Carbamazepine untuk sakit neuropatik kronik dan fibromialgia dalam kalangan dewasa
Abstract
Background
This is an update of a Cochrane review entitled 'Carbamazepine for acute and chronic pain in adults' published in Issue 1, 2011. Some antiepileptic medicines have a place in the treatment of neuropathic pain (pain due to nerve damage). This updated review considers the treatment of chronic neuropathic pain and fibromyalgia only, and adds no new studies. The update uses higher standards of evidence than the earlier review, which results in the exclusion of five studies that were previously included.
Objectives
To assess the analgesic efficacy of carbamazepine in the treatment of chronic neuropathic pain and fibromyalgia, and to evaluate adverse events reported in the studies.
Search methods
We searched for relevant studies in MEDLINE, EMBASE and CENTRAL up to February 2014. Additional studies were sought from clinical trials databases, and the reference list of retrieved articles and reviews.
Selection criteria
Randomised, double blind, active or placebo controlled trials (RCTs) investigating the use of carbamazepine (any dose, by any route, and for at least two weeks' duration) for the treatment of chronic neuropathic pain or fibromyalgia, with at least 10 participants per treatment group. Participants were adults aged 18 and over.
Data collection and analysis
Two study authors independently extracted data on efficacy, adverse events, and withdrawals, and examined issues of study quality. Numbers needed to treat for an additional beneficial effect (NNT) or harmful effect (NNH) with 95% confidence intervals (CIs) were calculated from dichotomous data.
We performed analysis using three tiers of evidence. First tier evidence derived from data meeting current best standards and subject to minimal risk of bias (outcome equivalent to substantial pain intensity reduction, intention‐to‐treat analysis without imputation for dropouts, at least 200 participants in the comparison, at least 8 weeks' duration, parallel design), second tier from data that failed to meet one or more of these criteria and were considered at some risk of bias but with adequate numbers in the comparison, and third tier from data involving small numbers of participants that was considered very likely to be biased or used outcomes of limited clinical utility, or both.
Main results
Ten included studies (11 publications) enrolled 480 participants with trigeminal neuralgia, diabetic neuropathy, and post stroke pain. Nine studies used a cross‐over design, and one a parallel group design. Most of the studies were of short duration, lasting four weeks or less.
No study provided first or second tier evidence for an efficacy outcome. Using third tier evidence, carbamazepine generally provided better pain relief than placebo in the three conditions studied, with some indication of pain improvement over mainly the short term, but with poorly defined outcomes, incomplete reporting, and in small numbers of participants. There were too few data in studies comparing carbamazepine with active comparators to draw any conclusions.
In four studies 65% (113/173) of participants experienced at least one adverse event with carbamazepine, and 27% (47/173) with placebo; for every five participants treated, two experienced an adverse event who would not have done so with placebo. In eight studies 3% (8/268) of participants withdrew due to adverse events with carbamazepine, and none (0/255) with placebo. Serious adverse events were not reported consistently; rashes were associated with carbamazepine. Four deaths occurred in patients on carbamazepine, with no obvious drug association.
Authors' conclusions
Carbamazepine is probably effective in some people with chronic neuropathic pain, but with caveats. No trial was longer than four weeks, had good reporting quality, nor used outcomes equivalent to substantial clinical benefit. In these circumstances, caution is needed in interpretation, and meaningful comparison with other interventions is not possible.
PICO
Ringkasan bahasa mudah
Carbamazepine untuk sakit neuropatik kronik dan fibromialgia dalam kalangan dewasa
Sakit neuropatik adalah sakit yang berpunca dari kecederaan saraf. Ia adalah berbeza dari mesej sakit yang dibawa oleh saraf sihat dari tisu tercedera (jatuh atau terpotong, atau lutut artritis). Sakit neuropatik dirawat dengan pelbagai ubat yang berbeza dengan ubat untuk tisu tercedera. Ubat‐ubat seperti paracetamol atau ibuprofen adalah tidak berkesan untuk sakit neuropatik, manakala ubat‐ubat yang kadangkala diguna untuk merawat kemurungan atau epilepsi boleh menjadi amat berkesan bagi sesetengah orang dengan sakit neuropatik. Kefahaman kami tentang fibromialgia (satu keadaan sakit berterusan, meluas dan nyeri, masalah tidur dan keletihan) adalah kurang, namun fibromialgia boleh dirawat dengan ubat‐ubatan yang sama bagi sakit neuropatik.
Carbamazepine digunakan untuk merawat epilepsi, tetapi ia kini digunakan untuk merawat pelbagai jenis sakit kronik. Kami melakukan carian (sehingga Februari 2014) untuk mencari kajian klinikal di mana carbamazepine digunakan untuk merawat sakit neuropatik atau fibromialgia. Kami mendapati 10 kajian melibatkan 418 orang yang terlibat dalam kajian carbamazepine. Kajian‐kajian umumnya tidak berkualiti baik. Kebanyakannya adalah kajian yang sangat kecil, serta tempoh yang singkat. Kajian yang bertempoh hanya satu atau dua minggu tidak membantu apabila sakit boleh bertahan bertahun‐tahun.
Tiada bukti berkualiti yang cukup untuk mengatakan bagaimana carbamazepine berkesan dalam merawat keadaan sakit neuropatik. Peggabungan empat kajian kecil menunjukkan ia lebih baik daripada plasebo, tetapi keputusan itu tidak boleh dipercayai. Tiada cukup maklumat dari kajian‐kajian tersebut untuk membuat sebarang komen yang boleh dipercayai mengenai kesan‐kesan buruk atau mudarat.
Carbamazepine mungkin membantu sesetengah dengan sakit neuropatik kronik. Tidak mungkin untuk mengetahui lebih awal tentang siapa yang akan mendapat manfaat dan siapa yang tidak akan mendapat manfaat.
