Zuclopenthixoldihydrochlorid zur Behandlung von Schizophrenie
Abstract
Background
Oral zuclopenthixol dihydrochloride (Clopixol) is an anti‐psychotic treatment for people with psychotic symptoms, especially those with schizophrenia. It is associated with neuroleptic malignant syndrome, a prolongation of the QTc interval, extra‐pyramidal reactions, venous thromboembolism and may modify insulin and glucose responses.
Objectives
To determine the effects of zuclopenthixol dihydrochloride for treatment of schizophrenia.
Search methods
We searched the Cochrane Schizophrenia Group's Trials Register (latest search 09 June 2015). There were no language, date, document type, or publication status limitations for inclusion of records in the register.
Selection criteria
All randomised controlled trials (RCTs) focusing on zuclopenthixol dihydrochloride for schizophrenia. We included trials meeting our inclusion criteria and reporting useable data.
Data collection and analysis
We extracted data independently. For binary outcomes, we calculated risk ratio (RR) and its 95% confidence interval (CI), on an intention‐to‐treat basis. For continuous data, we estimated the mean difference (MD) between groups and its 95% CI. We employed a random‐effect model for analyses. We assessed risk of bias for included studies and created 'Summary of findings' tables using GRADE.
Main results
We included 20 trials, randomising 1850 participants. Data were reported for 12 comparisons, predominantly for the short term (up to 12 weeks) and inpatient populations. Overall risk of bias for included studies was low to unclear.
Data were unavailable for many of our pre‐stated outcomes of interest. No data were available, across all comparisons, for death, duration of stay in hospital and general functioning.
Zuclopenthixol dihydrochloride versus:
1. placebo
Movement disorders (EPSEs) were similar between groups (1 RCT, n = 28, RR 6.07 95% CI 0.86 to 43.04 very low‐quality evidence). There was no clear difference in numbers leaving the study early (2 RCTs, n = 100, RR 0.29, 95% CI 0.01 to 6.60, very low‐quality evidence).
2. chlorpromazine
No clear differences were found for the outcomes of global state (average CGI‐SI endpoint score) (1 RCT, n = 60, MD 0.00, 95% CI ‐0.49 to 0.49) or movement disorders (EPSEs) (3 RCTs, n = 199, RR 0.94, 95% CI 0.61 to 1.45), both very low‐quality evidence. More people left the study early for any reason from the zuclopenthixol group (6 RCTs, n = 766, RR 0.54, 95% CI 0.36 to 0.81, low‐quality evidence).
3. chlorprothixene
There was no clear difference in numbers leaving the study early for any reason (1 RCT, n = 20, RR 1.00, 95% CI 0.34 to 2.93, very low‐quality evidence).
4. clozapine
No useable data were presented.
5. haloperidol
No clear differences between treatment groups were found for the outcomes global state score (average CGI endpoint score) (1 RCT, n = 49, MD 0.13, 95% CI ‐0.30 to 0.55) or leaving the study early (2 RCTs, n = 141, RR 0.99, 95% CI 0.72 to 1.35), both very low‐quality evidence.
6. perphenazine
Those receiving zuclopenthixol were more likely to require medication in the short term for EPSEs than perphenazine (1 RCT, n = 50, RR 1.90, 95% CI 1.12 to 3.22, very low‐quality evidence). Similar numbers left the study early (2 RCTs, n = 104, RR 0.63, 95% CI 0.27 to 1.47, very low‐quality evidence).
7. risperidone
Those receiving zuclopenthixol were more likely to require medications for EPSEs than risperidone (1 RCT, n = 98,RR 1.92, 95% CI 1.12 to 3.28, very low quality evidence). There was no clear difference in numbers leaving the study early ( 3 RCTs, n = 154, RR 1.30, 95% CI 0.84 to 2.02) or in mental state (average PANSS total endpoint score) (1 RCT, n = 25, MD ‐3.20, 95% CI ‐7.71 to 1.31), both very low‐quality evidence).
8. sulpiride
No clear differences were found for global state (average CGI endpoint score) ( 1 RCT, n = 61, RR 1.18, 95% CI 0.49 to 2.85, very low‐quality evidence), requiring hypnotics/sedatives (1 RCT, n = 61, RR 0.60, 95% CI 0.27 to 1.32, very low‐quality evidence) or leaving the study early (1 RCT, n = 61, RR 2.07 95% CI 0.97 to 4.40, very low‐quality evidence).
9. thiothixene
No clear differences were found for the outcomes of 'global state (average CGI endpoint score) (1 RCT, n = 20, RR 0.50, 95% CI 0.17 to 1.46) or leaving the study early (1 RCT, n = 20, RR 0.57, 95% CI 0.24 to 1.35), both very low‐quality evidence).
10. trifluoperazine
No useable data were presented.
11. zuclopenthixol depot
There was no clear difference in numbers leaving the study early (1 RCT, n = 46, RR 1.95, 95% CI 0.36 to 10.58, very low‐quality evidence).
12. Zuclopenthixol dihydrochloride (cis z isomer) versus zuclopenthixol (cis z/trans e isomer)
There were no clear differences in reported side‐effects ( 1 RCT, n = 57, RR 1.34, 95% CI 0.82 to 2.18, very low‐quality evidence) and in numbers leaving the study early (4 RCTs, n = 140, RR 2.15, 95% CI 0.49 to 9.41, very low‐quality evidence).
Authors' conclusions
Zuclopenthixol dihydrochloride appears to cause more EPSEs than clozapine, risperidone or perphenazine, but there was no difference in EPSEs when compared to placebo or chlorpromazine. Similar numbers required hypnotics/sedatives when zuclopenthixol dihydrochloride was compared to sulpiride, and similar numbers of reported side‐effects were found when its isomers were compared. The other comparisons did not report adverse‐effect data.
Reported data indicate zuclopenthixol dihydrochloride demonstrates no difference in mental or global states compared to placebo, chlorpromazine, chlorprothixene, clozapine, haloperidol, perphenazine, sulpiride, thiothixene, trifluoperazine, depot and isomers. Zuclopenthixol dihydrochloride, when compared with risperidone, is favoured when assessed using the PANSS in the short term, but not in the medium term.
The data extracted from the included studies are mostly equivocal, and very low to low quality, making it difficult to draw firm conclusions. Prescribing practice is unlikely to change based on this meta‐analysis. Recommending any particular course of action about side‐effect medication other than monitoring, using rating scales and clinical assessment, and prescriptions on a case‐by‐case basis, is also not possible.
There is a need for further studies covering this topic with more antipsychotic comparisons for currently relevant outcomes.
PICOs
Laienverständliche Zusammenfassung
Zuclopenthixoldihydrochlorid zur Behandlung von Schizophrenie
Hintergrund
Schizophrenie ist eine schwere psychische Erkrankung, bei der Menschen Wahnvorstellungen (seltsame Gedanken/Ideen) und/oder Halluzinationen (hören oder sehen von Dinge, die nicht real sind) haben. Diese werden oft als Positivsymptome oder akute Symptome bezeichnet. Auch Negativsymptome oder chronische Symptome werden erlebt, die den akuten Symptomen in der Regel folgen. Dazu zählen sozialer Rückzug, mangelndes Interesse an alltäglichen Aktivitäten, Depression, sowie Probleme mit dem Gedächtnis und der Gedankenverarbeitung.
Die Behandlung besteht in der Regel aus einem Bündel von Maßnahmen: Der Versorgung mit Medikamenten wie Antipsychotika (Neuroleptika), bei Bedarf zusätzliche Therapien wie kognitive Verhaltenstherapie, Psychoedukation oder Ergotherapie.
Es gibt viele verschiedene Antipsychotika; es ist wichtig zu wissen, wie wirksam sie jeweils sind, verglichen mit keiner Behandlung oder Placebo (Scheinbehandlung) und mit anderen Antipsychotika. Dieser Review vergleicht die orale Verabreichung des Antipsychotikums Zuclopenthixoldihydrochlorid mit anderen Antipsychotika und Placebo.
Suche nach Evidenz
Eine elektronische Suche nach Studien wurde am 9. Juni 2015 durchgeführt, die Menschen mit Schizophrenie in Behandlungsgruppen randomisierten, die entweder Zuclopenthixoldihydrochlorid oder Placebo oder ein anderes Antipsychotikum erhielten.
Gefundene Evidenz
Die Autoren des Reviews fanden 20 Studien mit 1850 Teilnehmern. Die meisten dieser Teilnehmer waren Patienten in psychiatrischen Kliniken.
Die Studien verglichen oral verabreichtes Zuclopenthixoldihydrochlorid mit Placebo und neun anderen oralen Antipsychotika (Chlorpromazin, Chlorprothixen, Clozapin, Haloperidol, Perphenazin, Risperidon, Sulpirid, Thiothixen und Trifluoperazin). Es gab auch Studien, die oral verabreichtes Zuclopenthixoldihydrochlorid mit einer Injektion von Zuclopenthixoldihydrochlorid verglichen und einige, die zwei verschiedene Präparate von Zuclopenthixoldihydrochlorid verglichen.
Die Review‐Autoren waren daran interessiert, Evidenz über die Wirkung von Zuclopenthixoldihydrochlorid bezüglich sieben Hauptendpunkten zu finden: Allgemeinzustand, psychische Verfassung, unerwünschte Wirkungen, Sterblichkeit, Dauer des Krankenhausaufenthalts, vorzeitiges Verlassen der Studie und allgemeine Funktionsfähigkeit. Leider waren viele Daten aus den Studien unbrauchbar, Daten waren nur für Allgemeinzustand, psychische Verfassung, vorzeitiges Verlassen der Studie und unerwünschte Wirkungen bezüglich Bewegungsstörungen verfügbar. Die Studien, die Zuclopenthixoldihydrochlorid mit Sulpirid und Trifluoperazin verglichen, enthielten keine nutzbaren Daten für irgendeinen dieser Hauptendpunkte.
Die gesamten Ergebnisse legen nahe, dass die Wirkung von Zuclopenthixoldihydrochlorid auf den Allgemeinzustand, die psychische Verfassung und die Anzahl der Teilnehmer, die die Studie vorzeitig verließen ähnlich groß ist, wie bei den anderen, oben aufgeführten Antipsychotika.
Zuclopenthixoldihydrochlorid könnte mehr Bewegungsstörungen auslösen als Clozapin, Risperidon oder Perphenazin, aber es gab keinen Unterschied im Vergleich mit anderen Medikamenten oder Placebo.
Schlussfolgerungen
Die derzeit verfügbare Evidenz ist von sehr niedriger bis niedriger Qualität und lässt keine eindeutigen Schlüsse zu. Für viele wichtige Endpunkte liegen keine Daten vor, so dass Rückschlüsse auf die Gesamtwirksamkeit von Zuclopenthixoldihydrochlorid schwierig sind.
Die verfügbare Evidenz legt nahe, dass Zuclopenthixoldihydrochlorid nicht schlechter als andere Antipsychotika bei Behandlung der Symptome von Schizophrenie ist. Weitere Studien, die Daten von guter Qualität liefern sind allerdings nötig, bevor belastbare Schlussfolgerungen gezogen werden können.
Authors' conclusions
Summary of findings
| Patient or population: people with schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with PLACEBO (short term) | Risk with ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Adverse effects: Clinically important general adverse effect (extrapyramidal effects ‐ UKU side effect rating scale) | 80 per 1000 | 486 per 1000 | RR 6.07 | 28 | ⊕⊝⊝⊝ | Risk assumed to be moderate and rounded from 7.69% to 8%. |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Service outcomes: Duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Leaving the study early (any reason) | 40 per 1000 | 12 per 1000 | RR 0.29 | 100 | ⊕⊝⊝⊝ | Risk assumed to be moderate and rounded from 3.85% to 4%. Low number of events in both RCTs. |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'very serious' ‐ possible selection bias, blinding may have been single, attrition bias and reporting bias. 2 Risk of bias: rated 'very serious' ‐ selection, attrition and reporting bias 3 Risk of inconsistency: rated 'not serious' ‐ suspected but not found. 4 Risk of publication bias: rated 'strongly suspected' ‐ multiple papers published with the same patient cohort. 5 Risk of large effect: rated 'very large' ‐ RR 6.07, small n number but result likely when versus placebo. 6 Risk of imprecision: rated 'very serious' ‐ low n numbers | ||||||
| Patient or population: schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with CHLORPROMAZINE (short term) | Risk with ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (CGI‐SI, high score not reported, average score = 2.2) | The mean CGI‐SI endpoint score in the intervention group (MD) was 0 (‐0.49 lower to 0.49 higher) | ‐ | 64 | ⊕⊝⊝⊝ | Translated study. | |
| Adverse effects: Clinically important general adverse effect (EPSEs) | 300 per 1000 | 282 per 1000 | RR 0.94 | 199 | ⊕⊝⊝⊝ | Risk control rounded to 30% and set to moderate. Mixture of inpatient and outpatient, though predominantly hospitalised patients. |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (BPRS, high score = 34.2) | The mean mental state: average endpoint score (BPRS, high score = 34.2) in the intervention group was 0.4 more (2.43 fewer to 3.23 more) | ‐ | 221 | ⊕⊕⊝⊝ | Mixture of inpatient and outpatient, though predominantly hospitalised patients. | |
| Leaving the study early (any reason) | 70 per 1000 | 38 per 1000 | RR 0.54 | 766 | ⊕⊕⊝⊝ | Mixture of inpatient and outpatient, though predominantly hospitalised patients. Risk control set to moderate and rounded to 7%; extreme values not likely. |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'serious' ‐ Selection and attrition bias likely. 2 Risk of bias: rated 'serious' ‐ Selection attrition bias. 3 Risk of bias: rated 'serious' ‐ Selection, attrition and reporting bias. 4 Risk of bias: rated 'serious' ‐ Selection, attrition and reporting bias. Risk of inconsistency: rated as 'serious' ‐ All three papers reported on differing population sizes and obtained different levels of EPSEs in the experimental and control groups. 5 Risk of imprecision: rated 'very serious' ‐ low n numbers 6 Risk of indirectness: rated 'very serious' ‐ mixed samples For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | ||||||
| Patient or population: schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with CHLORPROTHIXENE (medium term) | Risk with ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Adverse effects: Clinically important general adverse effect (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Leaving the study early (any reason) | 400 per 1000 | 400 per 1000 | RR 1.00 | 20 | ⊕⊝⊝⊝ | |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated as 'serious' ‐ selection, reporting bias likely. 2 Risk of publication bias: rated as 'strongly suspected' ‐ several papers published using the same cohort of patients. 3 Risk of imprecision: rated 'very serious' ‐ low n numbers For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | ||||||
| Patient or population: schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with CLOZAPINE (short term) | Risk with ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Adverse effects: Clinically important general adverse effect (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Leaving the study early (any reason) | 0 per 1000 | 0 per 1000 | not estimable | 407 | ⊕⊕⊝⊝ | Multi‐centre, multi‐drug trial with disproportionate numbers of people in different arms of the study. The authors did not report that anybody left the study in the Zuclopenthixol and clozapine arms. |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated as 'serious' ‐ selection, attrition, reporting and performance bias likely. 2 Risk of indirectness: rated 'serious' ‐ Multiple study arms For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | ||||||
| Patient or population: schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with HALOPERIDOL (short term) | Risk with ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (CGI, mean score = 1.25) | The mean CGI endpoint score in the intervention group (MD) was 0.13 more (‐0.3 fewer to 0.55 more) | ‐ | 49 | ⊕⊝⊝⊝ | Small study, multiple scales used (NOSIE30, BPRS, CGI). Paper only reported outcomes of some of these scales. | |
| Adverse effects: Clinically important general adverse effect (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Leaving the study early (any reason) | 300 per 1000 | 297 per 1000 | RR 0.99 | 141 | ⊕⊝⊝⊝ | Risk control rounded to 30% from 29.25% and, as extreme values unlikely, it was set to moderate. |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated as 'very serious' ‐ likely selection, attrition, reporting and diagnostic purity bias. 2 Risk of bias: rated as 'serious' ‐ likely selection, attrition, reporting and diagnostic purity bias. Risk of inconsistency: rated as 'serious' ‐ both papers generated differing values for people leaving the study and do not appear consistent (face validity). 3 Risk of imprecision: rated 'serious' ‐ low n numbers 4 Risk of indirectness: rated 'serious' ‐ not all scales reported For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | ||||||
| Patient or population: schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with PERPHENAZINE (short and medium term) | Risk with ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Adverse effects: Clinically important general adverse effect (EPSEs requiring medication ‐ medium term) | 400 per 1000 | 760 per 1000 | RR 1.90 | 50 | ⊕⊝⊝⊝ | |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Leaving the study early (any reason, short and medium term) | 207 per 1000 | 130 per 1000 | RR 0.63 | 104 | ⊕⊝⊝⊝ | |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated as 'serious' ‐ likely selection and reporting bias. 2 Risk of imprecision: rated 'very serious' ‐ low n numbers For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | ||||||
| Patient or population: schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with RISPERIDONE (short and medium term) | Risk with ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Adverse effects: Clinically important general adverse effect ‐ short term (EPSEs requiring medication) | 271 per 1000 | 520 per 1000 | RR 1.92 | 98 | ⊕⊝⊝⊝ | |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (PANSS, average score = 20.5) ‐ medium term | The mean PANNS endpoint score in the intervention group (MD) was 3.2 fewer (‐7.71 fewer to 1.31 more) | ‐ | 25 | ⊕⊝⊝⊝ | Small study. Standard deviations may be standard errors. Open label trial. | |
| Leaving the study early (any reason, short and medium term) | 310 per 1000 | 403 per 1000 | RR 1.30 | 154 | ⊕⊝⊝⊝ | Risk control taken as mean of extremes: high + low / 2 (changed from 21.43% to 31%) |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated as 'very serious' ‐ open label trial, likely selection bias. 2 Risk of imprecision: rated as 'very serious' ‐ strongly suspect reported standard deviations are standard errors, low n numbers 3 Risk of publication bias: rated as 'strongly suspected' ‐ all studies published findings across several consecutive years in different journals and at conferences. 4 Risk of bias: rated as 'very serious' ‐ study 1 (selection, reporting, diagnostic purity and attrition bias likely ‐ author emailed); study 2 (open label); study 3 (reporting and attrition bias). 5 Risk of publication bias: rated as 'strongly suspected' ‐ multiple papers over consecutive years published with the same data. 6 Risk of bias: rated as 'serious' ‐ diagnostic purity and attrition bias; likely selection and reporting bias (authors contacted) For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | ||||||
| Patient or population: schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with SULPIRIDE (short term) | Risk with ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (CGI ‐ unchanged/worse) | 226 per 1000 | 266 per 1000 | RR 1.18 | 61 | ⊕⊝⊝⊝ | The study did not report clinical improvement so unchanged/worse is reported for this outcome. Usually we report clinical improvement. |
| Adverse effects: Clinically important general adverse effect ‐ requiring hypnotics/sedatives | 419 per 1000 | 252 per 1000 | RR 0.60 | 61 | ⊕⊝⊝⊝ | |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (BPRS) | The mean BPRS endpoint score in the intervention group (MD) was 1.3 fewer (‐5.08 fewer to 2.48 more) | ‐ | 61 | ⊕⊝⊝⊝ | ||
| Leaving the study early (any reason) | 230 per 1000 | 476 per 1000 | RR 2.07 | 61 | ⊕⊝⊝⊝ | Control of risk rounded up from 22.58% to 23%. |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated as 'serious' ‐ diagnostic purity, attrition bias. Per‐Protocol analysis suspected. 2 Risk of imprecision: rated as ' very serious' ‐ percentages used to describe data and comparisons are made against an assumption of baseline measurements. Low n numbers. For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | ||||||
| Patient or population: schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with THIOTHIXENE (medium term) | Risk with ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (unchanged/worse ‐ CGI) | 600 per 1000 | 300 per 1000 | RR 0.50 | 20 | ⊕⊝⊝⊝ | The study did not report clinical improvement so unchanged/worse is reported for this outcome. Usually we report clinical improvement. |
| Adverse effects: Clinically important general adverse effect (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported on this outcome. | |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported on this outcome. | |
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported on this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported on this outcome. | |
| Leaving the study early (any reason) | 700 per 1000 | 399 per 1000 | RR 0.57 | 20 | ⊕⊝⊝⊝ | |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported on this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated as 'serious' ‐ likely selection and reporting bias. 2 Risk of publication bias: rated as 'strongly suspected' ‐ several papers published using the same cohort. 3 Risk of imprecision: rated as 'serious' ‐ low n numbers For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | ||||||
| Patient or population: schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with TRIFLUOPERAZINE (short term) | Risk with ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Adverse effects: Clinically important general adverse effect (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Leaving the study early (any reason) | 0 per 1000 | 0 per 1000 | not estimable | 72 | ⊕⊝⊝⊝ | Multi‐centre and multi‐drug trial with low numbers in each arm. |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated as 'serious' ‐ selection, attrition, reporting and performance bias likely. 2 Risk of imprecision: rated 'very serious' ‐ low n numbers 3 Risk of indirectness: rated 'very serious' ‐ multiple arms, some missing For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | ||||||
| Patient or population: schizophrenia | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | ||
| Risk with ZUCLOPENTHIXOL DEPOT (long term) | Risk with ZUCLOPENTHIXOL | ||||||
| Global state: Average endpoint global state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | ||
| Adverse effects: Clinically important general adverse effect (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | ||
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | ||
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | ||
| Mental state: Average endpoint general mental state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | ||
| Leaving the study early (any reason) | 80 per 1000 | 156 per 1000 | RR 1.95 | 46 | ⊕⊝⊝⊝ | Control of risk rounded from 7.69% to 8%. | |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1 Risk of bias: rated as 'very serious' ‐ single researcher did randomisation, selection bias likely, open label trial and incomplete outcome data. 2 Risk of publication bias: rated as 'strongly suspected' ‐ several papers published using the same data and cohort. 3 Risk of imprecision: rated 'very serious' ‐ low n numbers For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | |||||||
| Patient or population: schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with CIS(Z)/TRANS(E) ZUCLOPENTHIXOL (short term) | Risk with CIS‐(Z) ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Adverse effects: Clinically important general adverse effect | 470 per 1000 | 630 per 1000 | RR 1.34 | 57 | ⊕⊝⊝⊝ | Control of risk set at moderate and rounded up from 46.4% to 47%. |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Leaving the study early: Any reason | 28 per 1000 | 61 per 1000 | RR 2.15 | 140 | ⊕⊝⊝⊝ | |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated as 'serious' ‐ selection bias throughout. Very difficult to ascertain from published materials if selection bias has been minimised. 2 Risk of imprecision: rated 'very serious' ‐ low n numbers For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | ||||||
Background
Description of the condition
Schizophrenia afflicts about 1% of the world's population across geographical, racial and gender barriers (Jablensky 1992).
It is characterised by distortions in thinking and perception in the context of inappropriate and/or blunted affects. It often includes the following psychopathological phenomena:
-
thought echo, thought insertion/withdrawal, thought broadcasting;
-
delusional perception and delusions of control, influence or passivity;
-
hallucinations (often auditory);
-
thought disorders;
-
negative symptoms.
The course of the illness can be continuous or episodic and outcomes are polymorphic (WHO 2010).
Description of the intervention
There is one oral preparation (marketed as Cisordinol, Clopixol) and two depot forms of zuclopenthixol dihydrochloride: zuclopenthixol acetate (Cisordinol‐Acutard, Clopixol‐Acuphase, Clopixol‐Acutard), and zuclopenthixol decanoate (Cisordinol depot, Clopixol depot, Clopixol Inj.). Zuclopenthixol dihydrochloride (Clopixol tablets) was manufactured by Lundbeck Pharmaceuticals, licensed in 1982 and is currently approved for use in 71 countries.
How the intervention might work
Zuclopenthixol belongs to the class of thioxanthene derivatives. Zuclopenthixol is the cis (Z)‐isomer of clopenthixoland has a high affinity for both dopamine D1 and D2 receptors. It is rapidly absorbed from the gastrointestinal tract and maximum serum concentration occurs after about four hours following a single oral dose. The elimination half‐life is approximately 20 hours (range 12 to 28 hours). It has a moderately high pre‐systemic metabolism and about 40% of the drug is eliminated this way (Dollery 1999).
Why it is important to do this review
Antipsychotic medication remains a key means of treating people with schizophrenia (Awad 1997; Dally 1967). Since the introduction of chlorpromazine in the early 1950's numerous antipsychotic drugs have been developed. These include haloperidol, thioridazine, trifluoperazine and zuclopenthixol and they are now inexpensive and accessible to millions. Zuclopenthixol has been licensed since the early 1980's and is used in at least 71 countries including many European countries, Australia, New Zealand and Canada.
A newer generation of so called 'atypical' antipsychotics have been developed and are increasingly prevalent for treating schizophrenia, especially in high‐income countries (Taylor 2000). This increase in the use of newer drugs is mirrored by a decline in the use of the older generation antipsychotics such as zuclopenthixol dihydrochloride although they are still used for treating schizophrenia and related psychotic conditions (Kozyrev 2003; Mace 2005; Percudani 2005). However, we have found it difficult to identify reviews on the effects of the oral preparation and we found no relevant meta‐analyses. This Cochrane review is the third review relevant to this compound and concentrates on the oral form of zuclopenthixol dihydrochloride. The first review investigated the effects of zuclopenthixol acetate for rapid tranquillisation of disturbed people with serious mental illnesses (Jayakody 2012), and the second concentrates on the decanoate preparation of zuclopenthixol as this is used as a long‐acting depot preparation (da Silva Freire Coutinho 1999).
