Penambahan zink untuk pencegahan diabetes mellitus jenis 2 dalam kalangan dewasa dengan kerintangan insulin
Abstract
Background
Diabetes is associated with long‐term damage, dysfunction and failure of various organs, especially the eyes, kidneys, nerves, heart and blood vessels. The risk of developing type 2 diabetes increases with age, obesity and lack of physical activity. Insulin resistance is a fundamental aspect of the aetiology of type 2 diabetes. Insulin resistance has been shown to be associated with atherosclerosis, dyslipidaemia, glucose intolerance, hyperuricaemia, hypertension and polycystic ovary syndrome. The mineral zinc plays a key role in the synthesis and action of insulin, both physiologically and in diabetes mellitus. Zinc seems to stimulate insulin action and insulin receptor tyrosine kinase activity.
Objectives
To assess the effects of zinc supplementation for the prevention of type 2 diabetes mellitus in adults with insulin resistance.
Search methods
This review is an update of a previous Cochrane systematic review published in 2007. We searched the Cochrane Library (2015, Issue 3), MEDLINE, EMBASE, LILACS and the ICTRP trial register (from inception to March 2015). There were no language restrictions. We conducted citation searches and screened reference lists of included studies.
Selection criteria
We included studies if they had a randomised or quasi‐randomised design and if they investigated zinc supplementation compared with placebo or no intervention in adults with insulin resistance living in the community.
Data collection and analysis
Two review authors selected relevant trials, assessed risk of bias and extracted data.
Main results
We included three trials with a total of 128 participants in this review. The duration of zinc supplementation ranged between four and 12 weeks. Risk of bias was unclear for most studies regarding selection bias (random sequence generation, allocation concealment) and detection bias (blinding of outcome assessment). No study reported on our key outcome measures (incidence of type 2 diabetes mellitus, adverse events, health‐related quality of life, all‐cause mortality, diabetic complications, socioeconomic effects). Evaluation of insulin resistance as measured by the Homeostasis Model Assessment of Insulin Resistance (HOMA‐IR) showed neutral effects when comparing zinc supplementation with control (two trials; 114 participants). There were neutral effects for trials comparing zinc supplementation with placebo for total cholesterol, high‐density lipoprotein (HDL) cholesterol, low‐density lipoprotein (LDL) cholesterol and triglycerides (2 studies, 70 participants). The one trial comparing zinc supplementation with exercise also showed neutral effects for total cholesterol, HDL and LDL cholesterol, and a mean difference in triglycerides of ‐30 mg/dL (95% confidence interval (CI) ‐49 to ‐10) in favour of zinc supplementation (53 participants). Various surrogate laboratory parameters were also analysed in the included trials.
Authors' conclusions
There is currently no evidence on which to base the use of zinc supplementation for the prevention of type 2 diabetes mellitus. Future trials should investigate patient‐important outcome measures such as incidence of type 2 diabetes mellitus, health‐related quality of life, diabetic complications, all‐cause mortality and socioeconomic effects.
PICO
Ringkasan bahasa mudah
Penambahan zink untuk pencegahan diabetes mellitus jenis 2
Soalan ulasan
Apakah kesan penambahan zink berbanding plasebo atau tanpa rawatan untuk pencegahan penyakit diabetes jenis 2 dalam kalangan dewasa dengan kerintangan insulin?
Latar belakang
Beberapa kajian telah menunjukkan bahawa zink menambahbaik paras glukosa (kawalan glisemik) dalam penghidap diabetes. Akibat diabetes, komplikasi jangka panjang boleh terjadi seperti penyakit buah pinggang, saraf dan mata. Risiko komplikasi kardiovaskular seperti serangan jantung dan strok juga meningkat. Diabetes jenis 1 ialah satu bentuk diabetes di mana badan tidak boleh menghasilkan insulin. Risiko mendapat diabetes jenis 2 meningkat dengan usia, obesiti dan kekurangan aktiviti fizikal dan dicirikan oleh peningkatan ketidakmampuan badan untuk memanfaatkan insulin (kerintangan insulin). Mineral zink memain peranan penting dalam tindakan insulin dan secara teori penambahan zink digunakan oleh orang dengan kerintangan insulin dapat mencegah permulaan diabetes.
Ciri‐ciri kajian
Kami memasukkan tiga kajian rawak terkawal dengan sejumlah 128 peserta dalam ulasan ini. Tempoh penambahan zink adalah di antara empat dan 12 minggu.
Keputusan utama
Tiada kajian melaporkan hasil utama pesakit yang penting (permulaan baru diabetes jenis 2, kesan sampingan, kualiti hidup berkaitan dengan kesihatan, kematian semua punca, komplikasi diabetes, kesan sosioekonomi). Kesan penambahan zink adalah tidak pasti terhadap tahap kerintangan dan lipid dalam darah (terutamanya kolesterol dan trigliserida).
Kualiti bukti
Kualiti keseluruhan kajian tyang dimasukkan adalah tidak jelas kerana penulis kajian tidak memberi maklumat penting untuk kami menilai cara kajian dilaksanakan (risiko yang tidak jelas dalam kebanyakan kes). Tambahan pula, bilangan kajian dan peserta adalah rendah dan para pengarang kajian tidak menyiasat hasil penting seperti permulaan baru diabetes jenis 2 atau kesan sampingan penambahan zink.
Status bukti
Bukti ini adalah terkini sehingga Mac 2015.
