Dispositivos para reducir la presión en el tratamiento de las úlceras por presión del talón
Resumen
Antecedentes
Las úlceras por presión son áreas de daño localizado en la piel y el tejido subyacente causadas por la presión o el roce. Los dispositivos para redistribuir la presión se utilizan como parte del tratamiento para reducir la presión sobre la úlcera. La anatomía del talón y la susceptibilidad del pie a la vasculopatía hacen que las úlceras por presión que se localizan allí requieran de un enfoque particular para reducir la presión.
Objetivos
Determinar los efectos de las intervenciones para reducir la presión en el tratamiento de las úlceras por presión del talón.
Métodos de búsqueda
En mayo de 2013, para esta primera actualización, se hicieron búsquedas en el Registro Especializado del Grupo Cochrane de Heridas (Cochrane Wounds Group); el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials, CENTRAL) (la Cochrane Library); Ovid MEDLINE; Ovid EMBASE; Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations) y EBSCO CINAHL. No se aplicaron restricciones de idioma o de fecha de publicación.
Criterios de selección
Se incluyeron los ensayos controlados aleatorizados (ECA) que compararon los efectos de los dispositivos para reducir la presión sobre la cicatrización de las úlceras por presión del talón. Los pacientes se trataron en cualquier ámbito de atención. Las intervenciones incluyeron cualquier dispositivo para reducir la presión incluyendo colchones y dispositivos específicos para el talón.
Obtención y análisis de los datos
Ambos autores de la revisión, de forma independiente, revisaron los títulos y resúmenes y seleccionaron los estudios para inclusión. Ambos autores de la revisión, de forma independiente, extrajeron los datos y evaluaron el riesgo de sesgo de los estudios.
Resultados principales
En la revisión original, sólo un estudio cumplió con los criterios de inclusión. Este estudio (141 participantes) comparó dos sistemas de colchón; sin embargo, las pérdidas durante el seguimiento fueron demasiado grandes para poder establecer conclusiones confiables. No se encontraron más estudios relevantes durante esta primera actualización.
Conclusiones de los autores
Esta revisión identificó un estudio pequeño con un riesgo de sesgo de moderado a alto que no proporcionó evidencia para informar la práctica. Se necesitan más estudios de investigación.
PICO
Resumen en términos sencillos
Dispositivos para reducir la presión en el tratamiento de las úlceras por presión del talón
Las úlceras por presión (también conocidas como escaras por presión, úlceras de decúbito y escaras de decúbito) son áreas de daño localizado en la piel y el tejido subyacente causadas por presión, roce o fricción. Los dispositivos para reducir la presión como camas, colchones, soportes para el talón, férulas y almohadas se utilizan como parte del tratamiento para reducir o aliviar la presión sobre la úlcera. Se estudiaron específicamente las úlceras del talón porque la estructura del talón es muy diferente a la de los otros sitios corporales que son propensos a las úlceras por presión (como las nalgas), y además son más propensas a enfermedades (como la circulación deficiente) que no afectan a otros sitios donde se producen las úlceras por presión. Se identificó un estudio con riesgo de sesgo de moderado a alto. En este estudio se perdió alrededor de la mitad de los participantes durante el seguimiento. Se necesitan estudios de investigación de alta calidad para informar la selección de los dispositivos para reducir la presión en el tratamiento de las úlceras por presión del talón.
Authors' conclusions
Background
Description of the condition
Aetiology of pressure ulcers
Pressure ulcers (also known as pressure sores, decubitus ulcers and bed sores) are areas of localised damage to the skin and underlying tissue, believed to be caused by pressure, shear or friction (Allman 1997). They usually occur over bony prominences, such as the heel, where there is little soft tissue, in particular subcutaneous fat, to provide mobility and padding. Animal studies show the severity of tissue damage to be proportional to the time and intensity of the pressure (Kosiak 1959). This has also been shown to apply to humans in a study of people with spinal injuries (Reswick 1976).