Authors' conclusions
Summary of findings
| Carbamazepine compared with placebo for chronic neuropathic pain | ||||||
| Patient or population: adults with neuropathic pain (Trigeminal neuralgia, painful diabetic neuropathy, chronic post stroke pain) Settings: community Intervention: oral carbamazepine (100 mg to 2400 mg daily) Comparison: placebo | ||||||
| Outcomes | Probable outcome with placebo | Probable outcome with intervention | NNT or NNH and/or relative effect (95% CI) | No of participants | Quality of the evidence | Comments |
| "Substantial" benefit At least 50% reduction in pain or equivalent | 94 in 1000 | 608 in 1000 | RR 6.5 (3.4 to 12) NNT 1.9 (1.6 to 2.5) | 188 participants, 4 studies | Low | Mixed conditions and doses, small studies of short duration, imputation not reported |
| "Moderate" benefit At least 30% reduction in pain | No data | Very low | No data | |||
| Proportion below 30/100 mm on VAS | No data | Very low | No data | |||
| Patient Global Impression of Change much or very much improved | No data | Very low | No data | |||
| Any adverse event | 270 in 1000 | 660 in 1000 | RR 2.4 (1.9 to 3.2) NNH 2.6 (2.1 to 3.5) | 346 participants, 4 studies | Low | Cross‐over studies Denominator = all potentially exposed |
| Adverse event withdrawals | 0 in 1000 | 30 in 1000 | not calculated | 523 participants, 8 studies | Very low | Cross‐over studies Denominator = all potentially exposed |
| Serious adverse events | not reported | 3 | not calculated | 46 participants, 2 studies | Very low | Denominator = all potentially exposed |
| Death | not reported | 4 | not calculated | 44 participants 1 study | Very low | Denominator = all potentially exposed |
| GRADE Working Group grades of evidence | ||||||
| NNT: number needed to treat for an additional beneficial effect: NNH: number needed to treat for an additional harmful effect; RR: risk ratio; VAS: visual analogue scale. | ||||||
Background
This updated review was originally published in The Cochrane Library as 'Anticonvulsant drugs for acute and chronic pain' (Wiffen 2000). At the third update in 2003 (Wiffen 2003), 12 new included studies were identified mainly of the newer antiepileptics (anticonvulsants) gabapentin and lamotrigine. In total the included studies provided data on six different medicines used in at least six identified neuropathic pain conditions. Issues of dose response and trial design added to the complexity. A decision was therefore taken to split that review into a number of smaller reviews each covering one medicine (chemical entity). In 2010 a review of carbamazepine for acute and chronic pain was published, and in 2011 the authors reviewed the literature and concluded that there were unlikely to be any new studies, so the review was marked as stable (Wiffen 2011a).
The decision to update it now, and concentrate on chronic neuropathic pain and fibromyalgia, was made because there have been more advances in the rigour with which we assess studies and report data, and in order to conform with other reviews in the series on neuropathic pain and fibromyalgia. In particular we consider study size and duration, outcomes reported, and method of imputation for withdrawals, and report results in three tiers according to outcome and freedom from known sources of bias. We wanted to bring this review in line with a template protocol so that it can be easily included in overview of antiepileptics for chronic neuropathic pain and fibromyalgia in adults (Wiffen 2013a). Reviews of clonazepam (Corrigan 2012), gabapentin (Moore 2011), lacosamide (Hearn 2012), lamotrigine (Wiffen 2013b), oxcarbazepine (Zhou 2013), phenytoin (Birse 2012), pregabalin (Moore 2009a), topiramate (Wiffen 2013c), and valproic acid (Gill 2011) have been completed. All the reviews analyse results according to the particular conditions in which they have been studied, and it is expected that in future updates fibromyalgia, at least, will become the subject of separate reviews.
The aim is for all our reviews to use the same methods, based on new criteria for what constitutes reliable evidence in chronic pain (Moore 2010a; Moore 2012b; Appendix 1). A Cochrane review of pregabalin in neuropathic pain and fibromyalgia demonstrated different response rates for different types of chronic pain (higher in diabetic neuropathy and postherpetic neuralgia and lower in central pain and fibromyalgia) (Moore 2009a). This indicates that different neuropathic pain conditions should be treated separately from one another, and that pooling should not be done unless there are good grounds for doing so. While fibromyalgia is considered to have a different aetiology from chronic neuropathic pain, it is a condition that responds to the same therapies. Because of limitations in the number of available clinical trials, it is convenient to consider fibromyalgia together with neuropathic pain. We make no presumption to pool data across individual neuropathic pain conditions or fibromyalgia, but will consider each condition separately.
The small amount of information in the 2011 review relating to acute pain has been moved to Appendix 2.
Description of the condition
The 2011 International Association for the Study of Pain definition of neuropathic pain is "pain caused by a lesion or disease of the somatosensory system" (Jensen 2011) based on an earlier consensus meeting (Treede 2008). Neuropathic pain may be caused by nerve damage, but is often followed by changes in the central nervous system (CNS) (Moisset 2007). It is complex, and neuropathic pain features can be found in patients with joint pain (Soni 2013).
Fibromyalgia is defined as widespread pain for longer than three months with pain on palpation at 11 or more of 18 specified tender points (Wolfe 1990), and is frequently associated with other symptoms such as poor sleep, fatigue, and depression. More recently, a definition of fibromyalgia has been proposed based on symptom severity and the presence of widespread pain (Wolfe 2010). The cause, or causes, are not well understood, but it has features in common with neuropathic pain, including changes in the CNS. Moreover, patients with neuropathic pain and those with fibromyalgia experience similar sensory phenomena (Koroschetz 2011), and peripheral nerve fibre changes seen in neuropathic pain also occur in fibromyalgia (Oaklander 2013; Üçeyler 2013). Many people with these conditions are significantly disabled with moderate or severe pain for many years.
In primary care in the United Kingdom (UK), the incidences per 100,000 person‐years' observation have been reported as 28 (95% CI 27 to 30) for postherpetic neuralgia, 27 (26 to 29) for trigeminal neuralgia, 0.8 (0.6 to 1.1) for phantom limb pain, and 21 (20 to 22) for painful diabetic neuropathy (Hall 2008). Estimates vary between studies, often because of small numbers of cases. The incidence of trigeminal neuralgia has been estimated at 4 in 100,000 per year (Katusic 1991; Rappaport 1994), while more recently, a study of facial pain in The Netherlands found incidences per 100,000 person‐years of 12.6 for trigeminal neuralgia and 3.9 for postherpetic neuralgia (Koopman 2009). A systematic review of chronic pain demonstrated that some neuropathic pain conditions, such as painful diabetic neuropathy, can be more common, with prevalence rates up to 400 per 100,000 person‐years (McQuay 2007). The prevalence of neuropathic pain was reported as being 3.3% in Austria (Gustorff 2008), 6.9% in France (Bouhassira 2008) and as high as 8% in the UK (Torrance 2006), and about 7% in a systematic review of studies published since 2000 (Moore 2013a). The incidence of some forms of neuropathic pain, such as diabetic neuropathy and postherpetic neuralgia, is increasing (Hall 2013). Fibromyalgia is common, especially in women, with an all‐age prevalence of 12%, and a female to male ratio of 6:1 (McNally 2006).
