Oxigenoterapia en ámbitos prehospitalarios para pacientes con una exacerbación aguda de la enfermedad pulmonar obstructiva crónica
Información
- DOI:
- https://doi.org/10.1002/14651858.CD005534.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 14 enero 2020see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Vías respiratorias
- Copyright:
-
- Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
MA and RWB wrote and developed the initial protocol and published the first version of the review in 2008. ZK and KVC updated the protocol, screened literature, conducted data extraction, analysed the evidence and drafted the manuscript for the 2018 review update. MA and RWB provided clinical input and reviewed the draft manuscript for the 2019 update.
Contributions of editorial team
Rebecca Fortescue (Co‐ordinating Editor) edited the review and advised on methodology, interpretation and content.
Chris Cates (Co‐ordinating Editor) checked the data entry prior to the full write‐up of the review and approved the final review prior to publication.
Brian Rowe (Contact Editor) edited the review and advised on methodology, interpretation and content.
Emma Dennett (Managing Editor) co‐ordinated the editorial process; advised on interpretation and content; and edited the review.
Emma Jackson (Assistant Managing Editor) conducted peer review; obtained translations; and edited the 'Plain language summary' and 'References' sections of the protocol and the review.
Elizabeth Stovold (Information Specialist) designed the search strategy; ran the searches; and edited the 'Search methods' section.
Sources of support
Internal sources
-
The authors declare that no such funding was received for this systematic review, Other.
External sources
-
The authors declare that no such funding was received for this systematic review, Other.
Declarations of interest
MA and RWB were authors on the one included study (Austin 2010). Thus, they were not involved in any capacity for protocol revision, study screening and selection, data extraction and analysis of the included and excluded studies.
ZK: none known
KVC: none known
MAA: none known
RW‐B: none known
Acknowledgements
Many thanks to the Cochrane Airways Group editorial base for support: Elizabeth Stovold for conducting the electronic searches, Emma Dennett, Emma Jackson, Rebecca Normansell and Chris Cates for editorial support. We would also like to thank Dr Julia Walters for advice and support.
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
The authors acknowledge Brian J Smith (Bendigo Hospital, Australia) for his review of an early version of the discussion section of this update.
The authors and Cochrane Airways editorial team are grateful to the following peer reviewers for their time and comments.
-
Begum Ergan (Dokuz Eylul University Medical School, Turkey)
-
Josefin Sundh (Örebro University, Sweden)
-
Magnus Ekström (Lund University, Sweden)
-
Nausherwan K Burki (UConn Health, USA)
Version history
| Published | Title | Stage | Authors | Version |
| 2020 Jan 14 | Oxygen therapy in the pre‐hospital setting for acute exacerbations of chronic obstructive pulmonary disease | Review | Zoe Kopsaftis, Kristin V Carson‐Chahhoud, Michael A Austin, Richard Wood‐Baker | |
| 2006 Jul 19 | Oxygen therapy in the pre‐hospital setting for acute exacerbations of chronic obstructive pulmonary disease | Review | Michael A. Austin, Richard Wood‐Baker | |
| 2005 Oct 19 | Oxygen therapy in the pre‐hospital setting for people suffering from an acute exacerbation of chronic obstructive pulmonary disease | Protocol | Michael A. Austin, Richard Wood‐Baker | |
Differences between protocol and review
This review has undergone significant revisions to style and methodology from the original review (Austin 2006). This was undertaken to ensure the review reflects current Cochrane methodological standards.
For this review we used the Cochrane 'Risk of bias' tool instead of the Jadad scale, and added a PRISMA diagram and 'Summary of findings' table. We also refined and consolidated the list of outcomes. The original primary outcome was mortality from respiratory causes this was updated to include all causes. The secondary outcomes of mental status score, consciousness score and illness score were replaced with quality of life; intensive care unit admission, invasive ventilation and non‐invasive ventilation were consolidated under treatment failure and duration of hospitalisation was amended to reflect broader hospital utilisation. We further defined the lung function outcome to state FEV1/FVC as the measure. Further, standard care was added to the list of accepted comparison interventions. We also specified that a random‐effects model would be used for the primary analyses and compared to the fixed‐effect model in a sensitivity analysis, and we further clarified the criteria for the planned sensitivity analysis based on risk of bias. Data extraction methods were updated to reflect current practice, with two independent reviewers performing extractions for the included study instead of one.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Aged; Humans;
PICO

In the high‐dose oxygen group 9 people out of 100 died, compared to 2 (95% confidence interval 0 to 9) out of 100 for the titrated oxygen group.

