Sildenafil bei Neugeborenen mit Lungenhochdruck
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial | |
| Participants | 24 neonates born at 34 weeks or later with PPHN and OI > 20 Group 1: n = 13 Male: 7/13 (%) Male: 8/11 (%) | |
| Interventions | iNO starting dose 20 ppm, weaning 2% to 4% every hour Group 1: iNO with 2 mg/kg sildenafil q6hours via orogastric tube (50 mg tablet crushed and diluted with 10 mL Orabase to prepare 5 mg/mL) Group 2: iNO with placebo (saline via orogastric tube) | |
| Outcomes | Oxygen index (absolute values and change from baseline, after first dose and every 6 hours for 7 days; improvement in OI, defined as a decrease of 10% from previously calculated value) A‐a gradient Haemodynamic parameters Days of hospitalisation Mortality (all‐cause within 28 days of life) Inotropic agent ROP Length of stay | |
| Notes | Registered with clinicaltrials.gov NCT01558466 Trial was funded by an investigator grant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation (information obtained from author Salama) |
| Allocation concealment (selection bias) | Low risk | Randomisation by sealed envelope competed by pharmacy |
| Blinding of participants and personnel (performance bias) | Low risk | Treating physicians and nurses were unaware of treatment allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Respiratory therapists were unaware of treatment allocation |
| Incomplete outcome data (attrition bias) | Low risk | Data are reported on all 24 randomised neonates |
| Selective reporting (reporting bias) | Low risk | All registered outcomes were reported |
| Other bias | Low risk | Appears free of other bias |
| Methods | Randomised controlled trial | |
| Participants | 13 neonates > 35.5 weeks' PMA with persistent hypoxaemia despite mechanical ventilation (OI ≥ 40) and echocardiographic diagnosis of PPHN were enrolled, at < 3 days old Mean GA: 38.4 (SD 2.6) weeks Group 2: n = 6 | |
| Interventions | Group 1: sildenafil (via orogastric tube) first dose of 1 mg/kg (0.5 mL/kg), subsequent doses every 6 hours; could be doubled to 2 mg/kg (1 mL/kg) if OI did not improve and BP remained stable until participant received a maximum of 8 doses, or until OI improved to < 20 | |
| Outcomes | Mortality | |
| Notes | OI was determined for all 7 participants in sildenafil group for baseline and for first 6 doses. Oxygenation index was reported for only 4 participants for the seventh dose (2 met the pre‐set exit criteria for the study, and 1 died) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on sequence generation was reported |
| Allocation concealment (selection bias) | Low risk | Randomisation was by simple allocation of pre‐sealed numbers. No information is provided on whether envelopes were opaque or were sequentially numbered |
| Blinding of participants and personnel (performance bias) | Low risk | Bedside clinicians were masked to treatment |
| Blinding of outcome assessment (detection bias) | Low risk | Primary outcome measures are not subjective |
| Incomplete outcome data (attrition bias) | Low risk | Data on all enrolled participants were reported |
| Selective reporting (reporting bias) | Unclear risk | A protocol or trial registration was not available to assess selective reporting |
| Other bias | Unclear risk | Small sample size; study was terminated prematurely |
| Methods | Randomised controlled trial | |
| Participants | 24 term neonates with PPHN and OI > 25 Group 1: n = 13 Male: 5/13 (38%) Group 2: n = 11 Male: 6/11 (55%) | |
| Interventions | Group 1: sildenafil 2 mg/kg via orogastric tube (duration of administration ‐ 72 hours) | |
| Outcomes | Changes in OI, PaCO2, A‐a DO2, PaO2 Death | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported |
| Allocation concealment (selection bias) | Unclear risk | Reported as blinded study, but no information provided on how treatment allocation was concealed |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study was described as blinded, but no information was provided on how blinding was achieved |
