Corticosteroides para el tratamiento del daño nervioso en la lepra
Appendices
Appendix 1. Cochrane Neuromuscular Specialised Register (CRS) search strategy
#1 MeSH DESCRIPTOR Leprosy Explode All [REFERENCE] [STANDARD]
#2 hansen*:ti or hansen:ab [REFERENCE] [STANDARD]
#3 lepr*:ti or lepr*:ab [REFERENCE] [STANDARD]
#4 #1 or #2 or #3 [REFERENCE] [STANDARD]
#5 MeSH DESCRIPTOR Adrenal Cortex Hormones Explode All [REFERENCE] [STANDARD]
#6 prednisone* or prednisolone* or cortisone* or cyclosporine* or azathioprine* [REFERENCE] [STANDARD]
#7 methylprednisolone or glucocorticoid* or corticosteroid* or "cortical hormone" or "cortical hormones" [REFERENCE] [STANDARD]
#8 #5 or #6 or #7 [REFERENCE] [STANDARD]
#9 MeSH DESCRIPTOR Peripheral Nervous System Diseases Explode All [REFERENCE] [STANDARD]
#10 neuritis or neuropath* or "nerve damage" or "nerve involvement" [REFERENCE] [STANDARD]
#11 "nerve loss" or "nerve function impairment" or "nerve problem" or "nerve problems" [REFERENCE] [STANDARD]
#12 "sensory loss" or "motor loss" or "motor function loss" [REFERENCE] [STANDARD]
#13 "nerve pain" or "nerve tenderness" [REFERENCE] [STANDARD]
#14 reaction* [REFERENCE] [STANDARD]
#15 #9 or #10 or #11 or #12 or #13 or #14 [REFERENCE] [STANDARD]
#16 #4 and #8 and #15 [REFERENCE] [STANDARD]
#17 (#4 and #8 and #15) AND (INREGISTER) [REFERENCE] [STANDARD]
Appendix 2. CENTRAL search strategy
#1 leprosy
#2 MeSH descriptor Leprosy explode all trees
#3 "hansen disease"
#4 (#1 OR #2 OR #3)
#5 "peripheral nervous system diseases" or "peripheral nerves"
#6 MeSH descriptor Peripheral Nervous System Diseases explode all trees
#7 (neuritis or neuralgia or neuropath* or "nerve damage" or "nerve involvement" or "nerve loss" or "nerve function impairment" or "nerve problem" or "sensory loss" or "motor loss" or "motor function loss" or "nerve pain" or "nerve tenderness" or reaction$)
#8 (#5 OR #6 OR #7)
#9 MeSH descriptor Adrenal Cortex Hormones explode all trees
#10 (prednisolone or prednisone or cortisone or cyclosporin or ciclosporin or azathioprine or methylprednisolone or betamethasone)
#11 (glucocorticoid* or corticosteroid* or prednisolon* or prednison* or cortison* or "cortical hormones" or "cortical hormone" or cyclosporin* or ciclosporin* or azathioprin* or methylprednisolon* or betamethason*)
#12 (#9 OR #10 OR #11)
#13 (#4 AND #8 AND #12)
Appendix 3. MEDLINE (OvidSP) search strategy
Database: Ovid MEDLINE(R) <1946 to June Week 1 2015>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 randomized controlled trial.pt. (396862)
2 controlled clinical trial.pt. (89648)
3 randomized.ab. (293733)
4 placebo.ab. (152857)
5 drug therapy.fs. (1782093)
6 randomly.ab. (207091)
7 trial.ab. (303153)
8 groups.ab. (1318490)
9 or/1‐8 (3363492)
10 exp animals/ not humans.sh. (4057817)
11 9 not 10 (2863375)
12 exp Leprosy/ (20239)
13 hansen* disease.tw. (829)
14 12 or 13 (20301)
15 exp Adrenal Cortex Hormones/ (344770)
16 Prednisolone/ (29599)
17 Prednisone/ (35381)
18 Cortisone/ (15363)
19 Cyclosporine/ (26754)
20 Azathioprine/ (13448)
21 Methylprednisolone/ (16493)