Neuropathic pain and fibromyalgia are known to be difficult to treat effectively, with only a minority of individuals experiencing a clinically relevant benefit from any one intervention. A multidisciplinary approach is now advocated, with pharmacological interventions being combined with physical and/or cognitive interventions. Conventional analgesics are usually not effective. Some patients with neuropathic pain may derive some benefit from topical lidocaine patch or low concentration topical capsaicin, although evidence of benefit is uncertain (Derry 2012; Khaliq 2007). High concentration topical capsaicin may benefit some patients with postherpetic neuralgia (Derry 2013). Treatment is more usually by so‐called unconventional analgesics such as antidepressants like duloxetine and amitriptyline (Lunn 2009; Moore 2012a; Sultan 2008) or antiepileptics like gabapentin or pregabalin (Moore 2009a; Moore 2011). The proportion of patients who achieve worthwhile pain relief (typically defined as at least 50% pain intensity reduction (Moore 2013b)) is small, typically 10% to 25% more than with placebo, with numbers needed to treat for an additional beneficial outcome (NNTs) usually between 4 and 10.
Description of the intervention
Carbamazepine was first marketed in the early 1960s to treat trigeminal neuralgia, with its antiepileptic effects recognised soon afterwards. It is licensed in the United Kingdom and United States of America for paroxysmal pain of trigeminal neuralgia (dosage up to 1600 mg daily), but has been used off‐label for other types of neuropathic pain. It is usually prescribed as a tablet, but chewable tablet, liquid, and suppository formulations are manufactured.
Antiepileptic drug use is not without risk: serious adverse effects have been reported, including deaths from haematological reactions (blood dyscrasias; Sweetman 2005), and life‐threatening cutaneous problems (Chung 2010; Kulkantrakorn 2012). Carbamazepine is also known to stimulate synthesis of certain enzymes, which can interfere with other drug therapies (e.g. anticoagulants, antiretrovirals, statins, antihypertensives) causing clinical problems, particularly at initiation and withdrawal (Brodie 2013). The most common adverse effects are impaired mental and motor function, which may limit clinical use, particularly in older people (Grahame‐Smith 1992; Rall 1992; Sweetman 2005).
How the intervention might work
Pain that manifests in different diseases may operate through common mechanisms, but the same symptom in two patients may be caused by different mechanisms. It is therefore impossible to predict the mechanisms responsible for an individual's pain based on the aetiology of the neuropathy or on the distribution or nature of symptoms (Woolf 1999). Carbamazepine stabilizes the inactivated state of voltage‐gated sodium channels, so that fewer of these channels are available to open, and brain cells are less excitable and less likely to fire (Ambrósio 1999; Morisset 2013).
Why it is important to do this review
The standards used to assess evidence in chronic pain trials have changed substantially in recent years, with particular attention being paid to trial duration, withdrawals, and statistical imputation following withdrawal, all of which can substantially alter estimates of efficacy (Appendix 1). The most important change is the move from using average pain scores, or average change in pain scores, to using the number of patients who have a large decrease in pain (by at least 50%); this level of pain relief has been shown to correlate with improvements in comorbid symptoms, function, and quality of life. These standards are set out in the reference guide for pain studies (AUREF 2012) and reflect what patients with chronic pain want from treatment (Moore 2013a).
This Cochrane review will assess evidence in ways that make both statistical and clinical sense, and will use developing criteria for what constitutes reliable evidence in chronic pain (Moore 2010a). Trials included and analysed will need to meet a minimum of reporting quality (blinding, randomisation), validity (duration, dose and timing, diagnosis, outcomes, etc), and size (ideally at least 500 participants in a comparison in which the (NNT) is four or more (Moore 1998)).
Carbamazepine has been used to treat some types of neuropathic pain for about 50 years. It is important to know its place in the range of drugs used to treat the various types of neuropathic pain. This updated review brings the evidence for carbamazepine into line with that for other medicines used in these conditions, and will form part of an overview of antiepileptic drugs for chronic neuropathic pain and fibromyalgia.
Objectives
To assess the analgesic efficacy of carbamazepine in the treatment of chronic neuropathic pain and fibromyalgia, and to evaluate adverse events reported in the studies.
Methods
Criteria for considering studies for this review
Types of studies
We included studies if they were randomised controlled trials (RCTs) with at least 10 participants per treatment group and double‐blind (participant and observers) assessment of participant‐reported outcomes, following two weeks of treatment or longer, although the emphasis of the review is on studies of eight weeks or longer. Full journal publication was required, with the exception of extended abstracts of otherwise unpublished clinical trials (for example detailed information from PDFs of posters that typically include all important details of methodology used and results obtained). We did not include short abstracts (usually meeting reports with inadequate or no reporting of data). We excluded studies of experimental pain, case reports, and clinical observations.
Migraine and headache studies previously included in an earlier version of this review were excluded (Wiffen 2000). This subject is being dealt with in greater depth by the Cochrane Pain, Palliative Care and Supportive Care Review Group.
Types of participants
We included adult participants aged 18 years and above. Participants could have one or more of a wide range of chronic neuropathic pain conditions including (but not limited to):
-
painful diabetic neuropathy (PDN);
-
postherpetic neuralgia (PHN);
-
trigeminal neuralgia;
-
phantom limb pain;
-
postoperative or traumatic neuropathic pain;
-
complex regional pain syndrome (CRPS) Type II;
-
cancer‐related neuropathy;
-
HIV‐neuropathy;
-
spinal cord injury;
or
-
fibromyalgia;
-
complex regional pain syndrome (CRPS) Type I.
We also included studies of participants with more than one type of neuropathic pain. We analysed results according to the primary condition.
Types of interventions
Carbamazepine in any dose, by any route, administered for the relief of neuropathic pain or fibromyalgia, and compared to placebo, no intervention or any other active comparator. We did not include studies using carbamazepine to treat pain resulting from the use of other drugs.
