Carbamazepine untuk sakit neuropatik kronik dan fibromialgia dalam kalangan dewasa
Información
- DOI:
- https://doi.org/10.1002/14651858.CD005451.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 10 abril 2014see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Dolor y cuidados paliativos
- Copyright:
-
- Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
PW registered the title, wrote the protocol, carried out searching and identified studies for inclusion. PW & RAM carried out data extraction, analysis, and drafting. All authors contributed to the final draft and approved the published version.
For this update, RAM and SD searched for additional studies. The updated Methods are taken from a template protocol for antiepileptics in neuropathic pain and fibromyalgia. SD, RAM and PW reassessed studies for inclusion. All authors contributed to the final draft and approved the published version.
PW will be responsible for updates.
Sources of support
Internal sources
-
Oxford Pain Relief Trust, UK.
General institutional support for this update
External sources
-
No sources of support supplied
Declarations of interest
SD and PW have received research support from charities, government and industry sources at various times, but none relate to this review.
RAM has consulted for various pharmaceutical companies and received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions, including (in the past five years) AstraZeneca, Eli Lilly, Flynn Pharma, Furtura Medical, Grünenthal, GSK, Horizon Pharma, Lundbeck, Menarini, MSD, Pfizer, Reckitt Benckiser, Sanofi Aventis, Urgo, Astellas, and Vifor Pharma.
Acknowledgements
Support for earlier versions of this review came from Marie Curie Cancer Care and NHS R&D funds.
Henry McQuay was an author on the original review and the 2010 update. Support for the 2010 update came from Oxford Pain Relief Trust, the NHS Cochrane Collaboration Programme Grant Scheme, and NIHR Biomedical Research Centre Programme.
The Oxford Pain Relief Trust provided general institutional support for this update.
Version history
| Published | Title | Stage | Authors | Version |
| 2014 Apr 10 | Carbamazepine for chronic neuropathic pain and fibromyalgia in adults | Review | Philip J Wiffen, Sheena Derry, R Andrew Moore, Eija A Kalso | |
| 2011 Jan 19 | Carbamazepine for acute and chronic pain in adults | Review | Philip J Wiffen, Sheena Derry, R Andrew Moore, Henry J McQuay | |
| 2005 Jul 20 | Carbamazepine for acute and chronic pain in adults | Review | Philip J Wiffen, Sheena Derry, R Andrew Moore, Henry J McQuay | |
Differences between protocol and review
The major difference between the original protocol and the 2010 update was the concentration on issues of methodological validity and bias that have emerged subsequently ‐ namely on size, on duration, on outcome, and potentially on a dependence on cross‐over designs. These are commented on and referenced in this updated review.
For this update we changed the title to reflect the clinical use of carbamazepine for pain relief, and to bring it in line with other reviews of antiepileptic drugs used to treat neuropathic pain and fibromyalgia; these reviews are included in an overview (Wiffen 2013a). As part of an ongoing drive to improve the standard of evidence in reviews we chose to exclude studies that were not double blind and did not have at least 10 participants per treatment arm. We also considered the implications of incomplete outcome assessment, and have analysed results according to the strength of the evidence (in three tiers).
The small amount of information relating to acute pain from the earlier review has been moved to Appendix 2.
Notes
Review methods been substantially amended following a search for new trials up to June 2010. Methods used have been further amended in 2013 to bring it in line with current standards of evidence in chronic pain, and following new searches to bring the evidence up to date.
A restricted search in February 2018 did not identify any potentially relevant studies likely to change the conclusions. The authors and editors are confident that further research will not change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. If appropriate, we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Analgesics, Non‐Narcotic [adverse effects, *therapeutic use];
- Carbamazepine [adverse effects, *therapeutic use];
- Chronic Pain [*drug therapy, etiology];
- Diabetic Neuropathies [*drug therapy];
- Fibromyalgia [*drug therapy];
- Neuralgia [drug therapy];
- Randomized Controlled Trials as Topic;
- Stroke [complications];
- Trigeminal Neuralgia [*drug therapy];
Medical Subject Headings Check Words
Adult; Humans;
PICO