| Blinding of outcome assessment (detection bias) | Unclear risk | Study was described as blinded, but no information was provided on how blinding was achieved |
| Incomplete outcome data (attrition bias) | High risk | Newborns whose data were incomplete or who were transferred to other hospitals were excluded |
| Selective reporting (reporting bias) | Unclear risk | A protocol or trial registration was not available for assessment of selective reporting |
| Other bias | Low risk | Appears free of other bias |
| Methods | Randomised clinical trial | |
| Participants | 65 term and near‐term neonates (35 to 42 weeks' GA) with persistent hypoxaemic respiratory failure (PAP ≥ 40 mmHg, OI ≥ 30, and need for mechanical ventilation) associated with PPHN were enrolled Group 1: sildenafil (n = 31) Group 2: MgSo4 (n = 34) Male: 20/34 (59%) | |
| Interventions | Group 1: 25 mg tablet of sildenafil diluted with 25 mL sterile water for final concentration of 1 mg/mL. Dose of solution (0.5 mg/kg) was given via orogastric tube every 6 hours. Dose could be doubled (until maximum of 2 mg/kg) if OI did not improve. Sildenafil dose was tapered 50% after reaching OI level < 15 and PAP < 20 mmHg and was terminated in 1 day Group 2: MgSO4 loading dose of 200 mg/kg infused IV over 30 minutes followed by maintenance dose of 20 mg/kg/h. If OI did not improve sufficiently, MgSO4 infusion rate was increased slowly (10 mg/kg/h to maximum 100 mg/kg/h). When OI level was < 15 and PAP was < 20 mmHg, MgSO4 infusion was decreased gradually (10 mg/kg/h) and was terminated in 1 day | |
| Outcomes | Primary outcome: time of adequate clinical response (defined as a decrease in PAP to < 20 mmHg (improvement in PAP) and in OI to < 15 (improvement in OI)) Secondary outcomes: Duration of mechanical ventilation Support of inotropic agent Mortality rate Adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent researcher used a computer‐generated randomisation table |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed |
| Blinding of participants and personnel (performance bias) | High risk | As MgSO4 was given IV and sildenafil was given by orogastric tube, care providers could not be blinded to treatment groups |
| Blinding of outcome assessment (detection bias) | Low risk | Data collector nurse, paediatric cardiologist, and ultrasonographer were blinded. Only the neonatologist who gave medical care to participants was not blinded to the type of treatment received |
| Incomplete outcome data (attrition bias) | High risk | Data were not analysed as intent to treat. Data were incomplete (missing) for 7 neonates (2 MgSO4, 5 sildenafil), 3 of whom had gastrointestinal bleeding |
| Selective reporting (reporting bias) | Unclear risk | A protocol or trial registration was not available for assessment of selective reporting |
| Other bias | Low risk | Appears free of other bias |
| Methods | Randomised controlled trial | |
| Participants | 40 term neonates with PPHN and OI > 20 Group 1: n = 31 Male: 16/31(%) Male: 13/20 (%) | |
| Interventions | Group 1: sildenafil 2 mg/kg via orogastric tube 3 mg/kg/dose until OI < 10 | |
| Outcomes | Changes in OI, PaO2, A‐aDO2, PaCO2, mean airway pressure Mortality Time on mechanical ventilation | |
| Notes | Published manuscript described 31 participants in the sildenafil group (20 randomised to receive placebo, and another 11 randomised to receive nitric oxide). We contacted study authors and retrieved data for the first 40 infants (20 in each group). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence was generated by a random numbers table (information provided by study author). |
| Allocation concealment (selection bias) | Low risk | Allocation was generated by 2 nurses who were not involved with the study. |