Types of outcome measures
We anticipated that studies would use a variety of outcome measures, with the majority of studies using standard subjective scales (numerical rating scale (NRS) or visual analogue scale (VAS)) for pain intensity or pain relief, or both. We were particularly interested in Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) definitions for moderate and substantial benefit in chronic pain studies (Dworkin 2008). These are defined as at least 30% pain relief over baseline (moderate), at least 50% pain relief over baseline (substantial), much or very much improved on Patient Global Impression of Change (PGIC) (moderate), and very much improved on PGIC (substantial). These outcomes concentrate on dichotomous outcomes where pain responses do not follow a normal (Gaussian) distribution. People with chronic pain desire high levels of pain relief, ideally more than 50%, and with pain intensity not worse than mild (O'Brien 2010).
We include a Summary of findings table as set out in the Cochrane Pain, Palliative and Supportive Care Group author guide (AUREF 2012). The Summary of findings table includes outcomes of at least 30% and at least 50% pain intensity reduction, PGIC, at least one adverse event, adverse event withdrawals, serious adverse events and death.
Primary outcomes
-
Participant‐reported pain intensity reduction of 30% or greater.
-
Participant‐reported pain intensity reduction of 50% or greater.
-
Participant‐reported global impression of clinical change (PGIC) much or very much improved.
-
Participant‐reported global impression of clinical change (PGIC) very much improved.
Secondary outcomes
-
Any pain‐related outcome indicating some improvement.
-
Withdrawals due to lack of efficacy.
-
Participants experiencing any adverse event.
-
Participants experiencing any serious adverse event.
-
Withdrawals due to adverse events.
-
Specific adverse events, particularly somnolence and dizziness.
These outcomes are not eligibility criteria for this review, but are outcomes of interest within whichever studies are included.
Search methods for identification of studies
Studies were identified by several methods.
Electronic searches
For the earlier review, RCTs of antiepileptics in acute, chronic or cancer pain were identified by searching MEDLINE (originally via Silver Platter, then Ovid) from 1966 to June 2010, EMBASE 1994 to Dec 2009, SIGLE 1980 to July 1999, and CENTRAL (Issue 4, 2010).
When the review was split in to individual drugs, this search strategy was narrowed to include carbamazepine only. Appendix 3 has the search strategies for CENTRAL, MEDLINE and EMBASE.
For this update we searched:
-
Cochrane Central Register of Controlled Trials (CENTRAL, 2014 Issue 1 in The Cochrane Library);
-
MEDLINE (via Ovid) (January 2010 to 7 February 2014);
-
EMBASE (via Ovid) (January 2010 to 7 February 2014);
-
PhRMA clinical study results database (clinicaltrials.gov) to 7 February 2014;
-
WHO International Clinical Trials Registry Platform (apps.who.int/trialsearch) to 7 February 2014
Searching other resources
Additional studies were identified from the reference list of the retrieved papers. In the first version, a letter was sent to the first author of a report for further information on their published report (method of randomisation, double blinding, outcome measures and dropouts) and to ask if they knew of any other studies which met our inclusion criteria, either undertaken by them or by other investigators. In addition, 41 medical journals were hand searched, chosen from the 50 with the highest number of reports in MEDLINE, and nine specialist journals which were either not on that list or were not indexed (Jadad 1994). The search process included volumes published between 1950 and 1990. No further hand searching has been undertaken as the key journals are now being searched by the Cochrane Collaboration.
In an earlier version of this review data were requested from 19 authors but only one (Leijon 1989) was able to supply information relevant to this review. In the two updates, no further attempt was made to contact authors.
Data collection and analysis
Selection of studies
Two review authors independently read the titles and abstracts of all studies identified by the search, and the full text of all potentially relevant studies. Agreement on eligibility was reached by discussion. We did not anonymise the studies in any way before assessment.
Data extraction and management
Two review authors extracted data using a standard data extraction form, and agreed data before entry into RevMan (RevMan 2012) or any other analysis method. Data extracted included information about the pain condition and number of participants treated, drug and dosing regimen, study design, study duration and follow up, analgesic outcome measures and results, withdrawals and adverse events (participants experiencing any adverse event, or a serious adverse event).
Assessment of risk of bias in included studies
We independently scored each study for quality using a three‐item scale (Jadad 1996) and agreed a 'consensus' score for each study. Scores of two and below have been associated with greater estimates of efficacy than studies of higher quality (Khan 1996). Quality scores were not used to weight the results in any way.
We used the 'Risk of bias' tool to assess the likely impact on the strength of the evidence of various study characteristics relating to methodological quality (randomisation, allocation concealment, blinding, freedom from selective reporting), study validity (duration, outcome reporting, and handling of missing data), and size (Moore 2010a).
Two review authors independently assessed risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and adapted from those used by the Cochrane Pregnancy and Childbirth Group, with any disagreements resolved by discussion. We assessed the following for each study.
-
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, e.g. random number table; computer random number generator); unclear risk of bias (method used to generate sequence not clearly stated). We excluded studies using a non‐random process (e.g. odd or even date of birth; hospital or clinic record number).
-
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions before assignment determines whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment. We assessed the methods as: low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes); unclear risk of bias (method not clearly stated). We excluded studies that did not conceal allocation (e.g. open list).
-
Blinding of outcome assessment (checking for possible detection bias). We assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study states that it was blinded and describes the method used to achieve blinding, e.g. identical tablets; matched in appearance and smell); unclear risk of bias (study states that it was blinded but does not provide an adequate description of how this was achieved). We excluded studies that were not double‐blind.
-
Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk of bias (< 10% of participants did not complete the study and/or used ‘baseline observation carried forward’ analysis); unclear risk of bias (used 'last observation carried forward' analysis); high risk of bias (used 'completer' analysis).
-
Size of study (checking for possible biases confounded by small size). We assessed studies as being at low risk of bias (≥ 200 participants per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm); high risk of bias (< 50 participants per treatment arm).
Measures of treatment effect
Relative risk (or risk ratio, RR) was used to establish statistical difference. Numbers needed to treat (NNT) and pooled percentages were used as absolute measures of benefit or harm.
The following terms are used to describe adverse outcomes in terms of harm or prevention of harm:
-
When significantly fewer adverse outcomes occurred with carbamazepine than with control (placebo or active) we use the term the number needed to treat to prevent one event (NNTp).
-
When significantly more adverse outcomes occurred with carbamazepine compared with control (placebo or active) we use the term the number needed to harm or cause one event (NNH).