Size of the problem
Pressure ulcers cause major morbidity and reduce the quality of life for participants and their carers (Essex 2009). The financial costs to the UK National Health Service (NHS) are also substantial. In the UK, the cost of preventing and treating pressure ulcers in a 600‐bedded large general hospital was estimated at between GBP £600,000 and £3 million per year (Clark 1994). Most published data on the prevalence and incidence of pressure ulcers come from hospital populations. In the UK, pressure ulcer prevalence ranges from 5% to 32% and incidence from 2% to 29% depending on setting and case mix (Kaltenthaler 2001).
The majority of pressure ulcers are found on the lower body including the feet. Although pressure ulcers of the sacral or buttock region are the most common (Dealey 1991) the heels are frequently reported as affected (Dealey 1991a). In the UK most NHS Trusts collect data on the prevalence and incidence of pressure ulcers. Repeated annual prevalence surveys from one UK hospital revealed that up to one‐quarter of all pressure ulcers were located on the heel (based on 19% to 26% in Table 1).
| Year | Total no. PUs | No. of heel PUs | % of PUs on heel |
| 2006 | 274 | 60 | 22% |
| 2007 | 333 | 85 | 26% |
| 2008 | 336 | 74 | 22% |
| 2009 | 557 | 107 | 19% |
PU = pressure ulcer
Heel pressure ulcers
Heel ulcers are rarely the focus of research and furthermore most research is focused on reducing the risk of pressure ulcer development (i.e. prevention) rather than the treatment of active ulceration. Treatment strategies (for example, nutritional support) are generally based on the extrapolation of prevention research. This evidence is sparse and of generally poor quality and there is insufficient evidence to demonstrate the effectiveness of nutritional supplements in prevention, and particularly treatment, of pressure ulcers (Langer 2003). The use of local pressure relief through support surfaces has been studied as a preventative intervention (McInnes 2011). The continued use of support surfaces, even when a pressure ulcer has occurred, is advocated to prevent further damage (RCN 2005). It is thought that people with diabetes may be particularly susceptible to pressure ulcers of the foot and indeed diabetic foot ulcers are wounds which occur anywhere on the feet; they may occur on the heel and be pressure‐related. There are two Cochrane systematic reviews on preventing and treating diabetic foot ulcers but these do not define foot ulcer and so may include pressure ulcers (Spencer 2000; Valk 2010). Neither of the reviews looked at heel ulcers as a subgroup. Since heel ulcers may represent a distinct clinical entity, in terms of risk and responses to treatment, they are worthy of specific scrutiny.
The feet are distinct from other body sites for the following reasons:
-
They are designed for weight‐bearing, with thickened dermis on the sole of the foot. This means that the skin has a relatively high number of collagen and elastic fibres to allow extensibility and elasticity and Pacinian corpuscles to identify pressure (Thoolen 2000) compared to skin found on most other areas of the body.
-
The circulation to the lower limbs often becomes compromised due to arterial diseases such as atherosclerosis. Although associated with increasing age (Vogt 1992), poor circulation is seen in younger people, particularly in association with risk factors such as smoking, diabetes and hypertension (Vogt 1992). The internal capillary pressures reduce and if subjected to external pressure are not able to respond appropriately to prevent occlusion (Kannell 1973).
-
Neuropathy (reduced or altered sensation) has been identified as a risk factor for ulceration in the feet of people with diabetes (McNeeley 1995). Neuropathy is also known to be associated with other diseases such as stroke, pernicious anaemia, spina bifida and multiple sclerosis although its precise prevalence is unknown (Neale 1981). Although no published papers have been identified so far, data collected during a study on pain in leg ulcers (Briggs 2003) has shown that many elderly people have some degree of neuropathy of the lower limbs. The presence of neuropathy may result in a person being unaware of pressure and, therefore, not responding to it (Raney 1989).
-
Oedema is the presence of excess extracellular fluid which causes localised swelling. It is associated with peripheral vascular disease, the effects of gravity on a dependent limb and other physiological changes (Ciocon 1993). The presence of oedema compromises tissue perfusion and removal of waste products (Ryan 1969). Also, the weight of the extra fluid in the feet is likely to result in normal resting pressures being exceeded, which may have an impact on tissue tolerance of pressure.
-
The absence of sebaceous glands on the sole of the foot results in a lack of lubrication of the skin; this may increase the likelihood of friction damage (Tortora 1996).
