Antidepresivos para el dolor neuropático
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Double blind double dummy parallel design study, eight weeks four week titration to max tolerated dose of amitriptyline then four week stable dose Randomisation method not stated Inclusion criteria: age 21 to 85 years, duration of symptoms at least four months | |
| Participants | Painful diabetic neuropathy of three to five years. 235 participants (212 final number). Age range 21 to 85 years. Baseline pain score in amitriptyline group VAS 64.5 and in capsaicin cream group VAS 61.7 | |
| Interventions | Amitriptyline dose escalation from 25 mg to 125 mg daily orally + active placebo in first two weeks (methyl nicotinate). Capsaicin cream topically 4 x daily + active placebo (benzatropine dose escalation from 0.25 mg to 1.25 mg, and for first two weeks diazepam 2 mg to 6 mg | |
| Outcomes | Pain patients reported. 6‐item global improvement, VAS, pain relief by VAS (from no relief to complete relief) At least better on amitriptyline 79/108 (complete response 11, much better 35, better 33, no change 23, worse 5, much worse 1), on capsaicin cream 75/104 (complete response 8, much better 31, VAS decreased on amitriptyline Pain relief on amitriptyline Sleep improved on amitriptyline | |
| Notes | Dropouts: 9/117 Reason for withdrawal not stated QS = 4 (R2, DB1, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Double blind placebo controlled study for 90 days. Follow up between six to eight months | |
| Participants | Pre‐emptive treatment of PHN | |
| Interventions | Amitriptyline 25 mg at night or placebo for 90 days | |
| Outcomes | Complete pain relief, duration of pain, AEs Pain free at six months: 32/38 amitriptyline, 22/34 placebo | |
| Notes | Dropouts: eight ‐ either non compliant or lost to follow up QS = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Double blind placebo controlled crossover design four weeks. Two four weeks treatment period, no washout. No analyses of carry over effect. Patients with discogenic pain were excluded Patients used high carbohydrate, low protein and low fat diet during the study. Randomisation methods not stated | |
| Participants | Ten participants (eight final number). Any neuropathic pain: five with atypical facial pain, two with postherpetic neuralgia, one with trigeminal neuralgia and two with discogenic pain. Mean age 47.3 years (range 26 to 81), four males and four female patients | |
| Interventions | L‐tryptophan 4000 mg, or placebo daily orally | |
| Outcomes | Global improvement, pain rating index (PRI), present pain intensity (PPI), Beck depression inventory (BDI), Hamilton depression rating scale (HDRS), Hamilton anxiety scale (HAS) PRI: less pain in all patients during the active treatment than placebo. Pain scores on L‐tryptophan PRI 21.4, PI 2.9, on placebo PRI 31.0, 3.4 Depression scores on L‐tryptophan BDI 11.8, HDRS 9.2 and HAS 9.2; on placebo BDI 13.5, HDRS 12.8 and HAS 10.4 | |
| Notes | No dropouts, 2/10 patients with discogenic pain excluded from the review No withdrawals due to side‐effects QS = 3 (R1, DB1, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Methods | Parallel group single blind study, three months 67 patients included, 31 patients with tension headache were excluded from analysis. Numbers of patients are conflicting (36 randomised, results of 39 patients). Randomisation method not stated | |
| Participants | 36 participants. Trigeminal neuralgia in 17 patients and postherpetic neuralgia in 19 patients. Age range 35 to 70, 15 male and 21 female | |
| Interventions | Amitriptyline dose escalation from 30 mg to 110 mg, or clomipramine from 20 mg to 75 mg daily orally | |
| Outcomes | Pain patients reported, five‐item global improvement, patients global satisfaction with treatment (yes/no) At least moderate improvement on amitriptyline 8/39 (marked improvement 4, moderate 4, slight 4, no change 7, worse 0), on clomipramine 10/39 (marked improvement 4, moderate 6, slight 5, no change 4, worse 1) Patients with trigeminal neuralgia: at least moderate improvement on amitriptyline 3/9 (marked improvement 2, moderate 1, slight 2, no change 4, worse 0); on clomipramine 7/9 (marked improvement 3, moderate 4, slight 5, no change 1, worse 0) Patients with postherpetic neuralgia: at least moderate improvement on amitriptyline 5/10 (marked improvement 2, moderate 3, slight 2, no change 3, worse 0,); on clomipramine 3/11 (marked improvement 1, moderate 2, slight 4, no change 3, worse 1) 10 patients satisfied on amitriptyline, and 13 on clomipramine Three patients with trigeminal neuralgia satisfied on amitriptyline, 8 on clomipramine; 7 patients with postherpetic neuralgia satisfied on amitriptyline, 5 on clomipramine | |
| Notes | No dropouts QS = 1 (R1, DB0, W0) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Double blind placebo controlled parallel design, six weeks Inclusion criteria: age 18 to 65 years, duration of pain at least three months | |
| Participants | Central pain: spinal cord injury. 84 participants (84 final number). Age range 21 to 64, 67 male and 17 female patients Pain score in amitriptyline group NRS 5.5 (1.8) and MPQ 17.5 (9.8), in placebo group NRS 5.0 (1.7) and MPQ 15.7 (7.4). Depression score in amitriptyline group 17.1 (9.7) and in placebo group 13.3 (8.6) | |
| Interventions | Amitriptyline dose escalation form 10 mg to125 mg daily orally, median dose 50 mg / day; or active placebo benztropine 0.5 mg daily orally | |
| Outcomes | Pain patients reported, NRS (0‐10) and VRS (MPQ). 20‐item depression scale CES‐D Pain on amitriptyline NRS 4.5 (1.9) and MPQ 14.6 (9.7); on placebo Depression on amitriptyline On amitriptyline no patients reported poor sleep, on placebo three patients | |
| Notes | Dropouts: 8/44 on amitriptyline (7 SE, 1 failure to return week two medication), 3/40 on placebo (2 adverse events, 1 hospitalisation for an unrelated problem) SE: 43/44 on amitriptyline, 36/40 on placebo 7/44 withdrawn on amitriptyline (one constipation, three urinary retention and/or autonomic dysreflexia, three other systemic symptoms), 2/40 withdrawn on placebo (one constipation, one urinary retention and constipation) QS = 4 (R1, DB2, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Randomised parallel group study. Not blinded, two months with assessment at 14, 28 and 56 days | |
| Participants | 53 participants. Age 46 years(SD 12 years). Patients with depression and chronic pain. 14 complained of low back pain, 11 fibromyalgia, 5 PHN, 4 facial pain and 6 migraine | |
| Interventions | Fluoxetine 10 mg daily for two weeks then 20 mg daily or Fluvoxamine 50 mg daily then 100 mg daily | |
| Outcomes | Italian pain questionnaire, Pain rating index rank co‐efficient, Hamilton rating scale for depression Results: Both groups showed reduction in pain intensity. Fluvoxamine greater than fluoxetine (sig diff). Pain relief independent of any impact on depression | |
| Notes | Analysis per protocol (20 per group). Can't differentiate between those with neuropathic pain and non neuropathic. No evaluable data In first three days, 8/28 withdrew on fluvoxmine, 5/25 withdrew on fluoxetine due to nausea, somnolence and headache QS = 2 (R1, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Methods | Open label parallel design, 12 weeks (four week titration to max tolerated dose then eight week stable dose). Randomisation method not stated. | |
| Participants | Diabetic neuropathy of 8 to 48 months 25 participants (25 final number). Age range 61 to 83 years, 11 male and 14 female patients. Pain score in amitriptyline group 2.8 (0.8), in gabapentin group 2.9 (0.8) Duration of pain significantly longer in gabapentin group than in amitriptyline group | |
| Interventions | Amitriptyline dose escalation from 10 mg to 90 mg daily orally, median dose 53 mg (16 mg); or gabapentin dose escalation from 400 mg to 2400 mg daily orally, median dose 1785 mg (351 mg) | |
| Outcomes | Pain relief (pain score one or less), VRS (0 to 4) 7/12 on amitriptyline reported pain relief, 8/13 on gabapentin. | |
| Notes | No dropouts SE: 11/12 on amitriptyline, 4/13 on gabapentin; no withdrawals due to SE QS = 2 (R1, DB0, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Double blind placebo controlled parallel design, eight weeks (one dose escalation, thereafter stable dose). Randomisation method not stated Inclusion criteria: | |
| Participants | Traumatic myelopathy. 18 participants (18 final number). mean age 39 years, 16 male and 2 female patients Pain score in trazodone group by PRI 33.2 (6.9), by NWC 12.0 (1.7), by PPI 2.9 (0.6), by SPI day 58.2 (9.4), by SPI week 63.8 (7.0), by PAD 55.1 (4.6); in placebo group by PRI 31.2 (6.4), by NWC 12.3 (1.5), by PPI 2.1 (0.3), by SPI day 56.6 (8.7), by SPI week 62.6 (8.8), by PAD 55.8 (4.4) | |
| Interventions | Trazodone 150 mg or placebo daily orally | |
| Outcomes | Global assessment of efficacy (yes/no), MPQ: pain rating index (PRI), number of words (NWC), present pain intensity (PPI), Sternback pain intensity (0 to 100) day and week (SPI), Zung pain and distress index (PAD) Global improvement on trazodone 4/9 and on placebo 3/9 Pain on trazodone by PRI 33.5 (2.4), by NWC 14.0 (1.0), by PPI 2.6 (0.2), by SPI day 61.7 (6.8), by SPI week 73.9 (4.7), by PAD 67.2 (3.8); in placebo group by PRI 32.1 (3.5), by NWC 13.2 (1.5), by PPI 1.7 (0.2), by SPI day 63.4 (8.4), by SPI week 68.3 (6.9), by PAD 53.0 (3.2) | |
| Notes | Dropouts 6/18; 5/9 on trazodone, 1/9 on placebo Reasons for dropouts not stated SE: 4/9 on trazodone and 1/9 on placebo In placebo group there were more patients with sensory complete spinal cord injuries (four patients in placebo group, one in trazodone) QS = 2 (R1, DB1, W0) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Methods | Double blind placebo controlled parallel design, nine weeks, dothiepin versus dothiepin + nocturnal bite guards vs placebo vs placebo + nocturnal bite guards (analysed in two groups: dothiepin +/‐ bite guards and placebo +/‐ bite guards), 12 months follow‐up. Randomisation method not stated. | |
| Participants | Psychogenic facial pain of median 3.4 years (3 months to 30 years), 50 patients with facial arthro myalgia and 43 with atypical facial pain. 93 participants (93 final number). Age range 19 to 65, 20 male and 73 female patients Pain score in dothiepin group 2.2 (0.6), in placebo group 2.2 (0.6). Number of psychiatric cases 26/48 in dothiepin group and 27/45 in placebo group | |