Unit of analysis issues
The control treatment arm would be split between active treatment arms in a single study if the active treatment arms were not combined for analysis.
Dealing with missing data
We planned to use intention‐to‐treat (ITT) analysis wherever possible. The ITT population consisted of participants who were randomised, took the assigned study medication and provided at least one post‐baseline assessment. Missing participants were assigned zero improvement (baseline observation carried forward, BOCF) where this could be done. We were aware that imputation methods might be problematical and examined trial reports for information about them.
Assessment of heterogeneity
We planned to deal with clinical heterogeneity by combining studies that examined similar conditions. We assessed statistical heterogeneity visually (L'Abbe 1987) and with the use of the I2 statistic.
Assessment of reporting biases
The aim of this review was to use dichotomous data of known utility (Moore 2009b). The review did not depend on what authors of the original studies chose to report or not report, though clearly there were difficulties with studies failing to report any dichotomous results. Continuous data, which probably poorly reflect efficacy and utility, were extracted and used only when useful for illustrative purposes.
We undertook no statistical assessment of publication bias.
We looked for evidence of possible enrichment, either complete or partial, in enrolment of participants into the studies. Enrichment typically means including participants known to respond to a therapy, and excluding those known not to respond, or to suffer unacceptable adverse effects, though for gabapentin no significant effects have been shown from partial enrichment (Straube 2008). Enriched enrolment randomised withdrawal studies, known to produce higher estimates of efficacy, would not be pooled (McQuay 2008).
Data synthesis
We analysed data for each painful condition in three tiers, according to outcome and freedom from known sources of bias.
-
The first tier uses data meeting current best standards, where studies report the outcome of at least 50% pain intensity reduction over baseline (or its equivalent), without the use of last observation carried forward (LOCF) or other imputation method for dropouts, report an intention‐to‐treat (ITT) analysis, last eight or more weeks, have a parallel‐group design, and have at least 200 participants (preferably at least 400) in the comparison (Moore 2010a; Moore 2012b). These top‐tier results are reported first.
-
The second tier uses data from at least 200 participants but where one or more of the above conditions is not met (for example reporting at least 30% pain intensity reduction, using LOCF or a completer analysis, or lasting four to eight weeks).
-
The third tier of evidence relates to data from fewer than 200 participants, or where there are expected to be significant problems because, for example, of very short duration studies of less than four weeks, where there is major heterogeneity between studies, or where there are shortcomings in allocation concealment, attrition, or incomplete outcome data. For this third tier of evidence, no data synthesis is reasonable, and may be misleading, but an indication of beneficial effects might be possible
Subgroup analysis and investigation of heterogeneity
We planned for all analyses to be according to individual painful conditions, because placebo response rates with the same outcome can vary between conditions, as can the drug‐specific effects (Moore 2009a). We also planned subgroup analysis for dose of carbamazepine and duration of study.
Sensitivity analysis
We planned no sensitivity analyses because the evidence base was known to be too small to allow reliable analysis.
Results
Description of studies
Results of the search
New searches to February 2014 identified two potentially relevant studies (Salinas 2012; Shaikh 2011), but neither satisfied our inclusion criteria, so there were no new included studies in this update. Two studies that were identified in an earlier search remain unavailable (Badran 1975; Liebel 2001). Figure 1 shows the flow diagram for included studies.

Study flow diagram.
Included studies
This updated review includes 10 studies (11 publications) with 480 participants, 414 of whom were randomised to receive carbamazepine, although not all of them contributed to analyses. Three studies (Gomez‐Perez 1996; Jia 2006; Nicol 1969) did not report the age or sex, or both, of participants, but in the remaining studies the mean age was 52 to 59 years (range 20 to 84 years), and the majority of participants were female.
A wide range of carbamazepine doses, ranging from 100 mg to 2400 mg daily, were used in the studies. Cross‐over studies predominated; only one had a parallel group design (Jia 2006). Most of the studies were of short duration, lasting four weeks or less. Pain conditions studied were trigeminal neuralgia, painful diabetic neuropathy, and post stroke pain.
Many of the studies were relatively old, with five published in the 1960s. Only one study (Jia 2006) has been published in the last ten years. A consequence of the age of the studies is that outcomes ‐ pain, adverse event, and discontinuation ‐ were reported inconsistently. Pooling of trial data in meta‐analyses was therefore problematical, because few studies reported the same outcomes in the same way in the same condition.
Details of included studies are given in the 'Characteristics of included studies' table.
Excluded studies
Five studies that were included in the earlier reviews are now excluded:
-
one in acute herpes zoster (Keczkes 1980) due to the change of title to chronic pain and it was probably not blinded,
-
one in postherpetic neuralgia (Gerson 1977) because it was not blinded, and
-
three in trigeminal neuralgia: one (Rasmussen 1970) because it was only single blind, and two (Rockliff 1966; Vilming 1986) because they had fewer than 10 participants per treatment arm.
Details of included studies are given in the 'Characteristics of excluded studies' table.
Risk of bias in included studies
Out of a maximum of five points, two studies scored 5 points, five scored 4 points, one scored 3 points, and two scored 2 points on the Oxford Quality Scale. Points were lost due to failure to adequately report withdrawals or details of the randomisation and blinding processes. Scores for individual trials are reported in the notes section of 'Characteristics of included studies' table.
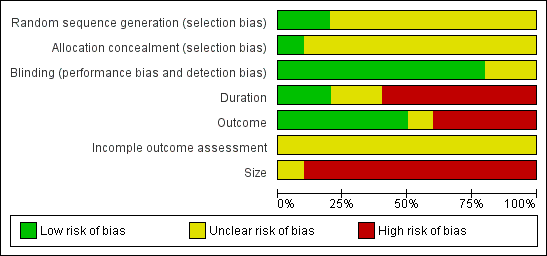
A risk of bias table was completed for randomisation, allocation concealment and blinding (Figure 2; Figure 3). All the included studies were judged to be at high risk of bias from at least one of three potential sources relating to size, duration, and outcome:

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Size. Treatment group sizes were small, ranging from a maximum of 12 to 66 (mean 33, median 33 for carbamazepine). Small studies can be associated with larger treatment effects than bigger studies (Counsell 1994; Moore 1998a; Moore 1998b; Nuesch 2010).
