Sildenafil w nadciśnieniu płucnym u noworodków
Abstract
Background
Persistent pulmonary hypertension in the neonate (PPHN) is associated with high mortality. Currently, the therapeutic mainstay for PPHN consists of assisted ventilation and administration of inhaled nitric oxide (iNO). However, nitric oxide is costly, and its use may not be appropriate in resource‐poor settings. Approximately 30% of patients fail to respond to iNO. High concentrations of phosphodiesterases in the pulmonary vasculature have led to the use of phosphodiesterase inhibitors such as sildenafil or milrinone.
Objectives
To assess the efficacy and safety of sildenafil for treatment of pulmonary hypertension in neonates.
Search methods
We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 3), MEDLINE via PubMed (1966 to 18 April 2017), Embase (1980 to 18 April 2017), and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to 18 April 2017). We searched clinical trials databases, conference proceedings, and reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials.
Selection criteria
We included randomised and quasi‐randomised controlled trials of sildenafil compared with placebo or other pulmonary vasodilators, irrespective of dose, route, and duration of administration, in neonates with pulmonary hypertension, if investigators reported any of the prespecified outcomes.
Data collection and analysis
We assessed the methodological quality of trials regarding how bias was minimised at study entry, during study intervention, and at outcomes measurement. We extracted data on relevant outcomes; we estimated the effect size and reported it as risk ratio (RR), risk difference (RD), or mean difference (MD), as appropriate. We applied the I2 test of heterogeneity and used GRADE to assess the quality of evidence.
Main results
For this update, we identified two additional studies, for a total of five eligible trials that enrolled 166 infants. The methodological quality of these studies ranged from low to high risk of bias. Three studies were performed in resource‐limited settings, where iNO and high‐frequency ventilation were not available at the time of the study. One study compared sildenafil versus active controls, and another study evaluated sildenafil as adjuvant therapy to iNO. When comparing sildenafil with placebo, investigators noted significant reduction in mortality in the sildenafil alone group (three studies, 77 participants; typical RR 0.20, 95% confidence interval (CI) 0.07 to 0.56; I2 = 0% ‐ none; typical RR ‐0.36, 95% CI ‐0.53 to ‐0.18; number needed to treat for an additional beneficial outcome 3, 95% CI 2 to 6; I2 = 39% ‐ low). Trials reported no significant differences in mortality upon comparison of the sildenafil group versus the active control group (one study, 65 participants; typical RR 0.55, 95% CI 0.05 to 5.75), or when iNO was administered to both groups (one study, 24 participants; typical RR 1.27, 95% CI 0.26 to 6.28). Physiological parameters of oxygenation (oxygenation index, partial pressure of oxygen in arterial blood (PaO2)) suggested steady improvement after the first dose of sildenafil. None of the included trials identified any clinically important side effects. We rated the quality of evidence as low to very low owing to imprecision related to small sample size and unclear methodological features.
Authors' conclusions
Sildenafil used for treatment of pulmonary hypertension has potential for reducing mortality and improving oxygenation in neonates, especially in resource‐limited settings where iNO is not available. However, large‐scale randomised trials comparing sildenafil versus active controls (other pulmonary vasodilators) and providing follow‐up for survivors are needed to assess the comparative effectiveness and long‐term safety of sildenafil versus other pulmonary vasodilators.
PICO
Streszczenie prostym językiem
Sildenafil w nadciśnieniu płucnym u noworodków
Pytanie badawcze
Czy sildenafil jest bezpieczny i skuteczny w leczeniu nadciśnienia płucnego u noworodków?
Wprowadzenie
Kiedy rodzi się dziecko, ciśnienie w naczyniach krwionośnych w płucach jest wysokie i w momencie ustabilizowania oddechu, ciśnienie to zaczyna spadać. U niektórych dzieci zjawisko to nie występuje i ciśnienie pozostaje wysokie, co nie pozwala dotrzeć krwi do płuc i dostarczyć odpowiedniej ilości tlenu. Stan ten nazywany jest przetrwałym nadciśnieniem płucnym u noworodków (ang. persistent pulmonary hypertension of the neonate, PPHN; przyp. tłum.). Inne sytuacje mogą prowadzić do rozwoju wysokiego ciśnienia w naczyniach krwionośnych w płucach w ciągu kilku dni po urodzeniu. Przetrwałe nadciśnienie w tych naczyniach może skutkować mniejszą ilością tlenu dostarczanego do wszystkich narządów. Lek o nazwie sildenafil może powodować rozszerzenie naczyń płucnych, poprawiając przepływ krwi oraz dostarczanie tlenu do wszystkich narządów.
Charakterystyka badania
Odnaleźliśmy pięć badań, które oceniały skuteczność sildenafilu: trzy badania porównywały sildenafil z placebo (leczenie pozorowane); jedno badanie porównywało sildenafil z innymi lekiem (siarczanem magnezu) oraz w jednym badaniu stosowano sildenafil w połączeniu z innym lekiem (tlenkiem azotu). Badania te obejmowały łącznie 166 noworodków i zostały przeprowadzone w Kolumbii, Meksyku, Turcji i Katarze.
Główne wyniki
Trzy badania, które porównywały sildenafil z placebo wykazały, że stosowanie sildenafilu zmniejsza liczbę zgonów. Badania, które porównywały sildenafil z innym lekiem lub połączeniem stosowania sildenafilu z inną metodą leczenia nie wykazały istotnych różnic w zmniejszeniu liczby zgonów. Sildenafil był bardziej skuteczny niż placebo w poprawie poziomu dotlenienia. Żadne z pięciu włączonych badań nie oceniało bezpieczeństwa leczenia. Jednak badania te obejmowały małą liczbę noworodków i większość z nich przeprowadzono w miejscach, gdzie inne leczenie nie jest dostępne. Sildenafil może być użyteczny w miejscach, w których inne metody leczenia nie są dostępne. Jednakże potrzebne są dodatkowe badania porównujące sildenafil z innymi stosowanymi metodami leczenia na obszarach rozwiniętych w celu oceny jego skuteczności i bezpieczeństwa.
Jakość danych naukowych
Jakość danych naukowych dotyczących zmniejszenia umieralności lub poprawy parametrów oddechowych była niska z powodu małej liczby włączonych badań oraz małej liczby objętych nimi dzieci. W niektórych włączonych badaniach wystąpiły problemy metodologiczne, skutkujące niską lub bardzo niską jakością danych naukowych.
Authors' conclusions
Summary of findings
| Sildenafil compared with placebo for pulmonary hypertension in neonates | ||||||
| Patient or population: pulmonary hypertension in neonates | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with sildenafil | |||||
| PaO2 in mmHg (absolute values) After 24‐25 hours | Mean PaO2 in mmHg (absolute values) | MD 15.31 higher | ‐ | 57 | ⊕⊕⊝⊝ | Evidence was downgraded due to unreported methodological features and imprecision (small sample size) |
| Change in oxygenation index | Mean change in oxygenation index | MD 38.79 lower | ‐ | 12 | ⊕⊕⊝⊝ | Evidence was downgraded due to unreported methodological features and imprecision (small sample size) |
| All‐cause mortality | Study population | RR 0.20 | 77 | ⊕⊕⊝⊝ | Evidence was downgraded due to unreported methodological features and imprecision (small sample size) | |
| 432 per 1000 | 77 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aImprecise due to small sample size bRisk of bias due to unclear randomisation allocation and lack of clinical trial registration | ||||||
| Sildenafil compared with active control for pulmonary hypertension in neonates | ||||||
| Patient or population: pulmonary hypertension in neonates | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with magnesium sulphate | Risk with sildenafil | |||||
| All‐cause mortality | Study population | RR 0.55 | 65 | ⊕⊝⊝⊝ | Evidence was downgraded due to very serious imprecision, as results from this single study have not been replicated and risk of bias is evident in study design (missing data) | |
| 59 per 1000 | 32 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aImprecise due to small sample size; only one included study bRisk of bias due to missing data (not analysed as intent to treat) | ||||||
| Sildenafil plus iNO compared with placebo plus iNO for pulmonary hypertension in neonates | ||||||
| Patient or population: pulmonary hypertension in neonates | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with placebo plus iNO | Risk with sildenafil plus iNO | |||||
| All‐cause mortality | Study population | RR 1.27 | 24 | ⊕⊕⊝⊝ | Evidence was downgraded due to imprecision . | |
| 182 per 1000 | 231 per 1000 | |||||
| Length of stay in hospital (days) | Mean length of stay in hospital was 17.81 days. | MD 5.39 higher | ‐ | 24 | ⊕⊕⊝⊝ | Evidence was downgraded due to imprecision . |
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aImprecise due to very small sample size; only one included study | ||||||
Background
Description of the condition
Neonatal pulmonary hypertension and persistent pulmonary hypertension of the newborn (PPHN) are terms that can be used interchangeably to describe a neonate who has cyanosis in the first few days of life in the absence of a structural congenital cardiac lesion or haemoglobinopathy (Gersony 1984). The clinical diagnosis of pulmonary hypertension is considered when hypoxaemia is refractory to oxygen therapy or to lung recruitment strategies (partial pressure of oxygen in arterial blood (PaO2) < 55 mmHg despite fraction of inspired oxygen (FiO2) of 1.0) (Roberts 1997; Shah 2004) associated with a preductal to postductal oxygen gradient greater than 20 mmHg (Walsh‐Sukys 2000). Clinicians make the echocardiographic diagnosis of PPHN by demonstrating the presence of extrapulmonary right‐to‐left shunting at the ductal or atrial level in the absence of severe pulmonary parenchymal disease with Doppler evidence of tricuspid regurgitation (Shah 2004; Wessel 1997). During cardiac catheterisation, pulmonary hypertension is defined as pulmonary arterial pressure (PAP) greater than 25 to 30 mmHg (Adatia 2002). The incidence of pulmonary hypertension among newborns has been reported as approximately 2/1000 live births, with a reported mortality rate at various centres in the United States of 4% to 33% (Walsh‐Sukys 2000). Pulmonary hypertension in the neonate can be primary (idiopathic) or can occur secondary to pulmonary parenchymal disease (such as meconium aspiration syndrome, surfactant deficiency, or alveolocapillary dysplasia), severe pulmonary hypoplasia (Adatia 2002; Gersony 1984), polycythaemia, hypoglycaemia, sepsis, or maternal ingestion of prostaglandin inhibitors.
