heart or intracranial surgery) requiring general or combined general‐regional anaesthesia and an overnight hospital stay. |
| Interventions | Experimental: Drug: Propranolol hydrochloride will be taken for a total of 14 days commencing on the morning of surgery Comparator: Placebo pill will be taken for a total of 14 days commencing on the morning of surgery |
| Outcomes | Primary outcomes: Postoperative delirium (assessed using CAM, CAM‐ICU), ICU length of stay, hospital length of stay, postoperative renal dysfunction Secondary outcomes: peri‐ and postoperative complications, pain intensity, PTSD symptoms, use of analgesics, length of mechanical ventilation, quality of life, functional status, sleep quality, depression symptoms, postoperative neurocognitive dysfunction score, mortality |
| Starting date | May 2012 |
| Contact information | |
| Notes | ClinicalTrials.gov identifier: NCT01555554 Estimated primary completion date December 2013 |
| Trial name or title | The effect of periarticular multi‐drug regimen on pain after partial hip replacement |
| Methods | Double‐blind randomised controlled trial |
| Participants | Patients admitted with femoral neck fracture, or for partial hip replacement |
| Interventions | Intervention: oral administration of oxycodone SR 10 mg and celecoxib 200 mg with 10 mL of water 1 hour before surgery and intraoperative periarticular injection of 50 mL solution containing ropivacaine 15 mg, epinephrine 0.3 mg, cefmetazole 1000 mg, ketorolac 30 mg and morphine HCL 10 mg before wound closure Control: no medication preoperatively or intraoperatively |
| Outcomes | Primary outcome: pain visual analogue scale (VAS) on postoperative days 1, 4 and 7 Secondary outcomes: opioid consumption on postoperative days 1, 4 and 7, frequency of use of patient controlled analgesia (PCA) on post operative days 1, 4 and 7, delirium (delirium rating scale) on postoperative days 1, 4 and 7 |
| Starting date | May 2010 |
| Contact information | Yong Chan Ha [email protected] |
| Notes | ClinicalTrials.gov identifier: NCT01112436 Correspondence with author suggests patients are assessed on surgical wards. Estimated final data collection for primary outcome April 2012 |
| Trial name or title | A randomised, double‐blind, placebo‐controlled trial to assess the safety and efficacy of the perioperative administration of pregabalin in reducing the incidence of postoperative delirium and improving acute postoperative pain management |
| Methods | Randomised double‐blind placebo‐controlled trial |
| Participants | Patients aged 60 years and older, admitted for major orthopaedic or vascular surgery with expected length of stay > 2 days |
| Interventions | Intervention: Pregabalin 75 mg given preoperatively, then either 50 mg or 25 mg every 8 hours for 3 days postoperatively (based on renal function) Control: Placebo |
| Outcomes | Primary outcome: Delirium (CAM‐ICU positive) Secondary outcomes: Interference with daily activities (BPI), pain at rest and on movement of the operative site (NRS), Narcotic analgesic requirements, Sedation (RSS), Narcotic‐related adverse effects (ORSDS), Recovery using the QoR, length of stay, Medical Outcome Study (MOS) sleep score |
| Starting date | May 2009 |
| Contact information | Dr. A. Chaput, Ottawa Hospital Research Institute |
| Notes | ClinicalTrials.gov identifier: NCT00819988 Correspondence with author suggests delirium assessed on wards. This study has been completed. |
| Trial name or title | An international, multi‐centre randomised controlled trial evaluating the effect of xenon on post‐operative delirium in elderly patients undergoing hip fracture surgery |
| Methods | Multi‐centre double‐blind randomised controlled trial |
| Participants | Patients aged 75 and over with hip fracture and surgery planned within 48 hours and able to provide informed consent |
| Interventions | Intervention: Xenon 60% (1 MAC) in oxygen (FiO2 0.35‐0.45) Control: Sevoflurane 1.1‐1.4%(1 MAC) in oxygen (FiO2 = 0.35‐0.45) and medical air |
| Outcomes | Primary outcome: Postoperative delirium (CAM) within four days post‐surgery Secondary outcomes: Postoperative delirium (CAM) from day 5 postoperatively until discharge, sequential organ failure assessment from day 1 to day 4 post‐surgery, recovery parameters, safety and health economic parameters |
| Starting date | September 2010 |
| Contact information | Steffen Rex |
| Notes | ClinicalTrials.gov identifier NCT01199276 Estimated completion date December 2013 |
| Trial name or title | Prevention of post‐operative delirium with donepezil |
| Methods | Double‐blind randomised controlled trial |
| Participants | Patients 70 Years and older, cognitively healthy, elective hip or knee replacement |
| Interventions | Intervention: Donepezil before (over 5‐7 days), during and after (over 7 days) surgery Control: Placebo |
| Outcomes | Primary outcome: Incidence of delirium Secondary outcome: Cognitive performance |
| Starting date | January 2006 |
| Contact information | Janine Diehl, M. D. Dept. of Psychiatry, Technische Universitaet Muenchen |
| Notes | ClinicalTrials.gov identifier NCT00220896 This study has now been completed |
| Trial name or title | Usefulness of bright light therapy in the prevention of delirium in patients undergoing Hematopoietic Stem Cell Transplant (HSCT) |
| Methods | Pilot double‐blind randomised placebo‐controlled study |
| Participants | Patients aged 18 and over undergoing HSCT |
| Interventions | Intervention: Bright light therapy (2500 Lux gaze directed every morning from 8 am until 8:30 am) |
| Outcomes | Primary outcome: Delirium incidence and time to development of delirium (Delirium Rating Scale‐Revised‐98 and/or Memorial Delirium Assessment Scale). Secondary outcomes: Length and severity of delirium episodes, dose of antipsychotic medications required to manage delirium, hospital length of stay, adverse events (falls, aspiration, infections, nutritional deficits). |
| Starting date | October 2012 |
| Contact information | Carlos Fernandez‐Robles Justin Eusebio |
| Notes | ClinicalTrials.gov identifier: NCT01700816 Estimated primary completion date April 2014 |
| Trial name or title | Tailored patient management guided with absolute cerebral oximetry to prevent neurocognitive injury in elderly patients undergoing cardiac surgery. |
| Methods | Double‐blind randomised controlled trial |
| Participants | Patients 65 and older admitted for elective cardiac or thoracic aortic surgery, able to provide informed consent |
| Interventions | Intervention: Optimisation of cerebral oxygenation within 5 minutes once cerebral desaturation (SctO2 < 60 %) has been established. Control: No intervention in this arm if the Sct02 falls below 60%. |
| Outcomes | Primary outcome: Postoperative delirium and postoperative cognitive dysfunction within 5 days of surgery. Secondary outcome: Postoperative morbidity and mortality |
| Starting date | September 2009 |
| Contact information | Gregory Fischer |
| Notes | ClinicalTrials.gov identifier: NCT00991328 Estimated Primary Completion Date: June 2010 |
| Trial name or title | Incidence of delirium in hip fracture patients randomized to regular hypnotics vs placebo |
| Methods | Randomised controlled trial |
| Participants | 70 years and older admitted for hip fracture |
| Interventions | Intervention: Zolpidem 5 mg daily in perioperative period Control: Placebo tablet in perioperative period |
| Outcomes | Primary outcome: Incidence and severity of postoperative delirium. Secondary outcomes: Sleep quality. mobilisation, loss of functional ability, length of stay, sedation, nocturnal nursing events. |
| Starting date | February 2004 |
| Contact information | Nicolai B Foss, MD, Hvidovre University Hospital |
| Notes | Clinical trials identifier: NCT00286936 |
| Trial name or title | Influence of multi‐modal analgesia with parecoxib and morphine on post‐surgical delirium in elderly patients |
| Methods | Single‐blind randomised controlled trial |
| Participants | Patients aged 60 years and over admitted for elective non‐cardiac surgery |
| Interventions | Intervention: multi‐modal analgesia with parecoxib and morphine PCA Control: opioid PCA |
| Outcomes | Primary outcomes: Pain at rest and on movement, delirium diagnosis with CAM‐ICU from 1 to 7 days after operation Secondary outcomes: adverse postoperative events, 28 day survival, hepatic and renal function at 48 hours, delirium (CAM‐ICU) assessed twice daily with CAM‐ICU |
| Starting date | December 2010 |
| Contact information | Zhen Hua: [email protected] |
| Notes | ChiCTR‐TRC‐10001063 |
| Trial name or title | Post‐operative melatonin administration and delirium prevention in patients undergoing vascular and cardiac surgery |
| Methods | Double‐blind randomised controlled trial |
| Participants | Patients over 60 admitted for non‐emergency vascular surgery with expected length of hospital stay > 48 hours, ASA category I to IV and able to provide informed consent |
| Interventions | Intervention: Melatonin 5 mg sublingually given at 9 pm for 5 days postoperatively or until discharge Control: placebo |
| Outcomes | Primary outcome: incidence of postoperative delirium (assessment up to day 7 postoperatively) Secondary outcome: pain visual analogue score |
| Starting date | August 2010 |
| Contact information | Rita Katznelson, Toronto General Hospital, UHN, Toronto, Ontario, Canada |
| Notes | ClinicalTrials.gov identifier: NCT01198938 Study completed February 2013 |
| Trial name or title | CONFUCIUS Study : Impact of a multi‐faceted program to prevent postoperative delirium in the elderly |
| Methods | Stepped wedge cluster‐randomised controlled trial |
| Participants | Patients aged over 75 admitted for scheduled surgery |
| Interventions | Intervention: Preoperative geriatric consultation performed by a mobile geriatric team, training of surgical ward staff and implementation of HELP (Hospital Elder Life Program), morbidity and mortality conferences related to delirium cases. Control: Usual care |
| Outcomes | Primary outcome: Postoperative delirium rate within 7 days after surgery (assessed using the CAM) Secondary outcomes: Mean delirium intensity, length of hospital stay, postoperative complications 30 days after surgery incidence, mortality 6 months after surgery, feasibility of the multi‐disciplinary prevention program |
| Starting date | March 2011 |
| Contact information | christelle.mouchoux@chu‐lyon.fr |
| Notes | ClinicalTrials.gov identifier: NCT01316965 Estimated primary completion date March 2013 Sponsors: Hospices Civils de Lyon |
| Trial name or title | Does positive airway pressure therapy reduce the incidence of post‐operative delirium in patients at risk for obstructive sleep apnoea? |
| Methods | Randomised controlled trial of continuous positive airways pressure |
| Participants | Patients at risk of obstructive sleep apnoea (OSA) (STOP‐BANG score>2, untreated for OSA undergoing elective joint replacement |
| Interventions | Continuous Positive Airway Pressure (CPAP) prior to surgery and on postoperative days 0, 1 and 2 vs. routine perioperative care |
| Outcomes | Incidence of delirium assessed using CAM and DRS‐R‐98 |
| Starting date | Not reported |
| Contact information | Not reported |
| Notes |
| Trial name or title | Early pharmacological intervention to prevent delirium: Haloperidol prophylaxis in older emergency department patients |
| Methods | Multi‐centre double‐blind randomised placebo‐controlled trial |
| Participants | Patients aged 70 or over, admitted to a medical or surgical specialty and at risk of delirium according to one or more positive answers on the VMS delirium‐risk questions |
| Interventions | Intervention: Haloperidol 1 mg twice daily at 12 am and 8 pm, orally Control: Placebo 1 mg twice‐daily at 12 am and 8 pm, orally |
| Outcomes | Primary outcome: Incident delirium and delirium duration (measured with Delirium Observation Screening (DOS) score) Secondary outcome Measures: Time to develop delirium, length of stay, ; The (mean) number of days participants are admitted to the hospital; change from baseline function at 3 and 6 months (ADL scale), change from baseline instrumental activities at 3 and 6 months (Instrumental ADL scale); mortality. |
| Starting date | November 2012 |
| Contact information | |
| Notes | ClinicalTrials.gov identifier: NCT01530308 Estimated primary completion date April 2014 |
| Trial name or title | Namenda to prevent post‐operative delirium |
| Methods | Double‐blind randomised placebo‐controlled trial |
| Participants | Patients over 50, medically stable admitted for elective joint replacement under general anaesthetic |
| Interventions | Intervention: Memantine 10 mg once daily orally 8 days prior to procedure and 4 days postoperatively Control: Placebo orally once daily 8 days prior to procedure and 4 days postoperatively |
| Outcomes | Incidence and severity of delirium measured with the Delirium Rating Scale Revised‐98, MMSE, CAM, Clock Drawing Test, DSM‐IV‐TR criteria for delirium |
| Starting date | March 2006 |
| Contact information | M Privitera, University of Rochester, USA |
| Notes | ClinicalTrials.gov identifier: NCT00303433 Terminated early December 2009 (under‐recruitment) |
| Trial name or title | Efficacy and safety of haloperidol prophylaxis for delirium prevention in older medical and surgical at‐risk patients acutely admitted to hospital through the emergency department: study protocol of a multicenter, randomised, double‐blind, placebo‐controlled clinical trial (HARPOON study) |
| Methods | Randomised controlled trial |
| Participants | 390 patients aged 70 years and older admitted through the emergency department for general medicine and surgical specialties |
| Interventions | Prophylactic haloperidol 1 mg or placebo twice daily for seven days |
| Outcomes | Incidence of delirium, severity of delirium, duration of delirium, adverse events, length of stay, all cause mortality, institutionalisation, instrumental ADL, cognitive function |
| Starting date | TBC |
| Contact information | Edmee Schrijver. [email protected] |
| Notes | ClinicalTrials.gov identifier NCT01530308 |
| Trial name or title | Perioperative cognitive function ‐ dexmedetomidine and cognitive reserve |
| Methods | Multi‐centre double‐blind randomised placebo‐controlled trial |
| Participants | 68 years and older, undergoing elective major surgery under general anaesthesia, ASA grade I‐III, MMSE >20 |
| Interventions | Intervention: Precedex (dexmedetomidine). 0.5/ug/kg/hr. Dexmedetomidine infusions will begin prior to the surgery (no loading dose), and will be maintained at 0.5 mcg/kg/hour throughout surgery and titrated postoperatively for 2 hrs postoperatively. Control: Placebo infusion. |
| Outcomes | Primary outcome: Delirium Battery post‐surgery and then daily for 5 days then at 3 and 6 months Secondary outcomes: Neuropsychological testing at 3 and 6 months |
| Starting date | February 2008 |
| Contact information | Jeff Silverstein, Mount Sinai School of Medicine |
| Notes | ClinicalTrials.gov identifier: NCT00561678 Estimated Primary Completion Date: June 2013 |
| Trial name or title | Perioperative physostigmine prophylaxis for liver resection patients at risk for delirium and postoperative cognitive dysfunction: a prospective, randomised, controlled, double‐blinded, two‐armed single‐centre trial |
| Methods | Phase IV double‐blind randomised placebo‐controlled trial |
| Participants | Patients over 18 undergoing elective liver resection with or without additional elective surgery in the same session, able to provide informed consent, negative pregnancy testing (beta‐human chorionic gonadotrophin [B‐HCG]). |
| Interventions | During liver resection: |
| Outcomes | Primary outcomes: Incident delirium (DSM‐IV criteria), measured preoperatively and up to hospital discharge, Cambridge Neurophysiological Test Automated Battery (CANTAB), measured preoperatively, on the 7th, 90th and 365th postoperative day Secondary outcomes: Delirium; Evaluation of intensive care unit performance, Length of postoperative hospital stay, Length of postoperative ICU stay, pain, postoperative complications and organ dysfunction, rate of systemic inflammatory response syndrome (SIRS) and infection, quality of life questionnaires, mortality, postoperative survival at 90 days, 6 months and one year, immune parameters, perioperative assessment of sleep stage, parameters of haematology, parameters of renal function. |
| Starting date | August 2009 |
| Contact information | |
| Notes | ISRCTN18978802 Anticipated end date: April 2016 |
| Trial name or title | Design and methods of the Hospital Elder Life Program (HELP), a multi component targeted intervention to prevent delirium in hospitalised older patients: efficacy and cost‐effectiveness in Dutch health care |
| Methods | Cluster‐randomised controlled trial (stepped wedge) |
| Participants | Patients aged 70 years and over at risk for delirium and admitted to cardiology, internal medicine, geriatrics, orthopedics and surgery |
| Interventions | Multi‐component targeted delirium prevention intervention (Hospital Elder Life Program) |
| Outcomes | Incidence of delirium, duration of delirium, severity of delirium, quality of life, length of stay, use of care services |
| Starting date | TBC |
| Contact information | |
| Notes | Netherlands trial register NTR3842 |
| Trial name or title | Does femoral nerve catheterization reduce the incidence of post‐operative delirium in patients presenting for hip fracture repair? |
| Methods | Randomised controlled trial |
| Participants | Patients aged 50 and over presenting with a hip fracture |
| Interventions | Intervention: Preoperative femoral nerve catheterisation Control: Intravenous opioids given postoperatively |
| Outcomes | Primary outcome: Rate of postoperative delirium up to 3 days Secondary outcomes: length of stay, pain score (VAS) and consumption of analgesic medication |
| Starting date | March 2012 |
| Contact information | |
| Notes | ClinicalTrials.gov identifier: NCT01547468 Estimated date of primary completion March 2015 |
| Trial name or title | Randomised double‐blind placebo‐controlled study of post‐operative haloperidol versus placebo for prevention of post‐operative delirium after acute hip surgery |
| Methods | Double‐blind randomised placebo‐controlled study |
| Participants | Patients aged 75 and over undergoing surgery for hip fracture |
| Interventions | Intervention: Haloperidol 1 mg twice daily for 72 hours Control: Placebo 1 mg twice daily for 72 hours |
| Outcomes | Primary outcomes: Incidence of postoperative delirium in 72 hours postoperative period |
| Starting date | November 2005 |
| Contact information | Boke Linso Sjirk Borger van der Burg, Department of Surgery, Bronovo Hospital |
| Notes | ClinicalTrials.gov identifier: NCT00250237 Study completed October 2008. Results not published. |
| Trial name or title | Effects of two different anaesthesia‐analgesia methods on the incidence of post‐operative delirium: a multi‐centre, randomized controlled trial |
| Methods | Multi‐centre randomised controlled trial |
| Participants | Patients aged 60‐90 years undergoing elective major (more than two hours) open abdominal or thoracic (non‐cardiovascular) surgery, able to provide informed consent. |
| Interventions | Intervention: Combined epidural and general anaesthesia (Epi‐GA) with postoperative patient controlled epidural analgesia (PGEA). Control: General anaesthesia and patient controlled intravenous analgesia (PCIA). |
| Outcomes | Primary outcome: Incidence of postoperative delirium. Secondary outcomes: Incidence of postoperative complications, 30‐day mortality, VAS pain score, duration of postoperative hospital stay, daily prevalence of postoperative delirium (7 days) |
| Starting date | November 2011 |
| Contact information | Yuan Zeng |
| Notes | ClinicalTrials.gov identifier: NCT01661907 Estimated primary completion date October 2014 |
| Trial name or title | Prevention of Delirium (POD) for older people in hospital: protocol for a randomised controlled feasibility study |
| Methods | Cluster‐randomised controlled trial |
| Participants | Patients, aged 65 years and over, admitted to a participating orthopaedic trauma or geriatric medicine. |
| Interventions | Intervention: A manualised, multi‐component intervention and systematic implementation process Control: Usual care |
| Outcomes | Primary outcome: New onset delirium Secondary outcomes: Number, severity and length of delirium episodes (including persistent delirium); length of stay in hospital; in‐hospital mortality; destination at discharge; health‐related quality of life and health resource use; physical and social independence; anxiety and depression; patient experience. |
| Starting date | 13/03/2014 |
| Contact information | Clinical Trials Research Unit, Leeds Institute of Clinical Trials Research, |
| Notes | Trial registration: ISRCTN01187372 |
ADL: activities of daily living; CAM: Confusion Assessment Method; DSM‐IIR: Diagnostic and Statistical Manual; ICU: Intensive Care Unit; MDAS:Memorial Delirium Assessment Scale; MMSE: Mini Mental State Examination; PCA: patient controlled analgesia; PTSD: post‐traumatic stress disorder
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 7 | 1950 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.59, 0.81] |
| Analysis 1.1  Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 1 Incident delirium. | ||||
| 1.1 Medical patients | 4 | 1365 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.43, 0.92] |
| 1.2 Surgical patients | 3 | 585 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.59, 0.85] |
| 2 Incidence of delirium in patients with dementia Show forest plot | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.59, 1.36] |
| Analysis 1.2  Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 2 Incidence of delirium in patients with dementia. | ||||
| 2.1 Surgical patients | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.59, 1.36] |
| 3 Duration of delirium Show forest plot | 4 | 244 | Mean Difference (IV, Random, 95% CI) | ‐1.16 [‐2.96, 0.64] |
| Analysis 1.3  Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 3 Duration of delirium. | ||||
| 3.1 Medical patients | 2 | 63 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐2.43, 1.13] |
| 3.2 Surgical patients | 2 | 181 | Mean Difference (IV, Random, 95% CI) | ‐2.40 [‐7.27, 2.46] |
| 4 Severity of delirium Show forest plot | 2 | 67 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.04 [‐1.65, ‐0.43] |
| Analysis 1.4 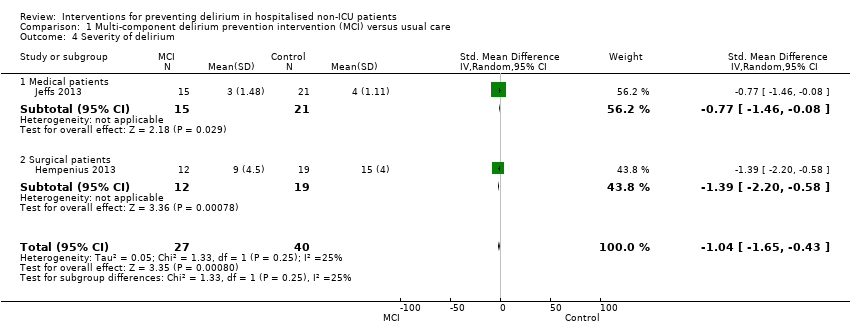 Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 4 Severity of delirium. | ||||
| 4.1 Medical patients | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.46, ‐0.08] |
| 4.2 Surgical patients | 1 | 31 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.39 [‐2.20, ‐0.58] |
| 5 Length of admission Show forest plot | 6 | 1920 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.48, 0.51] |
| Analysis 1.5 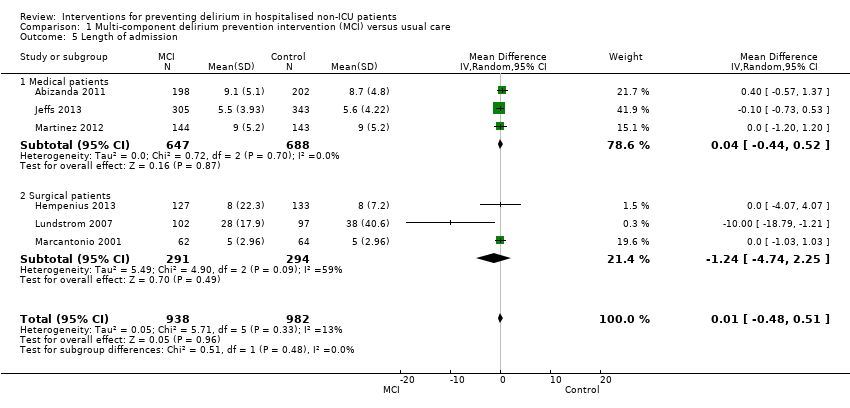 Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 5 Length of admission. | ||||
| 5.1 Medical patients | 3 | 1335 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.44, 0.52] |
| 5.2 Surgical patients | 3 | 585 | Mean Difference (IV, Random, 95% CI) | ‐1.24 [‐4.74, 2.25] |
| 6 Cognition Show forest plot | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 9.10 [7.20, 11.00] |
| Analysis 1.6  Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 6 Cognition. | ||||
| 6.1 Medical patients | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 9.10 [7.20, 11.00] |
| 7 Improvement in Activities of Daily Living Show forest plot | 1 | 341 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.91, 1.47] |
| Analysis 1.7 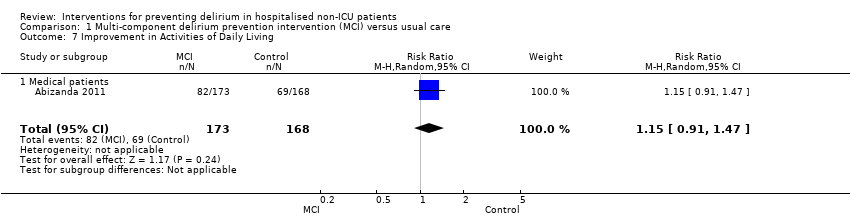 Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 7 Improvement in Activities of Daily Living. | ||||
| 7.1 Medical patients | 1 | 341 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.91, 1.47] |
| 8 Return to independent living Show forest plot | 4 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.85, 1.06] |
| Analysis 1.8 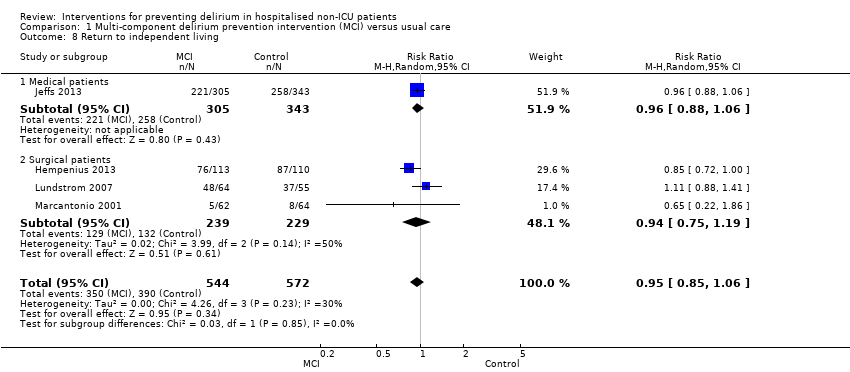 Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 8 Return to independent living. | ||||
| 8.1 Medical patients | 1 | 648 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| 8.2 Surgical patients | 3 | 468 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.75, 1.19] |
| 9 Depression Show forest plot | 1 | 149 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐0.44, 1.84] |
| Analysis 1.9 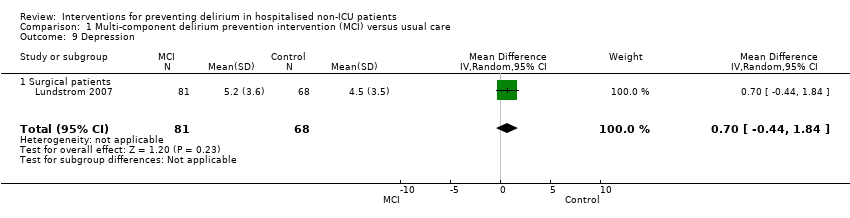 Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 9 Depression. | ||||
| 9.1 Surgical patients | 1 | 149 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐0.44, 1.84] |
| 10 Withdrawal from protocol Show forest plot | 1 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 1.10  Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 10 Withdrawal from protocol. | ||||
| 10.1 Surgical patients | 1 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Falls Show forest plot | 3 | 746 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.16, 2.01] |
| Analysis 1.11  Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 11 Falls. | ||||
| 11.1 Medical patients | 1 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 2.03] |
| 11.2 Surgical patients | 2 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.18, 3.46] |
| 12 Pressure ulcers Show forest plot | 2 | 457 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.26, 0.89] |
| Analysis 1.12 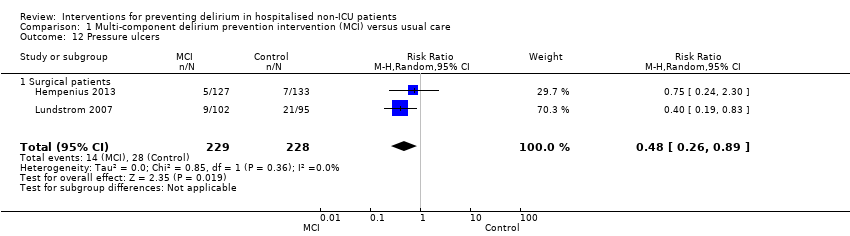 Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 12 Pressure ulcers. | ||||
| 12.1 Surgical patients | 2 | 457 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.26, 0.89] |
| 13 Inpatient mortality Show forest plot | 3 | 859 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.56, 1.43] |
| Analysis 1.13 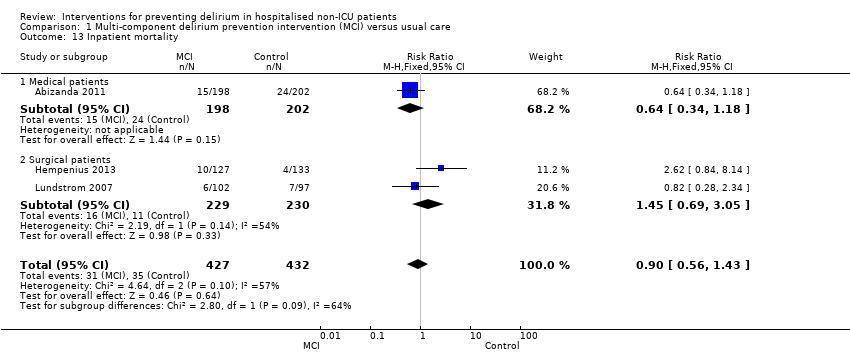 Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 13 Inpatient mortality. | ||||
| 13.1 Medical patients | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.34, 1.18] |
| 13.2 Surgical patients | 2 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.69, 3.05] |
| 14 12 month mortality Show forest plot | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.46, 1.56] |
| Analysis 1.14 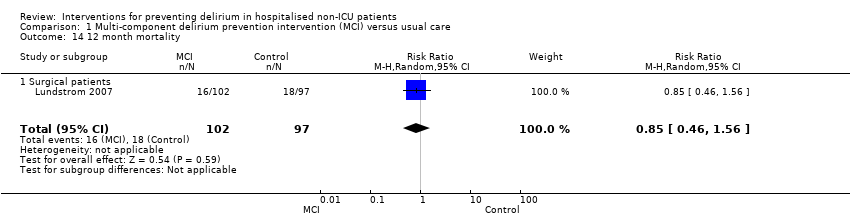 Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 14 12 month mortality. | ||||
| 14.1 Surgical patients | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.46, 1.56] |
| 15 Cardiovascular complication Show forest plot | 1 | 260 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.78, 1.65] |
| Analysis 1.15  Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 15 Cardiovascular complication. | ||||
| 16 Urinary tract infection Show forest plot | 1 | 260 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.45, 3.20] |
| Analysis 1.16  Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 16 Urinary tract infection. | ||||
| 17 Mental health worsened Show forest plot | 1 | 246 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.64, 1.20] |
| Analysis 1.17  Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 17 Mental health worsened. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 2 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.17, 2.62] |
| Analysis 2.1  Comparison 2 Prophylactic cholinesterase inhibitor versus placebo, Outcome 1 Incident delirium. | ||||
| 1.1 Donepezil | 2 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.17, 2.62] |
| 2 Duration of delirium Show forest plot | 1 | 15 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 2.2  Comparison 2 Prophylactic cholinesterase inhibitor versus placebo, Outcome 2 Duration of delirium. | ||||
| 2.1 Donepezil | 1 | 15 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Severity of delirium Show forest plot | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐4.17, 3.57] |
| Analysis 2.3 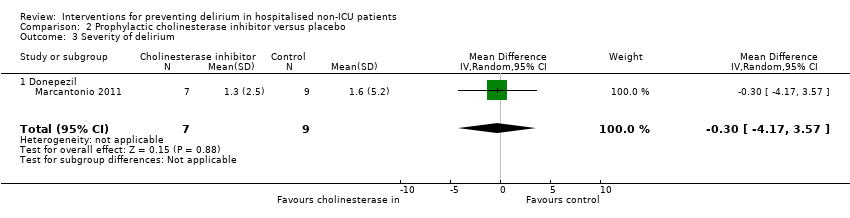 Comparison 2 Prophylactic cholinesterase inhibitor versus placebo, Outcome 3 Severity of delirium. | ||||
| 3.1 Donepezil | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐4.17, 3.57] |
| 4 Length of admission Show forest plot | 3 | 128 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐1.54, 0.86] |
| Analysis 2.4 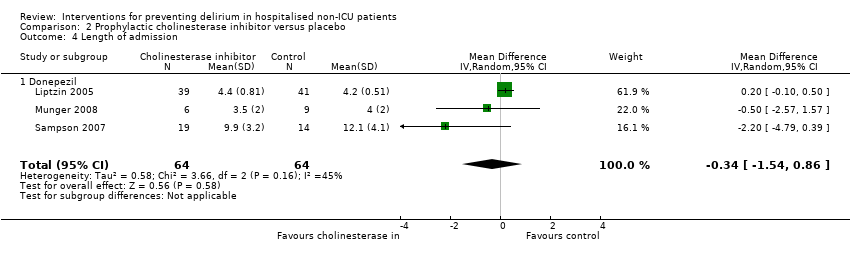 Comparison 2 Prophylactic cholinesterase inhibitor versus placebo, Outcome 4 Length of admission. | ||||
| 4.1 Donepezil | 3 | 128 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐1.54, 0.86] |
| 5 Cognition Show forest plot | 1 | 15 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐4.45, 1.65] |
| Analysis 2.5 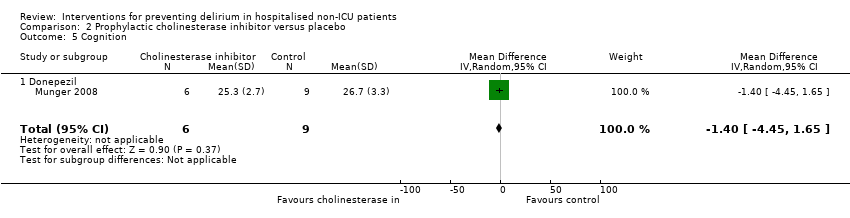 Comparison 2 Prophylactic cholinesterase inhibitor versus placebo, Outcome 5 Cognition. | ||||
| 5.1 Donepezil | 1 | 15 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐4.45, 1.65] |
| 6 Withdrawal from protocol Show forest plot | 2 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.49, 1.87] |
| Analysis 2.6 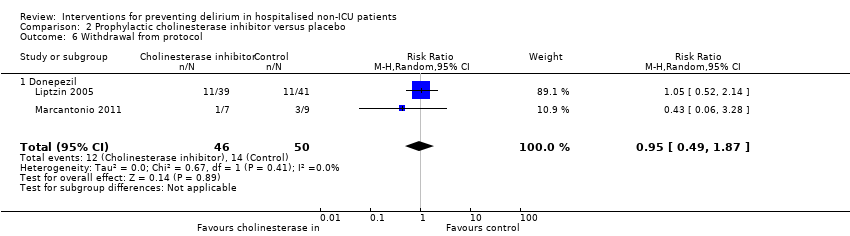 Comparison 2 Prophylactic cholinesterase inhibitor versus placebo, Outcome 6 Withdrawal from protocol. | ||||
| 6.1 Donepezil | 2 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.49, 1.87] |
| 7 Adverse events (continuous) Show forest plot | 1 | 33 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.26, 0.52] |
| Analysis 2.7  Comparison 2 Prophylactic cholinesterase inhibitor versus placebo, Outcome 7 Adverse events (continuous). | ||||