-
The superficial fascia is dense over the heel and contains loci of fat in facial pockets. Although this provides added protection from pressure, the effect of exceeding normal tissue tolerance has not been identified. This is likely to affect the response to pressure (Tortora 1996).
The presence of arterial disease and neuropathy may have an impact on the tolerance of the heel in terms of the extent and duration of applied pressure. Clearly these pathologies are less likely to influence other pressure sites such as the sacrum or ischial tuberosities (parts of the pelvis prone to pressure ulcers). The review of heel pressure ulcers as a separate entity is, therefore, a worthy study.
Description of the intervention
Once a heel pressure ulcer has occurred, treatment is concerned with reducing exposure of the heel to pressure and the management of the wound. The management of the wound is outside the scope of this review.
Reducing the extent and duration of applied pressure is achieved by changing a person's position, using equipment which provides an overall reduced pressure or alternating periods of low and high pressure or both. Pressure‐reducing supports can be for the whole body, for example mattresses, or specific to the body site such as foot protectors and booties. Most interventions are designed for pressure ulcer prevention; however, if an ulcer has occurred it is necessary to reduce the risks of further tissue breakdown.
Devices that reduce the magnitude of the applied pressure are thought to work by distributing body weight over a larger surface area, by conforming to the shape of the body generally or the heel specifically. These are known as constant low pressure devices (CLP). They vary in their construction, for example foam, gel, sheepskin, or an air‐filled or water‐filled device. Devices which reduce the duration of pressure use a system of air‐filled cells (AP) which alternate between high and low pressure by inflation and deflation of alternate cells. Other devices remove pressure from the body site at risk, for example by supporting the foot or calf in a splint, using a foot trough or pillow that leaves the heel with no surface contact.
Why it is important to do this review
Very little is known about the contribution of the reduction or relief of pressure to the wound‐healing process. The rationale for this review is based on the assumption that the reduction of external pressure on the pressure ulcer will have a positive effect on the wound‐healing process.
Objectives
To determine the effects of pressure‐relieving devices for treating pressure ulcers on the heel.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) which compare the effects of pressure‐relieving devices on the healing of heel pressure ulcers. RCTs focusing specifically on pressure relief for diabetic foot ulcers were included if heel ulcer data were separately identified. Controlled clinical trials (CCTs) were to be included in the absence of RCTs.
Types of participants
Studies involving participants with existing heel pressure ulcers of any grade (RCN 2005) and in any care setting.
Types of interventions
Pressure‐relieving, re‐distributing or reducing aids used alone or in combination. Pressure‐relieving aids include the following:
-
mattresses;
-
foam overlays;
-
foam mattress replacements;
-
alternating air‐filled overlays;
-
alternating air‐filled mattress replacements;
-
air overlays; and
-
air‐fluidised bead beds.
Heel‐specific aids:
-
air‐filled booties;
-
foam foot protectors;
-
gel foot protectors;
-
pillows and other aids positioned under the legs to redistribute pressure;
-
splints or other medical devices; and
-
sheepskins.
Eligible comparisons would be any of the interventions listed above compared with each other, no intervention or standard care as defined by the trialists. To be eligible for inclusion treatment arms differed only in the pressure‐relief; local wound care (which is usually used in combination with pressure relief) should not have differed systematically across treatment arms within a trial.
Types of outcome measures
Primary outcomes
Trials reporting any of the following outcomes at any endpoint were eligible.
-
Proportion of heel ulcers healed within a defined time period.
-
Time to complete healing of heel ulcers.
Secondary outcomes
-
Costs of pressure‐relieving device.
-
Total costs of interventions (including service and maintenance).
-
Patient comfort.
-
Ease of use.
-
Health‐related quality of life.
-
Adverse events.