| Blinding of participants and personnel (performance bias) | Low risk | Described as blinded. "Pharmacy prepared the solution and bedside clinicians were unaware of group assignment" (information provided by study author). |
| Blinding of outcome assessment (detection bias) | Low risk | Described as blinded. "Outcome assessment was masked as nurses were unaware of treatment allocation" (information provided by study author) |
| Incomplete outcome data (attrition bias) | High risk | Published report had combined data on 51 participants and did not include information on when the change in randomisation occurred |
| Selective reporting (reporting bias) | Unclear risk | Protocol or trial registration was not available for assessment of selective reporting |
| Other bias | Unclear risk | Published data included subsequent participants; therefore, it is difficult to assess other bias |
A‐a DO2 = alveolar‐arterial oxygen difference
BP = blood pressure
GA = gestational age
iNO = inhaled nitric oxide
MgSO4 = magnesium sulphate
OI = oxygenation index
PaCO2 = partial pressure of carbon dioxide in arterial blood
PaO2 = partial pressure of oxygen in arterial blood
PAP = pulmonary artery pressure
PMA = postmenstrual age
PPHN = persistent pulmonary hypertension of the newborn
ROP = retinopathy of prematurity
SD = standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Retrospective study design | |
| Unclear whether patients had persistent pulmonary hypertension in neonates (PPHN); newborns on nitric oxide (NO) were excluded | |
| Included patients whose age ranged from 0.1 years onwards | |
| Single‐arm study; no control arm | |
| Open‐label single‐arm study; no randomisation | |
| Only infants after cardiac surgery were included |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised controlled trial |
| Participants | 32 neonates born at 34 weeks or later with PPHN (cyanosis that continued 30 minutes after ventilation with 100% supplemental oxygen via hood) Group 1: n = 16 No participant demographics were reported No participant demographics were reported |
| Interventions | Tadalafil orally; 1 mg/kg/d Sildenafil orally; 1 mg/kg/dose 3 times daily |
| Outcomes | Tricuspid regurgitation (TR) severity assessed by echocardiography at baseline and after 6 months Mean pulmonary arterial pressure (MPAP) recorded via echocardiography based on gradient of pulmonary insufficiency or gradient TR on Doppler echocardiography Right ventricular end‐diastolic diameter (RVEDD) assessed by echocardiography at baseline and after 6 months Main pulmonary artery (MPA) diameter assessed by echocardiography at baseline and 6 months after treatment |
| Notes | Funding support, potential conflicts, and trial registration were not reported. This study was classified as awaiting classification, as the review authors required clarification of the time period of measurement. It is reported that echocardiography took place 6 months after treatment, which we believe could have taken hours. We have contacted the corresponding author multiple times |
| Methods | Randomised clinical trial |
| Participants | Inclusion criteria: Diagnosis of PPHN in the NICU, primary disease:neonate respiratory distress syndrome, meconium aspiration syndrome of newborn, severe neonatal infectious pneumonia. Pulmonary artery pressure > 50 mmHg, mechanical ventilation over 48 hours, primary OI (PO2/FiO2) < 300, difference of SpO2 between upper and lower limbs > 10%, high FiO2, oxygen inhalation test: positive Exclusion criteria: |
| Interventions | Nitric oxide inhalation continued with sildenafil vs inhaled nitric oxide alone |
| Outcomes | Primary outcome measures: Persistent normal pulmonary artery pressure Pulmonary artery pressure returned to a normal level (< 30 mmHg) and lasting over 48 hours Recovery without complication (time frame: 1 month after therapy) Incidence of pulmonary disease (chronic lung disease) Incidence of brain injury (hypoxic‐ischaemic encephalopathy) Heart structure change (right ventricle enlarged) |