| 7.1 Donepezil | 1 | 33 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.26, 0.52] |
| 8 Adverse events (binary) Show forest plot | 1 | 16 | Risk Ratio (M‐H, Random, 95% CI) | 6.25 [0.35, 112.52] |
| Analysis 2.8  Comparison 2 Prophylactic cholinesterase inhibitor versus placebo, Outcome 8 Adverse events (binary). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 3 | 916 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.33, 1.59] |
| Analysis 3.1 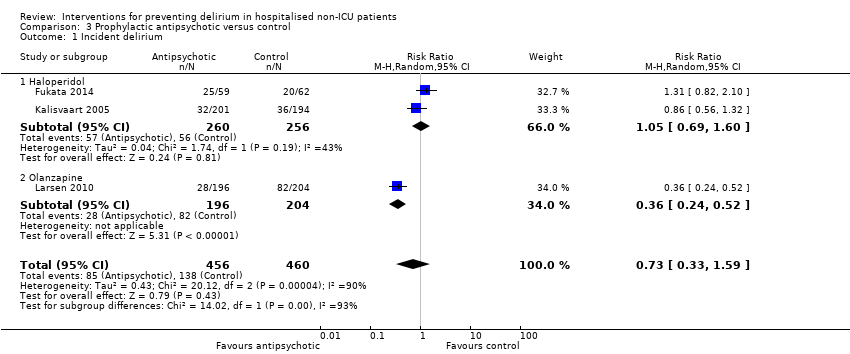 Comparison 3 Prophylactic antipsychotic versus control, Outcome 1 Incident delirium. | ||||
| 1.1 Haloperidol | 2 | 516 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.69, 1.60] |
| 1.2 Olanzapine | 1 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.24, 0.52] |
| 2 Duration of delirium Show forest plot | 2 | 178 | Mean Difference (IV, Random, 95% CI) | ‐2.74 [‐9.59, 4.11] |
| Analysis 3.2  Comparison 3 Prophylactic antipsychotic versus control, Outcome 2 Duration of delirium. | ||||
| 2.1 Haloperidol | 1 | 68 | Mean Difference (IV, Random, 95% CI) | ‐6.4 [‐9.38, ‐3.42] |
| 2.2 Olanzapine | 1 | 110 | Mean Difference (IV, Random, 95% CI) | 0.60 [0.10, 1.10] |
| 3 Severity of delirium Show forest plot | 2 | 178 | Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐6.80, 4.76] |
| Analysis 3.3 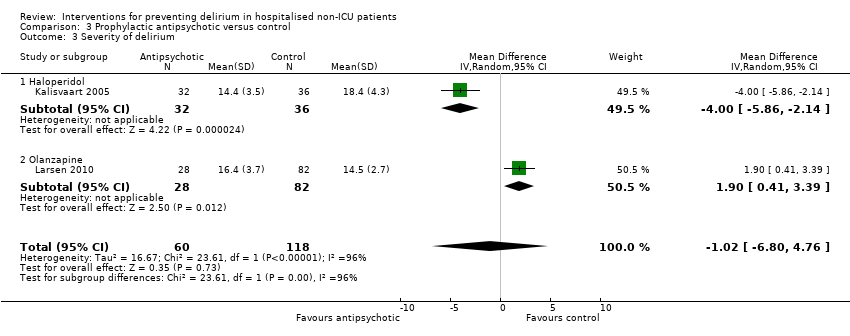 Comparison 3 Prophylactic antipsychotic versus control, Outcome 3 Severity of delirium. | ||||
| 3.1 Haloperidol | 1 | 68 | Mean Difference (IV, Random, 95% CI) | ‐2.00 [‐5.86, ‐2.14] |
| 3.2 Olanzapine | 1 | 110 | Mean Difference (IV, Random, 95% CI) | 1.90 [0.41, 3.39] |
| 4 Length of admission Show forest plot | 1 | 68 | Mean Difference (IV, Random, 95% CI) | ‐5.5 [‐12.17, 1.17] |
| Analysis 3.4  Comparison 3 Prophylactic antipsychotic versus control, Outcome 4 Length of admission. | ||||
| 4.1 Haloperidol | 1 | 68 | Mean Difference (IV, Random, 95% CI) | ‐5.5 [‐12.17, 1.17] |
| 5 Cognition Show forest plot | 1 | 110 | Mean Difference (IV, Random, 95% CI) | ‐4.90 [‐7.42, ‐2.38] |
| Analysis 3.5 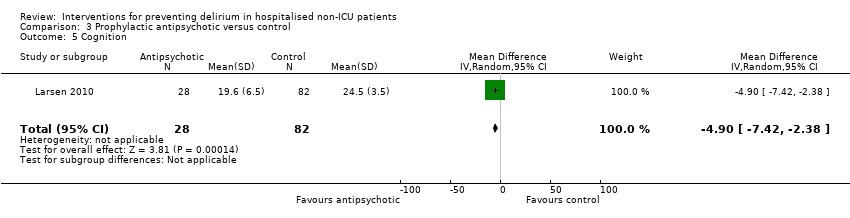 Comparison 3 Prophylactic antipsychotic versus control, Outcome 5 Cognition. | ||||
| 6 Withdrawal from protocol Show forest plot | 2 | 925 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.68, 1.24] |
| Analysis 3.6 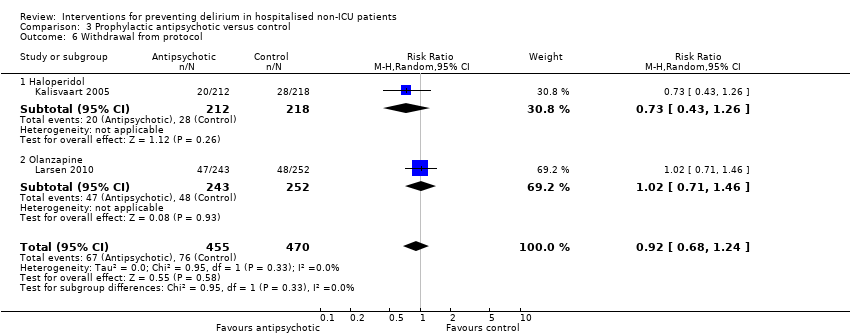 Comparison 3 Prophylactic antipsychotic versus control, Outcome 6 Withdrawal from protocol. | ||||
| 6.1 Haloperidol | 1 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.43, 1.26] |
| 6.2 Olanzapine | 1 | 495 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.71, 1.46] |
| 7 Adverse events Show forest plot | 1 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.10, 1.43] |
| Analysis 3.7 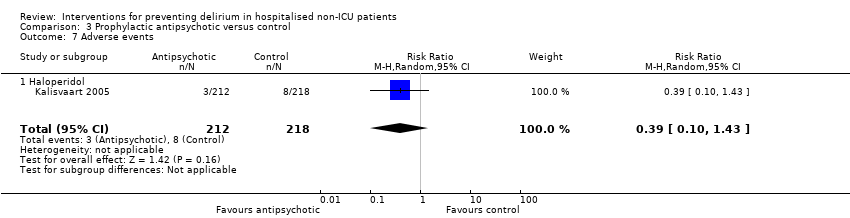 Comparison 3 Prophylactic antipsychotic versus control, Outcome 7 Adverse events. | ||||
| 7.1 Haloperidol | 1 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.10, 1.43] |
| 8 Pneumonia Show forest plot | 1 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 7.28 [0.38, 140.11] |
| Analysis 3.8  Comparison 3 Prophylactic antipsychotic versus control, Outcome 8 Pneumonia. | ||||
| 9 Urinary tract infection Show forest plot | 1 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.03, 2.31] |
| Analysis 3.9  Comparison 3 Prophylactic antipsychotic versus control, Outcome 9 Urinary tract infection. | ||||
| 10 Congestive heart failure Show forest plot | 1 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.07, 16.52] |
| Analysis 3.10  Comparison 3 Prophylactic antipsychotic versus control, Outcome 10 Congestive heart failure. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 3 | 529 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.09, 1.89] |
| Analysis 4.1 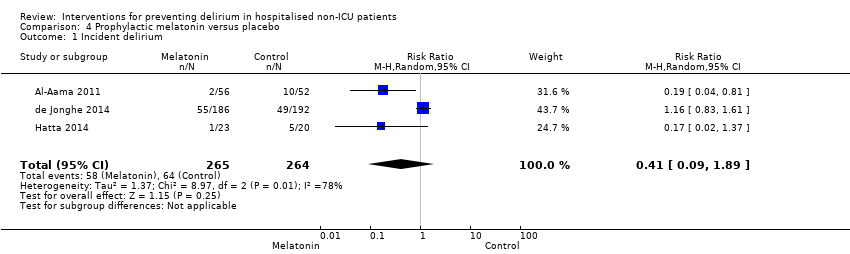 Comparison 4 Prophylactic melatonin versus placebo, Outcome 1 Incident delirium. | ||||
| 2 Duration of delirium Show forest plot | 1 | 104 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.57, 0.57] |
| Analysis 4.2 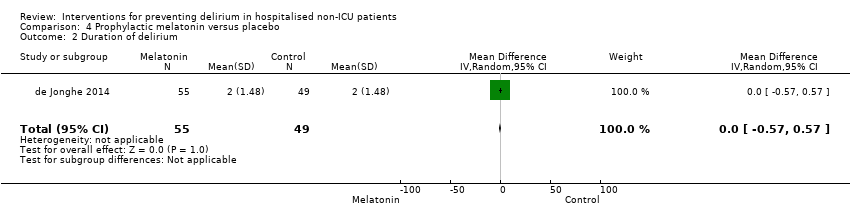 Comparison 4 Prophylactic melatonin versus placebo, Outcome 2 Duration of delirium. | ||||
| 3 Severity of delirium (binary severe vs. not severe) Show forest plot | 1 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.58, 1.27] |
| Analysis 4.3 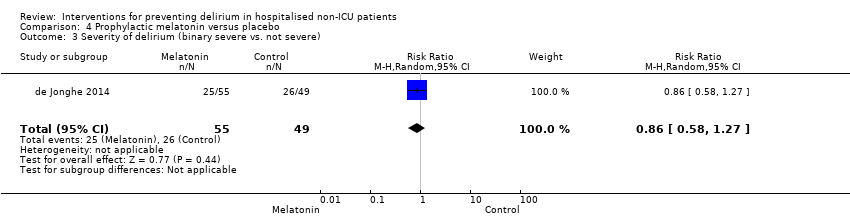 Comparison 4 Prophylactic melatonin versus placebo, Outcome 3 Severity of delirium (binary severe vs. not severe). | ||||
| 4 Severity of delirium (DRS‐R‐98) Show forest plot | 1 | 6 | Mean Difference (IV, Random, 95% CI) | ‐4.10 [‐19.47, 11.27] |
| Analysis 4.4 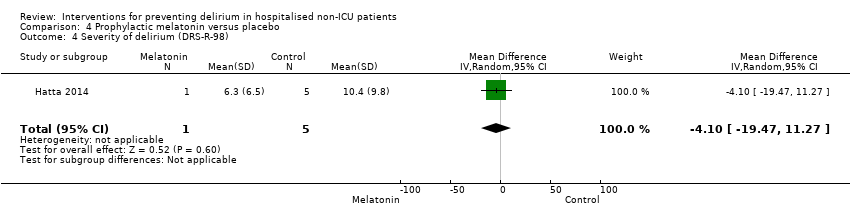 Comparison 4 Prophylactic melatonin versus placebo, Outcome 4 Severity of delirium (DRS‐R‐98). | ||||
| 5 Length of admission Show forest plot | 2 | 500 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐1.20, 1.39] |
| Analysis 4.5  Comparison 4 Prophylactic melatonin versus placebo, Outcome 5 Length of admission. | ||||
| 6 Cognitive impairment Show forest plot | 1 | 378 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.70, 1.04] |
| Analysis 4.6 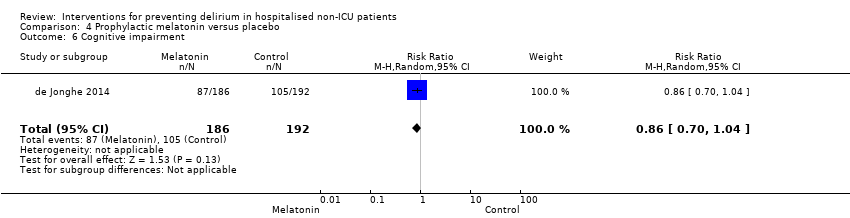 Comparison 4 Prophylactic melatonin versus placebo, Outcome 6 Cognitive impairment. | ||||
| 7 Activities of daily living Show forest plot | 1 | 369 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐1.20, 1.20] |
| Analysis 4.7 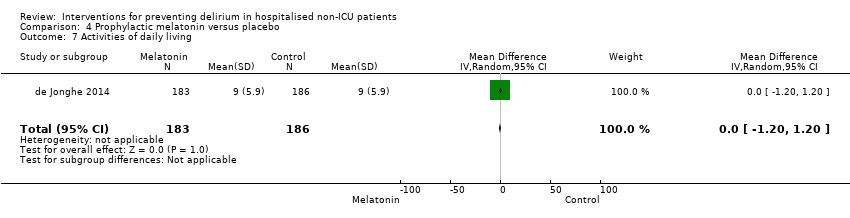 Comparison 4 Prophylactic melatonin versus placebo, Outcome 7 Activities of daily living. | ||||
| 8 Use of psychotropic medication (binary) Show forest plot | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.64, 1.18] |
| Analysis 4.8 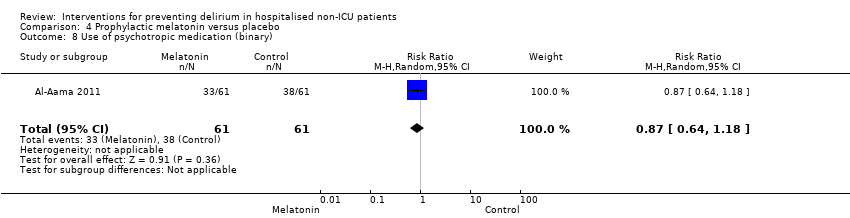 Comparison 4 Prophylactic melatonin versus placebo, Outcome 8 Use of psychotropic medication (binary). | ||||
| 9 Antipsychotic medication use (cumulative) Show forest plot | 1 | 378 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐1.79, ‐0.21] |
| Analysis 4.9 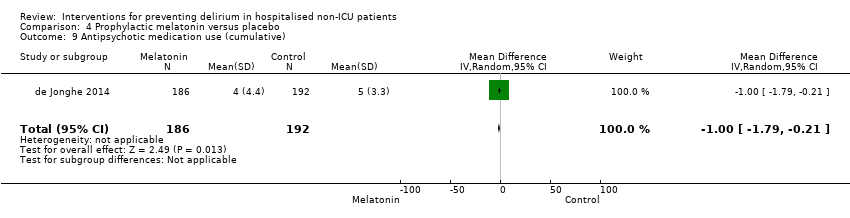 Comparison 4 Prophylactic melatonin versus placebo, Outcome 9 Antipsychotic medication use (cumulative). | ||||
| 10 Benzodiazepine use (cumulative) Show forest plot | 1 | 378 | Mean Difference (IV, Random, 95% CI) | ‐11.60 [‐24.34, 1.14] |
| Analysis 4.10  Comparison 4 Prophylactic melatonin versus placebo, Outcome 10 Benzodiazepine use (cumulative). | ||||
| 11 Withdrawal from study Show forest plot | 2 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.15, 6.87] |
| Analysis 4.11  Comparison 4 Prophylactic melatonin versus placebo, Outcome 11 Withdrawal from study. | ||||
| 12 In‐hospital mortality Show forest plot | 3 | 543 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.37, 1.88] |
| Analysis 4.12  Comparison 4 Prophylactic melatonin versus placebo, Outcome 12 In‐hospital mortality. | ||||
| 13 Mortality by 3 months Show forest plot | 1 | 378 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.67, 1.45] |
| Analysis 4.13  Comparison 4 Prophylactic melatonin versus placebo, Outcome 13 Mortality by 3 months. | ||||
| 14 Adverse events Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 4.14 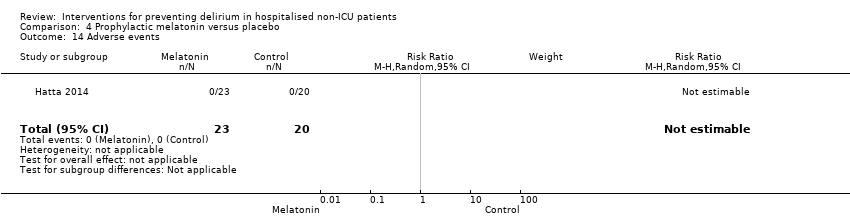 Comparison 4 Prophylactic melatonin versus placebo, Outcome 14 Adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.1  Comparison 5 Prophylactic citicoline versus placebo, Outcome 1 Incident delirium. | ||||
| 1.1 Incident delirium day 1 post surgery | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.22, 2.06] |
| 2 Cognitive status Show forest plot | 1 | 81 | Mean Difference (IV, Random, 95% CI) | ‐1.47 [‐3.85, 0.91] |
| Analysis 5.2  Comparison 5 Prophylactic citicoline versus placebo, Outcome 2 Cognitive status. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 6.1  Comparison 6 Oral premedication with diazepam and diphenhydramine, Outcome 1 Incident delirium. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 7507 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.87, 1.19] |
| Analysis 7.1 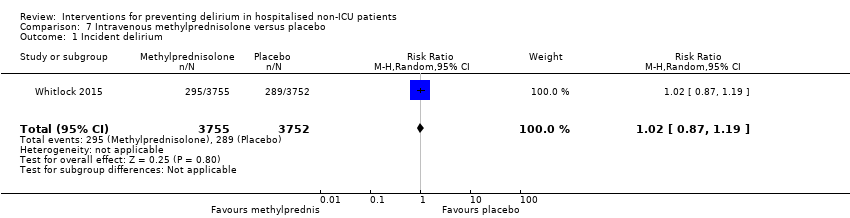 Comparison 7 Intravenous methylprednisolone versus placebo, Outcome 1 Incident delirium. | ||||
| 2 Length of admission Show forest plot | 1 | 7507 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.20, 0.20] |
| Analysis 7.2  Comparison 7 Intravenous methylprednisolone versus placebo, Outcome 2 Length of admission. | ||||
| 3 Mortality at 30 days Show forest plot | 1 | 7507 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.70, 1.07] |
| Analysis 7.3 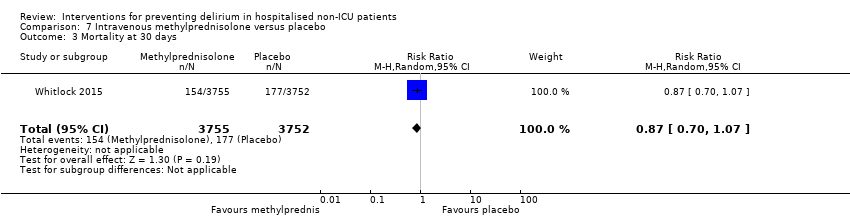 Comparison 7 Intravenous methylprednisolone versus placebo, Outcome 3 Mortality at 30 days. | ||||
| 4 Myocardial injury Show forest plot | 1 | 7507 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.07, 1.38] |
| Analysis 7.4  Comparison 7 Intravenous methylprednisolone versus placebo, Outcome 4 Myocardial injury. | ||||
| 5 Respiratory failure Show forest plot | 1 | 7507 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.80, 1.05] |
| Analysis 7.5 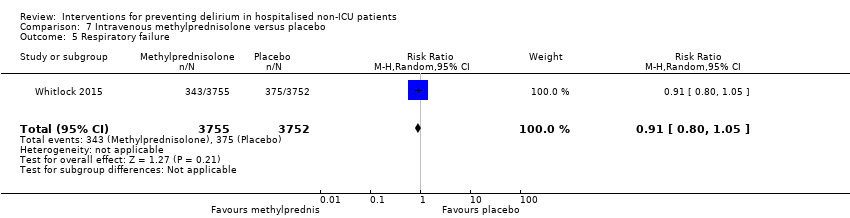 Comparison 7 Intravenous methylprednisolone versus placebo, Outcome 5 Respiratory failure. | ||||
| 6 Infection Show forest plot | 1 | 7507 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.84, 1.06] |
| Analysis 7.6 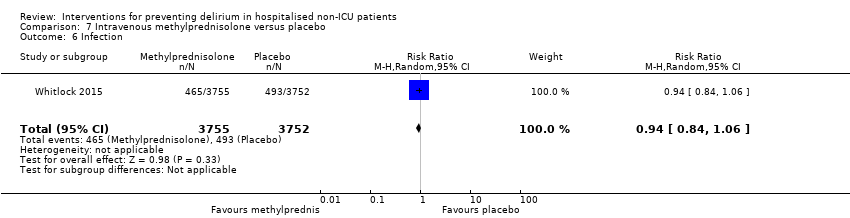 Comparison 7 Intravenous methylprednisolone versus placebo, Outcome 6 Infection. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 21 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.01, 1.90] |
| Analysis 8.1  Comparison 8 Gabapentinoids versus placebo, Outcome 1 Incident delirium. | ||||
| 2 Length of admission Show forest plot | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐2.12, 0.92] |
| Analysis 8.2 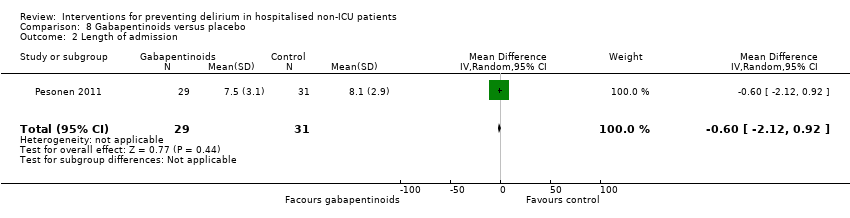 Comparison 8 Gabapentinoids versus placebo, Outcome 2 Length of admission. | ||||
| 3 Cognition Show forest plot | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐2.76, 4.76] |
| Analysis 8.3  Comparison 8 Gabapentinoids versus placebo, Outcome 3 Cognition. | ||||
| 4 Psychotropic Medication Use Show forest plot | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.21, 1.38] |
| Analysis 8.4  Comparison 8 Gabapentinoids versus placebo, Outcome 4 Psychotropic Medication Use. | ||||
| 5 Withdrawal from protocol Show forest plot | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 9.00 [0.50, 161.13] |
| Analysis 8.5  Comparison 8 Gabapentinoids versus placebo, Outcome 5 Withdrawal from protocol. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.21, 19.23] |
| Analysis 9.1  Comparison 9 Ketamine versus placebo, Outcome 1 Incident delirium. | ||||
| 2 Withdrawal from protocol Show forest plot | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 14.34] |
| Analysis 9.2 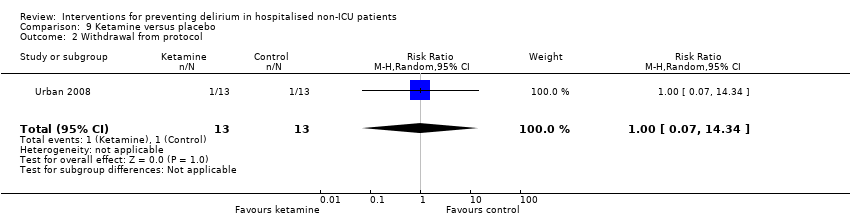 Comparison 9 Ketamine versus placebo, Outcome 2 Withdrawal from protocol. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.26, 0.98] |
| Analysis 10.1  Comparison 10 Intravenous parecoxib sodium analgesia versus Morphine and Saline, Outcome 1 Incident delirium. | ||||
| 2 Length of admission Show forest plot | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.58, ‐0.22] |
| Analysis 10.2 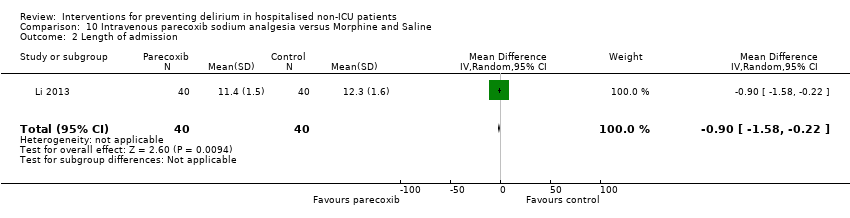 Comparison 10 Intravenous parecoxib sodium analgesia versus Morphine and Saline, Outcome 2 Length of admission. | ||||
| 3 Postoperative cognitive dysfunction at 3 days Show forest plot | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.21, 1.02] |
| Analysis 10.3  Comparison 10 Intravenous parecoxib sodium analgesia versus Morphine and Saline, Outcome 3 Postoperative cognitive dysfunction at 3 days. | ||||
| 4 Postoperative cognitive dysfunction at 1 week Show forest plot | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.15, 0.98] |
| Analysis 10.4  Comparison 10 Intravenous parecoxib sodium analgesia versus Morphine and Saline, Outcome 4 Postoperative cognitive dysfunction at 1 week. | ||||
| 5 Postoperative cognitive dysfunction at 3 months Show forest plot | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.09, 1.01] |
| Analysis 10.5 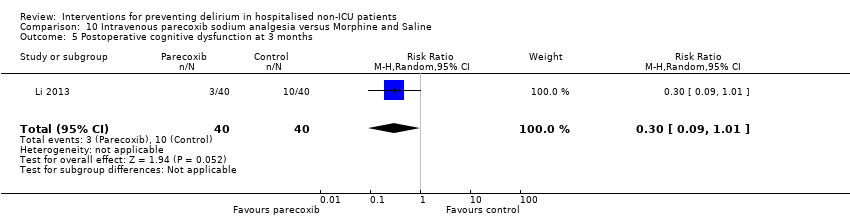 Comparison 10 Intravenous parecoxib sodium analgesia versus Morphine and Saline, Outcome 5 Postoperative cognitive dysfunction at 3 months. | ||||
| 6 Postoperative cognitive dysfunction at 6 months Show forest plot | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.02, 1.11] |
| Analysis 10.6  Comparison 10 Intravenous parecoxib sodium analgesia versus Morphine and Saline, Outcome 6 Postoperative cognitive dysfunction at 6 months. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.9 [0.44, 1.85] |
| Analysis 11.1  Comparison 11 Intrathecal morphine and PCA morphine versus PCA morphine, Outcome 1 Incident delirium. | ||||
| 2 Length of admission Show forest plot | 1 | 52 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.51, 0.51] |
| Analysis 11.2 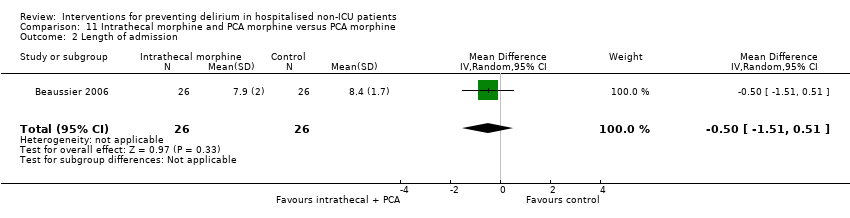 Comparison 11 Intrathecal morphine and PCA morphine versus PCA morphine, Outcome 2 Length of admission. | ||||
| 3 Cognition ‐ days for MMSE to return to preoperative level Show forest plot | 1 | 52 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐1.03, 1.43] |
| Analysis 11.3  Comparison 11 Intrathecal morphine and PCA morphine versus PCA morphine, Outcome 3 Cognition ‐ days for MMSE to return to preoperative level. | ||||
| 4 Withdrawal from protocol Show forest plot | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.19, 3.17] |
| Analysis 11.4  Comparison 11 Intrathecal morphine and PCA morphine versus PCA morphine, Outcome 4 Withdrawal from protocol. | ||||
| 5 Mortality Show forest plot | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.13] |
| Analysis 11.5  Comparison 11 Intrathecal morphine and PCA morphine versus PCA morphine, Outcome 5 Mortality. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.24, 0.87] |
| Analysis 12.1  Comparison 12 Fascia iliaca compartment block (FICB) versus placebo, Outcome 1 Incident delirium. | ||||
| 2 Severity of delirium Show forest plot | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐4.30 [‐6.81, ‐1.79] |
| Analysis 12.2 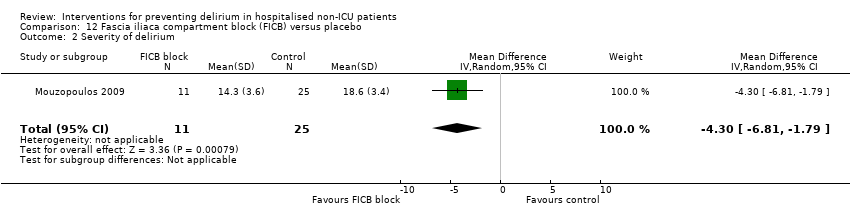 Comparison 12 Fascia iliaca compartment block (FICB) versus placebo, Outcome 2 Severity of delirium. | ||||
| 3 Duration of delirium Show forest plot | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐5.7 [‐9.50, ‐1.90] |
| Analysis 12.3  Comparison 12 Fascia iliaca compartment block (FICB) versus placebo, Outcome 3 Duration of delirium. | ||||
| 4 Mortality Show forest plot | 1 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.05, 5.58] |
| Analysis 12.4 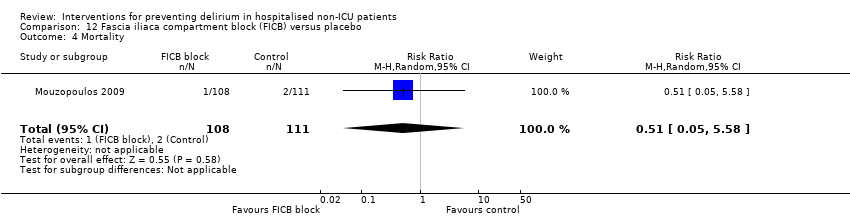 Comparison 12 Fascia iliaca compartment block (FICB) versus placebo, Outcome 4 Mortality. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.26, 0.89] |
| Analysis 13.1  Comparison 13 Light versus deep propofol sedation, Outcome 1 Incident delirium. | ||||
| 2 Duration of delirium Show forest plot | 1 | 34 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐3.30, 2.10] |
| Analysis 13.2 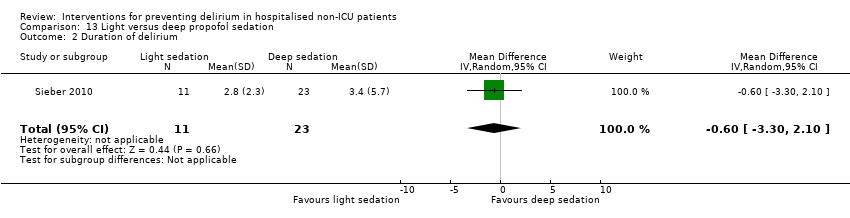 Comparison 13 Light versus deep propofol sedation, Outcome 2 Duration of delirium. | ||||
| 3 Length of admission Show forest plot | 1 | 114 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.80, 1.20] |
| Analysis 13.3  Comparison 13 Light versus deep propofol sedation, Outcome 3 Length of admission. | ||||
| 4 Cognition on day 2 Show forest plot | 1 | 114 | Mean Difference (IV, Random, 95% CI) | 3.10 [0.30, 5.90] |
| Analysis 13.4 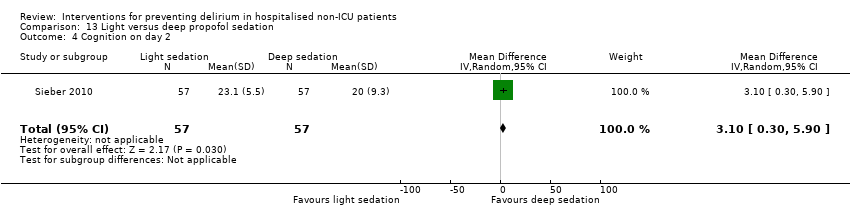 Comparison 13 Light versus deep propofol sedation, Outcome 4 Cognition on day 2. | ||||
| 5 In‐hospital mortality Show forest plot | 1 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.05, 5.36] |
| Analysis 13.5 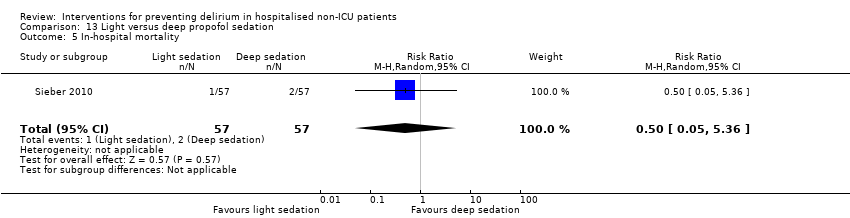 Comparison 13 Light versus deep propofol sedation, Outcome 5 In‐hospital mortality. | ||||
| 6 Postoperative complications (>=1) Show forest plot | 1 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.60, 1.26] |
| Analysis 13.6  Comparison 13 Light versus deep propofol sedation, Outcome 6 Postoperative complications (>=1). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 2 | 2057 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.60, 0.85] |
| Analysis 14.1  Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 1 Incident delirium. | ||||
| 2 Length of admission Show forest plot | 2 | 2057 | Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐1.45, ‐0.43] |
| Analysis 14.2 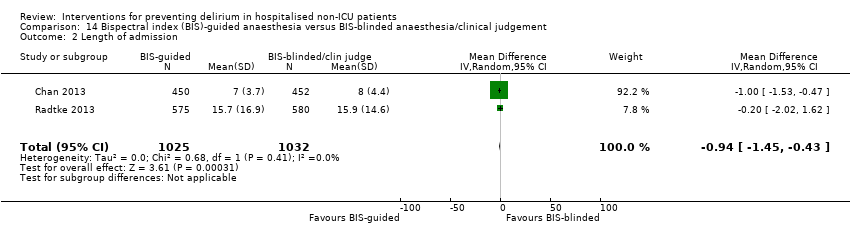 Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 2 Length of admission. | ||||
| 3 Cognition at 7 days Show forest plot | 2 | 1938 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.71, 1.05] |
| Analysis 14.3 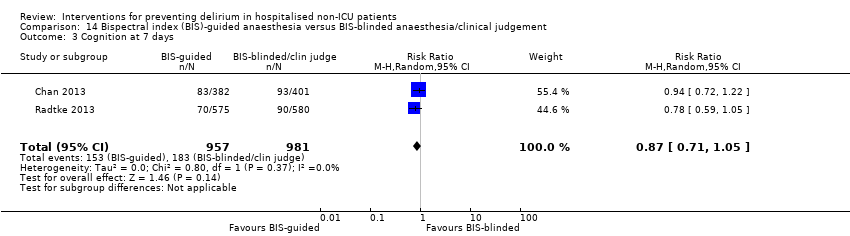 Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 3 Cognition at 7 days. | ||||
| 4 Cognition at 3 months Show forest plot | 2 | 1990 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.53, 0.97] |
| Analysis 14.4  Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 4 Cognition at 3 months. | ||||
| 5 SF‐36 mental summary score Show forest plot | 1 | 902 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐3.40, ‐0.40] |
| Analysis 14.5 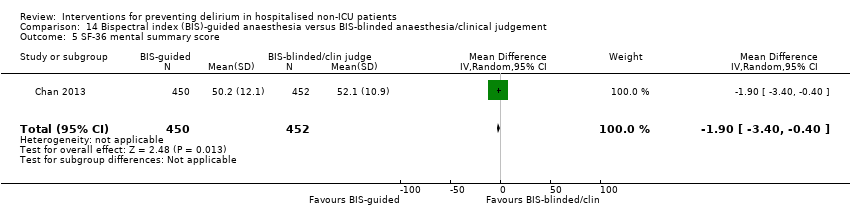 Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 5 SF‐36 mental summary score. | ||||
| 6 Mortality at 7 days Show forest plot | 1 | 921 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.42, 5.25] |
| Analysis 14.6  Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 6 Mortality at 7 days. | ||||
| 7 Mortality at 3 months Show forest plot | 2 | 1938 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.77, 1.59] |
| Analysis 14.7  Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 7 Mortality at 3 months. | ||||
| 8 Cardiac complications Show forest plot | 1 | 902 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.52, 1.39] |
| Analysis 14.8  Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 8 Cardiac complications. | ||||
| 9 Respiratory complications Show forest plot | 1 | 902 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.59, 1.07] |
| Analysis 14.9  Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 9 Respiratory complications. | ||||
| 10 Infective complications Show forest plot | 1 | 902 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.55, 0.95] |
| Analysis 14.10  Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 10 Infective complications. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.47, 1.34] |
| Analysis 15.1 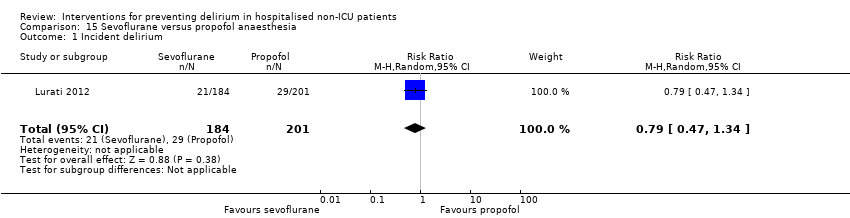 Comparison 15 Sevoflurane versus propofol anaesthesia, Outcome 1 Incident delirium. | ||||
| 2 Mortality at 12 months Show forest plot | 1 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.70, 2.02] |
| Analysis 15.2  Comparison 15 Sevoflurane versus propofol anaesthesia, Outcome 2 Mortality at 12 months. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.20, 2.79] |
| Analysis 16.1  Comparison 16 Xenon versus sevoflurane anaesthesia, Outcome 1 Incident delirium. | ||||
| 2 Length of admission Show forest plot | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 4.0 [‐1.72, 9.72] |
| Analysis 16.2  Comparison 16 Xenon versus sevoflurane anaesthesia, Outcome 2 Length of admission. | ||||
| 3 In‐hospital mortality Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 16.3  Comparison 16 Xenon versus sevoflurane anaesthesia, Outcome 3 In‐hospital mortality. | ||||
| 4 Adverse events Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.34, 1.64] |
| Analysis 16.4  Comparison 16 Xenon versus sevoflurane anaesthesia, Outcome 4 Adverse events. | ||||
| 5 Sepsis Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.5 [0.29, 7.73] |
| Analysis 16.5  Comparison 16 Xenon versus sevoflurane anaesthesia, Outcome 5 Sepsis. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 2 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.69, 2.03] |
| Analysis 17.1  Comparison 17 Epidural anaesthesia versus general anaesthesia, Outcome 1 Incident delirium. | ||||
| 2 Length of admission > 10 days Show forest plot | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.28, 1.24] |
| Analysis 17.2  Comparison 17 Epidural anaesthesia versus general anaesthesia, Outcome 2 Length of admission > 10 days. | ||||
| 3 Cognitive decline Show forest plot | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.02, 1.06] |
| Analysis 17.3  Comparison 17 Epidural anaesthesia versus general anaesthesia, Outcome 3 Cognitive decline. | ||||
| 4 Urinary tract infection Show forest plot | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.57, 3.09] |
| Analysis 17.4  Comparison 17 Epidural anaesthesia versus general anaesthesia, Outcome 4 Urinary tract infection. | ||||
| 5 Psychological morbidity Show forest plot | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.23, 4.71] |
| Analysis 17.5  Comparison 17 Epidural anaesthesia versus general anaesthesia, Outcome 5 Psychological morbidity. | ||||
| 5.1 Depression | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.23, 4.71] |
| 6 Postoperative complications Show forest plot | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.35, 2.39] |
| Analysis 17.6  Comparison 17 Epidural anaesthesia versus general anaesthesia, Outcome 6 Postoperative complications. | ||||
| 7 Pressure ulcer Show forest plot | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.16, 2.36] |
| Analysis 17.7  Comparison 17 Epidural anaesthesia versus general anaesthesia, Outcome 7 Pressure ulcer. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.45, 1.27] |
| Analysis 18.1 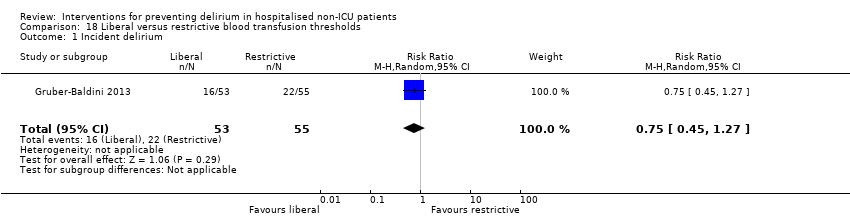 Comparison 18 Liberal versus restrictive blood transfusion thresholds, Outcome 1 Incident delirium. | ||||
| 2 Delirium severity Show forest plot | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐2.99, 2.79] |
| Analysis 18.2 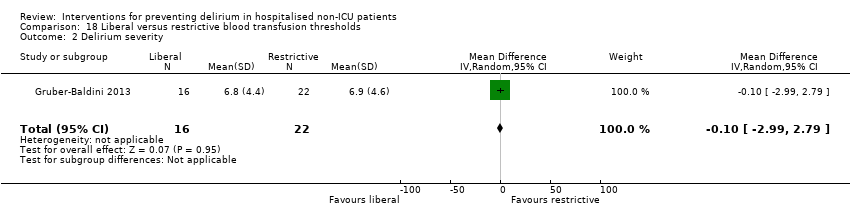 Comparison 18 Liberal versus restrictive blood transfusion thresholds, Outcome 2 Delirium severity. | ||||
| 3 Length of admission Show forest plot | 1 | 138 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐1.36, 1.16] |
| Analysis 18.3 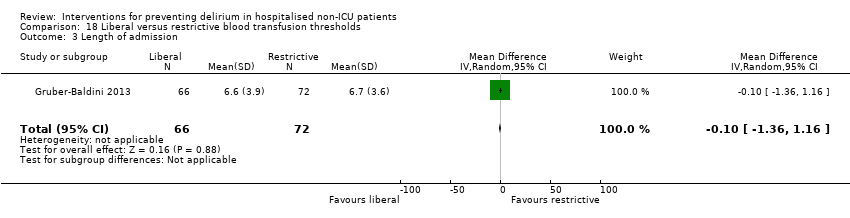 Comparison 18 Liberal versus restrictive blood transfusion thresholds, Outcome 3 Length of admission. | ||||
| 4 Psychoactive medication use Show forest plot | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.87, 1.12] |
| Analysis 18.4  Comparison 18 Liberal versus restrictive blood transfusion thresholds, Outcome 4 Psychoactive medication use. | ||||
| 5 Infection Show forest plot | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.23, 5.22] |
| Analysis 18.5  Comparison 18 Liberal versus restrictive blood transfusion thresholds, Outcome 5 Infection. | ||||
| 6 Congestive heart failure Show forest plot | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.05, 5.88] |
| Analysis 18.6  Comparison 18 Liberal versus restrictive blood transfusion thresholds, Outcome 6 Congestive heart failure. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.09, 0.77] |
| Analysis 19.1 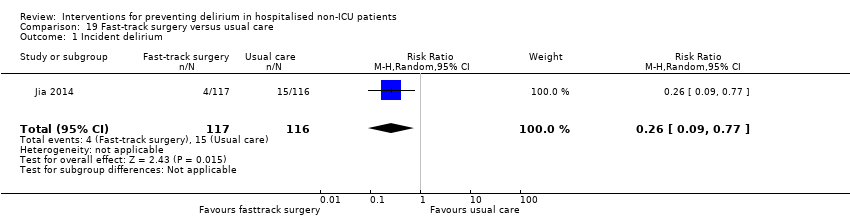 Comparison 19 Fast‐track surgery versus usual care, Outcome 1 Incident delirium. | ||||
| 2 Length of admission Show forest plot | 1 | 233 | Mean Difference (IV, Random, 95% CI) | ‐4.20 [‐4.60, ‐3.80] |
| Analysis 19.2 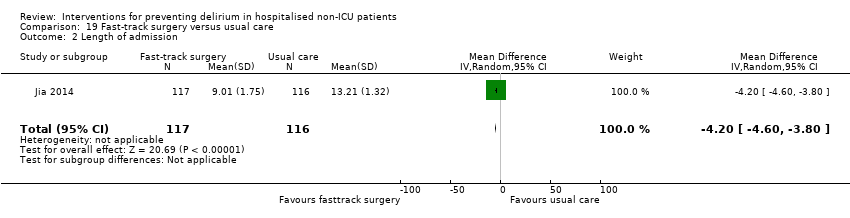 Comparison 19 Fast‐track surgery versus usual care, Outcome 2 Length of admission. | ||||
| 3 Urinary tract infection Show forest plot | 1 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.14, 1.04] |
| Analysis 19.3 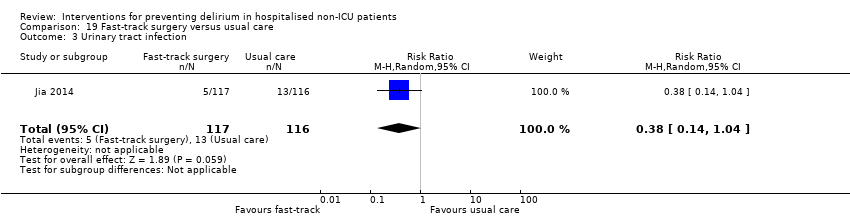 Comparison 19 Fast‐track surgery versus usual care, Outcome 3 Urinary tract infection. | ||||
| 4 Heart failure Show forest plot | 1 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.10, 0.91] |
| Analysis 19.4  Comparison 19 Fast‐track surgery versus usual care, Outcome 4 Heart failure. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.02, 1.06] |
| Analysis 20.1  Comparison 20 Postoperative delirium‐free protocol (DFP) versus usual care, Outcome 1 Incident delirium. | ||||
| 2 Length of admission Show forest plot | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐4.30 [‐12.51, 3.91] |
| Analysis 20.2  Comparison 20 Postoperative delirium‐free protocol (DFP) versus usual care, Outcome 2 Length of admission. | ||||
| 3 Behavioural disturbance Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.03, 1.56] |
| Analysis 20.3  Comparison 20 Postoperative delirium‐free protocol (DFP) versus usual care, Outcome 3 Behavioural disturbance. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.82, 1.43] |
| Analysis 21.1  Comparison 21 Computerised clinical decision support system (CCDS) versus usual care, Outcome 1 Incident delirium. | ||||
| 2 Length of admission Show forest plot | 1 | 424 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐0.35, 2.15] |
| Analysis 21.2 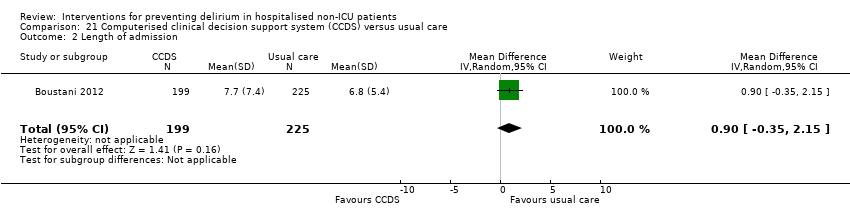 Comparison 21 Computerised clinical decision support system (CCDS) versus usual care, Outcome 2 Length of admission. | ||||
| 3 Mortality within 30 days of discharge Show forest plot | 1 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.49, 2.23] |
| Analysis 21.3  Comparison 21 Computerised clinical decision support system (CCDS) versus usual care, Outcome 3 Mortality within 30 days of discharge. | ||||
| 4 Falls Show forest plot | 1 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.39, 2.19] |
| Analysis 21.4 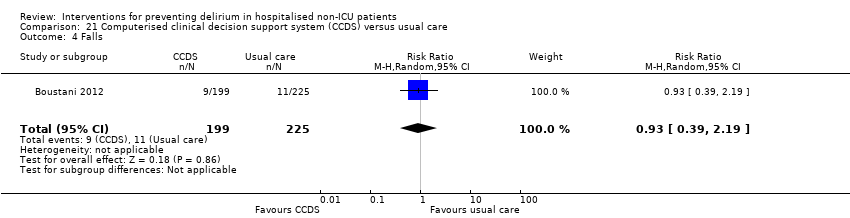 Comparison 21 Computerised clinical decision support system (CCDS) versus usual care, Outcome 4 Falls. | ||||
| 5 Pressure ulcers Show forest plot | 1 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.64, 1.84] |
| Analysis 21.5  Comparison 21 Computerised clinical decision support system (CCDS) versus usual care, Outcome 5 Pressure ulcers. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.79, 1.22] |
| Analysis 22.1  Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 1 Incident delirium. | ||||
| 2 Duration of delirium Show forest plot | 1 | 163 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐2.04, 0.04] |
| Analysis 22.2 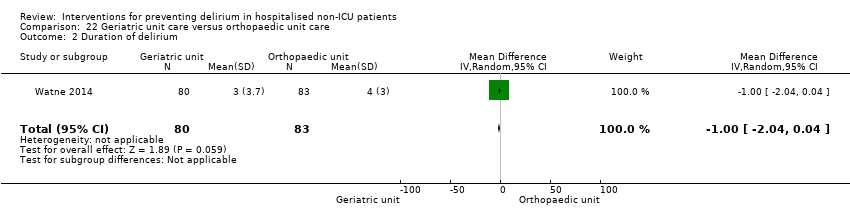 Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 2 Duration of delirium. | ||||
| 3 Severity of delirium Show forest plot | 1 | 163 | Mean Difference (IV, Random, 95% CI) | 1.5 [1.00, 4.00] |
| Analysis 22.3  Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 3 Severity of delirium. | ||||
| 4 Length of admission Show forest plot | 1 | 329 | Mean Difference (IV, Random, 95% CI) | 3.0 [1.94, 4.06] |
| Analysis 22.4  Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 4 Length of admission. | ||||
| 5 Cognitive function (composite score) at 4 months Show forest plot | 1 | 228 | Mean Difference (IV, Random, 95% CI) | 1.80 [‐5.92, 9.52] |
| Analysis 22.5 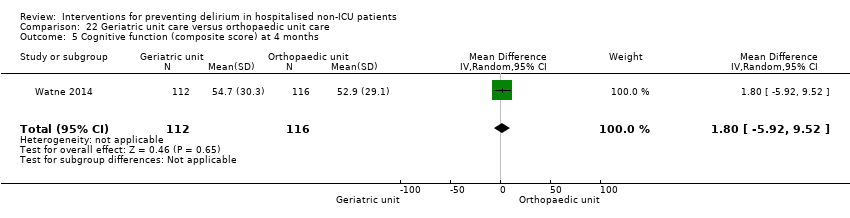 Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 5 Cognitive function (composite score) at 4 months. | ||||
| 6 Incident dementia at 12 months Show forest plot | 1 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 2.26 [0.60, 8.49] |
| Analysis 22.6 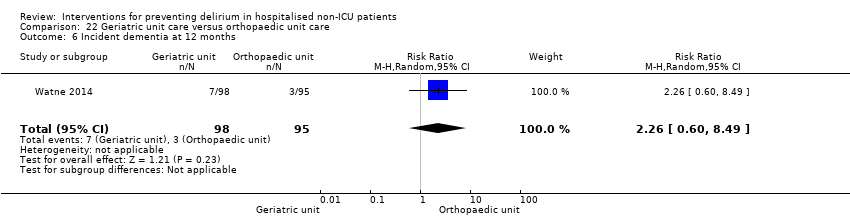 Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 6 Incident dementia at 12 months. | ||||
| 7 ADL function at 4 months Show forest plot | 1 | 239 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐0.70, 2.70] |
| Analysis 22.7  Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 7 ADL function at 4 months. | ||||
| 8 Institutionalisation at 4 months Show forest plot | 1 | 242 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.58, 1.91] |
| Analysis 22.8  Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 8 Institutionalisation at 4 months. | ||||
| 9 Institutionalisation at 12 months Show forest plot | 1 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.47, 1.59] |
| Analysis 22.9  Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 9 Institutionalisation at 12 months. | ||||
| 10 Inpatient mortality Show forest plot | 1 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.21, 1.47] |
| Analysis 22.10 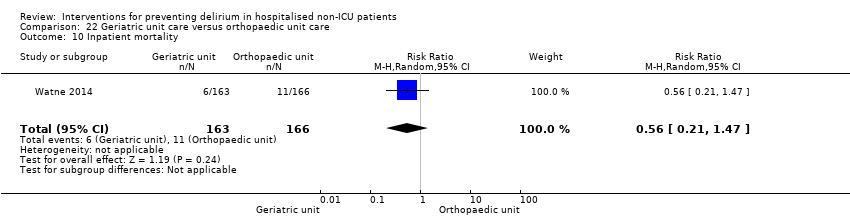 Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 10 Inpatient mortality. | ||||
| 11 Falls Show forest plot | 1 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.61, 2.77] |
| Analysis 22.11  Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 11 Falls. | ||||
| 12 Pressure ulcers Show forest plot | 1 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.10, 1.41] |
| Analysis 22.12 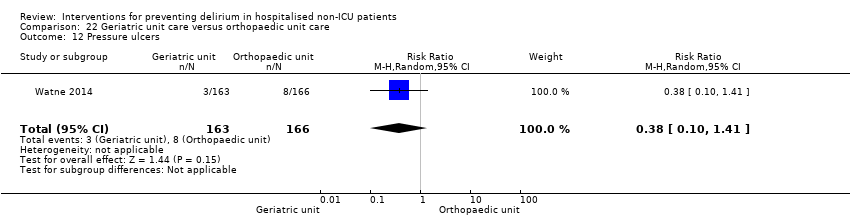 Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 12 Pressure ulcers. | ||||
| 13 Other medical adverse events Show forest plot | 1 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.76, 1.23] |
| Analysis 22.13 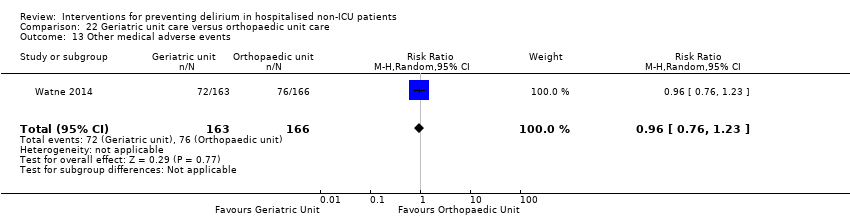 Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 13 Other medical adverse events. | ||||
| 14 Postoperative complications Show forest plot | 1 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.20, 2.36] |
| Analysis 22.14  Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 14 Postoperative complications. | ||||
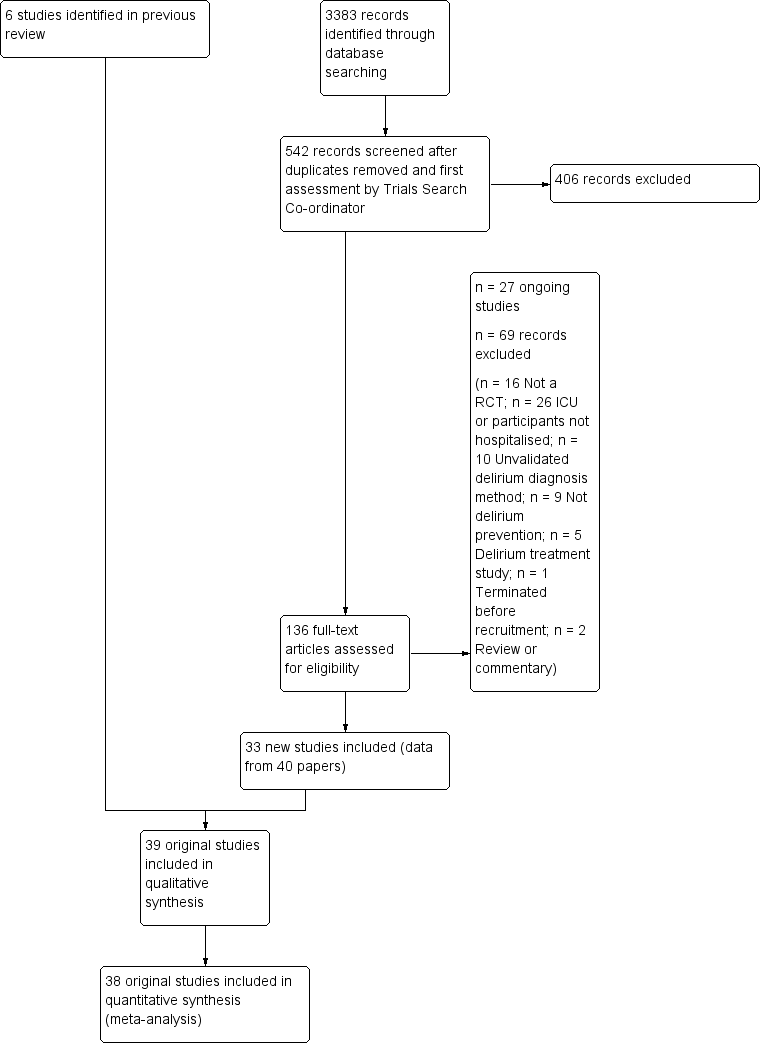
Study flow diagram
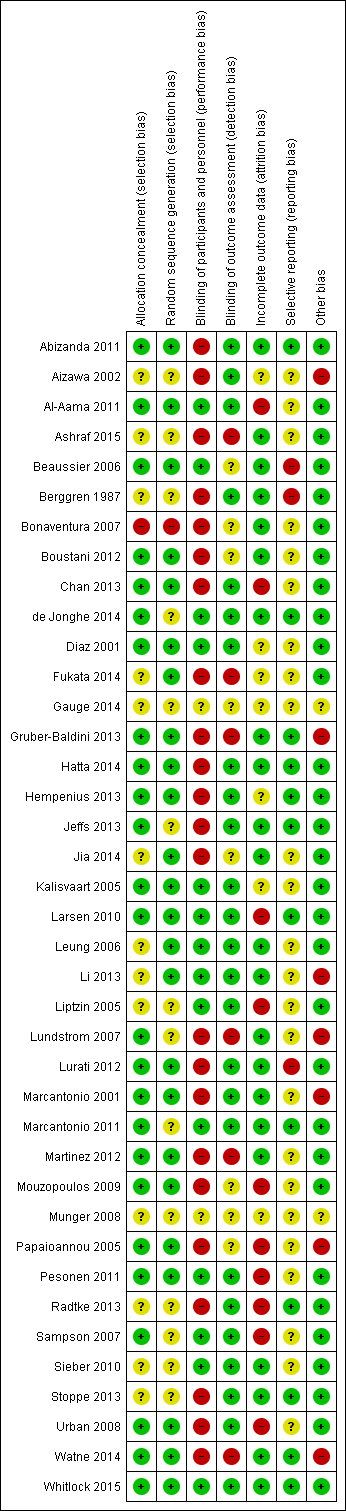
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Multi‐component delirium prevention intervention (MCI) versus usual care, outcome: 1.1 Incident delirium.