Authors' conclusions
Summary of findings
| Zinc supplementation for the prevention of type 2 diabetes mellitus | ||||||
| Population: non‐diabetic adults with insulin resistance Settings: not specified/outpatients Intervention: zinc supplementation Comparison: placebo or exercise | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or exercise | Zinc supplementation | |||||
| Incidence of type 2 diabetes mellitus | See comment | See comment | See comment | See comment | See comment | Outcome not reported |
| Diabetic complications | See comment | See comment | See comment | See comment | See comment | Outcome not reported |
| Adverse events | See comment | See comment | See comment | See comment | See comment | Outcome not reported |
| Health‐related quality of life | See comment | See comment | See comment | See comment | See comment | Outcome not reported |
| All‐cause mortality | See comment | See comment | See comment | See comment | See comment | Outcome not reported |
| Socioeconomic effects | See comment | See comment | See comment | See comment | See comment | Outcome not reported |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
Background
Description of the condition
This is an update of a Cochrane Review first published in the Cochrane Library in 2007 (Beletate 2007), which included one study that did not provide evidence on patient‐important outcome measures such as incidence of new‐onset type 2 diabetes (Marreiro 2006).
Diabetes mellitus is a metabolic disorder resulting from a defect in insulin secretion, insulin action, or both. A consequence of this is chronic hyperglycaemia (that is elevated levels of plasma glucose) with disturbances of carbohydrate, fat and protein metabolism. Long‐term complications of diabetes mellitus include retinopathy, nephropathy and neuropathy. The risk of cardiovascular disease is increased (ADA 1999). There are two major forms of diabetes: type 1 diabetes (insulin‐dependent diabetes mellitus (IDDM)) and type 2 diabetes (non‐insulin‐dependent diabetes mellitus (NIDDM) (ADA 1999). Type 2 diabetes mellitus, the most prevalent form of the disease, is often asymptomatic and may remain undiagnosed for many years (ADA 1998). Type 2 diabetes may be seen in children and young adults. In type 2 diabetes the pancreatic islet cells are capable of compensating insulin resistance (see below) and producing larger quantities of insulin, at least at the beginning of the disease (Chausmer 1998).
Prevalence and costs
The worldwide prevalence of diabetes has been estimated to be around 347 million people (Danaei 2011). In 2004, an estimated 3.4 million people died from consequences of high blood sugar (WHO 2009). The World Health Organization (WHO) projects that diabetes will be the 7th leading cause of death in 2030 (WHO 2011). Diabetes accounts for over EUR 77 billion (USD 98) in healthcare costs (Tracey 2003).
The risk of developing type 2 diabetes and insulin resistance
The risk of developing type 2 diabetes increases with age, obesity and lack of physical activity. Insulin resistance is a fundamental aspect of the aetiology of type 2 diabetes (Kahn 2000). The majority of people who develop type 2 diabetes are insulin resistant, a condition in which the pancreas produces insulin but the body's cells become increasingly resistant to the regular effects of insulin, which results in hyperglycaemia (Tracey 2003). Insulin resistance has been shown to be associated with atherosclerosis (Howard 1996), dyslipidaemia (Moro 2003), glucose intolerance, hyperuricaemia, hypertension (Bonora 1998), and polycystic ovary syndrome (Kahn 2000).
Insulin resistance can be measured by using the glucose clamp technique (DeFronzo 1979), which is considered the gold standard in the assessment of insulin sensitivity (Karelis 2004). However, this method is laborious, expensive and inadequate for large‐scale or epidemiological studies (Bonora 2000). Matthews et al developed the Homeostasis Model Assessment (HOMA) method, which derives an estimate of insulin sensitivity by taking fasting plasma glucose and insulin concentrations into account. HOMA is supposed to evaluate insulin resistance and the function of ß‐cells. Insulin resistance is calculated with the following formula: (fasting serum insulin (µU/ml) x fasting plasma glucose (mmol/L)/22.5 (Matthews 1985).
Impaired glucose tolerance (IGT) and impaired fasting glucose (IFG) are associated with insulin resistance. IFG and IGT refer to an intermediate metabolic stage between normal glucose homeostasis and diabetes. This stage includes individuals with fasting glucose levels equal to or greater than 110 mg/dL but lower than 126 mg/dL or after an orale glucose tolerance test with two‐hour values equal to or greater than 140 mg/dL but lower than 200 mg/dL.
Description of the intervention
Every single cell in the body needs zinc for structural and energy‐producing functions. Zinc is an essential trigger for many biochemical reactions and also for protein production. Zinc plays an important role in cell division, growth and repair. It helps with wound healing and maintaining a normal sense of taste and smell. Zinc works as an immune booster and can be instrumental in fighting colds, flu and other infections. Zinc is a component of more than 200 enzymes, most of them involved in protein and DNA synthesis. Zinc has beneficial effects on sex and thyroid hormones. The human body does not produce zinc on its own, so it must be obtained from outside sources. The mineral zinc can be found in both animal and plant food sources, but the richest source of zinc comes from animal food.
The daily recommended dose of zinc is 12 mg for women and 15 mg for men. Studies suggest oral supplementation of zinc sulphate from 30 mg to 200 mg per day. The effect of supplementation with zinc was assessed in 10 patients with liver cirrhosis (Marchesini 1998). The patients presented with impaired glucose tolerance and zinc deficiency. Supplementation was approximately 136 mg zinc per day. The study showed that zinc supplementation produced a significant improvement in glucose disposal. The action of zinc seemed to be related to the increased activities of insulin independent glucose transporters (Marchesini 1998).
Adverse effects of the intervention
Amounts of zinc of two or more grams per day can cause gastrointestinal irritation and vomiting (Marchesini 1998; Marreiro 2004).