22 Betamethasone/ (5331)
23 (glucocorticoid$ or corticosteroid$ or prednisolon$ or prednison$ or cortison$ or cortical hormones$ or cyclosporin$ or ciclosporin$ or azathioprin$ or methylprednisolon$ or betamethason$).tw. (216209)
24 or/15‐23 (451754)
25 11 and 14 and 24 (385)
26 exp Peripheral Nervous System Diseases/ (119912)
27 (neuritis or neuropath$ or nerve damage or nerve involvement or nerve loss or nerve function impairment or nerve problem or sensory loss or motor loss or motor function loss or nerve pain or nerve tenderness or reaction$ or reversal reaction$ or type 1 reaction or type 2 reaction).tw. (885639)
28 26 or 27 (969292)
29 11 and 14 and 24 and 28 (260)
30 remove duplicates from 29 (259)
Appendix 4. EMBASE (OvidSP) search strategy
Database: Embase <1980 to 2015 Week 24>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 crossover‐procedure/ (43171)
2 double‐blind procedure/ (121038)
3 randomized controlled trial/ (373903)
4 single‐blind procedure/ (20388)
5 (random$ or factorial$ or crossover$ or cross over$ or cross‐over$ or placebo$ or (doubl$ adj blind$) or (singl$ adj blind$) or assign$ or allocat$ or volunteer$).tw. (1464106)
6 clinical trial/ (845836)
7 or/1‐6 (2014785)
8 exp animals/ (19964586)
9 exp humans/ (15918733)
10 8 not (8 and 9) (4045853)
11 7 not 10 (1855155)
12 limit 11 to embase (1511872)
13 exp LEPROSY/ (23445)
14 hansen$ disease.tw. (1180)
15 leprosy.tw. (18747)
16 or/13‐15 (25694)
17 exp Peripheral Neuropathy/ (53096)
18 neuritis.mp. (16732)
19 neuropath$.mp. (224458)
20 nerve damage.mp. (5418)
21 nerve involvement.mp. (2736)
22 nerve loss.mp. (125)
23 nerve function impairment.mp. (110)
24 nerve problem$.mp. (142)
25 sensory loss.mp. (3119)
26 motor loss.mp. (240)
27 motor function loss.mp. (43)
28 nerve pain.mp. (268)
29 nerve tenderness.mp. (18)
30 reaction$.tw. (1029628)
31 reversal reaction.mp. (604)
32 type 1 reaction$.mp. (225)
33 type 2 reaction$.mp. (135)
34 erythema nodosum leprosum.mp. (1241)
35 or/17‐34 (1267303)
36 (steroid$ or glucocorticoid$ or corticosteroid$ or prednisolon$ or prednison$ or cortical hormone$ or cyclosporin A or azathioprin$ or methylprednisolon$ or betamethason$ or cortison$).mp. (840421)
37 exp decompression surgery/ (36094)
38 (necrolysis or epicondylectomy).mp. (6971)
39 or/36‐38 (878190)
40 exp peripheral neuropathy/ (53096)
41 (neuritis or neuropath$ or nerve damage or nerve involvement or nerve loss or nerve function impairment or nerve problem$).mp. (241878)
42 (sensory loss or motor loss or motor function loss or nerve pain or nerve tenderness or reaction$ or reversal reaction or type 1 reaction$ or type 2 reaction$ or erythema nodosum leprosum).mp. (1868057)
43 12 and 16 and 39 and 42 (119)
Appendix 5. LILACS (BIREME IAHx) search strategy