Objectives
To determine the effects of zuclopenthixol dihydrochloride for the treatment of schizophrenia.
Methods
Criteria for considering studies for this review
Types of studies
We included all relevant randomised controlled trials. Where a trial was described as 'double‐blind' but it was implied that the study was randomised, we included these trials in a sensitivity analysis. If there was no substantive difference within primary outcomes (see Types of outcome measures) when these 'implied randomisation' studies were added, then we included these in the final analysis. If there was a substantive difference, only clearly randomised trials were utilised and the results of the sensitivity analysis described in the text. We excluded quasi‐randomised studies such as those allocating by using alternate days of the week.
Types of participants
We included people with schizophrenia and schizophrenia‐like disorders such as schizophreniform disorder, delusional disorder or schizoaffective disorder, diagnosed by any criteria. We also included those with 'serious/chronic mental illness' or 'psychotic illness'. Where possible we excluded people with dementia, depression and problems primarily associated with substance misuse.
Types of interventions
1. Zuclopenthixol dihydrochloride administered in oral form, any dose.
2. Placebo or no treatment.
3. Other antipsychotic drugs: any dose, administered in depot form.
4. Other antipsychotic drugs: any dose, administered in oral form.
Types of outcome measures
All outcomes were reported for the short term (up to 12 weeks), medium term (13 to 26 weeks), and long term (more than 26 weeks). None of the included studies went beyond one year.
Primary outcomes
1. Global state: average endpoint global state score
2. Adverse effects: clinically important general adverse effect
Secondary outcomes
1. Death ‐ suicide and natural causes
2. Global state
2. 1 Relapse
2. 2 Time to relapse
2. 3 Clinically important change in global state
2. 4 Any change in global state
2. 5 Average change in global state scores
3. Service outcomes
3. 1 Hospitalisation
3. 2 Time to hospitalisation
3. 3 Duration of stay in hospital
4. Mental state
4. 1 Clinically important change in general mental state
4. 2 Any change in general mental state
4. 3 Average endpoint general mental state score
4. 4 Average change in general mental state scores
4. 5 Clinically important change in specific symptoms
4. 6 Any change in specific symptoms
4. 7 Average endpoint specific symptom score
4. 8 Average change in specific symptom scores
5. Leaving the study early
5. 1 For specific reasons
5. 2 For general reasons
6. General functioning
6. 1 Clinically important change in general functioning
6. 2 Any change in general functioning
6. 3 Average endpoint general functioning score
6. 4 Average change in general functioning scores
6. 5 Clinically important change in specific aspects of functioning, such as social or life skills
6. 6 Any change in specific aspects of functioning, such as social or life skills
6. 7 Average endpoint specific aspects of functioning, such as social or life skills
6. 8 Average change in specific aspects of functioning, such as social or life skills
7. Behaviour
7. 1 Clinically important change in general behaviour
7. 2 Any change in general behaviour
7. 3 Average endpoint general behaviour score
7. 4 Average change in general behaviour scores
7. 5 Clinically important change in specific aspects of behaviour
7. 6 Any change in specific aspects of behaviour
7. 7 Average endpoint specific aspects of behaviour
7. 8 Average change in specific aspects of behaviour
8. Adverse effects
8. 1 Any general adverse effects
8. 2 Average endpoint general adverse effect score
8. 3 Average change in general adverse effect scores
8. 4 Clinically important change in specific adverse effects
8. 5 Any change in specific adverse effects
8. 6 Average endpoint specific adverse effects
8. 7 Average change in specific adverse effects
9. Engagement with services
9. 1 Clinically important engagement
9. 2 Not any engagement
9. 3 Average endpoint engagement score
9. 4 Average change in engagement scores
10. Satisfaction with treatment
10. 1 Recipient of care satisfied with treatment
10. 2 Recipient of care average satisfaction score
10. 3 Recipient of care average change in satisfaction scores
10. 4 Carer satisfied with treatment
10. 5 Carer average satisfaction score
10. 6 Carer average change in satisfaction scores
11. Quality of life
11. 1 No clinically important change in quality of life
11. 2 Not any change in quality of life
11. 3 Average endpoint quality of life score
11. 4 Average change in quality of life scores
11. 5 No clinically important change in specific aspects of quality of life
11. 6 Not any change in specific aspects of quality of life
11. 7 Average endpoint specific aspects of quality of life
11. 8 Average change in specific aspects of quality of life
12. Economic outcomes
12. 1 Direct costs
12. 2 Indirect costs
Summary of findings
We used the GRADE approach to interpret findings (Schünemann 2011) and used the GRADE profiler to import data from Review Manager (RevMan) to create 'Summary of findings' tables. These tables provide outcome‐specific information concerning the overall quality of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on all outcomes we rated as important to patient‐care and decision making.
We selected the following main outcomes for inclusion in the 12 'Summary of findings' tables.
-
Global state: Average endpoint global state score (clinically improved) [primary outcome]
-
Adverse effects: Clinically important general adverse effect [primary outcome]
-
Death: Suicide and natural causes [secondary outcome]
-
Service outcome: Duration of stay in hospital [secondary outcome]
-
Mental state: Average endpoint general mental state score (clinically improved) [secondary outcome]
-
Leaving the study early: any reason [secondary outcome]
-
General functioning: Average endpoint general functioning score (clinically improved) [secondary outcome]
Search methods for identification of studies
Electronic searches
Cochrane Schizophrenia Group’s Trials Register
On June 09, 2015, the Trials Search Co‐ordinator (TSC) searched the Cochrane Schizophrenia Group’s Study‐Based Register of Trials using the following search strategy which has been developed based on literature review and consulting with the authors of the review:
*Zuclopenthixol* in Intervention of STUDY
In such a study‐based register, searching the major concept retrieves all the relevant keywords and studies because all the studies have already been organised based on their interventions and linked to the relevant topics.
The Cochrane Schizophrenia Group’s Register of Trials is compiled by systematic searches of major resources (including AMED, BIOSIS, CINAHL, Embase, MEDLINE, PsycINFO, PubMed, and registries of clinical trials) and their monthly updates, handsearches, grey literature, and conference proceedings (see Group Module). There is no language, date, document type, or publication status limitations for inclusion of records into the register.
For previous searches, please see Appendix 1.
Searching other resources
1. Reference searching
We inspected references of all included studies for further relevant studies.
2. Personal contact
Where necessary, we contacted the first author of each included study for information regarding unpublished trials. We noted the outcome of this contact in the included or awaiting assessment studies tables.
Data collection and analysis
For previous data collection and analysis see Appendix 2.
Selection of studies
For this update, review author EJB independently inspected citations from the searches and identified relevant abstracts. A random 20% sample was independently re‐inspected by Dr Marie Ann Purcell (MAP) to ensure reliability. Where disputes arose, the full report was acquired for more detailed scrutiny. Full reports of the abstracts meeting the review criteria were obtained and inspected by EJB. Again, a random 20% of full reports were‐inspected by MAP in order to ensure reliable selection. Where it was not possible to resolve disagreement by discussion, we attempted to contact the authors of the study for clarification.
Data extraction and management
1. Extraction
For this update, review author EJB extracted data from all included studies. In addition, to ensure reliability, JX (see Acknowledgements) independently extracted data from a random sample of these studies, comprising 10% of the total. Again, any disagreements were discussed, decisions documented and, if necessary, authors of studies contacted for clarification. With remaining problems MAP helped clarify issues and these final decisions were documented. Data presented only in graphs and figures were to be extracted whenever possible, but included only if two review authors independently had the same result. We attempted to contact authors through an open‐ended request in order to obtain missing information or for clarification whenever necessary. If studies were multi‐centre, where possible, we extracted data relevant to each component centre separately.
2. Management
2.1 Forms
We extracted data onto simple standard forms.
2.2 Scale‐derived data
We included continuous data from rating scales only if:
a) the psychometric properties of the measuring instrument have been described in a peer‐reviewed journal (Marshall 2000); and
b) the measuring instrument has not been written or modified by one of the trialists for that particular trial.
Ideally, the measuring instrument should either be i. a self‐report or ii. completed by an independent rater or relative (not the therapist). We realise that this is not often reported clearly, in Description of studies we noted if this was the case or not.
2.3 Endpoint versus change data
There are advantages of both endpoint and change data. Change data can remove a component of between‐person variability from the analysis. On the other hand, calculation of change needs two assessments (baseline and endpoint) which can be difficult in unstable and difficult to measure conditions such as schizophrenia. We decided primarily to use endpoint data, and only use change data if the former were not available. Endpoint and change data were combined in the analysis as we used mean differences (MD) rather than standardised mean differences (SMD) throughout (Higgins 2011).
2.4 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we aimed to apply the following standards to all data before inclusion:
Please note, we entered data from studies of at least 200 participants, for example, in the analysis irrespective of the following rules, because skewed data pose less of a problem in large studies. We also entered change data as when continuous data are presented on a scale that includes a possibility of negative values (such as change data), it is difficult to tell whether data are skewed or not. We presented and entered change data into the statistical analyses.
For endpoint data:
(a) when a scale starts from the finite number zero, we subtracted the lowest possible value from the mean, and divided this by the standard deviation (SD). If this value was lower than 1, it strongly suggests a skew and the study was excluded. If this ratio was higher than 1 but below 2, there is suggestion of skew. We entered the study and tested whether its inclusion or exclusion changed the results substantially. Finally, if the ratio was larger than 2, the study was included, because skew is less likely (Altman 1996; Higgins 2011).
b) if a scale starts from a positive value (such as the Positive and Negative Syndrome Scale (PANSS) (Kay 1987), which can have values from 30 to 210), the calculation described above was modified to take the scale starting point into account. In these cases skew is present if 2 SD > (S‐S min), where S is the mean score and 'S min' is the minimum score.
2.5 Common measure
To facilitate comparison between trials, we intended to convert variables that can be reported in different metrics, such as days in hospital (mean days per year, per week or per month) to a common metric (e.g. mean days per month).
2.6 Conversion of continuous to binary
Where possible, we made efforts to convert outcome measures to dichotomous data. This can be done by identifying cut‐off points on rating scales and dividing participants accordingly into 'clinically improved' or 'not clinically improved'. It is generally assumed that if there is a 50% reduction in a scale‐derived score such as the Brief Psychiatric Rating Scale (BPRS, Overall 1962) or the Positive and Negative Syndrome Scale (PANSS, Kay 1986), this could be considered as a clinically significant response (Leucht 2005; Leucht 2005a). If data based on these thresholds were not available, we used the primary cut‐off presented by the original authors.
2.7 Direction of graphs
Where possible, we entered data in such a way that the area to the left of the line of no effect indicates a favourable outcome for Zuclopenthixol dihydrochloride. Where keeping to this made it impossible to avoid outcome titles with clumsy double‐negatives, we reported data where the left of the line indicates an unfavourable outcome. This is noted in the relevant graphs.
Assessment of risk of bias in included studies
Again, review author EJB worked independently to assess risk of bias by using criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to assess trial quality. This set of criteria is based on evidence of associations between overestimate of effect and high risk of bias of the article such as sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting. A random 20% was screened for validity independently by MAP.
If the raters disagreed, the final rating was made by consensus, with the involvement of another member of the review group. Where inadequate details of randomisation and other characteristics of trials were provided, we contacted the authors of the studies in order to obtain further information. We reported non‐concurrence in quality assessment , but if disputes arose as to which category a trial was to be allocated, again, we resolved this by discussion.
We noted the level of risk of bias in both the text of the review and in the 'Summary of findings' tables.
Measures of treatment effect
1. Binary data
For binary outcomes we calculated a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI). It has been shown that RR is more intuitive (Boissel 1999) than odds ratios and that odds ratios tend to be interpreted as RR by clinicians (Deeks 2000). The number needed to treat for an additional beneficial outcome/number needed to treat for an additional harmful outcome (NNTB/NNTH) statistic with its confidence intervals is intuitively attractive to clinicians but is problematic both in its accurate calculation in meta‐analyses and interpretation (Hutton 2009). For binary data presented in the 'Summary of findings' tableS, where possible, we calculated illustrative comparative risks.
2. Continuous data
For continuous outcomes we estimated mean difference (MD) between groups. We preferred not to calculate effect size measures (standardised mean difference (SMD)). However, if scales of very considerable similarity were used, we presumed there was a small difference in measurement, and we calculated effect size and transformed the effect back to the units of one or more of the specific instruments.
Unit of analysis issues
1. Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice), but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intra‐class correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992) whereby P values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997; Gulliford 1999).
Where clustering was not accounted for in primary studies, we presented data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review we will seek to contact first authors of studies to obtain intra‐class correlation coefficients (ICCs) for their clustered data and to adjust for this by using accepted methods (Gulliford 1999). Where clustering had been incorporated into the analysis of primary studies, we presented these data as if from a non‐cluster randomised study, but adjusted for the clustering effect.
We sought statistical advice and have been advised that the binary data as presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the ICC [Design effect = 1+(m‐1)*ICC] (Donner 2002). If the ICC was not reported it was be assumed to be 0.1 (Ukoumunne 1999).
If cluster studies have been appropriately analysed taking into account ICCs and relevant data documented in the report, synthesis with other studies was possible using the generic inverse variance technique.
2. Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, on entry to the second phase the participants can differ systematically from their initial state despite a wash‐out phase. For the same reason cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in severe mental illness, we only used data of the first phase of cross‐over studies.
3. Studies with multiple treatment groups
Where a study involved more than two treatment arms, if relevant, the additional treatment arms were presented in the comparisons. If data were binary, we simply added and combined them within the two‐by‐two table. If data were continuous, we combined data following the formula in section 7.7.3.8 (Combining groups) of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Where the additional treatment arms were not relevant, we did not use these data.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss of follow‐up, data must lose credibility (Xia 2009). We chose that, for any particular outcome, should more than 50% of data be unaccounted for, we would not reproduce these data or use them within the analyses. If, however, more than 50% of those in one arm of a study were lost, but the total loss was less than 50%, we addressed this within the 'Summary of findings' tables by down‐rating quality. Finally, we also downgraded quality within the 'Summary of findings' tables should loss be 25% to 50% in total.
2. Binary
In the case where attrition for a binary outcome was between 0% and 50% and where these data were not clearly described, we presented data on a 'once‐randomised‐always‐analyse' basis (an intention‐to‐treat (ITT) analysis). Those leaving the study early were all assumed to have the same rates of negative outcome as those who completed, with the exception of the outcome of death and adverse effects. For these outcomes, the rate of those who stayed in the study ‐ in that particular arm of the trial ‐ were used for those who did not. We undertook a sensitivity analysis to test how prone the primary outcomes were to change when data only from people who completed the study to that point were compared to the ITT analysis using the above assumptions.
3. Continuous
3.1 Attrition
In the case where attrition for a continuous outcome was between 0% and 50%, and data only from people who completed the study to that point were reported, we reproduced these.
3.2 Standard deviations
If standard deviations (SDs) were not reported, we first tried to obtain the missing values from the authors. If not available, where there were missing measures of variance for continuous data, but an exact standard error (SE) and confidence intervals available for group means, and either 'P' value or 't' value available for differences in mean, we could calculate them according to the rules described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011): When only the SE is reported, SDs are calculated by the formula SD = SE * square root (n). Chapters 7.7.3 and 16.1.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) present detailed formulae for estimating SDs from P values, t or F values, confidence intervals, ranges or other statistics. If these formulae did not apply, we calculated the SDs according to a validated imputation method which is based on the SDs of the other included studies (Furukawa 2006). Although some of these imputation strategies can introduce error, the alternative would be to exclude a given study’s outcome and thus to lose information. We nevertheless examined the validity of the imputations in a sensitivity analysis excluding imputed values.
3.3 Assumptions about participants who left the trials early or were lost to follow‐up
Various methods are available to account for participants who left the trials early or were lost to follow‐up. Some trials just present the results of study completers, others use the method of last observation carried forward (LOCF), while more recently methods such as multiple imputation or mixed‐effects models for repeated measurements (MMRM) have become more of a standard. While the latter methods seem to be somewhat better than LOCF (Leon 2006), we feel that the high percentage of participants leaving the studies early and differences in the reasons for leaving the studies early between groups is often the core problem in randomised schizophrenia trials. We therefore did not exclude studies based on the statistical approach used. However, preferably we used the more sophisticated approaches. For example, MMRM or multiple‐imputation were preferred to LOCF and completer analyses were only presented if some kind of ITT data were not available at all. Moreover, we addressed this issue in the item "incomplete outcome data" of the 'Risk of bias' tool.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all included studies initially, without seeing comparison data, to judge clinical heterogeneity. We simply inspected all studies for clearly outlying people or situations which we had not predicted would arise. When such situations or participant groups arose, we fully discussed these.
2. Methodological heterogeneity
We considered all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We simply inspected all studies for clearly outlying methods which we had not predicted would arise. When such methodological outliers arose, we fully discussed these.
3. Statistical heterogeneity
3.1 Visual inspection
We visually inspected graphs to investigate the possibility of statistical heterogeneity.
3.2 Employing the I2 statistic
We investigated heterogeneity between studies by considering the I2 method alongside the Chi2 'P' value. The I2 provides an estimate of the percentage of inconsistency thought to be due to chance (Higgins 2003). The importance of the observed value of I2 depends on i. magnitude and direction of effects and ii. strength of evidence for heterogeneity (e.g. 'P' value from Chi2 test, or a confidence interval for I2). An I2 estimate greater than or equal to around 50% accompanied by a statistically significant Chi2 statistic, was interpreted as evidence of substantial levels of heterogeneity (Section 9.5.2 ‐ Higgins 2011). When substantial levels of heterogeneity were found in the primary outcome, we explored reasons for heterogeneity (Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
1. Protocol versus full study
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results. These are described in section 10.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We attempted to locate protocols of included randomised trials. If the protocol was available, we compared outcomes in the protocol and in the published report. If the protocol was not available, we compared outcomes listed in the methods section of the trial report with actually reported results.
2. Funnel plot
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). Again, these are described in Section 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small‐study effects. We did not use funnel plots for outcomes where there were 10 or fewer studies, or where all studies were of similar sizes. In other cases, where funnel plots were possible, we planned to seek statistical advice in their interpretation.
Data synthesis
We understand that there is no closed argument for preference for use of fixed‐effect or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This often seems to be true to us and the random‐effects model takes into account differences between studies, even if there is no statistically significant heterogeneity. There is, however, a disadvantage to the random‐effects model. It puts added weight onto small studies which often are the most biased ones. Depending on the direction of effect, these studies can either inflate or deflate the effect size. We chose a random‐effects model for all analyses. The reader is, however, able to choose to inspect the data using the fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
1. Subgroup analyses
1.1 Primary outcomes
No subgroup analysis was expected.
1.2 Clinical state, stage or problem
We proposed to undertake this review and provide an overview of the effects of zuclopenthixol dihydrochloride for people with schizophrenia in general. In addition, however, we tried to report data on subgroups of people in the same clinical state, stage and with similar problems. This was not done in this update.
2. Investigation of heterogeneity
If inconsistency was high, this was reported. First, we investigated whether data had been entered correctly. Second, if data were correct, the graph was visually inspected and outlying studies were successively removed to see if homogeneity was restored. For this review we decided that should this occur with data contributing to the summary finding of no more than around 10% of the total weighting, data were presented. If not, data were not pooled and issues discussed. We know of no supporting research for this 10% cut‐off but are investigating the use of prediction intervals as an alternative to this unsatisfactory state.
When unanticipated clinical or methodological heterogeneity were obvious we simply stated hypotheses regarding these for future reviews or versions of this review. We do not anticipate undertaking analyses relating to these.
Sensitivity analysis
1. Implication of randomisation
We aimed to include trials in a sensitivity analysis if they were described in some way as to imply randomisation. For the primary outcomes, we included these studies and if there was no substantive difference when the implied randomised studies were added to those with better description of randomisation, then all data were employed from these studies.
2. Assumptions for lost binary data
Where assumptions had to be made regarding people lost to follow‐up (see Dealing with missing data), we compared the findings of the primary outcomes when we used our assumption/s and when we used data only from people who completed the study to that point. If there was a substantial difference, we reported results and discussed them but continued to employ our assumption.