Duration. Studies were of generally of short duration, with individual treatment periods ranging from five‐days in a straightforward cross‐over study (Killian 1968) to 42 months in a partial cross‐over study in which participants stayed on the first treatment for a minimum of two weeks and switched only if or when treatment was 'unsatisfactory' (Nicol 1969). One study (Lechin 1989) used eight‐week treatment periods and three studies (Campbell 1966; Gomez‐Perez 1996; Leijon 1989) used four‐week periods, with the remainder using 5 to 14 day periods. Chronic pain studies of six weeks or less have been shown to manifest greater treatment effects than those of eight weeks or more (Moore 2009b).
Outcome. A variety of different pain outcomes was reported, including average pain scores, raw individual pain scores (though not always complete), dichotomous outcomes such as the proportion with any improvement or benefit, including scores (like global impression of change) equivalent to IMMPACT outcomes of moderate or substantial benefit (Dworkin 2008); some studies gave little or no indication of how many patients benefited from treatment. Four studies reported only group mean pain scores (Jia 2006; Lechin 1989; Wilton 1974) or a measure of 'upgrading', rather than absolute pain scores or amount of change (Campbell 1966). Higher levels of benefit (e.g. ≥ 50% pain relief rather than any pain relief) result in higher NNTs (Moore 2009b).
None of the included studies mentioned how missing data were handled, for example using last observation carried forward or baseline observation carried forward imputation for participants who withdrew from the studies. Some studies may have analysed only those participants who completed the study, or both phases of a cross‐over study.
Intention‐to‐treat analysis was not carried out and patients who dropped out of studies were not included in the analysis. This is likely to be the source of additional bias.
Exaggeration of treatment effects in cross‐over trials compared with parallel group designs has been seen in some circumstances (Khan 1996), but it is unclear whether this is a general effect (Elbourne 2002). The predominance of cross‐over trials in this review has to be considered as a possible source of additional bias. In these circumstances, caution is needed in interpreting the data as far as efficacy is concerned. In particular, meaningful comparison of efficacy with other interventions is not possible.
Effects of interventions
See: Summary of findings for the main comparison
Ten studies were suitable for inclusion. Studies enrolled participants with trigeminal neuralgia, diabetic neuropathy, or post stroke pain. There were no studies investigating carbamazepine in other neuropathic pain conditions or in fibromyalgia. Details of results in individual studies are presented in Appendix 4.
Trigeminal neuralgia
Five studies of carbamazepine in trigeminal neuralgia were included. One of these (Killian 1968) also recruited some participants with other neuralgias; 30 of 42 participants had trigeminal neuralgia.
There was no first or second tier evidence of efficacy.
Third tier evidence.
Three studies were placebo‐controlled (Campbell 1966; Killian 1968; Nicol 1969). Using dose titration to a maximum daily dose of 1000 mg, 19/27 participants had a complete or very good response with carbamazepine compared with minimal or no response with placebo on five days' treatment in a subset of patients with trigeminal neuralgia (Killian 1968). Again using dose titration and a cross‐over design, but to a maximum daily dose of 2400 mg, 15/20 participants randomised to initial carbamazepine had a good or excellent response after 14 days' treatment, compared with 6 of 24 reporting good or excellent response who started on placebo (Nicol 1969). There were too few data for analysis.
A study by Campbell 1966 reported results by the number of changes in the pain score; this study has been removed from the analyses in this version as the numbers presented in the paper are events rather than patient data. It had claimed a mean fall in maximum pain intensity of 58% after two weeks with carbamazepine 400 to 800 mg daily compared to 26% with placebo.
Two active controlled studies were included. Lindstrom 1987 compared carbamazepine (maximum tolerated dose) with tocainide (an antiarrhythmic drug; 20 mg/kg/day divided into three doses). All participants had baseline pain scores without treatment of ≥4/10. There was no difference between the treatments; 7/12 and 6/12 participants treated with carbamazepine and tocainide respectively had mean pain scores of ≤ 3/10 (no worse than mild pain) in the last 10 days of a two‐week treatment period. Only one participant responded to carbamazepine, but not tocainide.
Lechin 1989 compared carbamazepine (titrated to maximum 1200 mg/day) with pimozide (an antipsychotic drug; titrated to maximum 12 mg/day) over eight weeks. All 24 participants treated with pimizole, and 14/24 treated with carbamazepine in the first period of treatment "eventually improved", with maximal improvement after 6 weeks of treatment. Results for the second period of the cross‐over study were similar.
Diabetic neuropathy
Four studies evaluated carbamazepine in diabetic neuropathy (Gomez‐Perez 1996; Jia 2006; Rull 1969; Wilton 1974).
There was no first or second tier evidence of efficacy.
Third tier evidence
Two studies were placebo controlled (Rull 1969; Wilton 1974). In a complicated three‐way cross‐over, Rull 1969 found that, using the top two levels of pain improvement (3 points or better out of 5), in the first two of three cross‐over periods for each group, 17/30 improved with carbamazepine and 2/30 for placebo, over two weeks. The other study (Wilton 1974) reported only on preferences after one week of treatment with carbamazepine and placebo; 24/40 preferred carbamazepine, 14/40 preferred placebo, and 2/40 had no preference.
Two active controlled studies met the inclusion criteria. One compared carbamazepine 200 mg to a nortriptyline 10 mg plus fluphenazine 0.5 mg combination over four weeks (Gomez‐Perez 1996). No significant difference was found between carbamazepine and the nortriptyline combination; both treatments improved paraesthesia and pain. Jia 2006 compared venlafaxine with carbamazepine over two weeks in 132 participants, with both drugs given at fixed and relatively low dose. Both drugs demonstrated effect with venlafaxine showing a somewhat larger mean effect.
Post stroke pain
There was no first or second tier evidence.
Third tier evidence
A cross‐over study compared carbamazepine, amitriptyline, and placebo, with a treatment duration of four weeks for each (Leijon 1989). Global report of any improvement occurred in 10 of 15 on amitriptyline, 5 of 15 on carbamazepine, and 1 of 15 on placebo.
Overall estimation of efficacy
Although we specified that we would analyse different pain conditions separately, because carbamazepine is such a widely used drug for neuropathic pain (particularly trigeminal neuralgia) and because we found so little evidence, we did carry out a post‐hoc analysis combining pain conditions to look for a direction of effect.