Forest plot of comparison: 2 Prophylactic cholinesterase inhibitor versus placebo, outcome: 2.1 Incident delirium.
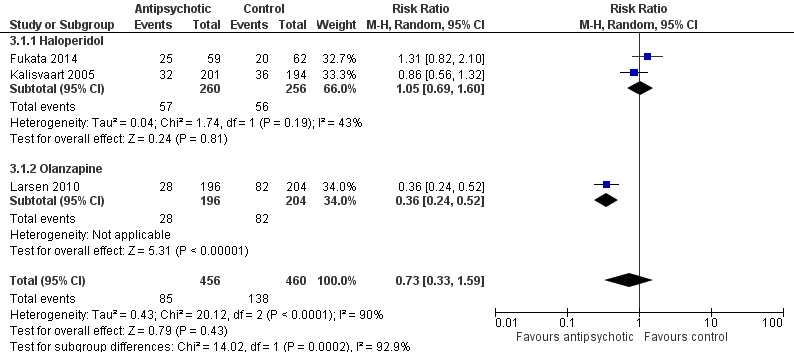
Figure 5Forest plot of comparison: 3 Prophylactic antipsychotic versus control, outcome: 3.1 Incidence of delirium.
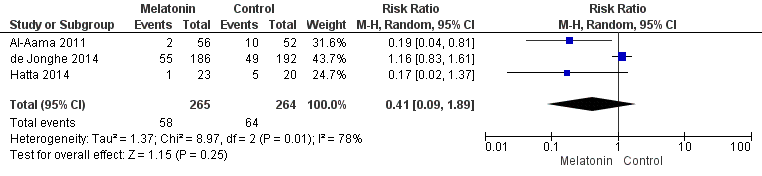
Forest plot of comparison: 4 Prophylactic melatonin versus placebo, outcome: 4.1 Incident delirium.
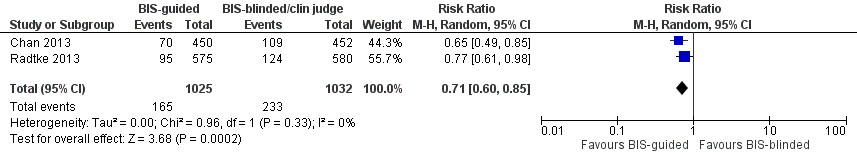
Forest plot of comparison: 11 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia, outcome: 11.1 Incident delirium.
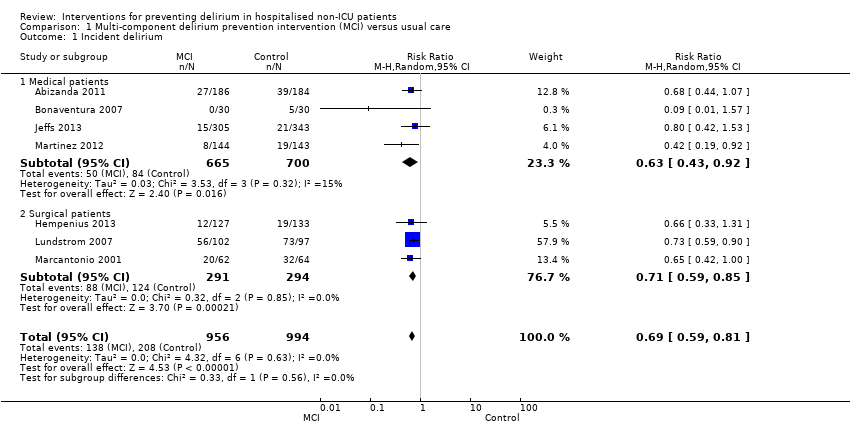
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 1 Incident delirium.
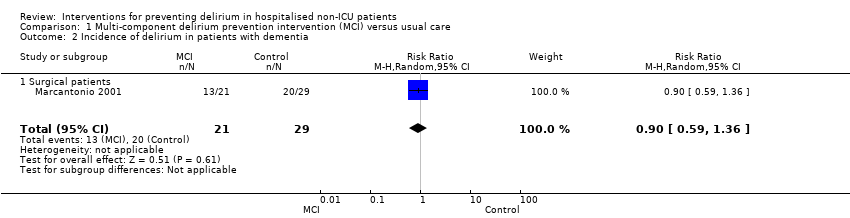
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 2 Incidence of delirium in patients with dementia.
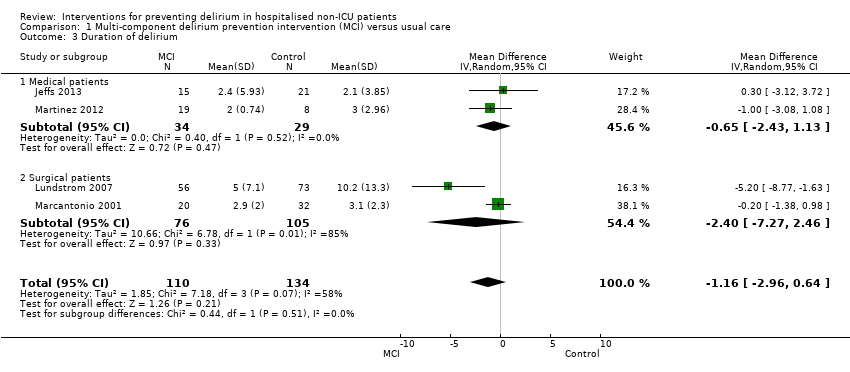
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 3 Duration of delirium.
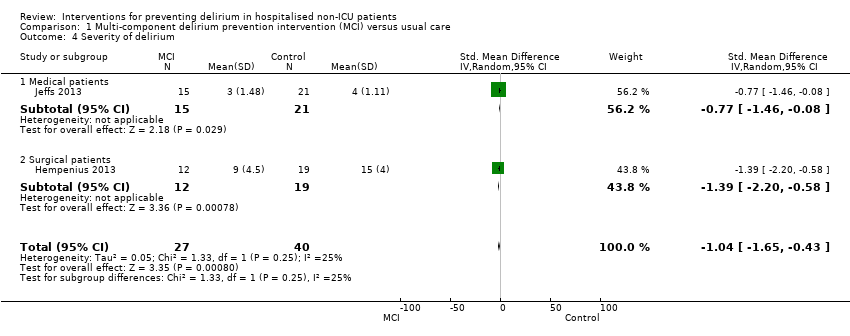
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 4 Severity of delirium.

Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 5 Length of admission.
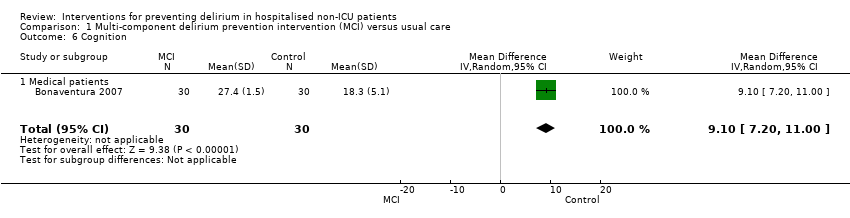
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 6 Cognition.
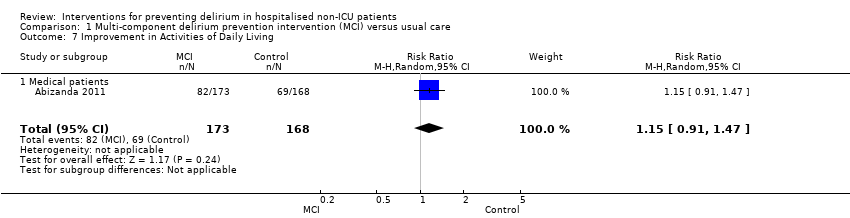
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 7 Improvement in Activities of Daily Living.

Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 8 Return to independent living.

Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 9 Depression.

Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 10 Withdrawal from protocol.
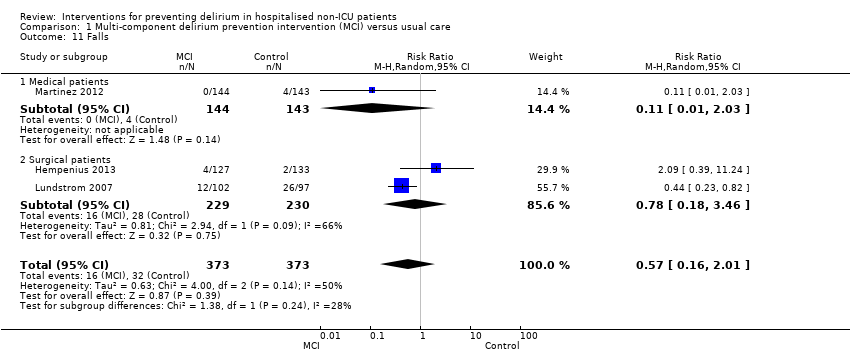
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 11 Falls.

Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 12 Pressure ulcers.

Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 13 Inpatient mortality.
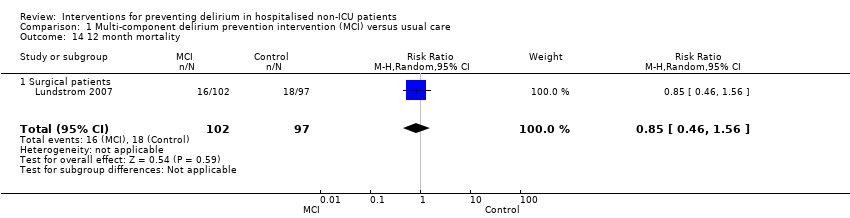
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 14 12 month mortality.
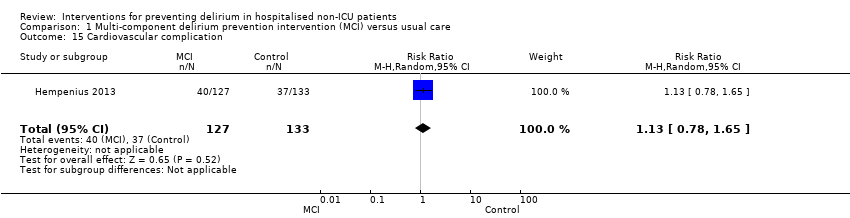
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 15 Cardiovascular complication.

Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 16 Urinary tract infection.
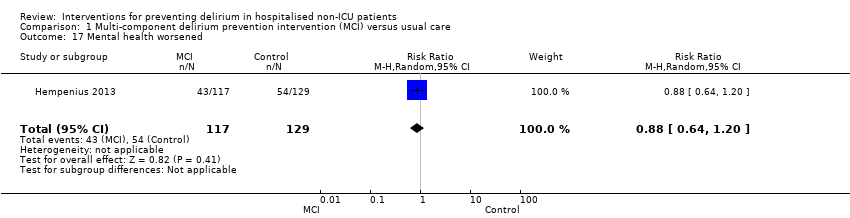
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 17 Mental health worsened.
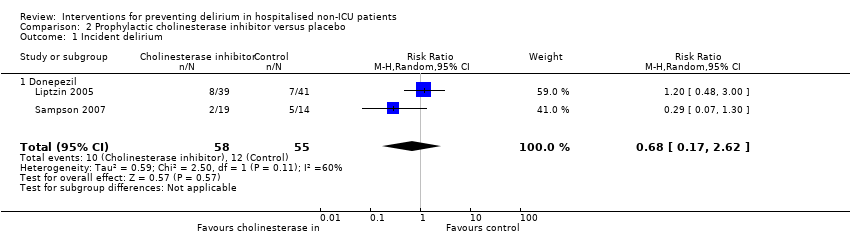
Comparison 2 Prophylactic cholinesterase inhibitor versus placebo, Outcome 1 Incident delirium.

Comparison 2 Prophylactic cholinesterase inhibitor versus placebo, Outcome 2 Duration of delirium.
Comparison 2 Prophylactic cholinesterase inhibitor versus placebo, Outcome 3 Severity of delirium.
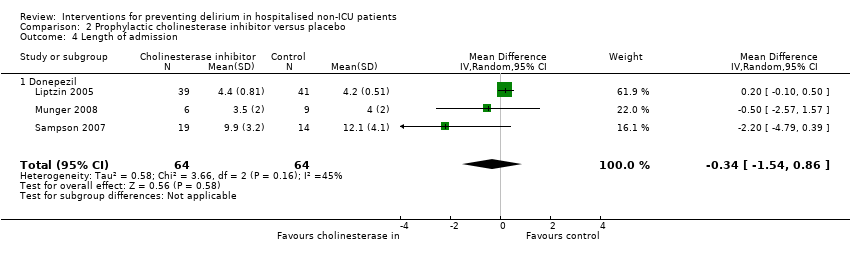
Comparison 2 Prophylactic cholinesterase inhibitor versus placebo, Outcome 4 Length of admission.
Comparison 2 Prophylactic cholinesterase inhibitor versus placebo, Outcome 5 Cognition.

Comparison 2 Prophylactic cholinesterase inhibitor versus placebo, Outcome 6 Withdrawal from protocol.

Comparison 2 Prophylactic cholinesterase inhibitor versus placebo, Outcome 7 Adverse events (continuous).

Comparison 2 Prophylactic cholinesterase inhibitor versus placebo, Outcome 8 Adverse events (binary).
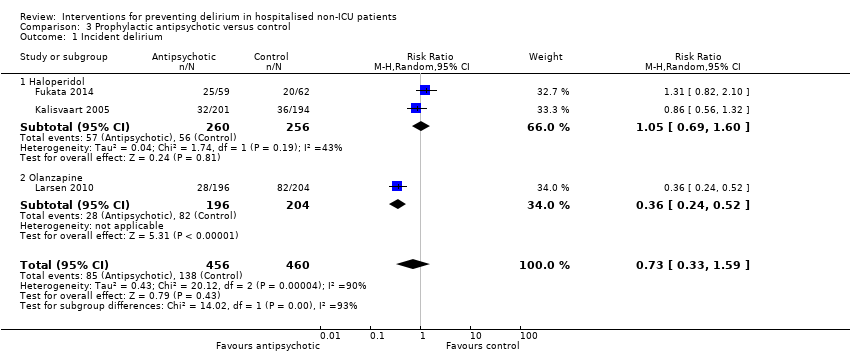
Comparison 3 Prophylactic antipsychotic versus control, Outcome 1 Incident delirium.

Comparison 3 Prophylactic antipsychotic versus control, Outcome 2 Duration of delirium.

Comparison 3 Prophylactic antipsychotic versus control, Outcome 3 Severity of delirium.
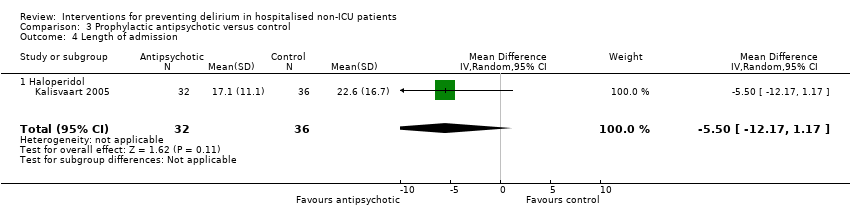
Comparison 3 Prophylactic antipsychotic versus control, Outcome 4 Length of admission.

Comparison 3 Prophylactic antipsychotic versus control, Outcome 5 Cognition.

