Infusión continua de insulina subcutánea versus inyecciones diarias múltiples de insulina para las mujeres embarazadas con diabetes
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | "Randomly divided". | |
| Participants | 10 women recruited from 8 weeks to 'term'. | |
| Interventions | CSII versus MDI. | |
| Outcomes | Mean gestational age at birth, rate of instrumental delivery. | |
| Notes | Single centre trial in Italy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding of assessors not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Pre‐specified outcomes not reported. |
| Selective reporting (reporting bias) | Unclear risk | Not reported. |
| Other bias | Unclear risk | Small trial group, limited reporting. |
| Methods | "Randomly assigned" no other information reported. | |
| Participants | 15 women with type 1 diabetes (13 on conventional insulin therapy, 2 on continuous insulin therapy) and 14 women with type 2 diabetes (4 were on oral hypoglycaemics, 10 diet‐controlled). Recruitment occurred in the first trimester, 2 women allocated to CSII had already been using a CSII pump pre‐conceptually. | |
| Interventions | CSII versus MDI. Participants were hospitalised initially in order to achieve optimal glycaemic control, diet was prescribed according to individual needs. A Microject MC 20 portable syringe pump was used with porcine insulin (Actrapid MC) 40 U/ml, adjustments were made to the dosage in order to obtain strict glycaemic control (fasting BG < 80 + 10 mg/dl, postprandial BG < 120 mg/dl). Participants randomised to the MDI dose were given Actrapid MC split into 4 boluses. | |
| Outcomes | Maternal and neonatal mortality, fetal anomaly and hypoglycaemia, mean 24‐hour BG, mean HbA1c, mean gestational age at birth, mean birthweight, rate of instrumental delivery. | |
| Notes | Single‐centre trial in Italy. Participants and their neonates were followed up at delivery and for the first 2 days postnatally. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding of assessors not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Pre‐specified outcomes not reported. |
| Selective reporting (reporting bias) | Unclear risk | Not reported. |
| Other bias | Unclear risk | Small trial group, limited reporting of methods. |
| Methods | "Randomly assigned'". | |
| Participants | 71 women with type 1 diabetes. | |
| Interventions | MDI versus CSII. | |
| Outcomes | Daily glucose levels, 24 hour glycaemic profiles, infant abdominal fat deposition. | |
| Notes | Raw and mean data not reported, therefore could not be included in the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding of assessors not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Pre‐specified outcomes not reported. |
| Selective reporting (reporting bias) | Unclear risk | Not reported. |
| Other bias | Unclear risk | Single centre trial in Italy, small trial group, limited reporting of methods. |
| Methods | "Randomly allocated", no other information reported . | |
| Participants | 31 women with type 1 insulin‐dependent diabetes included undergoing 32 pregnancies, 1 woman included twice for separate pregnancies. 4 women were recruited in the pre‐conception period, 28 women recruited during their first trimester. The women were described as highly motivated and referred to their centre for intensive therapy. | |
| Interventions | Allocated either CSII or MDI. Microject MC 20 and Daedi B.V. portable battery‐powered syringe infusion pumps, participants receiving MDI had 4 daily insulin injections (regular insulin at each meal and intermediate acting insulin at night, type of insulin used was not stated). | |
| Outcomes | Maternal and neonatal mortality, fetal anomaly and hypoglycaemia, mean 24‐hour BG, mean HbA1c, mean gestational age at birth, mean birthweight, instrumental delivery rate. | |
| Notes | No losses to follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding of assessors not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Pre‐specified outcomes not reported. |
| Selective reporting (reporting bias) | Unclear risk | No information provided. |
| Other bias | High risk | Small trial group, described as "highly motivated". Single‐centre trial in Italy, limited reporting of methods. |
| Methods | "Randomly assigned". | |
| Participants | 12 women with type 1 diabetes. | |
| Interventions | MDI versus CSII. | |
| Outcomes | Mean blood glucose, pregnancy weight gain. | |
| Notes | Several data outcomes described as comparable rather than reported as statistics. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding of assessors not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Pre‐specified outcomes not reported. |
| Selective reporting (reporting bias) | Unclear risk | Not reported. |
| Other bias | Unclear risk | Not reported. |
BG: blood glucose
CSII: continuous subcutaneous insulin infusion
HBA1c: glycated haemoglobin
MDI: multiple daily injection
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Exclusion of MDI group participants from analysis if normoglycaemia not achieved (number not reported). | |