Description of the intervention
By virtue of its selective pulmonary vasodilator effects, inhaled nitric oxide (iNO) is considered the mainstay for treatment of pulmonary hypertension among term or near‐term neonates (Barrington 2010). Approximately 30% of neonates with PPHN fail to respond to iNO (Goldman 1996). For some patients, nitric oxide therapy is associated with rebound pulmonary hypertension when therapy is discontinued, as the result of suppression of endogenous nitric oxide production (Kinsella 2000). Other potential complications include development of methaemoglobinaemia. In addition, iNO is a costly intervention (Subhedar 2002). The potential role of iNO in the treatment of preterm neonates with respiratory insufficiency remains unclear (Finer 2017).
How the intervention might work
Advances in our understanding of the physiology of vasoactive mediators have revealed a high concentration of phosphodiesterases in the pulmonary vasculature (Rabe 1994). Inhibition of phosphodiesterase‐5 leads to increased concentrations of cyclic adenosine monophosphate (AMP) and guanosine monophosphate (GMP) locally, which in turn leads to relaxation of pulmonary vascular smooth muscles (Humbert 2004). Phosphodiesterase‐5 inhibitors include dipyridamole, zaprinast, pentoxifylline, and sildenafil (Travadi 2003). Dipyridamole has a significant systemic vasodilatory effect (Dukarm 1998). Zaprinast and pentoxifylline have not been adequately studied. Sildenafil has been studied in neonatal animal models. A neonatal pig model of pulmonary hypertension induced secondary to meconium aspiration (Shekerdemian 2002) demonstrated marked improvement in pulmonary vascular resistance and cardiac output (without deterioration in systemic oxygenation) one hour after intravenous infusion of sildenafil compared with control. In a separate experiment, Shekerdemian and co‐workers observed improvement in pulmonary vascular resistance; however, improvement was associated with systemic vasodilation and deterioration of oxygenation when sildenafil (0.5 mg/kg) was administered along with 20 ppm of iNO (Shekerdemian 2004). The interaction of sildenafil with other selective pulmonary vasodilators warrants further study.
Sildenafil has been used for treatment of pulmonary hypertension in adults (Kanthapillai 2004; Sastry 2004) in intravenous, oral (Ikeda 2005), and inhaled (Ichinose 2001) forms. Uncontrolled experiments in children showed that sildenafil reduced pulmonary vascular resistance (Abrams 2000; Erickson 2002; Carroll 2003) and improved exercise capacity. Uncontrolled studies on the use of sildenafil in neonates have reported improved pulmonary vascular resistance and survival (Erickson 2002; Kumar 2002). These reports have evoked a mixed reaction from the scientific community (Kumar 2002; Lewin 2002; Oliver 2002; Patole 2002). Marsh 2004 reported severe retinopathy of prematurity following use of sildenafil in a neonate with severe pulmonary hypertension; however, another study did not report this complication (Pierce 2005). Use of sildenafil in adults is suspected to worsen proliferative diabetic retinopathy (Burton 2000; Behn 2001). Therefore, retinal vascular growth must be carefully observed, especially in preterm neonates. Sastry 2004 reported a slightly higher incidence of backache, headache, numbness of feet and hands, and constipation among adults who received sildenafil versus placebo for primary pulmonary hypertension.
Why it is important to do this review
A systematic review of sildenafil for pulmonary hypertension in adults and children identified four eligible studies including 77 participants. Review authors concluded that more studies of adequate size are necessary (Kanthapillai 2004). This review did not include neonates. The disease is more prevalent among neonates and, in most cases, has a different pathophysiology (in neonates, characterised by failure of the natural decrease in pulmonary vascular resistance; in children and adults, occurring as primary disease or secondary to various chronic illnesses such as collagen vascular disease, left heart disease, chronic obstructive pulmonary disease, interstitial disease, or chronic thromboembolic disorders). This review systematically evaluates the use of sildenafil for treatment of pulmonary hypertension in neonates.
Objectives
To assess the efficacy and safety of sildenafil for treatment of pulmonary hypertension in neonates.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised and quasi‐randomised controlled trials of sildenafil for treatment of pulmonary hypertension in neonates. We considered studies that used any route of administration (intravenous, inhaled, or oral), any dose of sildenafil, and any duration of administration. We did not include cross‐over studies owing to frequent resolution of the condition over a short time.
Types of participants
We included in the review both term and preterm infants (at postnatal age < 28 days after reaching 40 weeks' postmenstrual age (PMA)) with primary or secondary pulmonary hypertension. We included studies in which the diagnosis was based on clinical findings with or without echocardiographic confirmation. We excluded patients with known structural heart disease (other than patent foramen ovale or patent ductus arteriosus).
Types of interventions
We included the following interventions.
-
Sildenafil versus placebo or no treatment.
-
Sildenafil versus another pulmonary vasodilator.
-
Sildenafil and another pulmonary vasodilator versus another pulmonary vasodilator or placebo.
Types of outcome measures
Primary outcomes
-
Haemodynamic parameters (absolute values and change from baseline measured after the first dose, after 24 hours, after 30 hours, after 36 hours, after 42 hours, and at the end of treatment)
-
Pulmonary arterial pressure (PAP) in mmHg
-
Oxygenation (PaO2) or FiO2 requirement
-
Cardiac output in L/kg/min
-
Mean arterial blood pressure in mmHg
-
-
All‐cause mortality within the first 28 days of life (neonatal mortality)
Secondary outcomes
-
Changes in pulmonary vascular resistance index in Woods unit m2 (WUm2) (absolute values and change from baseline measured after first dose, after 24 hours, after 30 hours, after 36 hours, after 42 hours, and at the end of treatment)
-
Changes in systemic vascular resistance index in WUm2 (absolute values and change from baseline measured after first dose, after 24 hours, after 30 hours, after 36 hours, after 42 hours, and at the end of treatment)
-
Changes in oxygenation index (OI = PaO2 × FiO2/100) (absolute values and change from baseline measured after first dose, after 24 hours, after 30 hours, after 36 hours, after 42 hours, and at the end of treatment)
-
Rebound increase in PAP (dichotomous)
-
Decrease in cardiac output after weaning from sildenafil (dichotomous)
-
Extracorporeal membrane oxygenation (ECMO) treatment before discharge
-
All‐cause mortality before discharge
-
Length of hospitalisation (days)
-
Retinopathy of prematurity (among very preterm infants at < 32 weeks' gestation), any stage and stage 3 or greater
-
Intraventricular haemorrhage (any stage and grade 3 or greater)
-
Neurodevelopmental disability at 18 to 24 months (including cerebral palsy, cognitive impairment, deafness, and blindness)
-
Clinically important adverse effects reported by study authors (not prespecified)
-
Any other clinically important outcome reported by study authors (not prespecified)
-
Alveolar‐arterial oxygen difference (A‐a DO2)
-
Mean airway pressure
-
For all haemodynamic parameters, we planned to assess the change from baseline at 1, 2, 4, 6, 8, 12, 24, and 48 hours, or at nearest times reported by study authors.
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and the Cochrane Neonatal Review Group (CNRG) (see the Cochrane Neonatal search strategy for specialized register).
Electronic searches
We applied standard search strategies of the CNRG as outlined in the Cochrane Library. We conducted a comprehensive electronic search including the Cochrane Central Register of Controlled Trials (CENTRAL 2017; Issue 3) in the Cochrane Library; MEDLINE via PubMed (1966 to 18 April 2017); Embase (1980 to 18 April 2017); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to18 April 2017), using the following search terms: (Sildenafil[MeSH] OR sildenafil OR tadalafil OR Viagra OR Phosphodiesterase Inhibitors[MeSH] OR Phosphodiesterase V[MeSH] OR pulmonary vasodilator) AND (hypertension, pulmonary[MeSH] OR PPHN OR hypertension OR persistent fetal circulation syndrome[MeSH] OR rebound OR persistent fetal circulation syndrome), plus database‐specific limiters for randomised controlled trials (RCTs) and neonates (see Appendix 1 for full search strategies for each database). We did not apply language restrictions.
Searching other resources
We searched clinical trials registries for ongoing or recently completed trials ((clinicaltrials.gov); the World Health Organization International Trials Registry and Platform (www.whoint/ictrp/search/en/); the ISRCTN Registry)). We excluded the following types of articles: letters, editorials/commentaries, reviews, lectures, and commentaries. We manually searched the reference lists of full‐text versions (RCTs and reviews) identified during the primary literature search.
Data collection and analysis
We used standard review methods of the CNRG to select studies for inclusion, to extract study data, and to assess the methodological quality of identified studies.