| Interventions | Dothiepin dose escalation from 25 mg to 150 mg daily orally +/‐ nocturnal bite guard, mean dose 130 mg; or placebo daily orally +/‐ nocturnal bite guard | |
| Outcomes | Pain relief (yes or no), number of patients reduced analgesic use. Number of psychiatric cases Pain relief in 34/48 patients on dothiepin, 21/45 on placebo Reduction in analgesic use 40/48 patients on dothiepin, 19/45 on placebo Number of psychiatric cases 7/48 on dothiepin, 10/45 on placebo | |
| Notes | Dropouts: 1/48 on dothiepin (SE), 1/45 on placebo (SE) SE: 1/48 withdrawn on dothiepin (epilepsy), 1/45 on placebo (loss of consciousness). No effect of bite guard QS = 4 (R1, DB2, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Double blind placebo controlled crossover design 2 x 4 weeks. Two four week treatment periods, with two week washout Follow up for 12 weeks | |
| Participants | Atypical facial pain. Pain at least three on 11 point scale. 30 participants. Median age 52 (range 38 to 66) | |
| Interventions | Venlafaxine 37.5 mg vs placebo. Doses up to Venlafaxine 75 mg daily. NSAIDs and paracetamol allowed | |
| Outcomes | Pt reported VASPI, VRS, VASPR, anxiety, Beck depression, AEs and use of escape medication No significant difference between Venlafaxine and placebo for reduction in PI. > use of rescue meds in placebo group | |
| Notes | 10 dropouts. 8 due to AEs: 6 venlafaxine (nausea 5, fatigue 1), 2 placebo (rash 1, dizziness 1) 2 non compliant QS = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Open label placebo controlled parallel design, two weeks, carbamazepine + clomipramine vs transcutaneous electrical nerve stimulation. Randomisation method not stated. | |
| Participants | Postherpetic neuralgia, 29 participants (12 final number) Pain score in drug group 59.0 (9.2), in TENS group 27.0 | |
| Interventions | Clomipramine dose escalation from 10 mg to 75 mg daily orally and carbamazepine dose from 150 mg to 1000 mg daily orally; or transcutaneous electrical nerve stimulation (TENS) | |
| Outcomes | Global improvement, pain intensity VAS change, mental outlook‐VAS Marked pain relief in drug group 8/9 patients, in placebo 2/3 VAS degreased in drug group 42.3 (9.8), in TENS group 8.3 Improvement in mental outlook in drug group 29 (from 34 to 5), in TENS group 6 (from 17 to 11) | |
| Notes | Dropouts 17/29; in drug group dropouts and four crossed over to the other treatment group; in TENS group two dropouts and eight crossed over Side‐effects not reported QS = 2 (R1, DB 0, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Methods | Double blind placebo controlled crossover study, 30 days. Two 30 days periods, no washout. No analyses of carry over effect Dose escalation during the first week Randomisation method not stated | |
| Participants | Diabetic neuropathy. 24 participants (18 final number). Mean age 55 (range 30 to 73), 9 male and 9 female patients | |
| Interventions | Nortriptyline dose escalation from 30 mg to 60 mg and fluphenazine from 1.5 mg to 3 mg daily orally, or placebo daily orally | |
| Outcomes | Pain patients reported, pain relief 50% or more, VAS change from baseline Pain relief on active treatment 16/18 , on placebo 1/18 Pain decreased | |
| Notes | Dropouts 6/24 (one ketoacidosis, No withdrawals due to SE QS = 4 (R1, DB2, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Double blind double dummy crossover design study, 30 days. Two 30 day treatment periods (15 days titration to max dose then 15 days stable dose), two to four weeks washout, during which the symptoms returned to baseline level. During the washout period patient received placebos of both therapies Randomisation method not stated. First period results also available, but number of patients inadequate Inclusion criteria: | |
| Participants | Diabetic neuropathy of 2.15 years. 16 participants (14 final number). Mean age 47 years | |
| Interventions | Nortriptyline dose escalation from 10 mg to 60 mg and fluphenazine from 0.5 mg to 3 mg daily orally; or carbamazepine dose escalation from 100 mg to 600 mg daily orally | |
| Outcomes | Pain (VAS) change from baseline Pain decreased | |
| Notes | Dropouts: 2/16 (one upper GI bleeding ‐ alcohol gastritis related, one lack of adherence to the medication) SE: 8/16 on nortriptyline + fluphenazine; 3/16 on carbamazepine. 1/16 withdrawn due to alcohol related gastric bleeding on nortriptyline + fluphenazine QS = 4 (R1, DB2, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Double blind placebo controlled parallel design, eight weeks Randomisation method not stated Inclusion criteria: | |
| Participants | Postherpetic neuralgia of 33.4 (29.5) months. 50 participants (49 final number). Mean age 72.9 (10.1), 27 male and 22 female patients. Pain score VAS 55.22 (16.34) and MPQ 23.22 (13.23). Pain score per group: in amitriptyline VAS 55.9 (19.58) and MPQ 22.54 (13.95), in amitriptyline + fluphenazine VAS 47.6 (13.43) and MPQ 27.25 (17.71), in fluphenazine VAS 65.4 (10.87) and MPQ 21.75 (10.18), and in placebo group VAS 53.92 (17.05) and | |
| Interventions | Amitriptyline dose escalation from 12.5 mg to 200 mg, or amitriptyline from 12.5 mg to 200 mg + fluphenazine from 1 mg to 3 mg, or fluphenazine from 1 mg to 3 mg, or active placebo (glycopyrrolate or cellulose) daily orally | |
| Outcomes | Pain patients reported, VAS and MPQ. Beck Depression Inventory (BDI) Pain by VAS on amitriptyline Pain by MPQ on amitriptyline Depression by BDI on amitriptyline | |
| Notes | Dropouts: 1/12 on amitriptyline (SE), 0/12 on amitriptyline + fluphenazine, 0/13 on fluphenazine, 0/13 on placebo SE: one withdrawn on amitriptyline due to sedation Results of other depression scales also available QS = 4 (R1, DB2, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Parallel study design, six weeks (only three patients used levomepromazine). | |
| Participants | Postherpetic neuralgia. 35 participants (22 final number). Pain score in clomipramine group 4.1 (0.8) and in tramadol group 3.6 (0.7) | |
| Interventions | Clomipramine 100 mg +/‐ levomepromazine 100 mg, or | |
| Outcomes | 5‐item global improvement, 5‐item VRS At least satisfactory global improvement 6/11 on clomipramine, 9/10 on tramadol Pain on clomipramine 2.3, on tramadol 2.2 | |
| Notes | Dropouts: 7/18 on clomipramine, 7/17 on tramadol SE: 83.3 % on clomipramine, 76.5 % on tramadol. Withdrawn due to side effects QS = 2 (R1, DB0, W1) Pain results only in figures | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Parallel study design, five weeks (two weeks dose escalation of amitriptyline, one week of distigmine, thereafter stable dose). Patients were randomly allocated to three treatment groups, in addition fourth group of patients who have already taken amitriptyline were included in the study Patients in group four are excluded from the review as well as patients with low back pain and multiple sclerosis Randomisation methods not stated | |
| Participants | Any neuropathic pain. Duration of symptoms from four months to 13 years. Age range from 30 to 75 years. 65 participants (24 final number). Pain score in amitriptyline group 7.0, in distigmine group 6.8, and in placebo group 7.6 | |
| Interventions | Amitriptyline dose escalation from 25 to 75 mg; distigmine from 5 mg to 10 mg; or combination of amitriptyline and distigmine daily orally | |
| Outcomes | Pain intensity measured by VAS VAS on amitriptyline 4.9, on distigmine 4.5 and on combination therapy 4.2 | |
| Notes | Dropouts 41/65; 15/65 reason not stated, 14/65 in group four, 12/65 low back pain or multiple sclerosis. QS = 1 (R1, DB0, W0) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Methods | Double blind placebo controlled parallel design, 13 weeks. 178 patients included, 89 had also cognitive behavioral therapy, results of which was analysed separately (excluded) Randomisation methods not stated. | |
| Participants | Idiopathic facial pain. 98 participants (63 final number) Pain score 3.7 in fluoxetine group, 3.3 in placebo group | |
| Interventions | Fluoxetine 20 mg or placebo daily orally | |
| Outcomes | Pain patient reported, MPI (multidimensional pain inventory) Pain severity on fluoxetine 2.3, on placebo 2.7; | |
| Notes | Dropouts: 12/44 on fluoxetine, 14/45 on placebo Reason for withdrawal not stated Result presented only in figures QS = 4 (R1, DB2, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Methods | Double blind placebo controlled crossover design four weeks. Two four weeks treatment period and two weeks washout. No analyses of carry over effect | |
| Participants | Postoperative pain after breast cancer treatment in ipsilateral arm and scar area. 20 participants (13 final number). Mean age 56 years (range 39‐72), all females. Arm pain in 11, scar pain in 10 patients. Baseline pain score in arm MPQ words 8 (2 to 13), MPQ score 275 (49 to 654), VAS 5 (1.7 to 7.1) and VRS 4 (2 to 7); pain score in scar MPQ words 8 (5 to 15), MPQ score 326 (154 to 618), VAS 3.3 (1.4 to 6.2) and VRS 3 (2 to 6) Two patients were depressed, 8 patients in arm group had sleep disturbance and 6 in scar group Effect on daily life in arm group three (1 to 5), in scar group two (0 to 3) | |
| Interventions | Amitriptyline dose escalation from 5 mg to 100 mg daily orally (13 patients escalated up to 100 mg, two patients up to 50 mg), or placebo daily orally. | |
| Outcomes | Pain patients reported, VAS, VRS ( 0‐7), MPQ (number of words and score), pain relief (VRS 5‐item), Arm pain relief on amitriptyline Scar pain relief on amitriptyline Sleep disturbance in arm group on amitriptyline 1/13 and on placebo 6/13; in scar group on amitriptyline 0/13 and on placebo 6/13 patients. Effect on daily life in arm group one (0 to 4) on amitriptyline, 2 (0 to 4) on placebo; in scar group 0.5 (0 to 1) on amitriptyline and | |
| Notes | Dropouts 7/20 SE: 4/20 withdrawn due to SE (tiredness) QS = 3 (R1, DB1, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Double blind placebo controlled parallel design study, nine weeks (four week dose escalation to max tolerated dose then stable dose), follow up time 10 weeks | |