| Notes | Status verified as "recruiting" in January 2011 by Third Military Medical University |
| Methods | Randomised controlled trial |
| Participants | 40 neonates (GA > 34 weeks) admitted within 12 hours of delivery with diagnosis of MAS and development of PPHN Group 1: n = 20 Male: 9/20 (%) Group 2: n = 20 Male: 12/20 (%) |
| Interventions | Group 1: oral sildenafil (1 mg/kg/dose q6hours) administered through feeding tube for a total of 8 doses (treatment for 2 days) Group 2: placebo |
| Outcomes | Oxygen saturation Oxygenation index Length of hospitalisation Duration of mechanical ventilation Mortality |
| Notes | We identified study through ClinicalTrials.gov and contacted study authors, who stated that trial results are unavailable yet, but recruitment has been completed |
| Methods | Randomised clinical trial |
| Participants | 49 term neonates with PPHN and OI > 25 were included |
| Interventions | Randomised to placebo (n = 20) vs sildenafil (n = 29) |
| Outcomes | OI, mean blood pressure and mean airway pressure |
| Notes | Difficult to differentiate from included studies, as some study authors overlap |
FiO2 = fraction of inspired oxygen
GA = gestational age
MAS = meconium aspiration syndrome
MPA = mean pulmonary artery
MPAP = mean pulmonary arterial pressure
NICU = neonatal intensive care unit
OI = oxygenation index
PO2 = partial pressure of oxygen
PPHN = persistent pulmonary hypertension in the neonate
RVEDD = right ventricular end‐diastolic diameter
SD = standard deviation
SpO2 = peripheral capillary oxygen saturation
TR = tricuspid regurgitation
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A study to evaluate the safety and efficacy of IV sildenafil in the treatment of neonates with persistent pulmonary hypertension of the newborn |
| Methods | Multi‐centre randomised placebo‐controlled double‐blind 2‐armed parallel‐group study |
| Participants | Inclusion criteria: Neonates with persistent pulmonary hypertension of the newborn Age ≤ 96 hours and ≥ 34 weeks' gestational age Oxygenation index > 15 and < 60 Concurrent treatment with inhaled nitric oxide and ≥ 50% oxygen Exclusion criteria: Prior or immediate need for extracorporeal membrane oxygenation or cardiopulmonary resuscitation Expected duration of mechanical ventilation < 48 hours Profound hypoxaemia Life‐threatening or lethal congenital anomaly |
| Interventions | Treatment: intravenous (IV) sildenafil · Loading dose of 0.1 mg/kg over 30 minutes followed by maintenance dose of 0.03 mg/kg/h. To infuse minimum of 48 hours and maximum of 14 days Control: placebo IV placebo or 0.9% sodium chloride or 10% dextrose. Infusion rate based on weight |
| Outcomes | Primary outcome measures (at day 14 or until discharge): · Time on inhaled nitric oxide treatment after initiation of IV study drug · Treatment failure rate, defined as need for additional treatment targeting persistent pulmonary hypertension of the newborn Secondary outcome measures: · Time to final weaning off mechanical ventilation for persistent pulmonary hypertension of the newborn · Time from initiation of study drug to treatment failure · Change in oxygenation parameters at 6, 12, and 24 hours from baseline · Sildenafil plasma concentrations and corresponding pharmacokinetic (PK) parameters · Safety parameters: incidence and severity of adverse events and abnormal laboratory parameters Long‐term outcomes (12 and 24 months): · Developmental progress of participants as assessed by Bayley Scales of Infant Development and Behavior Questionnaire · Safety as assessed by adverse events and survival · Neurological progress of participants as assessed by the Neurology Optimality Score, also known as the Hammersmith Infant Neurological Examination · Visual status of participants as assessed by eye examination of anterior and posterior segments · Audiological status of participants as assessed by physiological and behavioural tests |