Selection of studies
We assessed for inclusion all published articles identified as potentially relevant by the literature search. We resolved discrepancies regarding inclusion/exclusion of studies by consensus.
Data extraction and management
Each review author extracted data separately using predesigned data abstraction forms. Review authors compared results and resolved differences. One review author entered data into RevMan 5.3 (RevMan 2014); the other review authors cross‐checked the printout against data entered into abstraction forms, and corrected errors by consensus.
If relevant articles were identified, review authors obtained data from study authors when published data provided inadequate information for the review, and when relevant data could not be abstracted.
Assessment of risk of bias in included studies
Three review authors (LEK, AO, PSS) used the Cochrane 'Risk of bias' tool (Higgins 2011) to independently assess risk of bias (low, high, or unclear) of all included trials for the following domains.
-
Sequence generation (selection bias).
-
Allocation concealment (selection bias).
-
Blinding of participants and personnel (performance bias).
-
Blinding of outcome assessment (detection bias).
-
Incomplete outcome data (attrition bias).
-
Selective reporting (reporting bias).
-
Any other bias.
We resolved disagreements by discussion. See Appendix 2 for a detailed description of risk of bias for each domain.
Measures of treatment effect
We performed statistical analyses in accordance with recommendations of the CNRG, using RevMan 5.3 software (RevMan 2014). Treatment effect estimates included typical risk ratio (RR), typical risk difference (RD), number needed to treat for an additional beneficial outcome (NNTB), or number needed to treat for an additional harmful outcome (NNTH) for dichotomous outcomes, and mean difference (MD) for continuous outcomes. We reported all estimates of treatment effects with 95% confidence intervals (CIs).
Unit of analysis issues
The unit of analysis in included trials was the individual randomised neonate. We planned to include cluster‐RCTs, if available. We planned to analyse cluster‐RCTs using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), along with an estimate of the intracluster correlation coefficient.
Dealing with missing data
As needed, we requested from corresponding study authors additional information regarding study design or outcome measures. We planned to include in an intention‐to‐treat analysis all infants for whom included studies reported outcomes.
Assessment of heterogeneity
Heterogeneity testing including the I2 test, which we performed to assess the appropriateness of pooling data by using RevMan 5.3 software. We roughly categorised degree of heterogeneity according to the recommendations of Higgins and coworkers (Higgins 2011). We followed CNRG recommendations by using the following criteria to describe percentage of heterogeneity: < 25% no heterogeneity, ≥ 25% to 49% low heterogeneity, ≥ 50% to 74% moderate heterogeneity, and ≥ 75% high heterogeneity.
We performed planned subgroup analyses according to the criteria listed below.
Assessment of reporting biases
We assessed reporting and publication biases by examining the degree of asymmetry on a funnel plot, using RevMan 5.3 software, when a comparison includes at least 10 clinical trials.
Data synthesis
We performed meta‐analyses using Review Manager software (RevMan 2014) supplied by the Cochrane Collaboration. For estimates of typical RR and RD, we used the Mantel‐Haenszel method; for measured quantities, the inverse variance method; and for all meta‐analyses, we used the fixed‐effect model.
Quality of evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: respiratory parameters (PaO2, OI, mean PAP), mortality; and length of hospital stay.
Two review authors independently assessed the quality of evidence for each of the outcomes above. We considered evidence from RCTs as high quality but downgraded evidence one level for serious (or two levels for very serious) limitations on the basis of the following: design (risk of bias), consistency across studies, directness of evidence, precision of estimates, and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of evidence.
The GRADE approach yields an assessment of the quality of a body of evidence according to one of four grades.
-
High: We are very confident that the true effect lies close to that of the estimate of the effect.
-
Moderate: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
-
Low: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect.
-
Very low: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
We planned to perform a priori subgroup analyses based on the following.
-
Gestational age (term and preterm ‐ defined as < 37 weeks' gestation).
-
Method of diagnosis of pulmonary hypertension (clinical or echocardiographic).
-
Route of administration of sildenafil (oral vs intravenous vs inhaled).
-
Primary or secondary cause of pulmonary hypertension.
-
Comparison 1: sildenafil versus control
-
Category 1: type of control intervention
-
Subgroups: A. Sildenafil versus placebo. B. Sildenafil versus no treatment
-
-
Category 2: gestational age
-
Subgroups: 1. Preterm. 2. Term
-
-
-
Comparison 2: sildenafil versus other pulmonary vasodilator
-
Category 1: type of control intervention
-
Subgroups: A. Sildenafil versus inhaled nitric oxide. B. Sildenafil versus other pulmonary vasodilator
-
-
Category 2: gestational age
-
Subgroups 1. Preterm. 2. Term
-
-
-
Comparison 3: sildenafil and other pulmonary vasodilator versus other pulmonary vasodilator
-
Category 1: type of control intervention
-
Subgroups: A. Sildenafil and other pulmonary vasodilator versus inhaled nitric oxide. B. Sildenafil and nitric oxide versus other pulmonary vasodilator. C. Sildenafil and nitric oxide versus placebo/no treatment
-
-
Category 2: gestational age
-
Subgroups 1. Preterm. 2. Term
-
-
Post hoc comparison modification
After performing a revised literature search, we made a post hoc modification. Comparison 3 now includes "sildenafil plus other vasodilator (iNO) versus control (placebo) plus iNO" to include studies that used sildenafil as adjuvant therapy (both groups received nitric oxide) and compared treatment versus a placebo control.
Sensitivity analysis
If needed, we planned to explore the impact of the level of bias by undertaking sensitivity analyses.
Results
Description of studies
In this update of our review, we evaluated 1674 citations (Figure 1). Review of 77 articles in full text yielded two additional studies that were eligible for inclusion (Al Omar 2016; Uslu 2011) and three studies (Baquero 2006; Herrera 2006; Vargas‐Origel 2010) that were included in a previous version of this review (Shah 2011). We excluded a total of six studies (Kahveci 2014; König 2014; Namachivayam 2006; Sayed 2015; Steinhorn 2009; Stocker 2003), we kept four studies (NCT01757782; NCT01373749; Soliz 2009; Alipour 2017) in awaiting assessment status, and we identified one ongoing study (NCT01720524).

Study flow diagram: review update.
We excluded Kahveci 2014, as it was a retrospective comparison of sildenafil and iloprost. König 2014 compared sildenafil versus placebo, but we excluded this study because it included neonates with bronchopulmonary dysplasia, and some patients may not have received a diagnosis of pulmonary hypertension. We excluded Namachivayam 2006 because the study included patients from 0.1 year of age (potentially > 1 month), and most (> 80%) participants had congenital heart disease. Sayed 2015 performed a single‐arm uncontrolled evaluation of sildenafil. Steinhorn 2009 was an open‐label dose‐escalating study. Stocker 2003 studied infants following cardiac surgery. We identified NCT01757782 and NCT01373749 through ClinicalTrials.Gov and placed these studies in the awaiting status category, as published results were not yet available. We excluded Soliz 2009 because the abstract was presented at a scientific meeting but information was inadequate to distinguish it as a separate study. We placed Alipour 2017 in the awaiting further information classification, as review authors did not present clear details of when outcome measures were assessed.
Our previous report included Herrera 2006 in abstract form; in the current version of our review, we have included this study as a full report published in Spanish. For Baquero 2006, we requested data regarding haemodynamic measurements taken before and during the intervention period, as well as incidence of rebound hypoxaemia, incidence of intraventricular haemorrhage, length of stay, and number of infants needing ECMO. Study authors provided data on FiO2 before the start of therapy, mean arterial BP before the start of therapy and at 36 hours after therapy among survivors, and the number of infants with grade 3 or 4 intraventricular haemorrhage. In addition, study authors reported follow‐up data in abstract form from three infants (one neonatal death, one death at five months of age, and one loss to follow‐up) (Baquero 2006). Vargas‐Origel 2010 published data on a total of 53 study participants. At first, investigators enrolled 20 participants in the placebo group and 20 in the sildenafil group. After 40 participants had been enrolled, the institutional ethics board prohibited use of placebo, and investigators thereafter randomised study participants to sildenafil versus nitric oxide. The published manuscript provides data on 33 participants in the sildenafil group and 20 in the placebo group. We contacted study authors and requested only data from comparison of the first 40 participants (20 in the sildenafil group and 20 in the placebo group). Newly added studies include Al Omar 2016 and Uslu 2011. Study authors for Al Omar 2016 provided clarification regarding mortality outcomes data, demographic data (including birth weight standard deviation), and OI mean values.
For additional details, see Characteristics of included studies.