| Participants | HIV related painful neuropathy. 145 participants (121 to 128 final number) Mean age 41 years, 139 male and 6 female patients Pain score 1.02 (0.05) in amitriptyline group, 1.06 (0.04) in mexiletine group, 1.13 (0.04) in placebo group | |
| Interventions | Amitriptyline dose escalation 25 mg to 100 mg + inactive placebo, or mexiletine dose escalation from 150 mg to 600 mg + active placebo (benztropine 0.125 mg ‐ 0.500 mg), or inactive + active placebo daily orally | |
| Outcomes | Pain patient reported, pain relief (0 to 6), Gracely verbal scale (VRS) 0 to 1.75, analgesic consumption Complete pain relief on amitriptyline in 3/34 patients, Chance in Gracely scale on amitriptyline +0.31, on mexiletine +0.23, on placebo +0.20 Analgesic consumption on amitriptyline decreased in 7/41 patients, no change in 22/41 and increased in 12/41; on mexiletine decreased 7/44, no change 23/44, increased 14/44; on placebo decreased 10/43, no change 20/43, increased 13/43 | |
| Notes | Dropouts: 14/47 on amitriptyline (3 toxicity, 4 investigations or patients request , 4 miscellaneous, 2 lost to follow up, 1 did not receive treatment); 14/48 on mexiletine (4 toxicity, 3 investigators or patients requests, 6 miscellaneous, 1 SE: 3 withdrew on amitriptyline, 4 on mexiletine, 1 on placebo QS = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Double blind placebo controlled crossover design, six weeks. Two six weeks treatment periods (four weeks titration to max dose then two weeks stable dose), no washout. No carry over effect Randomisation method not stated Inclusion criteria: | |
| Participants | Postherpetic neuralgia of 28.5 months (3 months to 8 years). 26 participants (19 final number). Mean age 62 years (range 38 to 79 years), 17 male and 9 female patients | |
| Interventions | Desipramine dose escalation 12.5 mg to 250 mg daily orally, | |
| Outcomes | Pain patients reported, 6‐item global improvement At least moderate improvement 12/19 on desipramine (complete improvement 1, a lot 7, moderate 4, slight 2, no change 4, | |
| Notes | Dropouts 7/26 (SE or intercurrent medical illnesses) SE 19/19 on desipramine, 15/19 on placebo. Withdrawn due to SE 5/19 on desipramine (1 syncope, 1 palpitation and left bundle branch block, 1 chest pain, 1 fever, 1 vertigo); 3/19 on placebo (1 vertigo and nausea, 1one skin rash, 1 feeling of unsteadiness) Pain results illustrated only in figures QS = 3 (R1, DB1, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Double blind placebo controlled crossover design, five weeks (one week titration of dose then four weeks stable dose). Randomisation method not stated. Data biased, carry over effect. First period analyses also available, but inadequate number of patients | |
| Participants | Diabetic neuropathy over two years. 15 participants (12 final number). Mean age 55 (range 30 to 75), five male and seven female patients | |
| Interventions | Imipramine dose escalation 50 mg to 100 mg, or placebo daily orally | |
| Outcomes | Symptoms patients reported, 3‐item global improvement of neuropathic symptoms (including pain) On imipramine symptoms improved 8/12, no change 4/12, worse 0/12; on placebo improved 1/12, no change 11/12, worse 0/12 | |
| Notes | Dropouts 3/15 (2 poor compliance, 1 SE) SE: withdrawn 1 on desipramine (dizziness) Pain not analysed separately, included in neuropathic score QS = 4 (R1, DB2, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Double blind placebo controlled parallel design study for one year | |
| Participants | Prophylaxis of central post stroke pain after thalamic stroke. 39 participants age 36 to 68 | |
| Interventions | Amitriptyline extended release, 10 to 75 mg daily or placebo for one year | |
| Outcomes | Time to event (pain), Pain intensity, type, site and distribution. Presence/absence of allodynia. AES | |
| Notes | Two moderate AEs in amitriptyline group requiring dose reduction. two withdrew due to protocol violations QS = 4 (R1, DB2, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Double blind crossover design, two weeks. Two 2 weeks treatment periods, one week washout (three days titration of dose). First period analyses Randomisation assured from author | |
| Participants | Neuropathy of traumatic, infectious or surgical origin. 48 participants (39 final number) | |
| Interventions | Clomipramine dose escalation from 50 mg to 150 mg, or aspirin dose escalation to 1500 mg daily orally | |
| Outcomes | Pain physicians reported, 4‐item global improvement On clomipramine complete improvement 1/19, good 9/19, partial relief 4/19, no change 5/19; on aspirin complete improvement 1/20, good 3/20, partial relief 5/20, no change 11/20 | |
| Notes | Dropouts: 5/24 on clomipramine, 4/24 on aspirin Reason for withdrawal not stated. Patients and physicians reported similar results QS = 2 (R1, DB1, W0) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Double blind placebo controlled crossover design, four weeks. Two four week treatment periods, no washout. No analyses of carry over effect. First period analyses | |
| Participants | Atypical facial pain. 40 participants (40 final number) | |
| Interventions | Phenelzine 45 mg or placebo daily orally | |
| Outcomes | 4‐item global improvement. On phenelzine markedly improved 6/20, improved 9/20, no change 5/20, worse 0/20; on placebo markedly improved 1/20, improved 6/20, no change 9/20, worse 4/20 On phenelzine depression improved 15/20, no change 5/20, worse 0/20; on placebo improved 5/20, no change 14/20,worse 17/20 | |
| Notes | No dropouts No withdrawal due to SE QS = 4 (R1, DB2, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Double blind placebo controlled crossover design, four weeks Three four week periods, two one week washout periods (final doses reached on day six for amitriptyline and on day 18 for carbamazepine) Randomisation method not stated | |
| Participants | Central post stroke pain of 54 months (range 11 to 154). 15 participants (15 final number). Mean age 66 years (range 53 to 74), 12 male and 3 female patients. Pain score in amitriptyline group 4.7 (1.3), in carbamazepine group 4.6 (1.2), in placebo group 5.5 (1.5) Depression score 2.9 (range 0 to 6.5) | |
| Interventions | Amitriptyline dose escalation from 25 mg to 75 mg (75 mg for all patients); or | |
| Outcomes | Pain patients reported, 5‐item global improvement, 10‐step VRS 10‐item comprehensive psychopathological rating scale (CPRS) At least improved 10/15 on amitriptyline (complete improvement 0, Pain on amitriptyline 4.2 (1.6), on carbamazepine 4.2 (1.7), on placebo 5.3 (2.0) Depression on amitriptyline | |
| Notes | Dropouts 1/15 on carbamazepine (drug interaction) SE: 14/15 on amitriptyline, 13/15 on carbamazepine, 7/15 on placebo. No patients were withdrawn due to SE QS = 4 (R1, DB2, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Double blind parallel design, eight weeks. | |
| Participants | Burning mouth syndrome of 1.4 years. 76 participants (68 final number). Mean age 63.5, 16 male and 60 female patients Pain score 7.2 (1.2) in amisulpride group, 7.0 (1.2) in paroxetine group, 7.2 (1.0) in sertraline group HAM for depression 10.5 ( 2.4) and HAM for anxiety 15.5 (8.2) in amisulpride group, HAM‐D 10.3 (2.4) and HAM‐A | |
| Interventions | Amisulpride 50 mg, or paroxetine 20 mg, or sertraline 50 mg daily orally | |
| Outcomes | Pain patients reported, global improvement (global improvement score <3 and VAS reduced >50 %), VAS Hamilton rating scale for depression (HAM‐D) and for anxiety (HAM‐A) Global improvement VAS 3.2 (1.7) on amisulpride, 3.2 (2.1) on paroxetine, 2.8 (2.4) on sertraline HAM‐D 7.2 ( 3.0) and HAM‐A 10.4 (7.0) on amisulpride, HAM‐D 7.2 (2.7) and HAM‐A 11.1 (6.1) on paroxetine, HAM‐D 7.4 (1.8) and HAM‐A 11.6 (7.4) on sertraline | |
| Notes | Dropouts: 0/27 on amisulpride, 3/26 on paroxetine (1 lack of compliance, 1 side effects, 1 lack of efficacy), 5/23 on sertraline (1 lack of compliance, 1 concurrent medication, 2 side effects, 1 lack of efficacy) SE: withdrawn 0/27 on amisulpride, 1/26 on paroxetine, 2/23 on sertraline QS = 2 (R1, DB0, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Double blind placebo controlled crossover design, six weeks. Two six week periods, no washout (3 weeks titration of dose then 3 weeks stable dose). Carry over effect. First period analyses. | |
| Participants | Diabetic neuropathy of 2 years. 37 participants (29 final number). Mean age 57 years, 17 male and 12 female patients Pain score in amitriptyline group 0.91, in placebo group 1.2 14 depressed and 15 non depressed | |
| Interventions | Amitriptyline dose escalation from 25 mg to 150 mg daily orally, mean dose 116 mg (for first period), or active placebo benztropine 1 mg daily orally + diazepam 5 mg for days 1to 18 | |
| Outcomes | Pain patients reported, VRS (13‐item word list) Pain on amitriptyline 0.45, on placebo 0.89 | |
| Notes | Dropouts: 8/37 SE: 28/37 on amitriptyline, 25/37 on placebo; withdrawn on amitriptyline 3/37 (2 dizziness, 1 syncope), on placebo 3/37 (1 dizziness, 1 abdominal pain, 1 forgetfulness and increased pain) Pain results illustrated in figures only QS = 4 (R1, DB2, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Double blind placebo controlled crossover design, six weeks. Two six week periods, one week washout (three week titration to max tolerated dose then stable dose) Randomisation groups: placebo followed by amitriptyline, placebo followed by lorazepam, amitriptyline followed by lorazepam, and lorazepam followed by amitriptyline Randomisation methods not stated. A significant drug‐time interaction Inclusion criteria: | |
| Participants | Postherpetic neuralgia of 19 months (range 3 months ‐ 25 years). 62 participants (41 final number). Mean age 72 (range 25 to 86), 31 male and 27 female patients, 15 depressed and 43 non depressed | |
| Interventions | Amitriptyline dose escalation from 12.5 mg to 150 mg, mean dose 65 mg; or lorazepam from 0.5 mg to 6 mg, mean dose 2.4 mg; or placebo (lactose 250 mg ‐ 1500 mg) daily orally | |
| Outcomes | Pain patients reported, 6‐item global improvement At least moderate improvement 16/34 on amitriptyline (complete improvement 1, a lot 12, moderate 3, slight 10, no change 6, | |