Comparison 3 Prophylactic antipsychotic versus control, Outcome 6 Withdrawal from protocol.
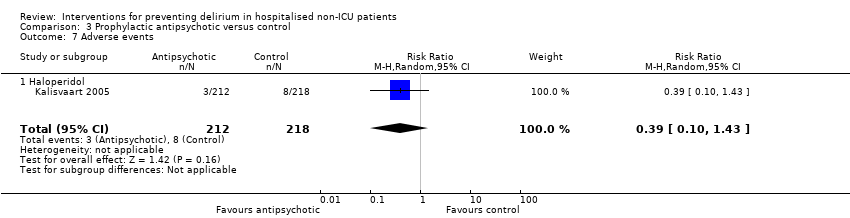
Comparison 3 Prophylactic antipsychotic versus control, Outcome 7 Adverse events.
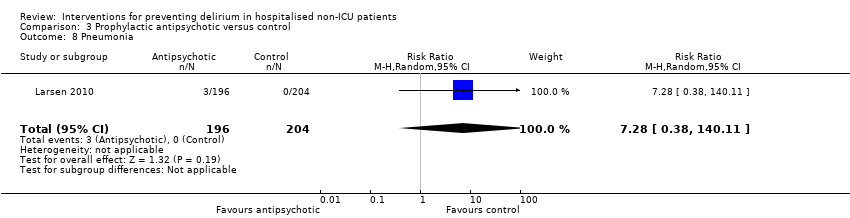
Comparison 3 Prophylactic antipsychotic versus control, Outcome 8 Pneumonia.
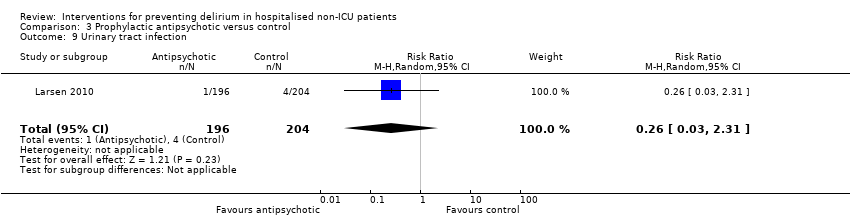
Comparison 3 Prophylactic antipsychotic versus control, Outcome 9 Urinary tract infection.
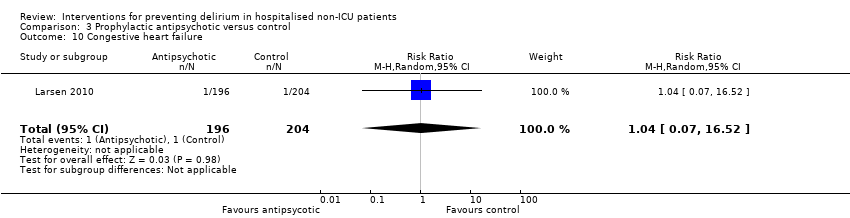
Comparison 3 Prophylactic antipsychotic versus control, Outcome 10 Congestive heart failure.
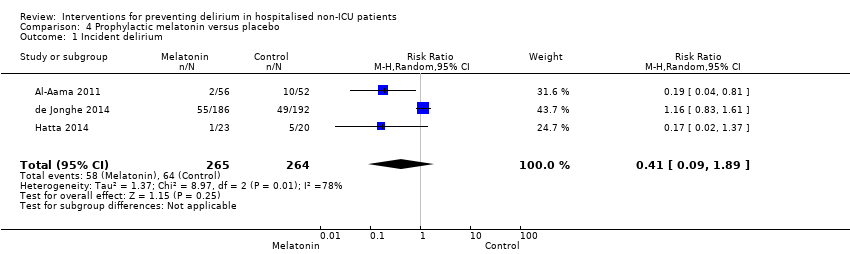
Comparison 4 Prophylactic melatonin versus placebo, Outcome 1 Incident delirium.

Comparison 4 Prophylactic melatonin versus placebo, Outcome 2 Duration of delirium.

Comparison 4 Prophylactic melatonin versus placebo, Outcome 3 Severity of delirium (binary severe vs. not severe).

Comparison 4 Prophylactic melatonin versus placebo, Outcome 4 Severity of delirium (DRS‐R‐98).

Comparison 4 Prophylactic melatonin versus placebo, Outcome 5 Length of admission.

Comparison 4 Prophylactic melatonin versus placebo, Outcome 6 Cognitive impairment.
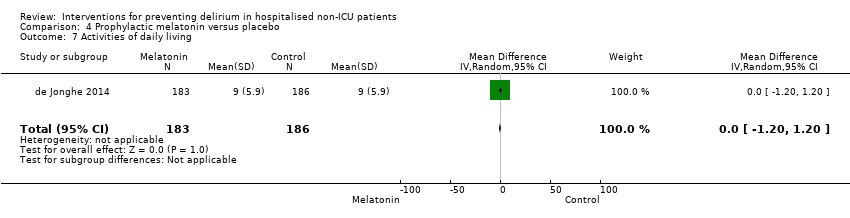
Comparison 4 Prophylactic melatonin versus placebo, Outcome 7 Activities of daily living.
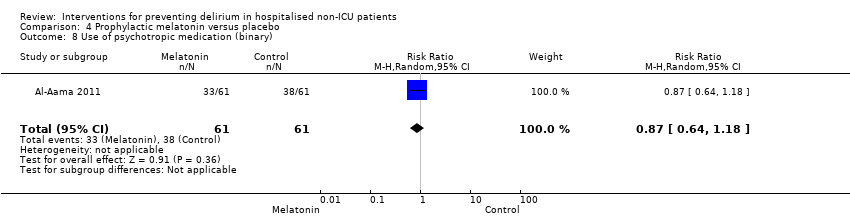
Comparison 4 Prophylactic melatonin versus placebo, Outcome 8 Use of psychotropic medication (binary).

Comparison 4 Prophylactic melatonin versus placebo, Outcome 9 Antipsychotic medication use (cumulative).

Comparison 4 Prophylactic melatonin versus placebo, Outcome 10 Benzodiazepine use (cumulative).
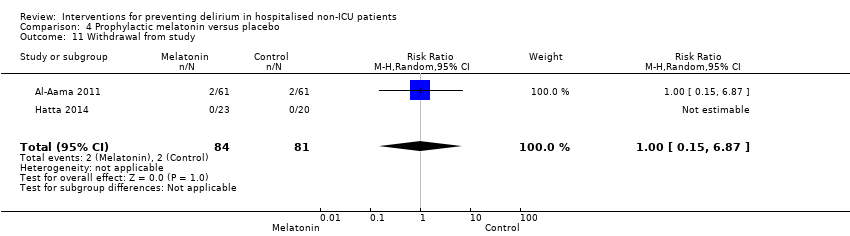
Comparison 4 Prophylactic melatonin versus placebo, Outcome 11 Withdrawal from study.
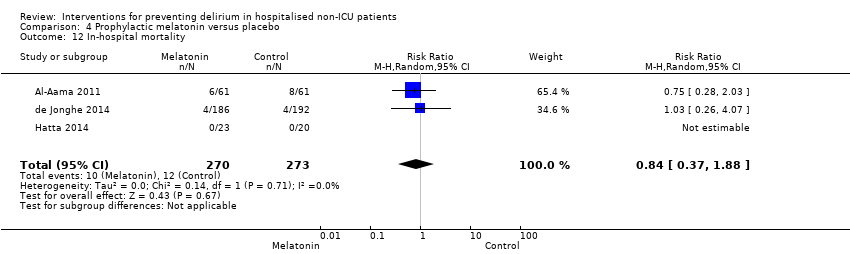
Comparison 4 Prophylactic melatonin versus placebo, Outcome 12 In‐hospital mortality.
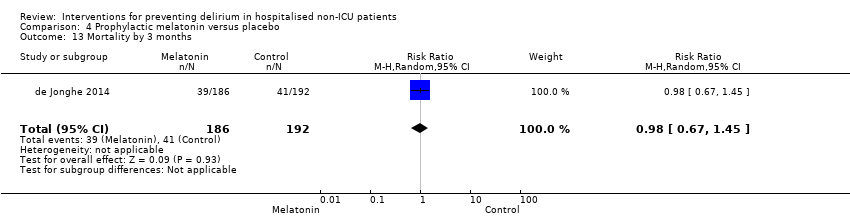
Comparison 4 Prophylactic melatonin versus placebo, Outcome 13 Mortality by 3 months.

Comparison 4 Prophylactic melatonin versus placebo, Outcome 14 Adverse events.

Comparison 5 Prophylactic citicoline versus placebo, Outcome 1 Incident delirium.

Comparison 5 Prophylactic citicoline versus placebo, Outcome 2 Cognitive status.
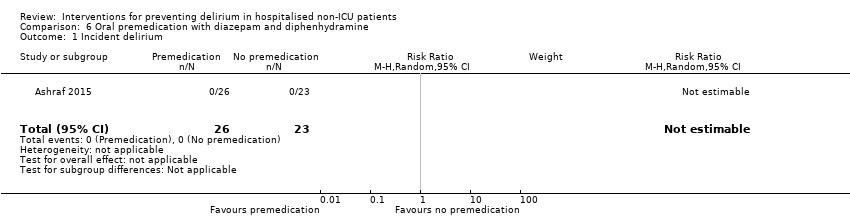
Comparison 6 Oral premedication with diazepam and diphenhydramine, Outcome 1 Incident delirium.

Comparison 7 Intravenous methylprednisolone versus placebo, Outcome 1 Incident delirium.

Comparison 7 Intravenous methylprednisolone versus placebo, Outcome 2 Length of admission.

Comparison 7 Intravenous methylprednisolone versus placebo, Outcome 3 Mortality at 30 days.

Comparison 7 Intravenous methylprednisolone versus placebo, Outcome 4 Myocardial injury.

Comparison 7 Intravenous methylprednisolone versus placebo, Outcome 5 Respiratory failure.

Comparison 7 Intravenous methylprednisolone versus placebo, Outcome 6 Infection.

Comparison 8 Gabapentinoids versus placebo, Outcome 1 Incident delirium.
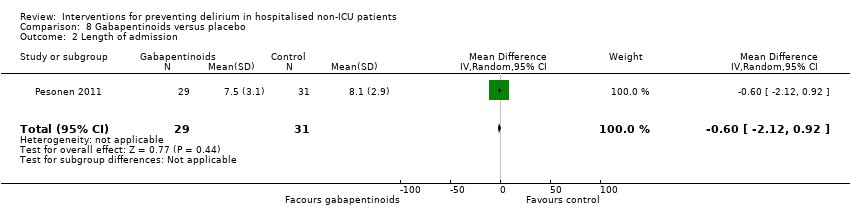
Comparison 8 Gabapentinoids versus placebo, Outcome 2 Length of admission.
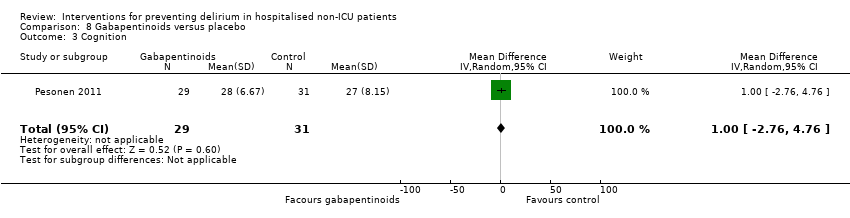
Comparison 8 Gabapentinoids versus placebo, Outcome 3 Cognition.
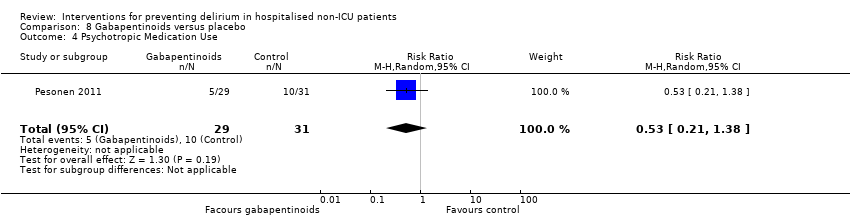
Comparison 8 Gabapentinoids versus placebo, Outcome 4 Psychotropic Medication Use.
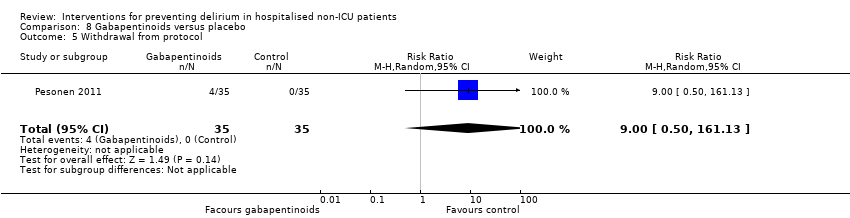
Comparison 8 Gabapentinoids versus placebo, Outcome 5 Withdrawal from protocol.

Comparison 9 Ketamine versus placebo, Outcome 1 Incident delirium.
Comparison 9 Ketamine versus placebo, Outcome 2 Withdrawal from protocol.
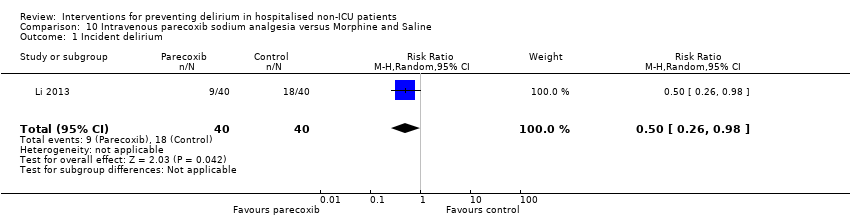
Comparison 10 Intravenous parecoxib sodium analgesia versus Morphine and Saline, Outcome 1 Incident delirium.
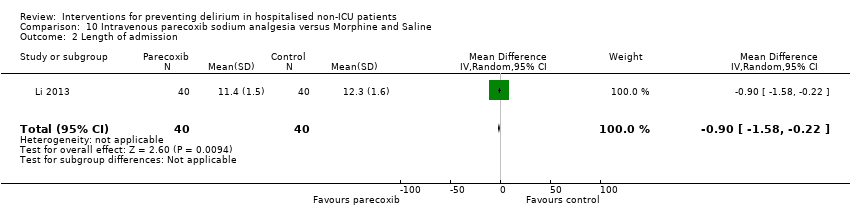
Comparison 10 Intravenous parecoxib sodium analgesia versus Morphine and Saline, Outcome 2 Length of admission.
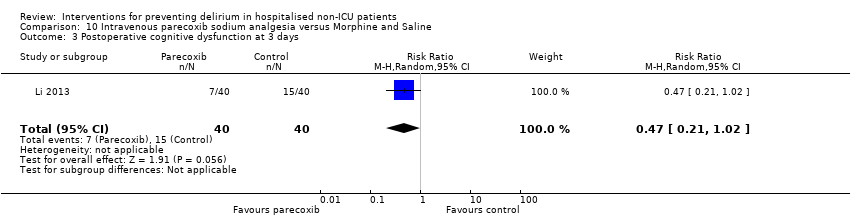
Comparison 10 Intravenous parecoxib sodium analgesia versus Morphine and Saline, Outcome 3 Postoperative cognitive dysfunction at 3 days.

Comparison 10 Intravenous parecoxib sodium analgesia versus Morphine and Saline, Outcome 4 Postoperative cognitive dysfunction at 1 week.
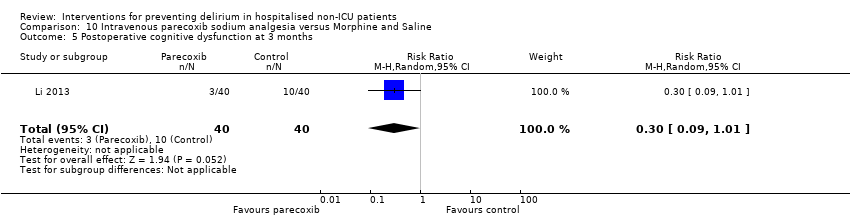
Comparison 10 Intravenous parecoxib sodium analgesia versus Morphine and Saline, Outcome 5 Postoperative cognitive dysfunction at 3 months.
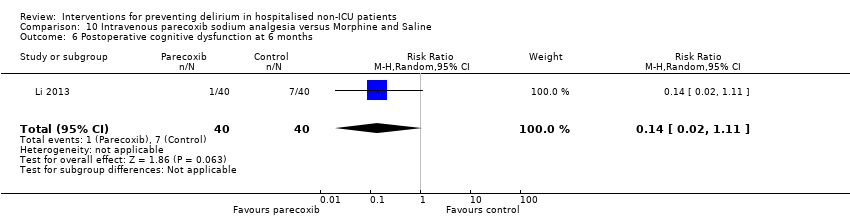
Comparison 10 Intravenous parecoxib sodium analgesia versus Morphine and Saline, Outcome 6 Postoperative cognitive dysfunction at 6 months.

Comparison 11 Intrathecal morphine and PCA morphine versus PCA morphine, Outcome 1 Incident delirium.

Comparison 11 Intrathecal morphine and PCA morphine versus PCA morphine, Outcome 2 Length of admission.
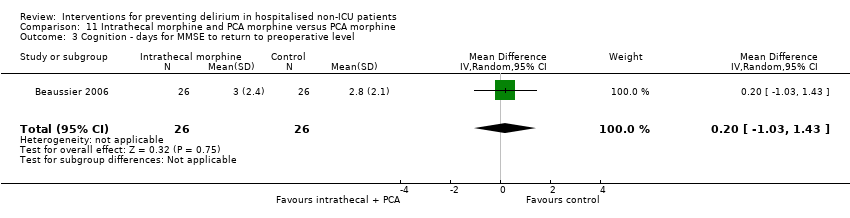
Comparison 11 Intrathecal morphine and PCA morphine versus PCA morphine, Outcome 3 Cognition ‐ days for MMSE to return to preoperative level.
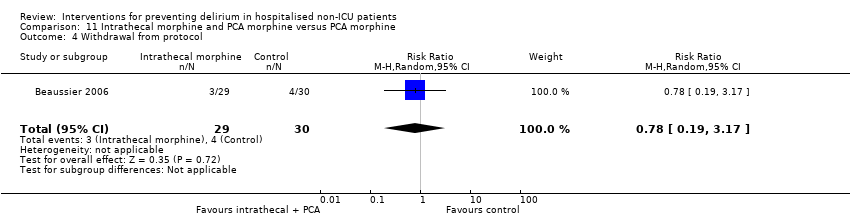
Comparison 11 Intrathecal morphine and PCA morphine versus PCA morphine, Outcome 4 Withdrawal from protocol.
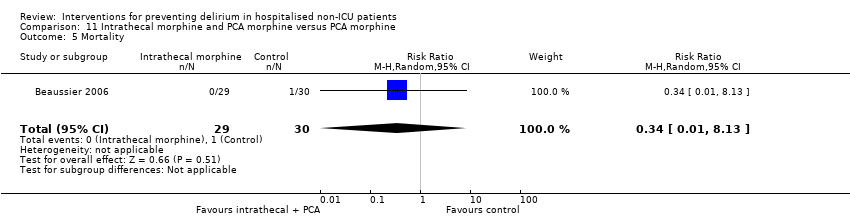
Comparison 11 Intrathecal morphine and PCA morphine versus PCA morphine, Outcome 5 Mortality.
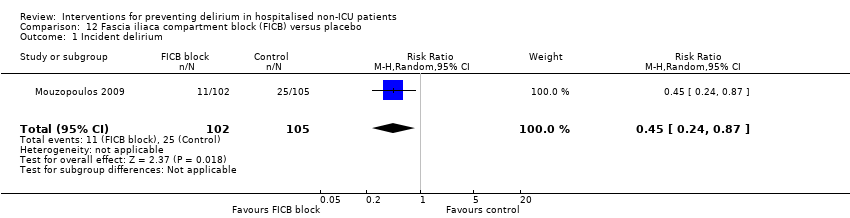
Comparison 12 Fascia iliaca compartment block (FICB) versus placebo, Outcome 1 Incident delirium.
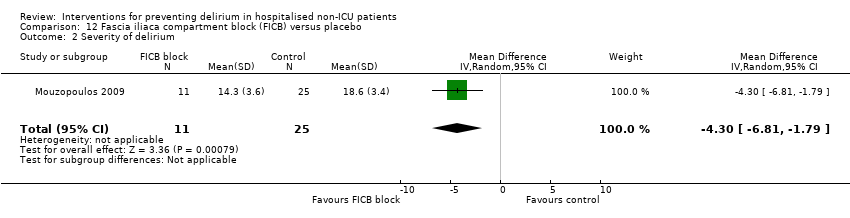
Comparison 12 Fascia iliaca compartment block (FICB) versus placebo, Outcome 2 Severity of delirium.
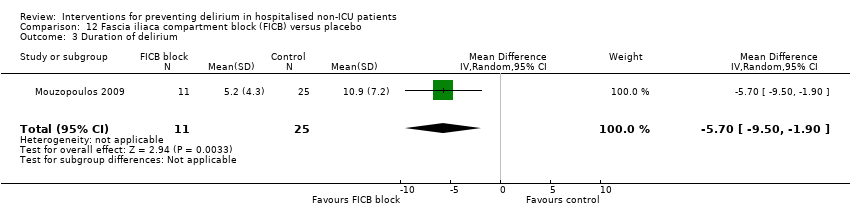
Comparison 12 Fascia iliaca compartment block (FICB) versus placebo, Outcome 3 Duration of delirium.

Comparison 12 Fascia iliaca compartment block (FICB) versus placebo, Outcome 4 Mortality.