Where assumptions had to be made regarding missing SDs data (see Dealing with missing data), we compared the findings of the primary outcomes when we used our assumption/s and when we used data only from people who completed the study to that point. A sensitivity analysis was undertaken to test how prone results were to change when completer‐only data only were compared to the imputed data using the above assumption. If there was a substantial difference, we reported results and discussed them but continued to employ our assumption.
3. Risk of bias
We analysed the effects of excluding trials that were judged to be at high risk of bias across one or more of the domains of randomisation (implied as randomised with no further details available) allocation concealment, blinding and outcome reporting for the meta‐analysis of the primary outcome. If the exclusion of trials at high risk of bias did not substantially alter the direction of effect or the precision of the effect estimates, then data from these trials were included in the analysis.
4. Imputed values
We also undertook a sensitivity analysis to assess the effects of including data from trials where we used imputed values for ICC in calculating the design effect in cluster‐randomised trials.
If substantial differences were noted in the direction or precision of effect estimates in any of the sensitivity analyses listed above, we did not pool data from the excluded trials with the other trials contributing to the outcome, but presented them separately.
5. Fixed and random effects
We synthesised all data using a random‐effects model, however, we also synthesised data for the primary outcome using a fixed‐effect model to evaluate whether this altered the significance of the results.
Results
Description of studies
Accross all comparisons there was a significant lack of data for the primary outcomes and the secondary outcomes. The majority of data were for the short term only, for inpatient populations and focused on adverse effects. Most studies were small with the exception of Fischer‐Cornelssen 1976.
Results of the search
Details of the search results are illustrated in the PRISMA tableFigure 1
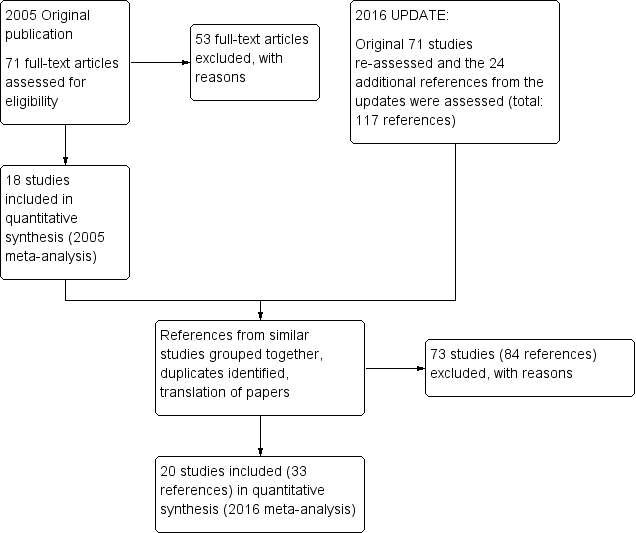
Study flow diagram ‐ update 2016.
After obtaining the initial results of the search, removing duplicates and clearly irrelevant material, the original review authors inspected 79 abstracts. Out of these 79 papers, they selected 71 full‐text papers to be assessed for eligibility. The original authors then grouped these into 'studies' where several of the reports referred to the same trial. Fifty‐three articles were excluded with reasons and 18 studies were included in the 2005 meta‐analysis.
During the update we identified 117 potential reports for the review. We had to exclude 73 (84 reports) of these studies. So, at the end of this review update we have identified 33 reports of 20 trials for inclusion in the 2016 meta‐analysis. At the time of writing zero studies are awaiting assessment and we know of no ongoing studies at the time of writing.
Included studies
We identified 20 studies (from 33 references) spanning the period 1968 to 2008 covering 12 comparisons for inclusion in this update. Fifteen were described as randomised and in the remaining five, randomisation was implied. One trial was a cross‐over trial and one was an open‐label trial. Two authors need to be contacted for additional information at the next update (Arango 2006 and Fagerlund 2004). Ban 1975 data has been updated to reflect the two‐week washout period not originally included in the initial publication.
The trial authors were not always explicit in their descriptions of randomisation or blinding and an assumption of either or both was made based on implication and context of the discussions and/or data. The majority of the data were short term and from inpatient samples. The studies used relatively small cohorts with the exception of Fischer‐Cornelssen 1976.
Where authors published research from the same cohort in several different reports the data were pooled and used only once in the meta‐analysis. Some degree of interpretation on our part was required during this process.
Further information on the included studies can be obtained in the Characteristics of included studies table.
Excluded studies
We excluded 73 studies: two had healthy volunteers, 42 used the wrong intervention (predominantly depot), four used the wrong type of participant, eight were not randomised, 16 did not have any useable data (either data that could be extracted for meta‐analysis or no data provided), and one duplicated data from elsewhere. Galdersi 1994 was originally included but excluded in this update because it was not a randomised trial.
Further information on the excluded studies can be obtained in theCharacteristics of excluded studies table.
Risk of bias in included studies
Most information is from studies at low or unclear risk of bias. It is difficult to provide a generalised assessment of the risk of bias given the low number of studies in this update. We would advise reviewing the 'Risk of bias' tables in the Characteristics of included studies table of the review.
Risk of bias overall: Unclear.
Allocation
Three studies were graded as low risk, 16 as unclear risk and one as high risk. All included studies were randomised or had implied randomisation. Most of the included studies did not explicitly describe the method by which randomisation was done.The majority of patients came from inpatient samples and there was a degree of diagnostic purity to the samples. Review of demographic tables (where published) indicated a roughly equal spread between different arms within the different studies.
Overall risk of selection bias: unclear.
Blinding
Thirteen studies were graded as low risk, four as unclear risk and three as high risk. Two of the studies were open‐label and all included studies used some form of established rating scale or modified rating scale to measure changes in global state, adverse effects and mental state. Several of the papers were vague on their methods for blinding, but it was generally implied in the majority. Only a few papers explicitly stated that blinding was conducted and this was usually mentioned in the title and/or abstract.
Overall risk of performance/detection bias: low risk.
Incomplete outcome data
Nine studies were graded as low risk, five as unclear risk and six as high risk. Reasons for loss to follow‐up are well‐reported and we have recorded these in the outcomes. Most studies, however, have not clearly described how they used data for people who were lost to follow‐up and we found no reporting of attempts to validate any assumptions by following up those who dropped out early. Per‐protocol analysis was used in eight studies.
Overall risk of attrition bias: low risk.
Selective reporting
Five studies were graded as low risk, five as unclear risk and 10 as high risk. Overall, due to poor reporting, we were unable to use a lot of the data (missing data were detected in nine of the studies). Findings presented as graphs, whether as percentiles or as inexact P values, are often of little use to a reviewer. Many studies failed to provide standard deviations when reporting mean changes. Three of the studies published the same data in multiple locations. One study presented its data inaccurately requiring EJB to make a professional judgement on what the authors intended.
Overall risk of reporting bias: high risk.
Other potential sources of bias
Two studies were graded as low risk, two as unclear risk and 16 as high risk. The predominant biases in this category are: response bias, instrument bias, interviewer bias and the Hawthorne effect. The authors of the included studies did not discuss these biases in any detail, if at all.
Overall risk of other bias: high risk.
Effects of interventions
See: Summary of findings for the main comparison Zuclopenthixol dihydrochloride versus placebo; Summary of findings 2 Zuclopenthixol dihydrochloride versus chlorpromazine; Summary of findings 3 Zuclopenthixol dihydrochloride versus chlorprothixene; Summary of findings 4 Zuclopenthixol dihydrochloride versus clozapine; Summary of findings 5 Zuclopenthixol dihydrochloride versus haloperidol; Summary of findings 6 Zuclopenthixol dihydrochloride versus perphenazine; Summary of findings 7 Zuclopenthixol dihydrochloride versus risperidone; Summary of findings 8 Zuclopenthixol dihydrochloride versus sulpiride; Summary of findings 9 Zuclopenthixol dihydrochloride versus thiothixene; Summary of findings 10 Zuclopenthixol dihydrochloride versus trifluoperazine; Summary of findings 11 Zuclopenthixol dihydrochloride versus zuclopenthixol depot; Summary of findings 12 Zuclopenthixol dihydrochloride (Cis Z isomer) versus Zuclopenthixol (Cis Z/Trans E isomer)
Studies relevant to this review fall into 12 comparisons. We identified 20 randomised trials from which it was possible to extract numerical data. These data can be compared to the original published data in Appendix 3.
1. ZUCLOPENTHIXOL versus PLACEBO ‐ all short term
1.1 Leaving the study early (any reason)
For this outcome we found two relevant studies, with a total of 100 people. There was not a clear difference between zuclopenthixol dihydrochloride and placebo (risk ratio (RR) 0.29, 95% confidence interval (CI) 0.01 to 6.60, very low‐quality evidence) (Analysis 1.1).
1.2 Adverse effects: 1. Clinically important change in specific adverse effects ‐ cardiovascular ‐ orthostatic
For this outcome we found a single study (total n = 28). We did not find evidence of a clear difference between the two treatments (RR 0.29, 95% CI 0.01 to 6.60) (Analysis 1.2).
1.3 Adverse effects: 2. Clinically important change in specific adverse effects ‐ central nervous system ‐ arousal state
We identified one study relevant to this outcome.
1.3.1 excitation
We found one trial to be relevant to this subgroup, which included a total of 28 participants. There was not a clear difference (RR 2.62, 95% CI 0.12 to 59.40) (Analysis 1.3).
1.3.2 sleepiness / sedation
We found one trial to be relevant to this subgroup (total n = 28). For this outcome, within this subgroup, we did find evidence that 'zuclopenthixol' was clearly different in its effects compared with 'placebo ‐ all short term' (RR 2.89, 95% CI 1.01 to 8.30). Zuclopenthixol increases the likelihood of sleepiness/sedation when compared to placebo (Analysis 1.3).
1.4 Adverse effects: 3. Clinically important change in specific adverse effects ‐ endocrine ‐ menstruation started
We identified one study relevant to this outcome involving 36 participants. There was not a clear difference (RR 7.00, 95% CI 0.39 to 126.48) (Analysis 1.4).
1.5 Adverse effects: 4a. Any general adverse effects ‐ movement disorders ‐ EPSEs (UKU side effect rating scale, no scores)
There is a single trial (total n = 28), we did not find evidence of a clear difference between the two treatments when measured on the UKU (Lingjaerde 1987) (RR 6.07, 95% CI 0.86 to 43.04, very low‐quality evidence ). (Analysis 1.5).
1.6 Adverse effects: 4b. Clinically important change in specific adverse effects ‐ movement disorders ‐ EPSEs
1.6.1 parkinsonism
There is a single trial in this subgroup, which included a total of 36 participants. For this subgroup, we did not find evidence of a clear difference between the two treatments (RR 5.00, 95% CI 0.26 to 97.37) (Analysis 1.6).
1.6.2 oculogyric crisis
We found one trial to be relevant to this subgroup (total n = 36). For this subgroup, we did not find evidence of a clear difference between the two treatments (RR 3.00, 95% CI 0.13 to 69.09) (Analysis 1.6).
1.6.3 tremor
We found one trial to be relevant to this subgroup (total n = 36). For this subgroup, we did not find evidence of a clear difference between the two treatments (RR 5.00, 95% CI 0.26 to 97.37) (Analysis 1.6).
2. ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term
This comparison has 15 outcomes.
2.1 Global state: 1. Average endpoint global state score ‐ Unchanged/worse (CGI, scores not reported)
For this outcome we found two relevant studies (total n = 135). There was not a clear difference in the CGI (Guy 1976) (RR 0.92, 95% CI 0.75 to 1.13) (Analysis 2.1).
2.2 Global state: 2. Average endpoint global state score ‐ no Recovery
We identified one study relevant to this outcome, with a total of 64 people. We did not find evidence of a clear difference between the two treatments in this comparison (RR 1.02, 95% CI 0.89 to 1.16) (Analysis 2.2).
2.3 Global state: 3a. Average endpoint global state score (GAS, high score not reported, average score = 63.4)
For this outcome we found a single study (total n = 60). We did not find evidence of a clear difference between the two treatments in this comparison (MD ‐0.60 95% CI ‐8.12 to 6.92) (Analysis 2.3).
2.4 Global state: 3b. Average endpoint global state score (CGI‐SI, high score not reported, average score = 2.2)
We identified one study relevant to this outcome, with a total of 60 people. We did not find evidence of a clear difference (mean difference (MD) 0.00, 95% CI ‐0.49 to 0.49, very low‐quality evidence) (Analysis 2.4).
2.5 Mental state: 1. No clinically important change in general mental state ‐ Not improved (PANSS, scores not reported)
We identified one study relevant to this outcome (total n = 120). There was not a clear difference (RR 0.98, 95% CI 0.81 to 1.18) (Analysis 2.5).
2.6 Mental state: 2. No clinically important change in general mental state ‐ No clinical response
We identified one study relevant to this outcome involving 64 participants. We did not find evidence of a clear difference between the two treatments in this comparison (RR 0.78, 95% CI 0.25 to 2.42) (Analysis 2.6).
2.7 Mental state: 3. Average endpoint general mental state score (BPRS, high score = 34.2)
For this outcome we found three relevant studies, with a total of 221 people. There was not a clear difference (MD 0.40, 95% CI ‐2.43 to 3.23) (Analysis 2.7).
2.8 Leaving the study early (any reason)
For this outcome we found six relevant studies (total n = 766). For this outcome, we did find evidence that 'zuclopenthixol' was clearly different in its effects compared with 'chlorpromazine ‐ all short term' (RR 0.54, 95% CI 0.36 to 0.81, low‐quality evidence). Chlorpromazine was more likely to cause patients to leave a study early for any reason (Analysis 2.8).
2.9 Adverse effects: 1. Any general adverse effects ‐ side‐effects (CGI, high score not reported)
For this outcome we found a single study involving 94 participants. For this outcome, we did find evidence that 'zuclopenthixol' was clearly different in its effects compared with 'chlorpromazine ‐ all short term' (RR 0.86, 95% CI 0.77 to 0.97). There is a lower risk of general side‐effects measured on the CGI with zuclopenthixol (Analysis 2.9).
2.10 Adverse effects: 2. Average endpoint general adverse effect score (TESS, high score not reported, average score = 12.00)
For this outcome we found a single study, with a total of 60 people. There was not a clear difference (MD 4.48, 95% CI ‐2.38 to 11.34) (Analysis 2.10).
2.11 Adverse effects: 3. Any change in specific adverse effects ‐ cardiovascular ‐ postural hypotension (dizziness/syncope)
For this outcome we found a single study (total n = 43). There was not a clear difference (RR 0.11, 95% CI 0.01 to 1.73) (Analysis 2.11).
2.12 Adverse effects: 4. Any change in specific adverse effects ‐ central nervous system ‐ arousal
2.12.1 excitation
There is a single trial in this subgroup (total n = 43). For this subgroup, we did not find evidence of a clear difference between the two treatments (RR 0.62, 95% CI 0.07 to 5.47) (Analysis 2.12).
2.12.2 sedation
There are two relevant trials in this subgroup (total n = 163). There was not a clear difference (RR 1.11, 95% CI 0.73 to 1.70) (Analysis 2.12).
2.13 Adverse effects: 5. Any change in specific adverse effects ‐ metabolic ‐ weight change ‐ loss or gain of weight of 10 pounds
There is a single study relating to this outcome (total n = 29). We did not find evidence of a clear difference between the two treatments (RR 0.62 95% CI 0.22 to 1.75) (Analysis 2.13).
2.14 Adverse effects: 6a. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs
For this outcome we found three relevant studies involving 199 participants. There was not a clear difference (RR 0.94, 95% CI 0.61 to 1.45, very low‐quality evidence) (Analysis 2.14).
2.15 Adverse effects: 6b. Any change in specific adverse effects ‐ movement disorders ‐ additional medication use
These data were presented in other data tables because it is not quantitative. The authors of all references did not specify which patient arms received which additional medication, benzhexol, diazepam, trihexphenidyl and scopolamine if necessary (Analysis 2.15).
3. ZUCLOPENTHIXOL versus CHLORPROTHIXENE ‐ all medium term
In this comparison, there were two outcomes.
3.1 Global state: Average endpoint global state score ‐ unchanged/worse (CGI)
For this outcome we found a single study (n = 20) that demonstrated no difference (RR 0.38, 95% CI 0.14 to 1.02) (Analysis 3.1).
3.2 Leaving the study early (any reason)
We identified one study relevant to this outcome (total n = 20). There was not a clear difference between 'zuclopenthixol' and 'chlorprothixene ‐ all medium term' (RR 1.00 95% CI 0.34 to 2.93, very low‐quality evidence) (Analysis 3.2).
4. ZUCLOPENTHIXOL versus CLOZAPINE ‐ all short term
4.1 Leaving the study early (any reason)
We identified one study relevant to this outcome, with a total of 407 people. We found no evidence of a clear difference between 'zuclopenthixol' and 'clozapine ‐ all short term' (RR not estimable) (Analysis 4.1).
4.2 Adverse effects: Any general adverse effects ‐ side‐effects ‐ frequency per day
These data were presented in other data tables because the information is a list with percentages only (Analysis 4.2). A single RCT reported the following results.
-
Drowsiness ‐ no difference (Zuclopenthixol 12% / Clozapine 12%)
-
Stimulation ‐ favours zuclopenthixol (14% / 21%)
-
Confusion ‐ favours zuclopenthixol (1% / 3%)
-
GI ‐ favours zuclopenthixol (1% / 8%)
-
Anticholinergic ‐ favours clozapine (14% / 8%)
-
Dizziness ‐ favours zuclopenthixol (6% / 15%)
-
Orthostatic reaction ‐ favours zuclopenthixol (1% / 6%)
-
Headache ‐ slightly favours zuclopenthixol (2% / 3%)
-
Hypersalivation ‐ slightly favours zuclopenthixol (2% / 4%)
-
Hypokinesia ‐ slightly favours clozapine (4% / 2%)
-
Hyperkinesia ‐ slightly favours zuclopenthixol(2% / 4%)
-
Dyskinesia ‐ no difference (0.4% / 0%)
-
Rigor ‐ favours clozapine (5% / 1%)
-
Tremor ‐ slightly favours clozapine (5% / 3%)
-
Akathisia ‐ no difference (3% / 3%)
5. ZUCLOPENTHIXOL versus HALOPERIDOL ‐ all short term
This particular comparison has seven outcomes.
5.1 Global state: 1. Average endpoint global state score ‐ unchanged/worse (CGI)
We identified one study relevant to this outcome. No clear difference was observed (n = 63, 1 RCT, RR 0.86, 95% CI 0.60 to 1.24) (Analysis 5.1).
5.2 Global state: 2. Average endpoint global state score (CGI, high score = 1.25)
For this outcome we found a single study (total n = 49). We found no evidence of a clear difference (MD 0.13, 95% CI ‐0.30 to 0.55, very low‐quality evidence) (Analysis 5.2).
5.3 Leaving the study early (any reason)
For this outcome we found two relevant studies involving 141 participants. We did not find evidence of a clear difference between the two treatments in this comparison (RR 0.99, 95% CI 0.72 to 1.35, very low‐quality evidence). For this outcome heterogeneity is high (Chi2 = 0.0; df = 0.0; P = 0.0; I2 = 100%) (Analysis 5.3).
5.4 Adverse effects: 1. Any change in specific adverse effects ‐ interference with functioning
There is a single trial in this subgroup (total n = 63). There was not a clear difference (RR 0.91 95% CI 0.64 to 1.29) (Analysis 5.4).
5.5 Adverse effects: 2. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs ‐ requiring medication
There is a single trial (total n = 63), we did not find evidence of a clear difference between the two treatments (RR 0.83, 95% CI 0.47 to 1.45) (Analysis 5.5).
5.6 Adverse effects: 3. Any change in specific adverse effects ‐ requiring additional medication
There is a single trial with a total of 63 people. We did not find evidence of a clear difference between the two treatments (RR 0.99, 95% CI 0.47 to 2.10) (Analysis 5.6).
5.7 Adverse effects: 4. Any change in specific adverse effects ‐ requiring hypnotics/sedatives
We found one trial to be relevant, which included a total of 63 participants. There was not a clear difference (RR 0.81, 95% CI 0.44 to 1.47) (Analysis 5.7).
6. ZUCLOPENTHIXOL versus PERPHENAZINE
In this comparison, there were four outcomes.
6.1 Global state: Average endpoint global state score ‐ unchanged/worse (global rating ‐ investigator opinion) ‐ medium term
For this outcome we found a single study (n = 50, 1 RCT, MD 1.50, 95% CI 0.48 to 4.68), which demonstrated no significant difference (Analysis 6.1).
6.2 Leaving the study early (any reason) ‐ short/medium term
For this outcome we found two relevant studies, with a total of 104 people. We did not find evidence of a clear difference between the two treatments in this comparison (RR 0.63, 95% CI 0.27 to 1.47, very low‐quality evidence) (Analysis 6.2).
6.3 Adverse effects: 1. Any change in specific adverse effects ‐ central nervous system ‐ arousal ‐ requiring medication ‐ medium term
There is a single trial with a total of 50 people. There was not a clear difference between 'zuclopenthixol' and 'perphenazine' (RR 2.00, 95% CI 0.98 to 4.10) (Analysis 6.3).
6.4 Adverse effects: 2. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs ‐ requiring medication ‐ medium term
We found one trial to be relevant, which included a total of 50 participants. We found evidence of a clear difference between 'zuclopenthixol' and 'perphenazine' (RR 1.90, 95% CI 1.12 to 3.22, very low‐quality evidence). Zuclopenthixol is more likely to require medication in the short term for EPSEs than perphenazine (Analysis 6.4).
7. ZUCLOPENTHIXOL versus RISPERIDONE
This particular comparison has nine outcomes.
7.1 Mental State: 1. Average endpoint general mental state score (PANSS, average score = 45.8) ‐ medium term
For this outcome we found a single study (total n = 25). We did not find evidence of a clear difference between the two treatments in this comparison (MD ‐3.20, 95% CI ‐7.71 to 1.31, very low‐quality evidence) (Analysis 7.1).
7.2 Mental State: 2. Average endpoint general mental state score (PANSS General, average score medium term = 20.5) ‐ short/medium term
For this outcome we found two relevant studies, the data from which we divided into two subgroups in accordance with our protocol.
7.2.1 short term
There is a single trial in this subgroup, with a total of 19 people. We found evidence of a clear difference between 'zuclopenthixol' and 'risperidone' within this subgroup (MD ‐2.40, 95% CI ‐4.52 to ‐0.28). PANSS scores were more likely to improve with zuclopenthixol than with risperidone (Analysis 7.2).
7.2.2 medium term
We found one trial to be relevant to this subgroup, with a total of 25 people. For this subgroup, we did not find evidence of a clear difference between the two treatments (MD ‐0.30, 95% CI ‐2.72 to 2.12) (Analysis 7.2).
7.3 Mental State: 3. Average endpoint general mental state score (PANSS positive, average score medium term = 9.8) ‐ medium term
We identified one study relevant to this outcome involving 25 participants. For this outcome, we did not find evidence that 'zuclopenthixol' was clearly different in its effects compared with 'risperidone' (MD ‐1.00, 95% CI ‐2.69 to 0.69) (Analysis 7.3).
7.4 Mental State: 4. Average endpoint general mental state score (PANSS negative, average score = 11.5) ‐ medium term
We identified one study relevant to this outcome (total n = 25). We did not find evidence of a clear difference between the two treatments in this comparison (MD ‐1.50, 95% CI ‐4.05 to 1.05) (Analysis 7.4).
7.5 Leaving the study early (any reason) ‐ short/medium term
For this outcome we found three relevant studies, with a total of 154 people. We did not find evidence of a clear difference between the two treatments in this comparison (RR 1.30, 95% CI 0.84 to 2.02, very low‐quality evidence) (Analysis 7.5).
7.6 Adverse Effects: 1. Any general adverse effects ‐ additional medication use ‐ short/medium term
For zuclopenthixol (n = 18, 1 RCT), benzodiazepines were used on seven occasions and anticholinergics were used on 11 occasions. For risperidone (n = 15, 1 RCT), benzodiazepines were used on eight occasions and anticholinergics on seven occasions.
One of the RCTs (Glenthoj 2007) did not differentiate between the two antipsychotics but reported combined totals of benzodiazepine use on 11 occasions, anticholinergic use on 10 occasions and antidepressant use on one occasion (Analysis 7.6).
7.7 Adverse effects: 2a. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs ‐ requiring medication ‐ short term
There is a single trial (total n = 98). We found evidence of a clear difference between 'zuclopenthixol' and 'risperidone' (RR 1.92, 95% CI 1.12 to 3.28, very low‐quality evidence). Zuclopenthixol was more likely to require medications fors EPSEs than risperidone (Analysis 7.7).
7.8 Adverse effects: 2b. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs (ESRS) ‐ short term
There is a single trial, which included a total of 19 participants. There was a clear difference between 'zuclopenthixol' and 'risperidone' (MD 4.50, 95% CI 0.67 to 8.33). Zuclopenthixol was more likely to cause EPSEs as measured on the ESRS (Chouinard 1980) than risperidone (Analysis 7.8).
7.9 Adverse effects: 3. Any change in specific adverse effects ‐ negative and cognitive symptoms of schizophrenia ‐ short term
We identified one study relevant to this outcome, the data from was used in one subgroup in accordance with our protocol.
7.9.1 UKU ‐ asthenia/lassitude/increased fatigability
We found one trial to be relevant to this subgroup, with a total of 98 people. There was not a clear difference between 'zuclopenthixol' and 'risperidone' within this subgroup (RR 0.82, 95% CI 0.67 to 1.01) (Analysis 7.9).
8. ZUCLOPENTHIXOL versus SULPIRIDE ‐ all short term
In this comparison, there were seven outcomes.
8.1 Global state: 1. Average endpoint global state score ‐ unchanged/worse (CGI)
We identified one study relevant to this outcome (n = 61). No clear difference was noted (RR 1.18, 95% CI 0.49 to 2.85, very low‐quality evidence) (Analysis 8.1).
8.2 Global State: 2. Average endpoint global state score ‐ moderately or severely ill (CGI)
We identified one study relevant to this outcome (total n = 61). We did not find evidence of a clear difference between the two treatments in this comparison (RR 0.99, 95% CI 0.75 to 1.30). This outcome had important levels of heterogeneity (Chi2 = 0.0; df = 0.0; P = 0.0; I2 = 100%) (Analysis 8.2).
8.3 Mental State: Average endpoint general mental state score (BPRS, average = 5.7)