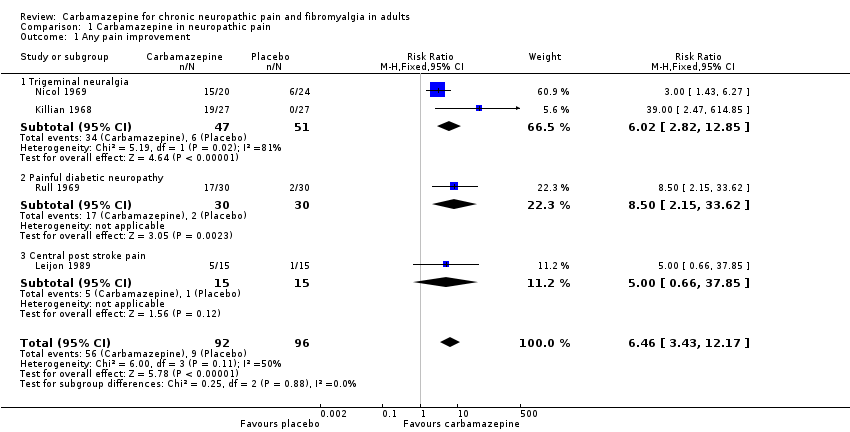
Four studies (188 participants) compared carbamazepine with placebo and provided dichotomous outcomes equivalent to the IMMPACT substantial improvement (Killian 1968; Leijon 1989; Nicol 1969; Rull 1969). Carbamazepine at any dose was consistently better than placebo (Figure 4) and overall 61% (56/92) of participants improved with carbamazepine compared with 9% (9/96) with placebo. The RR was 6.5 (3.4 to 12.2), and the NNT 1.9 (1.6 to 2.5) (Analysis 1.1; Figure 5).

Four studies showing percentage improvement (any definition) with carbamazepine (any dose) and placebo. Size of the study is proportional to the size of the symbol (inset scale). Yellow symbols = trigeminal neuralgia , blue = painful diabetic neuropathy, red = post stroke pain

Forest plot of comparison: 1 Carbamazepine in neuropathic pain, outcome: 1.1 Any pain improvement.
Withdrawals
Eight studies provided information on adverse event withdrawals with carbamazepine (Campbell 1966; Gomez‐Perez 1996; Killian 1968; Lechin 1989; Leijon 1989; Lindstrom 1987; Nicol 1969; Wilton 1974). In these studies, 8/268 (3%) of participants withdrew because of adverse events with carbamazepine, compared with 0/255 with placebo.
At least one adverse event
Four studies provided information on participants experiencing at least one adverse event (Campbell 1966; Lechin 1989; Leijon 1989; Wilton 1974). Adverse event experience was more common with carbamazepine (66% of participants) than placebo (27%), RR 2.4 (1.9 to 3.2), NNH 2.6 (2.1 to 3.5).
Serious adverse events
Serious adverse events were not reported consistently, and that included the absence of negative statements that there were no serious adverse events. Only one study (Gomez‐Perez 1996) reported an adverse event as serious, a case of upper gastrointestinal bleeding thought to be associated with alcohol rather than carbamazepine. Rashes associated with carbamazepine were reported in two participants in Rull 1969; these may be considered serious because of association with Stevens‐Johnson syndrome.
Deaths
Four deaths occurred on treatment with carbamazepine, all in one study (Nicol 1969), which had the longest follow‐up; two participants died suddenly, presumably of cardiovascular disease, one had a brain tumour, and one died of progressive debilitating disease.
Specific adverse effects
Specific adverse events reported at high incidence (> 10%) included giddiness, dizziness, unsteadiness, and somnolence. These were not reported in sufficient detail to be combined, but the incidence of somnolence and dizziness was as high as 40‐60% with carbamazepine.
Discussion
Much has been written about how to justify the use of our long‐established medical interventions. While the randomised controlled trial (RCT) is the gold standard for the assessment of health care technologies and interventions (DOH 1992), buttressed by double blinding when the outcome measures are subjective (Colditz 1989; Schulz 1994; Turner 1994), the fact remains that many interventions are time‐honoured rather than RCT‐honoured. On whom then does the burden of proof fall? (Eddy 1993). The aim of this systematic review was to review the effectiveness and safety of the antiepileptic drug carbamazepine in the management of neuropathic pain and fibromyalgia. Since the first version of this review, gabapentin has become established as a treatment for neuropathic pain, and is now licensed for this indication in a number of countries. The problem is that there is very little good evidence to support the received wisdom regarding carbamazepine and its use in neuropathic pain or fibromyalgia generally, or even in trigeminal neuralgia, where response to carbamazepine has in the past been regarded as almost pathognomic.
More recent antiepileptic drugs have good evidence of effect, as with gabapentin (Moore 2011), pregabalin (Moore 2009a), or oxcarbazepine (Zhou 2013), or good evidence of lack of effect as with lamotrigine (Wiffen 2013b) and topiramate (Wiffen 2013c). An updated version of Gabapentin is currently awaiting publication(April 2014) and an overview of these medicines titled 'Antiepileptic drugs for neuropathic pain and fibromyalgia‐an overview of Cochrane reviews' has been recently published (Wiffen 2013a).
Summary of main results
Carbamazepine generally provided better pain relief than placebo in a comparison that included three different chronic neuropathic pain conditions (trigeminal neuralgia, painful diabetic neuropathy, and central post stroke pain). There was some indication of pain improvement over mainly the short term, but with poorly defined outcomes, in fewer than 200 participants (less than a tenth of the number of participants available for one dose of pregabalin, for instance; (Moore 2009a)). The NNH for any adverse event was 4, though again reporting was neither consistent nor complete.
What we have is an indication that carbamazepine can produce good levels of pain relief for some patients with distressing chronic painful conditions.
Overall completeness and applicability of evidence
The evidence is far from complete, and any assessment of applicability resides less with the evidence than the long experience of using the drug in neuropathic pain. The major problems with the amount and quality of the evidence available include:
-
limited size, with all but one study involving fewer than 60 participants;
-
short duration, with all but two studies being four weeks or less in treatment duration;
-
inadequate outcomes, with inconsistent reporting not allowing outcomes equivalent to IMMPACT outcomes of at least moderate benefit to be assumed;
-
incomplete outcome assessment, with studies reporting on completers only, or not reporting on imputation methods.
Poor quality reporting limited the ability to combine data, because many studies reported insufficient information, used a variety of different outcome measures, and several studies used variable dosing. Although the authors of the original reports were originally contacted by letter, not all of them replied, and of those who did, only Leijon 1989 was able to provide additional data.
