Diclorhidrato de zuclopentixol para la esquizofrenia
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Allocation: randomised. Funding: Not mentioned. | |
| Participants | Diagnosis: paranoid schizophrenia. Exclusion: not mentioned. | |
| Interventions | 1. Cis(Z)‐clopenthixol hydrochloride: dose range 5 mg to 50 mg/day. Taken twice daily. N = 4. | |
| Outcomes | 5.2 Leaving the study early for General Reasons (zero people left the study early). Unable to use: Physiological ‐ serum concentrations of Cis(Z)‐clopenthixol and clopenthixol. Non‐clinical data all reported post‐cross‐over. | |
| Notes | Cross‐over trial. Patient 4 excluded during statistical analysis (also received multiple drugs). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Done "in a randomised way". Authors do not state how. Attempted to contact authors but Dikemark Hospital no longer exists. |
| Allocation concealment (selection bias) | Unclear risk | Authors do not comment. Attempted to contact authors but Dikemark Hospital no longer exists. |
| Blinding | Low risk | Authors state "Double blindly". |
| Incomplete outcome data (attrition bias) | Low risk | None detected. |
| Selective reporting (reporting bias) | Low risk | All data appear to have been reported that was sought by the study's aims. |
| Other bias | High risk | Selection bias (n = 9) ‐ diagnostic purity bias. |
| Methods | Allocation: randomised Blindness: none ‐ open‐label Duration: 1 year Funding: Theodore and Vada Stanley Foundation Grant Setting: 3 inpatient units | |
| Participants | Diagnosis: schizophrenia N = 46 Age: mean ˜34 years, range 24‐44 Sex: 38 male, 8 female Inclusion: DSM IV criteria for schizophrenia, a violent episode in the previous year with a score of greater than or equal to 3 on the physical aggression sub‐scale or the MOAS. A family member living with the patient and willing to collaborate with the researchers. Informed consent signed by the patient and the informant. Exclusion: Any other axis I disorders, including alcohol and/or drug abuse/dependence, mental retardation. | |
| Interventions | 1. Zuclopenthixol (oral) 35 mg/day n = 20 2. Zuclopenthixol (depot) 233 mg every 14 days (˜16.6 mg/day) n = 26 Additional medication: All received biperiden, other psychotropics were permitted. | |
| Outcomes | Useable: 4. Mental State ‐ PANSS positive only 5. Leaving the study Early 7. Behaviour ‐ number of violent episodes 8. Adverse Effects ‐ additional medication Unable to use: 7. Behaviour ‐ MOAS, baseline data only 8. Adverse effects ‐ UKU ‐ no data provided by group | |
| Notes | Madrid Inclusion criteria do not match included data and significant selective reporting. Authors will need to be contacted at next update to obtain clarification. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A researcher at one centre did the randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding | High risk | Open‐label. |
| Incomplete outcome data (attrition bias) | High risk | Missing UKU, missing PANSS. |
| Selective reporting (reporting bias) | High risk | MOAS not reported other than at baseline. |
| Other bias | High risk | Selection bias ‐ Berkson |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: acute functional psychosis (schizophrenia n = 68, schizoaffective n = 7, hypomania n = 4, unknown n = 4 and unreported n = 11. | |
| Interventions | 1. Zuclopenthixol: dose range 25 mg to 150 mg/day. N = 50. Additional medication: amitriptyline, temazepam, procyclidine. | |
| Outcomes | 2. Global state: CGI. Table 1 5. Leaving the study early. Zuclo n = 32 completed study, Chlor n = 21 completed the study. Table 1 Unable to use: | |
| Notes | Zuclopenthixol group previous episodes 0‐17. Chlorpromazine group previous episodes 0‐20. Most common zuclopenthixol dose 75 mg/day. Most common chlorpromazine dose 600 mg/day | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors do not mention. Lundbeck UK contacted to request information regarding potential biases 5th January 2016. |
| Allocation concealment (selection bias) | Unclear risk | Authors do not mention. |
| Blinding | Low risk | Clearly stated in the paper as double‐blind design. |
| Incomplete outcome data (attrition bias) | High risk | 11 patients were excluded and not analysed. Missing outcome data. |
| Selective reporting (reporting bias) | Low risk | All data appear to have been reported on. |
| Other bias | High risk | Attrition bias ‐ per protocol analysis. |
| Methods | Allocation: randomised. Funding: Public health service research grant ‐ Pfizer | |
| Participants | Diagnosis: schizophrenia. Exclusion criteria: not reported. | |
| Interventions | 1. Clopenthixol: dose range 50 mg to 200 mg/day. Average 150 mg/day. N = 10. | |
| Outcomes | Useable: 2. Global state: CGI. 5. Leaving the study early Unable to use: | |
| Notes | 15 chronic and 15 new admissions. Original Cochrane authors did not include the two‐week washout period in the duration of the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Suggested but not reported by authors. "10 week double blind." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding | Low risk | "10 week double blind." |
| Incomplete outcome data (attrition bias) | Low risk | None obvious and none mentioned by authors. |
| Selective reporting (reporting bias) | High risk | Multiple areas where data not reported. Several papers produced with same data. |
| Other bias | High risk | Selection bias ‐ sampling ‐ Berkson |
| Methods | Allocation: inpatient Blindness: randomised by specially assigned person Duraton:: 6 weeks | |
| Participants | Diagnosis: CCMD ‐ 3 N = 120 Age: mean ˜33 years, range 24‐42 Exclusion: severe physical diseases, alcohol and substance dependence | |
| Interventions | 1. Clopenthixol 40 mg to 70 mg/day. N = 60 2. Chlorpromazine 300 mg to 550 mg/day. N = 60 Other antipsychotics were not allowed during the treatment. Benzhexol and diazepam were used if necessary. | |
| Outcomes | Able to use: 4. Mental state: BPRS, PANSS 8. Adverse Effects: EPSEs and excessive sedation Unable to use: 4. Mentals State: SANS ‐ no useable data | |
| Notes | Paper kindly translated and data extracted by Jun Xia. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not mentioned. No detailed information was described in the study. No Concerns raised by the data extractor. |
| Allocation concealment (selection bias) | Low risk | Not mentioned. No detailed information was described in the study. No Concerns raised by the data extractor. |
| Blinding | Low risk | Randomised. No detailed information was described in the study. No Concerns raised by the data extractor. |
| Incomplete outcome data (attrition bias) | Low risk | All outcomes reported. |
| Selective reporting (reporting bias) | Low risk | None found. |
| Other bias | Low risk | None obvious. |
| Methods | Allocation: randomised. Funding: Not reported. | |
| Participants | Diagnosis: chronic schizophrenia. Exclusion criteria: not discussed. | |
| Interventions | 1. Clopenthixol: dose 132.5 mg/day, range 25 mg to 250 mg/day. N = 25. | |
| Outcomes | Useable: 2. Global state: CGI. 5. Leaving the study early. Unable to use: 8. Adverse Effects ‐ table 3 ‐ week 12 missing | |
| Notes | All but two patients were taking 1 or more psychoactive drugs (mostly phenothiazines) before starting the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported, suggested but not clear. |
| Allocation concealment (selection bias) | Unclear risk | Not reported, suggested but not clear. |
| Blinding | Low risk | Reasonable methods employed for blinding. |
| Incomplete outcome data (attrition bias) | Low risk | Reasons given by authors. |
| Selective reporting (reporting bias) | Low risk | Not detected. |
| Other bias | High risk | Per‐protocol analysis. |
| Methods | Allocation: Random Blindness: open‐label Duration: 13 weeks Funding: Danish Medical Counscil, H:S Research council, University of Copenhagen Faculty of Humanities Location: Copenhagen | |
| Participants | Diagnosis: ICD10 F20 Schizophrenia N = 31 Age: 27.3 years (+/‐5.9), range 19‐37 Sex: not discussed History: untreated psychosis 4‐78 months Inclusion: anti‐psychotic naive admitted for treatment and written informed consent Exclusion: known retardation, need acute medication, compulsorily hospitalised | |
| Interventions | 1. Zuclopenthixol 6 mg to 26 mg average 9.6 mg. N = 10 2. Risperidone 2 mg to 7 mg average 3.6 mg. N = 15 3: Healthy controls N = 25 Additional Medication: Benzodiazepiens and anticholinergics but not on the day of examination | |
| Outcomes | Usable: 4. Mental State: PANSS ‐ table 2 5. Leaving the study Early Not Useable: 6. General Functioning: Cognitive Functions (CANTAB, WCST, verbal fluency, Figural fluency, trail making tests A & B, DART) ‐ no useable data 8. Adverse Effects: ESRS ‐ no data | |
| Notes | Not all data published by authors. When review next updated the authors should be contacted to obtain this information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated but not elaborated on. |
| Allocation concealment (selection bias) | High risk | Open‐label. |
| Blinding | High risk | Open‐label. |
| Incomplete outcome data (attrition bias) | Unclear risk | Authors state they will publish other data elsewhere though not found in searches to date. |
| Selective reporting (reporting bias) | Unclear risk | Authors state they will publish other data elsewhere though not found in searches to date. |
| Other bias | High risk | Selection bias, Berkson |
| Methods | Allocation: randomised not clear. Funding: not stated | |
| Participants | Diagnosis: acute paranoid schizophrenia. | |
| Interventions | 1. Clopenthixol: dose 100 mg/day. N = 36. | |
| Outcomes | Useable: 8. Adverse effects: drowsiness, stimulation, confusion, gastrointestinal side‐effects, anticholinergic side‐effects, dizziness, orthostatic reaction, headache, hypersalivation, hypokinesia, hyperkinesia, dyskinesia, rigor, tremor, akathisia. Unable to use: | |
| Notes | All of the higher dose ranges are medians. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Sandoz UK contacted by online form to clarify bases and seek additional data 5th January 2016. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding | Unclear risk | Authors state double‐blind but do not say how. |
| Incomplete outcome data (attrition bias) | Unclear risk | Total figures are not provided. |
| Selective reporting (reporting bias) | High risk | Sandoz side‐effect check list not reported. |
| Other bias | High risk | Selection bias, performance bias, unclear if intention‐to‐treat analysis was used. |
| Methods | Allocation: random Blindness: not reported Duration: 12 weeks Funding: committee on medicine and science ‐ personal grant and an undisclosed non‐restricted grant | |
| Participants | Diagnosis: F20 schizophrenia n = 45 Age: ˜21‐33 years Sex: 25 male, 13 female, 7 unknown Included: drug naive first episode patients with schizophrenia, F20 ICD10 criteria for schizophrenia Excluded: mental retardation, severe somatic diseases, severe head trauma, pregnancy, presence of MRI contraindications, acute need of medication, compulsory hospitalisation in patients, presence of psychotic illness in controls or their first‐degree relatives. | |
| Interventions | 1. Zuclopenthixol (n = 8) 10.3 mg/day average (3.8 mg to 16.8 mg) 2. Risperidone (n = 11) 3.4 mg/day average (1.9 mg to 4.9 mg) 3. Control (n = 20) Of the original n = 45 it is unclear as to which groups the remaining n = 6 were attributed. Other medication: Anticholinergic n = 1‐, antidepressant n = 1, benzodiazepines n = 11 | |
| Outcomes | Usable: 4. Mental State: PANSS ‐ table 2 (PANSS general, mean and SD baseline and follow‐up). 5. Leaving the study early 8: Adverse effects ‐ ESRS ‐ table 2 | |
| Notes | 3 patients minimally medicated prior to the start of the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not reported. DRCMR research group contacted 5th January 2016 to clarify these biases and seek any missing information. |
| Allocation concealment (selection bias) | High risk | Not reported. |
| Blinding | High risk | Not reported. |
| Incomplete outcome data (attrition bias) | High risk | Conflicting reports from several papers using same population cohort. |
| Selective reporting (reporting bias) | High risk | Supplementary publications highlight dropout and inconsistencies in the randomisation. |
| Other bias | High risk | Berkson bias |
| Methods | Allocation: unclear. "In the investigation patients ... were allocated." Implied when table 1 reviewed. | |
| Participants | Diagnosis: acute and chronic psychoses, mainly schizophrenia Exclusion: below 15 years age, serious somatic disease, pathological laboratory findings, pregnant patients. | |
| Interventions | 1. Cis(Z)‐clopenthixol 48mg/day range 10 mg to 150 mg/day. N = 10. | |
| Outcomes | 2. 6 Average Endpoint in global state scores Table 7, CGI 5. Leaving the study early n = 2, (one for refusal due to side‐effects, oculogyric crisis and one for non‐compliance) Unable to use: 4. 4 Average endpoint general mental state score Table 3 and 4 ‐ CGI time 0, 2 weeks and 4 weeks no SD 4. 4 Average change in general mental state scores Table 5 ‐ BPRS ‐ total score time 0, 2 weeks and 4 weeks. no SD 4. 8 Average change in specific symptom scores Thinking disturbance: Table 5 ‐ BPRS ‐ time 0, 2 weeks and 4 weeks. no SD 4. 8 Average change in specific symptom scores Withdrawal ‐ retardation: Table 5 ‐ BPRS ‐ time 0, 2 weeks and 4 weeks. noS.D 4. 8 Average change in specific symptom scores Hostile‐suspiciousness: Table 5 ‐ BPRS ‐ time 0, 2 weeks and 4 weeks. no SD 4. 8 Average change in specific symptom scores Anxious‐depression: Table 5 ‐ BPRS ‐ time 0, 2 weeks and 4 weeks. no SD 8. 1 Clinically important general adverse effects Table 8, individual side‐effects and totals. Frequencies not patient numbers. | |
| Notes | none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported but implied; "A double blind clinical investigation". |
| Allocation concealment (selection bias) | Unclear risk | patients were "allocated" Attempted to contact authors but Dikemark Hospital no longer exists. Google search did not reveal any contact details of the authors. |
| Blinding | Low risk | Authors state double‐blinding but the details are not clear in the paper. |
| Incomplete outcome data (attrition bias) | Low risk | The two excluded patients had reasons given by the authors. |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes stated in the study aims. |
| Other bias | Unclear risk | Berkson and diagnostic purity bias. |