| Notes | Dropouts 21/62 SE: 55/62 on amitriptyline, 62/62 on lorazepam, 45/62 on placebo. Withdrawn due to SE 5 on amitriptyline (1 rash, 1 palpitation, 1 dizziness, 1 sedation, 1 urinary retention), 6 on lorazepam (4 acute depression, 1 ataxia, 1 nightmares), 3 on placebo (1 dizziness, 1 disorientation, 1 rash) Results illustrated only in figures QS = 3 (R1, DB1, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Double blind placebo controlled crossover design, six weeks. Two six week periods, no washout, no carry over effect (four week titration to max tolerated dose then stable dose) Randomisation methods not stated. | |
| Participants | Diabetic neuropathy of 24 months (range 5 to 120). 24 participants (20 final number). Mean age 62 years (range 21 to 71), 15 male and 9 female patients. 4 depressed and 16 non depressed by Hamilton; 7 depressed and 13 non depressed by psychiatrist's | |
| Interventions | Desipramine dose escalation from 12.5 mg 250 mg, mean dose 201 mg (87.5 to 250 mg), or active placebo (benztropine 0.5 mg ‐ 1 mg and lactose) daily orally | |
| Outcomes | Pain patients reported, 6‐item global improvement At least moderate improvement on desipramine 11/20 (complete improvement 0, a lot 4, moderate 7, slight 2, no change 5, worse 2), on placebo 2/20 (complete improvement 0, a lot 1, moderate 1, slight 3, no change 5, worse 10) | |
| Notes | Dropouts 4/24; on desipramine 2 (SE) , on placebo 2 (1 angina pectoris, 1 lack of effect) SE: 18/20 on desipramine, 17/20 on placebo. Withdrawn due to SE 2/20 on desipramine (1 seizure, 1 insomnia), 0/20 on placebo Results illustrated only in figures QS = 3 (R1, DB1, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Crossover design, six weeks. Two six weeks periods, two week washout, no carry over effect (four week titration to max tolerated dose then stable dose). Patients were randomised to two different studies (Max 1992a and b). 49 randomised + 5 additional patients. 5 additional patients were excluded from the review, only first period results available Randomisation methods not stated, blinding not clear Inclusion criteria: | |
| Participants | Diabetic neuropathy of 3 years (range 0.5‐12). 54 participants (25 final number in first period analyses: 12 in amitriptyline group and 13 on desipramine group). Mean age 58 years (range 20 to 84), 33 male and 21 female patients | |
| Interventions | Amitriptyline escalation from 12.5 mg to 150 mg, mean dose 105 mg (37 mg), or desipramine from 12.5 mg to 150 mg, mean dose 111 mg (39 mg) daily orally | |
| Outcomes | Pain patients reported, VRS Pain score decreased on amitriptyline 0.47 (0.09), on desipramine 0.45 (0.12) | |
| Notes | Dropouts 16/54 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Crossover design, six weeks. Two six week periods, two week washout, no carry over effect (four week titration to max tolerated dose then stable dose). Patients were randomised to two different studies (Max 1992a and b). 37 randomised + 17 additional non‐randomised patients. 17 non randomised patients were excluded from analyses, only first period results available Randomisation methods not stated, blinding not clear | |
| Participants | Diabetic neuropathy of 4 years (range 0.5 to 12). 54 participants (27 final number in first period analyses: 12 on in fluoxetine group and 15 in placebo group). Mean age 58 (range 25 to 84), 31 male and 23 female patients | |
| Interventions | Fluoxetine dose escalation from 20 mg to 40 mg daily orally (40 mg for all patients, except one); or active placebo benztropine from 0.125 mg to 1.5 mg daily orally, mean dose 1.3 (0.2 mg) | |
| Outcomes | Pain patients reported, VRS Pain score decreased on fluoxetine 0.35 (0.11), on placebo 0.15 (0.07) | |
| Notes | Dropouts 8/54(5 SE, others not reported) SE: 29/46 on fluoxetine, 31/46 on placebo Withdrawn due to SE 3/46 on fluoxetine (1 orthostatic hypotension, 1 headache, 1 rash), 2/46 on placebo (1 fatigue, 1 chest pain) QS = 1 (R1, DB0, W0) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Double blind parallel group four week study. | |
| Participants | Any neuropathic pain of 62.7 months. 200 participants (151 final number). Mean age 46 years, 63 male and 88 female patients. Pain score in doxepin group 7.29, in capsaicin group 7.11, in doxepin + capsaicin group 7.47, in placebo group 7.13 | |
| Interventions | 3.3 % doxepin hydrochloride x 3 / day topically, or 0.025 capsaicin cream x 3 / day, or 3.3% doxepin + 0.025% capsaicin x 3 / day, or placebo (aqueous cream) x 3 / day | |
| Outcomes | Pain patients reported, VAS, patients wish to continue therapy Number of patiens wished to continue doxepin 17/41, capsaicin 13/41, doxepin + capsaicin 9/33, placebo 1/36 VAS decreased on doxepin 0.9 (95% CI 0.34‐1.46), on capsaicin 1.12 (0.44‐1.8), on doxepin + capsaicin 1.07 (0.39‐1.75), no change on placebo | |
| Notes | Dropouts 49/200 Reason for withdrawal not stated. Duration of pain was significantly longer in the combination group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Double blind parallel group four week study of topical doxepin | |
| Participants | Any neuropathic pain of 69 months (range 3 to 324). 40 participants (30 final number). Mean age 52 years (range 27 to 80) Pain score in doxepin group 6.22 (2.51), in placebo group | |
| Interventions | 5% doxepin hydrochloride x 2/day topically, or placebo (aqueous cream) | |
| Outcomes | Pain patients reported, VAS VAS on doxepin 5.04 (2.61), on placebo 6.91 (2.15). VAS decreased on doxepin 1.18 (2.01), on placebo VAS increased 0.42 (1.5) | |
| Notes | Dropouts 10/30; 4/20 on doxepin, 6/20 on placebo Reason for withdrawal not stated QS = 4 (R2, DB2, W0) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Methods | Double blind placebo controlled crossover design, two week no washout, carry over effect not analysed | |
| Participants | Neuropathic cancer pain range 4 to 7 on 11 point scale. 16 advanced cancer patients on systemic morphine therapy. Age 55 to 78 | |
| Interventions | Amitriptyline up to 50 mg at night for patients < 65 yrs, Amitriptyline up to 30 mg at night for patients > 65 yrs. All patients used Morphine | |
| Outcomes | Opioid consumption, global pain intensity. | |
| Notes | No washout so likely to be significant carry over for in first phase Amitriptyline group AEs reported as drowsiness, confusion, dry mouth QS = 3 (R1, DB1, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Double blind double dummy crossover design six weeks, two six week periods, one week washout, no carry over effect (one week dose titration then stabile dose). First period results also available. | |
| Participants | Diabetic neuropathy of 5.7 (4.2) years. 25 participants (21 final number). Mean age 60.4 (10.8) years, 24 male and one female patients | |
| Interventions | Amitriptyline dose escalation from 12.5 mg to 75 mg orally, mean dose 59 mg; or gabapentin from 300 mg to 1800 mg orally, mean dose 1565 mg | |
| Outcomes | Pain patients reported, 6‐item global improvement , 13 words VRS At least moderate improvement on amitriptyline 14/21 (complete improvement 1, a lot 4, moderate 9, slight 4, no change 3, worse 0), on gabapentin 11/21 (complete improvement 1, a lot 5, moderate 5, slight 3, no change 6, Pain decreased 0.44 (0.089) in 9 patients on amitriptyline, 0.31 (0.064) in 10 patients on gabapentin during the first study period | |
| Notes | Dropouts 4/25, on amitriptyline 2 (1 protocol violation and 1 SE), on gabapentin 2 (1 SE and 1 SE + protocol violation) Early crossover from amitriptyline to gabapentin 1/13 (SE), from gabapentin to amitriptyline 2/12 (SE and lack of effect) SE: 17/21 on amitriptyline, 18/21 on gabapentin. Withdrawn due to SE 2/21 on amitriptyline, 3/21 on gabapentin QS = 4 (R1, DB2, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Double blind placebo controlled crossover design, three weeks (one week dose titration then stable dose) Three week periods, no washout, carry over effect not analysed Randomisation method not stated Inclusion criteria: age from 18 to 80 years, duration of symptoms at least six months | |
| Participants | Central pain: phantom or stump pain 28 patients, posttraumatic nerve lesions 7, postherpetic neuralgia 4. 39 participants (24 final number). Mean age 49 years, 22 male and 17 female patients. Mean duration of pain 20.6 months. Pain score in clomipramine group 49.1 (17.13), in nortriptyline group 45.9 (16.6), in placebo group 37.1 (13.13) In clomipramine group non‐depressed 3 (HAM score < 7), borderline depressed 1 (HAM 8‐13), moderate or severely depressed 4 (HAM > 13); in nortriptyline group | |
| Interventions | Clomipramine dose escalation from 25 mg to 100 mg, or | |
| Outcomes | Pain patients reported, VAS. HAM depression score VAS on clomipramine 12 (7), on nortriptyline 28 (16), on placebo 36.5 (16) In depressed patiens VAS on clomipramine 15 (2.5), on nortriptyline 24 (21), on placebo 30 (SD 17); in | |
| Notes | Dropouts 15/39; on clomipramine 1 (poor efficacy), on nortriptyline 7 (5 poor efficacy, 2 poor tolerability), on placebo 7 (6‐poor efficacy, 1 poor tolerability) SE: 23/39 on clomipramine, 22/39 on nortriptyline, 10/39 on placebo Withdrawn due to SE 2/39 on nortriptyline, 1/39 on placebo Results illustrated in figures QS = 2 (R1, DB0, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Double blind placebo controlled crossover design, six weeks (two week dose titration then stable dose). Two six week periods, no washout, carry over effect not analysed Randomisation method not stated | |
| Participants | Chronic intractable pain without specific organic cause. 52 participants (21 final number). | |
| Interventions | Amitriptyline dose escalation from 50 mg to 150 mg daily orally, or placebo | |
| Outcomes | Global improvement clinicians reported, VAS, Zung depression questionnaire Partial or complete pain relief 4/12 on amitriptyline, 3/12 on placebo VAS on amitriptyline 50.62, on placebo 53.03 Depression score 50.24 on amitriptyline, 49.38 on placebo | |
| Notes | Dropouts 20/52 (10 on amitriptyline and 10 on placebo, mainly related to side effects) Side effects reported in scores Clinicians and patients reported global improvement did not differ significantly QS = 3 (R1, DB1, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Double blind placebo controlled crossover design, three treatment periods of eight weeks. (four week dose titration two week maintenance, two to three week taper off),one week washout, carry over effect not analysed Randomisation method not stated | |