Doses of carbamazepine used in some of the studies were small; Gomez Perez, for example, used 200 mg daily, which while effective in some is not effective in all (Taylor 1981). Dose escalation was rapid in some studies, potentially resulting in adverse effects. Although carbamazepine takes two to four days to achieve its maximum effect, auto induction of enzymes that metabolise the drug, which is complete at three weeks, often means that late dose increases are needed. These factors are largely ignored, and this limits the applicability of the available evidence.
These studies do not provide adequate information about adverse events, and in particular serious adverse events. This is of particular importance for serious cutaneous adverse events in some parts of the world. A strong genetic association between HLA‐B*1502 and carbamazepine‐induced Stevens‐Johnson syndrome and toxic epidermal necrolysis has been shown in Han Chinese (Chung 2010), Indian (Mehta 2009), and Thai (Tassaneeyakul 2010) populations, and Asian populations generally may be more susceptible. While the frequency of this allele is low in Europe, its frequency in Asian populations is 5‐10% (Chung 2010). Carbamazepine is the most common causative agent for Stevens‐Johnson syndrome and toxic epidermal necrolysis in Europe (8% of total), rising to 26% in Taiwan, 36% in Malaysia and 28% in Singapore (Chung 2010).
There is also an interaction between carbamazepine and warfarin metabolism which can be of major clinical importance (Herman 2006). If treatment with carbamazepine cannot be avoided, patients taking warfarin should be frequently monitored, especially when initiating or stopping carbamazepine therapy.
Quality of the evidence
Studies were small, short, and had inadequate definitions of benefit, plus incomplete reporting. For each of these there is evidence that they could be the source of systematic bias, and this significantly reduces the weight we can give such evidence as we have. In particular, it limits comparability of carbamazepine results with results for other interventions obtained from larger, longer, and better studies and meta‐analyses.
In order to be sure that carbamazepine works in chronic painful conditions and to be confident of the magnitude of the effect, the ideal would be several large randomised double blind studies comparing carbamazepine at sensible doses with placebo, over 8 to 12 weeks, and using IMMPACT outcomes (perhaps at least moderate improvement or benefit) or their equivalent in each of several clinical conditions, as we have for pregabalin, for example (Moore 2009a). We actually have only one study of adequate duration; Lechin 1989 enrolled 59 participants with trigeminal neuralgia, had a quality score of 4 of 5, but reported only an undefined improvement for only 48 of 59 participants randomised (1 lost to follow‐up and 10 protocol deviations).
Potential biases in the review process
Criteria for assessing potential biases in chronic pain are becoming more stringent as new biases are being discovered (Moore 2010b; Moore 2012b). Potential biases in the review process derive from including studies with the potential for bias, though the review has sought to highlight the potential for bias when it occurs.
Using only criteria of sufficient stringency to avoid all these potential biases would reduce the pool of included studies to nil, which, given that carbamazepine is used to treat neuropathic pain, is less than helpful.
Agreements and disagreements with other studies or reviews
The results of this review are generally in agreement with the previous version. We are not aware of any other systematic reviews specifically concerning carbamazepine, but a broad overview of interventions for neuropathic pain (Finnerup 2005) had a combined NNT for efficacy of 2.0 (1.6 to 2.5), similar to our estimate of 1.7 (1.5 to 2.0). The source of the small difference cannot be ascertained from details provided.

Study flow diagram.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Four studies showing percentage improvement (any definition) with carbamazepine (any dose) and placebo. Size of the study is proportional to the size of the symbol (inset scale). Yellow symbols = trigeminal neuralgia , blue = painful diabetic neuropathy, red = post stroke pain

Forest plot of comparison: 1 Carbamazepine in neuropathic pain, outcome: 1.1 Any pain improvement.
Comparison 1 Carbamazepine in neuropathic pain, Outcome 1 Any pain improvement.

Comparison 1 Carbamazepine in neuropathic pain, Outcome 2 At least 1 adverse event.
| Carbamazepine compared with placebo for chronic neuropathic pain | ||||||
| Patient or population: adults with neuropathic pain (Trigeminal neuralgia, painful diabetic neuropathy, chronic post stroke pain) Settings: community Intervention: oral carbamazepine (100 mg to 2400 mg daily) Comparison: placebo | ||||||
| Outcomes | Probable outcome with placebo | Probable outcome with intervention | NNT or NNH and/or relative effect (95% CI) | No of participants | Quality of the evidence | Comments |
| "Substantial" benefit At least 50% reduction in pain or equivalent | 94 in 1000 | 608 in 1000 | RR 6.5 (3.4 to 12) NNT 1.9 (1.6 to 2.5) | 188 participants, 4 studies | Low | Mixed conditions and doses, small studies of short duration, imputation not reported |
| "Moderate" benefit At least 30% reduction in pain | No data | Very low | No data | |||
| Proportion below 30/100 mm on VAS | No data | Very low | No data | |||
| Patient Global Impression of Change much or very much improved | No data | Very low | No data | |||
| Any adverse event | 270 in 1000 | 660 in 1000 | RR 2.4 (1.9 to 3.2) NNH 2.6 (2.1 to 3.5) | 346 participants, 4 studies | Low | Cross‐over studies Denominator = all potentially exposed |
| Adverse event withdrawals | 0 in 1000 | 30 in 1000 | not calculated | 523 participants, 8 studies | Very low | Cross‐over studies Denominator = all potentially exposed |
| Serious adverse events | not reported | 3 | not calculated | 46 participants, 2 studies | Very low | Denominator = all potentially exposed |
| Death | not reported | 4 | not calculated | 44 participants 1 study | Very low | Denominator = all potentially exposed |
| GRADE Working Group grades of evidence | ||||||
| NNT: number needed to treat for an additional beneficial effect: NNH: number needed to treat for an additional harmful effect; RR: risk ratio; VAS: visual analogue scale. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any pain improvement Show forest plot | 4 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.46 [3.43, 12.17] |
| 1.1 Trigeminal neuralgia | 2 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.02 [2.82, 12.85] |
| 1.2 Painful diabetic neuropathy | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.5 [2.15, 33.62] |
| 1.3 Central post stroke pain | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.66, 37.85] |
| 2 At least 1 adverse event Show forest plot | 4 | 346 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [1.85, 3.12] |