How the intervention might work
Zinc ions have an insulin‐like effect. A particularly sensitive target of zinc ions is protein tyrosine phosphatase 1B, a key regulator of the phosphorylation state of the insulin receptor (Haase 2005). Several studies have investigated the role of zinc status in insulin secretion and metabolism. In vitro studies show that insulin may form a complex with zinc, improving the solubility of this hormone in the pancreatic β‐cells (Rossetti 1990). Moreover, the binding ability of insulin to its receptor may be increased (Rossetti 1990). Alterations in zinc concentration and distribution in tissues, as well as improvements in insulin sensitivity after supplementation with this element have been demonstrated (Marchesini 1998).
Why it is important to do this review
Given the worldwide public health relevance of type 2 diabetes mellitus any intervention thought to be associated with only minor or no adverse effects should be investigated by means of a systematic review of the available evidence.
Objectives
To assess the effects of zinc supplementation for the prevention of type 2 diabetes mellitus in adults with insulin resistance.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised and quasi‐randomised controlled clinical trials with a minimum duration of four weeks.
Types of participants
Non‐diabetic adults (18 years or older) living in the community with insulin resistance.
Diagnostic criteria
Insulin resistance had to be measured by the Homeostasis Model Assessment of Insulin Resistance (HOMA‐IR) or the glucose clamp technique.
To be consistent with changes in the classification of and diagnostic criteria for diabetes mellitus over the years, the diagnosis should have been established using the standard criteria valid at the time of the trial commencing (for example ADA 1999; WHO 1999). Ideally, the diagnostic criteria should have been described. If necessary, we would have used the study authors' definition of diabetes mellitus. We planned to subject diagnostic criteria to a sensitivity analysis.
Types of interventions
We planned to investigate the following comparisons of intervention versus control/comparator.
Intervention
-
Zinc
Comparator
-
Placebo.
-
No intervention.
-
Another dose of zinc.
Concomitant interventions had to be the same in the intervention and comparator groups to establish fair comparisons.
Types of outcome measures
Primary outcomes
-
Incidence of type 2 diabetes mellitus.
-
Adverse events.
Secondary outcomes
-
Insulin resistance.
-
Health‐related quality of life.
-
All‐cause mortality.
-
Diabetic complications.
-
Socioeconomic effects.
-
Lipid levels.
Method of outcome measurement
-
Incidence of type 2 diabetes mellitus: new onset of type 2 diabetes mellitus as defined by standard diagnostic criteria (such as ADA 1997; ADA 1999; ADA 2003; WHO 1980; WHO 1985).
-
Adverse events: for example, diarrhoea, nausea, fatigue, gastrointestinal discomfort, anaemia.
-
Insulin resistance: estimated by the HOMA‐IR or the glucose clamp technique.
-
Health‐related quality of life: measured with a validated instrument such as the Quality of Well‐Being Scale (QWB), Medical Outcomes Study 36‐item Short‐Form Health Survey (SF‐36), EuroQol (EQ‐5D) and specific instruments such as the Diabetes Care Profile (DCP) and the Diabetes Quality of Life Measure (DQOL).
-
All‐cause mortality: defined as death from any cause.
-
Diabetic complications: defined as hypoglycaemia, diabetic ketoacidosis, hyperosmolar hyperglycaemic state, retinopathy, coronary heart disease, nephropathy, neuropathy, stroke and amputation.
-
Socioeconomic effects: such as consequences on income, wealth, education, occupation.
-
Lipid levels: as measured by total cholesterol, LDL and HDL cholesterol, triglycerides and leptin concentration.
Timing of outcome measurement
We planned to collect data for all primary and secondary outcomes for any time of outcome measurement.
'Summary of findings' table
We present a 'Summary of findings' table reporting the following outcomes listed according to priority.
-
Incidence of type 2 diabetes mellitus.
-
Diabetic complications.
-
Adverse events.
-
Health‐related quality of life.
-
All‐cause mortality.
-
Socioeconomic effects.
Search methods for identification of studies
Electronic searches
We searched the following sources from inception to the specified date without language restriction.
-
Cochrane Library (2015, Issue 3)
-
Cochrane Database of Systematic Reviews (CDSR)
-
Cochrane Central Register of Controlled Trials (CENTRAL)
-
Database of Abstracts of Reviews of Effects (DARE)
-
Health Technology Assessment (HTA) reports
-
-
MEDLINE (until March 2015)
-
EMBASE (until March 2015)
-
LILACS (until March 2015)
-
International Clinical Trials Registry Platform (ICTRP) Search Portal (http://apps.who.int/trialsearch/):
-
Australian New Zealand Clinical Trials Registry (2 March 2015)
-
ClinicalTrials.gov (2 March 2015)
-
EU Clinical Trials Register (2 March 2015)
-
International Standard Randomised Controlled Trial Number (ISRCTN) (2 March 2015)
-
Brazilian Clinical Trials Registry (2 March 2015)
-
Chinese Clinical Trial Registry (2 March 2015)
-
Clinical Trials Registry ‐ India (2 March 2015)
-
Clinical Research Information Service ‐ Republic of Korea (2 March 2015)
-
Cuban Public Registry of Clinical Trials (2 March 2015)
-
German Clinical Trials Register (2 March 2015)
-
Iranian Registry of Clinical Trials (2 March 2015)
-
Japan Primary Registries Network (2 March 2015)
-
Pan African Clinical Trial Registry (2 March 2015)
-
Sri Lanka Clinical Trials Registry (2 March 2015)
-
The Netherlands National Trial Register (2 March 2015)
-
Thai Clinical Trials Register (2 March 2015)
-
For detailed search strategies see Appendix 1. We continuously applied a MEDLINE (via Ovid SP) email alert service to identify newly published studies (Appendix 1). If we detected new studies for inclusion we would have evaluated these and incorporated findings in our review before submission of the final review draft (Beller 2013).