Baquero 2006
-
Setting : single‐centre pilot randomised double‐blind controlled trial at a regional neonatal intensive care unit (NICU) in Colombia. In this unit, iNO, high‐frequency ventilation, and ECMO were not available
-
Objective : to evaluate the feasibility of using oral sildenafil and to determine the effects of oral sildenafil on oxygenation in term and near‐term infants with PPHN
-
Population : term and near‐term (≥ 35.5 weeks' gestation) infants with severe hypoxaemia (need for mechanical ventilation with OI ≥ 40) and echocardiographically confirmed PPHN (presence of right‐to‐left shunt and estimated PAP ≥ 40 mmHg)
-
Intervention : oral sildenafil or placebo (diluent). The solution for sildenafil was prepared by crushing a 50 mg tablet of sildenafil in Orabase (diluent) to achieve a concentration of 2 mg/mL. The protocol for dosing included (1) first dose of 1 mg/kg (0.5 mL/kg) within 30 minutes of randomisation, (2) dosing every six hours, (3) potential doubling of the dose (1 mL/kg) if OI did not improve and blood pressure remained stable, and (4) discontinuation of treatment if OI was < 20, or if participant had received eight doses. Other aspects of care management remained the same in both study arms
-
Outcomes : mortality; changes in OI, mortality, PaO2, and mean arterial blood pressure. OI was not reported for two neonates who improved to meet study exit criteria
-
Recruitment : eligibility criteria met by 22 patients. Of these, two patients died (before enrolment in the study), four parents refused consent, and three parents were not approached for consent. Researchers enrolled a total of 13 participants (six in the placebo group and seven in the treatment group). The institutional review board terminated the study owing to the death of six participants enrolled in the study
-
Follow‐up : data reported in abstract form on four survivors in the sildenafil group assessed at 18 months of age
Herrera 2006
-
Setting : single‐centre randomised controlled study in Mexico. The centre did not have the facility needed to administer iNO
-
Objective : to compare the efficacy of oral sildenafil therapy versus conventional therapy in term neonates with PPHN at a centre without iNO
-
Population : term neonates with a diagnosis of PPHN and OI > 25
-
Intervention : participants randomised to sildenafil (n = 13) 2 mg/kg via orogastric tube or distilled water (n = 11). Total duration of therapy was 72 hours. Sildenafil was administered at 2 mg/kg/dose via orogastric tube every six hours
-
Outcomes : mortality; changes in OI, PaO2, mean arterial blood pressure, PaCO2, and intubation days were compared
-
Recruitment : 13 of 24 recruited neonates randomly selected to receive sildenafil. Outcomes data were reported on all 24 enrolled neonates
Vargas‐Origel 2010
-
Setting : single‐centre randomised controlled trial in Mexico. In this unit, iNO was not available at the start of the trial. Inhaled nitric oxide became available during the study, and the ethics board prohibited use of placebo after 40 neonates had been randomised
-
Objective : to evaluate the efficacy of oral sildenafil in newborns with PPHN
-
Population : term and post‐term infants with PPHN diagnosed within first 48 hours who had OI > 20
-
Intervention : participants given either oral sildenafil or placebo (normal saline). Solution for sildenafil was prepared by crushing a 50‐mg tablet of sildenafil in 20 mL of water. Protocol for dosing was 3 mg/kg/dose every six hours via nasogastric tube. Treatment was continued until OI was < 10. Other aspects of management of infant care remained the same in both arms of the study
-
Outcomes : data on OI, mean airway pressure, mean arterial pressure, PaO2, and mortality for first 40 participants randomised to sildenafil (20) and placebo (20) provided by study authors
-
Recruitment : 51 enrolled neonates. Following an ethics board decision, only the first 40 participants were randomised to placebo or sildenafil. Full‐text article presents data on all enrolled neonates; however, study authors provided data from the first 40 randomised neonates for inclusion in this review
Uslu 2011
-
Setting : single‐centre randomised double‐blind controlled trial at a regional referral NICU in Turkey. In this unit, iNO, high‐frequency ventilation, and ECMO were not available
-
Objective : to determine and compare clinical efficacy and side effects of intravenous MgSO4 and oral sildenafil therapy in newborns with PPHN
-
Population : term and near‐term (35 to 42 weeks' gestation) infants with hypoxaemic respiratory failure associated with PPHN. Inclusion criteria included PAP ≥ 40 mmHg, OI ≥ 30, and the need for mechanical ventilation. Researchers excluded neonates with congenital heart disease, suspicion of sepsis (and other anomalies), gastric intolerance, or gastric bleeding
-
Intervention : oral sildenafil (0.5 mg/kg every 6 hours) or IV MgSO4 (200 mg/kg loading dose, followed by a maintenance dose of 20 mg/kg/h)
-
Outcomes : primary outcome was time of adequate clinical response, defined as a decrease in PAP to < 20 mmHg and OI to < 15. Secondary outcomes included duration of mechanical ventilation, support of inotropic agent, mortality rate, and adverse events
-
Recruitment : eligibility criteria met by 77 patients, 72 of whom were randomised following parental consent. A total of 36 participants were given oral sildenafil, and 36 were treated with MgSO4. Complete data were collected on 31/36 infants in the sildenafil group (3 gastric bleeding, 2 incomplete records) and on 34/36 in the MgSO4 group (2 incomplete records)
Al Omar 2016
-
Setting : single‐centre randomised clinical trial conducted at an NICU in Qatar over three years (September 2011 to September 2014)
-
Objectives : to evaluate the feasibility and effectiveness of adding sildenafil as adjuvant therapy together with iNO when treating newborns with PPHN and/or hypoxaemic respiratory failure; to assess whether this approach could improve oxygenation, decrease time on mechanical ventilation, and prevent rebound hypoxaemic episodes
-
Population : newborn infants born at gestational age of 34 weeks or greater who at less than 48 hours of age had an OI greater than or equal to 20 mmHg; radiological, clinical, and biochemical evidence of acute hypoxaemic respiratory failure; surfactant therapy established where indicated and an arterial line
-
Intervention : participants given either oral sildenafil (2 mg/kg/dose every 6 hours) or placebo via nasogastric tube. Starting dose of iNO was 20ppm in both groups; weaning from iNO was carried out at 2% to 4% per hour
-
Outcomes : primary outcome was OI absolute values and change from baseline measured after first dose, every 6 hours for 7 days, or until the infant was extubated. Improvement in OI was defined as a decrease of 10% from the previously calculated OI value. Secondary outcome measures included haemodynamic parameters, PAP (measured by echocardiography), practicality of administration, gastric tolerance, hypotension, renal function, liver function, and length of stay. Study authors provided data on all‐cause mortality within the first 28 days of life. We were unable to obtain information regarding the variability of reported means for OI and mean PAP
-
Recruitment : eligibility criteria met by a total of 51 infants. Following exclusions for hypoxic‐ischaemic encephalopathy (HIE), congenital diaphragmatic hernia (CDH), and surfactant protein B deficiency, and four refusals to participate, 24 cases (13 sildenafil, 11 placebo) were included.
Results of the search
We have provided results of the search in Figure 1. We screened a total of 1674 publication records for inclusion, of which we retrieved 77 in full text.
Included studies
A previous version of this review (Shah 2011) included three studies (Baquero 2006; Herrera 2006; Vargas‐Origel 2010) that evaluated sildenafil in neonates with PPHN. We identified two new studies that met our inclusion criteria (Al Omar 2016; Uslu 2011).
Excluded studies
We excluded 74 full‐text articles that did not meet our inclusion criteria; 65 were not randomised clinical trials, and 9 did not recruit neonates.
Risk of bias in included studies
We have presented details on risk of bias of included studies in Figure 2. Overall, these studies were at low to high risk of bias, which we have summarised in Figure 3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
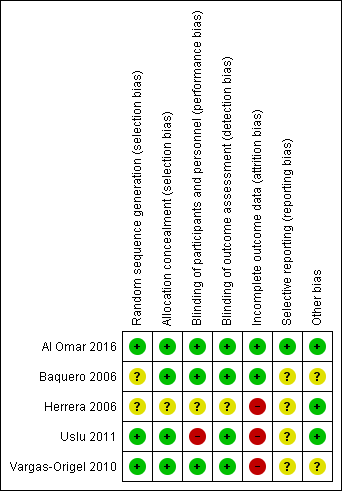
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Baquero 2006 was a randomised double‐blind placebo‐controlled trial. Investigators performed randomisation using presealed envelopes. The pharmacy prepared the solution in identical containers, and bedside clinicians were unaware of group assignment. Outcome assessment appears to be masked, as no one was aware of treatment allocation. Researchers terminated the study early on the basis of pre‐set criteria (which included hypotension, gastric intolerance or bleeding, renal failure, and death in six infants). Pre‐set criteria for discontinuation of dosing included OI < 20 or administration of a maximum of eight doses. Investigators used analysis of variance for repeated comparison of data on OI, blood pressure, and oxygen saturation. We assigned this study unclear risk of reporting bias, as we found no registration data to verify selective outcome reporting.
Herrera 2006 was a randomised placebo‐controlled trial that did not report the randomisation method used. Study authors described allocation as blinded. Investigators compared data using descriptive statistics and Student's t‐test. Data analysis excluded neonates who had been transferred. As we found no registration data from this trial to verify selective outcome reporting, we assessed risk of bias as unclear.
Vargas‐Origel 2010 was a randomised double‐blind placebo‐controlled trial. Two nurses who were not involved in the study generated randomisation using a random number table (information provided by study authors). The pharmacy prepared the solution, and bedside clinicians were unaware of group assignment. Outcome assessment was masked, as nurses were unaware of treatment allocation.
Uslu 2011 was a randomised controlled trial that evaluated sildenafil and magnesium sulphate (MgSO4; control). An independent researcher prepared a computer‐generated randomisation table and concealed allocation. Neonatologists could not be blinded owing to the nature of the interventions (orogastric vs intravenous). Study staff including nurses and data collectors were blinded. As this trial was not registered, we could not evaluate selective outcome reporting. We did not perform intention‐to‐treat analysis and noted that data were missing for seven neonates (five in the treatment group and two in the control group).
Al Omar 2016 was a randomised clinical trial that evaluated sildenafil and placebo in addition to "standard care", which included iNO. Investigators performed randomisation using an online sequence and concealed allocation using opaque envelopes distributed by the pharmacy (information provided by study authors). The treatment team was blinded to the study arm and reported outcomes data on all randomised neonates. Investigators reported all registered outcomes.