We identified one study relevant to this outcome involving 61 participants. We did not find evidence of a clear difference between the two treatments in this comparison (MD ‐1.30, 95% CI ‐5.08 to 2.48) (Analysis 8.3).
8.4 Leaving the study early (any reason)
For this outcome we found a single study involving 61 participants. There was not a clear difference between 'zuclopenthixol' and 'sulpiride ‐ all short term' (RR 2.07, 95% CI 0.97 to 4.40, very low‐quality evidence; ) (Analysis 8.4).
8.5 Adverse Effects: 1. Any general adverse effects ‐ additional medication use
See tables. Amitrypilline was used four times in each of the zuclopenthixol and sulpiride groups (Analysis 8.5).
8.6 Adverse Effects: 2. Any change in specific adverse effects ‐ metabolic ‐ weight change
We identified one study relevant to this outcome (total n = 61). There was not a clear difference between 'zuclopenthixol' and 'sulpiride ‐ all short term' (MD ‐1.60, 95% CI ‐8.35 to 5.15) (Analysis 8.6).
8.7 Adverse Effects: 3. Any change in specific adverse effects ‐ requiring additional medication ‐ hypnotics/sedative
There is a single trial which included a total of 61 participants. There was not a clear difference between 'zuclopenthixol' and 'sulpiride ‐ all short term' (RR 0.60, 95% CI 0.27 to 1.32) (Analysis 8.7).
9. ZUCLOPENTHIXOL versus THIOTHIXENE ‐ all medium term
This comparison has two outcomes.
9.1 Global state: Average endpoint global state score ‐ unchanged/worse (CGI)
For this outcome we found one study (n = 20). No significant difference was found (RR 0.50, 95% CI 0.17 to 1.46, very low‐quality evidence) (Analysis 9.1).
9.2 Leaving the study early (any reason)
For this outcome we found a single study (total n = 20). We did not find evidence of a clear difference between the two treatments in this comparison (RR 0.57, 95% CI 0.24 to 1.35, very low‐quality evidence) (Analysis 9.2).
10. ZUCLOPENTHIXOL versus TRIFLUOPERAZINE ‐ all short term
This comparison has a single outcome.
10.1 Leaving the study early (any reason)
We identified one study relevant to this outcome (total n = 72). We found no evidence of a clear difference between 'zuclopenthixol' and 'trifluoperazine ‐ all short term' (RR not estimable, no events were reported by the authors) (Analysis 10.1).
11. ZUCLOPENTHIXOL versus ZUCLOPENTHIXOL DEPOT ‐ all long term
This comparison has four outcomes.
11.1 Leaving the study early (any reason)
We identified one study relevant to this outcome involving 46 participants. There are no subgroups in this outcome. There was not a clear difference between 'zuclopenthixol' and 'zuclopenthixol depot ‐ all long term' (RR 1.95, 95% CI 0.36 to 10.58, very low‐quality evidence) (Analysis 11.1).
11.2 Behaviour: Average change in specific aspects of behaviour ‐ Violence during follow‐up
We identified one study relevant to this outcome involving 46 participants. We did not find evidence of a clear difference between the two treatments in this comparison (RR 0.87, 95% CI 0.44 to 1.71). This outcome had important levels of heterogeneity (Chi2 = 0.0; df = 0.0; P = 0.0; I2 = 100%) (Analysis 11.2).
11.3 Adverse Effects: 1a. Any general adverse effects ‐ additional medication use
One person in the zuclopenthixol group was prescribed propranolol (reasons not stated by the author). One person in the depot group was prescribed venlafaxine and one person prescribed lithium (Analysis 11.3).
11.4 Adverse Effects: 1b. Any change in specific adverse effects ‐ additional medication use ‐ benzodiazepine use at least once
For this outcome we found a single study (total n = 46). There was not a clear difference between 'zuclopenthixol' and 'zuclopenthixol depot ‐ all long term' (RR 1.30, 95% CI 0.59 to 2.86) (Analysis 11.4).
12. CIS‐(Z) ZUCLOPENTHIXOL versus CIS(Z)/TRANS(E) ZUCLOPENTHIXOL ‐ all short term
This particular comparison has five outcomes.
12.1 Global state: Average endpoint global state score ‐ Unwell
We identified three studies relevant to this outcome involving 131 participants. We did not find evidence of a clear difference between the two treatments in this comparison (RR 0.97, 95% CI 0.80 to 1.17) (Analysis 12.1).
12.2 Mental state: Average endpoint general mental state score ‐ not improved
We identified one study relevant to this outcome (total n = 57). We did not find evidence of a clear difference between the two treatments in this comparison (RR 0.97, 95% CI 0.45 to 2.07) (Analysis 12.2).
12.3 Leaving the study early (any reason)
We identified four studies relevant to this outcome (total n = 140). There was not a clear difference between 'cis‐(z) zuclopenthixol' and 'cis(z)/trans(e) zuclopenthixol ‐ all short term' (RR 2.15, 95% CI 0.49 to 9.41, very low‐quality evidence) (Analysis 12.3).
12.4 Adverse Effects: 1a. Any general adverse effects ‐ side‐effects reported
We identified one study relevant to this outcome (total n = 57). There was not a clear difference between 'cis‐(z) zuclopenthixol' and 'cis(z)/trans(e) zuclopenthixol ‐ all short term' (RR 1.34, 95% CI 0.82 to 2.18, very low‐quality evidence) (Analysis 12.4).
12.5 Adverse Effects: 1b. Any change in specific adverse effects ‐ individual side‐effects
EPSEs were the most frequent adverse effect (no data) and the authors report no significant difference between the two isomers.
For the Cis Z isomer: Dry mouth, Disturbance of accommodation, Disturbance of urination, Constipation, Dizziness, Headache, Increased sweating, Drowsiness, Anxiety, Parkinsonism, Akathisia, Tardive dyskinesia and others were reported.
For the Cis Z/Trans E isomer: Dry mouth, Disturbance of urination, Constipation, Dizziness, Drowsiness, Parkinsonism, Akathisia, Tardive dyskinesia and others were reported (Analysis 12.5).
Discussion
In the original publication the authors completed the following analyses (Appendix 4).
-
Zuclopenthixol versus placebo (only short term)
-
Zuclopenthixol versus other typical antipsychotics (only short term)
-
Zuclopenthixol versus atypical antipsychotics (only short term)
-
Cis (Z) zuclopenthixol versus cis (Z)/Trans (E) zuclopenthixol (only short term)
For this update the original four analyses were modified and adapted in an attempt to reflect the evidence base for realistic clinical questions e.g. should I use zuclopenthixol instead of clozapine? It was felt that the original four comparisons were misleading to clinicians, patients and policy makers as they suggested that zuclopenthixol had been compared to all other possible antipsychotics. This was not the original authors intent.
This review update identified comparisons against nine alternative antipsychotics, only two of which were newer atypical drugs. Comparisons one and four remain and have been updated (see below).
Summary of main results
Compared to placebo
Data were lacking for this comparison and where data were available, they were only for the short term. Participants did not leave a study earlier when prescribed oral zuclopenthixol (n = 100, 2 RCTs, RR 0.29, 95% CI 0.01 to 6.60) which is surprising given that we expect the side‐effects of being on medication to be more frequent than being on no medication. This information is probably only of use to researchers and caution should be used in transferring this result into community or inpatient settings.
Extrapyramidal effects detected using the UKU side effect rating scale also did not reveal any significant difference, which is again surprising as you would expect more EPSEs on antipsychotic medication than on placebo (n = 28, 1 RCT, RR 6.07, 95% CI 0.86 to 43.04). This may be a consequence of the low number of trials and low participant numbers in these trials (see summary of findings Table for the main comparison).
There was some evidence that zuclopenthixol increased the likelihood of patients experiencing more 'sleepiness/sedation' than placebo (n = 28, 1 RCT, RR 2.89 95% CI 1.01 to 8.3). There was little evidence on whether zuclopenthixol was different to non‐pharmacological treatment (e.g. psychotherapeutic methods, community care or no care at all) in terms of genuine improvements in patients' mental health.
The dose range of oral zuclopenthixol in these studies was 150 mg to 205 mg/day.
Compared to chlorpromazine
Again, despite a large number of participants in this comparison, data were lacking and only available for the short term. Chlorpromazine increases the likelihood of patients leaving a study early (n = 766, 6 RCTs, RR 0.54, 95% CI 0.36 to 0.81), though the reasons for this can only be hypothesised as being related to treatment as a whole as this information was not directly reported or commented on by the researchers. This information is probably only of use to researchers and caution should be used in transferring this result into community or inpatient settings (see summary of findings Table 2).
More side‐effects were reported in patients on chlorpromazine (identified using the CGI; n = 94, 1 RCT, RR 0.86, 95% CI 0.77 to 0.97). Additional medication was used for patients on chlorpromazine and zuclopenthixol (trihexphenidyl, scopolamine, benzhexol and diazepam), but there are no data on frequency so inferring likelihood of use in clinical practice is difficult. The evidence does not help us decide to prescribe outside the usual clinical need. There was no evidence that zuclopenthixol was different to chlorpromazine with regards to improvements in patients' mental health.
The dose range of oral zuclopenthixol in these studies was 25 mg to 600 mg/day and the dose range of chlorpromazine was 100 mg to 1800 mg/day.
Compared to chlorprothixene
The evidence for this comparison is severely limited (see summary of findings Table 3). More research is needed in the form of high‐quality randomised controlled trials.
The dose of oral zuclopenthixol in this single study was 50 mg to 200 mg/day and the dose range of chlorprothixene was 150 mg to 600 mg/day.
Compared to clozapine
Data were severely lacking for this comparison with no information about global state or mental state changes (see summary of findings Table 4). Zuclopenthixol appears to have lower likelihood (at face value) of patients reporting 'stimulation', 'confusion', 'GI', 'dizziness', 'orthostatic reaction', 'headache', 'hypersalivation' and 'hyperkinesia' side‐effects than clozapine. Conversely, there is an increased likelihood of reporting 'anticholinergic', 'hypokinesia', 'rigor' and 'tremor' side‐effects.
The dose range of oral zuclopenthixol in this single study was 100 mg/day and the dose range of clozapine was 200 mg to 300 mg/day.
Compared to haloperidol
summary of findings Table 5 demonstrates the poverty of information available for this comparison. No differences were detected for any of the outcomes when the dose range of oral zuclopenthixol was 40 mg to 205 mg/day and the dose range of haloperidol was4 mg to 12.3 mg/day. It is unlikely that there are no differences between the two medications, but we cannot infer any conclusions based on the current available evidence.
Compared to perphenazine
Patients did not leave a study any earlier whilst prescribed perphenazine or zuclopenthixol in the medium term (n = 104, 2 RCTs, RR 0.63, 95% CI 0.27 to 1.47). Caution should be used in translating this information into clinical practice, but this is useful to those carrying out research.
Zuclopenthixol has a greater likelihood of EPSEs that require medication (n = 50, 1 RCT, RR 1.90, 95% CI 1.12 to 3.22). The number needed to treat for an additional harmful outcome (NNTH) is 2.8, thus on average, 2.8 patients would have to receive zuclopenthixol treatment (instead of perphenazine treatment) for one additional patient to require medication for EPSEs. Any patients prescribed zuclopenthixol should be advised that this is the case and appropriate clinical caution should be taken (asking patients about EPSEs on each contact and/or consideration to EPSE rating scales). There is no evidence on how frequently this should be done.
The dose range of oral zuclopenthixol in this single study was 10 mg to 250 mg/day and the dose range of perphenazine was 8 mg to 72 mg/day. See summary of findings Table 6.
Compared to risperidone
The baseline average PANSS general score of 26.4 SD 5.5 reduced to an average score of 19.3 SD 1.8 for patients on zuclopenthixol. The patients in the risperidone arm scored higher baseline averages (32.4 SD 6.2) and experienced a reduction in average scores at follow‐up (21.7 SD 2.9) that were roughly equivalent to zuclopenthixol. Despite the disparity in the patient baseline mental states (worse mental states in the risperidone arm), in the short term, there was a clear difference in favour of zuclopenthixol in the PANSS General score (n = 19, 1 RCT, MD ‐2.40, 95% CI ‐4.52 to ‐0.28).
The baseline average PANSS positive score of 19.1 SD 3.0 reduced to an average score of 9.8 SD 1.9 for patients on zuclopenthixol. The patients in the risperidone arm had similar baseline averages (20.9 SD 4.4) and experienced a similar reduction in average scores at follow‐up (10.8 SD 2.4). In the medium term there was no difference seen (n = 25, 1 RCT, MD ‐1.00, 95% CI ‐2.69 to 0.69).
Additional medication use (benzodiazepines and anticholinergics) in the short and medium term was associated with both antipsychotics, but frequency of use was the only data reported by the RCTs. No clear difference is observed at face value.
In the short term, zuclopenthixol has a greater likelihood of EPSEs requiring medication than risperidone (n = 98, 1 RCT RR 1.92, 95% CI 1.12 to 3.28, NNTH = 4). Any patients prescribed zuclopenthixol should be advised that this is the case and appropriate clinical caution should be taken (asking patients about EPSEs on each contact and/or consideration to EPSE rating scales). There is no evidence on how frequently this should be done.
For the dose ranges (zuclopenthixol 3.8 mg to 38 mg/day, risperidone 1.9 mg to 8 mg/day) in the included trials, the evidence seems to suggest some clinical benefit for using zuclopenthixol over risperidone, in terms of mental state measured using the PANNS, both in the short and medium term. There is some indication in the short term at these doses that zuclopenthixol will lead to more EPSEs that need medication than risperidone, but both are associated with additional medication use, specifically benzodiazepines and anticholinergics (see summary of findings Table 7).
Compared to sulpiride
For the outcomes in the protocol, very limited evidence exists for this comparison. In the short term, no clear differences were detected causing patients to leave the study early for any reason, in the requirement of hypnotics and/or sedatives, in the global state measured using the CGI and in the mental state measured using the BPRS. Amitriptyline was used four times in the zuclopenthixol group and four times in the sulpiride group, suggesting that the occurrence of mood symptoms could be similar for patients prescribed both antipsychotics (see summary of findings Table 8).
More research is needed in the form of high‐quality randomised controlled trials.
The dose of oral zuclopenthixol in this single study was 25 mg to 150 mg/day and the dose range of sulpiride was 200 mg to 1200 mg/day.
Compared to thiothixene
Extremely limited evidence is available for this comparison and only in the medium term. No clear difference was detected causing patients to leave the study early for any reason (n = 20, 1 RCT, RR 0.57, 95% CI 0.24 to 1.35) or in global state scores using the CGI (n = 20, 1 RCT, RR 0.50, 95% CI 0.17 to 1.46). More research is needed in the form of high‐quality randomised controlled trials.
The dose of oral zuclopenthixol in this single study was 50 mg to 200 mg/day and the dose range of thiothixene was 10 mg to 40 mg/day (see summary of findings Table 9).
Compared to trifluoperazine
We did not identify any significant findings for this comparison (see summary of findings Table 10) for any of the protocol outcomes. More research is needed in the form of high‐quality randomised controlled trials.
The dose of oral zuclopenthixol in this single study was 100 mg/day and the dose range of trifluoperazine was 20 mg to 30 mg/day.
Compared to zuclopenthixol depot
Evidence for this comparison was limited only to the longer term, where there was no clear difference detected causing patients to leave the study early for any reason (n = 46, 1 RCT, RR 1.95, 95% CI 0.36 to 10.58). More research is needed in the form of high quality‐randomised controlled trials.
The dose of oral zuclopenthixol in this single study was 35 mg/day and the dose of depot was 233 mg every 14 days (see summary of findings Table 11).
Compared to zuclopenthixol isomer
In the short term, there was no clear difference detected causing patients to leave the study early for any reason (n = 140, 4 RCTs, RR 2.15, 95% CI 0.49 to 9.41), and no clear difference in general adverse effects (n = 57, 1 RCT, RR 1.34, 95% CI 0.82 to 2.18).
Dry mouth, disturbance of accommodation, disturbance of urination, constipation, dizziness, headache, increased sweating, drowsiness, anxiety, parkinsonism, akathisia, tardive dyskinesia and 'others' were reported for both isomers but no data were presented. This is perhaps useful only in as far as informing what side‐effects could develop in patients prescribed zuclopenthixol, but with no information on frequency, it is not possible to advise on how common these effects are.
More research is needed in the form of high quality‐randomised controlled trials.
The dose range of oral cis (Z) zuclopenthixol in these studies was 10 mg to 200 mg/day and the dose range of cis(Z)/trans(E) zuclopenthixol was 20 mg to 200 mg/day (see summary of findings Table 12).
Overall completeness and applicability of evidence
1. Completeness
1.1 Relevance of the evidence to the review question
Zuclopenthixol dihydrochloride is generally used for patients with a psychotic illness and predominantly those with schizophrenia‐spectrum diagnoses. The primary and secondary outcomes of this review (death, service outcomes, mental state, leaving the study early, general functioning, behaviour, adverse effects, engagement with services, satisfaction with treatment, quality of life and economic) were not generally the primary focus of the included evidence. The extracted data were predominantly around mental state, leaving the study early, general functioning and adverse effects. Much of the data were obtained from rating scales which are not always used in clinical practice but are heavily used in research.
The included papers focused on (1) behaviour and therapeutic effect (versus placebo); (2) efficacy, dosage, tolerance and safety (versus chlorpromazine); (3) efficacy, dosage, tolerance and safety (versus clozapine); (4) efficacy, dosage, tolerance and safety (versus haloperidol); (5) efficacy, dosage, tolerance and safety (versus trifluoperazine); (6) clinical profile (versus perphenazine); (7) efficacy, tolerability, baseline and follow‐up basal ganglia volumes, executive functions, selective attention and reaction times (versus risperidone); (8) no clear focus (versus thiothixene and chlorprothixene); (9) violence reduction (versus depot zuclopenthixol) and (10) serum levels and clinical effect (versus zuclopenthixol isomers).
The main interventions of the included studies were antipsychotics and the outcomes were generally related to this. None of the included studies covered other psycho‐social aspects.
1.2 Overall judgement of the external validity of the review
Data were obtained predominantly for the short term (up to 12 weeks) and for inpatient populations. Some data were obtained for the medium term (13 to 26 weeks). Longer‐term data (> 26 weeks) were obtained only for depot zuclopenthixol. The studies were conducted either in Europe or North America (though this update does add some Chinese research).
The majority of trials involved inpatient participants with little in the way of physical and psychiatric co‐morbidity and with well‐defined schizophrenia or schizoaffective disorder. Such people are a minority in everyday care, where it is the norm to find people (not in a hospital setting) who suffer from less well‐defined illnesses combined with problems such as depression, personality disorder and substance abuse/misuse. Much of the data were obtained from rating scales which are not always used in clinical practice but are heavily used in research.
The results of this study can be generalised in a limited capacity in the short term (up to 12 weeks) to inpatient populations based mainly in European and American healthcare systems that are similar to those during the period 1968 to 2008.
2. Applicability of findings
2.1 The results and current practice
Current practice is unlikely to be significantly changed in Europe or the USA by the results of this meta‐analysis. The results do continue to support the importance of reviewing patients for EPSEs when they are prescribed oral zuclopenthixol, but advice on frequency of monitoring cannot be drawn from the data included. Much of the data focus on older antipsychotics, which tend to be used as second‐ or third‐line agents currently (including zuclopenthixol), though the data on superiority/inferiority were lacking and limited to only nine comparisons.
Small but significant improvements in average endpoint mental states are seen with zuclopenthixol when compared to the newer risperidone, but at the cost of an increased chance of EPSEs requiring further medication in the form of anticholinergics and benzodiazepines. Again, of the newer agents, evidence was only found for risperidone and clozapine, thus not allowing us to comment on the wide range of other newer anti‐psychotics.
The evidence is primarily generated from inpatient samples and caution should be given to using the results for patients in the community, general hospital settings or outpatient departments. The dose range of oral zuclopenthixol dihydrochloride across all of the included studies was 10 mg to 600 mg/day.
2.2 The results and current international practice
The authors of this paper are not familiar with all areas of current international clinical practice and advise caution interpreting and generalising the findings of this update to areas outside of Europe and the USA where healthcare systems could be very different from those covered in the included studies.
Quality of the evidence
1. Possibility of a robust conclusion
The evidence identified in this review is only for 12 comparisons (n = 1850, 20 RCTs, 1968 to 2008), and most of these comparisons are for older antipsychotics and/or antipsychotics that are not used commonly in clinical practice currently.
The largest study had 723 participants but the majority of studies were small. Data were obtained for patients with a psychotic illness and predominantly those with schizophrenia‐spectrum diagnoses. The evidence was mainly for the short term (up to 12 weeks) and for inpatient populations. Some data were obtained for the medium term (13‐26 weeks) and longer‐term data (> 26 weeks) were obtained only for depot zuclopenthixol.
The included studies were all RCTs of varying degrees of quality though no significant key methodological flaws were detected. Inconsistency of results between RCTs was difficult to assess given the small number of included studies and the wide number of non‐equal comparisons.
This makes drawing robust conclusions about the use of zuclopenthixol dihydrochloride for schizophrenia in the broader sense more difficult.
2. Overall quality of the evidence
The overall quality of the evidence contributing to the findings of this review ranged from very low to medium, with much of the evidence falling into either very low to low quality using GRADE. The reasons for the down grading of the included evidence was mostly because of biases (reporting, selection, Berkson, publication and attrition), and these reasons are clarified in the footnote section of each 'Summary of findings' table.
Potential biases in the review process
1. Preventing biases: strengths
The Cochrane systematic review process is designed to prevent and minimise biases introduced by authors of reviews. A single review author (EJB), independently of the original co‐authors (AK and DS), and at a different point in time, re‐appraised the original papers for inclusion in the review update in addition to appraising the suitability of new evidence for inclusion.
EJB utilised a strict system of appraisal of evidence as guided by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and attempted to contact the original authors where possible/appropriate. At several stages in the review process, a 10% sample of appraised papers, included papers and extracted data was assessed by review author MAP to ensure consensus was reached.
EJB has no known conflicts of interest.
2. Preventing biases: limitations
A systematic review is only ever as good at preventing bias in the findings as the original evidence used in determining the findings. For example, the review data are predominantly from inpatient samples, so the review findings are predominantly focused on this patient population. The review cannot correct for many of the biases of the original evidence. There may also be biases introduced through the systematic approach that cannot be controlled/minimised. The methods used to identify the evidence could have introduced biases at different stages e.g. searching, study selection, data collection, analysis.
3. Missing relevant studies
Judging by the preponderance of small positive studies included in this review update, it is likely that not all evidence has been identified. Several of the papers included had results published that could not be extracted for meta‐analysis, even though they may have been relevant to the review question.
Agreements and disagreements with other studies or reviews
The previous incarnation of this review suggested oral zuclopenthixol dihydrochloride caused movement disorders, perhaps more so than newer antipsychotics, but no more frequently than other older antipsychotics. This update agrees partially with these conclusions (it does cause movement disorders), but restricts their breadth as data were only obtained for two newer agents (clozapine and risperidone) and only for seven older agents. The only two comparisons that suggested oral zuclopenthixol dihydrochloride caused more EPSEs was with perphenazine and risperidone. There was no significant evidence of more EPSEs being reported when compared to placebo. More side‐effects were reported with chlorpromazine than with zuclopenthixol.
Additionally, the previous version of this review indicated that oral zuclopenthixol dihydrochloride may offer a clinical advantage in terms of global state over other older alternatives in the short term, but would need to be taken with additional medication to temper the sequelae of EPSEs. The results of this update do not support this statement, at least for the comparisons where data were obtained.
This update suggests zuclopenthixol dihydrochloride only has a modest clinical advantage with regards to mental state (measured on the PANSS) when compared to risperidone only (short term).