Searching other resources
We tried to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials, (systematic) reviews, meta‐analyses and health technology assessment reports.
Data collection and analysis
Selection of studies
Two review authors (LFOG, MSPO) independently scanned the title or abstract of every record retrieved. We retrieved full articles for further assessment if the information given suggested that the study: 1) included patients with insulin resistance, 2) compared a zinc intervention with placebo. Where differences in opinion existed, they were resolved by a third party (RED). If resolving disagreement was not possible, we planned to add the article to those 'awaiting classification' and contact the study authors for clarification. We present an adapted Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram to show the process of study selection (Liberati 2009).
Data extraction and management
For studies that fulfilled the inclusion criteria, two review authors (LFOG, MSPO) independently abstracted relevant population and intervention characteristics, using standard data extraction templates as supplied by the Cochrane Metabolic and Endocrine Disorders (CMED) Group, with any disagreements to be resolved by discussion, or, if required, by a third review author (RED) (for details see Table 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6;Appendix 7; Appendix 8; Appendix 9; Appendix 10).
| Intervention(s) and comparator(s) | Sample sizea | Screened/eligible | Randomised | ITT | Analysed | Finishing study | Randomised finishing study | Follow‐up timeb | |
| (1) Gómez‐García 2006 | I: 100 mg/day zinc sulfate | 12 | 14 | 7 | ‐ | 7 | 7 | 100 | 1 month |
| C: placebo | 7 | ‐ | 7 | 7 | 100 | ||||
| total: | 14 | ‐ | 14 | 14 | 100 | ||||
| (2) Marreiro 2006 | I: 30 mg/day zinc amino chelate | ‐ | ‐ | 28 | ‐ | 28 | 28 | 100 | 1 month |
| C: placebo | 28 | ‐ | 28 | 28 | 100 | ||||
| total: | 56 | ‐ | 56 | 56 | 100 | ||||
| (3) Soheilykhah 2012 | I: 50 mg/day zinc sulfate | ‐ | ‐ | ‐ | ‐ | 28 | 28 | 100 | 12 weeks |
| C: regular exercise and weight control | ‐ | ‐ | 25 | 25 | 100 | ||||
| total: | 58 | ‐ | 53 | 53 | 91.4 | ||||
| Grand total | All interventions | 63 | 63 | ||||||
| All comparators | 60 | 60 | |||||||
| All interventions and comparators | 128 | 123 | |||||||
aAccording to power calculation in study publication or report
bDuration of intervention and/or follow‐up under randomised conditions until end of study
"‐" denotes not reported
C: comparator; I: intervention; ITT: intention‐to‐treat.
We planned to provide information including trial identifier about potentially relevant ongoing studies in the 'Characteristics of ongoing studies' table and in a joint appendix. We attempted to find the protocol for each included study and had planned to report primary, secondary and other outcomes in comparison with data in publications in a joint appendix 'Matrix of study endpoint (publications and trial documents)'.
We sent an email request to the authors of included studies to enquire whether they were willing to answer questions regarding their trials. Appendix 11 shows the results of this survey. Thereafter, we sought relevant missing information on the trial from the study authors of the article, if required.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents or multiple reports of a primary study, we maximised the yield of information by collating all available data. In case of doubt the publication reporting the longest follow‐up associated with our primary or secondary outcomes had priority.
Assessment of risk of bias in included studies
Two review authors (LFOG, MSPO) assessed each trial independently. We resolved possible disagreement by consensus, or with consultation of a third review author in case of disagreement (RED). In cases of disagreement, we consulted the rest of the group and made a judgement based on consensus.
We used the 'Risk of bias' assessment toll of the Cochrane Collaboration (Higgins 2011a; Higgins 2011b) and evaluated the following bias criteria:
-
Random sequence generation (selection bias)
-
Allocation concealment (selection bias)
-
Blinding of participants and personnel (performance bias)
-
Blinding of outcome assessment (detection bias)
-
Incomplete outcome data (attrition bias)
-
Selective reporting (reporting bias)
-
Other potential sources of bias
We judged the above 'Risk of bias' criteria as 'low risk', 'high risk' or 'unclear risk' and evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We have presented a 'Risk of bias' graph and a 'Risk of bias' summary. We assessed the impact of individual bias domains on study results at endpoint and study levels.
We planned to assess outcome reporting bias by integrating the results of the appendix 'Matrix of study endpoints (publications and trial documents)' and the appendix 'Examination of outcome reporting bias' (Kirkham 2010). This analysis would have formed the basis of the judgement of selective reporting (reporting bias).
For blinding of participants and personnel (performance bias), detection bias (blinding of outcome assessors) and attrition bias (incomplete outcome data) we intended to evaluate risk of bias separately for subjective and objective outcomes (Hróbjartsson 2013). We considered the implications of missing outcome data from individual participants.
We defined the following endpoints as subjective outcomes.
-
Adverse events
-
Health‐related quality of life
We defined the following endpoints as objective outcomes.
-
Incidence of type 2 diabetes mellitus
-
Diabetic complications
-
All‐cause mortality
-
Lipid levels
-
Socioeconomic effects
Measures of treatment effect
We planned to express dichotomous data as risk ratios (RRs) with 95% confidence intervals (CIs). We planned to express continuous data as mean differences (MDs) with 95% CIs.
Unit of analysis issues
We planned to take into account the level at which randomisation occurred, such as cross‐over trials, cluster‐randomised trials and multiple observations for the same outcome.
Dealing with missing data
We planned to obtain relevant missing data from authors, if feasible, and we evaluated important numerical data such as screened, eligible and randomised participants, as well as intention‐to‐treat (ITT), as‐treated and per‐protocol populations. We investigated attrition rates, for example drop‐outs, losses to follow‐up and withdrawals, and critically appraised issues of missing data and imputation methods (e.g. last observation carried forward (LOCF)).