Effects of interventions
See: Summary of findings for the main comparison Sildenafil compared with placebo for pulmonary hypertension in neonates; Summary of findings 2 Sildenafil compared with active control for pulmonary hypertension in neonates; Summary of findings 3 Sildenafil plus iNO compared with placebo plus iNO for pulmonary hypertension in neonates
Sildenafil versus placebo (Comparison 1)
Three studies (Baquero 2006; Herrera 2006; Vargas‐Origel 2010) compared sildenafil versus placebo in neonates with persistent pulmonary hypertension.
Primary outcomes
Haemodynamic parameters (absolute values and change from baseline)
Pulmonary arterial pressure (in mmHg) (Outcome 1.1)
Only one study (Vargas‐Origel 2010) measured PAP at baseline and revealed no significant differences between sildenafil and control (mean difference (MD) 1.10, 95% confidence interval (CI) ‐7.68 to 9.88; heterogeneity ‐ not applicable). Changes in PAP were not reported at a later stage (Analysis 1.1).
Oxygenation (PaO2 in mmHg) (Outcome 1.2)
Baquero 2006 reported that all participants in treatment and placebo groups required 100% oxygen before the start of therapy. Baquero 2006 reported that oxygen saturation (SaO2) steadily improved in the sildenafil group, was statistically significantly different from baseline in the sildenafil group at 12 hours (P < 0.03), and was significantly higher at 24 and 36 hours in the sildenafil group compared with the placebo group (P < 0.03).
The other two studies (Herrera 2006; Vargas‐Origel 2010) reported that PaO2 at baseline was higher in the sildenafil group than in the placebo group (MD 8.06 mmHg, 95% CI 1.58 to 14.54 mmHg; two studies, 64 participants; I2 = 43% ‐ low). This trend continued in both studies over the course of reporting: After the first dose (MD 11.09 mmHg, 95% CI 1.65 to 20.52 mmHg; two studies, 64 participants; I2 = 0% ‐ none), after 6 to 7 hours (MD 14.30 mmHg, 95% CI 5.25 to 23.34 mmHg; two studies, 63 participants; I2 = 0% ‐ none), and after 24 to 25 hours (MD 15.31 mmHg, 95% CI 6.49 to 24.13 mmHg; two studies, 58 participants; I2 = 0% ‐ none), PaO2 was higher in the sildenafil group than in the placebo group. Differences in PaO2 increased over time during the first 24 hours. Herrera 2006 reported results at 72 hours (MD 20.98, 95% CI 14.81 to 27.15; one study, 24 participants; I2 = not applicable), showing a significant increase in PaO2 in the sildenafil group.
Cardiac output in L/kg/min
No studies reported changes in cardiac output.
Mean arterial blood pressure (in mmHg) (Outcome 1.3)
One study (Baquero 2006) reported no statistically significant differences in mean arterial blood pressure between sildenafil and placebo groups and did not provide numerical data. Baquero 2006 and Vargas‐Origel 2010 provided data on mean arterial blood pressure before the start of therapy in all participants and at the end of therapy among survivors. Mean arterial blood pressure was higher at baseline in the sildenafil group than in the placebo group (MD 5.65, 95% CI 2.69 to 8.61 mmHg; two studies, 53 participants; I2 = 56% ‐ moderate). Values for mean arterial blood pressure after completion of therapy were available for only one of the survivors (Baquero 2006) in the placebo group (45.3 mmHg), whereas values were provided for six survivors in the sildenafil group: (mean + SD) 43.7 + 3.3 mmHg. Vargas‐Origel 2010 reported significantly higher mean arterial pressure in the sildenafil group than in the placebo group at completion of therapy (22.70 mmHg, 95% CI 1.23 to 44.17 mmHg; 40 participants; heterogeneity estimate ‐ not applicable) (Analysis 1.3).
All‐cause mortality within first 28 days of life (Outcome 1.4)
All three studies that evaluated sildenafil alone reported data on mortality, showing a statistically significant reduction in mortality rate for the sildenafil group compared with the placebo group (RR 0.20, 95% CI 0.07 to 0.56; RD ‐0.36, 95% CI ‐0.53 to ‐0.18; number needed to treat for an additional beneficial outcome (NNTB) 3, 95% CI 2 to 6; three studies, 77 participants; I2 = 39% ‐ low) (Analysis 1.4).
Secondary outcomes
Changes in pulmonary or systemic vascular resistance index in WUm2 (absolute values and change from baseline)
None of the three included studies that performed this comparison reported these data.
Changes in oxygenation index (absolute values and change from baseline)
Baquero 2006 reported these data for individual participants in a table format. We used these data to calculate OI for this study.
Oxygenation index (absolute values) (Outcome 1.5)
At baseline: Data show no statistically significant differences in OI at baseline between groups (MD ‐0.74, 95% CI ‐8.11 to 6.64; three studies, 77 participants; I2 = 28% ‐ low).
After administration of first dose: Results show a reduction in OI in the sildenafil alone group compared with the placebo group (MD ‐12.53, 95% CI ‐18.60 to ‐6.47; three studies, 77 participants; I2 = 27% ‐ low).
After 6 to 7 hours of treatment: Data show a statistically significant reduction in OI in the sildenafil alone group compared with the placebo group after 6 to 7 hours of treatment (MD ‐20.07, 95% CI ‐26.12 to ‐14.02; two studies, 63 participants; I2 = 0% ‐ none).
After 24 to 25 hours of treatment: Results show a statistically significant reduction in OI in the sildenafil alone group compared with the placebo group after 24 to 25 hours of treatment (MD ‐19.15, 95% CI ‐24.52 to ‐13.77; three studies, 69 participants; I2 = 0% ‐ none).
After administration of intervention for 30 hours: Data show a statistically significant reduction in OI in the sildenafil alone group compared with the placebo group after 30 hours of treatment (MD ‐45.46, 95% CI ‐61.87 to ‐29.05; one study, 11 participants; heterogeneity estimate ‐ not applicable).
After administration of intervention for 36 hours (completion of therapy): Results show a statistically significant reduction in OI in the sildenafil group compared with the placebo group after 36 hours of treatment (MD ‐32, 95% CI ‐45.74 to ‐17.76; one study, eight participants; heterogeneity estimates ‐ not applicable).
After 72 hours: Data show a statistically significant reduction in OI in the sildenafil alone group compared with the placebo group after 72 hours of treatment (MD ‐19, 95% CI ‐23.42 to ‐15.52; one study, 24 participants; heterogeneity estimates ‐ not applicable) (Analysis 1.5).
Changes in oxygenation index (Outcome 1.6)
We calculated these data from individual participant data provided in the original manuscript (Baquero 2006).
After administration of first dose: Results show a statistically significant reduction in OI two hours after administration of the first dose of sildenafil alone compared with placebo (MD ‐17.14, 95% CI ‐27.75 to ‐6.53; one study, 13 participants; heterogeneity estimates ‐ not applicable).
After administration of intervention for 24 hours: Data show a statistically significant reduction in OI after administration of five doses of sildenafil alone (at 24 hours after administration) compared with placebo (MD ‐38.79, 95% CI ‐56.97 to ‐20.61; one study, 12 participants; heterogeneity estimates ‐ not applicable).
After administration of intervention for 30 hours: Results show a statistically significant reduction in OI after administration of six doses of sildenafil alone (at 30 hours after administration) compared with placebo (MD ‐33.08, 95% CI ‐50.85 to ‐15.31; one study, 11 participants; heterogeneity estimates ‐ not applicable).
After administration of intervention for 36 hours (completion of therapy): Data show a statistically significant reduction in OI after administration of seven doses of sildenafil alone compared with placebo (MD ‐44.75, 95% CI ‐65.55 to ‐23.95; one study, eight participants; heterogeneity estimates ‐ not applicable). By 42 hours, one participant in the sildenafil group and two participants in the control group had died. Two participants in the control group had significantly reduced OI before the last dose and were not given the last dose (according to prespecified criteria).
Herrera 2006 reported that OI improved within the first hour of administration (Analysis 1.6).
Rebound increase in PAP or decrease in cardiac output after weaning from sildenafil (dichotomous)
Results show no evidence of rebound hypoxaemia in two participants for whom sildenafil was discontinued because of OI < 20 (Baquero 2006).
Decrease in cardiac output after weaning from sildenafil and the need for extracorporeal membrane oxygenation (ECMO) before discharge
No studies reported these outcomes.
Mortality before discharge
Investigators reported no additional mortality outside of the first 28 days.
Retinopathy of prematurity (among preterm infants at < 32 weeks' gestation)
None of the studies (Baquero 2006; Herrera 2006; Vargas‐Origel 2010) that compared sildenafil versus placebo enrolled preterm infants at risk for retinopathy of prematurity.
Intraventricular haemorrhage
Baquero 2006 reported no grade 3 or 4 intraventricular haemorrhage in any of the infants in either group.
Neurodevelopmental disability at 18 to 24 months (including cerebral palsy, cognitive impairment, deafness, and blindness)
Baquero 2006 reported data on neurodevelopmental follow‐up in abstract form. Investigators assessed only four out of six survivors in the sildenafil alone group at 18 months. One participant in the sildenafil group died during the neonatal period, one died at five months of age, and one was lost to follow‐up. All four participants had a normal neurological examination (Gessel scale of 100, 100, 100, and 111 points). All had normal magnetic resonance imaging (MRI), evoked potential, and electroencephalography (EEG) reading. Their growth parameters (weight, height, and head circumference) were within normal limits.