Study flow diagram ‐ update 2016.
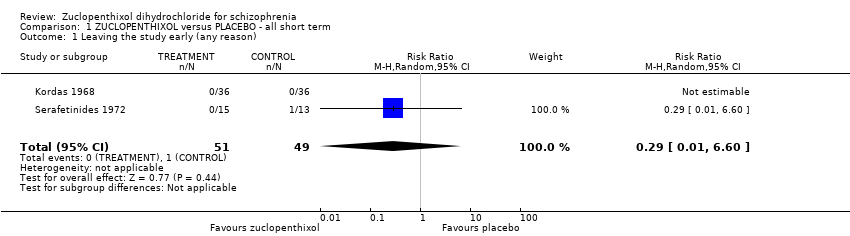
Comparison 1 ZUCLOPENTHIXOL versus PLACEBO ‐ all short term, Outcome 1 Leaving the study early (any reason).
Comparison 1 ZUCLOPENTHIXOL versus PLACEBO ‐ all short term, Outcome 2 Adverse effects: 1. Clinically important change in specific adverse effects ‐ cardiovascular ‐ orthostatic.

Comparison 1 ZUCLOPENTHIXOL versus PLACEBO ‐ all short term, Outcome 3 Adverse effects: 2. Clinically important change in specific adverse effects ‐ central nervous system ‐ arousal state.