Where standard deviations for outcomes were not reported, we planned to impute these values by assuming the standard deviation of the missing outcome to be the average of the standard deviations from those studies where this information was reported. We planned to investigate the impact of imputation on meta‐analyses by means of sensitivity analysis.
Assessment of heterogeneity
In the event of substantial clinical, methodological or statistical heterogeneity, we planned not to report study results as the pooled effect estimate in a meta‐analysis.
We wanted to identify heterogeneity by visual inspection of the forest plots and by using a standard Chi² test with a significance level of α = 0.1. In view of the low power of this test, we also planned to examine heterogeneity using the I² statistic, which quantifies inconsistency across studies to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003), where an I² statistic of 75% or more indicates a considerable level of inconsistency (Higgins 2011a).
Had we found heterogeneity, we would have attempted to determine potential reasons for it by examining individual study and subgroup characteristics.
Assessment of reporting biases
We planned to use funnel plots to assess small study effects, if there were at least 10 studies for a particular outcome. There can be several explanations for funnel plot asymmetry, including true heterogeneity of effect with respect to trial size, poor methodological design (and hence bias of small trials) and publication bias. We planned therefore to interpret the results carefully (Sterne 2011).
Data synthesis
Unless there was good evidence for homogeneous effects across studies we planned primarily to summarise low risk of bias data by means of a random‐effects model (Wood 2008). We planned to interpret random‐effects meta‐analyses with due consideration of the whole distribution of effects, ideally by presenting a prediction interval (Higgins 2009). A prediction interval specifies a predicted range for the true treatment effect in an individual study (Riley 2011). In addition, we planned to perform statistical analyses according to the statistical guidelines referenced in the latest version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Quality of evidence
We wanted to present the overall quality of the evidence for each outcome according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, which takes into account issues not only related to internal validity (risk of bias, inconsistency, imprecision, publication bias), but also to external validity such as directness of results. Two review authors (LFOG, MSPO) would have independently rated the quality of evidence for each outcome. We planned to present a summary of the evidence in a 'Summary of findings' table, which provides key information about the best estimate of the magnitude of the effect, in relative terms and as absolute differences, for each relevant comparison of alternative management strategies, numbers of participants, and studies addressing each important outcome and the rating of the overall confidence in effect estimates for each outcome. We created the 'Summary of findings' table based on the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We presented results for the outcomes as described in Types of outcome measures. If meta‐analysis was not possible, we planned to present the results in a narrative 'Summary of findings' table.
Subgroup analysis and investigation of heterogeneity
There were insufficient data to allow for any subgroup analyses. Should sufficient data in the future permit, we plan to carry out the following subgroup analyses and plan to investigate interaction.
-
Gender (female/male)
-
Age (depending on data but especially older versus younger participants)
-
Participants with or without comorbidities (for example, heart attack, stroke, peripheral vascular disease)
-
Duration of intervention
-
Different doses of zinc
Sensitivity analysis
There were insufficient data to allow for any sensitivity analyses to explore the influence of the following factors on effect size by restricting the analysis to:
-
Published studies.
-
Taking into account risk of bias, as specified in the 'Assessment of risk of bias in included studies' section.
-
Very long or large studies to establish how much they dominate the results.
-
Studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), or country.
We also planned to test the robustness of the results by repeating the analysis using different measures of effect size (RRs, ORs etc.) and different statistical models (fixed‐effect and random‐effects models).
Results
Description of studies
For a detailed description of studies, see the tables Characteristics of included studies, Characteristics of excluded studies and Characteristics of ongoing studies.
Results of the search
In the first version of the review (2007) we evaluated 192 records. Following the assessment of the full articles, we considered only two publications for inclusion. We excluded one study (Marchesini 1998), and included one study that met our inclusion criteria (Marreiro 2006). For this first update, our comprehensive literature searches identified 132 records; from these, we identified 14 full‐text publications for further examination. We excluded the other studies on the basis of their titles or abstracts, because they did not meet the inclusion criteria or were not relevant to the question under study (Figure 1). After screening the full text of the selected publications, two new studies (Gómez‐García 2006; Soheilykhah 2012), and a further unpublished manuscript of Marreiro 2006 met the inclusion criteria for this update. We excluded nine studies (Czernichow 2006; Czernichow 2009; Islam 2013; Kelishadi 2010; Kim 2012; Marreiro 2002; Marchesini 1998; Ramos 2007; Stewart 2011). One further study is an ongoing trial (Ranasinghe 2013).