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 High flow versus titrated oxygen therapy, Outcome 1 Mortality (respiratory‐related and all‐cause).

Comparison 1 High flow versus titrated oxygen therapy, Outcome 2 Arterial Blood Gas (pH).

Comparison 1 High flow versus titrated oxygen therapy, Outcome 3 Ventilation of any type.

Comparison 1 High flow versus titrated oxygen therapy, Outcome 4 Length of Stay (days).
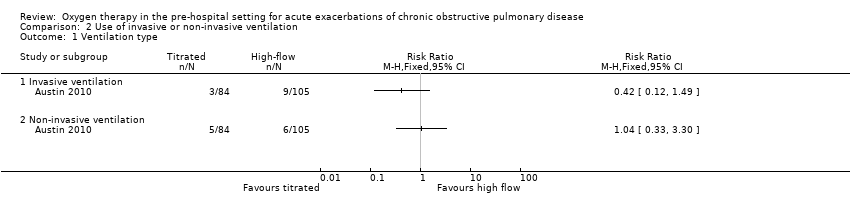
Comparison 2 Use of invasive or non‐invasive ventilation, Outcome 1 Ventilation type.
| Titrated oxygen therapy compared to high‐flow oxygen therapy for acute exacerbations of chronic obstructive pulmonary disease | ||||||
| Patient or population: adults with acute exacerbations of chronic obstructive pulmonary disease | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with high‐flow oxygen therapy | Risk with titrated oxygen therapy | |||||
| Mortality (respiratory‐ related and all‐cause | 94 per 1,000 | 21 per 1,000 | RR 0.22 | 214 | ⊕⊕⊝⊝ | A difference in mortality was observed, with 11 deaths in the high‐flow oxygen arm compared to two deaths in the titrated oxygen arm (P = 0.05). This translates to a number needed to treat for an additional beneficial outcome (NNTB) of 14 (95% CI 12 to 355) with administration of titrated oxygen therapy, and is shown as a Cates plot in Figure 1. All deaths occurred after arrival at the hospital; two were in intensive care. Respiratory failure was the cause of mortality in all cases, with approximately 70% of deaths occurring within the first five days following admission for both treatment arms. |
| Arterial blood gas (pH) | The mean arterial blood gas (pH) was 7.29 | MD 0.06 pH higher | ‐ | 214 | ⊕⊕⊝⊝ | Based on the intention‐to‐treat analysis for the COPD subgroup, no significant difference between treatment arms for blood gas measurements was observed between groups (P = 0.23). Only 11% of participants had this measurement performed according to protocol. |
| Ventilation of any type | 143 per 1,000 | 96 per 1000 | RR 0.67 | 189 | ⊕⊕⊝⊝ | No significant difference observed between treatment arms for ventilation requirement for per protocol or intention‐to‐treat analyses. |
| Length of hospital stay | The mean length of hospital stay was 6.3 days | MD 0.88 days lower | ‐ | 214 | ⊕⊕⊝⊝ | No significant difference was observed between treatment arms in length of hospital stay for the intention‐to‐treat analysis (P = 0.21). |
| Quality of life ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | This was not reported as an outcome in the single included study. |
| Lung function ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | This was not reported as an outcome in the single included study. |
| Dyspnoea score ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | This was not reported as an outcome in the single included study. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded once for indirectness because there was a single included study with moderate sample size (n = 214). The study was conducted in one state within Australia only. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality (respiratory‐related and all‐cause) Show forest plot | 1 | Risk Ratio (Fixed, 95% CI) | Totals not selected | |
| 2 Arterial Blood Gas (pH) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 3 Ventilation of any type Show forest plot | 1 | Risk Ratio (Fixed, 95% CI) | Totals not selected | |
| 4 Length of Stay (days) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ventilation type Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Invasive ventilation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Non‐invasive ventilation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