Any clinically important outcomes reported by study authors (not prespecified)
Alveolar‐arterial oxygen difference (A‐a DO2) (Outcome 1.7)
Two studies evaluating sildenafil alone reported on this outcome (Herrera 2006; Vargas‐Origel 2010).
At baseline: A‐a DO2 was not significantly different (MD 0.99, 95% CI ‐11.54 to 13.51; two studies, 64 participants; I2 = 0% ‐ none).
At 6 to 7 hours of age: A‐a DO2 was not significantly different (MD 0.01, 95% CI ‐27.72 to 27.74; one study, 24 participants; heterogeneity estimates ‐ not applicable).
At 24 to 25 hours of age: A‐a DO2 was not significantly different (MD 1.59, 95% CI ‐18.98 to 22.16; two studies, 57 participants; I2 = 74% ‐ moderate).
At 72 hours: A‐aDO2 was significantly lower in the sildenafil alone group (MD ‐18.34, 95% CI ‐26.59 to ‐10.09; one study, 24 participants; heterogeneity estimates ‐ not applicable) (Analysis 1.7).
Mean airway pressure (Outcome 1.8)
Two studies evaluating sildenafil alone reported on this outcome (Herrera 2006; Vargas‐Origel 2010).
At baseline: Mean airway pressure was lower in the sildenafil alone group than in the placebo group (MD ‐2.09, 95% CI ‐3.30 to ‐0.88 cm of H2O; two studies, 64 participants; I2 = 0% ‐ none).
At 6 to 7 hours after administration: Mean airway pressure was significantly lower in the sildenafil alone group than in the placebo group (MD ‐5.94, 95% CI ‐7.36 to ‐4.52 cm of H2O; two studies, 64 participants; I2 = 35% ‐ low).
At 24 to 25 hours after administration: Mean airway pressure was significantly lower in the sildenafil alone group than in the placebo group (MD ‐6.64, 95% CI ‐8.49 to ‐4.80 cm of H2O; two studies, 57 participants; I2 = 0% ‐ none).
At 72 hours after administration: Mean airway pressure was significantly lower in the sildenafil group than in the placebo group (MD ‐8.58, 95% CI ‐10.37 to ‐6.79 cm of H2O; one study, 24 participants; heterogeneity estimate ‐ not applicable) (Analysis 1.8).
Sildenafil versus active control (Comparison 2)
One study (Uslu 2011) evaluated sildenafil alone against an active control (MgSO4).
Primary outcomes
Haemodynamic parameters (absolute values and change from baseline)
Pulmonary arterial pressure in mmHg
Investigators reported these data in graphical format and did not provide mean and variance measures. Uslu 2011 reported PAP absolute values that were significantly lower with sildenafil than with MgSO4 on day 1 (P = 0.001), on day 2 (P = 0.0001), and on the third day following treatment (P = 0.007).
Oxygenation (PaO2) or FiO2 requirement, cardiac output (L/kg/min), and mean arterial blood pressure (mmHg)
Uslu 2011 did not report changes in oxygenation, cardiac output, and mean arterial blood pressure.
All‐cause mortality (Outcome 2.1)
Data show no differences in mortality rate between sildenafil and MgSO4 (RR 0.55, 95% CI 0.05 to 5.75; RD ‐0.03; 95% CI ‐0.13 to 0.07; one study, 65 participants; heterogeneity estimate ‐ not applicable) (Analysis 2.1).
Secondary outcomes
Oxygenation index
Researchers reported these data in graphical format and did not provide mean and variance measures. Uslu 2011 reported OI values that were significantly lower with sildenafil than with MgSO4 at 12 hours (P = 0.007), at 24 hours (P = 0.005), at 36 hours (P = 0.001), at 48 hours (P = 0.009), and at 60 hours (P = 0.01) after treatment. .
Time to adequate response (Outcome 2.2)
Data show no significant difference in the number of days to reach an adequate response between sildenafil and MgSO4 groups (MD ‐0.60 days, 95% CI ‐4.20 to 3.00; one study, 65 participants; heterogeneity estimate ‐ not applicable) (Analysis 2.2).
Duration of ventilation (Outcome 2.3)
Researchers reported no significant differences in the number of days on ventilation between sildenafil and MgSO4 groups (MD ‐1.30 days, 95% CI ‐4.54 to 1.94; one study, 65 participants; heterogeneity estimate ‐ not applicable) (Analysis 2.3).
Use of an inotropic agent (Outcome 2.4)
Despite similar numbers of neonates requiring inotropic support at baseline (sildenafil 9.1% (3/31) vs MgSO4 11.8% (4/34)), results show that significantly fewer neonates were receiving inotropic agents in the sildenafil group than in the MgSO4 group (RR 0.55, 95% CI 0.36 to 0.83; RD ‐0.37, 95% CI ‐0.59 to ‐0.15; NNTB 3, 95% CI 2 to 8; one study, 65 participants; heterogeneity estimate ‐ not applicable; P < 0.0008) (Analysis 2.4).
Retinopathy of prematurity (among preterm infants at < 32 weeks' gestation)
Uslu 2011 did not include extremely preterm infants at risk for retinopathy of prematurity.
Sildenafil plus iNO versus placebo plus iNO (Comparison 3)
One study (Al Omar 2016) evaluated sildenafil used as adjuvant therapy with nitric oxide against placebo with nitric oxide.
Primary outcomes
Haemodynamic parameters (absolute values and change from baseline) including PAP in mmHg, oxygenation (PaO2) or FiO2 requirement, cardiac output (L/kg/min), and mean arterial blood pressure (mmHg)
Study authors reported changes in mean PAP in graphical format and did not provide mean and variance measures for meta‐analysis. They did not report changes in PaO2 or FiO2, cardiac output, and mean arterial blood pressure.
All‐cause mortality (Outcome 3.1)
Al Omar 2016, which evaluated sildenafil as adjuvant therapy to iNo, reported no significant reduction in mortality (RR 1.27, 95% CI 0.26 to 6.28; RD 0.05, 95% CI ‐0.27 to 0.37; one study, 24 participants; heterogeneity estimate ‐ not applicable) (Analysis 3.1).
Secondary outcomes
Oxygenation index
Al Omar 2016 reported mean changes in OI at six‐hourly intervals but did not provide mean or variance measures; therefore, we could not include this study in this meta‐analysis. Investigators presented data in graphical format, and we could not abstract absolute values.
Length of stay in hospital (Outcome 3.2)
Data show no significant differences in the number of days in hospital between groups given sildenafil versus placebo as adjuvant to iNO therapy (MD 5.39, 95% CI ‐5.31 to 16.09; one study, 24 participants; heterogeneity estimate ‐ not applicable) (Analysis 3.2).
Use of an inotropic agent (Outcome 3.3)
Only one study included in this analysis (Al Omar 2016) evaluated use of an inotropic agent. Results show no significant differences in the need for an inotropic agent between groups given sildenafil or placebo adjuvant to iNO therapy (RR 1.06, 95% CI 0.37 to 3.00; RD 0.02, 95% CI ‐0.37 to 0.41; one study, 24 participants; heterogeneity estimate ‐ not applicable) (Analysis 3.3).
Duration of ventilation (Outcome 3.4)
Al Omar 2016 reported the duration of ventilation (days). Data show no significant differences in duration of ventilation among neonates treated with sildenafil or placebo given adjuvant to iNO therapy (MD 1.26, 95% CI ‐1.32 to 3.84; one study, 24 participants; heterogeneity estimate ‐ not applicable) (Analysis 3.4).
Retinopathy of prematurity (among preterm infants at < 32 weeks' gestation) (Outcome 3.5)
Al Omar 2016 did not report any cases of retinopathy of prematurity in either group (Analysis 3.5).
Summary of findings
This review evaluated three comparisons against sildenafil: placebo alone, active control, and placebo with iNO. Overall, when compared with placebo alone, sildenafil significantly reduced mortality (summary of findings Table for the main comparison). We graded the evidence as low owing to potential bias in outcome reporting/data analysis and imprecision related to a small number of participants.
When compared with an active control (summary of findings Table 2) or when used as adjuvant therapy (summary of findings Table 3), sildenafil did not show improved mortality among neonates with persistent pulmonary hypertension treated with sildenafil. We graded the evidence as low to very low owing to imprecision related to the small number of included studies.
Discussion
Summary of main results
Publications to date include five very small randomised controlled trials that evaluated sildenafil and included a total of 166 neonates. These trials were conducted in resource‐limited settings such as Columbia, Mexico, Qatar, and Turkey. The most commonly reported (40% to 45%) underlying diagnosis was meconium aspiration syndrome (MAS). All three studies that compared sildenafil versus placebo reported that intensive care units did not have facilities for providing high‐frequency ventilation or nitric oxide ‐ therapies that have shown promise in the treatment of persistent pulmonary hypertension in neonates (PPHN). Uslu 2011 compared sildenafil versus active control (magnesium sulphate; MgSO4). Al Omar 2016 evaluated sildenafil and placebo as adjuvant therapy to inhaled nitric oxide (iNO), as nitric oxide was routinely offered as standard of care.