| Participants | PHN with pain of at least three months after resolution of lesions. 76 participants. Median age 73 yrs (range 32 to 90) | |
| Interventions | Morphine up to 240 mg daily , nortriptyline up to 160 mg daily or placebo in 2 or 3 divided doses. Drugs in same class also offered (methadone or desipramine) | |
| Outcomes | Pt reported 11 point PI and PR, cognitive function, sleep, mood,. AEs and treatment preference Mean dose for morphine 91 mg (15 mg to 225 mg). Reduction in pain scores greater on Morphine: 2.2 (95%CI 1.6 to 2.7), nortriptyline 1.2 (95%CI 0.7 to 1.7), Treatment preference : opioids 54%, TCA 30%, Placebo 16% | |
| Notes | 50 completed 2 periods and 44 completed 3 periods. 20 dropouts on opioids, 6 TCA, 1 placebo QS = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Double blind placebo controlled parallel group study, two weeks treatment. Follow up for six months | |
| Participants | Pre‐emptive treatment of post mastectomy pain syndrome. 100 participants age 38 to 54 yrs | |
| Interventions | Venlafaxine 75mg SR at night for two weeks or placebo starting night prior to surgery. Post op PCA used | |
| Outcomes | VASPI , pain scores at four hours, one month and at six months. Pain at rest, movement, arm and chest wall pain. sensory tests. analgesic consumption. Axilla pain: 29/48 had pain in venlafaxine group, placebo 24/47 at six months | |
| Notes | 94 completed , no withdrawals for AEs QS = 4 (R2, DB1, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Randomised double blind placebo controlled parallel group study, six weeks treatment | |
| Participants | 39 participants age 22 to 65 years, Amputation related pain of > 6 months. Average pain at least 2 on 11 point scale | |
| Interventions | Amitriptyline 10 mg / day up to 125 mg/day. Active placebo (benztropine 0.5 mg) dose not escalated | |
| Outcomes | Pt reported average PI on 11 pt scale. SF McGill, unmodified BPI, depression scale. Functional ability assessment, satisfaction with life Amitriptyline was not different from placebo for phantom limb pain or residual limb pain. No sig diff in depression scores between amitriptyline and placebo | |
| Notes | Two withdrew in amitriptyline group due to AEs. Dry mouth, dizziness commonly reported QS = 4 (R1, DB2, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Multicentre randomised double blind placebo controlled parallel group study with dose escalation over first two weeks | |
| Participants | 245 participants with pain full diabetic neuropathy of at least moderate severity for three months or longer and metabolically stable (Type 1 or type 2 diabetes) | |
| Interventions | Placebo, venlafaxine 75 mg or venlafaxine 150 to 225 mg daily for six weeks followed by two week tapered dose | |
| Outcomes | VASPI, VASPR, clinical global impressions‐severity (CGI‐s) and CGI‐I (improvement)‐ both clinician assessed Patients global rating of pain relief Results: | |
| Notes | Withdrawals: totals 12/81 placebo, 12/81 Ven 75, 18/82 Ven150/225 QS = 4 (R1, DB2, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Double blind placebo controlled crossover design, six weeks (one week dose titration then stable dose). Two six week periods, no washout, no carry over effect Randomisation method not stated Inclusion criteria: age at least 18 years, duration of symptoms at least three months | |
| Participants | Any neuropathic pain of four years. 41 participants (41 final number) Mean age 60 years (range 23 to 88), 19 male and 22 female patients. Pain score 5.7 (0.26) | |
| Interventions | Bupropion dose escalation from 150 mg to 300 mg daily orally, or placebo | |
| Outcomes | Pain patients reported, 5‐item global improvement, Wisconsin Brief Pain Inventory (0 to 10). Sleep and mood (from 0 no problems to 10 major problems). Pain at least improved 30/41 on bupropion (complete improvement 1, much improved 14, improved 15, no change 8, Pain on bupropion Mood on bupropion 2.85 (0.44), on placebo 4.46 (0.41) Sleep on bupropion 2.93 (0.48), on placebo 4.15 (0.48) | |
| Notes | Dropouts 4/41; SE: 22/41 on bupropion, 8/41 on placebo. Withdrawn due to SE: 2/41 on bupropion (1 dizziness, 1 nausea and vomiting), 1/41 on placebo (nausea and vomiting) QS = 4 (R1, DB2, W10 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Double blinded placebo controlled crossover study four weeks. Randomly allocated to one of three groups: low dose amitriptyline versus placebo, high dose amitriptyline versus placebo, or high dose versus low dose Patients in group high vs low amitriptyline are excluded from the review. Two four week treatment periods and two weeks washout Randomisation method not stated Inclusion criteria: | |
| Participants | Chronic facial pain including both musculoskeletal and neurogenic origin. 32 participants (19 final number) Mean age 41.5 years, 6 male and 22 female patients | |
| Interventions | Amitriptyline low dose escalation from 10 mg to 30 mg daily orally, mean dose 23.6 mg; high dose from 50 to 150 mg, mean dose 129.4 mg; or placebo | |
| Outcomes | Change in pain intensity (VAS) and MPQ, pain relief‐VAS, Hamilton depression inventory (HDI) Change in pain intensity on amitriptyline 29, on placebo 5; change in MPQ on amitriptyline 11 and on placebo 4; pain relief on amitriptyline 32 and on placebo 19 | |
| Notes | Dropouts 19/32 (2 use of other drugs, 2 failure to complete the study, 9 low versus high amitriptyline comparison) Side effects not reported Results from figures QS = 4 (R1, DB2, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Methods | Double blind parallel group study, 14 weeks. Original study design: amitriptyline + standardised acupuncture regimen (SAR) vs amitriptyline + control points vs placebo + SAR vs placebo + control points (125 patients). Later additional 114 patients were randomised between SAR and control points and 11 patients between amitriptyline and placebo. From these patients 136 were able to comparison between amitriptyline +/‐ SAR or control points and placebo +/‐ SAR or control points Inclusion criteria: | |
| Participants | HIV associated peripheral neuropathy. 136 participants (101 final number). Mean age in amitriptyline group 40.1 (7.1) years, in placebo group 39.9 (5.9), 124 male and 12 female patients. Pain score in amitriptyline group 1.10 (0.3), in placebo group 1.13 (0.3) | |
| Interventions | Amitriptyline dose escalation from 25 mg to 75 mg daily orally, | |
| Outcomes | 6‐item global improvement, Gracely verbal scale (0.0 to 1.75), 39‐item QOL assessment tool At least moderate pain relief 31/61 on amitriptyline (complete improvement 3, a lot 6, moderate 22, slight 14, none 11, worse 5), 28/60 on placebo (complete improvement 3, a lot 10, moderate 15, slight 13, none 11, worse 8) Gracely score decreased 0.26 on amitriptyline, 0.30 on placebo Mean change in QOL 7.1 on amitriptyline, 0.6 on placebo | |
| Notes | Dropouts 35/136; 22/71 on amitriptyline, 13/65 on placebo Reasons for dropout not stated QS = 3 (R1, DB2, W0) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Methods | Double blind placebo controlled parallel group study, eight weeks Gabapentin non‐responding patients were randomised to gabapentin + venlafaxine or gabapentin + placebo (dose escalation during the first three weeks, thereafter stabile dose Randomisation methods not stated | |
| Participants | Diabetic neuropathy. 11 participants (7 final number) Pain score in venlafaxine group 6.4, in placebo group 6.5 | |
| Interventions | Gabapentin dose escalation from 300 to 3600 mg + venlafaxine dose escalation from 37.5 mg to 150 mg daily orally; or maximal tolerated dose of gabapentin + placebo | |
| Outcomes | Global improvement, pain score (0 to 10) Much or moderate pain relief on venlafaxine 3/4 patients , on placebo 1/3 patients Pain score on venlafaxine 4.4, on placebo 6.1 | |
| Notes | Dropouts 4/11; 2/6 on venlafaxine (1 treatment failure, 1 side‐effects); 2 on placebo (treatment failure) Withdrawn due to side‐effects 1/6 on venlafaxine QS = 4 (R1, DB2, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Methods | Double blind placebo controlled crossover design, three weeks (dose finding before randomisation according to plasma levels). Two three week periods, no washout, no carry over effect Randomisation method not stated Inclusion criteria: duration of symptoms at least one year | |
| Participants | Diabetic neuropathy. 13 participants (9 final number) Mean age 49, 4 male and 5 female patients | |
| Interventions | Imipramine dose escalation from 125 mg to 200 mg daily orally, | |
| Outcomes | Pain patients reported, 6‐item neuropathic scale including pain, global improvement in neuropathic score 8/9 patients preferred imipramine, 1/9 preferred placebo Neuropathic score lower on imipramine 8/9 patients, on placebo 0/9, no difference in one patient Mean neuropathic score 2.2 on imipramine, 5 on placebo | |
| Notes | Dropouts 4/13; 1/13 on imipramine (SE), 2/13 on placebo (SE), 1/13 group is not known (acute myocardial infarction) SE: withdrawn due to SE 1/9 on imipramine (dizziness), 2/9 on placebo (dizziness) Pain not analysed separately,included in 6‐item neuropathic score QS = 4 (R1, DB2, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Double blind placebo controlled crossover design, two weeks (imipramine dose finding before randomisation according to plasma levels). Three two week periods, two to four weeks washout for slow metabolizers when needed, no washout for extensive metabolizers, no analyses of carry over effect Randomisation method not stated Inclusion criteria: duration of symptoms at least one year | |
| Participants | Diabetic neuropathy of 4.75 years (range 1 to 12 years) 26 participants (20 final number). Mean age 46.9 years (range 28 to 75), 10 male and 10 female patients | |
| Interventions | Paroxetine 40 mg; or imipramine from 25 mg to 350 mg (mean dose 197.5 mg); or | |
| Outcomes | Pain patients reported, 5‐item pain score (0 to 2) Pain score on paroxetine 0.49, on imipramine 0.52, on placebo 1.47 | |