Comparison 13 Light versus deep propofol sedation, Outcome 1 Incident delirium.
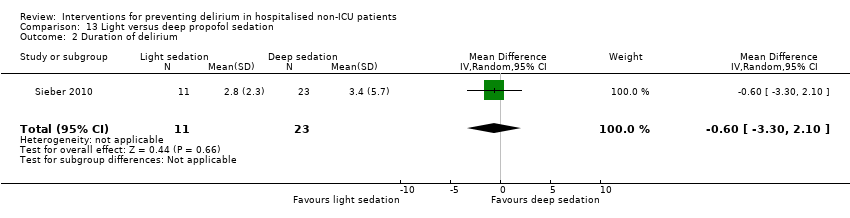
Comparison 13 Light versus deep propofol sedation, Outcome 2 Duration of delirium.
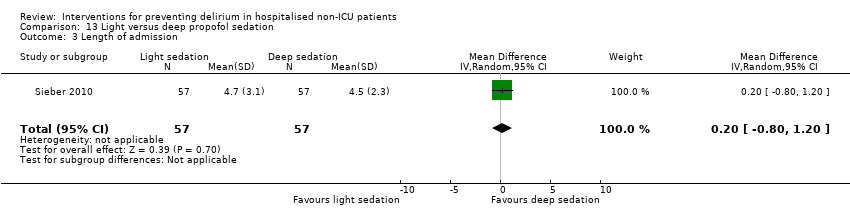
Comparison 13 Light versus deep propofol sedation, Outcome 3 Length of admission.
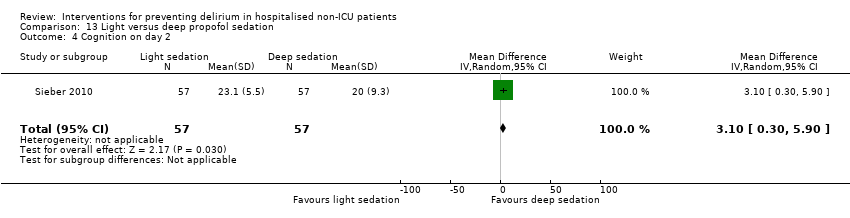
Comparison 13 Light versus deep propofol sedation, Outcome 4 Cognition on day 2.
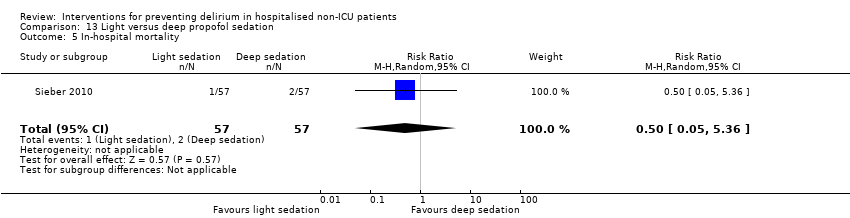
Comparison 13 Light versus deep propofol sedation, Outcome 5 In‐hospital mortality.

Comparison 13 Light versus deep propofol sedation, Outcome 6 Postoperative complications (>=1).
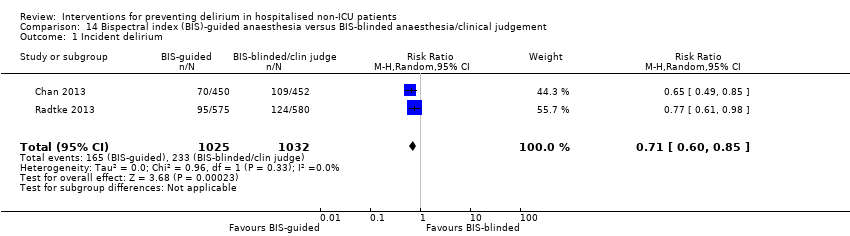
Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 1 Incident delirium.
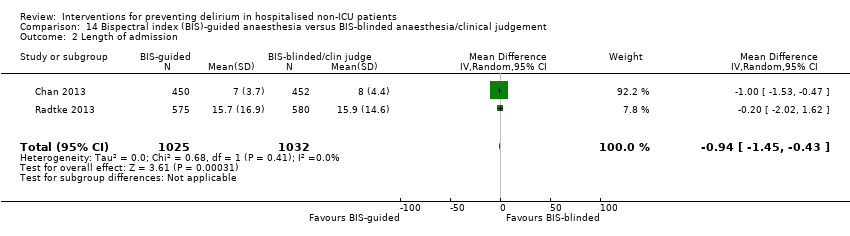
Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 2 Length of admission.
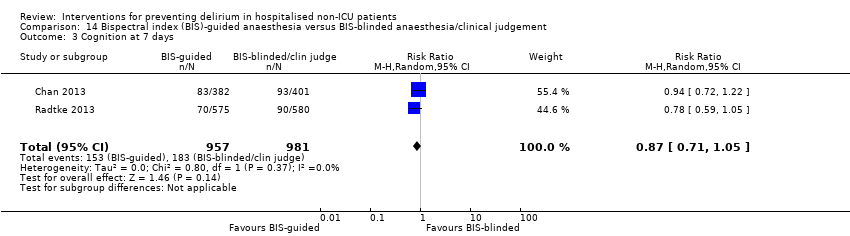
Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 3 Cognition at 7 days.

Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 4 Cognition at 3 months.

Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 5 SF‐36 mental summary score.

Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 6 Mortality at 7 days.

Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 7 Mortality at 3 months.

Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 8 Cardiac complications.

Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 9 Respiratory complications.

Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 10 Infective complications.
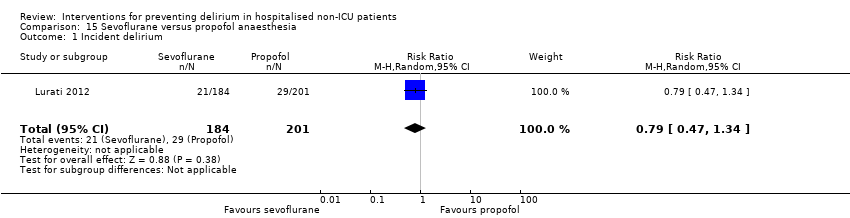
Comparison 15 Sevoflurane versus propofol anaesthesia, Outcome 1 Incident delirium.
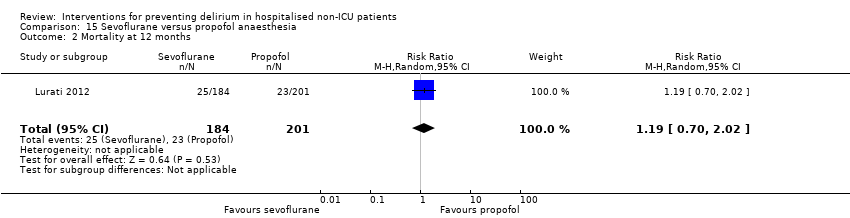
Comparison 15 Sevoflurane versus propofol anaesthesia, Outcome 2 Mortality at 12 months.

Comparison 16 Xenon versus sevoflurane anaesthesia, Outcome 1 Incident delirium.
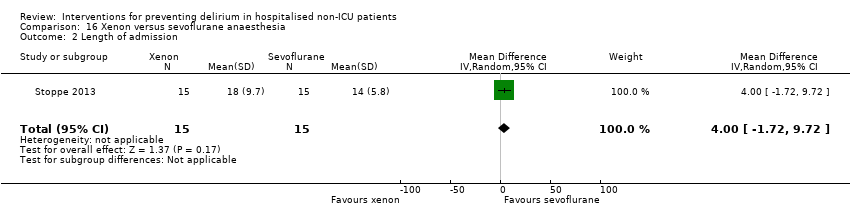
Comparison 16 Xenon versus sevoflurane anaesthesia, Outcome 2 Length of admission.

Comparison 16 Xenon versus sevoflurane anaesthesia, Outcome 3 In‐hospital mortality.

Comparison 16 Xenon versus sevoflurane anaesthesia, Outcome 4 Adverse events.

Comparison 16 Xenon versus sevoflurane anaesthesia, Outcome 5 Sepsis.

Comparison 17 Epidural anaesthesia versus general anaesthesia, Outcome 1 Incident delirium.
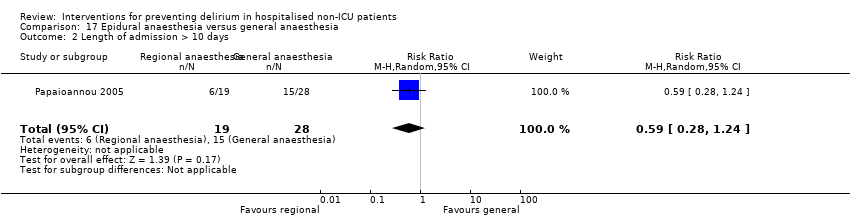
Comparison 17 Epidural anaesthesia versus general anaesthesia, Outcome 2 Length of admission > 10 days.
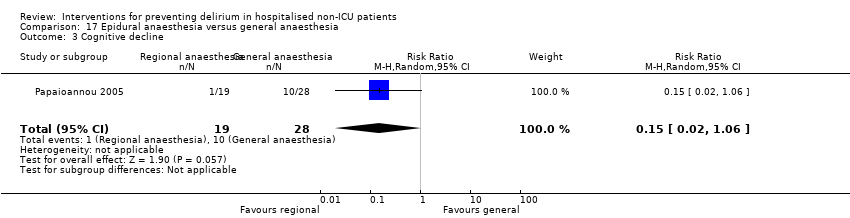
Comparison 17 Epidural anaesthesia versus general anaesthesia, Outcome 3 Cognitive decline.

Comparison 17 Epidural anaesthesia versus general anaesthesia, Outcome 4 Urinary tract infection.
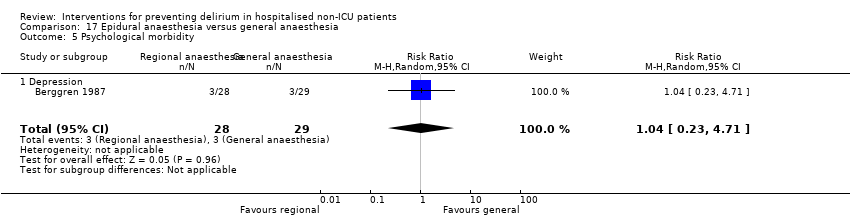
Comparison 17 Epidural anaesthesia versus general anaesthesia, Outcome 5 Psychological morbidity.

Comparison 17 Epidural anaesthesia versus general anaesthesia, Outcome 6 Postoperative complications.

Comparison 17 Epidural anaesthesia versus general anaesthesia, Outcome 7 Pressure ulcer.

Comparison 18 Liberal versus restrictive blood transfusion thresholds, Outcome 1 Incident delirium.

Comparison 18 Liberal versus restrictive blood transfusion thresholds, Outcome 2 Delirium severity.
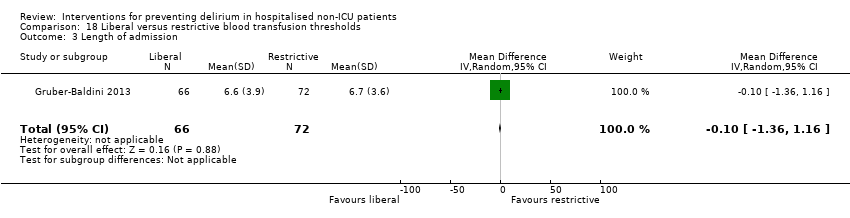
Comparison 18 Liberal versus restrictive blood transfusion thresholds, Outcome 3 Length of admission.

Comparison 18 Liberal versus restrictive blood transfusion thresholds, Outcome 4 Psychoactive medication use.
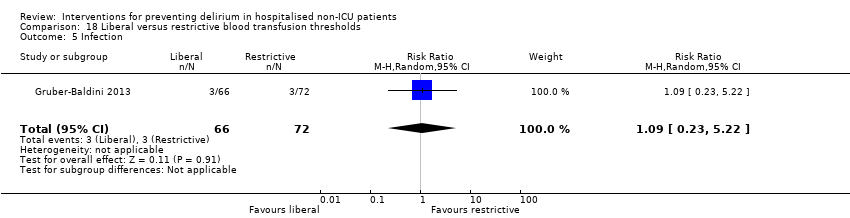
Comparison 18 Liberal versus restrictive blood transfusion thresholds, Outcome 5 Infection.
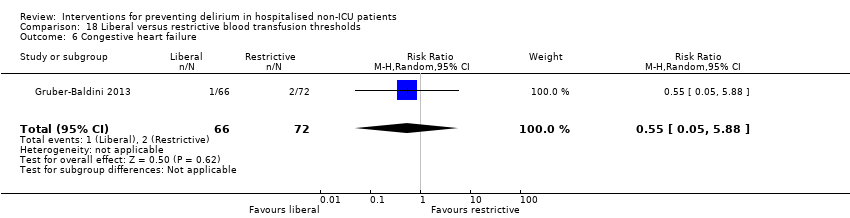
Comparison 18 Liberal versus restrictive blood transfusion thresholds, Outcome 6 Congestive heart failure.

Comparison 19 Fast‐track surgery versus usual care, Outcome 1 Incident delirium.

Comparison 19 Fast‐track surgery versus usual care, Outcome 2 Length of admission.
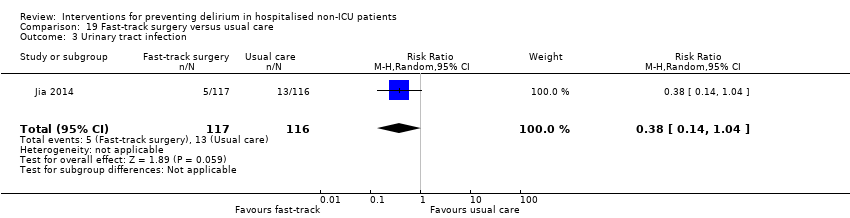
Comparison 19 Fast‐track surgery versus usual care, Outcome 3 Urinary tract infection.

Comparison 19 Fast‐track surgery versus usual care, Outcome 4 Heart failure.
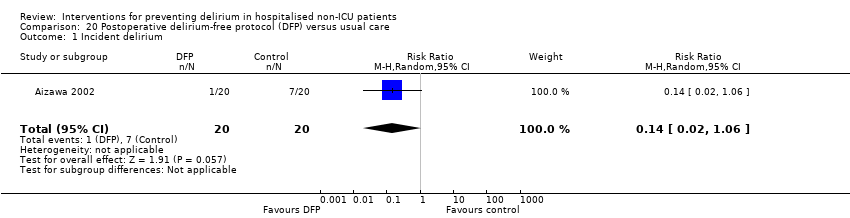
Comparison 20 Postoperative delirium‐free protocol (DFP) versus usual care, Outcome 1 Incident delirium.
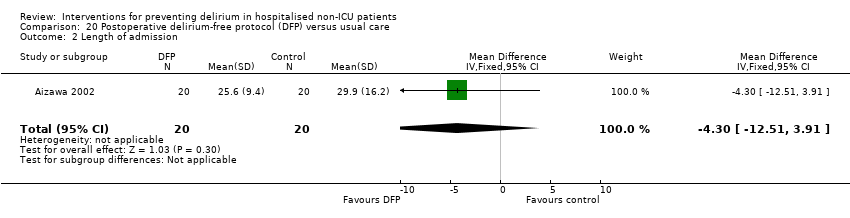
Comparison 20 Postoperative delirium‐free protocol (DFP) versus usual care, Outcome 2 Length of admission.
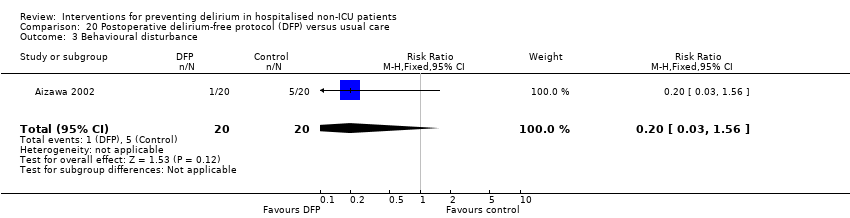
Comparison 20 Postoperative delirium‐free protocol (DFP) versus usual care, Outcome 3 Behavioural disturbance.
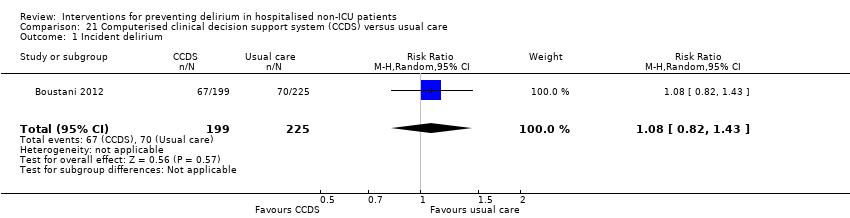
Comparison 21 Computerised clinical decision support system (CCDS) versus usual care, Outcome 1 Incident delirium.
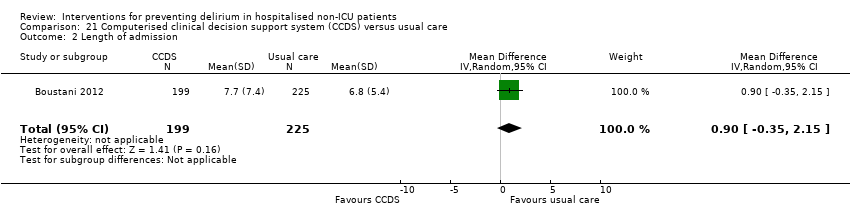
Comparison 21 Computerised clinical decision support system (CCDS) versus usual care, Outcome 2 Length of admission.
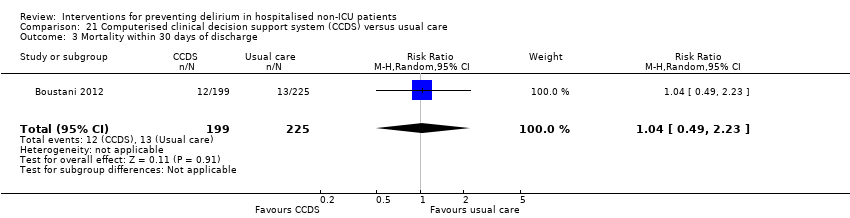
Comparison 21 Computerised clinical decision support system (CCDS) versus usual care, Outcome 3 Mortality within 30 days of discharge.
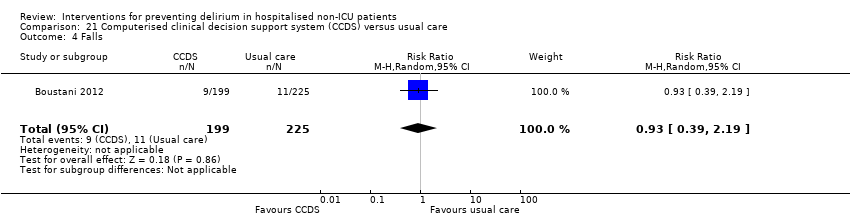
Comparison 21 Computerised clinical decision support system (CCDS) versus usual care, Outcome 4 Falls.

Comparison 21 Computerised clinical decision support system (CCDS) versus usual care, Outcome 5 Pressure ulcers.

Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 1 Incident delirium.
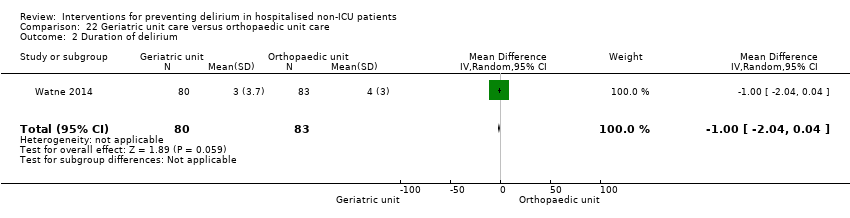
Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 2 Duration of delirium.
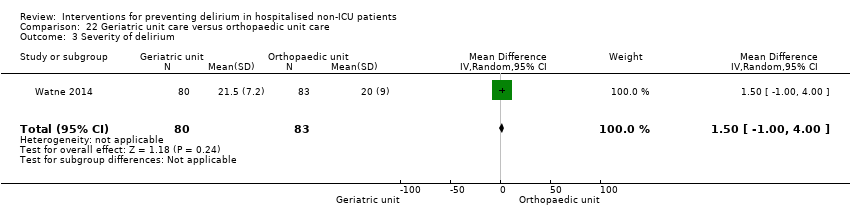
Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 3 Severity of delirium.
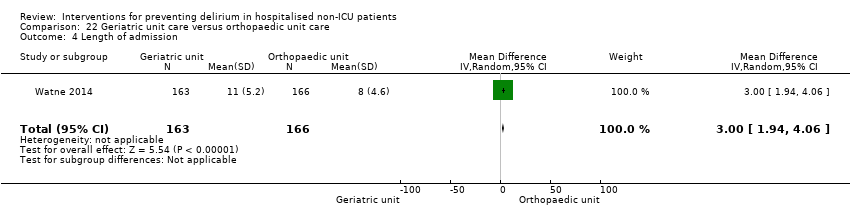
Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 4 Length of admission.

Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 5 Cognitive function (composite score) at 4 months.
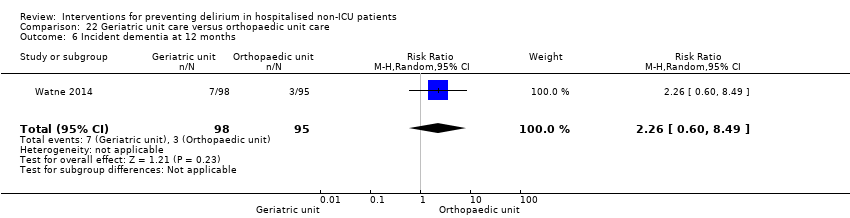
Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 6 Incident dementia at 12 months.

Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 7 ADL function at 4 months.

Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 8 Institutionalisation at 4 months.

Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 9 Institutionalisation at 12 months.

Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 10 Inpatient mortality.

Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 11 Falls.

Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 12 Pressure ulcers.
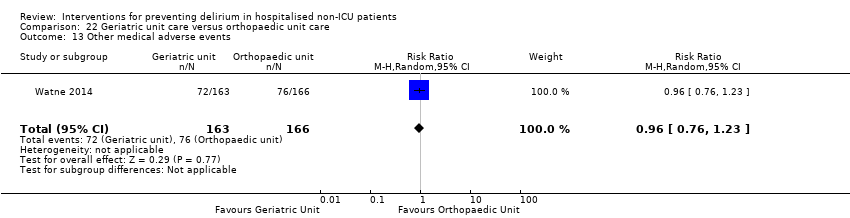
Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 13 Other medical adverse events.
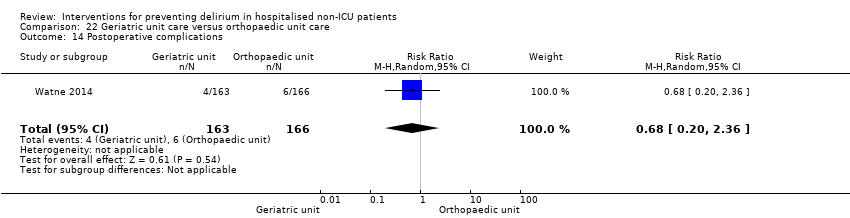
Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 14 Postoperative complications.
| Multi‐component delirium prevention intervention compared to usual care for hospitalised non‐ICU patients | ||||||
| Intervention: A multi‐component delirium prevention intervention versus usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| A multi‐component delirium prevention intervention | ||||||
| Incidence of delirium | 209 per 10002 | 144 per 1000 | RR 0.69 | 1950 | ⊕⊕⊕⊝ | |
| Duration of delirium | The mean duration of delirium in the control groups ranged from | The mean duration of delirium in the intervention groups was | 244 | ⊕⊝⊝⊝ | ||
| Severity of delirium | The standardised mean severity of delirium in the intervention groups was | 67 | ⊕⊕⊝⊝ | |||
| Length of admission | The mean length of admission in the control groups ranged from | The mean length of admission in the intervention groups was | 1920 | ⊕⊕⊕⊝ | ||
| Return to independent living | 682 per 10002 | 648 per 1000 | RR 0.95 | 1116 | ⊕⊕⊕⊝ | |
| Inpatient mortality | 81 per 10002 | 73 per 1000 | RR 0.90 | 859 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Three validated methods for delirium detection used ‐ the CAM, OBS and DRS 6 Higher baseline prevalence of dementia in the control groups of two studies compared to the intervention groups causing risk of bias 9 Downgraded because inconsistent results 10 Delirium Rating Scale‐Revised‐98 (0 to 46) and Confusion Assessment Method‐Severity (0 to 10) | ||||||
| Prophylactic cholinesterase inhibitor versus placebo for preventing delirium in hospitalised non‐ICU patients | ||||||
| Intervention: Prophylactic cholinesterase inhibitor versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Prophylactic cholinesterase inhibitors | |||||
| Incidence of delirium | 218 per 10001 | 148 per 1000 | RR 0.68 | 113 | ⊕⊝⊝⊝ | |
| Duration of delirium ‐ not measured | N/A | N/A | N/A | N/A | ||
| Severity of delirium | The mean severity of delirium in the control groups was | The mean severity of delirium in the intervention groups was | 16 | ⊕⊕⊝⊝ | ||
| Length of admission | The mean length of admission ranged across control groups from | The mean length of admission in the intervention groups was | 128 | ⊕⊕⊝⊝ | ||
| Return to independent living ‐ not measured | N/A | N/A | N/A | N/A | ||
| Inpatient mortality ‐ not measured | N/A | N/A | N/A | N/A | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The assumed risk is the risk in the control group 2 Both studies are at high risk of attrition bias and have incomplete outcome data. 3 Downgraded because inconsistent results 4 Estimate of effect includes 'no benefit' and both appreciable benefit and appreciable harm. 5 Estimate of effect includes both 'no effect' and minimally important difference, downgraded two levels due to serious imprecision 6 Risk of bias unclear in all domains in one study (abstract only available). Remaining two studies have incomplete outcome reporting and are at risk of attrition bias 7 Downgraded due to imprecision in result | ||||||
| Prophylactic antipsychotic medications for preventing delirium in hospitalised non‐ICU patients | ||||||
| Intervention: Prophylactic antipsychotic medications versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Prophylactic antipsychotic medications | |||||
| Incidence of delirium | 300 per 10001 | 165 per 1000 | RR 0.55 | 916 | ⊕⊝⊝⊝ | |
| Duration of delirium | The mean duration of delirium in the control groups ranged from 2.2 to 5.4 days | The mean duration of delirium in the intervention groups was | 178 | ⊕⊝⊝⊝ | ||
| Severity of delirium | The mean severity of delirium in the control groups ranged from 14.4 to 16.4 points | The mean severity of delirium in the intervention groups was | 178 | ⊕⊝⊝⊝ | ||
| Length of admission | The mean length of admission in the control group was 17.1 days | The mean length of admission in the intervention groups was | 68 | ⊕⊕⊝⊝ | ||
| Return to independent living ‐ not measured | N/A | N/A | N/A | N/A | ||
| Inpatient mortality ‐ not measured | N/A | N/A | N/A | N/A | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The assumed risk is the risk in the control group 2Downgraded because inconsistent results 3 Downgraded because of imprecision in results 4 Downgraded due to risk of bias 5 Downgraded two levels because very imprecise results | ||||||
| Prophylactic melatonin for preventing delirium in hospitalised non‐ICU patients | ||||||
| Intervention: Prophylactic melatonin versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Prophylactic melatonin | |||||
| Incidence of delirium | 242 per 10001 | 128 per 1000 | RR 0.53 | 529 | ⊕⊝⊝⊝ | |
| Duration of delirium | The mean duration of delirium in the control group was 2 days | The mean duration of delirium in the intervention groups was | 104 | ⊕⊕⊕⊝ | ||
| Severity of delirium (binary severe vs. not severe) | 531 per 1000 | 457 per 1000 | RR 0.86 | 104 | ⊕⊕⊕⊝ | |
| Severity of delirium DRS‐R‐98 score | The mean severity of delirium in the control group was 6.3 points | The mean severity of delirium in the intervention group was 4.1 points lower (19.47 points lower to 11.27 points higher) | 6 (1 study) | ⊕⊕⊝⊝ | ||
| Length of admission | The mean length of admission in the control groups ranged from 11 to 18.5 days | The mean length of admission in the intervention groups was | 500 | ⊕⊕⊕⊝ | ||
| Return to independent living ‐ not measured | N/A | N/A | N/A | N/A | ||
| In‐hospital mortality | 47 per 10001 | 39 per 1000 | RR 0.84 | 543 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The assumed risk is the risk in the control group 2 Downgraded because inconsistent results 3 Downgraded because imprecise results 4 Downgraded due to risk of bias 5 Downgraded because imprecise results and very small number of events | ||||||
| Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement for preventing delirium in hospitalised non‐ICU patients | ||||||
| Intervention: Bispectral index (BIS)‐guided anaesthesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| BIS‐blinded/clinical judgement | BIS‐guided | |||||
| Incidence of delirium CAM, DSM‐IV | 226 per 10001 | 160 per 1000 | RR 0.71 | 2057 | ⊕⊕⊕⊝ | |
| Duration of delirium ‐ not measured | N/A | N/A | N/A | N/A | ||
| Severity of delirium ‐ not measured | N/A | N/A | N/A | N/A | ||
| Length of admission Days | The mean length of admission in the control groups ranged from 7 to 15.7 days | The mean length of admission in the intervention group was 0.94 days shorter (0.43 days shorter to 1.45 days shorter) | ‐ | 2057 | ⊕⊕⊕⊝ | |
| Return to independent living ‐ not measured | N/A | N/A | N/A | N/A | ||
| In‐hospital mortality ‐ not measured | N/A | N/A | N/A | N/A | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The assumed risk is the risk in the control group (BIS‐blinded/clinical judgement) | ||||||
| Study | Intervention Components | |||||||||||||||||||
| Individualised care | Checklists/ protocols | Education/ training1 | Re‐orientation | Attention to sensory deprivation | Familiar objects | Cognitive stimulation | Nutrition/ hydration | Identification of infection | Mobilisation | Sleep hygiene | MDTcare2 | CGA3 | Oxygenation | Electrolytes | Pain control | Medication review | Mood4 | Bowel/ bladder care | Postoperative complications | |
| Abizanda 2011 | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||
| Bonaventura 2007 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||||||
| Jeffs 2013 | ✔ | ✔ | ||||||||||||||||||
| Martinez 2012 | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||
| Hempenius 2013 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||
| Lundstrom 2006 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||
| Marcantonio 2001 | ✔ | |||||||||||||||||||
| 1Education/training: structured education/training of staff or carers; 2MDT Multidisciplinary Team; 3CGA Comprehensive Geriatric Assessment; 4Mood: assessment for depression/anxiety | ||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 7 | 1950 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.59, 0.81] |
| 1.1 Medical patients | 4 | 1365 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.43, 0.92] |
| 1.2 Surgical patients | 3 | 585 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.59, 0.85] |
| 2 Incidence of delirium in patients with dementia Show forest plot | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.59, 1.36] |
| 2.1 Surgical patients | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.59, 1.36] |
| 3 Duration of delirium Show forest plot | 4 | 244 | Mean Difference (IV, Random, 95% CI) | ‐1.16 [‐2.96, 0.64] |
| 3.1 Medical patients | 2 | 63 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐2.43, 1.13] |
| 3.2 Surgical patients | 2 | 181 | Mean Difference (IV, Random, 95% CI) | ‐2.40 [‐7.27, 2.46] |
| 4 Severity of delirium Show forest plot | 2 | 67 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.04 [‐1.65, ‐0.43] |
| 4.1 Medical patients | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.46, ‐0.08] |
| 4.2 Surgical patients | 1 | 31 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.39 [‐2.20, ‐0.58] |
| 5 Length of admission Show forest plot | 6 | 1920 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.48, 0.51] |
| 5.1 Medical patients | 3 | 1335 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.44, 0.52] |
| 5.2 Surgical patients | 3 | 585 | Mean Difference (IV, Random, 95% CI) | ‐1.24 [‐4.74, 2.25] |
| 6 Cognition Show forest plot | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 9.10 [7.20, 11.00] |
| 6.1 Medical patients | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 9.10 [7.20, 11.00] |
| 7 Improvement in Activities of Daily Living Show forest plot | 1 | 341 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.91, 1.47] |
| 7.1 Medical patients | 1 | 341 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.91, 1.47] |
| 8 Return to independent living Show forest plot | 4 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.85, 1.06] |
| 8.1 Medical patients | 1 | 648 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| 8.2 Surgical patients | 3 | 468 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.75, 1.19] |
| 9 Depression Show forest plot | 1 | 149 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐0.44, 1.84] |
| 9.1 Surgical patients | 1 | 149 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐0.44, 1.84] |
| 10 Withdrawal from protocol Show forest plot | 1 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Surgical patients | 1 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Falls Show forest plot | 3 | 746 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.16, 2.01] |
| 11.1 Medical patients | 1 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 2.03] |
| 11.2 Surgical patients | 2 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.18, 3.46] |
| 12 Pressure ulcers Show forest plot | 2 | 457 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.26, 0.89] |
| 12.1 Surgical patients | 2 | 457 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.26, 0.89] |
| 13 Inpatient mortality Show forest plot | 3 | 859 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.56, 1.43] |
| 13.1 Medical patients | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.34, 1.18] |
| 13.2 Surgical patients | 2 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.69, 3.05] |
| 14 12 month mortality Show forest plot | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.46, 1.56] |
| 14.1 Surgical patients | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.46, 1.56] |
| 15 Cardiovascular complication Show forest plot | 1 | 260 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.78, 1.65] |
| 16 Urinary tract infection Show forest plot | 1 | 260 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.45, 3.20] |
| 17 Mental health worsened Show forest plot | 1 | 246 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.64, 1.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 2 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.17, 2.62] |
| 1.1 Donepezil | 2 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.17, 2.62] |
| 2 Duration of delirium Show forest plot | 1 | 15 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Donepezil | 1 | 15 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Severity of delirium Show forest plot | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐4.17, 3.57] |
| 3.1 Donepezil | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐4.17, 3.57] |
| 4 Length of admission Show forest plot | 3 | 128 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐1.54, 0.86] |
| 4.1 Donepezil | 3 | 128 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐1.54, 0.86] |
| 5 Cognition Show forest plot | 1 | 15 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐4.45, 1.65] |
| 5.1 Donepezil | 1 | 15 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐4.45, 1.65] |
| 6 Withdrawal from protocol Show forest plot | 2 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.49, 1.87] |
| 6.1 Donepezil | 2 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.49, 1.87] |
| 7 Adverse events (continuous) Show forest plot | 1 | 33 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.26, 0.52] |
| 7.1 Donepezil | 1 | 33 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.26, 0.52] |
| 8 Adverse events (binary) Show forest plot | 1 | 16 | Risk Ratio (M‐H, Random, 95% CI) | 6.25 [0.35, 112.52] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 3 | 916 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.33, 1.59] |
| 1.1 Haloperidol | 2 | 516 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.69, 1.60] |
| 1.2 Olanzapine | 1 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.24, 0.52] |
| 2 Duration of delirium Show forest plot | 2 | 178 | Mean Difference (IV, Random, 95% CI) | ‐2.74 [‐9.59, 4.11] |
| 2.1 Haloperidol | 1 | 68 | Mean Difference (IV, Random, 95% CI) | ‐6.4 [‐9.38, ‐3.42] |
| 2.2 Olanzapine | 1 | 110 | Mean Difference (IV, Random, 95% CI) | 0.60 [0.10, 1.10] |
| 3 Severity of delirium Show forest plot | 2 | 178 | Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐6.80, 4.76] |
| 3.1 Haloperidol | 1 | 68 | Mean Difference (IV, Random, 95% CI) | ‐2.00 [‐5.86, ‐2.14] |
| 3.2 Olanzapine | 1 | 110 | Mean Difference (IV, Random, 95% CI) | 1.90 [0.41, 3.39] |
| 4 Length of admission Show forest plot | 1 | 68 | Mean Difference (IV, Random, 95% CI) | ‐5.5 [‐12.17, 1.17] |
| 4.1 Haloperidol | 1 | 68 | Mean Difference (IV, Random, 95% CI) | ‐5.5 [‐12.17, 1.17] |
| 5 Cognition Show forest plot | 1 | 110 | Mean Difference (IV, Random, 95% CI) | ‐4.90 [‐7.42, ‐2.38] |
| 6 Withdrawal from protocol Show forest plot | 2 | 925 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.68, 1.24] |
| 6.1 Haloperidol | 1 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.43, 1.26] |
| 6.2 Olanzapine | 1 | 495 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.71, 1.46] |
| 7 Adverse events Show forest plot | 1 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.10, 1.43] |
| 7.1 Haloperidol | 1 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.10, 1.43] |
| 8 Pneumonia Show forest plot | 1 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 7.28 [0.38, 140.11] |
| 9 Urinary tract infection Show forest plot | 1 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.03, 2.31] |
| 10 Congestive heart failure Show forest plot | 1 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.07, 16.52] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 3 | 529 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.09, 1.89] |
| 2 Duration of delirium Show forest plot | 1 | 104 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.57, 0.57] |
| 3 Severity of delirium (binary severe vs. not severe) Show forest plot | 1 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.58, 1.27] |
| 4 Severity of delirium (DRS‐R‐98) Show forest plot | 1 | 6 | Mean Difference (IV, Random, 95% CI) | ‐4.10 [‐19.47, 11.27] |
| 5 Length of admission Show forest plot | 2 | 500 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐1.20, 1.39] |
| 6 Cognitive impairment Show forest plot | 1 | 378 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.70, 1.04] |
| 7 Activities of daily living Show forest plot | 1 | 369 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐1.20, 1.20] |
| 8 Use of psychotropic medication (binary) Show forest plot | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.64, 1.18] |
| 9 Antipsychotic medication use (cumulative) Show forest plot | 1 | 378 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐1.79, ‐0.21] |
| 10 Benzodiazepine use (cumulative) Show forest plot | 1 | 378 | Mean Difference (IV, Random, 95% CI) | ‐11.60 [‐24.34, 1.14] |
| 11 Withdrawal from study Show forest plot | 2 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.15, 6.87] |
| 12 In‐hospital mortality Show forest plot | 3 | 543 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.37, 1.88] |
| 13 Mortality by 3 months Show forest plot | 1 | 378 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.67, 1.45] |
| 14 Adverse events Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Incident delirium day 1 post surgery | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.22, 2.06] |
| 2 Cognitive status Show forest plot | 1 | 81 | Mean Difference (IV, Random, 95% CI) | ‐1.47 [‐3.85, 0.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 7507 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.87, 1.19] |
| 2 Length of admission Show forest plot | 1 | 7507 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.20, 0.20] |
| 3 Mortality at 30 days Show forest plot | 1 | 7507 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.70, 1.07] |
| 4 Myocardial injury Show forest plot | 1 | 7507 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.07, 1.38] |
| 5 Respiratory failure Show forest plot | 1 | 7507 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.80, 1.05] |
| 6 Infection Show forest plot | 1 | 7507 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.84, 1.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 21 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.01, 1.90] |
| 2 Length of admission Show forest plot | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐2.12, 0.92] |
| 3 Cognition Show forest plot | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐2.76, 4.76] |
| 4 Psychotropic Medication Use Show forest plot | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.21, 1.38] |
| 5 Withdrawal from protocol Show forest plot | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 9.00 [0.50, 161.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.21, 19.23] |
| 2 Withdrawal from protocol Show forest plot | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 14.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.26, 0.98] |
| 2 Length of admission Show forest plot | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.58, ‐0.22] |
| 3 Postoperative cognitive dysfunction at 3 days Show forest plot | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.21, 1.02] |
| 4 Postoperative cognitive dysfunction at 1 week Show forest plot | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.15, 0.98] |
| 5 Postoperative cognitive dysfunction at 3 months Show forest plot | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.09, 1.01] |
| 6 Postoperative cognitive dysfunction at 6 months Show forest plot | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.02, 1.11] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.9 [0.44, 1.85] |
| 2 Length of admission Show forest plot | 1 | 52 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.51, 0.51] |
| 3 Cognition ‐ days for MMSE to return to preoperative level Show forest plot | 1 | 52 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐1.03, 1.43] |
| 4 Withdrawal from protocol Show forest plot | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.19, 3.17] |
| 5 Mortality Show forest plot | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.24, 0.87] |
| 2 Severity of delirium Show forest plot | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐4.30 [‐6.81, ‐1.79] |
| 3 Duration of delirium Show forest plot | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐5.7 [‐9.50, ‐1.90] |
| 4 Mortality Show forest plot | 1 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.05, 5.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.26, 0.89] |
| 2 Duration of delirium Show forest plot | 1 | 34 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐3.30, 2.10] |
| 3 Length of admission Show forest plot | 1 | 114 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.80, 1.20] |
| 4 Cognition on day 2 Show forest plot | 1 | 114 | Mean Difference (IV, Random, 95% CI) | 3.10 [0.30, 5.90] |
| 5 In‐hospital mortality Show forest plot | 1 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.05, 5.36] |
| 6 Postoperative complications (>=1) Show forest plot | 1 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.60, 1.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 2 | 2057 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.60, 0.85] |
| 2 Length of admission Show forest plot | 2 | 2057 | Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐1.45, ‐0.43] |
| 3 Cognition at 7 days Show forest plot | 2 | 1938 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.71, 1.05] |
| 4 Cognition at 3 months Show forest plot | 2 | 1990 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.53, 0.97] |
| 5 SF‐36 mental summary score Show forest plot | 1 | 902 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐3.40, ‐0.40] |
| 6 Mortality at 7 days Show forest plot | 1 | 921 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.42, 5.25] |
| 7 Mortality at 3 months Show forest plot | 2 | 1938 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.77, 1.59] |
| 8 Cardiac complications Show forest plot | 1 | 902 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.52, 1.39] |
| 9 Respiratory complications Show forest plot | 1 | 902 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.59, 1.07] |
| 10 Infective complications Show forest plot | 1 | 902 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.55, 0.95] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.47, 1.34] |
| 2 Mortality at 12 months Show forest plot | 1 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.70, 2.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.20, 2.79] |
| 2 Length of admission Show forest plot | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 4.0 [‐1.72, 9.72] |
| 3 In‐hospital mortality Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Adverse events Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.34, 1.64] |
| 5 Sepsis Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.5 [0.29, 7.73] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 2 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.69, 2.03] |
| 2 Length of admission > 10 days Show forest plot | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.28, 1.24] |
| 3 Cognitive decline Show forest plot | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.02, 1.06] |
| 4 Urinary tract infection Show forest plot | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.57, 3.09] |
| 5 Psychological morbidity Show forest plot | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.23, 4.71] |
| 5.1 Depression | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.23, 4.71] |
| 6 Postoperative complications Show forest plot | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.35, 2.39] |
| 7 Pressure ulcer Show forest plot | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.16, 2.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.45, 1.27] |
| 2 Delirium severity Show forest plot | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐2.99, 2.79] |
| 3 Length of admission Show forest plot | 1 | 138 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐1.36, 1.16] |
| 4 Psychoactive medication use Show forest plot | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.87, 1.12] |
| 5 Infection Show forest plot | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.23, 5.22] |
| 6 Congestive heart failure Show forest plot | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.05, 5.88] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.09, 0.77] |
| 2 Length of admission Show forest plot | 1 | 233 | Mean Difference (IV, Random, 95% CI) | ‐4.20 [‐4.60, ‐3.80] |
| 3 Urinary tract infection Show forest plot | 1 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.14, 1.04] |
| 4 Heart failure Show forest plot | 1 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.10, 0.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.02, 1.06] |
| 2 Length of admission Show forest plot | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐4.30 [‐12.51, 3.91] |
| 3 Behavioural disturbance Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.03, 1.56] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.82, 1.43] |
| 2 Length of admission Show forest plot | 1 | 424 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐0.35, 2.15] |
| 3 Mortality within 30 days of discharge Show forest plot | 1 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.49, 2.23] |
| 4 Falls Show forest plot | 1 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.39, 2.19] |
| 5 Pressure ulcers Show forest plot | 1 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.64, 1.84] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.79, 1.22] |
| 2 Duration of delirium Show forest plot | 1 | 163 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐2.04, 0.04] |
| 3 Severity of delirium Show forest plot | 1 | 163 | Mean Difference (IV, Random, 95% CI) | 1.5 [1.00, 4.00] |
| 4 Length of admission Show forest plot | 1 | 329 | Mean Difference (IV, Random, 95% CI) | 3.0 [1.94, 4.06] |
| 5 Cognitive function (composite score) at 4 months Show forest plot | 1 | 228 | Mean Difference (IV, Random, 95% CI) | 1.80 [‐5.92, 9.52] |
| 6 Incident dementia at 12 months Show forest plot | 1 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 2.26 [0.60, 8.49] |
| 7 ADL function at 4 months Show forest plot | 1 | 239 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐0.70, 2.70] |
| 8 Institutionalisation at 4 months Show forest plot | 1 | 242 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.58, 1.91] |
| 9 Institutionalisation at 12 months Show forest plot | 1 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.47, 1.59] |
| 10 Inpatient mortality Show forest plot | 1 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.21, 1.47] |
| 11 Falls Show forest plot | 1 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.61, 2.77] |
| 12 Pressure ulcers Show forest plot | 1 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.10, 1.41] |
| 13 Other medical adverse events Show forest plot | 1 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.76, 1.23] |
| 14 Postoperative complications Show forest plot | 1 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.20, 2.36] |