| Starting date | 17 September 2012 |
| Contact information | Pfizer CT.gov call centre: 1‐800‐718‐1021 |
| Notes | Includes a long‐term follow‐up investigation of developmental progress at 12 and 24 months |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
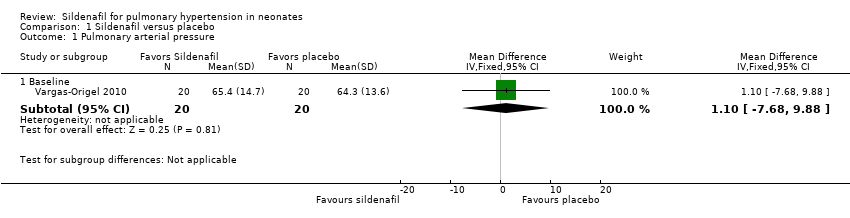
| 1 Pulmonary arterial pressure Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1 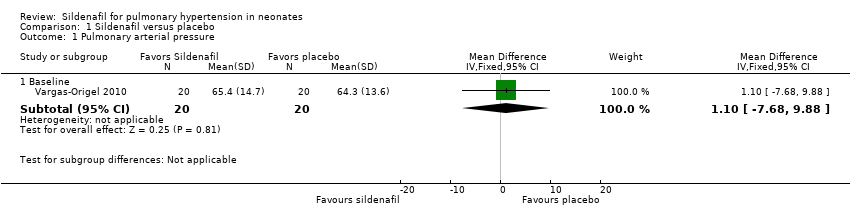 Comparison 1 Sildenafil versus placebo, Outcome 1 Pulmonary arterial pressure. | ||||
| 1.1 Baseline | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [‐7.68, 9.88] |
| 2 PaO2 in mmHg (absolute values) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2 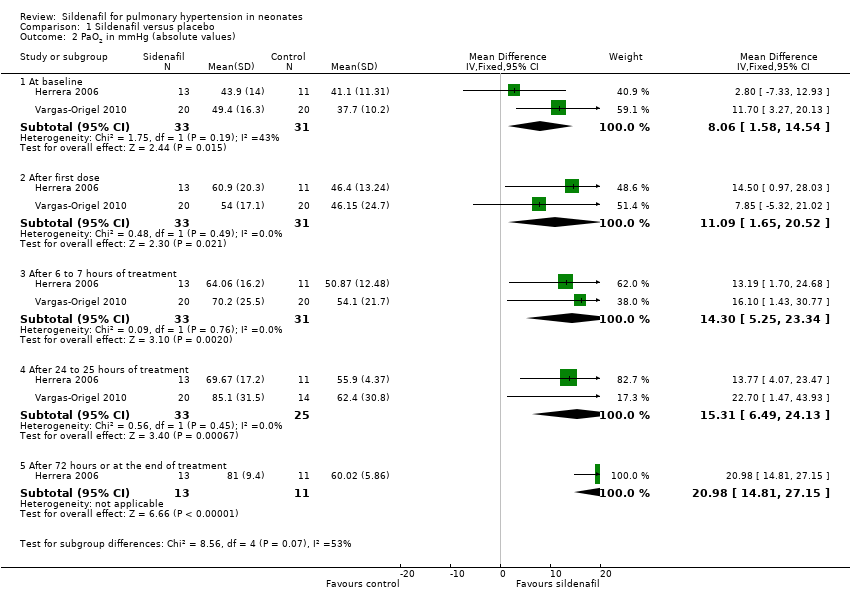 Comparison 1 Sildenafil versus placebo, Outcome 2 PaO2 in mmHg (absolute values). | ||||
| 2.1 At baseline | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | 8.06 [1.58, 14.54] |
| 2.2 After first dose | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | 11.09 [1.65, 20.52] |
| 2.3 After 6 to 7 hours of treatment | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | 14.30 [5.25, 23.34] |
| 2.4 After 24 to 25 hours of treatment | 2 | 58 | Mean Difference (IV, Fixed, 95% CI) | 15.31 [6.49, 24.13] |
| 2.5 After 72 hours or at the end of treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 20.98 [14.81, 27.15] |
| 3 Mean arterial blood pressure in mmHg Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3 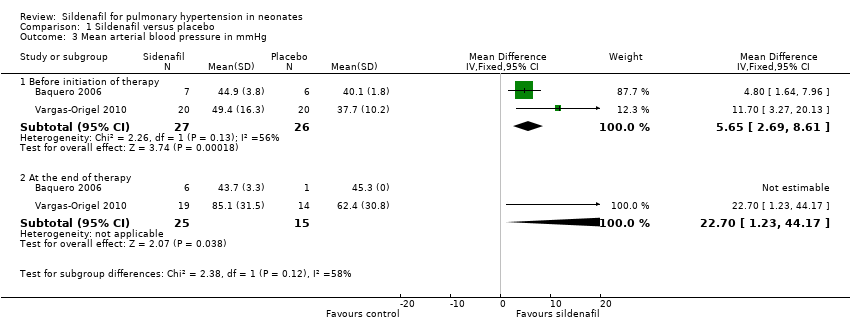 Comparison 1 Sildenafil versus placebo, Outcome 3 Mean arterial blood pressure in mmHg. | ||||
| 3.1 Before initiation of therapy | 2 | 53 | Mean Difference (IV, Fixed, 95% CI) | 5.65 [2.69, 8.61] |
| 3.2 At the end of therapy | 2 | 40 | Mean Difference (IV, Fixed, 95% CI) | 22.70 [1.23, 44.17] |
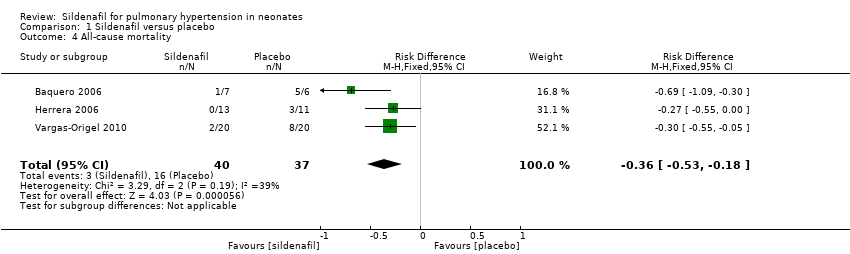
| 4 All‐cause mortality Show forest plot | 3 | 77 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.36 [‐0.53, ‐0.18] |
| Analysis 1.4  Comparison 1 Sildenafil versus placebo, Outcome 4 All‐cause mortality. | ||||
| 5 Oxygenation index (absolute values) Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Sildenafil versus placebo, Outcome 5 Oxygenation index (absolute values). | ||||
| 5.1 At baseline | 3 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐0.74 [‐8.11, 6.64] |
| 5.2 After first dose | 3 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐12.53 [‐18.60, ‐6.47] |
| 5.3 After 6 to 7 hours of treatment | 2 | 75 | Mean Difference (IV, Fixed, 95% CI) | ‐20.07 [‐26.12, ‐14.02] |
| 5.4 After 24 to 25 hours of treatment | 3 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐19.15 [‐24.52, ‐13.77] |
| 5.5 After 30 hours of treatment | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | ‐45.46 [‐61.87, ‐29.05] |
| 5.6 After 36 hours of treatment | 1 | 8 | Mean Difference (IV, Fixed, 95% CI) | ‐31.75 [‐45.74, ‐17.76] |
| 5.7 After 72 hours or at the end of treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐19.47 [‐23.42, ‐15.52] |
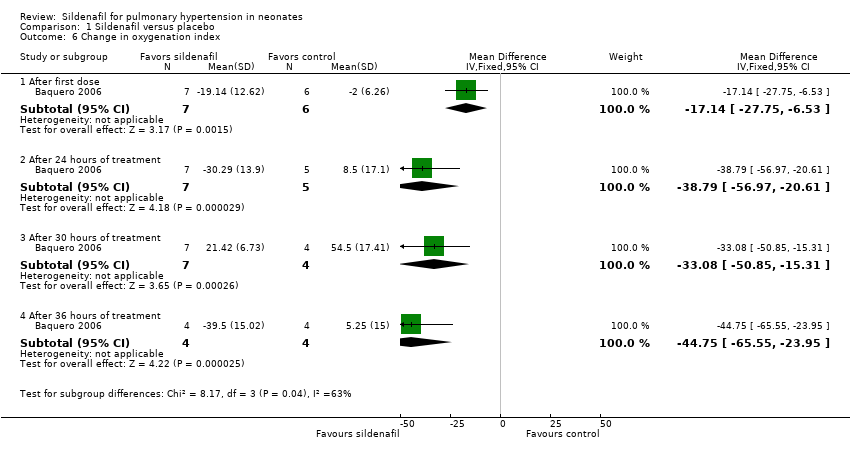
| 6 Change in oxygenation index Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Sildenafil versus placebo, Outcome 6 Change in oxygenation index. | ||||
| 6.1 After first dose | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | ‐17.14 [‐27.75, ‐6.53] |
| 6.2 After 24 hours of treatment | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐38.79 [‐56.97, ‐20.61] |
| 6.3 After 30 hours of treatment | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | ‐33.08 [‐50.85, ‐15.31] |
| 6.4 After 36 hours of treatment | 1 | 8 | Mean Difference (IV, Fixed, 95% CI) | ‐44.75 [‐65.55, ‐23.95] |
| 7 A‐a DO2 difference Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Sildenafil versus placebo, Outcome 7 A‐a DO2 difference. | ||||
| 7.1 Baseline | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | 0.99 [‐11.54, 13.51] |