| Notes | Dropouts 7/26 (four SE, two need of analgesia not related to neuropathy, SE: five patients withdrawn on imipramine QS = 4 (R1, DB2, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Double blind placebo controlled crossover design, two weeks Three two week periods, at least one week washout for extensive metabolisers and at least three weeks for poor metabolisers. Some residual effect after clomipramine. | |
| Participants | Diabetic neuropathy of 3.5 years (range 1 to 20). 26 participants (19 final number). Mean age 54.7 years (range 29 to 78), 9 male and 10 female patients | |
| Interventions | Clomipramine 50 mg for poor metabolisers and 75 mg for extensive metabolisers; or | |
| Outcomes | 5‐item VRS (0 to 2) Pain on clomipramine 0.99 (range 0 to 2.0), on desipramine 1.02 (range 0 to 2.0), on placebo 1.5 (range 0.5 to 2) | |
| Notes | Dropouts 7/26; on clomipramine 4 (3 SE, 1 lack of effect), on desipramine 3 (SE) SE: withdrawn on clomipramine 3/26 (nausea, tiredness, dizziness, confusion), on desipramine 3/26 (1 nausea, 1 tiredness, 1 dizziness) QS = 4 (R1, DB2, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Double blind placebo controlled crossover design, three weeks. Two three week periods, one week washout, no carry over effect. First period results also available, but number of patients inadequate Randomisation method not stated | |
| Participants | Diabetic neuropathy of four years (range 1 to 17). 18 participants (15 final number). Mean age 56 years (range 31 to 66), 12 male and 3 female patients Neuropathic score in citalopram group 6.0, in placebo group 6.2 | |
| Interventions | Citalopram 40 mg daily orally, or placebo | |
| Outcomes | Symptoms patients reported, 6‐item neuropathic score (0 to 2 each) Neuropathic score on citalopram 4.5, on placebo 7.0 | |
| Notes | Dropouts 3/18; (1 SE, 1 poor control of diabetes, 1 measurable level of citalopram during both treatment periods) SE: withdrawn 2/18 on citalopram (1 nausea and vomiting, 1 gastric upset and diarrhoea) Pain not reported separately, only neuropathic score available QS = 4 (R1, DB2, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Double blind placebo controlled crossover design, two weeks Three two week periods, one to three weeks washout, no carry over effect. First period results available, but number of patients inadequate Randomisation method not stated | |
| Participants | Diabetic neuropathy of 3.7 years (range 1 to 11). 22 participants (18 final number). mean age 55.8 years (range 29 to 80), 9 male and 9 female patients | |
| Interventions | Mianserin 60 mg; or | |
| Outcomes | Symptoms patients reported, 6‐item neuropathic score (0 to 2 each item) Neuropathic score on mianserin 5.5, on imipramine 4.0, on placebo 5.0 | |
| Notes | Dropouts: 4/22; 1 on mianserin (personal reasons), 1 on imipramine (SE), 2 on placebo (1 SE and 1 persona reasons) SE: withdrawn 1/22 on imipramine, 1/1 on placebo Pain not analysed separately, only neuropathic score available QS = 4 (R1, DB2, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Double blind placebo controlled crossover design, five weeks. Two five week periods, at least one week washout, no carry over effect Inclusion criteria: age at least 20 years, duration of symptoms at least six months | |
| Participants | Polyneuropathy (diabetic 18, non‐diabetic 29). 54 participants (47 final number) Mean age 58 years (range 30 to 82), 31 male and 16 female patients Pain score 14 (25 to 75 % CI: 9 to 19), mean consumption of paracetamol 500 mg six tablets / week (25 to 75 % CI: 0 to 22) | |
| Interventions | St.John's wort (total hypericin) 2700 mcg daily orally, or placebo | |
| Outcomes | Pain patients reported, 6‐item global improvement, sum pain score (0 to 40), paracetamol weekly consumption (number of 500 mg tablets), overall period reference On St.John's complete or good improvement 6/47, moderate 3/47, slight 4/47, no change 22/47, worse 12/47: on placebo complete or good improvement Pain on St.John's 14 (25 to 75 % CI: 7 to 21), on placebo 15 (9 to 19) Paracetamol consumption on St.John's 4 (25 to 75 % CI: 0 to 21), on placebo 5 (0 to18) Overall period preference 25 for St.John's, 16 for placebo, 6 no difference | |
| Notes | Dropouts 7/54; SE: 13/54 on St. Johns, 15/54 on placebo. Withdrawn due to SE 1/54 on St. johns, 1/54 on placebo QS = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Double blind placebo controlled crossover design, three way crossover, 3 x 4 week periods, one week washout | |
| Participants | Painfull polyneuropathy of > 6 months duration. 40 participants mean age 56, range 31 to 69 yrs | |
| Interventions | Venlafaxine 225 mg, imipramine 150 mg or placebo | |
| Outcomes | Patient rated pain paroxysms, constant pain, touch and pressure evoked pain; all on 11 point VAS. Global impression of pain relief 6 point (none to complete 5 pt or worse). AEs, rescue medication NNTs for moderate or better pain relief Venlafaxine 5.2 (2.7 to 5.9), imipramine 2.7 (1.8 to 5.5). For moderate or better pain relief: 2/33 placebo, 8/33 venlafaxine, 14/33 imipramine | |
| Notes | 33 completed all 3 arms. 7 withdrew due to AEs. (2 placebo, 4 venlafaxine, 1 imipramine) one lost to follow up QS = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Double blind placebo controlled parallel group design, eight weeks (dose titration during the first five days). Pain more intensive in trazodone group at baseline. | |
| Participants | Burning mouth pain from six months to 20 years. 37 participants (28 final number). Mean age 58.6 years (range 39 to 71), all females. Pain score in trazodone group 59.2 by VAS and 8.2 by MPQ; in placebo group VAS 46.6 and MPQ 7.5. 17 patients depressed | |
| Interventions | Trazodone dose escalation from 100 mg to 200 mg daily orally, or placebo | |
| Outcomes | Pain patients reported, 3‐item global improvement, VAS, VRS (MPQ) On trazodone pain improved 8/11, no change 2/11, worse 1/11; on placebo improved 13/17, no change 4/17, VAS on trazodone 45.3, on placebo 34.3 Benefit in relation to side‐effects: on trazodone | |
| Notes | Dropouts 9/37; SE: 16/18 on trazodone, 11/19 on placebo Withdrawn due to SE 7/18 on trazodone, 2/19 on placebo QS = 4 (R1, DB2, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Double blind placebo controlled crossover design, four weeks (four weeks dose titration to max tolerated dose). Two four week periods, two weeks washout, no carry over effect | |
| Participants | Postoperative neuropathic pain in breast cancer patients. 15 participants (13 final number). Mean age 55 years (range 37 to 72), all females. Pain score by VRS (0 to 7) 3 (range 3 to 4), depression score 10 (range 1 to 28) | |
| Interventions | Venlafaxine dose escalation from 18.75 mg to 75 mg daily orally, (11 had 75 mg); or placebo | |
| Outcomes | Pain patients reported, pain relief (VRS 0 to 4), pain intensity VRS (0 to 7). Beck's Depression Inventory (0 to 63) Pain relief on venlafaxine 2 (range 0 to 4), on placebo 0 (range 0 to 4) Pain intensity on venlafaxine 1 (range 0 to 3), on placebo 2 (range 0 to 4) Depression score on venlafaxine 7 (range 1 to 39), on placebo 7 (range 1 to 11) | |
| Notes | Dropouts 2/15 (1 SE and 1 no compliance) SE: withdrawn 1/13 on venlafaxine (nausea, sweating, headache) QS = 4 (R2, DB1, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Double blind placebo controlled parallel group study, three months Randomisation methods not stated Inclusion criteria: | |
| Participants | Diabetic neuropathy. 59 participants (59 final number). Age range 20 to 59 years, 27 male and 32 female patients. All patients had pain at baseline Depression score 8.4 (0.6) in imipramine group, 7.8(0.4) Sleep was disturbed in all patients at baseline | |
| Interventions | Imipramine 100 mg, or amitriptyline 100 mg, or placebo daily orally | |
| Outcomes | Number of patients with painful legs. Pain free legs on imipramine 20/20, on amitriptyline 19/19, on placebo 0/20 Depression on imipramine 3.9 (0.3), on amitriptyline 3.7 (0.4), on placebo 8.2 (0.6) Sleep disturbance on imipramine 0/20, on amitriptyline 0/19, on placebo 20/20 | |
| Notes | No dropouts SE: no withdrawals due to SE QS = 3 (R1, DB1, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Double blind parallel group study, 15 days (dose escalation during three days) Randomisation not stated | |
| Participants | Any neuropathy (27 cancer related peripheral nerve lesions, 9 non‐cancer related nerve lesions, 6 postherpetic neuralgia, 3 other). 45 participants (31 final number). Age range 34 to 79 years. Pain score in amitriptyline group 66, in trazodone group 46 | |
| Interventions | Amitriptyline dose escalation from 25 mg to 75 mg, or trazodone from 75 mg to 225 mg daily orally | |
| Outcomes | Pain score 0 to 240 (intensity and duration of daily pain) Pain score 26 on amitriptyline, on trazodone 31. Pain score decreased on amitriptyline 40, on trazodone 15 | |
| Notes | Dropouts 14/45; 4/22 on amitriptyline (2 death, 2 lack of compliance), 10/23 on trazodone (6 SE, 1 no effect, 3 lack of compliance) SE: withdrawn 0/22 on amitriptyline, 6/23 on trazodone Results illustrated only in figures QS = 3 (R1, DB1, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Methods | Double blind placebo controlled crossover design, four weeks (one week dose titration then stable dose) Three 3 week periods, one week washout, no carry over effect Randomisation method not stated Inclusion criteria: | |
| Participants | Polyneuropathy (19 diabetic, 18 non‐diabetic). 37 participants (33 final number). Mean pain duration 48 months, 17 male and 19 female patients Pain score for diabetics 5.0 (1.4) and for non‐diabetic 4.1 (1.9). Depression score for diabetic 2.8 (range 0 to18.0) and non‐diabetic 2.9 (range 0 to 22.5) | |
| Interventions | Amitriptyline dose escalation from 25 mg to 75 mg, or maprotiline from 25 mg to 75 mg, or placebo daily orally | |
| Outcomes | Pain patients reported, 5‐item global improvement, more than 20 % pain decrease Comprehensive Psychopathological Rating Scale Number of patients with improved sleep On amitriptyline pain completely improved Pain reduced at least 20% on amitriptyline 20/33, on maprotiline 15/33, on placebo 7/33 Depression score in diabetic patients on amitriptyline 1.2 (range 0‐12.5), on maprotiline 2.4 (range 0‐145), on placebo 2.3 (range 0‐12.5); Sleep improved on amitriptyline | |