Search methods for identification of studies
For the search methods used in the original version of this review see Appendix 1
Electronic searches
In May 2013, for this first update, we searched the following electronic databases to find reports of relevant RCTs:
-
The Cochrane Wounds Group Specialised Register (searched 10 May 2013);
-
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 4);
-
Ovid MEDLINE (2011 to May Week 1 2013);
-
Ovid EMBASE (2010 to 2013 Week 18);
-
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations, May 09 2013);
-
EBSCO CINAHL (2011 to 2 May 2013)
We used the following search strategy in the Cochrane Central Register of Controlled Trials (CENTRAL):
#1 MeSH descriptor: [Beds] explode all trees251
#2 (bed or beds):ti,ab,kw 4202
#3 (mattress* or cushion* or pillow*):ti,ab,kw 662
#4 ("foam" or cutfoam or overlay*):ti,ab,kw 1010
#5 ("pad" or "pads" or padding):ti,ab,kw 1201
#6 ("gel" or "gels"):ti,ab,kw 5206
#7 (pressure next relie*):ti,ab,kw 111
#8 (pressure next device*):ti,ab,kw 80
#9 (pressure next redistribution*):ti,ab,kw 3
#10 (low next pressure next support*):ti,ab,kw 2
#11 ((constant or alternat*) next pressure*):ti,ab,kw 78
#12 ((air or water) next suspension*):ti,ab,kw 10
#13 (sheepskin* or (sheep next skin*)):ti,ab,kw 20
#14 "foot waffle":ti,ab,kw 2
#15 (air next bag*):ti,ab,kw 2
#16 (elevat* near/2 device*):ti,ab,kw 6
#17 "static air":ti,ab,kw 3
#18 MeSH descriptor: [Shoes] explode all trees246
#19 ("shoe" or "shoes" or "boot" or "boots" or booties):ti,ab,kw 558
#20 (footwear or "foot wear"):ti,ab,kw 123
#21 MeSH descriptor: [Orthotic Devices] explode all trees753
#22 (orthotic next (device* or therapy)):ti,ab,kw 476
#23 (orthos* or insole*):ti,ab,kw 1856
#24 ((contact or walk*) near/1 ("cast" or "casts")):ti,ab,kw 39
#25 (aircast or scotchcast):ti,ab,kw 57
#26 ((foot or feet) near/2 pressure):ti,ab,kw 85
#27 ((foot or feet) near/2 protect*):ti,ab,kw 5
#28 ((foot or feet) near/2 device*):ti,ab,kw 26
#29 (heel* near/2 pressure*):ti,ab,kw 33
#30 (heel* near/2 protect*):ti,ab,kw 8
#31 (heel* near/2 device*):ti,ab,kw 19
#32 (heel* near/2 (lift* or float* or splint* or glove* or suspension or elevat*)):ti,ab,kw 27
#33 (trough* near/2 (leg* or "foot" or "feet" or heel*)):ti,ab,kw 1
#34 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 14442
#35 MeSH descriptor: [Pressure Ulcer] explode all trees510
#36 pressure next (ulcer* or sore*):ti,ab,kw 916
#37 decubitus next (ulcer* or sore*):ti,ab,kw 92
#38 (bed next sore*) or bedsore:ti,ab,kw 51
#39 #35 or #36 or #37 or #38 983
#40 #34 and #39 332
The search strategies for Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL can be found in Appendix 2; Appendix 3 and Appendix 4 respectively. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). We combined the Ovid EMBASE and EBSCO CINAHL searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (SIGN 2010). There were no restrictions on the basis of date or language of publication.
Searching other resources
We searched the bibliographies of all retrieved and relevant publications identified by these strategies for further studies. We contacted experts in the field and asked them if they had been involved in any further studies or were aware of recently completed or ongoing studies. We contacted manufacturers of pressure‐relieving equipment to request any studies they may have conducted which include heel pressure ulcers. Other journals (Phlebology and Diabetic Foot), which we had originally intended to handsearch, are now indexed in MEDLINE, therefore we undertook no additional handsearching.
Data collection and analysis
Selection of studies
Both review authors independently examined the titles and abstracts of citations generated by the search to identify those likely to meet the inclusion criteria and retrieved these in full. We resolved disagreements by consensus. The two review authors independently assessed the full‐text articles against the inclusion criteria.