| Methods | Allocation: patients are allocated to each group and it is unclear if a process of randomisation occurred. A review of table one suggests an element of randomisation ‐ implied randomisation. Funding: not declared. | |
| Participants | Diagnosis: chronic schizophrenia. Exclusion: not mentioned | |
| Interventions | 1. Cis(Z)‐clopenthixol hydrochloride, n = 29 average dose 47.6 mg/day Table 2 dosing reported by authors is unclear. | |
| Outcomes | 2.6 Global State ‐ Average change in global state scores (Table 1) 4.3 Average endpoint general mental state score 5.2 Leaving the study early (for general reasons) ‐ zero people left the study 6.3 General Functioning ‐ average endpoint general functioning scores (Table 4) Unable to use: Physiological ‐ serum concentrations of Cis(Z)‐clopenthixol and clopenthixol (non‐clinical data all reported post‐cross‐over). | |
| Notes | none. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported but implied; "A double blind clinical investigation". |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding | Low risk | Stated in paper. "The drugs were given double blindly in a randomised way." |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | None noted. |
| Other bias | Unclear risk | Berkson and diagnostic purity bias. |
| Methods | Allocation: "The patients were allocated..." Implied randomisation. Funding: not stated | |
| Participants | Diagnosis: 40 patients with schizophrenia and 14 patients with oligophrenia. | |
| Interventions | 1. Cis(Z)‐clopenthixol 73 mg/day (10 mg to 150 mg/day). N = 26 | |
| Outcomes | 2. 5 Average endpoint global state score Table 3 and 5: Severity of illness, scale unclear 5. Leaving the study early Table 3 and 5: n = 5 left, reasons not stated in the article. 8. 3 Average endpoint general adverse effect score Table 7: Single side‐effects week 8 total Unable to use: 8. 4 Average change in general adverse effect scores Table 6: Side‐effects interfering with patient's functioning ‐ CGI. No SD. 8. 8 Average change in specific adverse effects Table 7: Single side‐effects. No SD. | |
| Notes | none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported ‐ Finland Central Hospital contacted by email 5th January 2016. |
| Allocation concealment (selection bias) | Unclear risk | Not reported ‐ Finland Central Hospital contacted by email 5th January 2016. |
| Blinding | Unclear risk | Reported but not detailed |
| Incomplete outcome data (attrition bias) | High risk | At week 8, five patients were not included in the outcome data and not accounted for by the authors. |
| Selective reporting (reporting bias) | Unclear risk | Not enough information to make a clear decision. |
| Other bias | Low risk | Per protocol analysis, attrition bias |
| Methods | Allocation: not stated Funding: Not stated | |
| Participants | Diagnosis: chronic schizophrenia (n = 58) or other psychotic (n = 5, paranoic state, depressive/PD) | |
| Interventions | 1. Cis(Z)‐zuclopenthixol: dose 40 mg/day. N = 30. | |
| Outcomes | Useable: 2. Global state: CGI. 5. Leaving the study early [n = 27 left (13 Zuclo and 14 Halo) ‐ only 13 accounted for: n = 1 discharge, n = 9 insufficient effect and side‐effects, n = 3 reasons unrelated to treatment] Unable to use ‐ | |
| Notes | Previous neuroleptic medication: 14 different neuroleptics. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. Hospital District of South West Finland contacted to seek further information on biases 5th January 2016. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding | Low risk | Double‐blind. |
| Incomplete outcome data (attrition bias) | High risk | 27 patients dropped out and only 12 accounted for by the authors. |
| Selective reporting (reporting bias) | Unclear risk | Not able to ascertain from paper. |
| Other bias | High risk | Berkson bias, diagnostic purity bias |
| Methods | Allocation: randomised. Funding: not stated | |
| Participants | Diagnosis: chronic schizophrenia or schizophreniform disorder with an acute exacerbation (DSM‐III‐R). N = 98. Inclusion criteria: acute exacerbations. 18‐65y, acute psychotic symptoms necessitating antipsychotic treatment. Diagnosis of chronic or sub‐chronic schizophrenia or schizophreniform disorder according to DSM III‐R Exclusion criteria: patients with clinically significant organic or neurological disorder, serious psychotic disorder other than schizophrenia or schizophreniform disorder, clinically relevant abnormalities in laboratory tests, patients likely to be noncompliant, as well as pregnant or lactating women and those of reproductive age without adequate contraception. Setting: community. | |
| Interventions | 1. Zuclopenthixol: dose 38 mg/day, range 10 mg to 100 mg/day. N = 50. Authors aimed to avoid anti‐parkinsonism medication and benzodiazepines. | |
| Outcomes | Useable: 5. Leaving the study early (n = 98 to n = 40) 8. Adverse effects: use of antiparkinsonian medication. 8. Adverse effects: UKU side effect rating scale Unable to use 1. Death: n = 1 patient on risperidone. died 1 month after the trial. This patient was only on the drug for one week and the pathology diagnosis was viral myocarditis. The authors deemed this to not be related to risperidone. 4. Mental state: PANSS score, BPRS (originally included but upon review data is unusable) 8. Adverse effects: extrapyramidal symptom rating scale (data not useable) | |
| Notes | Discontinuation of anti‐parkinsonism and other psychotropic medication. Author contacted to seek clarification of some data: no reply at the time of writing. Information from multiple papers. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but not stated how. E‐mailed M.O Huttunen Summer 2015 with no response to date. |
| Allocation concealment (selection bias) | Unclear risk | Randomised but not stated how. E‐mailed M.O Huttunen Summer 2015 with no response to date. |
| Blinding | Unclear risk | Mentioned but not clear how. E‐mailed M.O Huttunen Summer 2015 with no response to date. |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | High risk | Report mean for non‐normal data instead of median. Only reported CGI data on comparison to previous neuroleptic treatment. |
| Other bias | High risk | Diagnostic purity and attrition biases. |
| Methods | Allocation: randomised. Funding: Ayerst, McKenna and Harrison LTD | |
| Participants | Diagnosis: schizophrenic reaction (different subtypes) plus one extreme incapacitating anxiety in a schizoid personality. N = 41 Age: 18‐65 years, mean ˜31 years Sex: 16 male, 25 female History: Duration of illness 0.4 ‐ 5.3 years Included: acute psychotic symptomatology Excluded: not mentioned. | |
| Interventions | 1. Clopenthixol (N = 20) 122 mg/day (75 mg to 600 mg), n = 5 additional medication 2. Chlorpromazine (N = 21) 435 mg/day (150 mg to 1800 mg), n = 2 additional medication All patients had previous treatment. | |
| Outcomes | Used: 2. Global state ‐ Global assessment ‐ table 2 4. Mental state ‐ BPRS ‐ table 1 5. Leaving the study early n = 4 (Chlorpromazine, 1 exhausted tablets and 3 due to side‐effects). n = 1 (zuclopenthixol, side‐effects). 8. Adverse effects ‐ table 3 and additional medication n = 7 (authors do not mention which additional medication) Unable to use: 5. Leaving the study early "Several patients ... diagnosis changed ... to a non‐psychotic disorder or to a brain syndrome were dropped from the study." No data. | |
| Notes | none. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Implied randomisation. Response: Patients assigned a number belonging to a bottle of medications. Research unit at the Royal Victoria Hospital in Quebec contacted for more information on biases and missing patient information 5th January 2016. |
| Allocation concealment (selection bias) | Low risk | Contents of bottles not known to anyone in the study until the end. |
| Blinding | Low risk | Study states "double blind". |
| Incomplete outcome data (attrition bias) | High risk | Several patients not included in the study because of a change in diagnosis. The numbers were not given and not included in the analysis. |
| Selective reporting (reporting bias) | High risk | Missing data, areas not reported but alluded to in discussion. |
| Other bias | High risk | Berkson bias, attrition bias, per protocol analysis |
| Methods | Allocation: "Patients were subsequently allocated" ‐ randomisation implied | |
| Participants | Diagnosis: chronic schizophrenia. Exclusion criteria: not reported. | |
| Interventions | 1. Clopenthixol: dose 150 mg/day. N = 18. Additional Medication: Artane and Phenergan. | |
| Outcomes | Used: 5. Leaving the study early n = 0 8. Adverse effects: number of patients experiencing side‐effects. (all on clopenthixol, n = 3 restarted menstruation, n = 2 parkinsonism, n = 1 oculogyric crisis Not used: 7. Behaviour: behavioural scale, Aof V (table III) ‐ no usable data | |
| Notes | none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned. Contacted Dromokaītion Hospital Athens to obtain further information on bias 5th January 2016. |
| Allocation concealment (selection bias) | Unclear risk | "...patients were subsequently allocated into three groups..." Unclear how. |
| Blinding | Unclear risk | "He was the only person knowing the identity of the tablets and to which group the patients belonged." Unclear if single‐ or double‐blind. |
| Incomplete outcome data (attrition bias) | Unclear risk | First rating deleted from analysis as was considered to be training by the researchers. |
| Selective reporting (reporting bias) | High risk | See above |
| Other bias | High risk | Hospitalised patients used ‐ Berkson bias. |
| Methods | Allocation: randomised. Funding: not declared. | |
| Participants | Diagnosis: acute schizophrenia (RDC). Inclusion criteria: aged 18‐65 years inclusive, were experiencing an acute schizophrenic episode according to RDC and minimum BPRS score of 15. Exclusion criteria: concurrent serious physical illness, patients who had previously demonstrated intolerance to neuroleptics, received a depot neuroleptic in the previous 2 weeks, or were suffering from a serious additional psychiatric or neurological disorder, patients who were pregnant or lactating; dependence or addiction to drugs or alcohol. Setting: hospital. | |
| Interventions | 1. Zuclopenthixol dihydrochloride: dose range 25 mg to 150 mg/day. N = 30. Additional medication: temazepam (insomnia), procyclidine (EPSEs), amitriptyline (depression). | |
| Outcomes | usable: 2. Global state: CGI. 5. Leaving the study Early | |
| Notes | Multicentre | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised but not elaborated on by authors. Bolton General Hospital contacted 5th January 2016 to seek clarification from the first author regarding biases. |
| Allocation concealment (selection bias) | Low risk | Randomly allocated but not elaborated on. |
| Blinding | Low risk | Clearly stated. |
| Incomplete outcome data (attrition bias) | Low risk | Authors discuss |
| Selective reporting (reporting bias) | High risk | Not all data was reported in the paper. |
| Other bias | High risk | Diagnostic purity bias, attrition bias, per‐protocol analysis |
| Methods | Randomised, double‐blind, 12 weeks | |
| Participants | Acute psychosis N = 54 Age: 20‐60 years (mean 38) Sex: 25 female, 15 male, 14 unspecified Inclusion: hospitalised in the preceding 7 days, acute psychosis, exacerbation of chronic psychosis, neuroleptic treatment was anticipated to be at least 3 weeks, informed consent. Excluded: mania/depression, serious somatic disease, organic brain damage, pregnant, history of abuse, previous good response to neuroleptic | |
| Interventions | 1. Zuclopenthixol 37 mg/day (10 mg to 20 mg) n = 27 2. Perphenazine 30 mg/day (8 mg to 72 mg) n = 27 Additional medication: benzodiazepines, methotrimeprazine, anti‐parkinsonism medication | |
| Outcomes | 5. Leaving the study early Unable to use: 2. Global state: CGI 4. Mental state: CPRS 8 Adverse effects | |
| Notes | Multicentre | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. Glostrup hospital contacted 5th January 2016 to obtain further information to clarify biases and seek additional outcome data if available. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding | Low risk | Double‐blind stated. |
| Incomplete outcome data (attrition bias) | Low risk | Not detected. |
| Selective reporting (reporting bias) | High risk | Multiple data not reported and often unclear. |
| Other bias | High risk | Berkson bias evident. |
| Methods | Allocation: randomised. Funding: United states Public health service grant | |
| Participants | Diagnosis: chronic schizophrenia. Exclusion: no complicating organic illness or known brain damage | |
| Interventions | 1. Clopenthixol: dose 205 mg/day. N = 15. Additional medication: antiparkinsonian medication, sedative. | |
| Outcomes | Useable: 5. Leaving the study early: n = 4 (behavioural deterioration n = 2, 1 Chlorpromazine and 1 placebo, Intestinal obstruction secondary faecal impaction n = 2, both chlorpromazine) Unable to use ‐ 4. Mental state: BPRS (no SD). Short‐term data not reported (first 12 weeks medication free) | |
| Notes | Originally reported as a 12‐week study in previous review. Combination of several papers (6 in total) all reporting on the same cohort. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "In a double blind placebo controlled trial..." Implied but not clear. |
| Allocation concealment (selection bias) | High risk | Not specifically discussed by the authors but "subjects were housed together on a special two‐wing ward, males in one wing and females in the other" so it is unlikely that allocation concealment was maintained. |
| Blinding | Low risk | Authors state double‐blind. Not discussed how. |
| Incomplete outcome data (attrition bias) | Unclear risk | Incomplete data described by the authors but not used in outcome data. |
| Selective reporting (reporting bias) | High risk | Multiple papers, same patient cohort. |
| Other bias | High risk | Selection bias (Berkson), Suspected data mining, likely per‐protocol analysis. |
| Methods | Allocation: Quote” Patients were randomly divided into two groups ”(p.4) Blinding: Double‐blind Duration: Quote”8 weeks”(p. 4) | |
| Participants | Schizophrenia, CCMD‐3; Inclusion and exclusion criteria: | |
| Interventions | 1. Zuclopenthixol Group: (n = 33) 2. Chlorpromazine Group: (n = 27) No other antipsychotic drugs were allowed during the treatment; Trihexyphenidyl or scopolamine was used when necessary; | |
| Outcomes | 2. Global State ‐ Clinical response Unable to use: electrocardiogram; electroencephalogram; liver function; blood routine examination. | |