Study flow diagram.
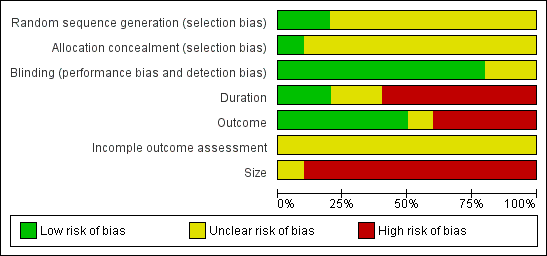
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Four studies showing percentage improvement (any definition) with carbamazepine (any dose) and placebo. Size of the study is proportional to the size of the symbol (inset scale). Yellow symbols = trigeminal neuralgia , blue = painful diabetic neuropathy, red = post stroke pain

Forest plot of comparison: 1 Carbamazepine in neuropathic pain, outcome: 1.1 Any pain improvement.
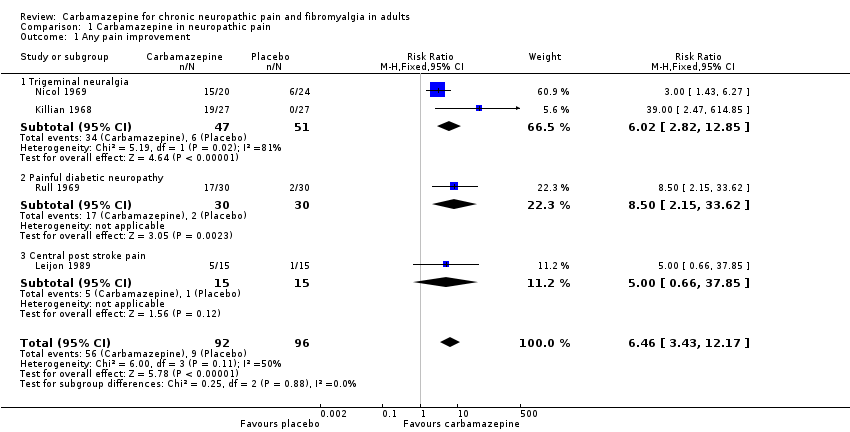
Comparison 1 Carbamazepine in neuropathic pain, Outcome 1 Any pain improvement.

Comparison 1 Carbamazepine in neuropathic pain, Outcome 2 At least 1 adverse event.
| Carbamazepine compared with placebo for chronic neuropathic pain | ||||||
| Patient or population: adults with neuropathic pain (Trigeminal neuralgia, painful diabetic neuropathy, chronic post stroke pain) Settings: community Intervention: oral carbamazepine (100 mg to 2400 mg daily) Comparison: placebo | ||||||
| Outcomes | Probable outcome with placebo | Probable outcome with intervention | NNT or NNH and/or relative effect (95% CI) | No of participants | Quality of the evidence | Comments |
| "Substantial" benefit At least 50% reduction in pain or equivalent | 94 in 1000 | 608 in 1000 | RR 6.5 (3.4 to 12) NNT 1.9 (1.6 to 2.5) | 188 participants, 4 studies | Low | Mixed conditions and doses, small studies of short duration, imputation not reported |
| "Moderate" benefit At least 30% reduction in pain | No data | Very low | No data | |||
| Proportion below 30/100 mm on VAS | No data | Very low | No data | |||
| Patient Global Impression of Change much or very much improved | No data | Very low | No data | |||
| Any adverse event | 270 in 1000 | 660 in 1000 | RR 2.4 (1.9 to 3.2) NNH 2.6 (2.1 to 3.5) | 346 participants, 4 studies | Low | Cross‐over studies Denominator = all potentially exposed |
| Adverse event withdrawals | 0 in 1000 | 30 in 1000 | not calculated | 523 participants, 8 studies | Very low | Cross‐over studies Denominator = all potentially exposed |
| Serious adverse events | not reported | 3 | not calculated | 46 participants, 2 studies | Very low | Denominator = all potentially exposed |
| Death | not reported | 4 | not calculated | 44 participants 1 study | Very low | Denominator = all potentially exposed |
| GRADE Working Group grades of evidence | ||||||
| NNT: number needed to treat for an additional beneficial effect: NNH: number needed to treat for an additional harmful effect; RR: risk ratio; VAS: visual analogue scale. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any pain improvement Show forest plot | 4 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.46 [3.43, 12.17] |
| 1.1 Trigeminal neuralgia | 2 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.02 [2.82, 12.85] |
| 1.2 Painful diabetic neuropathy | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.5 [2.15, 33.62] |
| 1.3 Central post stroke pain | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.66, 37.85] |
| 2 At least 1 adverse event Show forest plot | 4 | 346 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [1.85, 3.12] |