Data extraction and management
We extracted and summarised details of eligible trials using a data extraction sheet. We extracted the following data:
-
author, title, date of study and publication;
-
sample size;
-
participant inclusion and exclusion criteria;
-
care setting;
-
baseline variables, for example age, sex, diagnosis, co‐morbidity, baseline risk, details of existing ulcers;
-
description of interventions;
-
numbers of participants ‐ both randomised and analysed;
-
description of any co‐interventions;
-
follow‐up period;
-
results;
-
outcome measures;
-
adverse events;
-
use of intention‐to‐treat analysis; and
-
trialists' conclusions.
We made attempts to obtain any missing data by contacting the study authors. We included data from studies that had been published more than once only once, however, where trials were published more than once then the data extraction process utilised all available sources to facilitate the retrieval of the maximum amount of trial data possible. Two review authors independently undertook data extraction. We resolved disagreement by consensus.
Assessment of risk of bias in included studies
Two review authors independently assessed each included study using the Cochrane Collaboration tool for assessing risk of bias (Higgins 2011). This tool addresses six specific domains, namely sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues (e.g. extreme baseline imbalance) (see Appendix 5) for details of criteria on which the judgement is based). We assessed blinding and completeness of outcome data for each outcome separately. We completed a 'Risk of bias' table for each eligible study and discussed any disagreement to achieve a consensus.
We presented an assessment of risk of bias using a 'Risk of bias' summary figure (Figure 1), which presents all of the judgements in a cross‐tabulation of study by entry. This display of internal validity indicates the weight the reader may give the results of each study.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Assessment of heterogeneity
We intended to estimate the extent of heterogeneity between study results using the I2 statistic (Higgins 2003). This examines the percentage of total variation across studies due to heterogeneity rather than to chance. Due to the small number of studies heterogeneity was not assessed.
Data synthesis
We presented a narrative summary of results. The planned method of synthesising the studies depended upon the quality, design and heterogeneity of studies identified. As only one study was identified, it was not possible to consider conducting a meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses to assess whether the presence of a wound in a specific condition, for example in a person with diabetes, has any effect on treatment. We had also planned subgroup analysis according to grade of ulcer; it is known that the reliability of pressure ulcer diagnosis and classification is particularly poor with grade 1 pressure ulcers (Nixon 2005). Due to the lack of data, we performed no subgroup analyses.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies.
Results of the search
The total number of citations retrieved by all the searches was 628 (467 after duplicates were removed). Following independent review of the abstracts by the two review authors, we thought 75 potentially met the inclusion criteria or contained useful references and retrieved these in full (two review papers were not retrieved due to translation difficulties). The two review authors independently assessed the studies for inclusion according to the selection criteria. Several studies would have been eligible if the original data were available and heel outcomes could be analysed separately. We wrote to or contacted 21 authors; we received three responses: one stating no heel ulcers were included, one stating that no separate data were available and one providing the original thesis with full methods and results (Russell 2000). We sent letters or emails to 10 wound care experts and received three replies. We sent 15 letters to manufacturers of pressure‐relieving devices and received two responses. The letters did not identify any further trials.
Included studies
We identified one study which met the criteria for inclusion (Russell 2000). This study was carried out in an elderly care setting in the UK, with participants having illnesses such as dementia, neurological disabilities or cardiovascular disease.Russell 2000 included 141 participants (113 with heel pressure ulcers), average age of 84.7 years who were randomised to either one of the alternating air‐filled mattress replacement and cushion systems. Outcomes measures were "completed study", defined as healed, discharged or died. See Characteristics of included studies.
Excluded studies
We excluded 71 studies: 10 were reviews, 18 studies were not RCTs, 18 studies were concerned with prevention (not treatment) of the ulcer, nine considered treatment of the ulcers on body sites other than heels, 15 considered treatment of the ulcer on various body sites including heels but data could not be analysed separately and two were reviews in another language. See Characteristics of excluded studies.
Risk of bias in included studies
See Characteristics of included studies and Figure 1.
In the Russell 2000 study there was adequate sequence generation through computer‐generated random numbers and allocation was concealed. Blinding of the participant and caregiver to interventions is not possible when equipment (from which the patient cannot be moved) is used as the allocation is visually obvious. Russell stated that to prevent bias, the monitoring of the data collection and protocol compliance was carried out by a team member who was 'blinded' to the statistical analysis, however data were collected by nurses who would not have been blind to the intervention. Incomplete outcome data were not adequately addressed; 72 out of the 113 patients (64%) died, were discharged or did not heal. The outcomes were ulcer healed, patients discharged or died but these were reported together as "completed study", which made interpretation difficult. The study reported baseline comparability of the two groups with respect to age, risk of developing pressure ulcers, grade and severity of pressure ulcer but did not report gender and duration of the pressure ulcers. Overall this study should be judged as at moderate risk of bias; many of the bias domains are satisfied but the very high drop‐out rate would moderate the overall judgement.
Effects of interventions
1. Comparison of two different alternating mattress replacement and cushion systems (one RCT, 141 people)
This study (Russell 2000) randomised 141 people of whom 113 had heel pressure ulcers to one of two different alternating mattress replacement systems either the Huntleigh Nimbus 3 mattress (this has a 1 in 2 alternating cycle) and Aura cushion system (n = 70) or the Pegasus Cairwave (this has a 1 in 3 alternating cycle) mattress and Proactive cushion (n = 71). This study included outcomes for sacral and heel pressure ulcers. As a result of communication with the author, heel data were provided and could be analysed separately.
Primary outcome
The proportion of heel ulcers healed was not reported in the published study, therefore we have analysed data using both the study outcome of "completed study" and heel ulcers healed (based on additional data provided by the author). We have generated a flow chart from both the published data and data from personal communication with the author (Figure 2).
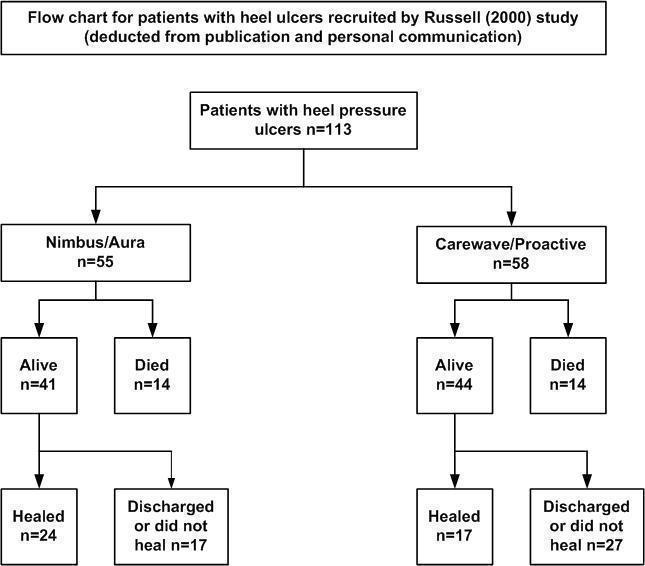
Flow chart for patients with heel ulcers recruited by Russell (2000) in the study
The outcome of "completed study" is defined as 'healed, discharged or died' at the end of the 18‐month follow‐up period. In the Nimbus group 24/55 participants and in the Cairwave group 17/58 participants healed. There was no significant difference when using either mattress system in the number of heel ulcers healed (RR 1.49; 95% CI 0.90 to 2.45)(Analysis 1.1).
There is a high risk of attrition bias as many people were lost to follow up: n = 17/55 (56%) in the Nimbus group and n = 27/58 (71%) in the Cairwave group were discharged or lost to follow up. Fourteen participants died in each group.
Secondary outcomes
Cost of pressure‐relieving device
Not reported.
Total costs of interventions (including service and maintenance) where given
Not reported (economic analysis was planned but not reported in the documents available).