Compared with placebo, sildenafil alone was associated with a significant reduction in mortality among neonates with PPHN. Respiratory parameters (oxygenation index (OI) and partial pressure of oxygen in arterial blood (PaO2)) showed significant improvement in the sildenafil group when compared with the placebo group. We did not identify a significant difference in mortality or respiratory parameters when sildenafil was compared with an active control or was used as adjuvant therapy with iNO. We noted heterogeneity in severity of illness at the time of entry in different studies; however, all enrolled participants who had moderate to severe hypoxaemic respiratory failure. Most studies reported steady improvement in oxygenation starting from the first dose of sildenafil. In one study of only 65 participants, sildenafil was effective in reducing the need for inotropes.
Overall completeness and applicability of evidence
Although studies comparing sildenafil versus placebo consistently reported improved respiratory outcomes with sildenafil, it must be noted that these studies had several limitations. The number of enrolled participants was small, reporting of various important outcomes was inadequate, and evidence showed heterogeneity between studies. Mortality was significantly reduced in our analyses when sildenafil alone was compared with placebo. This is clinically very significant and may influence treatment decisions made in these units, especially units in resource‐limited settings. Long‐term effects of sildenafil use remain unknown. Furthermore, data showed no significant improvement in mortality when sildenafil was compared with active control (MgSO4) or was used adjuvant to iNO therapy.
Included studies used heterogeneous sildenafil doses (range 0.5 to 3.0 mg/kg every 6 hours) and variable loading doses. Optimum dose, optimum route of administration, incidence of rebound pulmonary hypertension, and effectiveness in reducing rebound pulmonary hypertension remain unknown. Sildenafil may not be as effective in PPHN resulting from causes such as sepsis (where overproduction of nitric oxide leading to systemic vasodilation may be the major mechanism) but may be effective when PPHN has other causes such as chronic lung disease (Mesubi 2009; Mourani 2009). Concerns regarding retinal vascular growth (Kehat 2010) and lack of convincing data in preterm infants may preclude the use of sildenafil in preterm infants.
These five randomised clinical trials and several case reports, including recent reports from both resource‐limited settings (Juliana 2005; Simiyu 2006; Shivanna 2009; Sayed 2015) and resourceful settings (Steinhorn 2009), justify the call for a larger multi‐centre randomised controlled study. Studies of this type can be challenging. In addition to requiring multi‐centre collaboration, such studies would require that sildenafil be compared with other established therapies such as iNO and/or an optimal ventilatory strategy such as high‐frequency ventilation for PPHN. Resource‐limited settings could provide a platform for comparison of sildenafil versus placebo, and developed countries could provide settings for comparison with other management approaches. A large multi‐centre multi‐national trial in high‐income countries undertaken to evaluate the effectiveness of intravenous sildenafil versus placebo in terms of duration of nitric oxide treatment and treatment failure (additional treatment required for PPHN) is currently ongoing and may shed further light on this topic (NCT01720524). ClinicalTrials.gov (NCT01720524; updated 27 June 2016) reports an estimated enrolment of 64 and includes a plan for 12‐month and 24‐month safety and neurodevelopmental follow‐up. The estimated study completion date for this trial is December 2019. A pharmacokinetics study (NCT01670136; updated 17 March 2016) is currently recruiting preterm newborns receiving sildenafil as standard of care in the United States.
Quality of the evidence
We scored evidence quality using the GRADE PRO GDT online tool GRADEpro GDT. We graded the quality of evidence as low to very low, stemming from imprecision (small number of studies and small number of participants), lack of harmonised outcome measurement, and unclear methodological features due to inability to evaluate protocols because of lack of clinical trial registration. Without prospective registration, selective outcome reporting could not be addressed.
Potential biases in the review process
Variation in study design, including the addition of a post hoc adjuvant therapy comparison group, could have introduced a potential source of bias. After searching the literature, we believe that addition of this subgroup was warranted to account for future studies that are currently ongoing. Outcomes evaluated in the post hoc subgroup analysis did not influence our overall recommendations.
Agreements and disagreements with other studies or reviews
Case reports have described the efficacy of sildenafil for treatment of PPHN in neonates. A retrospective comparative assessment of iloprost versus sildenafil (Kahveci 2014) concluded that iloprost may be more effective than sildenafil in decreasing treatment time and improving ventilatory parameters for neonates with PPHN. As this review includes no published prospective randomised clinical trials of iloprost, we cannot confirm or disagree with these findings. Previous literature reviews (Spillers 2010; Iacovidou 2012) have presented information consistent with our finding that sildenafil may be effective in PPHN when other standard treatments such as iNO are not available.

Study flow diagram: review update.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
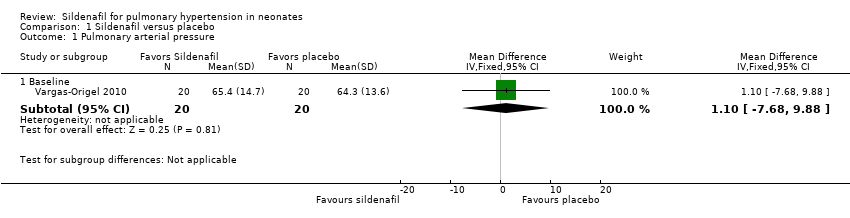
Comparison 1 Sildenafil versus placebo, Outcome 1 Pulmonary arterial pressure.

Comparison 1 Sildenafil versus placebo, Outcome 2 PaO2 in mmHg (absolute values).

Comparison 1 Sildenafil versus placebo, Outcome 3 Mean arterial blood pressure in mmHg.
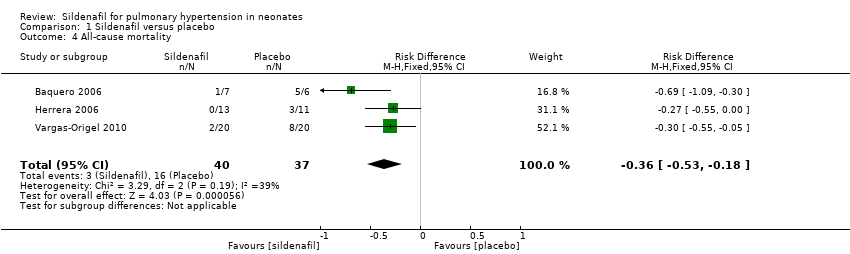
Comparison 1 Sildenafil versus placebo, Outcome 4 All‐cause mortality.

Comparison 1 Sildenafil versus placebo, Outcome 5 Oxygenation index (absolute values).
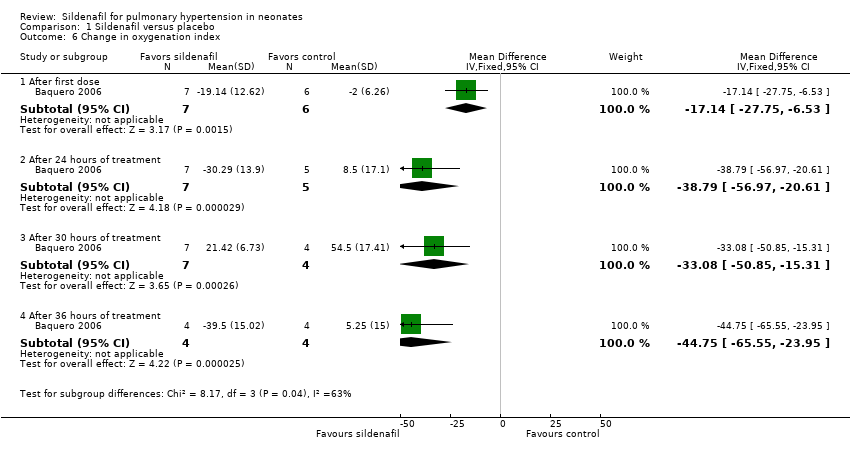
Comparison 1 Sildenafil versus placebo, Outcome 6 Change in oxygenation index.

Comparison 1 Sildenafil versus placebo, Outcome 7 A‐a DO2 difference.

Comparison 1 Sildenafil versus placebo, Outcome 8 Mean airway pressure.
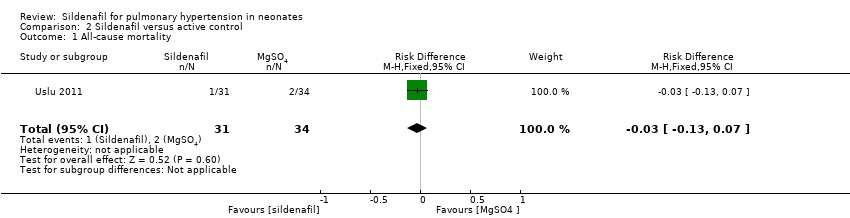
Comparison 2 Sildenafil versus active control, Outcome 1 All‐cause mortality.
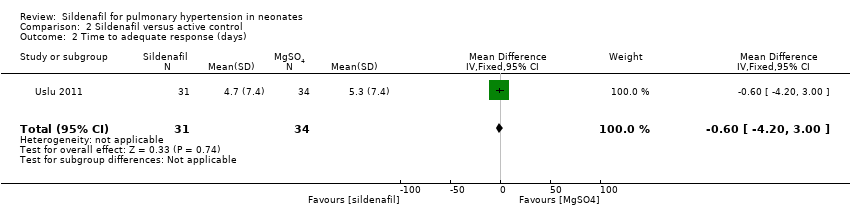
Comparison 2 Sildenafil versus active control, Outcome 2 Time to adequate response (days).