| Notes | We have noticed that the N number does not always add up in this study, for example, the total sample size randomised was claimed to be 64, and 61 completed, but the number randomised to each group was reported as 33 and 27. Author to be contacted at next update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors state patients randomised, not how |
| Allocation concealment (selection bias) | Unclear risk | Not reported after translation and data extraction |
| Blinding | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Unclear risk | N number unclear |
| Selective reporting (reporting bias) | Unclear risk | Not reported after translation and data extraction |
| Other bias | High risk | Berkson bias |
BPRS ‐ Brief Psychiatric Rating Scale, CCMD‐3 ‐ Chinese classification of mental disorders,CGI ‐ Clinical Global Impression, CPRS (Montgomery 1979) ‐ Comprehensive Psychopathological Rating Scale, DSM ‐ Diagnostic and Statistical Manual of Mental Disorders, EPSEs ‐ extrapyramidal side‐effects, ESRS ‐ Extrapyramidal Symptom Rating Scale, ICD 10 ‐ International Classification of Diseases, RCT ‐ randomised controlled trial, MRI ‐ magnetic resonance imaging, NOSIE ‐ Nourses' Observation Scale for Inpatient Evaluation, PANSS ‐ Positive and Negative Syndrome Scale, PIP ‐ Psychotic Inpatient Profile, RCT ‐ randomised controlled trial, RDC ‐ Research Diagnostic Criteria, SANS ‐ Scale for the Assessment of Negative Symptoms, SD ‐ standard deviation, TESS ‐ Treatment Emergent Symptom Scale, UKU ‐ side effects scale.
Where possible, contact has been attempted with authors and/or institutions to seek clarification of bias and to obtain missing and/or unclear outcome data.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: not randomised, case‐series. | |
| Allocation: not randomised, case series. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: not clear, double‐blind. | |
| Wrong intervention. | |
| Allocation: not clear, double‐blind. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised, blindness not clear. | |
| Not clear if oral or depot. Authors need to be contacted at next update. | |
| Allocation: randomised, double‐blind. | |
| Depot not oral intervention. | |
| Originally included but excluded at this update. It is not an RCT and blindness is not mentioned. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: not randomised. | |
| Allocation: randomised. | |
| Not randomised. Not blinded. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| No data. | |
| Not oral form. | |
| Study duplicates data presented by authors already included in review. | |
| injection not oral. | |
| Injection not oral. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: not randomised, case series. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: not randomised. | |
| Allocation: randomised. | |
| No data. | |
| Wrong intervention, no data. | |
| Allocation: randomised. | |
| Not oral form. | |
| Zuclopenthixol depot. | |
| Not oral form. | |
| Not oral form. | |
| Depot zuclopenthixol. | |
| Allocation: not clear, double ‐blind. | |
| Allocation: randomised. | |
| Allocation: randomisation not clear. | |
| Allocation: randomised. | |
| Depot zuclopenthixol. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: not randomised, case series. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Depot zuclopenthixol. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: not clear. |
BPRS ‐ Brief Psychiatric Rating Scale, CGI ‐ Clinical Global Impression, ESRS ‐ Extrapyramidal Symptom Rating Scale, i.m. ‐ intramuscular, MRI ‐ magnetic resonance imaging, PANSS ‐ Positive and Negative Syndrome Scale, RCT ‐ randomised controlled trial, SANS ‐ Scale for the Assessment of Negative Symptoms, SAPS ‐ Scale for the Assessment of Positive Symptoms, UKU ‐ side effects scale.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Leaving the study early (any reason) Show forest plot | 2 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.01, 6.60] |
| Analysis 1.1 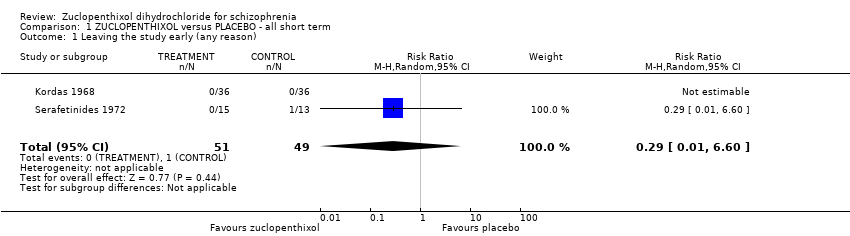 Comparison 1 ZUCLOPENTHIXOL versus PLACEBO ‐ all short term, Outcome 1 Leaving the study early (any reason). | ||||
| 2 Adverse effects: 1. Clinically important change in specific adverse effects ‐ cardiovascular ‐ orthostatic Show forest plot | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.01, 6.60] |
| Analysis 1.2 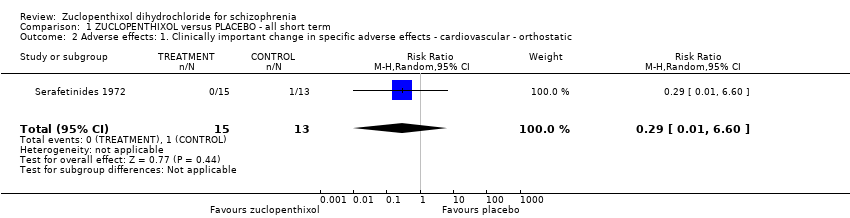 Comparison 1 ZUCLOPENTHIXOL versus PLACEBO ‐ all short term, Outcome 2 Adverse effects: 1. Clinically important change in specific adverse effects ‐ cardiovascular ‐ orthostatic. | ||||
| 3 Adverse effects: 2. Clinically important change in specific adverse effects ‐ central nervous system ‐ arousal state Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 ZUCLOPENTHIXOL versus PLACEBO ‐ all short term, Outcome 3 Adverse effects: 2. Clinically important change in specific adverse effects ‐ central nervous system ‐ arousal state. | ||||
| 3.1 excitation | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 2.62 [0.12, 59.40] |
| 3.2 sleepiness / sedation | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 2.89 [1.01, 8.30] |
| 4 Adverse effects: 3. Clinically important change in specific adverse effects ‐ endocrine ‐ menstruation started Show forest plot | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 7.0 [0.39, 126.48] |
| Analysis 1.4  Comparison 1 ZUCLOPENTHIXOL versus PLACEBO ‐ all short term, Outcome 4 Adverse effects: 3. Clinically important change in specific adverse effects ‐ endocrine ‐ menstruation started. | ||||
| 5 Adverse effects: 4a. Any general adverse effects ‐ movement disorders ‐ EPSEs (UKU side effect rating scale, no scores) Show forest plot | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 6.07 [0.86, 43.04] |
| Analysis 1.5  Comparison 1 ZUCLOPENTHIXOL versus PLACEBO ‐ all short term, Outcome 5 Adverse effects: 4a. Any general adverse effects ‐ movement disorders ‐ EPSEs (UKU side effect rating scale, no scores). | ||||
| 6 Adverse effects: 4b. Clinically important change in specific adverse effects ‐ movement disorders ‐ EPSEs Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 ZUCLOPENTHIXOL versus PLACEBO ‐ all short term, Outcome 6 Adverse effects: 4b. Clinically important change in specific adverse effects ‐ movement disorders ‐ EPSEs. | ||||
| 6.1 parkinsonism | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 5.00 [0.26, 97.37] |
| 6.2 oculogyric crisis | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 69.09] |
| 6.3 tremor | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 5.00 [0.26, 97.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | |||||||||||||||
| 1 Global state: 1. Average endpoint global state score ‐ Unchanged/worse (CGI, scores not reported) Show forest plot | 2 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.75, 1.13] | |||||||||||||||
| Analysis 2.1  Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 1 Global state: 1. Average endpoint global state score ‐ Unchanged/worse (CGI, scores not reported). | |||||||||||||||||||
| 2 Global state: 2. Average endpoint global state score ‐ No Recovery Show forest plot | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.89, 1.16] | |||||||||||||||
| Analysis 2.2  Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 2 Global state: 2. Average endpoint global state score ‐ No Recovery. | |||||||||||||||||||
| 3 Global state: 3a. Average endpoint global state score (GAS, high score not reported, average score = 63.4) Show forest plot | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐8.12, 6.92] | |||||||||||||||
| Analysis 2.3  Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 3 Global state: 3a. Average endpoint global state score (GAS, high score not reported, average score = 63.4). | |||||||||||||||||||
| 4 Global state: 3b. Average endpoint global state score (CGI‐SI, high score not reported, average score = 2.2) Show forest plot | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.49, 0.49] | |||||||||||||||
| Analysis 2.4 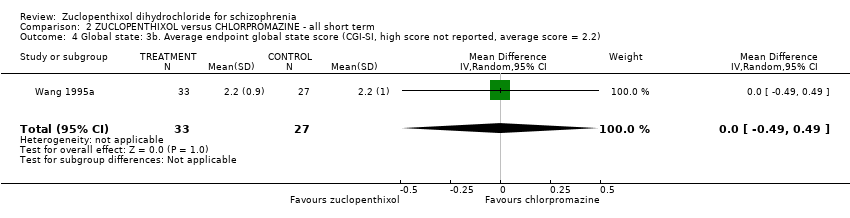 Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 4 Global state: 3b. Average endpoint global state score (CGI‐SI, high score not reported, average score = 2.2). | |||||||||||||||||||
| 5 Mental state: 1. No clinically important change in general mental state ‐ Not improved (PANSS, scores not reported) Show forest plot | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.81, 1.18] | |||||||||||||||
| Analysis 2.5  Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 5 Mental state: 1. No clinically important change in general mental state ‐ Not improved (PANSS, scores not reported). | |||||||||||||||||||
| 6 Mental state: 2. No clinically important change in general mental state ‐ No clinical response Show forest plot | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.25, 2.42] | |||||||||||||||
| Analysis 2.6 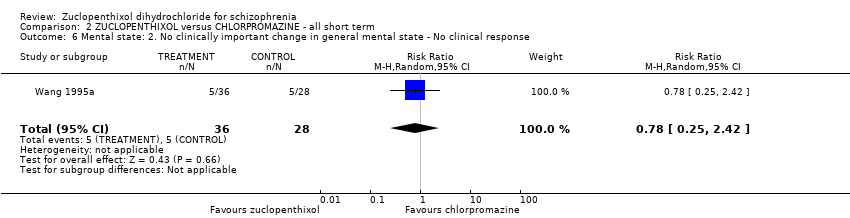 Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 6 Mental state: 2. No clinically important change in general mental state ‐ No clinical response. | |||||||||||||||||||
| 7 Mental state: 3. Average endpoint general mental state score (BPRS, high score = 34.2) Show forest plot | 3 | 221 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐2.43, 3.23] | |||||||||||||||
| Analysis 2.7  Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 7 Mental state: 3. Average endpoint general mental state score (BPRS, high score = 34.2). | |||||||||||||||||||
| 8 Leaving the study early (any reason) Show forest plot | 6 | 766 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.36, 0.81] | |||||||||||||||
| Analysis 2.8  Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 8 Leaving the study early (any reason). | |||||||||||||||||||
| 9 Adverse effects: 1. Any general adverse effects ‐ side effects (CGI, high score not reported) Show forest plot | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.77, 0.97] | |||||||||||||||
| Analysis 2.9 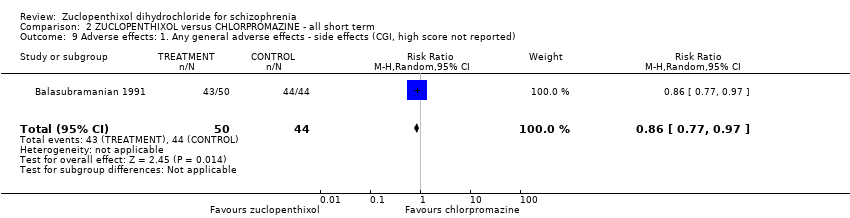 Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 9 Adverse effects: 1. Any general adverse effects ‐ side effects (CGI, high score not reported). | |||||||||||||||||||
| 10 Adverse effects: 2. Average endpoint general adverse effect score ‐ average score (TESS, high score not reported, average score = 12.00) Show forest plot | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 4.48 [‐2.38, 11.34] | |||||||||||||||
| Analysis 2.10 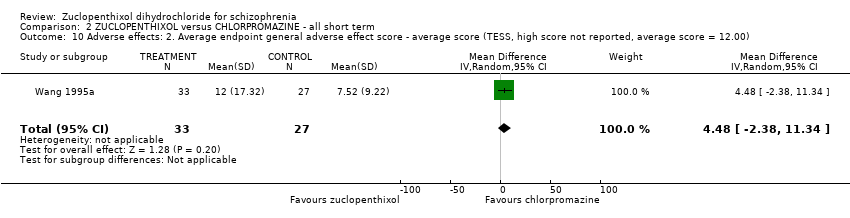 Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 10 Adverse effects: 2. Average endpoint general adverse effect score ‐ average score (TESS, high score not reported, average score = 12.00). | |||||||||||||||||||
| 11 Adverse effects: 3. Any change in specific adverse effects ‐ cardiovascular ‐ postural hypotension (dizziness/syncope) Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 1.73] | |||||||||||||||
| Analysis 2.11 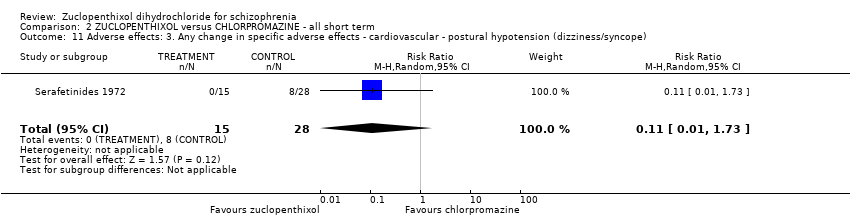 Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 11 Adverse effects: 3. Any change in specific adverse effects ‐ cardiovascular ‐ postural hypotension (dizziness/syncope). | |||||||||||||||||||
| 12 Adverse effects: 4. Any change in specific adverse effects ‐ central nervous system ‐ arousal Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | ||||||||||||||||
| Analysis 2.12  Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 12 Adverse effects: 4. Any change in specific adverse effects ‐ central nervous system ‐ arousal. | |||||||||||||||||||
| 12.1 excitation | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.07, 5.47] | |||||||||||||||
| 12.2 sedation | 2 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.73, 1.70] | |||||||||||||||
| 13 Adverse effects: 5. Any change in specific adverse effects ‐ metabolic ‐ weight change ‐ loss or gain of weight of 10 pounds Show forest plot | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.22, 1.75] | |||||||||||||||
| Analysis 2.13  Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 13 Adverse effects: 5. Any change in specific adverse effects ‐ metabolic ‐ weight change ‐ loss or gain of weight of 10 pounds. | |||||||||||||||||||
| 14 Adverse effects: 6a. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs Show forest plot | 3 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.61, 1.45] | |||||||||||||||
| Analysis 2.14  Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 14 Adverse effects: 6a. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs. | |||||||||||||||||||
| 15 Adverse effects: 6b. Any change in specific adverse effects ‐ movement disorders ‐ additional medication use Show forest plot | Other data | No numeric data | |||||||||||||||||
| Analysis 2.15
Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 15 Adverse effects: 6b. Any change in specific adverse effects ‐ movement disorders ‐ additional medication use. | |||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: Average endpoint global state score ‐ Unchanged/worse (CGI) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.1 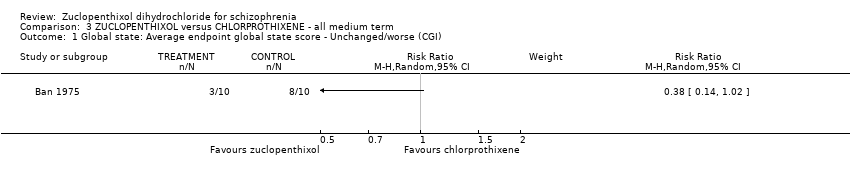 Comparison 3 ZUCLOPENTHIXOL versus CHLORPROTHIXENE ‐ all medium term, Outcome 1 Global state: Average endpoint global state score ‐ Unchanged/worse (CGI). | ||||
| 2 Leaving the study early (any reason) Show forest plot | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.34, 2.93] |
| Analysis 3.2  Comparison 3 ZUCLOPENTHIXOL versus CHLORPROTHIXENE ‐ all medium term, Outcome 2 Leaving the study early (any reason). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | |||||||||
| 1 Leaving the study early (any reason) Show forest plot | 1 | 407 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||
| Analysis 4.1  Comparison 4 ZUCLOPENTHIXOL versus CLOZAPINE ‐ all short term, Outcome 1 Leaving the study early (any reason). | |||||||||||||
| 2 Adverse effects: Any general adverse effects ‐ side effects ‐ frequency per day Show forest plot | Other data | No numeric data | |||||||||||
| Analysis 4.2
Comparison 4 ZUCLOPENTHIXOL versus CLOZAPINE ‐ all short term, Outcome 2 Adverse effects: Any general adverse effects ‐ side effects ‐ frequency per day. | |||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: 1. Average endpoint global state score ‐ Unchanged/worse (CGI) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.1 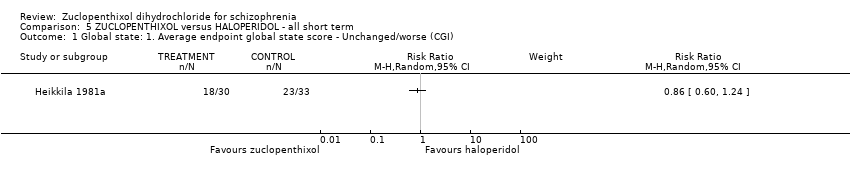 Comparison 5 ZUCLOPENTHIXOL versus HALOPERIDOL ‐ all short term, Outcome 1 Global state: 1. Average endpoint global state score ‐ Unchanged/worse (CGI). | ||||