| 7.2 At 6 to 7 hours of treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐27.72, 27.74] |
| 7.3 At 24 to 25 hours of treament | 2 | 57 | Mean Difference (IV, Fixed, 95% CI) | 1.59 [‐18.98, 22.16] |
| 7.4 At 72 hours or at the end of treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐18.34 [‐26.59, ‐10.09] |
| 8 Mean airway pressure Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.8 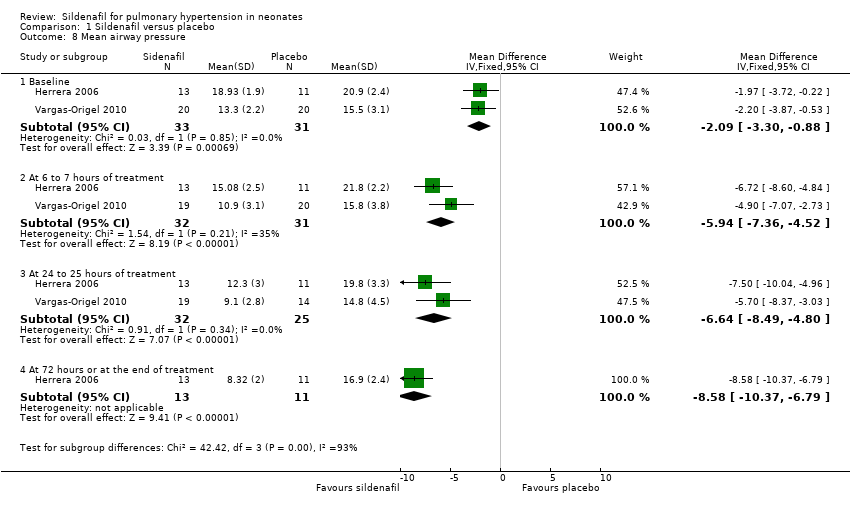 Comparison 1 Sildenafil versus placebo, Outcome 8 Mean airway pressure. | ||||
| 8.1 Baseline | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐2.09 [‐3.30, ‐0.88] |
| 8.2 At 6 to 7 hours of treatment | 2 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐5.94 [‐7.36, ‐4.52] |
| 8.3 At 24 to 25 hours of treatment | 2 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐6.64 [‐8.49, ‐4.80] |
| 8.4 At 72 hours or at the end of treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐8.58 [‐10.37, ‐6.79] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 1 | 65 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.03 [‐0.13, 0.07] |
| Analysis 2.1  Comparison 2 Sildenafil versus active control, Outcome 1 All‐cause mortality. | ||||
| 2 Time to adequate response (days) Show forest plot | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐4.20, 3.00] |
| Analysis 2.2  Comparison 2 Sildenafil versus active control, Outcome 2 Time to adequate response (days). | ||||
| 3 Duration of ventilation (days) Show forest plot | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐4.54, 1.94] |
| Analysis 2.3 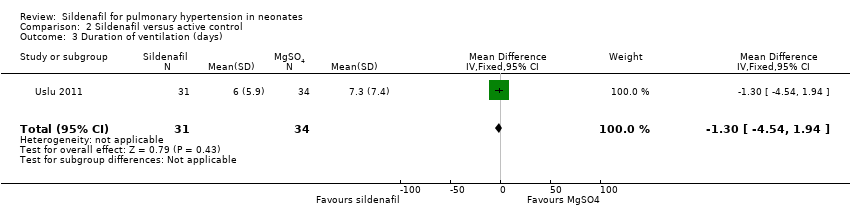 Comparison 2 Sildenafil versus active control, Outcome 3 Duration of ventilation (days). | ||||
| 4 Inotropic agent Show forest plot | 1 | 65 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.37 [‐0.59, ‐0.15] |
| Analysis 2.4  Comparison 2 Sildenafil versus active control, Outcome 4 Inotropic agent. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 1 | 24 | Risk Difference (M‐H, Fixed, 95% CI) | 0.05 [‐0.27, 0.37] |
| Analysis 3.1  Comparison 3 Sildenafil plus iNO versus placebo plus iNO, Outcome 1 All‐cause mortality. | ||||
| 2 Length of stay in hospital (days) Show forest plot | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 5.39 [‐5.31, 16.09] |
| Analysis 3.2  Comparison 3 Sildenafil plus iNO versus placebo plus iNO, Outcome 2 Length of stay in hospital (days). | ||||
| 3 Inotropic agent Show forest plot | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.37, 3.00] |
| Analysis 3.3  Comparison 3 Sildenafil plus iNO versus placebo plus iNO, Outcome 3 Inotropic agent. | ||||
| 4 Duration of ventilation (days) Show forest plot | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 1.26 [‐1.32, 3.84] |
| Analysis 3.4  Comparison 3 Sildenafil plus iNO versus placebo plus iNO, Outcome 4 Duration of ventilation (days). | ||||
| 5 Retinopathy of prematurity Show forest plot | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 3.5  Comparison 3 Sildenafil plus iNO versus placebo plus iNO, Outcome 5 Retinopathy of prematurity. | ||||

Study flow diagram: review update.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Comparison 1 Sildenafil versus placebo, Outcome 1 Pulmonary arterial pressure.

Comparison 1 Sildenafil versus placebo, Outcome 2 PaO2 in mmHg (absolute values).

Comparison 1 Sildenafil versus placebo, Outcome 3 Mean arterial blood pressure in mmHg.
Comparison 1 Sildenafil versus placebo, Outcome 4 All‐cause mortality.

Comparison 1 Sildenafil versus placebo, Outcome 5 Oxygenation index (absolute values).
Comparison 1 Sildenafil versus placebo, Outcome 6 Change in oxygenation index.

Comparison 1 Sildenafil versus placebo, Outcome 7 A‐a DO2 difference.

Comparison 1 Sildenafil versus placebo, Outcome 8 Mean airway pressure.
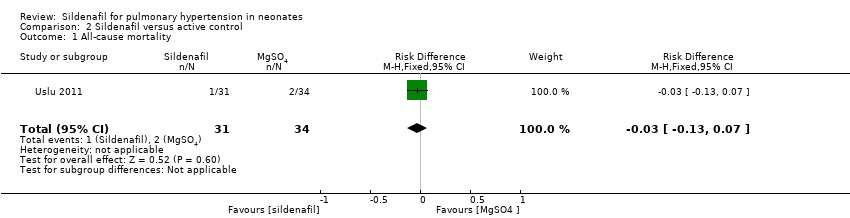
Comparison 2 Sildenafil versus active control, Outcome 1 All‐cause mortality.