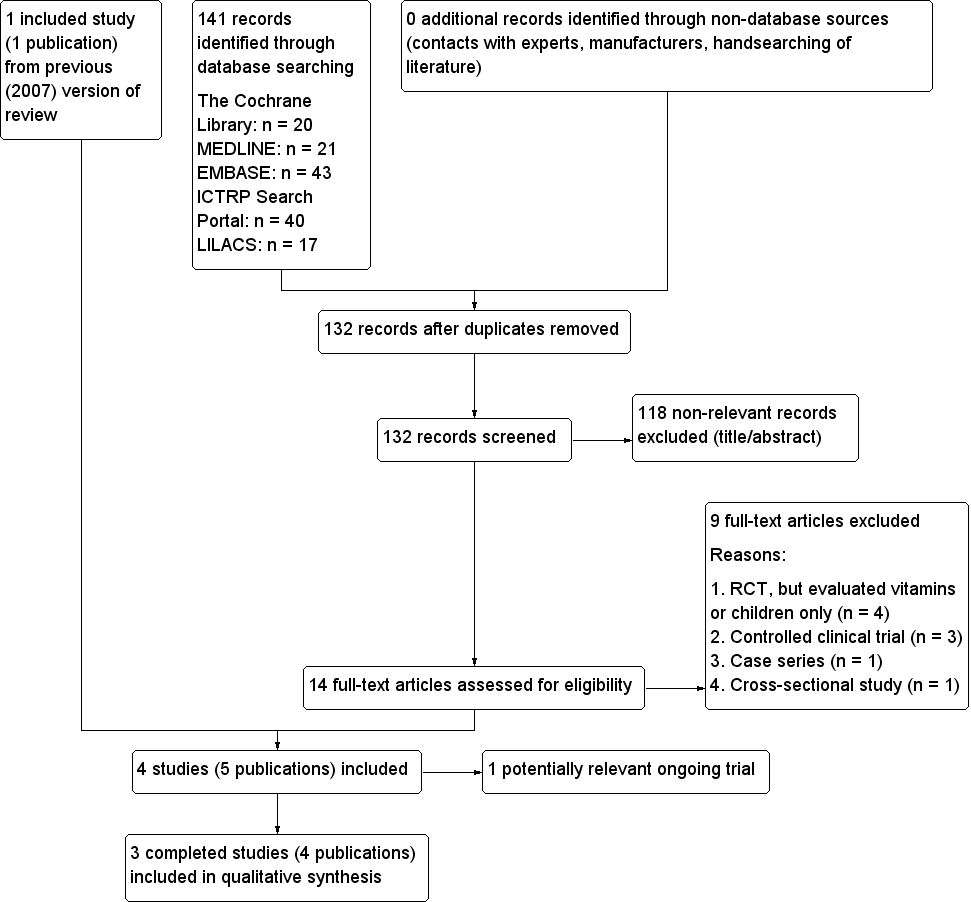
Study flow diagram.
Included studies
In this first update we included two published studies (Gómez‐García 2006; Soheilykhah 2012), and a further manuscript (Marreiro 2006), in addition to the original included study (Marreiro 2002), with a total of 128 randomised participants.
Overview of study populations
The Gómez‐García 2006 study enrolled 14 obese men. The Marreiro 2006 study included 56 normal glucose‐tolerant obese women, while the Soheilykhah 2012 study assessed 58 overweight or obese participants with a normal oral glucose tolerance test.
Study design
All included studies claimed to be randomised controlled trials.
Settings
Two of the included studies did not report the setting where the trials were conducted (Gómez‐García 2006; Soheilykhah 2012). One study involved outpatients visiting hospital clinics (Marreiro 2006).
Participants
Gómez‐García 2006 evaluated 14 men between 21 and 30 years old, while Marreiro 2006 assessed 56 women aged 25 to 45 years. The mean age of participants in Soheilykhah 2012 was 38 years.
Gómez‐García 2006 recruited study participants with a prediabetic state and stable weight for at least three months before the beginning of the study. Marreiro 2006 recruited glucose‐tolerant obese women. Soheilykhah 2012 recruited overweight or obese participants. The mean body mass index (BMI) at baseline ranged from 31 kg/m² in Gómez‐García 2006 to 36 kg/m² in Marreiro 2006. Soheilykhah 2012 evaluated 58 first degree relatives of diabetic people with a normal oral glucose tolerance test and a BMI > 25 kg/m²; the mean BMI was 29 kg/m².
Interventions
In the Gómez‐García 2006 study participants received 100 mg zinc per day for four weeks (n = 7) or a matching placebo (n = 7). In the Marreiro 2006 study treatment with zinc consisted of 30 mg per day for four weeks (n = 28) or a matching placebo (n = 28). In the Soheilykhah 2012 study 28 participants received 50 mg zinc per day for 12 weeks and for 25 participants regular exercise and weight control were recommended.
Outcomes
None of the included studies investigated the incidence of type 2 diabetes mellitus, adverse events, health‐related quality of life, all‐cause mortality, diabetic complications or socioeconomic effects.
In the Gómez‐García 2006 study the authors evaluated insulin sensitivity, leptin levels, biochemical profile and androgens. Outcome measures in the Marreiro 2006 study were insulin resistance, anthropometric parameters, diet parameters, leptin levels, insulin levels, zinc levels, lipid metabolism and fasting plasma glucose. In the Soheilykhah 2012 study adiponectin, fasting blood glucose, insulin, insulin resistance and lipid profile were assessed.
Excluded studies
Nine studies are described in the Characteristics of excluded studies (Czernichow 2006; Czernichow 2009; Islam 2013; Kelishadi 2010; Kim 2012; Marreiro 2002; Marchesini 1998; Ramos 2007; Stewart 2011). The main reason for exclusion was that the study was not a randomised controlled trial (RCT) or it was a RCT evaluating a combination of another oxidant and vitamins or included only children.
The Ranasinghe 2013 study is described in detail under Characteristics of ongoing studies. This is a RCT performed in Sri Lanka that evaluated participants in a prediabetic stage. The anticipated end of study was the end of June 2012. Participants received 20 mg zinc per day or a matching placebo for a period of 12 months. The objectives of this study were as follows: "The study aims to evaluate the effects of zinc supplementation on the progression of disease in patients with pre‐diabetes from Sri Lanka and determine the metabolic effects of zinc supplementation on glycemic control. Furthermore, we aim to evaluate the effects of zinc supplementation on appetite and body weight in patients with pre‐diabetes" (Ranasinghe 2013). None of our review's primary or secondary outcome measures were planned to be analysed.
Risk of bias in included studies
For details on the risk of bias of the included studies see Characteristics of included studies. For an overview of review authors' judgements about each risk of bias item for individual studies and across all studies see Figure 2 and Figure 3.