| Wrong intervention: Conventional versus intensive therapy, rather than CSII versus MDI. Women using CSII and MDI therapy included in the intensive therapy group. | |
| 21 women randomised antenatally (1 randomised twice for separate pregnancies), gestation at randomisation not stated, therefore length of treatment may be inconsistent. 7 women randomised in the pre‐conceptual period, conceiving between 2 weeks and 1 year. The differences in time to conceive and treatment length is likely to lead to inconsistency of treatment effects. | |
| Probably not a randomised study: 41 women were "divided" into 2 groups. Number of women included in the study and by study group inconsistently reported (41 women included and 21 in each of the 2 groups). | |
| 30 women randomised, 9 declined CSII, so did not receive the allocated intervention and were reported as a separate group, thus potentially confounding the results. | |
| No methods or data provided by trial authors, therefore assessment of the trial is not possible. |
CSII: continuous subcutaneous insulin infusion
MDI: multiple daily injection
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rate of caesarean section Show forest plot | 3 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.66, 1.77] |
| Analysis 1.1  Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 1 Rate of caesarean section. | ||||
| 2 Perinatal mortality Show forest plot | 3 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [0.38, 14.32] |
| Analysis 1.2  Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 2 Perinatal mortality. | ||||
| 3 Fetal anomaly Show forest plot | 2 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.07, 15.54] |
| Analysis 1.3  Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 3 Fetal anomaly. | ||||
| 4 Maternal hypoglycaemia Show forest plot | 2 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.35, 25.87] |
| Analysis 1.4  Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 4 Maternal hypoglycaemia. | ||||
| 5 Maternal hyperglycaemia Show forest plot | 2 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.39, 125.44] |
| Analysis 1.5  Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 5 Maternal hyperglycaemia. | ||||
| 6 Maternal 24 hour mean blood glucose (mg/dl) first trimester Show forest plot | 3 | 67 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐7.19, 7.43] |
| Analysis 1.6  Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 6 Maternal 24 hour mean blood glucose (mg/dl) first trimester. | ||||
| 7 Macrosomia Show forest plot | 2 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.2 [0.14, 72.62] |
| Analysis 1.7  Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 7 Macrosomia. | ||||
| 8 Gestation at delivery Show forest plot | 3 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐1.18 [‐2.92, 0.57] |
| Analysis 1.8  Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 8 Gestation at delivery. | ||||
| 9 Neonatal hypoglycaemia Show forest plot | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.64] |
| Analysis 1.9  Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 9 Neonatal hypoglycaemia. | ||||
| 10 Small‐for‐gestational age Show forest plot | 2 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.10, 18.71] |
| Analysis 1.10  Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 10 Small‐for‐gestational age. | ||||
| 11 Mean HbA1c first trimester Show forest plot | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐2.13, 1.73] |
| Analysis 1.11  Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 11 Mean HbA1c first trimester. | ||||
| 12 Mean HbA1c second trimester Show forest plot | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐2.29, 3.69] |
| Analysis 1.12  Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 12 Mean HbA1c second trimester. | ||||
| 13 Mean HbA1c third trimester Show forest plot | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐2.38, 2.58] |
| Analysis 1.13  Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 13 Mean HbA1c third trimester. | ||||
| 14 Mean birthweight Show forest plot | 2 | 61 | Mean Difference (IV, Fixed, 95% CI) | 220.56 [‐2.09, 443.20] |
| Analysis 1.14  Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 14 Mean birthweight. | ||||
| 15 Maternal 24 hour mean blood glucose (mg/dl) second trimester Show forest plot | 3 | 73 | Mean Difference (IV, Fixed, 95% CI) | 1.77 [‐5.02, 8.56] |
| Analysis 1.15  Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 15 Maternal 24 hour mean blood glucose (mg/dl) second trimester. | ||||
| 16 Maternal 24 hour mean blood glucose (mg/dl) third trimester Show forest plot | 3 | 69 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐5.57, 5.72] |
| Analysis 1.16  Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 16 Maternal 24 hour mean blood glucose (mg/dl) third trimester. | ||||
| 17 days hospitalised Show forest plot | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 9.40 [‐6.04, 24.84] |
| Analysis 1.17  Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 17 days hospitalised. | ||||

Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 1 Rate of caesarean section.

Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 2 Perinatal mortality.

Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 3 Fetal anomaly.

Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 4 Maternal hypoglycaemia.

Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 5 Maternal hyperglycaemia.

Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 6 Maternal 24 hour mean blood glucose (mg/dl) first trimester.

Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 7 Macrosomia.
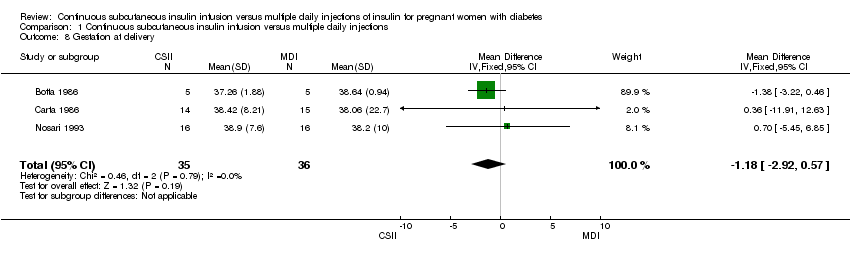
Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 8 Gestation at delivery.

Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 9 Neonatal hypoglycaemia.
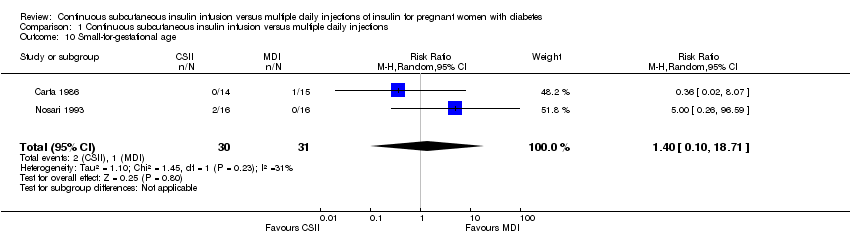
Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 10 Small‐for‐gestational age.

Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 11 Mean HbA1c first trimester.

Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 12 Mean HbA1c second trimester.

Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 13 Mean HbA1c third trimester.

Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 14 Mean birthweight.
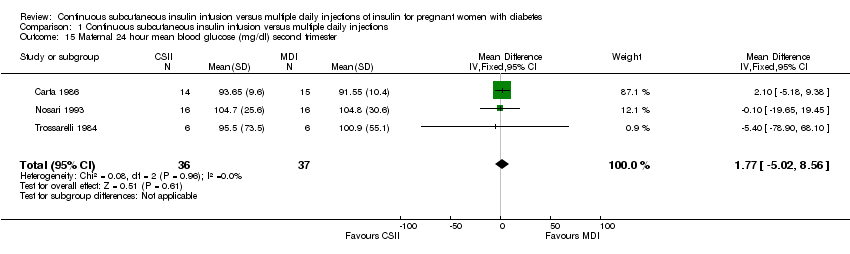
Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 15 Maternal 24 hour mean blood glucose (mg/dl) second trimester.
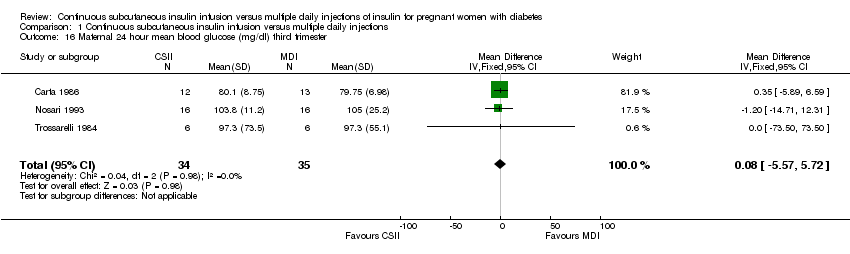
Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 16 Maternal 24 hour mean blood glucose (mg/dl) third trimester.

Comparison 1 Continuous subcutaneous insulin infusion versus multiple daily injections, Outcome 17 days hospitalised.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rate of caesarean section Show forest plot | 3 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.66, 1.77] |
| 2 Perinatal mortality Show forest plot | 3 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [0.38, 14.32] |
| 3 Fetal anomaly Show forest plot | 2 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.07, 15.54] |
| 4 Maternal hypoglycaemia Show forest plot | 2 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.35, 25.87] |
| 5 Maternal hyperglycaemia Show forest plot | 2 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.39, 125.44] |
| 6 Maternal 24 hour mean blood glucose (mg/dl) first trimester Show forest plot | 3 | 67 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐7.19, 7.43] |
| 7 Macrosomia Show forest plot | 2 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.2 [0.14, 72.62] |
| 8 Gestation at delivery Show forest plot | 3 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐1.18 [‐2.92, 0.57] |
| 9 Neonatal hypoglycaemia Show forest plot | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.64] |
| 10 Small‐for‐gestational age Show forest plot | 2 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.10, 18.71] |
| 11 Mean HbA1c first trimester Show forest plot | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐2.13, 1.73] |
| 12 Mean HbA1c second trimester Show forest plot | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐2.29, 3.69] |
| 13 Mean HbA1c third trimester Show forest plot | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐2.38, 2.58] |
| 14 Mean birthweight Show forest plot | 2 | 61 | Mean Difference (IV, Fixed, 95% CI) | 220.56 [‐2.09, 443.20] |
| 15 Maternal 24 hour mean blood glucose (mg/dl) second trimester Show forest plot | 3 | 73 | Mean Difference (IV, Fixed, 95% CI) | 1.77 [‐5.02, 8.56] |
| 16 Maternal 24 hour mean blood glucose (mg/dl) third trimester Show forest plot | 3 | 69 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐5.57, 5.72] |
| 17 days hospitalised Show forest plot | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 9.40 [‐6.04, 24.84] |

