Eingeschränkte Flüssigkeitsgabe zur Behandlung von Frühgeborenen mit chronischer Lungenerkrankung
Appendices
Appendix 1. Standard search methodology
Cochrane Library: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomised controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomised [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh]))
Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomised controlled trial or controlled clinical trial or randomised or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomised controlled trial OR controlled clinical trial OR randomised OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)

Study flow diagram.

Comparison 1 Fluid restricted compared to liberal fluids, Outcome 1 Duration of hospitalisation.
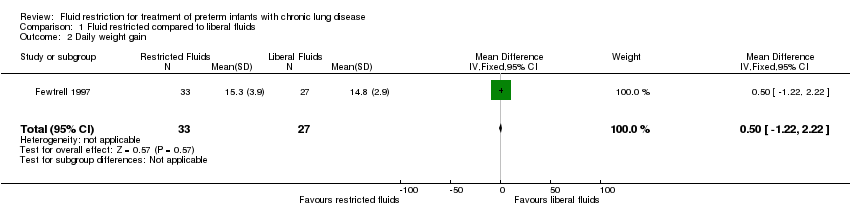
Comparison 1 Fluid restricted compared to liberal fluids, Outcome 2 Daily weight gain.
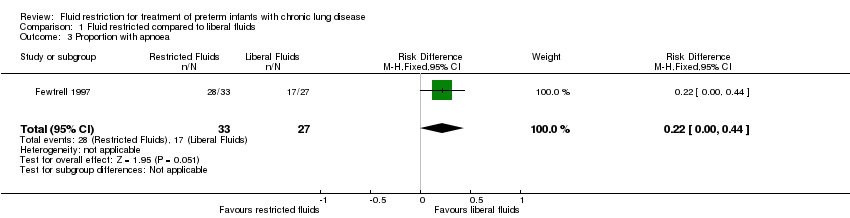
Comparison 1 Fluid restricted compared to liberal fluids, Outcome 3 Proportion with apnoea.
| Fluid restricted compared to liberal fluids compared to placebo for treatment of preterm babies with chronic lung disease | ||||||
| Participant or population: treatment of preterm babies with chronic lung disease | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with fluid restricted compared to liberal fluids | |||||
| Duration of oxygen therapy | The mean duration of oxygen therapy was 27.5 days | median 0.5 days higher | ‐ | 60 | ⊕⊕⊝⊝ | |
| Duration of hospitalisation | The mean duration of hospitalisation was 85 days | MD 3 Days higher | ‐ | 60 | ⊕⊕⊝⊝ | |
| Daily weight gain | The mean daily weight gain was 14.8 g/kg/d | MD 0.5 g/kg/d higher | ‐ | 60 | ⊕⊕⊝⊝ | |
| Proportion with apnoea | Study population | RR 1.35 | 60 | ⊕⊕⊝⊝ | ||
| 630 per 1000 | 850 per 1000 | |||||
| Moderate | ||||||
| 630 per 1000 | 850 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Unmasked study 2 Wide confidence limits | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of hospitalisation Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐7.64, 13.64] |
| 2 Daily weight gain Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐1.22, 2.22] |
| 3 Proportion with apnoea Show forest plot | 1 | 60 | Risk Difference (M‐H, Fixed, 95% CI) | 0.22 [‐0.00, 0.44] |