Comparison 1 ZUCLOPENTHIXOL versus PLACEBO ‐ all short term, Outcome 4 Adverse effects: 3. Clinically important change in specific adverse effects ‐ endocrine ‐ menstruation started.

Comparison 1 ZUCLOPENTHIXOL versus PLACEBO ‐ all short term, Outcome 5 Adverse effects: 4a. Any general adverse effects ‐ movement disorders ‐ EPSEs (UKU side effect rating scale, no scores).

Comparison 1 ZUCLOPENTHIXOL versus PLACEBO ‐ all short term, Outcome 6 Adverse effects: 4b. Clinically important change in specific adverse effects ‐ movement disorders ‐ EPSEs.

Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 1 Global state: 1. Average endpoint global state score ‐ Unchanged/worse (CGI, scores not reported).

Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 2 Global state: 2. Average endpoint global state score ‐ No Recovery.

Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 3 Global state: 3a. Average endpoint global state score (GAS, high score not reported, average score = 63.4).
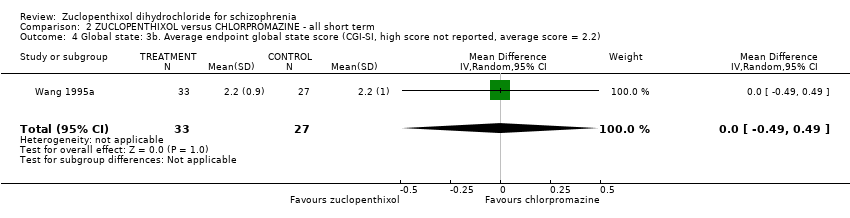
Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 4 Global state: 3b. Average endpoint global state score (CGI‐SI, high score not reported, average score = 2.2).

Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 5 Mental state: 1. No clinically important change in general mental state ‐ Not improved (PANSS, scores not reported).
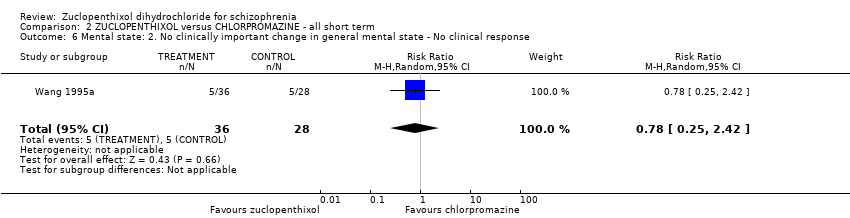
Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 6 Mental state: 2. No clinically important change in general mental state ‐ No clinical response.

Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 7 Mental state: 3. Average endpoint general mental state score (BPRS, high score = 34.2).

Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 8 Leaving the study early (any reason).
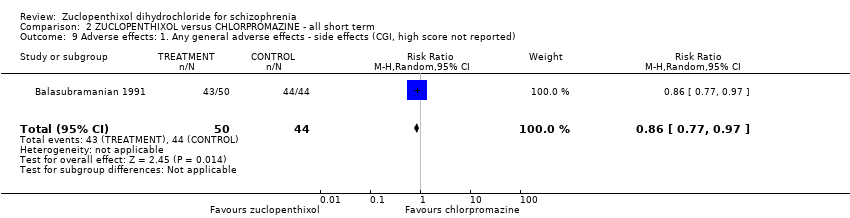
Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 9 Adverse effects: 1. Any general adverse effects ‐ side effects (CGI, high score not reported).
Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 10 Adverse effects: 2. Average endpoint general adverse effect score ‐ average score (TESS, high score not reported, average score = 12.00).
Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 11 Adverse effects: 3. Any change in specific adverse effects ‐ cardiovascular ‐ postural hypotension (dizziness/syncope).

Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 12 Adverse effects: 4. Any change in specific adverse effects ‐ central nervous system ‐ arousal.

Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 13 Adverse effects: 5. Any change in specific adverse effects ‐ metabolic ‐ weight change ‐ loss or gain of weight of 10 pounds.

Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 14 Adverse effects: 6a. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs.
| Study | Zuclopenthixol | Chlorpromazine |
| Kingstone 1970 | n = 5, authors do not state which additional medication. | n = 2, authors do not state which additional medication. |
| Kordas 1968 | Benzhexol and diazepam if necessary. | Benzhexol and diazepam if necessary. |
| Wang 1995a | Trihexphenidyl or scopolamine used if necessary. Authors do not report frequency of use or which group used which. | Trihexphenidyl or scopolamine used if necessary. Authors do not report frequency of use or which group used which. |
Comparison 2 ZUCLOPENTHIXOL versus CHLORPROMAZINE ‐ all short term, Outcome 15 Adverse effects: 6b. Any change in specific adverse effects ‐ movement disorders ‐ additional medication use.
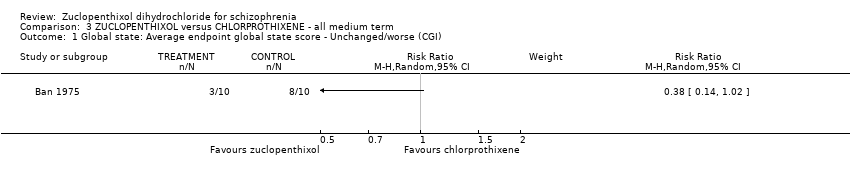
Comparison 3 ZUCLOPENTHIXOL versus CHLORPROTHIXENE ‐ all medium term, Outcome 1 Global state: Average endpoint global state score ‐ Unchanged/worse (CGI).

Comparison 3 ZUCLOPENTHIXOL versus CHLORPROTHIXENE ‐ all medium term, Outcome 2 Leaving the study early (any reason).

Comparison 4 ZUCLOPENTHIXOL versus CLOZAPINE ‐ all short term, Outcome 1 Leaving the study early (any reason).
| Study | Zuclopenthixol (n = 36) | Clozapine (n = 38) |
| Fischer‐Cornelssen 1976 | Drowsiness (12%); Stimulation (14%); Confusion (1%); GI (1%); anticholinergic (14%); dizziness (6%); Orthostatic reaction (1%); Headache (2%); Hypersalivation (2%); hypokinesia (4%); hyperkinesia (2%); dyskinesia (0.4%); rigor (5%); tremor (5%); akathisia (3%) | Drowsiness (12%); Stimulation (21%); Confusion (3%); GI (8%); anticholinergic (8%); dizziness (15%); Orthostatic reaction (6%); Headache (3%); Hypersalivation (4%); hypokinesia (2%); hyperkinesia (4%); dyskinesia (0%); rigor (1%); tremor (3%); akathisia (3%) |
Comparison 4 ZUCLOPENTHIXOL versus CLOZAPINE ‐ all short term, Outcome 2 Adverse effects: Any general adverse effects ‐ side effects ‐ frequency per day.
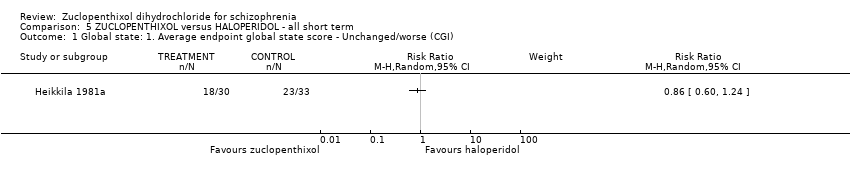
Comparison 5 ZUCLOPENTHIXOL versus HALOPERIDOL ‐ all short term, Outcome 1 Global state: 1. Average endpoint global state score ‐ Unchanged/worse (CGI).

Comparison 5 ZUCLOPENTHIXOL versus HALOPERIDOL ‐ all short term, Outcome 2 Global state: 2. Average endpoint global state score (CGI, mean score = 1.25).
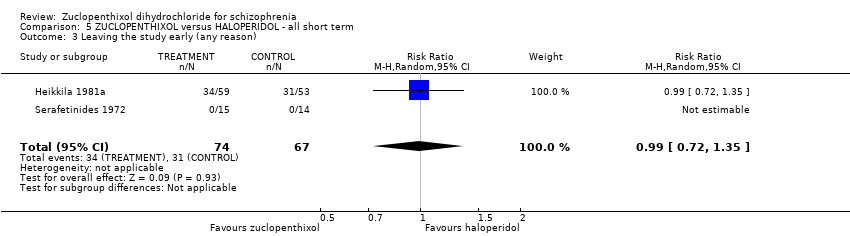
Comparison 5 ZUCLOPENTHIXOL versus HALOPERIDOL ‐ all short term, Outcome 3 Leaving the study early (any reason).

Comparison 5 ZUCLOPENTHIXOL versus HALOPERIDOL ‐ all short term, Outcome 4 Adverse effects: 1. Any change in specific adverse effects ‐ interference with functioning.

Comparison 5 ZUCLOPENTHIXOL versus HALOPERIDOL ‐ all short term, Outcome 5 Adverse effects: 2. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs ‐ requiring medication.
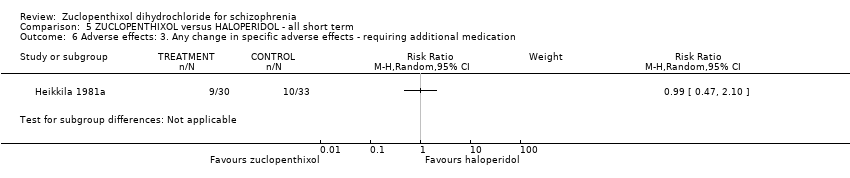
Comparison 5 ZUCLOPENTHIXOL versus HALOPERIDOL ‐ all short term, Outcome 6 Adverse effects: 3. Any change in specific adverse effects ‐ requiring additional medication.