(MH:C01.252.410.040.552.386$ or leprosy or lepra or hanseniase) and (prednisone or prednisona or prednisolone or prednisolona or cortisone or cortisona or cyclosporine or ciclosporina or azathioprine or azatioprina or methylprednisolone or metilprednisolona or betamethasone or betametasona or glucocorticoid$ or corticosteroid$ or corticoesteroid$) and ((PT:"Randomized Controlled Trial" or "Randomized Controlled trial" or "Ensayo Clínico Controlado Aleatorio" or "Ensaio Clínico Controlado Aleatório" or PT:"Controlled Clinical Trial" or "Ensayo Clínico Controlado" or "Ensaio Clínico Controlado" or "Random allocation" or "Distribución Aleatoria" or "Distribuição Aleatória" or randon$ or Randomized or randomly or "double blind" or "duplo‐cego" or "duplo‐cego" or "single blind" or "simples‐cego" or "simples cego" or placebo$ or trial or groups) AND NOT (B01.050$ AND NOT (humans or humanos or humanos)))
Appendix 6. CINAHL (EBSCOhost) search strategy
Tuesday, June 16, 2015 11:00:19 AM
S34 S15 and S33 7
S33 S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 752,583
S32 ABAB design* 93
S31 TI random* or AB random* 151,010
S30 ( TI (cross?over or placebo* or control* or factorial or sham? or dummy) ) or ( AB (cross?over or placebo* or control* or factorial or sham? or dummy) ) 301,434
S29 ( TI (clin* or intervention* or compar* or experiment* or preventive or therapeutic) or AB (clin* or intervention* or compar* or experiment* or preventive or therapeutic) ) and ( TI (trial*) or AB (trial*) ) 105,987
S28 ( TI (meta?analys* or systematic review*) ) or ( AB (meta?analys* or systematic review*) ) 36,870
S27 ( TI (single* or doubl* or tripl* or trebl*) or AB (single* or doubl* or tripl* or trebl*) ) and ( TI (blind* or mask*) or AB (blind* or mask*) ) 23,445
S26 PT ("clinical trial" or "systematic review") 127,929
S25 (MH "Factorial Design") 945
S24 (MH "Concurrent Prospective Studies") or (MH "Prospective Studies") 264,883
S23 (MH "Meta Analysis") 22,461
S22 (MH "Solomon Four‐Group Design") or (MH "Static Group Comparison") 48
S21 (MH "Quasi‐Experimental Studies") 7,381
S20 (MH "Placebos") 9,272
S19 (MH "Double‐Blind Studies") or (MH "Triple‐Blind Studies") 31,799
S18 (MH "Clinical Trials+") 188,614
S17 (MH "Crossover Design") 13,034
S16 (MH "Random Assignment") or (MH "Random Sample") or (MH "Simple Random Sample") or (MH "Stratified Random Sample") or (MH "Systematic Random Sample") 69,594
S15 S3 and S14 28
S14 S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 32,265
S13 (cortison*) or (MH "Cortisone") 389
S12 (betamethason*) or (MH "Betamethasone") 540
S11 (methylprednisolon*) or (MH "Methylprednisolone") 2,083
S10 (azathioprin*) or (MH "Azathioprine") 1,245
S9 (cyclosporin A) or (MH "Cyclosporine") 1,689
S8 prednison* 3,674
S7 (prednisolon*) or (MH "Prednisolone") 2,393
S6 corticosteroid* 9,046
S5 (glucocorticoid*) or (MH "Glucocorticoids") 6,518
S4 (steroid) or (MH "Steroids") 11,910
S3 S1 or S2 1,210
S2 hansen disease 11
S1 (leprosy) or (MH "Leprosy") 1,210

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
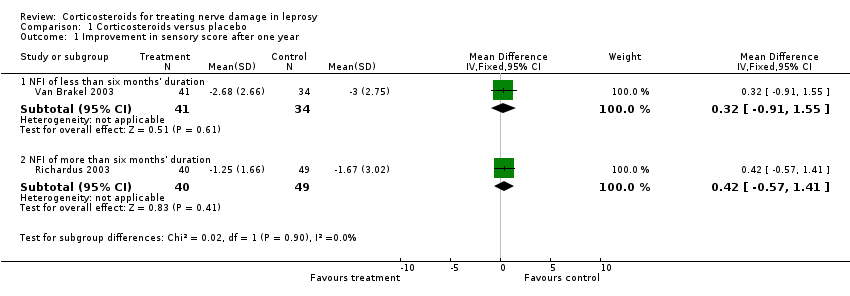
Comparison 1 Corticosteroids versus placebo, Outcome 1 Improvement in sensory score after one year.
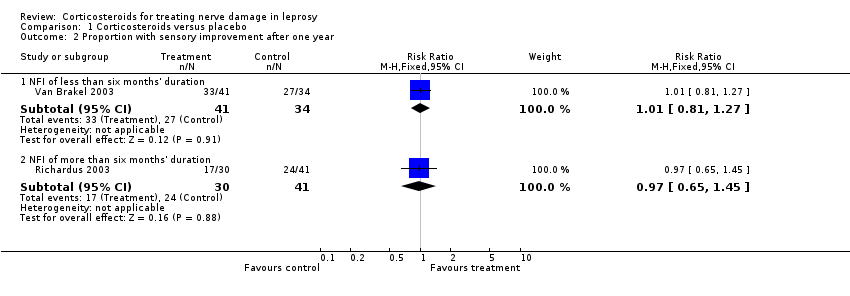
Comparison 1 Corticosteroids versus placebo, Outcome 2 Proportion with sensory improvement after one year.