Patient comfort
Patient comfort was measured using a digital analogue scale taken from Gray 1994; data were collected by members of the audit department. It contained questions which assessed mattress comfort, sleep and cushion comfort. Statistical analyses were carried out only on data from participants who completed the trial; results were not separated out for participants with sacral or heel pressure ulcers. Mean comfort scores were calculated for each question and did not show any statistically significant difference between the two groups (Table 2).
| Q1a | Q1b | Q2 | N | |
| Nimbus | 1.71 (± 0.68) | 2.48 (± 1.32) | 2.81 (± 0.96) | 29 |
| Cairwave | 1.94 (± 0.82) | 2.04 (± 1.30) | 2.46 (± 0.92) | 24 |
| Nimbus | 2.33 (± 1.81) | 2.25 (± 1.89) | 2.50 (± 1.0) | 4 |
| Cairwave | 2.51 (± 1.04) | 3.50 (± 1.69) | 3.56 (± 1.12) | 8 |
Q1 a and b refer to mattress comfort, Q2 asked about sleep. Results are mean comfort scores from visual analogue scale (± standard deviation). Results are from both heel ulcer and sacral ulcer participants. All results were not significant.
Ease of use
No differences reported.
Health‐related quality of life
Not reported.
Adverse events
No adverse events were reported.
Subgroup analysis, e.g. co‐morbidity or grade of ulcer
Russell 2000 used the Torrance scale to grade the pressure ulcers and only recruited those with Grade 2 and above. However, Grade 2 includes non‐blanching erythema with and without epidermal loss. The data available for heels has insufficient detail of Grades of ulcer to perform any subgroup analysis.
Discussion
Summary of main results
Overall, there is insufficient evidence to determine the relative effects of pressure‐relieving devices for healing pressure ulcers of the heel. Only one RCT met the inclusion criteria, it had high losses to follow up therefore findings need to be viewed with caution. The use of the outcome "completed study" made interpretation of data difficult since it included healed, discharged and died. There was no reported difference in the outcome of comfort between the two groups.
Overall completeness and applicability of evidence
Overall the evidence base is lacking.
Potential biases in the review process
Two non‐English review papers were identified by the search process but no translation was requested as only the bibliographies of reviews were being checked for further citations. There may be a potential risk of language bias with this process. There could be a risk of publication bias as studies of pressure‐relieving devices are frequently sponsored by the manufacturers. Results which show no difference or no benefit in their product may not be published. We contacted product manufacturers and experts in the field to reduce this risk.
Comments on search strategy
The search strategy was developed to include studies of participants with diabetic foot ulcers. There was a possibility that such studies may have included heel pressure ulcers. The list of titles generated did not include any diabetic foot ulcer papers. On reflection this may have been related to the fact that the strategy did not include the free‐text term '(heel or foot or feet) near ulcer'. Discussion took place with the Trials Search Co‐ordinator of the Cochrane Wounds Group and we informally examined citations of treatment studies for foot ulcers in people with diabetes which were identified in other systematic reviews. Most of these were found to include only ulcers on the plantar surface of the foot or forefoot and some specifically excluded ulcers on the heels. The potential benefit of re‐writing and running the strategy for all the years, in all the databases at this moment was not thought to justify the work entailed. This would, however, be a consideration for the future.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Flow chart for patients with heel ulcers recruited by Russell (2000) in the study

Comparison 1 Nimbus system compared with Cairwave system, Outcome 1 Heel ulcer healed.
| Year | Total no. PUs | No. of heel PUs | % of PUs on heel |
| 2006 | 274 | 60 | 22% |
| 2007 | 333 | 85 | 26% |
| 2008 | 336 | 74 | 22% |
| 2009 | 557 | 107 | 19% |
| PU = pressure ulcer | |||
| Q1a | Q1b | Q2 | N | |
| Nimbus | 1.71 (± 0.68) | 2.48 (± 1.32) | 2.81 (± 0.96) | 29 |
| Cairwave | 1.94 (± 0.82) | 2.04 (± 1.30) | 2.46 (± 0.92) | 24 |
| Nimbus | 2.33 (± 1.81) | 2.25 (± 1.89) | 2.50 (± 1.0) | 4 |
| Cairwave | 2.51 (± 1.04) | 3.50 (± 1.69) | 3.56 (± 1.12) | 8 |
| Q1 a and b refer to mattress comfort, Q2 asked about sleep. Results are mean comfort scores from visual analogue scale (± standard deviation). Results are from both heel ulcer and sacral ulcer participants. All results were not significant. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Heel ulcer healed Show forest plot | 1 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.90, 2.45] |