| 2 Global state: 2. Average endpoint global state score (CGI, mean score = 1.25) Show forest plot | 1 | 49 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.30, 0.55] |
| Analysis 5.2  Comparison 5 ZUCLOPENTHIXOL versus HALOPERIDOL ‐ all short term, Outcome 2 Global state: 2. Average endpoint global state score (CGI, mean score = 1.25). | ||||
| 3 Leaving the study early (any reason) Show forest plot | 2 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.72, 1.35] |
| Analysis 5.3 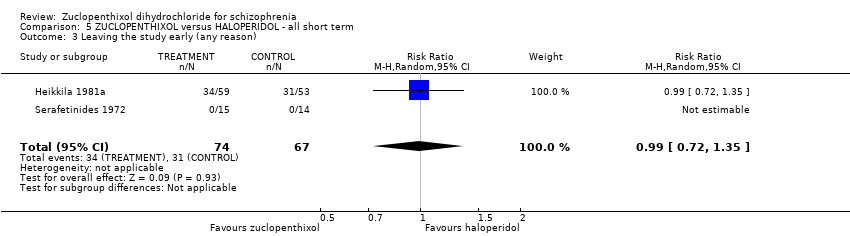 Comparison 5 ZUCLOPENTHIXOL versus HALOPERIDOL ‐ all short term, Outcome 3 Leaving the study early (any reason). | ||||
| 4 Adverse effects: 1. Any change in specific adverse effects ‐ interference with functioning Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.4  Comparison 5 ZUCLOPENTHIXOL versus HALOPERIDOL ‐ all short term, Outcome 4 Adverse effects: 1. Any change in specific adverse effects ‐ interference with functioning. | ||||
| 5 Adverse effects: 2. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs ‐ requiring medication Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.5  Comparison 5 ZUCLOPENTHIXOL versus HALOPERIDOL ‐ all short term, Outcome 5 Adverse effects: 2. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs ‐ requiring medication. | ||||
| 6 Adverse effects: 3. Any change in specific adverse effects ‐ requiring additional medication Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.6 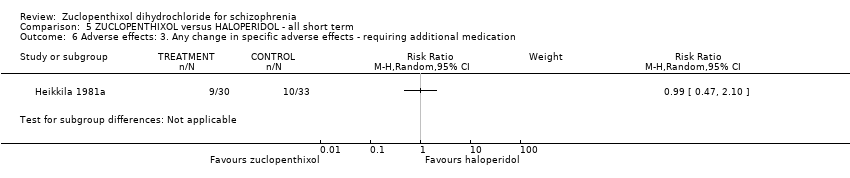 Comparison 5 ZUCLOPENTHIXOL versus HALOPERIDOL ‐ all short term, Outcome 6 Adverse effects: 3. Any change in specific adverse effects ‐ requiring additional medication. | ||||
| 7 Adverse effects: 4. Any change in specific adverse effects ‐ requiring hypnotics/sedatives Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.7  Comparison 5 ZUCLOPENTHIXOL versus HALOPERIDOL ‐ all short term, Outcome 7 Adverse effects: 4. Any change in specific adverse effects ‐ requiring hypnotics/sedatives. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: Average endpoint global state score ‐ unchanged/worse (global rating ‐ investigator opinion) ‐ medium term Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 6.1 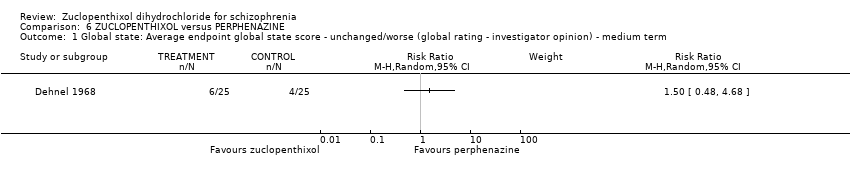 Comparison 6 ZUCLOPENTHIXOL versus PERPHENAZINE, Outcome 1 Global state: Average endpoint global state score ‐ unchanged/worse (global rating ‐ investigator opinion) ‐ medium term. | ||||
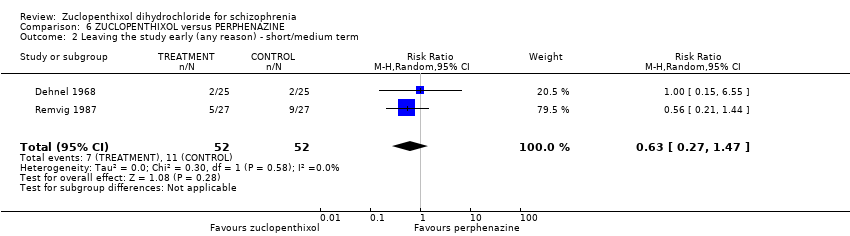
| 2 Leaving the study early (any reason) ‐ short/medium term Show forest plot | 2 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.27, 1.47] |
| Analysis 6.2  Comparison 6 ZUCLOPENTHIXOL versus PERPHENAZINE, Outcome 2 Leaving the study early (any reason) ‐ short/medium term. | ||||
| 3 Adverse effects: 1. Any change in specific adverse effects ‐ central nervous system ‐ arousal ‐ requiring medication ‐ medium term Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 6.3  Comparison 6 ZUCLOPENTHIXOL versus PERPHENAZINE, Outcome 3 Adverse effects: 1. Any change in specific adverse effects ‐ central nervous system ‐ arousal ‐ requiring medication ‐ medium term. | ||||
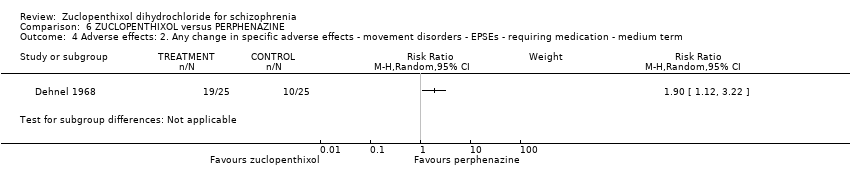
| 4 Adverse effects: 2. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs ‐ requiring medication ‐ medium term Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 6.4 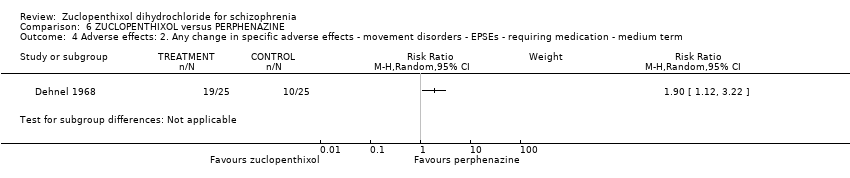 Comparison 6 ZUCLOPENTHIXOL versus PERPHENAZINE, Outcome 4 Adverse effects: 2. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs ‐ requiring medication ‐ medium term. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||
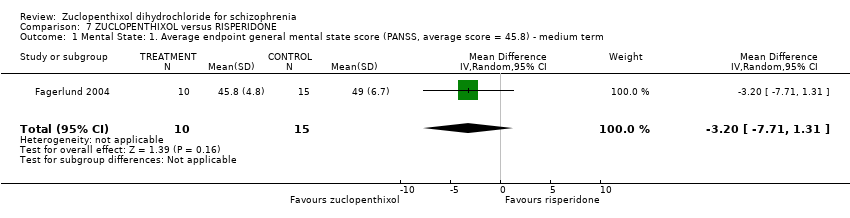
| 1 Mental State: 1. Average endpoint general mental state score (PANSS, average score = 45.8) ‐ medium term Show forest plot | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐3.20 [‐7.71, 1.31] | ||||||||||||
| Analysis 7.1  Comparison 7 ZUCLOPENTHIXOL versus RISPERIDONE, Outcome 1 Mental State: 1. Average endpoint general mental state score (PANSS, average score = 45.8) ‐ medium term. | ||||||||||||||||
| 2 Mental State: 2. Average endpoint general mental state score (PANSS General, average score medium term = 20.5) ‐ short/medium term Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |||||||||||||
| Analysis 7.2 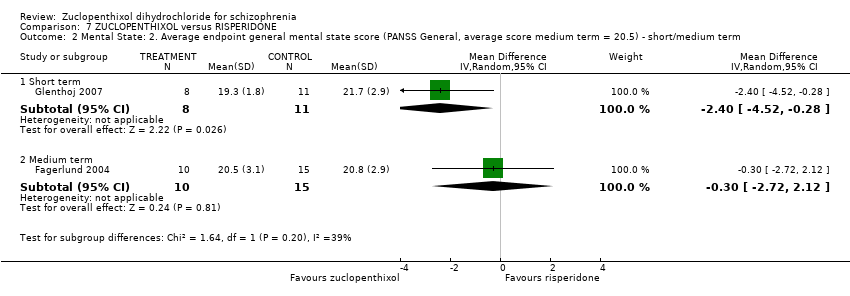 Comparison 7 ZUCLOPENTHIXOL versus RISPERIDONE, Outcome 2 Mental State: 2. Average endpoint general mental state score (PANSS General, average score medium term = 20.5) ‐ short/medium term. | ||||||||||||||||
| 2.1 Short term | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐2.40 [‐4.52, ‐0.28] | ||||||||||||
| 2.2 Medium term | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐2.72, 2.12] | ||||||||||||
| 3 Mental State: 3. Average endpoint general mental state score (PANSS Positive, average score = 9.8) ‐ medium term Show forest plot | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐2.69, 0.69] | ||||||||||||
| Analysis 7.3 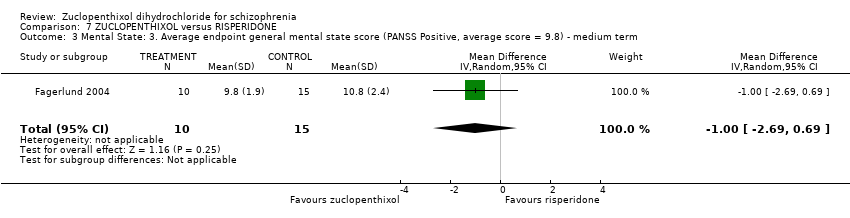 Comparison 7 ZUCLOPENTHIXOL versus RISPERIDONE, Outcome 3 Mental State: 3. Average endpoint general mental state score (PANSS Positive, average score = 9.8) ‐ medium term. | ||||||||||||||||
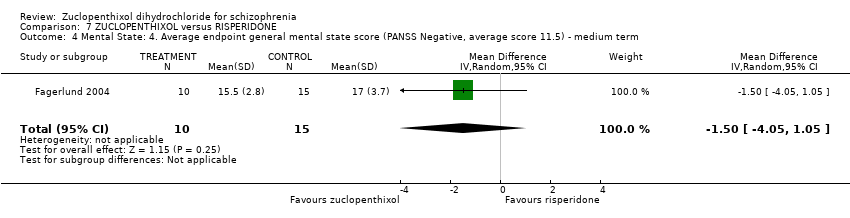
| 4 Mental State: 4. Average endpoint general mental state score (PANSS Negative, average score 11.5) ‐ medium term Show forest plot | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐1.5 [‐4.05, 1.05] | ||||||||||||
| Analysis 7.4  Comparison 7 ZUCLOPENTHIXOL versus RISPERIDONE, Outcome 4 Mental State: 4. Average endpoint general mental state score (PANSS Negative, average score 11.5) ‐ medium term. | ||||||||||||||||
| 5 Leaving the study early (any reason) ‐ short/medium term Show forest plot | 3 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.84, 2.02] | ||||||||||||
| Analysis 7.5  Comparison 7 ZUCLOPENTHIXOL versus RISPERIDONE, Outcome 5 Leaving the study early (any reason) ‐ short/medium term. | ||||||||||||||||
| 6 Adverse Effects: 1. Any change in general adverse effects ‐ additional medication use ‐ short/medium term Show forest plot | Other data | No numeric data | ||||||||||||||
| Analysis 7.6
Comparison 7 ZUCLOPENTHIXOL versus RISPERIDONE, Outcome 6 Adverse Effects: 1. Any change in general adverse effects ‐ additional medication use ‐ short/medium term. | ||||||||||||||||
| 7 Adverse effects: 2a. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs ‐ requiring medication ‐ short term Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |||||||||||||
| Analysis 7.7  Comparison 7 ZUCLOPENTHIXOL versus RISPERIDONE, Outcome 7 Adverse effects: 2a. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs ‐ requiring medication ‐ short term. | ||||||||||||||||
| 8 Adverse Effects: 2b. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs (ESRS) ‐ short term Show forest plot | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 4.5 [0.67, 8.33] | ||||||||||||
| Analysis 7.8  Comparison 7 ZUCLOPENTHIXOL versus RISPERIDONE, Outcome 8 Adverse Effects: 2b. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs (ESRS) ‐ short term. | ||||||||||||||||
| 9 Adverse effects: 3. Any change in specific adverse effects ‐ negative and cognitive symptoms of schizophrenia ‐ short term Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |||||||||||||
| Analysis 7.9 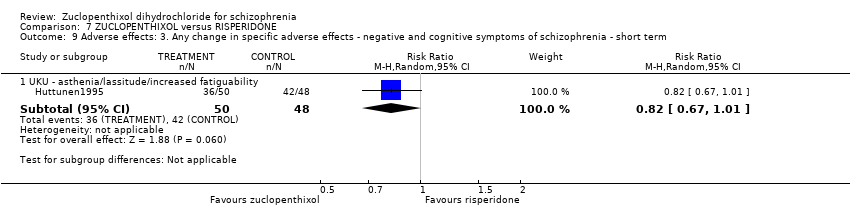 Comparison 7 ZUCLOPENTHIXOL versus RISPERIDONE, Outcome 9 Adverse effects: 3. Any change in specific adverse effects ‐ negative and cognitive symptoms of schizophrenia ‐ short term. | ||||||||||||||||
| 9.1 UKU ‐ asthenia/lassitude/increased fatiguability | 1 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.67, 1.01] | ||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | |||||||||
| 1 Global state: 1. Average endpoint global state score ‐ Unchanged/worse (CGI) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | ||||||||||
| Analysis 8.1  Comparison 8 ZUCLOPENTHIXOL versus SULPIRIDE ‐ all short term, Outcome 1 Global state: 1. Average endpoint global state score ‐ Unchanged/worse (CGI). | |||||||||||||
| 2 Global State: 2. Average endpoint global state score ‐ Moderately or severely ill (CGI) Show forest plot | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.75, 1.30] | |||||||||
| Analysis 8.2  Comparison 8 ZUCLOPENTHIXOL versus SULPIRIDE ‐ all short term, Outcome 2 Global State: 2. Average endpoint global state score ‐ Moderately or severely ill (CGI). | |||||||||||||
| 3 Mental State: Average endpoint general mental state score (BPRS, average = 5.7) Show forest plot | 1 | 61 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐5.08, 2.48] | |||||||||
| Analysis 8.3  Comparison 8 ZUCLOPENTHIXOL versus SULPIRIDE ‐ all short term, Outcome 3 Mental State: Average endpoint general mental state score (BPRS, average = 5.7). | |||||||||||||
| 4 Leaving the study early (any reason) Show forest plot | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 2.07 [0.97, 4.40] | |||||||||
| Analysis 8.4 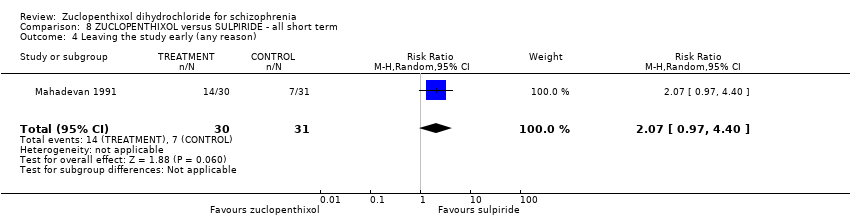 Comparison 8 ZUCLOPENTHIXOL versus SULPIRIDE ‐ all short term, Outcome 4 Leaving the study early (any reason). | |||||||||||||
| 5 Adverse Effects: 1. Any change in general adverse effects ‐ additional medication use Show forest plot | Other data | No numeric data | |||||||||||
| Analysis 8.5
Comparison 8 ZUCLOPENTHIXOL versus SULPIRIDE ‐ all short term, Outcome 5 Adverse Effects: 1. Any change in general adverse effects ‐ additional medication use. | |||||||||||||
| 6 Adverse Effects: 2. Any change in specific adverse effects ‐ metabolic ‐ weight change Show forest plot | 1 | 61 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐8.35, 5.15] | |||||||||
| Analysis 8.6 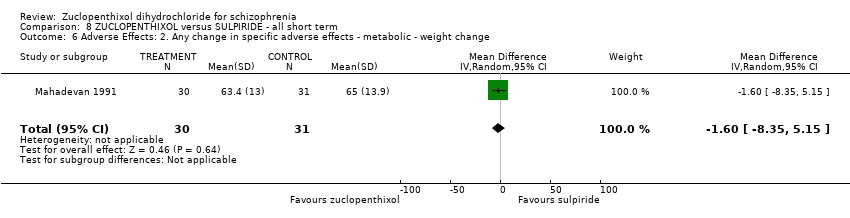 Comparison 8 ZUCLOPENTHIXOL versus SULPIRIDE ‐ all short term, Outcome 6 Adverse Effects: 2. Any change in specific adverse effects ‐ metabolic ‐ weight change. | |||||||||||||
| 7 Adverse effects: 3. Any change in specific adverse effects ‐ requiring additional medication ‐ hypnotics/sedatives Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | ||||||||||
| Analysis 8.7  Comparison 8 ZUCLOPENTHIXOL versus SULPIRIDE ‐ all short term, Outcome 7 Adverse effects: 3. Any change in specific adverse effects ‐ requiring additional medication ‐ hypnotics/sedatives. | |||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: Average endpoint global state score ‐ unchanged/worse (CGI) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 9.1  Comparison 9 ZUCLOPENTHIXOL versus THIOTHIXENE ‐ all medium term, Outcome 1 Global state: Average endpoint global state score ‐ unchanged/worse (CGI). | ||||
| 2 Leaving the study early (any reason) Show forest plot | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.24, 1.35] |
| Analysis 9.2 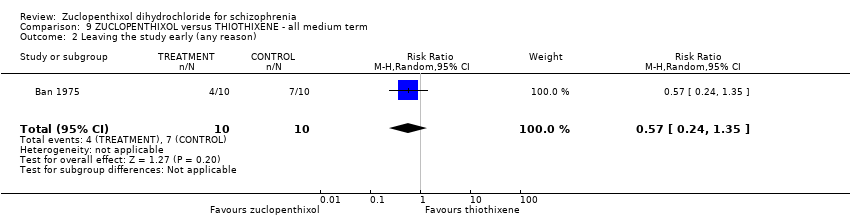 Comparison 9 ZUCLOPENTHIXOL versus THIOTHIXENE ‐ all medium term, Outcome 2 Leaving the study early (any reason). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Leaving the study early (any reason) Show forest plot | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 10.1 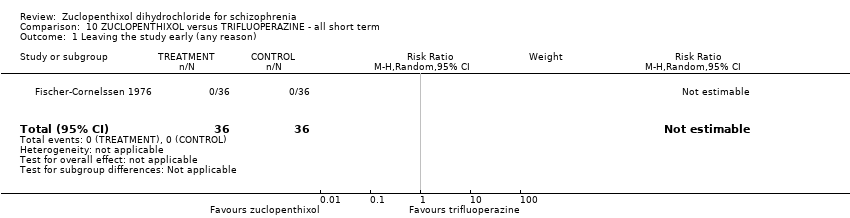 Comparison 10 ZUCLOPENTHIXOL versus TRIFLUOPERAZINE ‐ all short term, Outcome 1 Leaving the study early (any reason). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | |||||||||
| 1 Leaving the study early (any reason) Show forest plot | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [0.36, 10.58] | |||||||||
| Analysis 11.1  Comparison 11 ZUCLOPENTHIXOL versus ZUCLOPENTHIXOL DEPOT ‐ all long term, Outcome 1 Leaving the study early (any reason). | |||||||||||||
| 2 Behaviour: Average change in specific aspects of behaviour ‐ Violence during follow‐up Show forest plot | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.44, 1.71] | |||||||||
| Analysis 11.2  Comparison 11 ZUCLOPENTHIXOL versus ZUCLOPENTHIXOL DEPOT ‐ all long term, Outcome 2 Behaviour: Average change in specific aspects of behaviour ‐ Violence during follow‐up. | |||||||||||||
| 3 Adverse Effects: 1a. Any general adverse effects ‐ additional medication use Show forest plot | Other data | No numeric data | |||||||||||
| Analysis 11.3
Comparison 11 ZUCLOPENTHIXOL versus ZUCLOPENTHIXOL DEPOT ‐ all long term, Outcome 3 Adverse Effects: 1a. Any general adverse effects ‐ additional medication use. | |||||||||||||
| 4 Adverse Effects: 1b. Any change in specific adverse effects ‐ additional medication use ‐ benzodiazepine use at least once Show forest plot | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.3 [0.59, 2.86] | |||||||||
| Analysis 11.4  Comparison 11 ZUCLOPENTHIXOL versus ZUCLOPENTHIXOL DEPOT ‐ all long term, Outcome 4 Adverse Effects: 1b. Any change in specific adverse effects ‐ additional medication use ‐ benzodiazepine use at least once. | |||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||
| 1 Global state: Average endpoint global state score ‐ Unwell Show forest plot | 3 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.80, 1.17] | ||||||||||||
| Analysis 12.1  Comparison 12 CIS‐(Z) ZUCLOPENTHIXOL versus CIS(Z)/TRANS(E) ZUCLOPENTHIXOL ‐ all short term, Outcome 1 Global state: Average endpoint global state score ‐ Unwell. | ||||||||||||||||
| 2 Mental state: Average endpoint general mental state score ‐ Not improved Show forest plot | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.45, 2.07] | ||||||||||||
| Analysis 12.2  Comparison 12 CIS‐(Z) ZUCLOPENTHIXOL versus CIS(Z)/TRANS(E) ZUCLOPENTHIXOL ‐ all short term, Outcome 2 Mental state: Average endpoint general mental state score ‐ Not improved. | ||||||||||||||||
| 3 Leaving the study early (any reason) Show forest plot | 4 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 2.15 [0.49, 9.41] | ||||||||||||
| Analysis 12.3  Comparison 12 CIS‐(Z) ZUCLOPENTHIXOL versus CIS(Z)/TRANS(E) ZUCLOPENTHIXOL ‐ all short term, Outcome 3 Leaving the study early (any reason). | ||||||||||||||||
| 4 Adverse Effects: 1a. Any general adverse effects ‐ side effects reported Show forest plot | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.82, 2.18] | ||||||||||||
| Analysis 12.4  Comparison 12 CIS‐(Z) ZUCLOPENTHIXOL versus CIS(Z)/TRANS(E) ZUCLOPENTHIXOL ‐ all short term, Outcome 4 Adverse Effects: 1a. Any general adverse effects ‐ side effects reported. | ||||||||||||||||
| 5 Adverse Effects: 1b. Any change in specific adverse effects ‐ individual side effects Show forest plot | Other data | No numeric data | ||||||||||||||
| Analysis 12.5
Comparison 12 CIS‐(Z) ZUCLOPENTHIXOL versus CIS(Z)/TRANS(E) ZUCLOPENTHIXOL ‐ all short term, Outcome 5 Adverse Effects: 1b. Any change in specific adverse effects ‐ individual side effects. | ||||||||||||||||

Study flow diagram ‐ update 2016.

Comparison 1 ZUCLOPENTHIXOL versus PLACEBO ‐ all short term, Outcome 1 Leaving the study early (any reason).

Comparison 1 ZUCLOPENTHIXOL versus PLACEBO ‐ all short term, Outcome 2 Adverse effects: 1. Clinically important change in specific adverse effects ‐ cardiovascular ‐ orthostatic.
Comparison 1 ZUCLOPENTHIXOL versus PLACEBO ‐ all short term, Outcome 3 Adverse effects: 2. Clinically important change in specific adverse effects ‐ central nervous system ‐ arousal state.

Comparison 1 ZUCLOPENTHIXOL versus PLACEBO ‐ all short term, Outcome 4 Adverse effects: 3. Clinically important change in specific adverse effects ‐ endocrine ‐ menstruation started.

Comparison 1 ZUCLOPENTHIXOL versus PLACEBO ‐ all short term, Outcome 5 Adverse effects: 4a. Any general adverse effects ‐ movement disorders ‐ EPSEs (UKU side effect rating scale, no scores).
Comparison 1 ZUCLOPENTHIXOL versus PLACEBO ‐ all short term, Outcome 6 Adverse effects: 4b. Clinically important change in specific adverse effects ‐ movement disorders ‐ EPSEs.
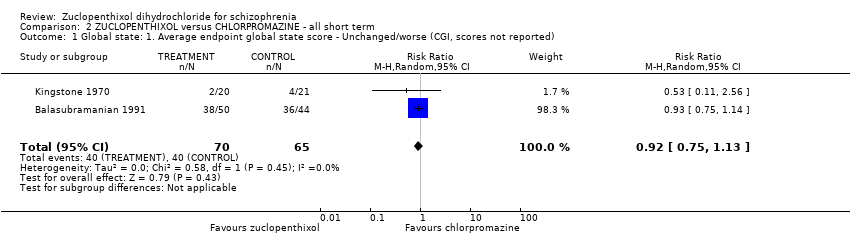
Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 1 Global state: 1. Average endpoint global state score ‐ Unchanged/worse (CGI, scores not reported).

Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 2 Global state: 2. Average endpoint global state score ‐ No Recovery.

Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 3 Global state: 3a. Average endpoint global state score (GAS, high score not reported, average score = 63.4).
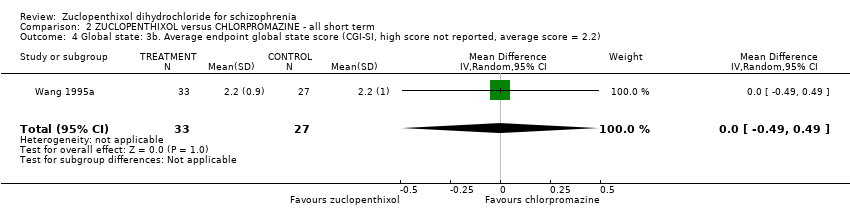
Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 4 Global state: 3b. Average endpoint global state score (CGI‐SI, high score not reported, average score = 2.2).

Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 5 Mental state: 1. No clinically important change in general mental state ‐ Not improved (PANSS, scores not reported).
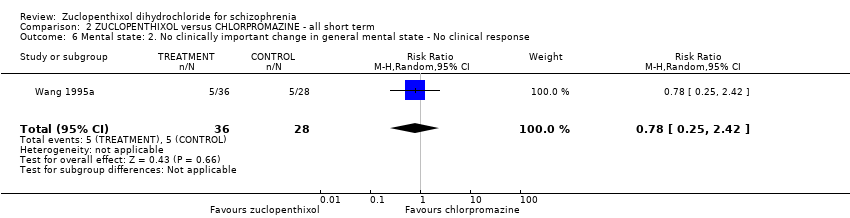
Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 6 Mental state: 2. No clinically important change in general mental state ‐ No clinical response.

Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 7 Mental state: 3. Average endpoint general mental state score (BPRS, high score = 34.2).

Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 8 Leaving the study early (any reason).

Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 9 Adverse effects: 1. Any general adverse effects ‐ side effects (CGI, high score not reported).
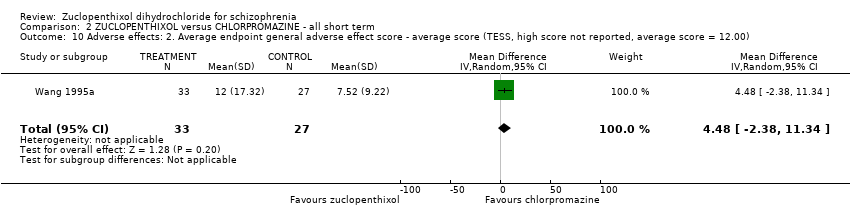
Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 10 Adverse effects: 2. Average endpoint general adverse effect score ‐ average score (TESS, high score not reported, average score = 12.00).
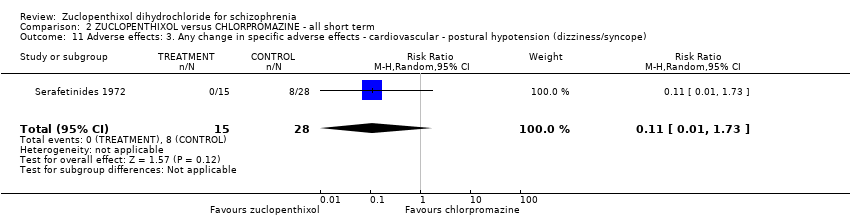
Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 11 Adverse effects: 3. Any change in specific adverse effects ‐ cardiovascular ‐ postural hypotension (dizziness/syncope).

Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 12 Adverse effects: 4. Any change in specific adverse effects ‐ central nervous system ‐ arousal.
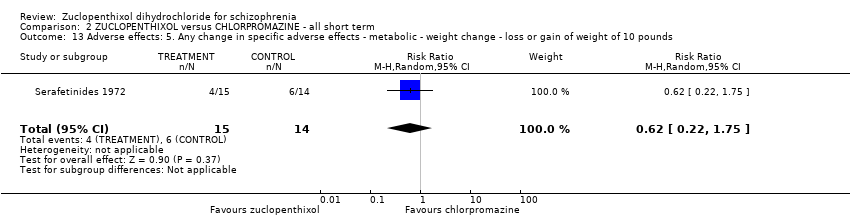
Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 13 Adverse effects: 5. Any change in specific adverse effects ‐ metabolic ‐ weight change ‐ loss or gain of weight of 10 pounds.
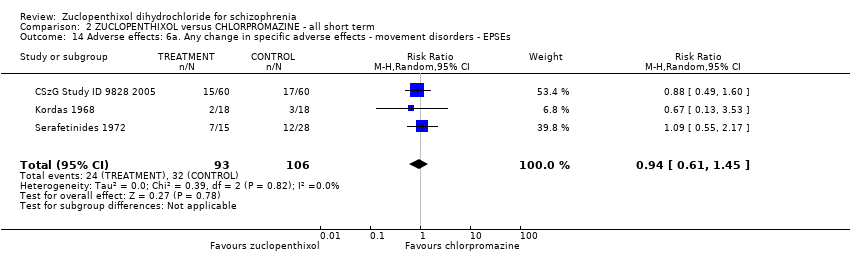
Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 14 Adverse effects: 6a. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs.
| Study | Zuclopenthixol | Chlorpromazine |
| Kingstone 1970 | n = 5, authors do not state which additional medication. | n = 2, authors do not state which additional medication. |
| Kordas 1968 | Benzhexol and diazepam if necessary. | Benzhexol and diazepam if necessary. |
| Wang 1995a | Trihexphenidyl or scopolamine used if necessary. Authors do not report frequency of use or which group used which. | Trihexphenidyl or scopolamine used if necessary. Authors do not report frequency of use or which group used which. |
Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 15 Adverse effects: 6b. Any change in specific adverse effects ‐ movement disorders ‐ additional medication use.
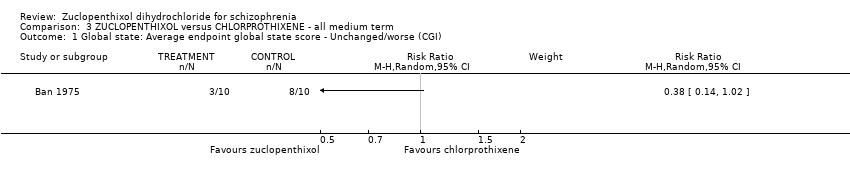
Comparison 3 ZUCLOPENTHIXOL versus CHLORPROTHIXENE ‐ all medium term, Outcome 1 Global state: Average endpoint global state score ‐ Unchanged/worse (CGI).

Comparison 3 ZUCLOPENTHIXOL versus CHLORPROTHIXENE ‐ all medium term, Outcome 2 Leaving the study early (any reason).

Comparison 4 ZUCLOPENTHIXOL versus CLOZAPINE ‐ all short term, Outcome 1 Leaving the study early (any reason).
| Study | Zuclopenthixol (n = 36) | Clozapine (n = 38) |
| Fischer‐Cornelssen 1976 | Drowsiness (12%); Stimulation (14%); Confusion (1%); GI (1%); anticholinergic (14%); dizziness (6%); Orthostatic reaction (1%); Headache (2%); Hypersalivation (2%); hypokinesia (4%); hyperkinesia (2%); dyskinesia (0.4%); rigor (5%); tremor (5%); akathisia (3%) | Drowsiness (12%); Stimulation (21%); Confusion (3%); GI (8%); anticholinergic (8%); dizziness (15%); Orthostatic reaction (6%); Headache (3%); Hypersalivation (4%); hypokinesia (2%); hyperkinesia (4%); dyskinesia (0%); rigor (1%); tremor (3%); akathisia (3%) |
Comparison 4 ZUCLOPENTHIXOL versus CLOZAPINE ‐ all short term, Outcome 2 Adverse effects: Any general adverse effects ‐ side effects ‐ frequency per day.
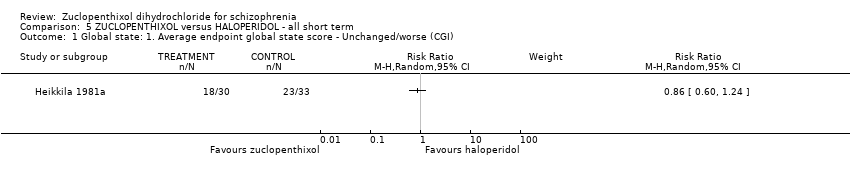
Comparison 5 ZUCLOPENTHIXOL versus HALOPERIDOL ‐ all short term, Outcome 1 Global state: 1. Average endpoint global state score ‐ Unchanged/worse (CGI).
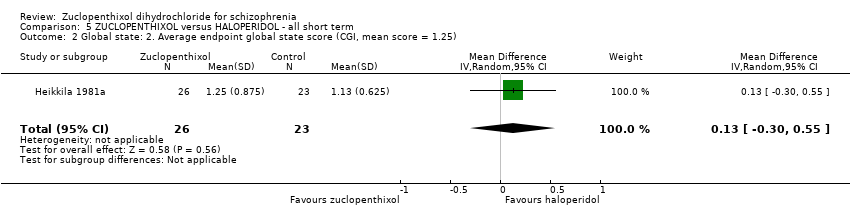
Comparison 5 ZUCLOPENTHIXOL versus HALOPERIDOL ‐ all short term, Outcome 2 Global state: 2. Average endpoint global state score (CGI, mean score = 1.25).

Comparison 5 ZUCLOPENTHIXOL versus HALOPERIDOL ‐ all short term, Outcome 3 Leaving the study early (any reason).
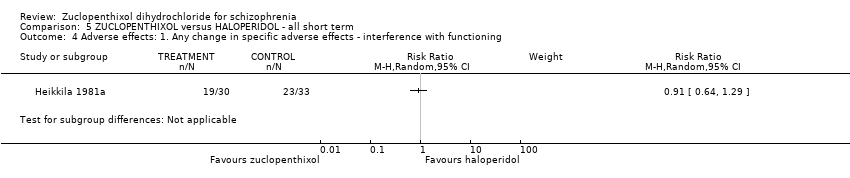
Comparison 5 ZUCLOPENTHIXOL versus HALOPERIDOL ‐ all short term, Outcome 4 Adverse effects: 1. Any change in specific adverse effects ‐ interference with functioning.

Comparison 5 ZUCLOPENTHIXOL versus HALOPERIDOL ‐ all short term, Outcome 5 Adverse effects: 2. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs ‐ requiring medication.

Comparison 5 ZUCLOPENTHIXOL versus HALOPERIDOL ‐ all short term, Outcome 6 Adverse effects: 3. Any change in specific adverse effects ‐ requiring additional medication.
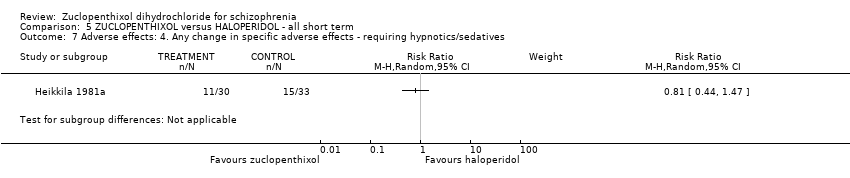
Comparison 5 ZUCLOPENTHIXOL versus HALOPERIDOL ‐ all short term, Outcome 7 Adverse effects: 4. Any change in specific adverse effects ‐ requiring hypnotics/sedatives.

Comparison 6 ZUCLOPENTHIXOL versus PERPHENAZINE, Outcome 1 Global state: Average endpoint global state score ‐ unchanged/worse (global rating ‐ investigator opinion) ‐ medium term.
Comparison 6 ZUCLOPENTHIXOL versus PERPHENAZINE, Outcome 2 Leaving the study early (any reason) ‐ short/medium term.

Comparison 6 ZUCLOPENTHIXOL versus PERPHENAZINE, Outcome 3 Adverse effects: 1. Any change in specific adverse effects ‐ central nervous system ‐ arousal ‐ requiring medication ‐ medium term.
Comparison 6 ZUCLOPENTHIXOL versus PERPHENAZINE, Outcome 4 Adverse effects: 2. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs ‐ requiring medication ‐ medium term.
Comparison 7 ZUCLOPENTHIXOL versus RISPERIDONE, Outcome 1 Mental State: 1. Average endpoint general mental state score (PANSS, average score = 45.8) ‐ medium term.

Comparison 7 ZUCLOPENTHIXOL versus RISPERIDONE, Outcome 2 Mental State: 2. Average endpoint general mental state score (PANSS General, average score medium term = 20.5) ‐ short/medium term.

Comparison 7 ZUCLOPENTHIXOL versus RISPERIDONE, Outcome 3 Mental State: 3. Average endpoint general mental state score (PANSS Positive, average score = 9.8) ‐ medium term.
Comparison 7 ZUCLOPENTHIXOL versus RISPERIDONE, Outcome 4 Mental State: 4. Average endpoint general mental state score (PANSS Negative, average score 11.5) ‐ medium term.

Comparison 7 ZUCLOPENTHIXOL versus RISPERIDONE, Outcome 5 Leaving the study early (any reason) ‐ short/medium term.
| Study | Zuclopenthixol | Risperidone |
| Fagerlund 2004 | Benzodiazepines n = 7 Anticholinergics n = 11 | Benzodiazepines n = 8 Anticholinergics n = 7 |
| Glenthoj 2007 | Anticholinergic n = 10, Benzodiazepines n = 11, Antidepressant n = 1 | Authors do not differentiate the use of additional medication between the two groups, totals only given. |
Comparison 7 ZUCLOPENTHIXOL versus RISPERIDONE, Outcome 6 Adverse Effects: 1. Any change in general adverse effects ‐ additional medication use ‐ short/medium term.
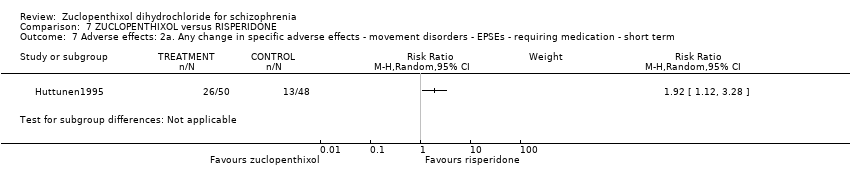
Comparison 7 ZUCLOPENTHIXOL versus RISPERIDONE, Outcome 7 Adverse effects: 2a. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs ‐ requiring medication ‐ short term.

Comparison 7 ZUCLOPENTHIXOL versus RISPERIDONE, Outcome 8 Adverse Effects: 2b. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs (ESRS) ‐ short term.
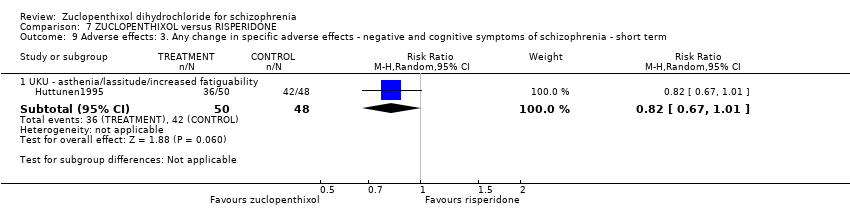
Comparison 7 ZUCLOPENTHIXOL versus RISPERIDONE, Outcome 9 Adverse effects: 3. Any change in specific adverse effects ‐ negative and cognitive symptoms of schizophrenia ‐ short term.

Comparison 8 ZUCLOPENTHIXOL versus SULPIRIDE ‐ all short term, Outcome 1 Global state: 1. Average endpoint global state score ‐ Unchanged/worse (CGI).

Comparison 8 ZUCLOPENTHIXOL versus SULPIRIDE ‐ all short term, Outcome 2 Global State: 2. Average endpoint global state score ‐ Moderately or severely ill (CGI).
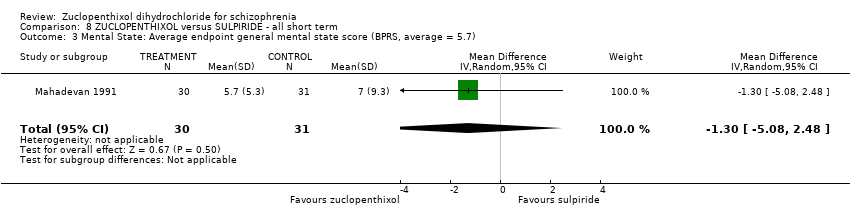
Comparison 8 ZUCLOPENTHIXOL versus SULPIRIDE ‐ all short term, Outcome 3 Mental State: Average endpoint general mental state score (BPRS, average = 5.7).
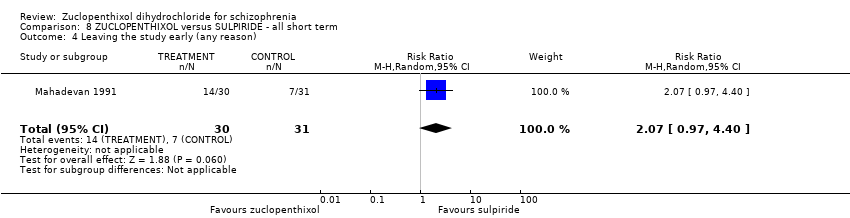
Comparison 8 ZUCLOPENTHIXOL versus SULPIRIDE ‐ all short term, Outcome 4 Leaving the study early (any reason).
| Study | Zuclopenthixol | Sulpiride |
| Mahadevan 1991 | n = 4 Amitriptyline | n = 4 Amitryptyline |
Comparison 8 ZUCLOPENTHIXOL versus SULPIRIDE ‐ all short term, Outcome 5 Adverse Effects: 1. Any change in general adverse effects ‐ additional medication use.

Comparison 8 ZUCLOPENTHIXOL versus SULPIRIDE ‐ all short term, Outcome 6 Adverse Effects: 2. Any change in specific adverse effects ‐ metabolic ‐ weight change.

Comparison 8 ZUCLOPENTHIXOL versus SULPIRIDE ‐ all short term, Outcome 7 Adverse effects: 3. Any change in specific adverse effects ‐ requiring additional medication ‐ hypnotics/sedatives.

Comparison 9 ZUCLOPENTHIXOL versus THIOTHIXENE ‐ all medium term, Outcome 1 Global state: Average endpoint global state score ‐ unchanged/worse (CGI).
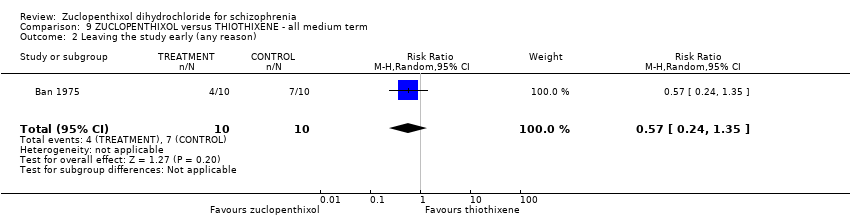
Comparison 9 ZUCLOPENTHIXOL versus THIOTHIXENE ‐ all medium term, Outcome 2 Leaving the study early (any reason).

Comparison 10 ZUCLOPENTHIXOL versus TRIFLUOPERAZINE ‐ all short term, Outcome 1 Leaving the study early (any reason).

Comparison 11 ZUCLOPENTHIXOL versus ZUCLOPENTHIXOL DEPOT ‐ all long term, Outcome 1 Leaving the study early (any reason).

Comparison 11 ZUCLOPENTHIXOL versus ZUCLOPENTHIXOL DEPOT ‐ all long term, Outcome 2 Behaviour: Average change in specific aspects of behaviour ‐ Violence during follow‐up.
| Study | Zuclopenthixol | Depot |
| Arango 2006 | n = 1 Propanolol | n = 1 venlafaxine n = 1 Lithium |
Comparison 11 ZUCLOPENTHIXOL versus ZUCLOPENTHIXOL DEPOT ‐ all long term, Outcome 3 Adverse Effects: 1a. Any general adverse effects ‐ additional medication use.

Comparison 11 ZUCLOPENTHIXOL versus ZUCLOPENTHIXOL DEPOT ‐ all long term, Outcome 4 Adverse Effects: 1b. Any change in specific adverse effects ‐ additional medication use ‐ benzodiazepine use at least once.

Comparison 12 CIS‐(Z) ZUCLOPENTHIXOL versus CIS(Z)/TRANS(E) ZUCLOPENTHIXOL ‐ all short term, Outcome 1 Global state: Average endpoint global state score ‐ Unwell.

Comparison 12 CIS‐(Z) ZUCLOPENTHIXOL versus CIS(Z)/TRANS(E) ZUCLOPENTHIXOL ‐ all short term, Outcome 2 Mental state: Average endpoint general mental state score ‐ Not improved.

Comparison 12 CIS‐(Z) ZUCLOPENTHIXOL versus CIS(Z)/TRANS(E) ZUCLOPENTHIXOL ‐ all short term, Outcome 3 Leaving the study early (any reason).