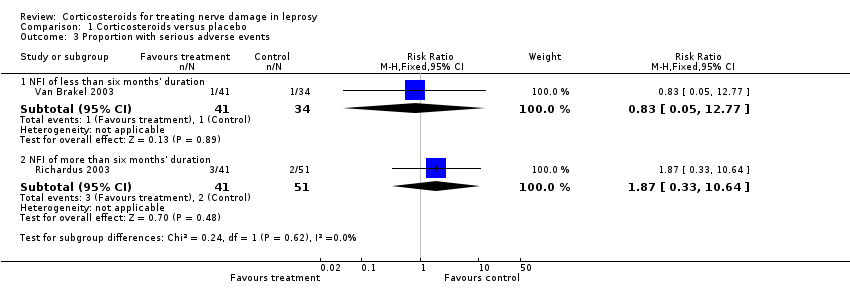
Comparison 1 Corticosteroids versus placebo, Outcome 3 Proportion with serious adverse events.
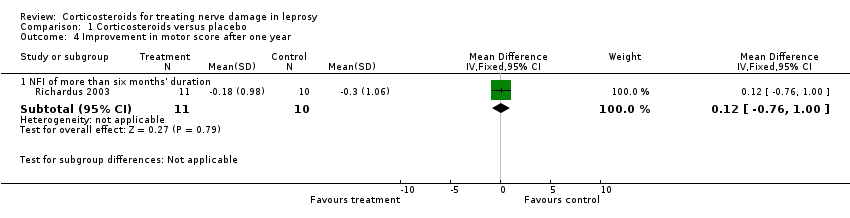
Comparison 1 Corticosteroids versus placebo, Outcome 4 Improvement in motor score after one year.

Comparison 1 Corticosteroids versus placebo, Outcome 5 Proportion with motor improvement after one year.
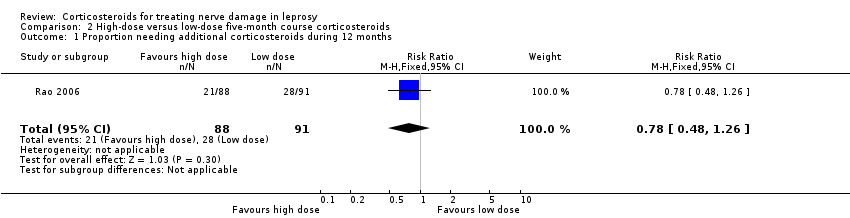
Comparison 2 High‐dose versus low‐dose five‐month course corticosteroids, Outcome 1 Proportion needing additional corticosteroids during 12 months.
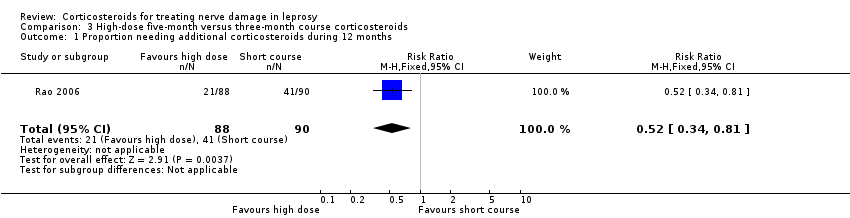
Comparison 3 High‐dose five‐month versus three‐month course corticosteroids, Outcome 1 Proportion needing additional corticosteroids during 12 months.
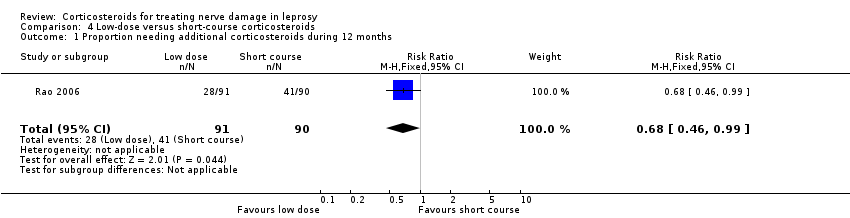
Comparison 4 Low‐dose versus short‐course corticosteroids, Outcome 1 Proportion needing additional corticosteroids during 12 months.

Comparison 5 Intravenous methylprednisolone and oral prednisolone versus intravenous normal saline and oral prednisolone, Outcome 1 Adverse events.
| Corticosteroids compared with placebo for treating nerve damage (< 6 months' duration) in leprosy | ||||||
| Patient or population: people with nerve damage (< 6 months' duration) in leprosy Settings: Nepal and Bangladesh Intervention: corticosteroids (prednisolone started at 40 mg/day then gradually tapered) Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with corticosteroids | |||||
| Improvement in sensory nerve function at 1 year Defined as a reduction in sensory score by 3 or more points from baseline | Study population | RR 1.01 (0.81 to 1.27) | 75 nerves | ⊕⊕⊕⊝ | ‐ | |
| 794 per 1000 | 802 per 1000 (643 to 1000) | |||||
| Improvement in motor nerve function at 1 year ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Change in nerve pain and in nerve tenderness at 1 year ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Limitations in activities of daily living at 1 year ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Limitations in participation at 1 year ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Adverse events Assessed as: occurrence of one or more major adverse events requiring withdrawal of treatment | Study population | RR 0.83 (0.05 to 12.77) | 75 nerves (1 RCT) | ⊕⊕⊕⊝ | ‐ | |
| 29 per 1000 | 24 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Small sample size. | ||||||
| Corticosteroids compared to placebo for treating nerve damage (6 to 24 months' duration) in leprosy | ||||||
| Patient or population: people with nerve damage (6 to 24 months' duration) in leprosy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with corticosteroids | |||||
| Improvement in sensory nerve function at 1 year Defined as a reduction by 3 or more points from baseline | Study population | RR 0.97 (0.65 to 1.45) | 71 | ⊕⊕⊕⊝ | ‐ | |
| 585 per 1000 | 568 per 1000 | |||||
| Improvement in motor nerve function at 1 year | The mean improvement in motor nerve function at 1 year was ‐0.30 ± 1.6 points | The mean improvement in motor nerve function at 1 year in the intervention group was 0.12 points higher (0.76 lower to 1.00 higher) | ‐ | 21 | ⊕⊕⊝⊝ | The MD slightly favoured the placebo group, but not significantly (MD 0.12, 95% CI ‐0.76 to 1.00) |
| Change in nerve pain and in nerve tenderness at 1 year ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Limitations in activities of daily living at 1 year ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Limitations in participation at 1 year ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Adverse events | Study population | RR 1.87 (0.33 to 10.64) | 92 | ⊕⊕⊕⊝ | ‐ | |
| 39 per 1000 | 73 per 1000 (13 to 417) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Small sample size. 2Very small sample size: 21 participants with motor nerve function impairment. | ||||||
| Ridley‐Jopling | WHO |
| TT: tuberculoid leprosy | PB: paucibacillary leprosy |
| BT: borderline tuberculoid leprosy | PB |
| BB: borderline leprosy | MB: multibacillary leprosy |
| BL: borderline lepromatous leprosy | MB |
| LL: lepromatous leprosy | MB |
| Colour | Approximate force | Score for hand | Score for foot |
| Blue filament felt | 200 mg | 0 | |
| Purple filament felt | 2 g | 1 | 0 |
| Red filament felt | 4 g | 2 | 1 |
| Orange filament felt | 10 g | 3 | 2 |
| Pink filament felt | 300 g | 4 | 3 |
| Pink filament not felt | ‐ | 5 | 4 |
| Grade | Definition |
| 5 | Full range of movement of the joint on which the muscle or muscle group is acting. Normal resistance can be given |
| 4 | Full range of movement but less than normal resistance |
| 3 | Full range of movement but no resistance |
| 2 | Partial range of movement with no resistance |
| 1 | Perceptible contraction of the muscle(s) not resulting in joint movement |
| 0 | Complete paralysis |
| MRC: Medical Research Council | |
| Score | Grade |
| Nerve pain | |
| 3 | Absent |
| 2 | Mild (only aware intermittently and does not limit activity) |
| 1 | Moderate (sleep disturbed, activities diminished, work efficiency diminished) |
| 0 | Severe (incapacitating) |
| Nerve tenderness | |
| 3 | Absent |
| 2 | Mild (absent if person's attention is distracted) |
| 1 | Moderate (present if attention is distracted) |
| 0 | Severe (very tender and person withdraws the arm forcibly) |
| High‐dose corticosteroids compared to low‐dose corticosteroids for treating nerve damage in leprosy (5‐month regimens) | ||||||
| Patient or population: people with nerve damage in leprosy (severe type 1 leprosy reaction) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with low‐dose corticosteroids | Risk with high‐dose corticosteroids | |||||
| Improvement in sensory nerve function at 1 year ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Improvement in motor nerve function at 1 year ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Change in nerve pain and in nerve tenderness at 1 year ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Limitations in activities of daily living at 1 year ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Limitations in participation at 1 year ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Adverse events | The trial assessing this comparison reported no serious adverse events from routine clinical examination during follow‐up | Not estimable | 179 | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Low‐dose corticosteroids compared to short‐course corticosteroids for treating nerve damage in leprosy | ||||||
| Patient or population: people with nerve damage in leprosy (severe type 1 leprosy reaction) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with short‐course corticosteroids | Risk with low‐dose corticosteroids | |||||
| Improvement in sensory nerve function at 1 year ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Improvement in motor nerve function at 1 year ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Change in nerve pain and in nerve tenderness at 1 year ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Limitations in activities of daily living at 1 year ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Limitations in participation at 1 year ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse events | The trial assessing this comparison reported no serious adverse events from routine clinical examination during follow‐up | Not estimable | 181 | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| High‐dose corticosteroids compared to short‐course corticosteroids for treating nerve damage in leprosy | ||||||
| Patient or population: people with nerve damage in leprosy (severe type 1 leprosy reaction) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with short‐course corticosteroids | Risk with high‐dose corticosteroids | |||||
| Improvement in sensory nerve function at 1 year ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Improvement in motor nerve function at 1 year ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Change in nerve pain and in nerve tenderness at 1 year ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Limitations in activities of daily living at 1 year ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Limitations in participation at 1 year ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Adverse events | The trial assessing this comparison reported no serious adverse events from routine clinical examination during follow‐up | Not estimable | 178 | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| High‐dose prednisone compared to low‐dose prednisone for treating nerve damage in leprosy | ||||||
| Patient or population: people with nerve damage in leprosy (ulnar neuropathy due to type 1 or type 2 leprosy reaction) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with low‐dose corticosteroids | Risk with high‐dose corticosteroids | |||||
| Improvement in sensory nerve function at 1 year ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Measured by microfilament test but summarised in a composite clinical score |
| Improvement in motor nerve function at 1 year ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Measured by MRC scale but summarised in a composite clinical score |
| Change in nerve pain and in nerve tenderness at 1 year ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Measured by visual analogue scale but summarised in a composite clinical score |
| Limitations in activities of daily living at 1 year ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Limitations in participation at 1 year ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Adverse events | 2 participants reported major adverse events, both in the high‐dose group | Not estimable | 21 (1 RCT) | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Intravenous methylprednisolone and oral prednisolone compared to intravenous normal saline and oral prednisolone for treating nerve damage in leprosy | ||||||
| Patient or population: people with nerve damage in leprosy type 1 reaction of less than 6 months' duration or with new nerve function impairment of less than 6 months' duration | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with intravenous normal saline and oral prednisolone | Risk with intravenous methylprednisolone and oral prednisolone | |||||
| Improvement in nerve function | There was a downward trend in the total Clinical Severity Scores of both groups. There were no statistically significant differences between the prednisolone‐alone and prednisolone plus methylprednisolone groups at any time point | ‐ | 42 (1 RCT) | ⊕⊕⊝⊝ | The proportion of participants with improvement in motor and sensory nerve function was not reported | |
| Change in nerve pain and in nerve tenderness at 1 year ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Limitations in activities of daily living at 1 year ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Limitations in participation at 1 year ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Adverse events | 545 per 1000 | 551 per 1000 | RR 1.01 (95% CI 0.58 to 1.75) | 42 | ⊕⊕⊕⊝ | 2 people (1 from each arm of the study) experienced at least 1 adverse event |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Outcome measure only narratively described. Significant improvement not defined. 2Small sample size, wide CI (imprecision). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Improvement in sensory score after one year Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 NFI of less than six months' duration | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐0.91, 1.55] |
| 1.2 NFI of more than six months' duration | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 0.42 [‐0.57, 1.41] |
| 2 Proportion with sensory improvement after one year Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 NFI of less than six months' duration | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.81, 1.27] |
| 2.2 NFI of more than six months' duration | 1 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.65, 1.45] |
| 3 Proportion with serious adverse events Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 NFI of less than six months' duration | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.05, 12.77] |
| 3.2 NFI of more than six months' duration | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.33, 10.64] |
| 4 Improvement in motor score after one year Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 NFI of more than six months' duration | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.76, 1.00] |
| 5 Proportion with motor improvement after one year Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 NFI of more than six months' duration | 1 | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.5 [0.32, 63.94] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion needing additional corticosteroids during 12 months Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.48, 1.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion needing additional corticosteroids during 12 months Show forest plot | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.34, 0.81] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion needing additional corticosteroids during 12 months Show forest plot | 1 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.46, 0.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse events Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.58, 1.75] |