| Notes | Dropouts 7/37 (5 SE, 1 depression, 1 early drop out) SE: 21/33 on amitriptyline, on 21/33 on maprotiline, 6/33 on placebo. Withdrawn due to SE 3 on amitriptyline (1 severe thirst, 1 urinary retention, 1 hyperglycaemia), 2 on maprotiline (1 sedation and vertigo, 1 urticaria) QS = 4 (R1, DB2, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Double blind placebo controlled crossover design, three weeks Two three week periods, one to two weeks washout, no analyses of carry over effect Randomisation method not stated | |
| Participants | Postherpetic neuralgia of 3.8 years (range 4 months to 9 years). 24 participants (24 final number). Mean age 66 years (range 49 to 81), 8 male and 16 female patients. 9 patients depressed | |
| Interventions | Amitriptyline dose escalation from 12.5 mg to 25 mg daily orally, dose range 25 mg ‐ 137.5 mg; or placebo | |
| Outcomes | 4‐item global improvement At least good on amitriptyline 16/24 (excellent improvement 3, good 13, | |
| Notes | Dropouts 6/24; 1 on amitriptyline (SE), 5 on placebo (SE, pain, depression) QS = 3 (R1, DB1, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Double blind crossover design, five weeks. Two five week periods, 2 weeks washout, no carry over effect Randomisation method not stated Inclusion criteria: duration of symptoms at least three months | |
| Participants | Postherpetic neuralgia of 14 months (range 4 months to 7 years). 35 participants (32 final number). Mean age 71 years (range 55 to 85), 18 male and 17 female patients 11 depressed. Pain score in amitriptyline group: steady pain 61.6, jabbing pain 58.3 and skin pain 71.1; in maprotiline group steady pain 56.3, jabbing pain 41.9 and skin pain 59.4 | |
| Interventions | Amitriptyline dose escalation from 12.5 mg to 25 mg + placebo daily orally, median dose 100 mg (range 37.5 to 150 mg); or | |
| Outcomes | Pain patients reported, 4‐item global improvement, 4‐item scale for effectiveness (including pain relief, side effects, sleep and satisfaction), VAS. The Bock Depression Inventory On amitriptyline Effectiveness on amitriptyline Amitriptyline better than maprotiline in 11/32 patients, maprotiline better than amitriptyline in 9/32 patients, no difference in 12/32 patients On amitriptyline steady pain VAS 41.4, jabbing pain 23.7 and skin pain 42.7; on maprotiline steady pain 17.7, jabbing pain 11.4 and skin pain 25.6 9/32 depressed on amitriptyline, 12/32 on maprotiline | |
| Notes | Dropouts 3/35; on amitriptyline 2 (1 SE and 1 pain didn't return after washout period), on maprotiline 1 (pain didn't return after washout period) SE: 20/35 on amitriptyline, 28/35 on maprotiline. Withdrawn 3 on amitriptyline (dry mouth and constipation, dizziness, sedation, lethargy, mouth ulceration or nausea), 3 on maprotiline (1 dry mouth and nausea, 1 nausea and vomiting, 1 restless legs) QS = 4 (R1, DB2, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Double blind crossover design, five weeks. Two five week periods, 2 weeks washout, no carry over effect Inclusion criteria: duration of symptoms at least three months | |
| Participants | Postherpetic neuralgia | |
| Interventions | Amitriptyline dose escalation from 10 mg to 20 mg daily orally, | |
| Outcomes | Pain patients reported, satisfied or unsatisfied (pain relief and side‐effects) On amitriptyline satisfied 17/31 and unsatisfied 14/31; on nortriptyline satisfied 15/31 and unsatisfied 16/32 | |
| Notes | Dropouts 2/33 (SE) SE: 31/33 on amitriptyline, 31/33 on nortriptyline Withdrawn 1 on amitriptyline (slurred speech, urinary retention), 1 on nortriptyline (increased pain, bad dreams, fever, perspiration, epigastric pain) QS = 4 (R1, DB2, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Double blind placebo controlled trial for eight weeks | |
| Participants | 60 participants aged 33 to 69 years. Neuropathic pain for longer than six months of at least four on 11 pt VASPI Patients subjected to experimentally induced pain | |
| Interventions | Venlafaxine 75 mg /day, venlafaxine 150 mg/ or placebo for eight weeks . paracetamol 500 mg 3/4 time daily for rescue. Antidepressants or anticonvulsants nota allowed | |
| Outcomes | VASPI, Patient satisfaction, activities of daily , AEs, global impression of change. | |
| Notes | 5/60 withdrew: 1 placebo, 1 venlafaxine 75 mg, 3 venlafaxine 150 mg QS = 3 (R1, DB1, W1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
AEs‐adverse events
BDI ‐ Beck depression inventory
HAS‐ Hamilton anxiety scale
HDRS ‐ Hamilton depression rating scale
MPQ ‐ McGill Pain Questionairre
NRS‐ numerical rating score
PAD‐ Zung pain and distress index
PCA‐ patient controlled analgesia
PHN‐ post herpetic neuralgia
PI‐ pain intensity
PPI ‐ present pain intensity
PR ‐ pain relief
PRI ‐ pain rating index
QOL ‐ quality of life
QS ‐ quality score
SD ‐ standard deviation
SE ‐ side effects
TCA ‐ tricyclic andidepressants.
VAS‐ visual analogue scale
VRS ‐ verbal rating scale
yrs ‐ years
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Somatoform pain disorder‐not neuropathic pain | |
| Review/not a study | |
| not RCT | |
| Terminal pain not neuropathic pain | |
| not RCT | |
| not RCT, follow up toBlumer 1980 | |
| RCT but pain not assessed | |
| Dose finding study | |
| Not RCT | |
| Dose finding not RCT | |
| Idiopathic pain study | |
| Not RCT | |
| Chronically ill patients, not neuropathic pain | |
| Case report, not neuropathic pain | |
| Chronic pain study | |
| Spinal pain study | |
| Spinal pain study | |
| Dual publication of Hameroff 1984 | |
| Chronic pain study | |
| Depressive patients with somatic symptoms | |
| Not RCT | |
| Comparison between electrotherapy and amitriptyline, and sham treatment and amitriptyline | |
| Chronic idiopathic pain study | |
| Chronic pain study, not neuropathic pain. | |
| Dose finding study, not neuropathic pain | |
| Inadequate number of patients (6) | |
| Chronic cancer pain study | |
| Chronic pain study | |
| Psychogenic pain study | |
| Comparison between cognitive‐behavioural therapy alone and with amitriptyline | |
| Not RCT | |
| Not RCT | |
| Appraisal of Morello 1999 | |
| Not RCT | |
| Concentration‐response pharmacokinetic study | |
| Concentration‐response pharmacokinetic study | |
| No pain outcome | |
| Case report | |
| Chronic myofascial pain study | |
| Not RCT | |
| Chronic idiopathic pain, "masked" depression | |
| Somatoform pain study | |
| Review | |
| Chronic pain study‐ not neuropathic pain | |
| Chronic pain secondary publication of von Knorring 1979 | |
| Not RCT | |
| Inadequate number of patients (6) | |
| Chronic pain study | |
| Somatoform pain study |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Amitriptyline versus placebo Show forest plot | 10 | 588 | Risk Ratio (M‐H, Random, 95% CI) | 2.23 [1.35, 3.69] |
| Analysis 1.1  Comparison 1 Global improvement ‐ number of patients with moderate pain relief or better, Outcome 1 Amitriptyline versus placebo. | ||||
| 2 Desipramine vs placebo Show forest plot | 2 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.75 [2.19, 15.08] |
| Analysis 1.2  Comparison 1 Global improvement ‐ number of patients with moderate pain relief or better, Outcome 2 Desipramine vs placebo. | ||||
| 3 Imipramine vs placebo Show forest plot | 2 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 19.0 [3.97, 90.84] |
| Analysis 1.3  Comparison 1 Global improvement ‐ number of patients with moderate pain relief or better, Outcome 3 Imipramine vs placebo. | ||||
| 4 Other antidepressants vs placebo Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Global improvement ‐ number of patients with moderate pain relief or better, Outcome 4 Other antidepressants vs placebo. | ||||
| 5 Tricyclics versus anticonvulsants Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.5  Comparison 1 Global improvement ‐ number of patients with moderate pain relief or better, Outcome 5 Tricyclics versus anticonvulsants. | ||||
| 6 Venlafaxine vs placebo Show forest plot | 3 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.16 [1.50, 3.11] |
| Analysis 1.6  Comparison 1 Global improvement ‐ number of patients with moderate pain relief or better, Outcome 6 Venlafaxine vs placebo. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressant vs placebo. Number of patients with moderate pain relief or better Show forest plot | 5 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 12.41 [5.27, 29.21] |
| Analysis 2.1  Comparison 2 Diabetic neuropathy, Outcome 1 Antidepressant vs placebo. Number of patients with moderate pain relief or better. | ||||
| 2 Changes in pain intensity: Desipramine vs placebo Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Diabetic neuropathy, Outcome 2 Changes in pain intensity: Desipramine vs placebo. | ||||
| 3 Changes in pain intensity: Amitriptyline vs placebo Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 Diabetic neuropathy, Outcome 3 Changes in pain intensity: Amitriptyline vs placebo. | ||||
| 4 Changes in pain intensity: Fluoxetine vs placebo Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.4  Comparison 2 Diabetic neuropathy, Outcome 4 Changes in pain intensity: Fluoxetine vs placebo. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressant vs placebo Show forest plot | 4 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [1.70, 3.19] |
| Analysis 3.1  Comparison 3 Postherpetic neuralgia‐ number of patients with moderate pain relief or better, Outcome 1 Antidepressant vs placebo. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressant vs placebo Show forest plot | 2 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.5 [1.31, 9.33] |
| Analysis 4.1  Comparison 4 Central pain‐ number of patients with moderate pain relief or better, Outcome 1 Antidepressant vs placebo. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressant vs placebo Show forest plot | 2 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.22, 2.29] |
| Analysis 5.1  Comparison 5 Atypical facial pain‐ number of patients with moderate pain relief or better, Outcome 1 Antidepressant vs placebo. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean change scores ‐ doxepin vs placebo Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.1  Comparison 7 Topical Doxepin, Outcome 1 Mean change scores ‐ doxepin vs placebo. | ||||

Comparison 1 Global improvement ‐ number of patients with moderate pain relief or better, Outcome 1 Amitriptyline versus placebo.