Comparison 12 CIS‐(Z) ZUCLOPENTHIXOL versus CIS(Z)/TRANS(E) ZUCLOPENTHIXOL ‐ all short term, Outcome 4 Adverse Effects: 1a. Any general adverse effects ‐ side effects reported.
| Study | Cis Z | Cis(Z)/Trans(E) |
| Gravem 1981 | EPSEs most frequent. Authors state no significant difference between two isomers. | EPSEs most frequent. |
| Heikkila 1981 | Dry mouth, Disturbance of accommodation, Disturbance of urination, Constipation, Dizziness, Headache, Increased sweating, Drowsiness, Anxiety, Parkinsonism, Akathisia, Tardive dyskinesia and others | Dry mouth, Disturbance of urination, Constipation, Dizziness, Drowsiness, Parkinsonism, Akathisia, Tardive dyskinesia, others |
Comparison 12 CIS‐(Z) ZUCLOPENTHIXOL versus CIS(Z)/TRANS(E) ZUCLOPENTHIXOL ‐ all short term, Outcome 5 Adverse Effects: 1b. Any change in specific adverse effects ‐ individual side effects.
| Patient or population: people with schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with PLACEBO (short term) | Risk with ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Adverse effects: Clinically important general adverse effect (extrapyramidal effects ‐ UKU side effect rating scale) | 80 per 1000 | 486 per 1000 | RR 6.07 | 28 | ⊕⊝⊝⊝ | Risk assumed to be moderate and rounded from 7.69% to 8%. |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Service outcomes: Duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Leaving the study early (any reason) | 40 per 1000 | 12 per 1000 | RR 0.29 | 100 | ⊕⊝⊝⊝ | Risk assumed to be moderate and rounded from 3.85% to 4%. Low number of events in both RCTs. |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'very serious' ‐ possible selection bias, blinding may have been single, attrition bias and reporting bias. 2 Risk of bias: rated 'very serious' ‐ selection, attrition and reporting bias 3 Risk of inconsistency: rated 'not serious' ‐ suspected but not found. 4 Risk of publication bias: rated 'strongly suspected' ‐ multiple papers published with the same patient cohort. 5 Risk of large effect: rated 'very large' ‐ RR 6.07, small n number but result likely when versus placebo. 6 Risk of imprecision: rated 'very serious' ‐ low n numbers | ||||||
| Patient or population: schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with CHLORPROMAZINE (short term) | Risk with ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (CGI‐SI, high score not reported, average score = 2.2) | The mean CGI‐SI endpoint score in the intervention group (MD) was 0 (‐0.49 lower to 0.49 higher) | ‐ | 64 | ⊕⊝⊝⊝ | Translated study. | |
| Adverse effects: Clinically important general adverse effect (EPSEs) | 300 per 1000 | 282 per 1000 | RR 0.94 | 199 | ⊕⊝⊝⊝ | Risk control rounded to 30% and set to moderate. Mixture of inpatient and outpatient, though predominantly hospitalised patients. |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (BPRS, high score = 34.2) | The mean mental state: average endpoint score (BPRS, high score = 34.2) in the intervention group was 0.4 more (2.43 fewer to 3.23 more) | ‐ | 221 | ⊕⊕⊝⊝ | Mixture of inpatient and outpatient, though predominantly hospitalised patients. | |
| Leaving the study early (any reason) | 70 per 1000 | 38 per 1000 | RR 0.54 | 766 | ⊕⊕⊝⊝ | Mixture of inpatient and outpatient, though predominantly hospitalised patients. Risk control set to moderate and rounded to 7%; extreme values not likely. |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'serious' ‐ Selection and attrition bias likely. 2 Risk of bias: rated 'serious' ‐ Selection attrition bias. 3 Risk of bias: rated 'serious' ‐ Selection, attrition and reporting bias. 4 Risk of bias: rated 'serious' ‐ Selection, attrition and reporting bias. Risk of inconsistency: rated as 'serious' ‐ All three papers reported on differing population sizes and obtained different levels of EPSEs in the experimental and control groups. 5 Risk of imprecision: rated 'very serious' ‐ low n numbers 6 Risk of indirectness: rated 'very serious' ‐ mixed samples For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | ||||||
| Patient or population: schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with CHLORPROTHIXENE (medium term) | Risk with ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Adverse effects: Clinically important general adverse effect (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Leaving the study early (any reason) | 400 per 1000 | 400 per 1000 | RR 1.00 | 20 | ⊕⊝⊝⊝ | |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated as 'serious' ‐ selection, reporting bias likely. 2 Risk of publication bias: rated as 'strongly suspected' ‐ several papers published using the same cohort of patients. 3 Risk of imprecision: rated 'very serious' ‐ low n numbers For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | ||||||
| Patient or population: schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with CLOZAPINE (short term) | Risk with ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Adverse effects: Clinically important general adverse effect (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Leaving the study early (any reason) | 0 per 1000 | 0 per 1000 | not estimable | 407 | ⊕⊕⊝⊝ | Multi‐centre, multi‐drug trial with disproportionate numbers of people in different arms of the study. The authors did not report that anybody left the study in the Zuclopenthixol and clozapine arms. |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated as 'serious' ‐ selection, attrition, reporting and performance bias likely. 2 Risk of indirectness: rated 'serious' ‐ Multiple study arms For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | ||||||
| Patient or population: schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with HALOPERIDOL (short term) | Risk with ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (CGI, mean score = 1.25) | The mean CGI endpoint score in the intervention group (MD) was 0.13 more (‐0.3 fewer to 0.55 more) | ‐ | 49 | ⊕⊝⊝⊝ | Small study, multiple scales used (NOSIE30, BPRS, CGI). Paper only reported outcomes of some of these scales. | |
| Adverse effects: Clinically important general adverse effect (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Leaving the study early (any reason) | 300 per 1000 | 297 per 1000 | RR 0.99 | 141 | ⊕⊝⊝⊝ | Risk control rounded to 30% from 29.25% and, as extreme values unlikely, it was set to moderate. |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated as 'very serious' ‐ likely selection, attrition, reporting and diagnostic purity bias. 2 Risk of bias: rated as 'serious' ‐ likely selection, attrition, reporting and diagnostic purity bias. Risk of inconsistency: rated as 'serious' ‐ both papers generated differing values for people leaving the study and do not appear consistent (face validity). 3 Risk of imprecision: rated 'serious' ‐ low n numbers 4 Risk of indirectness: rated 'serious' ‐ not all scales reported For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | ||||||
| Patient or population: schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with PERPHENAZINE (short and medium term) | Risk with ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Adverse effects: Clinically important general adverse effect (EPSEs requiring medication ‐ medium term) | 400 per 1000 | 760 per 1000 | RR 1.90 | 50 | ⊕⊝⊝⊝ | |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Leaving the study early (any reason, short and medium term) | 207 per 1000 | 130 per 1000 | RR 0.63 | 104 | ⊕⊝⊝⊝ | |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated as 'serious' ‐ likely selection and reporting bias. 2 Risk of imprecision: rated 'very serious' ‐ low n numbers For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | ||||||
| Patient or population: schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with RISPERIDONE (short and medium term) | Risk with ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Adverse effects: Clinically important general adverse effect ‐ short term (EPSEs requiring medication) | 271 per 1000 | 520 per 1000 | RR 1.92 | 98 | ⊕⊝⊝⊝ | |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (PANSS, average score = 20.5) ‐ medium term | The mean PANNS endpoint score in the intervention group (MD) was 3.2 fewer (‐7.71 fewer to 1.31 more) | ‐ | 25 | ⊕⊝⊝⊝ | Small study. Standard deviations may be standard errors. Open label trial. | |
| Leaving the study early (any reason, short and medium term) | 310 per 1000 | 403 per 1000 | RR 1.30 | 154 | ⊕⊝⊝⊝ | Risk control taken as mean of extremes: high + low / 2 (changed from 21.43% to 31%) |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated as 'very serious' ‐ open label trial, likely selection bias. 2 Risk of imprecision: rated as 'very serious' ‐ strongly suspect reported standard deviations are standard errors, low n numbers 3 Risk of publication bias: rated as 'strongly suspected' ‐ all studies published findings across several consecutive years in different journals and at conferences. 4 Risk of bias: rated as 'very serious' ‐ study 1 (selection, reporting, diagnostic purity and attrition bias likely ‐ author emailed); study 2 (open label); study 3 (reporting and attrition bias). 5 Risk of publication bias: rated as 'strongly suspected' ‐ multiple papers over consecutive years published with the same data. 6 Risk of bias: rated as 'serious' ‐ diagnostic purity and attrition bias; likely selection and reporting bias (authors contacted) For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | ||||||
| Patient or population: schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with SULPIRIDE (short term) | Risk with ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (CGI ‐ unchanged/worse) | 226 per 1000 | 266 per 1000 | RR 1.18 | 61 | ⊕⊝⊝⊝ | The study did not report clinical improvement so unchanged/worse is reported for this outcome. Usually we report clinical improvement. |
| Adverse effects: Clinically important general adverse effect ‐ requiring hypnotics/sedatives | 419 per 1000 | 252 per 1000 | RR 0.60 | 61 | ⊕⊝⊝⊝ | |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (BPRS) | The mean BPRS endpoint score in the intervention group (MD) was 1.3 fewer (‐5.08 fewer to 2.48 more) | ‐ | 61 | ⊕⊝⊝⊝ | ||
| Leaving the study early (any reason) | 230 per 1000 | 476 per 1000 | RR 2.07 | 61 | ⊕⊝⊝⊝ | Control of risk rounded up from 22.58% to 23%. |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated as 'serious' ‐ diagnostic purity, attrition bias. Per‐Protocol analysis suspected. 2 Risk of imprecision: rated as ' very serious' ‐ percentages used to describe data and comparisons are made against an assumption of baseline measurements. Low n numbers. For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | ||||||
| Patient or population: schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with THIOTHIXENE (medium term) | Risk with ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (unchanged/worse ‐ CGI) | 600 per 1000 | 300 per 1000 | RR 0.50 | 20 | ⊕⊝⊝⊝ | The study did not report clinical improvement so unchanged/worse is reported for this outcome. Usually we report clinical improvement. |
| Adverse effects: Clinically important general adverse effect (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported on this outcome. | |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported on this outcome. | |
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported on this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported on this outcome. | |
| Leaving the study early (any reason) | 700 per 1000 | 399 per 1000 | RR 0.57 | 20 | ⊕⊝⊝⊝ | |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported on this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated as 'serious' ‐ likely selection and reporting bias. 2 Risk of publication bias: rated as 'strongly suspected' ‐ several papers published using the same cohort. 3 Risk of imprecision: rated as 'serious' ‐ low n numbers For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | ||||||
| Patient or population: schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with TRIFLUOPERAZINE (short term) | Risk with ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Adverse effects: Clinically important general adverse effect (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Leaving the study early (any reason) | 0 per 1000 | 0 per 1000 | not estimable | 72 | ⊕⊝⊝⊝ | Multi‐centre and multi‐drug trial with low numbers in each arm. |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated as 'serious' ‐ selection, attrition, reporting and performance bias likely. 2 Risk of imprecision: rated 'very serious' ‐ low n numbers 3 Risk of indirectness: rated 'very serious' ‐ multiple arms, some missing For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | ||||||
| Patient or population: schizophrenia | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | ||
| Risk with ZUCLOPENTHIXOL DEPOT (long term) | Risk with ZUCLOPENTHIXOL | ||||||
| Global state: Average endpoint global state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | ||
| Adverse effects: Clinically important general adverse effect (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | ||
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | ||
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | ||
| Mental state: Average endpoint general mental state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | ||
| Leaving the study early (any reason) | 80 per 1000 | 156 per 1000 | RR 1.95 | 46 | ⊕⊝⊝⊝ | Control of risk rounded from 7.69% to 8%. | |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1 Risk of bias: rated as 'very serious' ‐ single researcher did randomisation, selection bias likely, open label trial and incomplete outcome data. 2 Risk of publication bias: rated as 'strongly suspected' ‐ several papers published using the same data and cohort. 3 Risk of imprecision: rated 'very serious' ‐ low n numbers For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | |||||||
| Patient or population: schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with CIS(Z)/TRANS(E) ZUCLOPENTHIXOL (short term) | Risk with CIS‐(Z) ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Adverse effects: Clinically important general adverse effect | 470 per 1000 | 630 per 1000 | RR 1.34 | 57 | ⊕⊝⊝⊝ | Control of risk set at moderate and rounded up from 46.4% to 47%. |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Leaving the study early: Any reason | 28 per 1000 | 61 per 1000 | RR 2.15 | 140 | ⊕⊝⊝⊝ | |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated as 'serious' ‐ selection bias throughout. Very difficult to ascertain from published materials if selection bias has been minimised. 2 Risk of imprecision: rated 'very serious' ‐ low n numbers For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Leaving the study early (any reason) Show forest plot | 2 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.01, 6.60] |
| 2 Adverse effects: 1. Clinically important change in specific adverse effects ‐ cardiovascular ‐ orthostatic Show forest plot | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.01, 6.60] |
| 3 Adverse effects: 2. Clinically important change in specific adverse effects ‐ central nervous system ‐ arousal state Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 excitation | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 2.62 [0.12, 59.40] |
| 3.2 sleepiness / sedation | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 2.89 [1.01, 8.30] |
| 4 Adverse effects: 3. Clinically important change in specific adverse effects ‐ endocrine ‐ menstruation started Show forest plot | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 7.0 [0.39, 126.48] |
| 5 Adverse effects: 4a. Any general adverse effects ‐ movement disorders ‐ EPSEs (UKU side effect rating scale, no scores) Show forest plot | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 6.07 [0.86, 43.04] |
| 6 Adverse effects: 4b. Clinically important change in specific adverse effects ‐ movement disorders ‐ EPSEs Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 parkinsonism | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 5.00 [0.26, 97.37] |
| 6.2 oculogyric crisis | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 69.09] |
| 6.3 tremor | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 5.00 [0.26, 97.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: 1. Average endpoint global state score ‐ Unchanged/worse (CGI, scores not reported) Show forest plot | 2 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.75, 1.13] |
| 2 Global state: 2. Average endpoint global state score ‐ No Recovery Show forest plot | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.89, 1.16] |
| 3 Global state: 3a. Average endpoint global state score (GAS, high score not reported, average score = 63.4) Show forest plot | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐8.12, 6.92] |
| 4 Global state: 3b. Average endpoint global state score (CGI‐SI, high score not reported, average score = 2.2) Show forest plot | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.49, 0.49] |
| 5 Mental state: 1. No clinically important change in general mental state ‐ Not improved (PANSS, scores not reported) Show forest plot | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.81, 1.18] |
| 6 Mental state: 2. No clinically important change in general mental state ‐ No clinical response Show forest plot | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.25, 2.42] |
| 7 Mental state: 3. Average endpoint general mental state score (BPRS, high score = 34.2) Show forest plot | 3 | 221 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐2.43, 3.23] |
| 8 Leaving the study early (any reason) Show forest plot | 6 | 766 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.36, 0.81] |
| 9 Adverse effects: 1. Any general adverse effects ‐ side effects (CGI, high score not reported) Show forest plot | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.77, 0.97] |
| 10 Adverse effects: 2. Average endpoint general adverse effect score ‐ average score (TESS, high score not reported, average score = 12.00) Show forest plot | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 4.48 [‐2.38, 11.34] |
| 11 Adverse effects: 3. Any change in specific adverse effects ‐ cardiovascular ‐ postural hypotension (dizziness/syncope) Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 1.73] |
| 12 Adverse effects: 4. Any change in specific adverse effects ‐ central nervous system ‐ arousal Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 excitation | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.07, 5.47] |
| 12.2 sedation | 2 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.73, 1.70] |
| 13 Adverse effects: 5. Any change in specific adverse effects ‐ metabolic ‐ weight change ‐ loss or gain of weight of 10 pounds Show forest plot | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.22, 1.75] |
| 14 Adverse effects: 6a. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs Show forest plot | 3 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.61, 1.45] |
| 15 Adverse effects: 6b. Any change in specific adverse effects ‐ movement disorders ‐ additional medication use Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: Average endpoint global state score ‐ Unchanged/worse (CGI) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Leaving the study early (any reason) Show forest plot | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.34, 2.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Leaving the study early (any reason) Show forest plot | 1 | 407 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Adverse effects: Any general adverse effects ‐ side effects ‐ frequency per day Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: 1. Average endpoint global state score ‐ Unchanged/worse (CGI) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Global state: 2. Average endpoint global state score (CGI, mean score = 1.25) Show forest plot | 1 | 49 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.30, 0.55] |
| 3 Leaving the study early (any reason) Show forest plot | 2 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.72, 1.35] |
| 4 Adverse effects: 1. Any change in specific adverse effects ‐ interference with functioning Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5 Adverse effects: 2. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs ‐ requiring medication Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6 Adverse effects: 3. Any change in specific adverse effects ‐ requiring additional medication Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7 Adverse effects: 4. Any change in specific adverse effects ‐ requiring hypnotics/sedatives Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: Average endpoint global state score ‐ unchanged/worse (global rating ‐ investigator opinion) ‐ medium term Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Leaving the study early (any reason) ‐ short/medium term Show forest plot | 2 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.27, 1.47] |
| 3 Adverse effects: 1. Any change in specific adverse effects ‐ central nervous system ‐ arousal ‐ requiring medication ‐ medium term Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4 Adverse effects: 2. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs ‐ requiring medication ‐ medium term Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mental State: 1. Average endpoint general mental state score (PANSS, average score = 45.8) ‐ medium term Show forest plot | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐3.20 [‐7.71, 1.31] |
| 2 Mental State: 2. Average endpoint general mental state score (PANSS General, average score medium term = 20.5) ‐ short/medium term Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Short term | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐2.40 [‐4.52, ‐0.28] |
| 2.2 Medium term | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐2.72, 2.12] |
| 3 Mental State: 3. Average endpoint general mental state score (PANSS Positive, average score = 9.8) ‐ medium term Show forest plot | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐2.69, 0.69] |
| 4 Mental State: 4. Average endpoint general mental state score (PANSS Negative, average score 11.5) ‐ medium term Show forest plot | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐1.5 [‐4.05, 1.05] |
| 5 Leaving the study early (any reason) ‐ short/medium term Show forest plot | 3 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.84, 2.02] |
| 6 Adverse Effects: 1. Any change in general adverse effects ‐ additional medication use ‐ short/medium term Show forest plot | Other data | No numeric data | ||
| 7 Adverse effects: 2a. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs ‐ requiring medication ‐ short term Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8 Adverse Effects: 2b. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs (ESRS) ‐ short term Show forest plot | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 4.5 [0.67, 8.33] |
| 9 Adverse effects: 3. Any change in specific adverse effects ‐ negative and cognitive symptoms of schizophrenia ‐ short term Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 UKU ‐ asthenia/lassitude/increased fatiguability | 1 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.67, 1.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: 1. Average endpoint global state score ‐ Unchanged/worse (CGI) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Global State: 2. Average endpoint global state score ‐ Moderately or severely ill (CGI) Show forest plot | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.75, 1.30] |
| 3 Mental State: Average endpoint general mental state score (BPRS, average = 5.7) Show forest plot | 1 | 61 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐5.08, 2.48] |
| 4 Leaving the study early (any reason) Show forest plot | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 2.07 [0.97, 4.40] |
| 5 Adverse Effects: 1. Any change in general adverse effects ‐ additional medication use Show forest plot | Other data | No numeric data | ||
| 6 Adverse Effects: 2. Any change in specific adverse effects ‐ metabolic ‐ weight change Show forest plot | 1 | 61 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐8.35, 5.15] |
| 7 Adverse effects: 3. Any change in specific adverse effects ‐ requiring additional medication ‐ hypnotics/sedatives Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: Average endpoint global state score ‐ unchanged/worse (CGI) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Leaving the study early (any reason) Show forest plot | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.24, 1.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Leaving the study early (any reason) Show forest plot | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Leaving the study early (any reason) Show forest plot | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [0.36, 10.58] |
| 2 Behaviour: Average change in specific aspects of behaviour ‐ Violence during follow‐up Show forest plot | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.44, 1.71] |
| 3 Adverse Effects: 1a. Any general adverse effects ‐ additional medication use Show forest plot | Other data | No numeric data | ||
| 4 Adverse Effects: 1b. Any change in specific adverse effects ‐ additional medication use ‐ benzodiazepine use at least once Show forest plot | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.3 [0.59, 2.86] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: Average endpoint global state score ‐ Unwell Show forest plot | 3 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.80, 1.17] |
| 2 Mental state: Average endpoint general mental state score ‐ Not improved Show forest plot | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.45, 2.07] |
| 3 Leaving the study early (any reason) Show forest plot | 4 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 2.15 [0.49, 9.41] |
| 4 Adverse Effects: 1a. Any general adverse effects ‐ side effects reported Show forest plot | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.82, 2.18] |
| 5 Adverse Effects: 1b. Any change in specific adverse effects ‐ individual side effects Show forest plot | Other data | No numeric data | ||