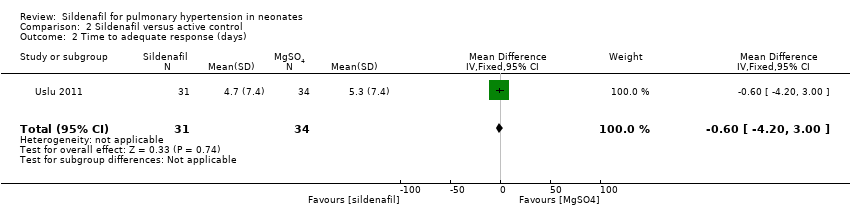
Comparison 2 Sildenafil versus active control, Outcome 2 Time to adequate response (days).
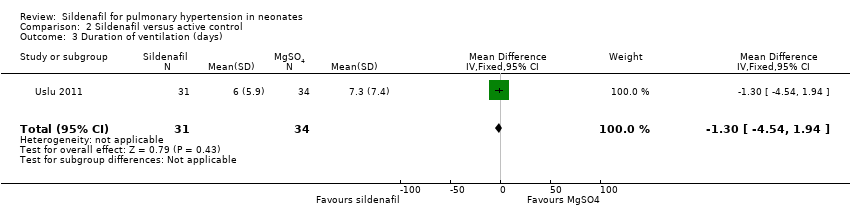
Comparison 2 Sildenafil versus active control, Outcome 3 Duration of ventilation (days).

Comparison 2 Sildenafil versus active control, Outcome 4 Inotropic agent.
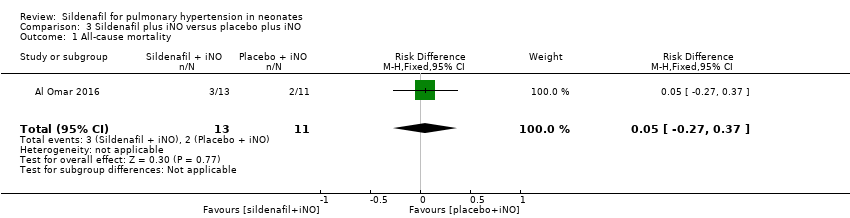
Comparison 3 Sildenafil plus iNO versus placebo plus iNO, Outcome 1 All‐cause mortality.
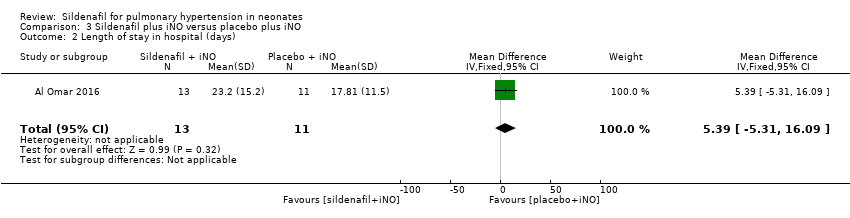
Comparison 3 Sildenafil plus iNO versus placebo plus iNO, Outcome 2 Length of stay in hospital (days).

Comparison 3 Sildenafil plus iNO versus placebo plus iNO, Outcome 3 Inotropic agent.
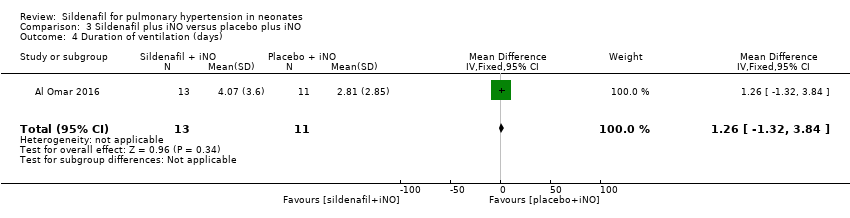
Comparison 3 Sildenafil plus iNO versus placebo plus iNO, Outcome 4 Duration of ventilation (days).

Comparison 3 Sildenafil plus iNO versus placebo plus iNO, Outcome 5 Retinopathy of prematurity.
| Sildenafil compared with placebo for pulmonary hypertension in neonates | ||||||
| Patient or population: pulmonary hypertension in neonates | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with sildenafil | |||||
| PaO2 in mmHg (absolute values) After 24‐25 hours | Mean PaO2 in mmHg (absolute values) | MD 15.31 higher | ‐ | 57 | ⊕⊕⊝⊝ | Evidence was downgraded due to unreported methodological features and imprecision (small sample size) |
| Change in oxygenation index | Mean change in oxygenation index | MD 38.79 lower | ‐ | 12 | ⊕⊕⊝⊝ | Evidence was downgraded due to unreported methodological features and imprecision (small sample size) |
| All‐cause mortality | Study population | RR 0.20 | 77 | ⊕⊕⊝⊝ | Evidence was downgraded due to unreported methodological features and imprecision (small sample size) | |
| 432 per 1000 | 77 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aImprecise due to small sample size bRisk of bias due to unclear randomisation allocation and lack of clinical trial registration | ||||||
| Sildenafil compared with active control for pulmonary hypertension in neonates | ||||||
| Patient or population: pulmonary hypertension in neonates | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with magnesium sulphate | Risk with sildenafil | |||||
| All‐cause mortality | Study population | RR 0.55 | 65 | ⊕⊝⊝⊝ | Evidence was downgraded due to very serious imprecision, as results from this single study have not been replicated and risk of bias is evident in study design (missing data) | |
| 59 per 1000 | 32 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aImprecise due to small sample size; only one included study bRisk of bias due to missing data (not analysed as intent to treat) | ||||||
| Sildenafil plus iNO compared with placebo plus iNO for pulmonary hypertension in neonates | ||||||
| Patient or population: pulmonary hypertension in neonates | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with placebo plus iNO | Risk with sildenafil plus iNO | |||||
| All‐cause mortality | Study population | RR 1.27 | 24 | ⊕⊕⊝⊝ | Evidence was downgraded due to imprecision . | |
| 182 per 1000 | 231 per 1000 | |||||
| Length of stay in hospital (days) | Mean length of stay in hospital was 17.81 days. | MD 5.39 higher | ‐ | 24 | ⊕⊕⊝⊝ | Evidence was downgraded due to imprecision . |
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aImprecise due to very small sample size; only one included study | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pulmonary arterial pressure Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Baseline | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [‐7.68, 9.88] |
| 2 PaO2 in mmHg (absolute values) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At baseline | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | 8.06 [1.58, 14.54] |
| 2.2 After first dose | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | 11.09 [1.65, 20.52] |
| 2.3 After 6 to 7 hours of treatment | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | 14.30 [5.25, 23.34] |
| 2.4 After 24 to 25 hours of treatment | 2 | 58 | Mean Difference (IV, Fixed, 95% CI) | 15.31 [6.49, 24.13] |
| 2.5 After 72 hours or at the end of treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 20.98 [14.81, 27.15] |
| 3 Mean arterial blood pressure in mmHg Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Before initiation of therapy | 2 | 53 | Mean Difference (IV, Fixed, 95% CI) | 5.65 [2.69, 8.61] |
| 3.2 At the end of therapy | 2 | 40 | Mean Difference (IV, Fixed, 95% CI) | 22.70 [1.23, 44.17] |
| 4 All‐cause mortality Show forest plot | 3 | 77 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.36 [‐0.53, ‐0.18] |
| 5 Oxygenation index (absolute values) Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 At baseline | 3 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐0.74 [‐8.11, 6.64] |
| 5.2 After first dose | 3 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐12.53 [‐18.60, ‐6.47] |
| 5.3 After 6 to 7 hours of treatment | 2 | 75 | Mean Difference (IV, Fixed, 95% CI) | ‐20.07 [‐26.12, ‐14.02] |
| 5.4 After 24 to 25 hours of treatment | 3 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐19.15 [‐24.52, ‐13.77] |
| 5.5 After 30 hours of treatment | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | ‐45.46 [‐61.87, ‐29.05] |
| 5.6 After 36 hours of treatment | 1 | 8 | Mean Difference (IV, Fixed, 95% CI) | ‐31.75 [‐45.74, ‐17.76] |
| 5.7 After 72 hours or at the end of treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐19.47 [‐23.42, ‐15.52] |
| 6 Change in oxygenation index Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 After first dose | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | ‐17.14 [‐27.75, ‐6.53] |
| 6.2 After 24 hours of treatment | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐38.79 [‐56.97, ‐20.61] |
| 6.3 After 30 hours of treatment | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | ‐33.08 [‐50.85, ‐15.31] |
| 6.4 After 36 hours of treatment | 1 | 8 | Mean Difference (IV, Fixed, 95% CI) | ‐44.75 [‐65.55, ‐23.95] |
| 7 A‐a DO2 difference Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Baseline | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | 0.99 [‐11.54, 13.51] |
| 7.2 At 6 to 7 hours of treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐27.72, 27.74] |
| 7.3 At 24 to 25 hours of treament | 2 | 57 | Mean Difference (IV, Fixed, 95% CI) | 1.59 [‐18.98, 22.16] |
| 7.4 At 72 hours or at the end of treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐18.34 [‐26.59, ‐10.09] |
| 8 Mean airway pressure Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Baseline | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐2.09 [‐3.30, ‐0.88] |
| 8.2 At 6 to 7 hours of treatment | 2 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐5.94 [‐7.36, ‐4.52] |
| 8.3 At 24 to 25 hours of treatment | 2 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐6.64 [‐8.49, ‐4.80] |
| 8.4 At 72 hours or at the end of treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐8.58 [‐10.37, ‐6.79] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 1 | 65 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.03 [‐0.13, 0.07] |
| 2 Time to adequate response (days) Show forest plot | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐4.20, 3.00] |
| 3 Duration of ventilation (days) Show forest plot | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐4.54, 1.94] |
| 4 Inotropic agent Show forest plot | 1 | 65 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.37 [‐0.59, ‐0.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 1 | 24 | Risk Difference (M‐H, Fixed, 95% CI) | 0.05 [‐0.27, 0.37] |
| 2 Length of stay in hospital (days) Show forest plot | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 5.39 [‐5.31, 16.09] |
| 3 Inotropic agent Show forest plot | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.37, 3.00] |
| 4 Duration of ventilation (days) Show forest plot | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 1.26 [‐1.32, 3.84] |
| 5 Retinopathy of prematurity Show forest plot | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |