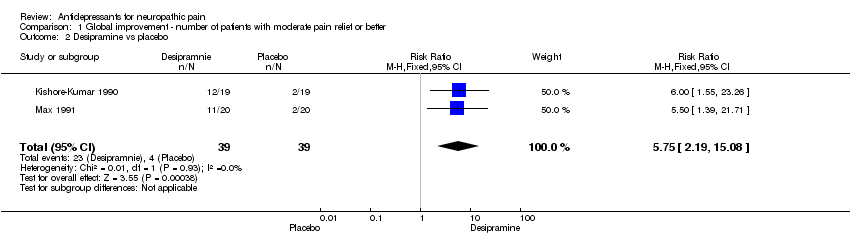
Comparison 1 Global improvement ‐ number of patients with moderate pain relief or better, Outcome 2 Desipramine vs placebo.

Comparison 1 Global improvement ‐ number of patients with moderate pain relief or better, Outcome 3 Imipramine vs placebo.

Comparison 1 Global improvement ‐ number of patients with moderate pain relief or better, Outcome 4 Other antidepressants vs placebo.

Comparison 1 Global improvement ‐ number of patients with moderate pain relief or better, Outcome 5 Tricyclics versus anticonvulsants.
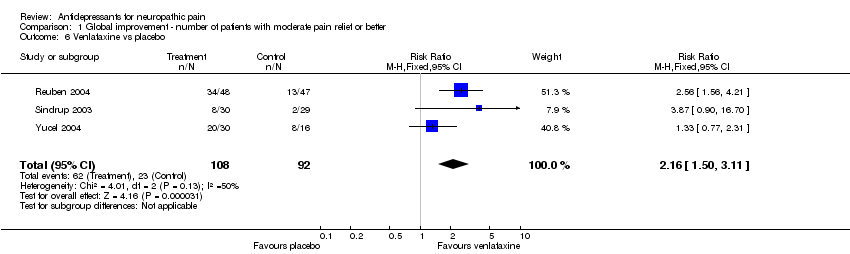
Comparison 1 Global improvement ‐ number of patients with moderate pain relief or better, Outcome 6 Venlafaxine vs placebo.
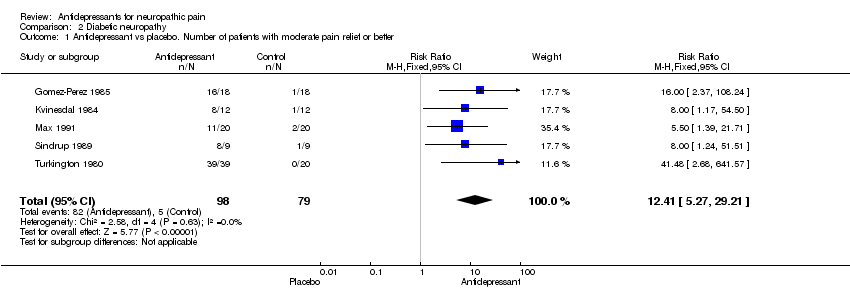
Comparison 2 Diabetic neuropathy, Outcome 1 Antidepressant vs placebo. Number of patients with moderate pain relief or better.
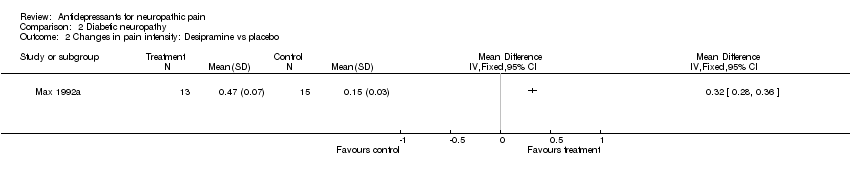
Comparison 2 Diabetic neuropathy, Outcome 2 Changes in pain intensity: Desipramine vs placebo.
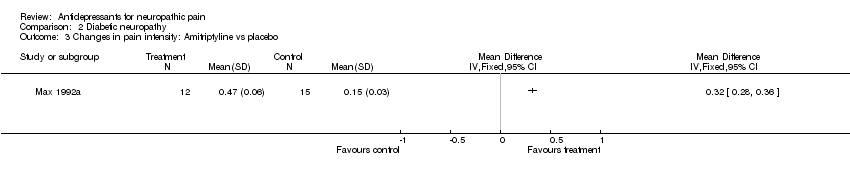
Comparison 2 Diabetic neuropathy, Outcome 3 Changes in pain intensity: Amitriptyline vs placebo.

Comparison 2 Diabetic neuropathy, Outcome 4 Changes in pain intensity: Fluoxetine vs placebo.

Comparison 3 Postherpetic neuralgia‐ number of patients with moderate pain relief or better, Outcome 1 Antidepressant vs placebo.
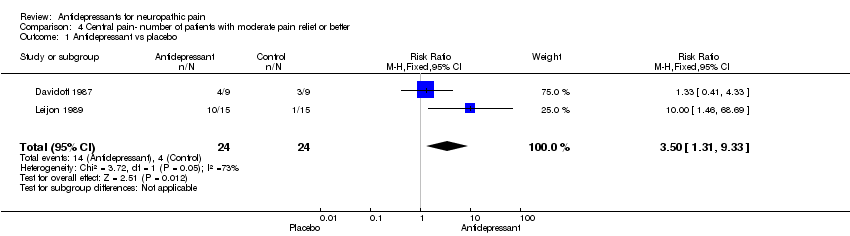
Comparison 4 Central pain‐ number of patients with moderate pain relief or better, Outcome 1 Antidepressant vs placebo.

Comparison 5 Atypical facial pain‐ number of patients with moderate pain relief or better, Outcome 1 Antidepressant vs placebo.
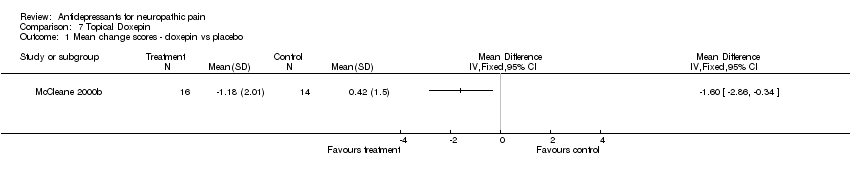
Comparison 7 Topical Doxepin, Outcome 1 Mean change scores ‐ doxepin vs placebo.
| Study ID | Condition | Medicines used | Continuous data |
| Cardenas 2002 | Spinal cord injury | Amitriptyline versus benztropine (active placebo) | No significant difference between acive and placebo |
| Gomez Perez 1996 | Diabetic Neuropathy | Nortriptyline‐Fluphenazine versus carbamazepine | Mean percent change but no standard deviations (SD) |
| Graff Radford 2000 | Post herpetic neuralgia | Amitiptyline and Fluphenazine | VAS change scores‐ see Metaview |
| Hampf 1989 | Mixed pain but only neuropathic pain analysed for this review | Distigmine and Amitriptyline | VAS change scores but no SDs |
| Harrison 1997 | Chronic Idiopathic Facial Pain | Fluoxetine | No evaluable data |
| Kalso 1995 | Post mastectomy pain | Amitriptyline | Pre and post VAS with Median and range scores. |
| Max 1987 | Diabetic Neuropathy | Amitriptyline versus benztropine (active placebo) | No evaluable data |
| Max 1992a | Diabetic neuropathy | Desipramine, amitriptyline and fluoxetine | Mean change scores with standard error. See metaview |
| McCleane 2000a | Neuropathic pain | Topical doxepin, topical capsaicin, and combination of both | Change scores with 95% CI |
| McCleane 2000b | Neuropathic pain | Topical doxepin | Mean plus SD for change in pain scores. See metaview |
| Panerai 1990 | Central pain | Clomipramine, nortriptyline | No evaluable data |
| Sharav 1987 | Chronic facial pain | Amitriptyline | Change VAS data with range |
| Sindrup 1990a | Diabetic neuropathy | Paroxetine, imipramine | No evaluable data |
| Sindrup 1990b | Diabetic neuropathy | Clomipramine, desipramine | No evaluable data |
| Sindrup 1992a | Diabetic neuropathy | Citalopram | No mean scores or SD |
| Sindrup 1992b | Diabetic neuropathy | Mianserin | No mean scores or SD |
| Tasmuth 2002 | Neuropathic pain following treatment for breast cancer | Venlafaxine | Medians for pain relief with ranges |
| Ventafridda 1987 | Neuropathic cancer pain | Amitriptyline, trazodone | No evaluable data. |
| Rowbotham 2004 | Diabetic Neuropathy | Venlafaxine | Means but no SD |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Amitriptyline versus placebo Show forest plot | 10 | 588 | Risk Ratio (M‐H, Random, 95% CI) | 2.23 [1.35, 3.69] |
| 2 Desipramine vs placebo Show forest plot | 2 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.75 [2.19, 15.08] |
| 3 Imipramine vs placebo Show forest plot | 2 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 19.0 [3.97, 90.84] |
| 4 Other antidepressants vs placebo Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Tricyclics versus anticonvulsants Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Venlafaxine vs placebo Show forest plot | 3 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.16 [1.50, 3.11] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressant vs placebo. Number of patients with moderate pain relief or better Show forest plot | 5 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 12.41 [5.27, 29.21] |
| 2 Changes in pain intensity: Desipramine vs placebo Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Changes in pain intensity: Amitriptyline vs placebo Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Changes in pain intensity: Fluoxetine vs placebo Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressant vs placebo Show forest plot | 4 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [1.70, 3.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressant vs placebo Show forest plot | 2 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.5 [1.31, 9.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antidepressant vs placebo Show forest plot | 2 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.22, 2.29] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean change scores ‐ doxepin vs placebo Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |