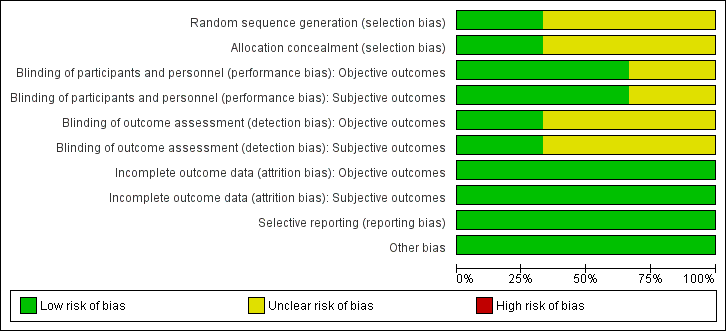
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
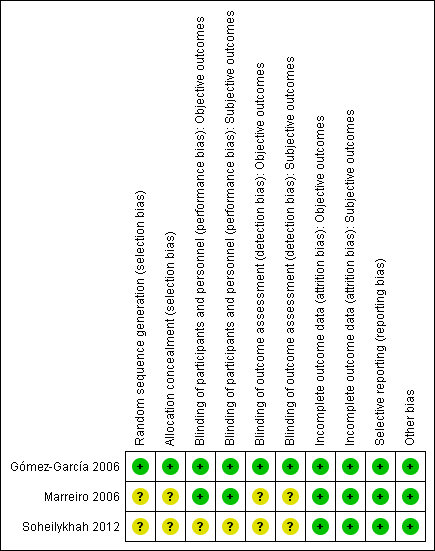
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All included studies had an unclear risk of selection bias with the exception of Gómez‐García 2006 that used opaque and sealed envelopes in a simple randomisation process.
Blinding
Two studies were double‐blinded for both investigators and participants resulting in a low risk of performance bias (Gómez‐García 2006; Marreiro 2006). Two of the included studies did not report on blinding of outcome assessors (Marreiro 2006; Soheilykhah 2012) and were ranked as unclear risk of bias for this domain. However, blinding of outcome assessors in Gómez‐García 2006 was done for both objective and subjective outcomes resulting in a low risk of detection bias.
Incomplete outcome data
All participants in the Gómez‐García 2006 and Marreiro 2006 studies completed the trial. In the Soheilykhah 2012 study 53 of 58 participants completed the study. Altogether we ranked attrition bias as low risk for all studies.
Selective reporting
We noted no evidence of selective reporting in any of the included studies.
Other potential sources of bias
We noted no evidence of other potential sources of bias in any of the included studies.
Effects of interventions
See: Summary of findings for the main comparison
Zinc supplementation versus placebo or no intervention
Primary outcomes
Incidence of type 2 diabetes mellitus
No study reported this outcome.
Adverse events
No study reported this outcome.
Secondary outcomes
Insulin resistance
Marreiro 2006 reported neutral effects for the Homeostasis Model Assessment of Insulin Resistance (HOMA‐IR), showing a mean difference (MD) of ‐0.10 (95% confidence interval (CI) ‐1.28 to 1.08), P = 0.87; 56 participants; Analysis 1.1 when comparing zinc supplementation with placebo. Soheilykhah 2012 reported a MD in the HOMA‐IR of ‐0.27 (95% CI ‐1.02 to 0.48), P = 0.48; 58 participants; Analysis 2.2 for zinc supplementation versus exercise.
Health‐related quality of life
No study reported this outcome.
All‐cause mortality
No study reported this outcome.
Diabetic complications
No study reported this outcome.
Socioeconomic effects
No study reported this outcome.
Lipid levels
All included studies reported on this outcome (Gómez‐García 2006; Marreiro 2006; Soheilykhah 2012). There were neutral effects for studies comparing zinc supplementation with placebo for total cholesterol, high‐density lipoprotein (HDL) cholesterol, low‐density lipoprotein (LDL) cholesterol and triglycerides (Gómez‐García 2006; Marreiro 2006) (Analysis 1.2). The one study comparing zinc supplementation with exercise, Soheilykhah 2012, also showed neutral effects for total, HDL andLDL cholesterol, and a MD in triglycerides of ‐30 mg/dL (95% CI ‐49 to ‐10) in favour of zinc supplementation (Analysis 2.2).
Additional parameters evaluated in the included studies
One study reported on body mass index, body perimeter, body constitution, skinfold thickness below the scapula, skinfold thickness above the hip bone, skinfold thickness of the upper arm, body mass index, waist, waist‐hip ratio, energy and nutrient intake, and percentage of fat as obtained by bioimpedance (Marreiro 2006). Marreiro 2006 also measured zinc concentrations in plasma, erythrocytes and urine.
One study reported on insulin, glucose, creatinine, uric acid, leptin, testosterone and sex hormone binding globulin (Gómez‐García 2006).
One study measured adiponectin, insulin and fasting blood sugar (FBS) (Soheilykhah 2012).
Discussion
Summary of main results
This systematic review offers up‐to‐date but very limited evidence supported by only three small randomised controlled trials regarding the effects of zinc supplementation for the prevention of type 2 diabetes mellitus. The relationship between obesity and insulin resistance is seen across all ethnic groups and is evident across the full range of body weights. Insulin resistance is thought to be a major feature of type 2 diabetes, particularly since high basal plasma insulin concentrations are often found in obese type 2 diabetic people. Unfortunately, none of our patient‐important outcome measures were reported in the included studies. Only parameters of insulin resistance and lipid levels were reported, showing neutral effects.
Overall completeness and applicability of evidence
None of the included studies investigated our predefined outcome measure, therefore the body of evidence is very limited. Marreiro 2006 suggested future trials with higher doses of zinc and longer follow‐up intervals, since four weeks and a dose of 30 mg of zinc are not sufficient to assess a long‐term process like the development of glucose intolerance and diabetes.
Quality of the evidence
The methodological quality of the included studies could not be judged according to the GRADE approach because none of our key endpoints were evaluated in the included studies.
Potential biases in the review process
Despite our thorough search in various databases we might have overlooked trials, especially with regard to the grey literature. However, we contacted the authors of the included studies to ask whether they had done another trial dealing with zinc supplementation for the prevention of type 2 diabetes mellitus in adults with insulin resistance.
Agreements and disagreements with other studies or reviews
The results of our review reflect those of the previous version of this review (Beletate 2007). Another systematic review of randomised controlled trials evaluated the effects of zinc supplementation on biomarkers of glycaemic control included type 1 and type 2 diabetic participants, participants with metabolic syndrome, participants with obesity and healthy persons (Capdor 2013). When comparing participants with metabolic conditions to healthy volunteers the study authors showed that zinc supplementation produced a greater reduction in glucose concentrations.

Study flow diagram.
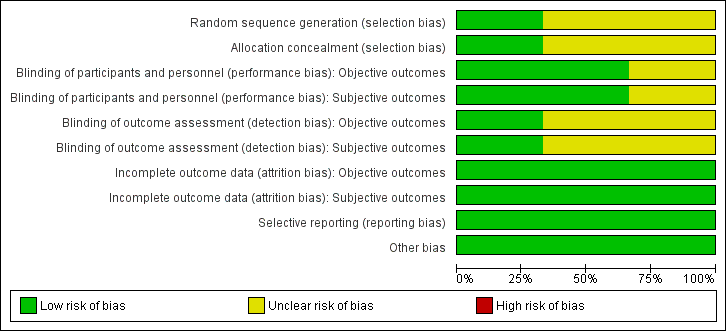
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Zinc supplementation versus placebo, Outcome 1 HOMA‐IR.

Comparison 1 Zinc supplementation versus placebo, Outcome 2 Lipids.
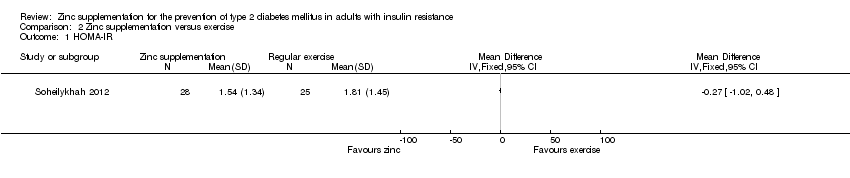
Comparison 2 Zinc supplementation versus exercise, Outcome 1 HOMA‐IR.

Comparison 2 Zinc supplementation versus exercise, Outcome 2 Lipids.
| Zinc supplementation for the prevention of type 2 diabetes mellitus | ||||||
| Population: non‐diabetic adults with insulin resistance Settings: not specified/outpatients Intervention: zinc supplementation Comparison: placebo or exercise | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or exercise | Zinc supplementation | |||||
| Incidence of type 2 diabetes mellitus | See comment | See comment | See comment | See comment | See comment | Outcome not reported |
| Diabetic complications | See comment | See comment | See comment | See comment | See comment | Outcome not reported |
| Adverse events | See comment | See comment | See comment | See comment | See comment | Outcome not reported |
| Health‐related quality of life | See comment | See comment | See comment | See comment | See comment | Outcome not reported |
| All‐cause mortality | See comment | See comment | See comment | See comment | See comment | Outcome not reported |
| Socioeconomic effects | See comment | See comment | See comment | See comment | See comment | Outcome not reported |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Intervention(s) and comparator(s) | Sample sizea | Screened/eligible | Randomised | ITT | Analysed | Finishing study | Randomised finishing study | Follow‐up timeb | |
| (1) Gómez‐García 2006 | I: 100 mg/day zinc sulfate | 12 | 14 | 7 | ‐ | 7 | 7 | 100 | 1 month |
| C: placebo | 7 | ‐ | 7 | 7 | 100 | ||||
| total: | 14 | ‐ | 14 | 14 | 100 | ||||
| (2) Marreiro 2006 | I: 30 mg/day zinc amino chelate | ‐ | ‐ | 28 | ‐ | 28 | 28 | 100 | 1 month |
| C: placebo | 28 | ‐ | 28 | 28 | 100 | ||||
| total: | 56 | ‐ | 56 | 56 | 100 | ||||
| (3) Soheilykhah 2012 | I: 50 mg/day zinc sulfate | ‐ | ‐ | ‐ | ‐ | 28 | 28 | 100 | 12 weeks |
| C: regular exercise and weight control | ‐ | ‐ | 25 | 25 | 100 | ||||
| total: | 58 | ‐ | 53 | 53 | 91.4 | ||||
| Grand total | All interventions | 63 | 63 | ||||||
| All comparators | 60 | 60 | |||||||
| All interventions and comparators | 128 | 123 | |||||||
| aAccording to power calculation in study publication or report "‐" denotes not reported C: comparator; I: intervention; ITT: intention‐to‐treat. | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HOMA‐IR Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 HOMA‐IR | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Lipids Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Total cholesterol | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Total cholesterol | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 HDL cholesterol | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 HDL cholesterol | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 LDL cholesterol | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 LDL cholesterol | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 VLDL cholesterol | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 VLDL lipoprotein | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Triglycerides | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.10 Triglycerides | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HOMA‐IR Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Lipids Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Total cholesterol | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 HDL cholesterol | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 LDL cholesterol | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Triglycerides | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