Comparison 5 ZUCLOPENTHIXOL versus HALOPERIDOL ‐ all short term, Outcome 7 Adverse effects: 4. Any change in specific adverse effects ‐ requiring hypnotics/sedatives.
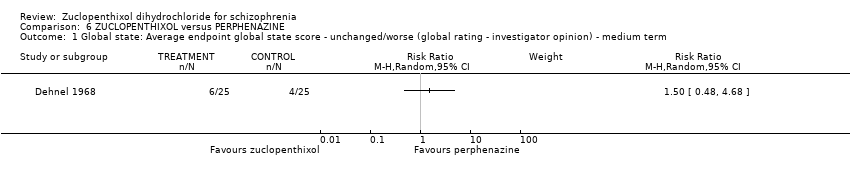
Comparison 6 ZUCLOPENTHIXOL versus PERPHENAZINE, Outcome 1 Global state: Average endpoint global state score ‐ unchanged/worse (global rating ‐ investigator opinion) ‐ medium term.

Comparison 6 ZUCLOPENTHIXOL versus PERPHENAZINE, Outcome 2 Leaving the study early (any reason) ‐ short/medium term.

Comparison 6 ZUCLOPENTHIXOL versus PERPHENAZINE, Outcome 3 Adverse effects: 1. Any change in specific adverse effects ‐ central nervous system ‐ arousal ‐ requiring medication ‐ medium term.
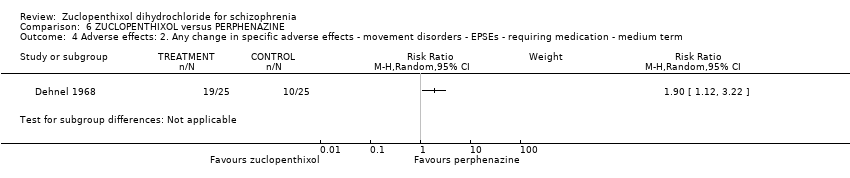
Comparison 6 ZUCLOPENTHIXOL versus PERPHENAZINE, Outcome 4 Adverse effects: 2. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs ‐ requiring medication ‐ medium term.

Comparison 7 ZUCLOPENTHIXOL versus RISPERIDONE, Outcome 1 Mental State: 1. Average endpoint general mental state score (PANSS, average score = 45.8) ‐ medium term.
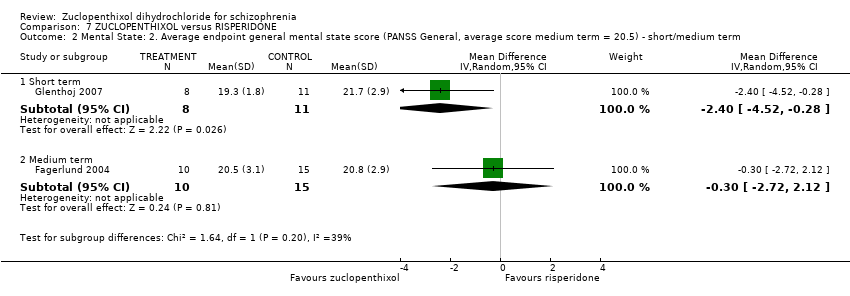
Comparison 7 ZUCLOPENTHIXOL versus RISPERIDONE, Outcome 2 Mental State: 2. Average endpoint general mental state score (PANSS General, average score medium term = 20.5) ‐ short/medium term.
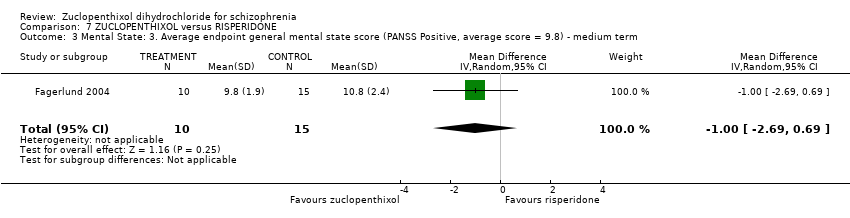
Comparison 7 ZUCLOPENTHIXOL versus RISPERIDONE, Outcome 3 Mental State: 3. Average endpoint general mental state score (PANSS Positive, average score = 9.8) ‐ medium term.

Comparison 7 ZUCLOPENTHIXOL versus RISPERIDONE, Outcome 4 Mental State: 4. Average endpoint general mental state score (PANSS Negative, average score 11.5) ‐ medium term.

Comparison 7 ZUCLOPENTHIXOL versus RISPERIDONE, Outcome 5 Leaving the study early (any reason) ‐ short/medium term.
| Study | Zuclopenthixol | Risperidone |
| Fagerlund 2004 | Benzodiazepines n = 7 Anticholinergics n = 11 | Benzodiazepines n = 8 Anticholinergics n = 7 |
| Glenthoj 2007 | Anticholinergic n = 10, Benzodiazepines n = 11, Antidepressant n = 1 | Authors do not differentiate the use of additional medication between the two groups, totals only given. |
Comparison 7 ZUCLOPENTHIXOL versus RISPERIDONE, Outcome 6 Adverse Effects: 1. Any change in general adverse effects ‐ additional medication use ‐ short/medium term.

Comparison 7 ZUCLOPENTHIXOL versus RISPERIDONE, Outcome 7 Adverse effects: 2a. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs ‐ requiring medication ‐ short term.

Comparison 7 ZUCLOPENTHIXOL versus RISPERIDONE, Outcome 8 Adverse Effects: 2b. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs (ESRS) ‐ short term.
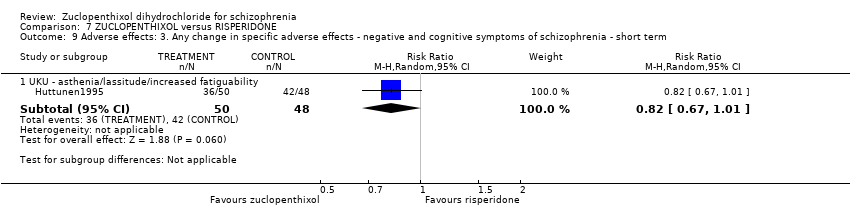
Comparison 7 ZUCLOPENTHIXOL versus RISPERIDONE, Outcome 9 Adverse effects: 3. Any change in specific adverse effects ‐ negative and cognitive symptoms of schizophrenia ‐ short term.

Comparison 8 ZUCLOPENTHIXOL versus SULPIRIDE ‐ all short term, Outcome 1 Global state: 1. Average endpoint global state score ‐ Unchanged/worse (CGI).

Comparison 8 ZUCLOPENTHIXOL versus SULPIRIDE ‐ all short term, Outcome 2 Global State: 2. Average endpoint global state score ‐ Moderately or severely ill (CGI).

Comparison 8 ZUCLOPENTHIXOL versus SULPIRIDE ‐ all short term, Outcome 3 Mental State: Average endpoint general mental state score (BPRS, average = 5.7).
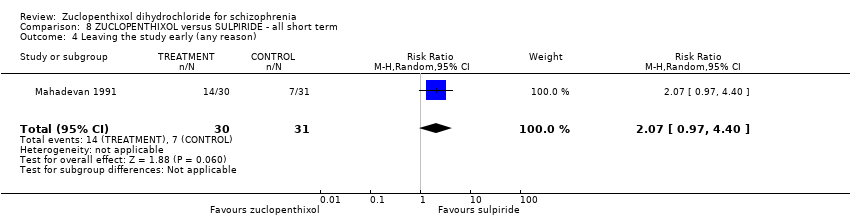
Comparison 8 ZUCLOPENTHIXOL versus SULPIRIDE ‐ all short term, Outcome 4 Leaving the study early (any reason).
| Study | Zuclopenthixol | Sulpiride |
| Mahadevan 1991 | n = 4 Amitriptyline | n = 4 Amitryptyline |
Comparison 8 ZUCLOPENTHIXOL versus SULPIRIDE ‐ all short term, Outcome 5 Adverse Effects: 1. Any change in general adverse effects ‐ additional medication use.
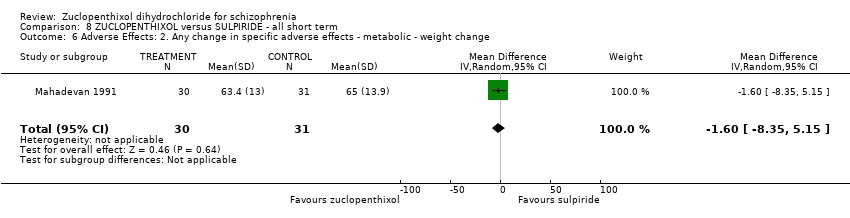
Comparison 8 ZUCLOPENTHIXOL versus SULPIRIDE ‐ all short term, Outcome 6 Adverse Effects: 2. Any change in specific adverse effects ‐ metabolic ‐ weight change.

Comparison 8 ZUCLOPENTHIXOL versus SULPIRIDE ‐ all short term, Outcome 7 Adverse effects: 3. Any change in specific adverse effects ‐ requiring additional medication ‐ hypnotics/sedatives.

Comparison 9 ZUCLOPENTHIXOL versus THIOTHIXENE ‐ all medium term, Outcome 1 Global state: Average endpoint global state score ‐ unchanged/worse (CGI).
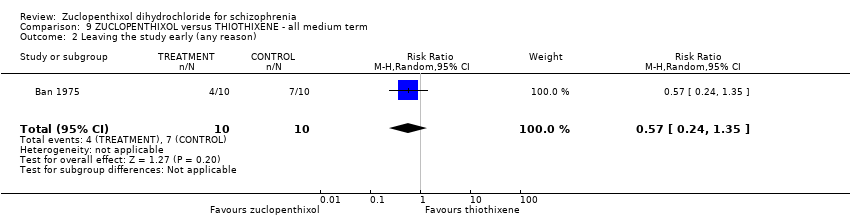
Comparison 9 ZUCLOPENTHIXOL versus THIOTHIXENE ‐ all medium term, Outcome 2 Leaving the study early (any reason).
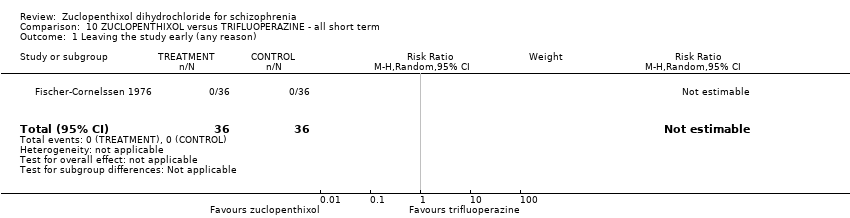
Comparison 10 ZUCLOPENTHIXOL versus TRIFLUOPERAZINE ‐ all short term, Outcome 1 Leaving the study early (any reason).
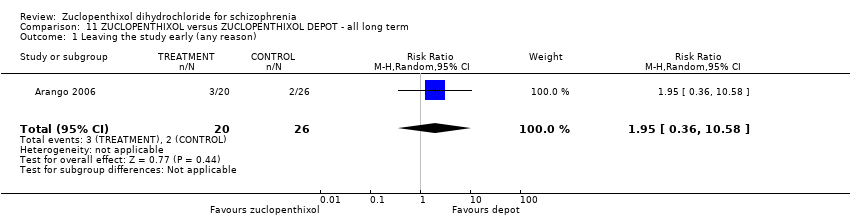
Comparison 11 ZUCLOPENTHIXOL versus ZUCLOPENTHIXOL DEPOT ‐ all long term, Outcome 1 Leaving the study early (any reason).
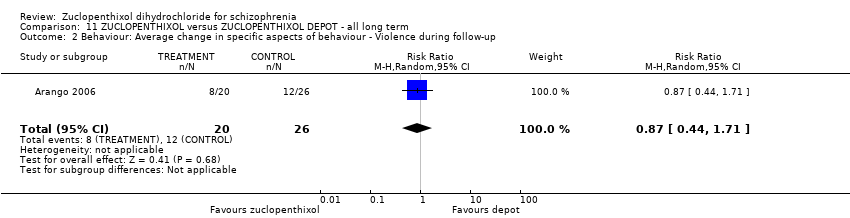
Comparison 11 ZUCLOPENTHIXOL versus ZUCLOPENTHIXOL DEPOT ‐ all long term, Outcome 2 Behaviour: Average change in specific aspects of behaviour ‐ Violence during follow‐up.
| Study | Zuclopenthixol | Depot |
| Arango 2006 | n = 1 Propanolol | n = 1 venlafaxine n = 1 Lithium |
Comparison 11 ZUCLOPENTHIXOL versus ZUCLOPENTHIXOL DEPOT ‐ all long term, Outcome 3 Adverse Effects: 1a. Any general adverse effects ‐ additional medication use.

Comparison 11 ZUCLOPENTHIXOL versus ZUCLOPENTHIXOL DEPOT ‐ all long term, Outcome 4 Adverse Effects: 1b. Any change in specific adverse effects ‐ additional medication use ‐ benzodiazepine use at least once.

Comparison 12 CIS‐(Z) ZUCLOPENTHIXOL versus CIS(Z)/TRANS(E) ZUCLOPENTHIXOL ‐ all short term, Outcome 1 Global state: Average endpoint global state score ‐ Unwell.

Comparison 12 CIS‐(Z) ZUCLOPENTHIXOL versus CIS(Z)/TRANS(E) ZUCLOPENTHIXOL ‐ all short term, Outcome 2 Mental state: Average endpoint general mental state score ‐ Not improved.

Comparison 12 CIS‐(Z) ZUCLOPENTHIXOL versus CIS(Z)/TRANS(E) ZUCLOPENTHIXOL ‐ all short term, Outcome 3 Leaving the study early (any reason).

Comparison 12 CIS‐(Z) ZUCLOPENTHIXOL versus CIS(Z)/TRANS(E) ZUCLOPENTHIXOL ‐ all short term, Outcome 4 Adverse Effects: 1a. Any general adverse effects ‐ side effects reported.
| Study | Cis Z | Cis(Z)/Trans(E) |
| Gravem 1981 | EPSEs most frequent. Authors state no significant difference between two isomers. | EPSEs most frequent. |
| Heikkila 1981 | Dry mouth, Disturbance of accommodation, Disturbance of urination, Constipation, Dizziness, Headache, Increased sweating, Drowsiness, Anxiety, Parkinsonism, Akathisia, Tardive dyskinesia and others | Dry mouth, Disturbance of urination, Constipation, Dizziness, Drowsiness, Parkinsonism, Akathisia, Tardive dyskinesia, others |
Comparison 12 CIS‐(Z) ZUCLOPENTHIXOL versus CIS(Z)/TRANS(E) ZUCLOPENTHIXOL ‐ all short term, Outcome 5 Adverse Effects: 1b. Any change in specific adverse effects ‐ individual side effects.
| Patient or population: people with schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with PLACEBO (short term) | Risk with ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Adverse effects: Clinically important general adverse effect (extrapyramidal effects ‐ UKU side effect rating scale) | 80 per 1000 | 486 per 1000 | RR 6.07 | 28 | ⊕⊝⊝⊝ | Risk assumed to be moderate and rounded from 7.69% to 8%. |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Service outcomes: Duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Leaving the study early (any reason) | 40 per 1000 | 12 per 1000 | RR 0.29 | 100 | ⊕⊝⊝⊝ | Risk assumed to be moderate and rounded from 3.85% to 4%. Low number of events in both RCTs. |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'very serious' ‐ possible selection bias, blinding may have been single, attrition bias and reporting bias. 2 Risk of bias: rated 'very serious' ‐ selection, attrition and reporting bias 3 Risk of inconsistency: rated 'not serious' ‐ suspected but not found. 4 Risk of publication bias: rated 'strongly suspected' ‐ multiple papers published with the same patient cohort. 5 Risk of large effect: rated 'very large' ‐ RR 6.07, small n number but result likely when versus placebo. 6 Risk of imprecision: rated 'very serious' ‐ low n numbers | ||||||
| Patient or population: schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with CHLORPROMAZINE (short term) | Risk with ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (CGI‐SI, high score not reported, average score = 2.2) | The mean CGI‐SI endpoint score in the intervention group (MD) was 0 (‐0.49 lower to 0.49 higher) | ‐ | 64 | ⊕⊝⊝⊝ | Translated study. | |
| Adverse effects: Clinically important general adverse effect (EPSEs) | 300 per 1000 | 282 per 1000 | RR 0.94 | 199 | ⊕⊝⊝⊝ | Risk control rounded to 30% and set to moderate. Mixture of inpatient and outpatient, though predominantly hospitalised patients. |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (BPRS, high score = 34.2) | The mean mental state: average endpoint score (BPRS, high score = 34.2) in the intervention group was 0.4 more (2.43 fewer to 3.23 more) | ‐ | 221 | ⊕⊕⊝⊝ | Mixture of inpatient and outpatient, though predominantly hospitalised patients. | |
| Leaving the study early (any reason) | 70 per 1000 | 38 per 1000 | RR 0.54 | 766 | ⊕⊕⊝⊝ | Mixture of inpatient and outpatient, though predominantly hospitalised patients. Risk control set to moderate and rounded to 7%; extreme values not likely. |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'serious' ‐ Selection and attrition bias likely. 2 Risk of bias: rated 'serious' ‐ Selection attrition bias. 3 Risk of bias: rated 'serious' ‐ Selection, attrition and reporting bias. 4 Risk of bias: rated 'serious' ‐ Selection, attrition and reporting bias. Risk of inconsistency: rated as 'serious' ‐ All three papers reported on differing population sizes and obtained different levels of EPSEs in the experimental and control groups. 5 Risk of imprecision: rated 'very serious' ‐ low n numbers 6 Risk of indirectness: rated 'very serious' ‐ mixed samples For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | ||||||
| Patient or population: schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with CHLORPROTHIXENE (medium term) | Risk with ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Adverse effects: Clinically important general adverse effect (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Leaving the study early (any reason) | 400 per 1000 | 400 per 1000 | RR 1.00 | 20 | ⊕⊝⊝⊝ | |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated as 'serious' ‐ selection, reporting bias likely. 2 Risk of publication bias: rated as 'strongly suspected' ‐ several papers published using the same cohort of patients. 3 Risk of imprecision: rated 'very serious' ‐ low n numbers For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | ||||||
| Patient or population: schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with CLOZAPINE (short term) | Risk with ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Adverse effects: Clinically important general adverse effect (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Leaving the study early (any reason) | 0 per 1000 | 0 per 1000 | not estimable | 407 | ⊕⊕⊝⊝ | Multi‐centre, multi‐drug trial with disproportionate numbers of people in different arms of the study. The authors did not report that anybody left the study in the Zuclopenthixol and clozapine arms. |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated as 'serious' ‐ selection, attrition, reporting and performance bias likely. 2 Risk of indirectness: rated 'serious' ‐ Multiple study arms For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | ||||||
| Patient or population: schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with HALOPERIDOL (short term) | Risk with ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (CGI, mean score = 1.25) | The mean CGI endpoint score in the intervention group (MD) was 0.13 more (‐0.3 fewer to 0.55 more) | ‐ | 49 | ⊕⊝⊝⊝ | Small study, multiple scales used (NOSIE30, BPRS, CGI). Paper only reported outcomes of some of these scales. | |
| Adverse effects: Clinically important general adverse effect (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Leaving the study early (any reason) | 300 per 1000 | 297 per 1000 | RR 0.99 | 141 | ⊕⊝⊝⊝ | Risk control rounded to 30% from 29.25% and, as extreme values unlikely, it was set to moderate. |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated as 'very serious' ‐ likely selection, attrition, reporting and diagnostic purity bias. 2 Risk of bias: rated as 'serious' ‐ likely selection, attrition, reporting and diagnostic purity bias. Risk of inconsistency: rated as 'serious' ‐ both papers generated differing values for people leaving the study and do not appear consistent (face validity). 3 Risk of imprecision: rated 'serious' ‐ low n numbers 4 Risk of indirectness: rated 'serious' ‐ not all scales reported For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | ||||||
| Patient or population: schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with PERPHENAZINE (short and medium term) | Risk with ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Adverse effects: Clinically important general adverse effect (EPSEs requiring medication ‐ medium term) | 400 per 1000 | 760 per 1000 | RR 1.90 | 50 | ⊕⊝⊝⊝ | |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Leaving the study early (any reason, short and medium term) | 207 per 1000 | 130 per 1000 | RR 0.63 | 104 | ⊕⊝⊝⊝ | |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated as 'serious' ‐ likely selection and reporting bias. 2 Risk of imprecision: rated 'very serious' ‐ low n numbers For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | ||||||
| Patient or population: schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with RISPERIDONE (short and medium term) | Risk with ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Adverse effects: Clinically important general adverse effect ‐ short term (EPSEs requiring medication) | 271 per 1000 | 520 per 1000 | RR 1.92 | 98 | ⊕⊝⊝⊝ | |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (PANSS, average score = 20.5) ‐ medium term | The mean PANNS endpoint score in the intervention group (MD) was 3.2 fewer (‐7.71 fewer to 1.31 more) | ‐ | 25 | ⊕⊝⊝⊝ | Small study. Standard deviations may be standard errors. Open label trial. | |
| Leaving the study early (any reason, short and medium term) | 310 per 1000 | 403 per 1000 | RR 1.30 | 154 | ⊕⊝⊝⊝ | Risk control taken as mean of extremes: high + low / 2 (changed from 21.43% to 31%) |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated as 'very serious' ‐ open label trial, likely selection bias. 2 Risk of imprecision: rated as 'very serious' ‐ strongly suspect reported standard deviations are standard errors, low n numbers 3 Risk of publication bias: rated as 'strongly suspected' ‐ all studies published findings across several consecutive years in different journals and at conferences. 4 Risk of bias: rated as 'very serious' ‐ study 1 (selection, reporting, diagnostic purity and attrition bias likely ‐ author emailed); study 2 (open label); study 3 (reporting and attrition bias). 5 Risk of publication bias: rated as 'strongly suspected' ‐ multiple papers over consecutive years published with the same data. 6 Risk of bias: rated as 'serious' ‐ diagnostic purity and attrition bias; likely selection and reporting bias (authors contacted) For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | ||||||
| Patient or population: schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with SULPIRIDE (short term) | Risk with ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (CGI ‐ unchanged/worse) | 226 per 1000 | 266 per 1000 | RR 1.18 | 61 | ⊕⊝⊝⊝ | The study did not report clinical improvement so unchanged/worse is reported for this outcome. Usually we report clinical improvement. |
| Adverse effects: Clinically important general adverse effect ‐ requiring hypnotics/sedatives | 419 per 1000 | 252 per 1000 | RR 0.60 | 61 | ⊕⊝⊝⊝ | |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (BPRS) | The mean BPRS endpoint score in the intervention group (MD) was 1.3 fewer (‐5.08 fewer to 2.48 more) | ‐ | 61 | ⊕⊝⊝⊝ | ||
| Leaving the study early (any reason) | 230 per 1000 | 476 per 1000 | RR 2.07 | 61 | ⊕⊝⊝⊝ | Control of risk rounded up from 22.58% to 23%. |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated as 'serious' ‐ diagnostic purity, attrition bias. Per‐Protocol analysis suspected. 2 Risk of imprecision: rated as ' very serious' ‐ percentages used to describe data and comparisons are made against an assumption of baseline measurements. Low n numbers. For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | ||||||
| Patient or population: schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with THIOTHIXENE (medium term) | Risk with ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (unchanged/worse ‐ CGI) | 600 per 1000 | 300 per 1000 | RR 0.50 | 20 | ⊕⊝⊝⊝ | The study did not report clinical improvement so unchanged/worse is reported for this outcome. Usually we report clinical improvement. |
| Adverse effects: Clinically important general adverse effect (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported on this outcome. | |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported on this outcome. | |
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported on this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported on this outcome. | |
| Leaving the study early (any reason) | 700 per 1000 | 399 per 1000 | RR 0.57 | 20 | ⊕⊝⊝⊝ | |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No studies reported on this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated as 'serious' ‐ likely selection and reporting bias. 2 Risk of publication bias: rated as 'strongly suspected' ‐ several papers published using the same cohort. 3 Risk of imprecision: rated as 'serious' ‐ low n numbers For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | ||||||
| Patient or population: schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with TRIFLUOPERAZINE (short term) | Risk with ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Adverse effects: Clinically important general adverse effect (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Leaving the study early (any reason) | 0 per 1000 | 0 per 1000 | not estimable | 72 | ⊕⊝⊝⊝ | Multi‐centre and multi‐drug trial with low numbers in each arm. |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated as 'serious' ‐ selection, attrition, reporting and performance bias likely. 2 Risk of imprecision: rated 'very serious' ‐ low n numbers 3 Risk of indirectness: rated 'very serious' ‐ multiple arms, some missing For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | ||||||
| Patient or population: schizophrenia | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | ||
| Risk with ZUCLOPENTHIXOL DEPOT (long term) | Risk with ZUCLOPENTHIXOL | ||||||
| Global state: Average endpoint global state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | ||
| Adverse effects: Clinically important general adverse effect (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | ||
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | ||
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | ||
| Mental state: Average endpoint general mental state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | ||
| Leaving the study early (any reason) | 80 per 1000 | 156 per 1000 | RR 1.95 | 46 | ⊕⊝⊝⊝ | Control of risk rounded from 7.69% to 8%. | |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1 Risk of bias: rated as 'very serious' ‐ single researcher did randomisation, selection bias likely, open label trial and incomplete outcome data. 2 Risk of publication bias: rated as 'strongly suspected' ‐ several papers published using the same data and cohort. 3 Risk of imprecision: rated 'very serious' ‐ low n numbers For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | |||||||
| Patient or population: schizophrenia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with CIS(Z)/TRANS(E) ZUCLOPENTHIXOL (short term) | Risk with CIS‐(Z) ZUCLOPENTHIXOL | |||||
| Global state: Average endpoint global state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Adverse effects: Clinically important general adverse effect | 470 per 1000 | 630 per 1000 | RR 1.34 | 57 | ⊕⊝⊝⊝ | Control of risk set at moderate and rounded up from 46.4% to 47%. |
| Death: Suicide and natural causes (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Service outcomes: duration of stay in hospital (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Mental state: Average endpoint general mental state score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| Leaving the study early: Any reason | 28 per 1000 | 61 per 1000 | RR 2.15 | 140 | ⊕⊝⊝⊝ | |
| General functioning: Average endpoint general functioning score (clinically improved) (no data) | not pooled | not pooled | not estimable | (0 studies) | No study reported this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated as 'serious' ‐ selection bias throughout. Very difficult to ascertain from published materials if selection bias has been minimised. 2 Risk of imprecision: rated 'very serious' ‐ low n numbers For risks rated as serious, we downgraded by 1. For risks rated as very serious we downgraded by 2. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Leaving the study early (any reason) Show forest plot | 2 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.01, 6.60] |
| 2 Adverse effects: 1. Clinically important change in specific adverse effects ‐ cardiovascular ‐ orthostatic Show forest plot | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.01, 6.60] |
| 3 Adverse effects: 2. Clinically important change in specific adverse effects ‐ central nervous system ‐ arousal state Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 excitation | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 2.62 [0.12, 59.40] |
| 3.2 sleepiness / sedation | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 2.89 [1.01, 8.30] |
| 4 Adverse effects: 3. Clinically important change in specific adverse effects ‐ endocrine ‐ menstruation started Show forest plot | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 7.0 [0.39, 126.48] |
| 5 Adverse effects: 4a. Any general adverse effects ‐ movement disorders ‐ EPSEs (UKU side effect rating scale, no scores) Show forest plot | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 6.07 [0.86, 43.04] |
| 6 Adverse effects: 4b. Clinically important change in specific adverse effects ‐ movement disorders ‐ EPSEs Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 parkinsonism | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 5.00 [0.26, 97.37] |
| 6.2 oculogyric crisis | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 69.09] |
| 6.3 tremor | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 5.00 [0.26, 97.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: 1. Average endpoint global state score ‐ Unchanged/worse (CGI, scores not reported) Show forest plot | 2 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.75, 1.13] |
| 2 Global state: 2. Average endpoint global state score ‐ No Recovery Show forest plot | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.89, 1.16] |
| 3 Global state: 3a. Average endpoint global state score (GAS, high score not reported, average score = 63.4) Show forest plot | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐8.12, 6.92] |
| 4 Global state: 3b. Average endpoint global state score (CGI‐SI, high score not reported, average score = 2.2) Show forest plot | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.49, 0.49] |
| 5 Mental state: 1. No clinically important change in general mental state ‐ Not improved (PANSS, scores not reported) Show forest plot | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.81, 1.18] |
| 6 Mental state: 2. No clinically important change in general mental state ‐ No clinical response Show forest plot | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.25, 2.42] |
| 7 Mental state: 3. Average endpoint general mental state score (BPRS, high score = 34.2) Show forest plot | 3 | 221 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐2.43, 3.23] |
| 8 Leaving the study early (any reason) Show forest plot | 6 | 766 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.36, 0.81] |
| 9 Adverse effects: 1. Any general adverse effects ‐ side effects (CGI, high score not reported) Show forest plot | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.77, 0.97] |
| 10 Adverse effects: 2. Average endpoint general adverse effect score ‐ average score (TESS, high score not reported, average score = 12.00) Show forest plot | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 4.48 [‐2.38, 11.34] |
| 11 Adverse effects: 3. Any change in specific adverse effects ‐ cardiovascular ‐ postural hypotension (dizziness/syncope) Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 1.73] |
| 12 Adverse effects: 4. Any change in specific adverse effects ‐ central nervous system ‐ arousal Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 excitation | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.07, 5.47] |
| 12.2 sedation | 2 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.73, 1.70] |
| 13 Adverse effects: 5. Any change in specific adverse effects ‐ metabolic ‐ weight change ‐ loss or gain of weight of 10 pounds Show forest plot | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.22, 1.75] |
| 14 Adverse effects: 6a. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs Show forest plot | 3 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.61, 1.45] |
| 15 Adverse effects: 6b. Any change in specific adverse effects ‐ movement disorders ‐ additional medication use Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: Average endpoint global state score ‐ Unchanged/worse (CGI) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Leaving the study early (any reason) Show forest plot | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.34, 2.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Leaving the study early (any reason) Show forest plot | 1 | 407 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Adverse effects: Any general adverse effects ‐ side effects ‐ frequency per day Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: 1. Average endpoint global state score ‐ Unchanged/worse (CGI) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Global state: 2. Average endpoint global state score (CGI, mean score = 1.25) Show forest plot | 1 | 49 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.30, 0.55] |
| 3 Leaving the study early (any reason) Show forest plot | 2 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.72, 1.35] |
| 4 Adverse effects: 1. Any change in specific adverse effects ‐ interference with functioning Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5 Adverse effects: 2. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs ‐ requiring medication Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6 Adverse effects: 3. Any change in specific adverse effects ‐ requiring additional medication Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7 Adverse effects: 4. Any change in specific adverse effects ‐ requiring hypnotics/sedatives Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: Average endpoint global state score ‐ unchanged/worse (global rating ‐ investigator opinion) ‐ medium term Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Leaving the study early (any reason) ‐ short/medium term Show forest plot | 2 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.27, 1.47] |
| 3 Adverse effects: 1. Any change in specific adverse effects ‐ central nervous system ‐ arousal ‐ requiring medication ‐ medium term Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4 Adverse effects: 2. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs ‐ requiring medication ‐ medium term Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mental State: 1. Average endpoint general mental state score (PANSS, average score = 45.8) ‐ medium term Show forest plot | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐3.20 [‐7.71, 1.31] |
| 2 Mental State: 2. Average endpoint general mental state score (PANSS General, average score medium term = 20.5) ‐ short/medium term Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Short term | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐2.40 [‐4.52, ‐0.28] |
| 2.2 Medium term | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐2.72, 2.12] |
| 3 Mental State: 3. Average endpoint general mental state score (PANSS Positive, average score = 9.8) ‐ medium term Show forest plot | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐2.69, 0.69] |
| 4 Mental State: 4. Average endpoint general mental state score (PANSS Negative, average score 11.5) ‐ medium term Show forest plot | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐1.5 [‐4.05, 1.05] |
| 5 Leaving the study early (any reason) ‐ short/medium term Show forest plot | 3 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.84, 2.02] |
| 6 Adverse Effects: 1. Any change in general adverse effects ‐ additional medication use ‐ short/medium term Show forest plot | Other data | No numeric data | ||
| 7 Adverse effects: 2a. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs ‐ requiring medication ‐ short term Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8 Adverse Effects: 2b. Any change in specific adverse effects ‐ movement disorders ‐ EPSEs (ESRS) ‐ short term Show forest plot | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 4.5 [0.67, 8.33] |
| 9 Adverse effects: 3. Any change in specific adverse effects ‐ negative and cognitive symptoms of schizophrenia ‐ short term Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 UKU ‐ asthenia/lassitude/increased fatiguability | 1 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.67, 1.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: 1. Average endpoint global state score ‐ Unchanged/worse (CGI) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Global State: 2. Average endpoint global state score ‐ Moderately or severely ill (CGI) Show forest plot | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.75, 1.30] |
| 3 Mental State: Average endpoint general mental state score (BPRS, average = 5.7) Show forest plot | 1 | 61 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐5.08, 2.48] |
| 4 Leaving the study early (any reason) Show forest plot | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 2.07 [0.97, 4.40] |
| 5 Adverse Effects: 1. Any change in general adverse effects ‐ additional medication use Show forest plot | Other data | No numeric data | ||
| 6 Adverse Effects: 2. Any change in specific adverse effects ‐ metabolic ‐ weight change Show forest plot | 1 | 61 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐8.35, 5.15] |
| 7 Adverse effects: 3. Any change in specific adverse effects ‐ requiring additional medication ‐ hypnotics/sedatives Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: Average endpoint global state score ‐ unchanged/worse (CGI) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Leaving the study early (any reason) Show forest plot | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.24, 1.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Leaving the study early (any reason) Show forest plot | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Leaving the study early (any reason) Show forest plot | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [0.36, 10.58] |
| 2 Behaviour: Average change in specific aspects of behaviour ‐ Violence during follow‐up Show forest plot | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.44, 1.71] |
| 3 Adverse Effects: 1a. Any general adverse effects ‐ additional medication use Show forest plot | Other data | No numeric data | ||
| 4 Adverse Effects: 1b. Any change in specific adverse effects ‐ additional medication use ‐ benzodiazepine use at least once Show forest plot | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.3 [0.59, 2.86] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: Average endpoint global state score ‐ Unwell Show forest plot | 3 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.80, 1.17] |
| 2 Mental state: Average endpoint general mental state score ‐ Not improved Show forest plot | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.45, 2.07] |
| 3 Leaving the study early (any reason) Show forest plot | 4 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 2.15 [0.49, 9.41] |
| 4 Adverse Effects: 1a. Any general adverse effects ‐ side effects reported Show forest plot | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.82, 2.18] |
| 5 Adverse Effects: 1b. Any change in specific adverse effects ‐ individual side effects Show forest plot | Other data | No numeric data | ||