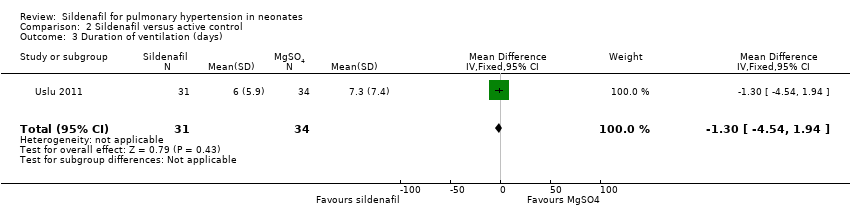
Comparison 2 Sildenafil versus active control, Outcome 3 Duration of ventilation (days).

Comparison 2 Sildenafil versus active control, Outcome 4 Inotropic agent.
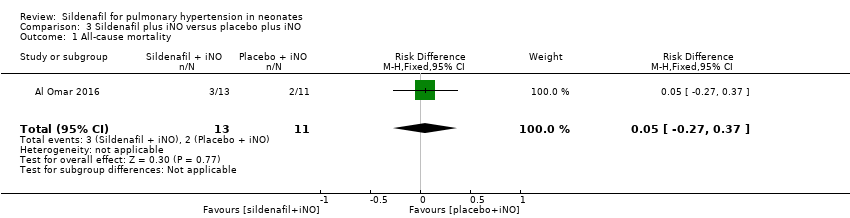
Comparison 3 Sildenafil plus iNO versus placebo plus iNO, Outcome 1 All‐cause mortality.
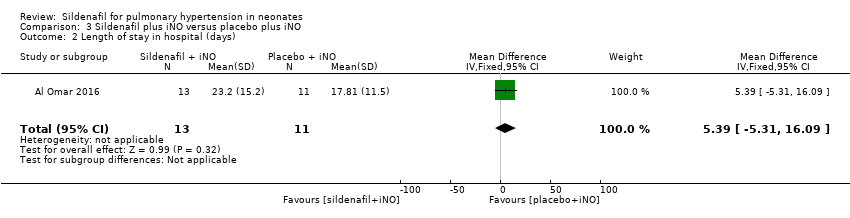
Comparison 3 Sildenafil plus iNO versus placebo plus iNO, Outcome 2 Length of stay in hospital (days).

Comparison 3 Sildenafil plus iNO versus placebo plus iNO, Outcome 3 Inotropic agent.
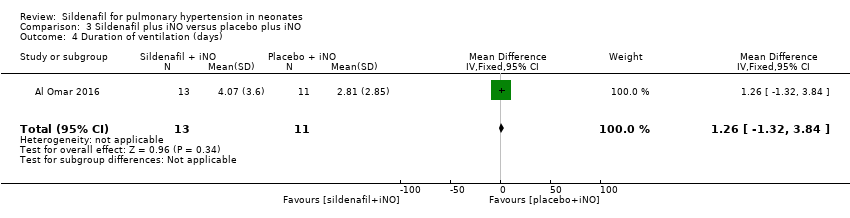
Comparison 3 Sildenafil plus iNO versus placebo plus iNO, Outcome 4 Duration of ventilation (days).

Comparison 3 Sildenafil plus iNO versus placebo plus iNO, Outcome 5 Retinopathy of prematurity.
| Sildenafil compared with placebo for pulmonary hypertension in neonates | ||||||
| Patient or population: pulmonary hypertension in neonates | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with sildenafil | |||||
| PaO2 in mmHg (absolute values) After 24‐25 hours | Mean PaO2 in mmHg (absolute values) | MD 15.31 higher | ‐ | 57 | ⊕⊕⊝⊝ | Evidence was downgraded due to unreported methodological features and imprecision (small sample size) |
| Change in oxygenation index | Mean change in oxygenation index | MD 38.79 lower | ‐ | 12 | ⊕⊕⊝⊝ | Evidence was downgraded due to unreported methodological features and imprecision (small sample size) |
| All‐cause mortality | Study population | RR 0.20 | 77 | ⊕⊕⊝⊝ | Evidence was downgraded due to unreported methodological features and imprecision (small sample size) | |
| 432 per 1000 | 77 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aImprecise due to small sample size bRisk of bias due to unclear randomisation allocation and lack of clinical trial registration | ||||||
| Sildenafil compared with active control for pulmonary hypertension in neonates | ||||||
| Patient or population: pulmonary hypertension in neonates | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with magnesium sulphate | Risk with sildenafil | |||||
| All‐cause mortality | Study population | RR 0.55 | 65 | ⊕⊝⊝⊝ | Evidence was downgraded due to very serious imprecision, as results from this single study have not been replicated and risk of bias is evident in study design (missing data) | |
| 59 per 1000 | 32 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aImprecise due to small sample size; only one included study bRisk of bias due to missing data (not analysed as intent to treat) | ||||||
| Sildenafil plus iNO compared with placebo plus iNO for pulmonary hypertension in neonates | ||||||
| Patient or population: pulmonary hypertension in neonates | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with placebo plus iNO | Risk with sildenafil plus iNO | |||||
| All‐cause mortality | Study population | RR 1.27 | 24 | ⊕⊕⊝⊝ | Evidence was downgraded due to imprecision . | |
| 182 per 1000 | 231 per 1000 | |||||
| Length of stay in hospital (days) | Mean length of stay in hospital was 17.81 days. | MD 5.39 higher | ‐ | 24 | ⊕⊕⊝⊝ | Evidence was downgraded due to imprecision . |
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aImprecise due to very small sample size; only one included study | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pulmonary arterial pressure Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Baseline | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [‐7.68, 9.88] |
| 2 PaO2 in mmHg (absolute values) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At baseline | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | 8.06 [1.58, 14.54] |
| 2.2 After first dose | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | 11.09 [1.65, 20.52] |
| 2.3 After 6 to 7 hours of treatment | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | 14.30 [5.25, 23.34] |
| 2.4 After 24 to 25 hours of treatment | 2 | 58 | Mean Difference (IV, Fixed, 95% CI) | 15.31 [6.49, 24.13] |
| 2.5 After 72 hours or at the end of treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 20.98 [14.81, 27.15] |
| 3 Mean arterial blood pressure in mmHg Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Before initiation of therapy | 2 | 53 | Mean Difference (IV, Fixed, 95% CI) | 5.65 [2.69, 8.61] |
| 3.2 At the end of therapy | 2 | 40 | Mean Difference (IV, Fixed, 95% CI) | 22.70 [1.23, 44.17] |
| 4 All‐cause mortality Show forest plot | 3 | 77 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.36 [‐0.53, ‐0.18] |
| 5 Oxygenation index (absolute values) Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 At baseline | 3 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐0.74 [‐8.11, 6.64] |
| 5.2 After first dose | 3 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐12.53 [‐18.60, ‐6.47] |
| 5.3 After 6 to 7 hours of treatment | 2 | 75 | Mean Difference (IV, Fixed, 95% CI) | ‐20.07 [‐26.12, ‐14.02] |
| 5.4 After 24 to 25 hours of treatment | 3 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐19.15 [‐24.52, ‐13.77] |
| 5.5 After 30 hours of treatment | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | ‐45.46 [‐61.87, ‐29.05] |
| 5.6 After 36 hours of treatment | 1 | 8 | Mean Difference (IV, Fixed, 95% CI) | ‐31.75 [‐45.74, ‐17.76] |
| 5.7 After 72 hours or at the end of treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐19.47 [‐23.42, ‐15.52] |
| 6 Change in oxygenation index Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 After first dose | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | ‐17.14 [‐27.75, ‐6.53] |
| 6.2 After 24 hours of treatment | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐38.79 [‐56.97, ‐20.61] |
| 6.3 After 30 hours of treatment | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | ‐33.08 [‐50.85, ‐15.31] |
| 6.4 After 36 hours of treatment | 1 | 8 | Mean Difference (IV, Fixed, 95% CI) | ‐44.75 [‐65.55, ‐23.95] |
| 7 A‐a DO2 difference Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Baseline | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | 0.99 [‐11.54, 13.51] |
| 7.2 At 6 to 7 hours of treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐27.72, 27.74] |
| 7.3 At 24 to 25 hours of treament | 2 | 57 | Mean Difference (IV, Fixed, 95% CI) | 1.59 [‐18.98, 22.16] |
| 7.4 At 72 hours or at the end of treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐18.34 [‐26.59, ‐10.09] |
| 8 Mean airway pressure Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Baseline | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐2.09 [‐3.30, ‐0.88] |
| 8.2 At 6 to 7 hours of treatment | 2 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐5.94 [‐7.36, ‐4.52] |
| 8.3 At 24 to 25 hours of treatment | 2 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐6.64 [‐8.49, ‐4.80] |
| 8.4 At 72 hours or at the end of treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐8.58 [‐10.37, ‐6.79] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 1 | 65 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.03 [‐0.13, 0.07] |
| 2 Time to adequate response (days) Show forest plot | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐4.20, 3.00] |
| 3 Duration of ventilation (days) Show forest plot | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐4.54, 1.94] |
| 4 Inotropic agent Show forest plot | 1 | 65 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.37 [‐0.59, ‐0.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 1 | 24 | Risk Difference (M‐H, Fixed, 95% CI) | 0.05 [‐0.27, 0.37] |
| 2 Length of stay in hospital (days) Show forest plot | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 5.39 [‐5.31, 16.09] |
| 3 Inotropic agent Show forest plot | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.37, 3.00] |
| 4 Duration of ventilation (days) Show forest plot | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 1.26 [‐1.32, 3.84] |
| 5 Retinopathy of prematurity Show forest plot | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |

