Местные анестетики для контроля боли во время восстановления после глубокого кожного пореза
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Single‐centre RCT, paediatric emergency department, United States | |
| Participants | 151 patients younger than 18 years old with lacerations on the scalp (n = 31), face (n = 79) or extremity (n = 41) | |
| Interventions | 1. Topical TAC solution (tetracaine 0.5%, epinephrine 1:2000, cocaine 11.8%), applied for 5 to 10 minutes (n = 56) | |
| Outcomes | 1. Before laceration repair, the physician probed the wound with a 25‐gauge needle to determine adequacy of initial anaesthesia. Results of topical TAC versus topical placebo include the following. Results of topical TAC versus infiltrated local anaesthetic include the following. | |
| Intervention dates | August 1986 to May 1987 | |
| Declaration of interest | Not reported | |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The last digit of the patient's medical record number was used to enter patients into either the intradermal or topical group". Comment: probably not done |
| Allocation concealment (selection bias) | High risk | Quote: "The last digit of the patient's medical record number was used to enter patients into either the intradermal or topical group". Comment: probably not done |
| Blinding (performance bias and detection bias) | High risk | Quote: "Individual study vials containing 5ml of TAC or placebo were prepared in the pharmacy of University of Massachusetts Medical Center following a standard protocol and assigned numbers"; "The ED staff member evaluating and suturing the patient were blind to the solution contained in the vials". Comment: Comparisons of topical TAC and topical placebo were probably blinded. However, comparisons between lidocaine infiltration and topical TAC were probably unblinded. |
| Incomplete outcome data (attrition bias) | Low risk | 153 eligible patients, 2 refused to participate. 151 randomized, no missing outcome data |
| selective reporting of outcomes | Unclear risk | All outcomes discussed in Methods section reported in Results. Subgroup analysis based on location of laceration was not prespecified. |
| Other bias (sample size) | High risk | 56 TAC: 53 lidocaine 42 placebo |
| Methods | Single‐centre RCT, emergency department, community‐based teaching hospital, United States | |
| Participants | 35 adult and paediatric patients (minimum age of 2 years) with facial and scalp lacerations, ≤ 6 cm in length | |
| Interventions | 1. Topical TAC solution (tetracaine 0.5%, epinephrine 1:2000, cocaine 10.4%), applied for 20 minutes (n = 18) | |
| Outcomes | 1. The participant reported discomfort using a facial effective pain scale (1‐9), which consisted of 9 faces with various emotional expressions. However, in a few cases, the participant was too young to use the pain scale, so the physician estimated the participant's pain using the same Faces scale. The study combined self‐reported and surrogate Faces pain scale scores in the final results. Results included the following. | |
| Intervention dates | May to August 1992 | |
| Declaration of interest | Not reported | |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The TAC and TLE solutions were arbitrarily assigned to a single‐dose (10ml), sequentially numbered vials by the pharmacist. The vials, with the specific contents unknown to the emergency physician, were forwarded to the ED as requested". Comment: probably not done |
| Allocation concealment (selection bias) | Low risk | Quote: "The solutions were made visibly identical by adding methylene blue to the TLE solution so that it matched the intrinsic blue colour of TAC". "The vials, with the specific contents unknown to the emergency physician, were forwarded to the ED as requested". Comment: probably done |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The solutions were made visibly identical by adding methylene blue to the TLE solution so that it matched the intrinsic blue colour of TAC". Comment: probably done |
| Incomplete outcome data (attrition bias) | Unclear risk | 35 participants in study but reporting of attrition or exclusions insufficient to permit judgement |
| selective reporting of outcomes | Low risk | All outcomes described in Methods were fully reported in Results section. Adverse events noted |
| Other bias (sample size) | High risk | Total N = 35: 17 participants in the TLE group; 18 in the TAC group |
| Methods | Single‐centre RCT, Department of Emergency Medicine, Children’s Hospital Wisconsin, Milwaukee, Wisconsin, United States | |
| Participants | 55 paediatric patients with facial lacerations | |
| Interventions | 1. Topical TAC solution (tetracaine 0.5%, epinephrine 1:2000, cocaine 11.8%), applied for 10 to 15 minutes (n = 24) | |
| Outcomes | 1. The physician calculated the total number of 'sutures eliciting pain' using the following system. Each suture placed involved 2 points; an entrance and an exit piercing of the wound tissue with the needle. A painful response consisted of a verbal participant experiencing a painful sensation or a non‐verbal participant beginning to cry, or crying with greater intensity. The total number of 'sutures placed eliciting pain' was calculated by dividing the total number of painful responses by 2. Results included the following. | |
| Intervention dates | Not reported | |
| Declaration of interest | No explicit documentation regarding conflicts of interest. | |
| Notes | Source of funding: general academic paediatric development fellowship from The Robert Wood Johnson Foundation; and Grant 10066 from The Robert Wood Foundation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "...as in each case an assistant randomly selected one of the two solutions for physician application..." Comment: probably not done |
| Allocation concealment (selection bias) | High risk | Quote: "an assistant randomly selected one of the two solutions for physician application..." Comment: probably not done |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The managing physician was 'blind' to which preparation was being administered...the physician was informed of the solution composition only after the suturing procedure and pain scoring were completed". Comment: probably done, assuming the 2 solutions were visually identical |
| Incomplete outcome data (attrition bias) | Unclear risk | 55 participants in study but reporting of attrition or exclusions insufficient to permit judgement |
| selective reporting of outcomes | Low risk | The study protocol is available, and all of the study’s prespecified outcomes have been reported in the prespecified way. |
| Other bias (sample size) | High risk | 55 paediatric participants: 1. Topical TAC solution, n = 24 2. Topical AC solution, n = 31 |
| Methods | RCT, single centre, emergency department, United States | |
| Participants | 139 adult and paediatric patients older than 5 years of age, with laceration of the face (n = 53), scalp (n = 33), extremity (n = 52) or trunk (n = 1), measuring < 5 cm in length | |
| Interventions | 1. Topical TAC solution (tetracaine 0.5%, epinephrine 1:2000, cocaine 11.8%), applied for 5 to 10 minutes (n = 69) | |
| Outcomes | 1. The physician assessed the adequacy of initial anaesthesia by pricking the wound with a pin. If pain was elicited with pinprick, then 1% lidocaine was infiltrated, and the participant was assigned to the 'poor anaesthesia' group. 3. Investigators reported acute adverse reactions directly related to the anaesthetic. Results include the following. | |
| Intervention dates | Not reported | |
| Declaration of interest | Not reported | |
| Notes | Source of funding: Saint Francis Hospital and Medical Center Study author contacted for additional information but did not reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "TAC and cocaine solutions were randomly distributed with only a number from 1‐150 appearing on each vial". Comment: unclear; exact mechanism of randomization not described |
| Allocation concealment (selection bias) | Unclear risk | Quote: "TAC and cocaine solutions were randomly distributed with only a number from 1‐150 appearing on each vial". "The investigator was blinded as to the identity of the agent. The code was kept in the pharmacy and was available to the investigators only in case of emergency". Comment: unclear; allocation concealment possible if a pharmacy‐controlled randomization process was used. However, this is not explicitly reported, so we decided upon unclear risk. |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The investigator was blinded as to the identity of the agent. The code was kept in the pharmacy and was available to the investigators only in case of emergency". Comment: probably done, assuming local anaesthetic solutions are identical in colour |
| Incomplete outcome data (attrition bias) | Low risk | 148 participants were enrolled and 9 were excluded from the study before unblinding and analysis (4 improper application, 4 participant younger than 5 years and one with laceration too large). We concluded low risk of bias because the number of excluded participants was balanced between the 2 interventions, and reasons for exclusion are unlikely to be related to pain scores during suturing. |
| selective reporting of outcomes | Unclear risk | All outcomes described in Methods section were reported in Results. Subgroup analyses by site and age were not prespecified. |
| Other bias (sample size) | Unclear risk | Total N = 139: 70 in the cocaine‐treated group 69 in the TAC‐treated group |
| Methods | Single‐centre RCT, Department of Medicine, Section of Emergency Medicine, Louisiana State University, New Orleans, Louisiana, United States | |
| Participants | 95 patients age 5 to 17 years with lacerations on the face (n = 64) or scalp (n = 31), ≤ 7 cm in length | |
| Interventions | 1. Topical LAT gel (lidocaine 4%, epinephrine 1:2000, tetracaine 0.5%), applied for 10 to 30 minutes (n = 48) | |
| Outcomes | 1. Participant‐rated modified multi‐dimensional pain scale (0‐10) Results include the following. | |
| Intervention dates | Not reported | |
| Declaration of interest | Not reported | |
| Notes | Funding not reported ‐Study author contacted for additional information but did not reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Gels were randomized according to a random numbers table". Comment: probably done |
| Allocation concealment (selection bias) | High risk | Quote: "randomized according to a random numbers table" Comment: probably not done |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Patients and physicians performing suturing were blinded to which gels were being used. Only the numbers 1‐100 appeared on the capped syringes". Comment: probably done, assuming the 2 gels were visually identical |
| Incomplete outcome data (attrition bias) | Low risk | 100 participants entered into the trial, but 5 were excluded before statistical analysis because topical anaesthesia was inadequate and lidocaine infiltration was required. Two participants in the LAT group and 3 in the TAC group were excluded. We judged low risk of bias because the number of excluded participants was balanced between the 2 interventions. |
| selective reporting of outcomes | Low risk | All prespecified primary outcomes were reported: Physicians and participants or parents rated anaesthesia effectiveness during suturing utilizing a modified multi‐dimensional pain scale. Prespecified secondary outcomes were also reported: Participants or parents reported the number of sutures causing pain, which was analysed as percent of total sutures placed. Quote: “Both physician and patient or parent rated the anaesthesia effectiveness during suturing utilizing a modified multidimensional pain scale…. Patients or parents reported the number of sutures causing pain, which was analysed as percent of total sutures placed”. Table 1 lists demographics (age, sex), wound size, location, amount of anaesthetic used and number of sutures placed. Table 2 reports percent of sutures causing pain in each topical anaesthesia group. Table 3 reports physician vs participant rating for pain scores for each topical anaesthesia group. |
| Other bias (sample size) | High risk | LAT GEL = 48 participants TAC gel = 47 participants |
| Methods | Single‐centre RCT, Department of Medicine, Section of Emergency Medicine, Louisiana State University, New Orleans, Louisiana, United States | |
| Participants | 95 adult patients with laceration of the face (n = 81) or scalp (n = 13) ≤ 7 cm in length | |
| Interventions | 1. Topical LAT solution (lidocaine 4%, epinephrine 1:2000, tetracaine 0.5%), applied for 10‐30 minutes (n = 48) | |
| Outcomes | 1. Participant‐rated VAS (100 mm) pain score Results include the following. | |
| Intervention dates | ||
| Declaration of interest | Not reported | |
| Notes | Funding resource: supported by a grant from the Louisiana State University Emergency Medicine Residency Grant Fund. Study author contacted for additional information but did not reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Solutions were randomized according to a random numbers table". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "The solutions were prepared by a pharmacist and were available in coded sterile, capped 3ml syringes". "Both TAC and LAT were clear solutions..." "Patients and physicians performing wound closure were blinded". Comment: probably done |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The solutions were prepared by a pharmacist and were available in coded sterile, capped 3ml syringes with a cotton ball for application". "Both TAC and LAT were clear solutions mixed from powders". "Patients and physicians performing wound closure were blinded". Comment: probably done |
| Incomplete outcome data (attrition bias) | High risk | 100 total participants enrolled but only 95 were included in final data analysis. Four participants were excluded because they required additional injected lidocaine (1 LAT group, 3 in TAC group), and 1 because of improper data collection. We judged 'no' (high risk of bias) because requirement of additional lidocaine is directly related to pain intensity during laceration repair. |
| selective reporting of outcomes | Low risk | All outcomes described in Methods reported fully in Results. Adverse events reported |
| Other bias (sample size) | Unclear risk | 47 receiving TAC and 48 receiving LAT. Total N = 95 |
| Methods | Single‐centre RCT, urban emergency department, United States | |
| Participants | 66 paediatric and adult patients, older than 5 years of age with laceration on the face (n = 30), scalp (n = 10) or extremity (n = 24), 1.5 to 10 cm in length | |
| Interventions | 1. Topical LAT gel (lidocaine 4%, epinephrine 1:2000, tetracaine 0.5%), applied for 10 to 20 minutes (n = 33) | |
| Outcomes | 1. Participant‐rated VAS (100 mm) pain scale scores Results included the following. | |
| Intervention dates | Not reported | |
| Declaration of interest | Not reported | |
| Notes | Funding not reported Study author contacted for additional information but did not reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The doses of anaesthetic were numbered 1‐66 according to a computer generated random table of numbers prepared before the study". Comment: probably done |
| Allocation concealment (selection bias) | High risk | Quote: "physicians and patients were not blinded to the form of anaesthesia". Comment: probably not done |
| Blinding (performance bias and detection bias) | High risk | Quote: "Because of the obvious differences in form and application, physicians and patients were not blinded to the form of anaesthesia". Comment: probably not done |
| Incomplete outcome data (attrition bias) | Unclear risk | 66 participants included in study but reporting of attrition or exclusions insufficient to permit judgement |
| selective reporting of outcomes | Low risk | All prespecified primary outcomes were reported: Participant and physician ranked pain of suturing with validated linear visual analogue scale. Prespecified secondary outcomes were also reported: Necessity for additional lidocaine and treatment success or failure were recorded at the time of the procedure. Quote: “The primary endpoints were patient and physician perception of application or injection pain and anaesthesia effectiveness…. Patients and physicians ranked the pain of injection or application and the pain of suturing using a previously validated linear visual analog scale so that each laceration had four associated measurements of pain”. Quote: “The length of the laceration, location, length of time anaesthesia lasted, amount of anaesthesia used, necessity for additional lidocaine, and treatment success or failure were recorded at the time of the procedure, along with any complications”. Table 1 lists demographics (age, sex), wound size, initial amount of anaesthesia, need for more anaesthesia and location. Table 2 reports physician and participant ratings of pain of local and topical anaesthetic application (VAS) ‐ effectiveness. Table 3 reports physician vs participant rating for pain scores of suturing (VAS). Table 4 reports percent of sutures causing pain per participant. |
| Other bias (sample size) | High risk | Quote: “66 subjects were entered in the study. Topical LAT = 33, infiltrated lidocaine = 33”. |
| Methods | Single‐centre RCT, community teaching hospital emergency department, United States | |
| Participants | 100 adult patients older than 18 years of age with lacerations involving scalp (n = 15), face (n = 15), lower extremity (n = 13), upper extremity (n = 15) or hands (n = 42) Laceration length ranged from < 1 cm to > 5 cm. | |
| Interventions | 1. Topical LE solution (lidocaine 5%, epinephrine 0.025%), applied for 10 to 15 minutes for 1 to 4 sequential layered applications (n = 50) 2. Intradermal infiltration with lidocaine (n = 50) | |
| Outcomes | 1. Participant‐rated VAS (100 mm) pain scale scores 2. Amount of lidocaine required (mg) 3. Number of applications of topical anaesthetic 4. Difficulty with wound healing or infection Results included the following. 1. Participant‐reported VAS (100 mm) pain scores (mean score ± SD: topical TLE = 0.16 ± 0.46 vs infiltrated lidocaine = 0.20 ± 0.49; P = 0.59) 2. Amount of lidocaine required (mean score: TLE = 135 mg vs infiltrated lidocaine = 124 mg; P = 0.90, SD not reported) 3. Number of anaesthetic applications of TLE (mean score = 2.7; 2 participants (4%) required 1 layer, 17 (34%) required 2 layers, 26 (52%) required 3 layers, 5 (10%) required 4 layers) 4. No participants had poor wound healing or infection. | |
| Intervention dates | Not reported | |
| Declaration of interest | Not reported | |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "We performed a prospective, randomized controlled trial.." Comment: unclear; study reported to be randomized but method of sequence generation not described |
| Allocation concealment (selection bias) | High risk | Comment: probably not done. Interventions of topical anaesthesia vs infiltrated anaesthesia are visually different. No mechanism used to conceal the intervention from participants or study personnel was described. |
| Blinding (performance bias and detection bias) | High risk | Quote: "100 patient[s] were enrolled in a randomized controlled trial..." Comment: probably not done, as study did not report blinding and compared topical vs infiltrated forms of anaesthesia |
| Incomplete outcome data (attrition bias) | Low risk | 100 enrolled participants in study, no missing outcome data or exclusions |
| selective reporting of outcomes | Low risk | All prespecified primary outcomes were reported: patient‐reported VAS pain scores Prespecified secondary outcomes were also reported: amount of lidocaine required, number of applications of topical anaesthetic and difficulty with wound healing. Quote: “The effectiveness of anaesthesia was assessed by the patient immediately after the procedure using a 1‐10 visual analog pain scale administered by a third‐party. The subject was instructed to assess the pain from application or anaesthesia, and the pain from suturing the wound”. Table 2. Application of anaesthesia Table 3. Pain during application of anaesthetic Table 4. Effectiveness of anaesthesia during wound repair Table 5. Follow‐up interview after wound repair for 79 participants |
| Other bias (sample size) | Unclear risk | Infiltrated lidocaine = 50 participants Topical TLE = 50 participants |
| Methods | Two‐centre RCT, emergency departments, Uunited States | |
| Participants | 467 patients, 18 years of age or younger, with dermal lacerations on the face, scalp, extremity and trunk | |
| Interventions | 1. TAC solution (tetracaine 0.5%, epinephrine 1:2000, cocaine 11.8%), 2. Intradermal infiltration with lidocaine 1% (n = 205) | |
| Outcomes | Pain during the suturing process was not directly assessed. Results include the following. | |
| Intervention dates | December 1986 to November 1987 | |
| Declaration of interest | Not reported | |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Randomization of anaesthetic treatment was determined by the final digit of the patients medical record number, with odd numbers receiving lidocaine and even numbers receiving TAC". Comment: probably not done |
| Allocation concealment (selection bias) | High risk | Quote: "Randomization of anaesthetic treatment was determined by the final digit of the patient's medical record number". "unblinded study" Comment: probably not done |
| Blinding (performance bias and detection bias) | High risk | Quote: "We conducted a prospective, randomized, unblinded study..." Comment: probably not done |
| Incomplete outcome data (attrition bias) | Unclear risk | 467 participants included in the study but reporting of attrition or exclusions insufficient to permit judgement |
| selective reporting of outcomes | Low risk | All outcomes described in Methods section were fully reported in Results, including subgroup analyses by area of laceration. |
| Other bias (sample size) | Low risk | 262 children received TAC (218 facial or scalp and 44 extremity or trunk wounds), and 205 received lidocaine (158 facial or scalp and 47 extremity or trunk wounds). |
| Methods | RCT, single‐centre, hospital emergency department, Northern Ireland | |
| Participants | 110 (54 topical anaesthetic putty, 56 lidocaine infiltration), median age (range): infiltration 35 (18‐84), topical anaesthetic putty 35 (20‐81) Male: 94 (85.5%), female: 16 (14.5%). Topical anaesthetic putty group had 10 F, 44 M; lidocaine infiltration group had 6 F, 50 M. Wounds: < 8 cm long and needing suturing or stapling | |
| Interventions | 1. Topical anaesthetic putty (containing 4.94% w/w lidocaine hydrochloride, 2. Lidocaine infiltration (1% w/v) | |
| Outcomes | Primary outcomes: Participant‐reported 0‐10 VAS during sensory testing with a 21‐gauge needle “directly after treatment”. Mean pain score was 0.78 + 1.12 (SD) after lidocaine infiltration, 1.49 + 1.76 after topical anaesthetic putty. Overlapping 1‐sided 95% CI limits plus (because data were not normally distributed) non‐parametric contrasting of median scores; both showed non‐inferiority of topical anaesthetic putty c/w infiltrated lidocaine Secondary outcomes: Need for rescue anaesthesia (required by 3 in infiltration group and 4 in topical anaesthetic putty group), “wound evaluation score” obtained 7‐10 days after treatment (12 in topical anaesthetic putty group had less than perfect scores vs 5 in infiltration group), presence of wound infection (4 in infiltration group vs 2 in topical anaesthetic putty group), dehiscence (2 in topical anaesthetic putty group) and adverse effects (1 inflamed wound in topical anaesthetic putty group, 1 required resuturing in each group) No anaesthetic toxicity reported | |
| Intervention dates | Not reported | |
| Declaration of interest | The wound putty used in this study was not a proprietary product and was not produced commercially. The putty was manufactured by 2 of the study authors ‐ Drs. Murphy and McCarron. After the success of this trial, Drs. Jenkins and McCarron sought to protect certain aspects of the putty formulation in both the United States and Europe. This patent application was pending at the time of publication and was related to a certain aspect of the formulation that enables lidocaine to be included. The authors of this study received no funding from commercial sources to support the study. Funding for this study was obtained through a peer‐reviewed competitive process from the Public Health Agency in Northern Ireland. Drs. Jenkins and McCarron were pursuing sources of capital to commercialise the putty but had not yet secured this funding. | |
| Notes | Sourse of funding: supported by the Research and Development Office (Northern Ireland) Trauma and Rehabilitation Recognised Research Group (RRG 8.46 RRG/3273/06) Rescue medication: no systemic anaesthesia or analgesia mentioned. However, “The decision to offer or use rescue anaesthesia rested with the treating investigator”. Rescue = wound margin infiltration with a further dose of 1% lidocaine for the 7 (4 in the topical anaesthetic putty group, 3 in the lidocaine infiltration group) who received it | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization sequence generated by Microsoft Excel version 14.3.9 through a permuted block randomization technique, with a block size of 8 |
| Allocation concealment (selection bias) | Low risk | Randomization sequence provided in opaque, serially numbered envelopes |
| Blinding (performance bias and detection bias) | High risk | Open label Quote: “Because of the nature of the treatment, it was not feasible to blind either the participants or the investigators to the treatment received". [Extractor’s note: not necessarily true, could have used placebo infiltration and placebo topical putty] |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed the first, acute part of the study; 19 did not complete the follow‐up wound assessment. |
| selective reporting of outcomes | Low risk | All outcome‐related data collected during the acute phase were complete. |
| Other bias (sample size) | Unclear risk | 54 topical anaesthetic putty 56 lidocaine infiltration |
| Methods | Single‐centre RCT, Accident and Emergency Department of Gloucestershire Royal Hospital, United Kingdom | |
| Participants | 107 paediatric patients, 3‐16 years old, with lacerations < 4 cm in length, located anywhere on the body except mucous membranes or digits | |
| Interventions | 1. Topical AC solution (epinephrine 1:2000, cocaine 4.7%), applied for 10‐15 minutes (n = 51) | |
| Outcomes | 1. Children younger than 10 years of age rated pain during both laceration repair and anaesthetic application using the Wong‐Baker Faces Scale. Patients 10 years of age or older used a VAS (10 cm) score to rate pain during suturing and anaesthetic administration. Results include the following. | |
| Intervention dates | January to November 1994 | |
| Declaration of interest | No explicit documentation regarding conflicts of interest | |
| Notes | No sources of funding mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Children presenting with an appropriate laceration were consecutively assigned to receive either conventional intradermal lignocaine or topical AC preparation". Comment: probably not done |
| Allocation concealment (selection bias) | High risk | Quote: "consecutively assigned to receive either conventional intradermal lignocaine or topical AC preparation" "Groups could not be blinded". Comment: probably not done |
| Blinding (performance bias and detection bias) | High risk | Quote: "The nature of the trial meant that the two groups could not be blinded". Comment: probably not done |
| Incomplete outcome data (attrition bias) | Low risk | 120 participants were enrolled but 13 were excluded before data analysis (incomplete data collection for 8, 2 received Steristrips and not sutures, 3 did not attend follow‐up). We concluded low risk of bias because reasons for exclusion were unlikely to be related to pain scores during laceration repair. |
| selective reporting of outcomes | Low risk | The study protocol is available, and all of the study’s prespecified outcomes have been reported in the prespecified way. |
| Other bias (sample size) | Unclear risk | 1. Topical AC solution, n = 56 2. Intradermal infiltration with lidocaine, n = 51 |
| Methods | RCT (unclear if single centre or multi‐centre) | |
| Participants | 41 adult and paediatric patients, 5 to 23 years of age, with simple lacerations < 5 cm in length | |
| Interventions | 1. Topical LET gel (lidocaine, epinephrine, tetracaine), applied for 60 minutes (n = 22) 2. EMLA cream (lidocaine 2.5%, prilocaine 2.5%), applied for 60 minutes (n = 19) | |
| Outcomes | 1. Participant‐rated VAS (100 mm) pain scale scores 3. Physician‐rated VAS (100 mm) pain scale scores 4. Requirement of supplemental lidocaine infiltration Pain scores were obtained at 4 points in time: after irrigation, first suture or staple placement, last suture or staple placement and during supplemental infiltration of lidocaine (if applicable). Results include the following. 2. Legal guardian‐reported VAS (100 mm) pain scores were not significantly different between the 2 groups (mean pain scores not provided; P > 0.05). 3. Physician‐reported VAS (100 mm) pain scores were greater in the EMLA group during irrigation (mean VAS EMLA = 21.4 mm vs LET gel = 10.1 mm; P = 0.3) and during first suture/staple placement (mean VAS EMLA = 41.7 mm vs LET gel = 14.0 mm; P = 0.004). 4. Requirement of supplemental infiltrated anaesthesia: 13/19 participants in the EMLA group required infiltrated lidocaine (68%) compared with 5/22 in the LET group (23%) (P = 0.005%) | |
| Intervention dates | Not reported | |
| Declaration of interest | Not reported | |
| Notes | Trial published as an abstract only. Source of funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "We conducted a double‐blind, randomized trial...". Comment: unclear, as method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "We conducted a double‐blind, randomized trial..." Comment: unclear, as reported to be double‐blind but no details provided |
| Incomplete outcome data (attrition bias) | Unclear risk | 41 participants included in the study but reporting of attrition or exclusions insufficient to permit judgement |
| selective reporting of outcomes | Low risk | The study protocol is available, and all of the study’s prespecified outcomes have been reported. |
| Other bias (sample size) | High risk | 41 participants: 1. Topical LET gel, n = 22 2. EMLA cream, n = 19 |
| Methods | Single‐centre (2 hospitals) RCT, emergency departments of 2 tertiary referral hospitals (1 paediatric), Adelaide South Australia | |
| Participants | 180 adult and paediatric patients, 6 years of age or older, with lacerations 3‐7 cm in length, located on the head (n = 114) or extremity (n = 66) | |
| Interventions | 1. Topical MAC solution (bupivacaine 0.5%, epinephrine 1:2000, cocaine 10.0%), applied for at least 10 to 15 minutes for head lacerations and for 30 minutes for extremity wounds (n = 92) | |
| Outcomes | 1. Children younger than 12 years of age rated pain during laceration repair using the Wong‐Baker Faces scale. Results include the following. 3. Adequacy of initial anaesthesia (topical MAC = 73% vs topical TAC = 74%; P = 0.87) | |
| Intervention dates | Feburary 1992 to April 1994 | |
| Declaration of interest | No explicit documentation regarding conflicts of interest | |
| Notes | Source of funding: grant from Society of Hospital Pharmacists of Australia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study was a double‐blinded, randomized, prospective trial.." Comment: unclear, as study reported to be randomized but method of sequence generation was not described |
| Allocation concealment (selection bias) | Low risk | Quote: "Solutions of MAC and modified TAC were prepared and placed in syringes marked A or B by a pharmacist not involved in study. All study participants remained blinded throughout the trial". Comment: probably done, assuming solutions were visually identical |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Solutions of MAC and modified TAC were prepared and placed in syringes marked A or B by a pharmacist not involved in study. All study participants remained blinded throughout the trial". Comment: probably done, assuming solutions were visually identical |
| Incomplete outcome data (attrition bias) | Low risk | 191 participants were enrolled but 10 were excluded before data analysis (5 younger than 6 years of age, 2 had wounds greater than 5 mm deep, 2 were not sutured, 1 had a digital laceration). We concluded low risk of bias because reasons for exclusion were unlikely to be related to pain scores during laceration repair. |
| selective reporting of outcomes | Unclear risk | The study protocol did not describe prespecified outcomes. |
| Other bias (sample size) | Unclear risk | 180 participants: 1. Topical MAC solution, n = 92 2. Topical TAC solution, n = 88 |
| Methods | Single‐centre RCT, Department of Emergency Medicine, Singapore General Hospital | |
| Participants | n = 40, > 1 year to 70 years (only 1 patient > 10 years old was included in the study), 29 males (72.5%), 11 females (27.5%). Length of the wounds was 3.1 cm for the LG group and 3.5 cm for the LI group. Depth of the wounds was 0.5 cm and 0.57 cm, respectively. | |
| Interventions | 1. LAT gel (n = 23): mean length of wound/cm (SE) 3.1 cm (SE 0.31). Mean depth of wound/cm (SE) 0.5 (0.07). Location of wound: head 17/23 (74.0%), trunk 0/23 (0%) and limb 6/23 (26%) 2. Infiltrated lidocaine (n = 17): mean length of wound/cm (SE) 3.5 cm (SE 0.36). Mean depth of wound/cm (SE) 0.57(0.08). Location of wound: head 11/17 (64.7%), trunk 0/17 (0%) and limb 6/17 (35.3%) | |
| Outcomes | 1. LAT gel: a. Efficacy: 10 cm VAS pain score by participant (mean ± SE) = 2.5 (0.52) b. Pain during application (mean ± SE): 1.5 (0.40) Pain score by parents, clinician or participants younger than 10 years old; results not provided 2. Lignocaine infiltration: a. Efficacy: 10 cm VAS pain score by participant (mean ± SE) = 2.6 (0.58) b. Pain during application (mean ± SE): 3.5 (0.46) Pain score by parents, clinician or participants younger than 10 years old; results not provided Complications: 1. No acute anaesthetic complications in either group 2. One week later, assessed for wound complications 1. LAT gel (study lists 25 but probably typographical error because only 23 participants in this treatment arm) a. Wound Infection, 5/25 (5/23?) b. Wound dehiscence = 1/25 (1/23?) c. Stitches lost = 1/25 (1/23?) 2. Lignocaine infiltration a. Wound Infection, 2/14 b. Wound dehiscence, 0/14 c. Stitches lost, 0/14 | |
| Intervention dates | Janurary to April 2003 | |
| Declaration of interest | None. | |
| Notes | Souce of funding: none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Suitable participants were assigned to 2 arms of treatment via sealed envelopes. However, precise method of sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Use of assigned envelopes described but information proved insufficient to allow a decision between low risk and high risk |
| Blinding (performance bias and detection bias) | High risk | Not blinded and outcome could be affected by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | 40 patients recruited and no drop‐outs mentioned |
| selective reporting of outcomes | Low risk | All prespecified primary outcomes were reported. |
| Other bias (sample size) | High risk | LAT gel = 23 participants Infiltrated lidocaine = 17 participants |
| Methods | Single‐centre RCT, Army Medical Center emergency department, United States | |
| Participants | 158 adult and paediatric patients, range 10 months to 53 years old (mean = 9 years old) | |
| Interventions | 1. Topical TAC solution (tetracaine 0.5%, epinephrine 1:2000, cocaine 11.8%), applied for minimum of 10 minutes | |
| Outcomes | 1. Participants 10 years of age or older rated anaesthetic efficacy (complete, partial or none) depending on whether they experienced pain during laceration repair. Results include the following. | |
| Intervention dates | October to December 1979 | |
| Declaration of interest | Not reported | |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "A prospective study of topical TAC and lidocaine infiltration was taken with the last digit of the patients military sponsor's social security number used as the selection variable, odd numbered patients were anaesthetised with topical TAC; even numbered patients were anaesthetised with lidocaine". Comment: probably not done |
| Allocation concealment (selection bias) | High risk | Quote: "the last digit of the patient's military sponsor's social security number used as the selection variable" Comment: probably not done. Anaesthetic agents visually different, and no mention of safeguards to prevent concealment of identity |
| Blinding (performance bias and detection bias) | High risk | Quote: none Comment: probably not blinded, as the paper did not state whether participants or clinicians were blinded between topical and infiltrated anaesthetics |
| Incomplete outcome data (attrition bias) | Low risk | A total of 158 participants enrolled with no drop‐outs or exclusions. |
| selective reporting of outcomes | High risk | All outcomes described in Methods section were reported in Results, but method of assessing anaesthetic adequacy appears inconsistent between Methods and Results sections. Subgroup analysis by age was described in Methods, but results were not presented for all subgroups for each outcome. Wound complications were measured at 3 time points, but results were presented only for overall rate. No adverse events due to anaesthetic administration were reported. Some results are presented only graphically. |
| Other bias (sample size) | Unclear risk | 82 received topical TAC and 76 received lidocaine infiltration for anaesthesia. |
| Methods | Single‐centre RCT, emergency department, University of Minnesota‐affiliated Children’s Hospital, Minneapolis, Minnesota, United States | |
| Participants | 194 paediatric patients with lacerations of the face and scalp | |
| Interventions | 1. Topical LAT solution (lidocaine 4%, epinephrine 1:2000, tetracaine 0.5%), applied for 20 minutes (n = 103) | |
| Outcomes | 1. The physician assessed the adequacy of initial anaesthesia by probing the wound with a 27‐gauge needle. If any pain was elicited with probing, the anaesthetic was considered 'inadequate' and infiltrated lidocaine was given. Results include the following. | |
| Intervention dates | March 1995 to March 1996 | |
| Declaration of interest | Not reported | |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated random number table was used by a hospital pharmacy personnel to label standard amber vials from 1 to 200". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: :hospital pharmacy personnel to label standard amber vials from 1 to 200" "it was required that the study medication be applied by a nurse not involved in the suturing" Comment: probably done |
| Blinding (performance bias and detection bias) | Low risk | Quote: "To ensure blinding of suture personnel, in the trial, it was required that the study medication be applied by a nurse not involved in the suturing" Comment: probably done |
| Incomplete outcome data (attrition bias) | Unclear risk | 200 participants enrolled and 3 withdrawn before test of initial anaesthesia because participants were unco‐operative or complicated laceration did not meet inclusion criteria. Of the 197 available for analysis, 3 data sheets were inadvertently lost. We concluded low risk of bias because plausible effect size among missing outcomes was not enough to have a clinically relevant impact on observed effect size. |
| selective reporting of outcomes | Unclear risk | All prespecified primary and secondary outcomes were reported: physician determination of adequacy of anaesthetic before repair and anaesthetic effectiveness during repair. Adverse effects also reported Quote: “Pain assessment was a 2‐stage process that evaluated adequacy of anaesthesia before suturing and effectiveness of anaesthesia during suturing”. “Effectiveness of anaesthesia during suturing was divided into 3 categories: complete, partial, and incomplete”. “Complications assessed were redness, drainage, fever, tenderness, swelling, or contact with medical personnel for wound‐related issues other than suture removal”. Quote: “Of the 194 patients, 162 (83.5%) obtained adequate anaesthesia as determined by the 27‐gague needle test”. Table 3. Efficacy of LET solution versus LET gel for topical anaesthesia of face and scalp (includes information on complete, partial and Incomplete effectiveness) Complications: “No adverse effects were noted in the 194 patients during the procedure. 13 patients who were not able to be contacted… one patient in each study arm sought medical care for a wound infection". |
| Other bias (sample size) | Unclear risk | Quote: “LET solution = 103 subjects, LET gel = 91 subjects” |
| Methods | Single‐centre RCT, Spokane Minor Emergency Centers, Spokane, Washington, United States | |
| Participants | 107 paediatric patients 10 years of age or younger, with laceration on the face (n = 84) or scalp (n = 23) | |
| Interventions | 1. Topical TAC solution (tetracaine 0.5%, epinephrine 1:2000, cocaine 11.8%), applied for 10 minutes (n = 56) | |
| Outcomes | 1. The physician rated anaesthetic effectiveness (complete, partial or inadequate) according to the ability of the participant to tolerate manipulation of the wound during repair. The anaesthesia was 'complete' if the participant did not cry, complain or wince during suturing. The anaesthesia was 'partial' if the participant had some discomfort but did have an avoidance reaction. 'Inadequate' anaesthesia was defined as obvious discomfort with minimal manipulation of the wound. Results include the following. | |
| Intervention dates | January to July 1983 | |
| Declaration of interest | Not reported | |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients who received topical anaesthesia were randomized by alternating between A and B solutions". Comment: probably not done |
| Allocation concealment (selection bias) | High risk | Quote: "...randomized by alternating between A and B solutions" Comment: probably not done |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Neither patients nor treating physicians were informed of the composition of the anaesthetic solutions". Comment: probably done, assuming topical TAC and TA were visually identical |
| Incomplete outcome data (attrition bias) | Unclear risk | 107 participants included in study but reporting of attrition or exclusions insufficient to permit judgement |
| selective reporting of outcomes | Low risk | All prespecified primary outcomes were reported: Treating physician rated anaesthetic effectiveness on the basis of participant tolerance of manipulation of wound during suturing (complete, partial, inadequate). The only prespecified secondary outcome was wound infection, which was reported. Quote: “The relative effectiveness of anaesthesia was assessed subjectively by treating physician based on ability of patient to tolerate manipulation of would during repair”. Table 1. Anesthesia effectiveness (treatment) Table 2. Wound location (initial examination) Table 3. Signs of wound infection (follow‐up visits) |
| Other bias (sample size) | Unclear risk | Quote: “Topical TAC = 56 patients, topical TA = 51 patients” |
| Methods | Single‐centre RCT, emergency department of a university‐affiliated private children's hospital, United States | |
| Participants | 151 patients, age 1 to 17 years, with facial (69.6%) and scalp (30.4%) lacerations | |
| Interventions | 1. Topical TAC solution (tetracaine 0.5%, epinephrine 1:2000, cocaine 11.8%), applied for 15 minutes (n = 73) | |
| Outcomes | 1. The physician assessed the adequacy of initial anaesthesia by probing the wound with a 27‐gauge needle. Results include the following. | |
| Intervention dates | June 1992 to May 1993 | |
| Declaration of interest | Not reported | |
| Notes | Source of funding: financial support provided by the FA Bean Education and Research Fund, Minneapolis Children's Medical Center | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...vials of the anaesthetic solutions were assigned random numbers.." Comment: unclear, as study was reported to be randomized, but method of sequence generation was not described |
| Allocation concealment (selection bias) | Low risk | Quote: "Both TAC and LET solutions are aqueous and have the same blue tint and viscosity". "labelled to ensure appropriate blindness of suture personnel" "A double blind topical application using 3ml of the test solutions was performed [at] study entry". Comment: probably done |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Both TAC and LET solutions are aqueous and have the same blue tint and viscosity. Unit‐dose, amber vials of the anaesthetic solutions were assigned random numbers; labelled to ensure appropriate blindness of suture personnel; and stored under refrigeration in the ED. A double blind topical application using 3ml of the test solutions was performed [at] study entry". Comment: probably done |
| Incomplete outcome data (attrition bias) | Low risk | 171 participants were initially enrolled, but data analysis was performed for only 151 participants. Five participants were excluded after consent was obtained (1 sedated before anaesthetic administration, 2 topical anaesthetics applied for inappropriate duration, 2 data sheets lost). 15 additional participants were withdrawn before evaluation of anaesthetic effectiveness because participants were unco‐operative or because it was discovered that the wound involved deeper tissue layers than inclusion criteria permitted. We concluded low risk of bias because reasons for exclusion were unlikely to be related to pain scores during laceration repair |
| selective reporting of outcomes | Unclear risk | All outcomes described in Methods were fully reported in Results section, but subgroup analyses (area of face, age of participant) were not prespecified. Adverse events were reported |
| Other bias (sample size) | Unclear risk | 73 participants were treated with TAC; 78 participants received LET |
| Methods | Single‐centre RCT, emergency department, Children’s Hospital, Columbus, Ohio, United States | |
| Participants | 240 patients, 2 to 17 years old, with lacerations ≤ 5 cm | |
| Interventions | 1. Bupivanor (BN) solution (0.48% bupivacaine with 1:26,000 norepinephrine), applied for 20 minutes (n = 30) | |
| Outcomes | 1. Participants 5 years of age or older, with reported discomfort on the VAS (100 mm) pain scale Results (topical BN vs topical EN vs topical MN vs topical PN vs topical TAC vs infiltrated local anaesthetic) include the following. | |
| Intervention dates | July to December 1992 | |
| Declaration of interest | No explicit documentation regarding conflicts of interest | |
| Notes | Source of funding: Ohio State University Seed Grant Program, Bremer Research Foundation, Ohio State University and Samuel J. Roessler Memorial Scholarship Fund Study author contacted to request additional study data; study author replied but unable to provide the missing information. High risk of bias for local anaesthetic vs topical anaesthetic, as this comparison was not blinded. However,unclear risk of bias in 3 domains for comparisons of different topical anaesthetics because of appropriate blinding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Study patients were assigned to one of six anaesthetic treatment groups using block randomization". Comment: unclear, as exact method of selecting the blocks was not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Comparisons among the five topical preparations were double blinded. Because lidocaine was given as an injection, its identity was not blinded"; "Anesthetics were prepared in advance by Children's Hospital pharmacy, sealed in envelopes labelled with a study identification number, and stored in a locked cabinet in the emergency department". Comment: probably blinded between comparisons of different topical agents, but probably not blinded between comparisons of infiltrated lidocaine and topical anaesthetic |
| Incomplete outcome data (attrition bias) | Unclear risk | 240 participants included in the study but reporting of attrition or exclusions insufficient to permit judgement |
| selective reporting of outcomes | Low risk | The study protocol is available, and all of the study’s prespecified outcomes have been reported in the prespecified way. |
| Other bias (sample size) | Unclear risk | 240 participants enrolled: 1. Bupivanor (BN) solution, n = 30 2. Etidonor (EN) solution, n = 30 3. Mepivanor (MN), n = 30 4. Prilonor (PN) solution, n = 30 5. TAC solution, n = 60 6. Infiltrated lidocaine, n = 60 |
| Methods | Single‐centre RCT, emergency department or a large children’s hospital, United States | |
| Participants | 71 patients, 2‐16 years old, with lacerations ≤ 5 cm in length located on the face (n = 43) or scalp (n = 28) | |
| Interventions | 1. Mepivanor (MN) solution (mepivacaine 2%, norepinephrine 1:100,000), applied for 20 minutes (n = 24) | |
| Outcomes | 1. Observer‐reported VAS (100 mm) pain scale scores (suture technicians, research assistants and videotape reviewers) Results (topical MN vs topical TAC vs infiltrated local anaesthetic) include the following. | |
| Intervention dates | Not reported | |
| Declaration of interest | Not reported | |
| Notes | Source of funding: Support was provided by a grant from the Children’s Hopsital Research Foundation, Columbus, Ohio (Grant #020‐876). Obtained additional study data by directly contacting study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Enrolled patients were assigned to receive one of three anaesthetic preparations by block randomization". Comment: unclear, as exact method of selecting the blocks not described in the study |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Comparions between topical Mepivanor and TAC were blinded to all observers. Since lidocaine was given as an injection, its identity was not blinded to those present for the procedure. However, after the anaesthetic was administered, suturing procedures were videotaped. These videotapes were later reviewed by an observer who was completely blinded to which local anaesthetic the patient had received". Comment: probably done |
| Incomplete outcome data (attrition bias) | Unclear risk | 71 participants included in the study but reporting of attrition or exclusions insufficient to permit judgement |
| selective reporting of outcomes | Low risk | All prespecified primary outcomes were reported: observer‐reported VAS pain score by suture technicians, research assistants ascertained at the end of the suturing procedure. Also, Lickert pain scale scores (participant, suture technician) Prespecified secondary outcomes were also reported: pain during application of anaesthesia and requirement for supplemental lidocaine infiltration. Quote: “Pain perceptions of suture technicians, research assistants were ascertained at the end of the suturing procedure by means of the visual analogue scale (VAS)… Pain perceptions of the parents and suture technicians were also measured using a seven‐point Likert scale…Observers were instructed to base their pain scores on the pain experienced as the needle pierced the skin in order to measure actual anaesthetic performance”. Figure 1. Mean VAS pain score by anaesthetic treatment group for suture technicians compared with research assistants compared with videotape reviewer. Figure 2. Mean Likert scale to rate the amount of pain they thought the child experienced during suturing by each anaesthetic treatment group for suture technicians compared with parents for all laceration types of repair. Additional reporting: “Suture technicians were instructed to give additional lidocaine by infiltration.. if they felt that the child had inadequate wound anaesthesia. Two patients received lidocaine rescue in the TAC group compared to 9 patients in the Mepivanor group”. “..Sixty six patients returned within 48 hours for a wound check. All wounds were healing without complication at that time, except for one patient…. There was one additional complication reported at the 2‐week follow up for a patient”. |
| Other bias (sample size) | High risk | Quote: “Seventy‐one patients were enrolled in the study. 23 received lidocaine, 24 received TAC, 24 were given Mepivanor”. |
| Methods | Single‐centre RCT, emergency department, Children’s Hospital, Columbus, Ohio, United States | |
| Participants | 240 patients, 1 to 18 years of age, with lacerations ≤ 5 cm in length, located on the face (51%), scalp (30%), extremity (18%) or other site (1%) | |
| Interventions | 1. Prilophen (PP) solution (prilocaine 3.56%, phenylephrine 0.99%), applied for 20 minutes (n = 60) | |
| Outcomes | 1. Participants 5 years of age or older reported VAS (100 mm) pain scale scores. Results (topical PP vs topical TP vs topical TLP vs topical TAC) include the following. | |
| Intervention dates | June to September 1994 | |
| Declaration of interest | No explicit documentation regarding conflicts of interest | |
| Notes | Source of funding: Grant 020‐898 from Children’s Hospital Research Foundation and Samuel J. Roessler Memorial Scholarship Fund Study author contacted to request additional study data; study author replied but unable to provide missing information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to one of four anaesthetic treatment groups.." Comment: unclear, as study was reported to be randomized but method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "using a prospective, randomized, double‐blind design..." "Anesthetic agents were sealed in envelopes labelled with a study identification number and stored in a locked cabinet in the emergency department". Comment: probably done, assuming topical solutions visually identical |
| Incomplete outcome data (attrition bias) | Unclear risk | 240 participants included in the study but reporting of attrition or exclusions insufficient to permit judgement |
| selective reporting of outcomes | Low risk | The study protocol is available, and all of the study’s prespecified outcomes have been reported in the prespecified way. |
| Other bias (sample size) | Unclear risk | 240 children enrolled: 1. Prilophen (PP) solution, n = 60 2. Tetraphen (TP) solution, n = 60 3. Tetralidophen (TLP) solution, n = 60 4. TAC solution, n = 60 |
| Methods | Single‐centre RCT, emergency department or a large children’s hospital, United States | |
| Participants | 180 patients, 1 to 18 years old, with lacerations ≤ 5 cm, located on the face (n = 76), scalp (n = 59), extremity (n = 43) or other (n = 2) | |
| Interventions | 1. Prilophen (PP) solution (3.56% prilocaine, 0.10% phenylephrine), applied for 20 minutes (n = 60) | |
| Outcomes | 1. Participants 5 years of age and older self‐reported pain using a VAS (100 mm) scale. Results (topical PP vs topical BP vs topical TAC) included the following. | |
| Intervention dates | Not reported | |
| Declaration of interest | Not reported | |
| Notes | Funding source: supported by Grant 020‐898 from the Children’s Hospital Research Foundation, Columbus, Ohio. Stipend support for medical students was provided by the Samuel L. Roessler Memorial Medical Scholarship Fund. Study author contacted to request additional study data; study author replied but unable to provide missing information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "68 patients were assigned to each of the three anaesthetic treatment groups using block randomization". Comment: unclear, as exact method of selecting the blocks not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "using a prospective, randomized, double‐blind design..." "Anesthetics were sealed in envelopes labelled with a study identification number and stored in a locked cabinet in the ED". Comment: probably done, assuming solutions visually identical |
| Incomplete outcome data (attrition bias) | Unclear risk | 180 participants included in the study but reporting of attrition or exclusions insufficient to permit judgement |
| selective reporting of outcomes | Low risk | All prespecified primary and secondary outcomes were reported: VAS pain scores during suturing by participants and observers (suture technicians, research assistants, parents) Quote: “Pain perceptions of suture technicians, research assistants, parents and patients 5 years of age and older were ascertained using a visual analogue scale (VAS)… Observers based pain scores on the pain experienced as the needle pierced the skin in order to measure actual anaesthetic performance”. Figure 1. Mean VAS pain score by anaesthetic treatment group for suture technicians compared with research assistants for all types of laceration of repair. Figure 2. Mean VAS pain score by anaesthetic treatment group for participants compared with parents for all types of laceration repair. Figure 3. Mean VAS pain score by anaesthetic treatment group for suture technicians compared with research assistants for only face and scalp laceration repairs. Figure 4. Mean VAS pain score by anaesthetic treatment group for participants compared with parents for face and scalp lacerations only. Additional reporting: 1. Complications at follow‐up were listed as “2 wound infections, 1 case of wound drainage that resolved without antibiotics, 3 cases of lost stitches, and 3 cases of wound dehiscence”. |
| Other bias (sample size) | Unclear risk | Quote: “Participants were 180 children. Three groups each of 60 subjects each: TAC vs Prilophen vs Bupivaphen". |
| Methods | Single‐centre RCT, urban paediatric emergency department, Boston, Massachusetts, United States | |
| Participants | 156 patients, 3 to 18 years old, with lacerations on the face/scalp (n = 102), extremity (n = 47) or trunk (n = 7) | |
| Interventions | 1. TAC 1 solution (tetracaine 0.5%, epinephrine 1:2000, cocaine 11.8%), applied for 15 to 30 minutes (n = 49) | |
| Outcomes | 1. Physician rating of anaesthetic effectiveness (complete, partial or no anaesthesia). Anaesthesia was 'complete' if the participant did not move, flinch or grimace during repair. Anaesthesia was 'partial' if the participant complained of pain, moved or grimaced. If supplemental lidocaine infiltration was required, then 'no anaesthesia' was given. Results for TAC 1 (standard formulation) vs TAC 3 (tetracaine‐cocaine) include the following. Results for TAC 2 (higher concentration tetracaine, lower concentration cocaine) vs TAC 3 (tetracaine‐cocaine) include the following. | |
| Intervention dates | Not reported | |
| Declaration of interest | No explicit documentation regarding conflicts of interest | |
| Notes | Source of funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The solutions were batched in lots of 10 doses to limit expiration of the study drugs. The order of batching was generated using a standard table of random numbers". Comment: probably done |
| Allocation concealment (selection bias) | High risk | Quote: "The order of batching was generated using a standard table of random numbers". Comment: probably not done |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "...we conducted a randomized, prospective, double‐blind, clinical trial comparing three different formulations of cocaine‐containing topical anaesthetics". Unclear: In the Introduction section, reported to be a double‐blind study, but no details provided in Methods or any other sections |
| Incomplete outcome data (attrition bias) | Low risk | A total of 165 participants were randomized in the study, and no missing outcome data or exclusions |
| selective reporting of outcomes | Low risk | The study protocol is available, and all of the study’s prespecified outcomes have been reported in the prespecified way. |
| Other bias (sample size) | High risk | 165 participants: 1. TAC 1 solution, n = 49 2. TAC 2 solution, n = 49 3. TAC 3 solution, n = 58 |
| Methods | Single‐centre RCT, emergency department at Arizona Health Sciences Center, Arizona, United States | |
| Participants | 68 adult patients, older than 18 years of age, with lacerations < 5 cm in length, located on the face (n = 22) or non‐facial (n = 46) | |
| Interventions | 1. TAC solution (tetracaine 0.5%, epinephrine 1:2000, cocaine 10.0%), applied for 5 to 10 minutes (n = 36) | |
| Outcomes | 1. Participant‐rated numerical pain scale score (0‐10) Results include the following. | |
| Intervention dates | Not reported | |
| Declaration of interest | No explicit documentation regarding conflicts of interest | |
| Notes | Source of funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Prior to delivery to the emergency department, the TAC and tetracaine solutions were assigned odd or even numbers"; "Randomization was achieved by matching the vials to the odd or even numbers at the end of the hospital number". Comment: probably not done |
| Allocation concealment (selection bias) | High risk | Quote: "Randomization was achieved by matching the vials to the odd or even numbers at the end of the hospital number". Comment: probably not done |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Only the pharmacist preparing the solutions knew which vials contained tetracaine and which contained TAC". Comment: probably done, assuming visually identical solutions |
| Incomplete outcome data (attrition bias) | Unclear risk | 68 patients participated in the study. It is not clear whether the same number were randomized, or whether any were withdrawn. |
| selective reporting of outcomes | Low risk | The study protocol is available, and all of the study’s prespecified outcomes have been reported in the prespecified way. |
| Other bias (sample size) | High risk | Total N = 68: 1. TAC solution, n = 36 2. Tetracaine solution, n = 32 |
| Methods | Single‐centre RCT, emergency department of Children’s Hospital of Pittsburgh, Pittsburgh, Pennsylvania, United States | |
| Participants | 32 patients, 5 to 18 years old, with lacerations < 5 cm long, located on the extremity (n = 32) | |
| Interventions | 1. EMLA cream (lidocaine 2.5%, prilocaine 2.5%), applied for maximum of 60 minutes (n = 16) | |
| Outcomes | 1. Participant‐rated VAS (100 mm) pain scores Results included the following. | |
| Intervention dates | April to December 1994 | |
| Declaration of interest | Not reported | |
| Notes | Funding source: supported by Grant 5M01 RR00084 from the General Clinical Research Center, Children’s Hospital of Pittsburgh | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...the patient was randomized into one of the two study groups by a table of random numbers" Comment: probably done |
| Allocation concealment (selection bias) | High risk | Quote: "...the patient was randomized into one of the two study groups by a table of random numbers" Comment: probably not done |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The suturers, who were blinded to the patients' assignments, were not investigators in the study and were not allowed to see the patient until the anaesthetic had been removed and the wound irrigated" Comment: probably done |
| Incomplete outcome data (attrition bias) | Low risk | A total of 32 participants enrolled with no drop‐outs or exclusions |
| selective reporting of outcomes | Low risk | All prespecified primary outcomes were reported: observer‐ or participant‐reported VAS pain scores during suturing One prespecified secondary outcome was also reported: need for supplemental infiltrated lidocaine Quote: “Assessment of pain associated with the entire procedure was conducted independently by the suturing physician, the patient, and the parent or guardian on the 10‐cm visual analogue scale (VAS)” Table. Pain scores on a 10‐cm VAS contains participant, parent and physician VAS scores Figure. Efficacy of EMLA and TAC demonstrates efficacy adequacy of anaesthesia after the procedure began Additional reporting: Complications were listed with “one case of wound dehiscence before suture removal in each group and no wound infections were seen in either group" |
| Other bias (sample size) | High risk | Quote: “a convenience sample of 32 patients were enrolled in our study group: EMLA cream 16 subjects and TAC solution 16 patients” |
AC: epinephrine (adrenaline) and cocaine; BN: bupivacaine‐noradrenaline; BP:blood pressure; CI: confidence interval; cm: centimetre; c/w: compared with; ED: emergency department; EMLA: Eutectic Mixture of Local Anesthetics (lidocaine and prilocaine); EN: etidocaine‐noradrenaline; LAT: lidocaine, epinephrine and tetracaine (same as LET); LE: lidocaine and epinephrine; LET: same as LAT; LG: local gel; LI: local infiltration; MAC: bupivacaine 0.5%, epinephrine 1:2000, cocaine 10.0%; mm: milli‐metre; MN: mepivacaine‐noradrenaline; PN: prilocaine‐noradrenaline; N: number; NS: not significant; P = P value; PP: prilocaine, phenylephrine; RCT: randomized controlled trial; RICDRS: Restrained Infants and Children Distress Rating Scale; SD: standard deviation; SE: standard error; TA: tetracaine and epinephrine; TAC: tetracaine, epinephrine and cocaine; TLE: topical lidocaine and epinephrine; TLP: tetracaine, lidocaine and phenylephrine; TP; tetracaine and phenylephrine; VAS: visual analogue scale; vs: versus; w/w: weight per weight.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Study compared topical lidocaine‐epinephrine‐tetracaine (LET) only vs placebo. No comparison with infiltrated local anaesthetics or other topical anaesthetics | |
| Topical xylocaine was not the primary anaesthetic for repair of the dermal injury. Instead the topical anaesthetic was only pretreatment given before infiltration with local anaesthetic. | |
| Stimulus was breast surgery, not laceration repair. Also, deep tissue may be involved. | |
| Stimulus was a cosmetic procedure, not dermal laceration repair. | |
| Review article, not a trial | |
| Topical agent was not the primary anaesthetic for repair of the dermal injury. Topical agent was only pretreatment given before infiltration with local anaesthetic. | |
| Topical agent was not the primary anaesthetic for repair of the dermal injury. Topical agent was only pretreatment given before infiltration with local anaesthetic. | |
| Not a randomized controlled trial. No controls, and all participants received topical lignocaine‐adrenaline‐cocaine | |
| Procedure is minimally invasive genealogical procedure, not dermal laceration repair. | |
| Not a randomized controlled trial. No controls, and all participants received topical TAC | |
| Not a randomized controlled trial. No controls, and all participants received topical TAC | |
| Not a randomized controlled trial. No controls, and all participants received TAC gel | |
| Study evaluated participants with lacerations located on mucous membranes. | |
| Compared local anaesthetic vs digital anaesthesia. All lacerations were pretreated with topical anaesthetic, but this was done only to reduce pain from local anaesthetic infiltration. Topical anaesthesia was not used to reduce pain from repair of lacerations. | |
| Not a randomized controlled trial. No controls, and all participants received topical LAT | |
| Procedure is wound VAC change, not laceration repair. Also, local anaesthetic was injected into the wound VAC sponge rather than into the skin. | |
| Stimulus was minor surgery on adult penis, not laceration repair. | |
| Not a randomized controlled trial. Instead, this is a review article. | |
| Outcomes of interest not measured; some lacerations repaired by non‐invasive procedures with additional analgesia/anaesthesia administrated to some participants. | |
| Not a study on repair of lacerations | |
| Topical anaesthetic was not the primary anaesthetic. Study compares topical local anaesthetics plus infiltration vs infiltration only. | |
| Not a randomized controlled trial; this is a retrospective study. Also, outcomes were not relevant to this review. | |
| Outcomes of interest were not measured. | |
| Not an RCT | |
| Intervention was blepharoplasty rather then laceration repair. | |
| Topical anaesthetic was only a pretreatment given before infiltration with local anaesthetic. Also, some wound closures were performed with adhesives. | |
| Topical anaesthetic was only a pretreatment given before infiltration with local anaesthetic. Also, some wound closures were performed with adhesives. | |
| Some participants (12) were sedated with chloral hydrate. | |
| Study evaluated participants with lacerations located on mucous membranes. | |
| Study evaluated patients with lacerations located on mucous membranes. | |
| This is a review article. | |
| This is a review article, not a trial. | |
| Outcomes of interest were not measured. | |
| Topical agent was not the primary anaesthetic for repair of the dermal injury. Topical agent was only a pretreatment given before lidocaine infiltration. | |
| Not a randomized controlled trial. No controls, and all participants received LAT gel | |
| Not a randomized controlled trial |
LAT: lidocaine, adrenaline, and tetracaine; LET: lidocaine‐epinephrine‐tetracaine; TAC: tetracaine‐adrenaline‐cocaine; VAC: vacuum.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Patient self‐reported VAS (0‐100 mm) pain scores Show forest plot | 2 | 240 | Mean Difference (IV, Random, 95% CI) | 5.59 [‐2.16, 13.35] |
| Analysis 1.1  Comparison 1 Topical prilocaine‐phenylephrine (PP) versus topical tetracaine‐epinephrine‐cocaine (TAC), Outcome 1 Patient self‐reported VAS (0‐100 mm) pain scores. | ||||

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
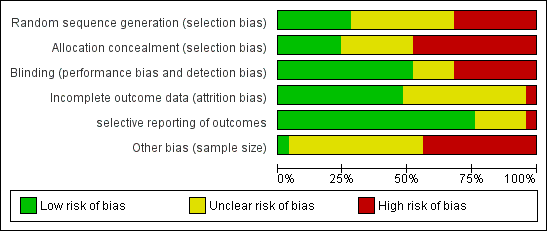
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
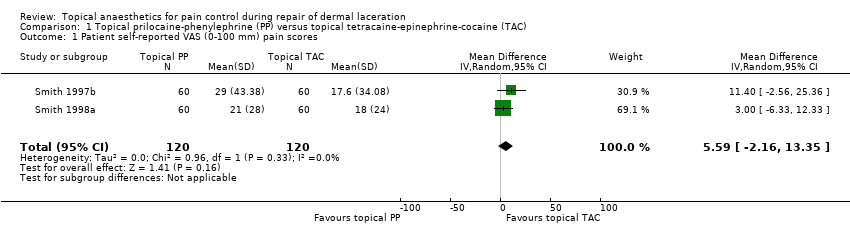
Comparison 1 Topical prilocaine‐phenylephrine (PP) versus topical tetracaine‐epinephrine‐cocaine (TAC), Outcome 1 Patient self‐reported VAS (0‐100 mm) pain scores.
| Pain control using topical local anaesthetics compared with infiltrated local anaesthetics or other topical agents for pain control during repair of dermal lacerations | ||||||
| Patient or population: adults and paediatric patients with dermal laceration Settings: any medical setting Intervention: topical local anaesthetics for pain control during repair of dermal laceration Comparison: infiltrated local anaesthetics or other topical agents for pain control during repair of dermal lacerations | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| (Infiltrated local anaesthetics or other topical agents) | (Topical local anaesthetics) | |||||
| Pain intensity measures Cocaine‐containing topical anaesthetics vs infiltrated local anaesthetics | See comment | See comment | Not estimable | 1006 | ⊕⊕⊝⊝ | Unable to mathematically combine results because of heterogeneity of outcome measures |
| Pain intensity measures Comparisons between different cocaine‐containing topical anaesthetics | See comment | See comment | Not estimable | 530 | ⊕⊕⊝⊝ | Unable to mathematically combine results because each topical anaesthetic comparison was limited to a single study |
| Pain intensity measures Cocaine‐free topical anaesthetics compared with infiltrated local anaesthetics | See comment | See comment | Not estimable | 543 | ⊕⊕⊝⊝ | Unable to mathematically combine results because of heterogeneity of outcome measures |
| Pain intensity measures Cocaine‐fee topical anaesthetics compared with cocaine‐containing topical anaesthetics | See comment | See comment | Not estimable | 1231 | ⊕⊕⊝⊝ | Two of the 11 trials studied a common topical anaesthetic and could be mathematically combined. |
| Pain intensity measures Comparisons between different cocaine‐free topical anaesthetics | See comment | See comment | Not estimable | 656 | ⊕⊕⊝⊝ | Trials could not be mathematically combined because each study compared a different cocaine‐free topical anaesthetic. |
| Anaesthetic‐related adverse effects | Study population | RR 0 (0 to 0) | 1686 | |||
| 1 per 1000 | 0 per 1000 | |||||
| Medium‐risk population | ||||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aEach of the trials had high risk of bias in multiple domains or unclear risk of bias in three domains. bTwo of the four trials had at least one domain that was at high risk of bias. cTwo of the trials had unclear risk of bias in multiple domains, and the other two studies had high risk of bias in two domains. dSix of the studies had high risk of bias for at least one domain, and the other five studies had unclear risk of bias for one or more domains. eEach of the five trials had unclear risk of bias in one or more domains. However, no trials contained any domains that were clearly at high risk | ||||||
| Primary outcome subanalysis: pain intensity measures of topical prilocaine‐phenylephrine (PP) and topical tetracaine‐epinephrine‐cocaine (TAC) | ||||||
| Patient or population: treatment repair of dermal laceration Setting: any medical setting | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with topical tetracaine‐epinephrine‐cocaine (TAC) | Risk with topical prilocaine‐phenylephrine (PP) | |||||
| Participant self‐reported VAS (0‐100 mm) pain scores | Mean participant self‐reported VAS (0‐100 mm) pain score was 0. | Mean participant self‐reported VAS (0‐100 mm) pain scores in the intervention group was 5.59. | ‐ | 240 | Lowa | 5.59 (95% CI for effect estimate, 2.16 to 13.35) |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aEach of the trials had unclear risk of bias in one or more domains. However, no trials included any domains that were clearly at high risk. | ||||||
| Study | Anaesthetics | Participant self‐reported pain scores | Secondary outcome measures | Incidence of anaesthetic toxicity |
| Topical tetracaine‐epinephrine‐cocaine (TAC) vs infiltrated lidocaine | None | 1) Adequate initial anaesthesia (TAC = 89% vs infiltrated local anaesthetic = 79%; P = non‐significant) | Not reported | |
| Topical TAC vs infiltrated lidocaine | None | 1) Adequate initial anaesthesia for facial and scalp lacerations (topical TAC = 81% vs infiltrated local anaesthetic = 87%; P = 0.005). Adequate initial anaesthesia for extremity and trunk wounds (topical TAC = 43% vs infiltrated local anaesthetic = 89%; P < 0.0001) | 0/467 | |
| Topical TAC vs infiltrated lidocaine | None | 1) Verbal rating of anaesthetic efficacy (complete: TAC = 84% vs infiltrated local anaesthetic = 88%; P = not reported) | Not reported | |
| Topical TAC vs infiltrated lidocaine | Patient‐reported VAS (100 mm) pain scores (mean scores: topical TAC = 12.0 vs infiltrated local anaesthetic = 26.3; P = NS) | 1) Observer‐reported VAS pain scores | Not reported | |
| Topical TAC vs infiltrated lidocaine | None | 1) Observer‐reported VAS pain scores (suture technicians, research assistants, videotape reviewers) 2) Observer‐reported Lickert (1‐7) pain scores (parents, suture technicians) 3) Requirement for supplemental lidocaine infiltration (See Characteristics of included studies for data.) | Not reported | |
| Topical (epinephrine‐cocaine) AC vs infiltrated lidocaine | The study pooled patient‐reported VAS and Wong‐Baker Faces pain scores (mean score: topical AC = 4.50 vs infiltrated local anaesthetic = 4.40; P = NS) | 1) Physician‐rated VAS pain scores | 0/107 | |
| AC: epinephrine (adrenaline) and cocaine; BN: bupivacaine‐noradrenaline; BP: blood pressure; cm: centimetre; c/w:compared with; ED: emergency department; EMLA: Eutectic Mixture of Local Anesthetics (lidocaine and prilocaine); EN: etidocaine‐noradrenaline; LAT: lidocaine, epinephrine and tetracaine (same as LET); LE: lidocaine and epinephrine; LET: same as LAT; LG: local gel; LI: local infiltration; MAC: bupivacaine 0.5%, epinephrine 1:2000, cocaine 10.0%; mm: milli‐metre; MN: mepivacaine‐noradrenaline; PN: prilocaine‐noradrenaline; N: number; NS: not significant; P = P value; PP: prilocaine, phenylephrine; RCT: randomized controlled trial; RICDRS: Restrained Infants and Children Distress Rating Scale; SD: standard deviation; SE: standard error; TA: tetracaine and epinephrine; TAC: tetracaine, epinephrine and cocaine; TLP: tetracaine, lidocaine and phenylephrine; TP: tetracaine and phenylephrine; VAS: visual analogue scale; vs: versus; w/w: weight per weight. | ||||
| Study | Topical Anaesthetics | Patient self‐reported pain scores | Secondary outcome measures | Incidence anaesthetic toxicity |
| Bupivacaine‐adrenaline‐cocaine (MAC) vs tetracaine‐epinephrine‐cocaine (TAC) | 1) In children < 12 years of age: Wong‐Baker Faces (1‐9) Scale (mean score ± SD: topical MAC = 2.35 ± .50 vs topical TAC = 2.46 ± 2.34; P = 0.96) 2) Participants 12 years of age or older: VAS (100 mm) pain scale (mean score ± SD: topical MAC = 6.9 ± 10.9 vs topical TAC = 12.0 ± 14.5; P = 0.16) | 1) Adequacy of initial anaesthesia 2) Participant preference for topical anaesthesia in the future | 0/180 | |
| TAC vs adrenaline‐cocaine (AC) | None | 1) Physician calculated total number of 'sutures eliciting pain' (topical AC = 7/103 (4%) vs topical TAC = 7/151 (7%); P = not reported) | 0/55 | |
| TAC vs cocaine (C) | None | 1) Incidence of 'poor anaesthesia' (topical cocaine = 20% vs topical TAC = 12%; P = not reported) 2) Physician numerical rating of anaesthetic effectiveness (0 = least effective to 10 = most effective) (mean scores ± SD: topical cocaine = 6.44 ± 3.48 vs topical TAC = 7.74 ± 3.03; P = 0.005) | 0/139 | |
| TAC (two different strengths) vs tetracaine‐cocaine (TC) | None | Topical TAC 1 vs topical TC: 2) Requirement for second dose of topical anaesthetic (TAC 1 = 30% vs topical TC = 66%; P < 0.003) 3) Requirement for supplemental lidocaine infiltration (TAC 1 = 6% vs topical TC = 9%; P = not reported) Topical TAC 2 vs topical TC: 2) Requirement for second dose of topical anaesthetic (TAC 2 = 46% vs TC = 66%; P < 0.003) 3) Requirement for supplemental lidocaine infiltration (TAC 2 = 2% vs TC = 9%; P = not reported) | 1/156 (erythematous rash 1 day after application of standard topical TAC) |
| Study | Anaesthetics | Participant self‐reported pain scores | Secondary outcome measures | Incidence of anaesthetic toxicity |
| Topical lidocaine‐epinephrine‐tetracaine (LAT) vs infiltrated lidocaine | VAS (100 mm) pain scores (median values: topical LAT = 0 vs infiltrated local anaesthetic = 0; P = 0.48) | 1) Physician‐rated VAS pain scores 2) Requirement for supplemental lidocaine infiltration 3) Percentage of painful sutures | Not reported | |
| Topical lidocaine‐epinephrine (LE) vs infiltrated lidocaine | VAS (100 mm) pain scores (mean score ± SD: topical TLE = 0.16 ± 0.46 vs infiltrated lidocaine = 0.20 ± 0.49; P = 0.59) | 1) Amount of lidocaine required (mg) 2) Total number of topical anaesthetic applications | Not reported | |
| Topical bupivacaine‐norepinephrine (BN), topical etidocaine‐norepinephrine (EN), topical mepivacaine‐norepinephrine (MN) and topical prilocaine‐norepinephrine (PN) vs infiltrated lidocaine | VAS (100 mm) pain scores (mean scores: BN = 18.3, EN = 46.5, MN = 27.0, PN = 36.0 vs infiltrated anaesthetic = 26.3, standard deviations not reported) (no significant difference between any of the cocaine‐free topical agents and infiltrated lidocaine) | 1) Observer‐reported VAS pain scores 2) Observer‐reported Likert pain scores 3) Oberver‐rated Restrained Infants and Children Disress Rating Scale 4) Suture technician‐rated anaesthetic effectiveness | Not reported | |
| Topical mepivacaine‐norepinephrine (MN) vs infiltrated lidocaine | None | 1) Observer‐reported VAS pain scale scores 2) Observer‐reported Lickert pain scores 3) Requirement for supplemental lidocaine infiltration (See characteristics of included studies for data) | Not reported | |
| Topical anaesthetic putty (containing 4.94% w/w lidocaine hydrochloride, equivalent to 4% w/w lidocaine base) vs lidocaine infiltration (1% w/v) | Mean pain score was 0.78 + 1.12 (SD) after lidocaine infiltration, 1.49 + 1.76 after topical anaesthetic putty. | 1) Need for rescue anaesthesia 2) Wound evaluation score 7‐10 days after treatment 3) Wound infection 4) Wound dehiscence 5) Adverse effects (inflamed wound or resuturing). | No anaesthetic toxicity reported | |
| Topical anaesthetic lidocaine, adrenaline and tetracaine (LAT) (4% lidocaine, 1:2 000 adrenaline, 1% tetracaine) vs lidocaine infiltration. Dosage of neither group was reported. | LAT gel group reported mean (± SE) pain intensity of 2.5 (0.52) vs 2.6 (0.58) for the lidocaine infiltration group. Pain during LAT application was 1.5 (0.40) vs 2.6 (0.58) during lidocaine infiltration (P ≤ 0.01). | 1) Pain score by parents or clinicians (intended to be gathered for children < 10 years old but such data were not reported) 2) Wound complications (infection, dehiscence, missing sutures) | None reported | |
| AC: epinephrine (adrenaline) and cocaine; BN: bupivacaine‐noradrenaline; BP: blood pressure; cm: centimetre; c/w: compared with; ED: emergency department; EMLA: Eutectic Mixture of Local Anesthetics (lidocaine and prilocaine); EN: etidocaine‐noradrenaline; LAT: lidocaine, epinephrine and tetracaine (same as LET); LE: lidocaine and epinephrine; LET: same as LAT; LG: local gel; LI: local infiltration; MAC: bupivacaine 0.5%, epinephrine 1:2000, cocaine 10.0%; mm: milli‐metre; MN: mepivacaine‐noradrenaline; PN: prilocaine‐noradrenaline; N: number; NS: not significant; P = P value; PP: prilocaine, phenylephrine; RCT: randomized controlled trial; RICDRS: Restrained Infants and Children Distress Rating Scale; SD: standard deviation; SE: standard error; TA: tetracaine and epinephrine; TAC: tetracaine, epinephrine and cocaine; TLP: tetracaine, lidocaine and phenylephrine; TP: tetracaine and phenylephrine; VAS: visual analogue scale; vs: versus; w/w: weight per weight. | ||||
| Study | Topical anaesthetics | Participant self‐reported pain scores | Secondary outcome measures | Incidence of anaesthetic toxicity |
| Bupivacaine‐norepinephrine (BN), etidocaine‐norepinephrine (EN), mepivacaine‐norepinephrine (MN) and prilocaine‐norepinephrine (PN) vs tetracaine‐epinephrine‐cocaine (TAC) | Participant‐reported VAS (100 mm) pain scores (mean scores: BN = 18.3, EN = 46.5, MN, PN = 36.0 vs TAC = 12.0, standard deviations not reported) (TAC significantly outperformed EN; no significant differences between any other groups) | 1) Observer‐reported VAS and Likert pain scale scores 2) Observer‐rated Restrained Infants and Children Disress Rating Scale 3) Suture technician‐rated anaesthetic effectiveness | Not reported | |
| Mepivacaine‐norepinephrine (MN) vs | None | 1) Observer‐reported VAS pain scores (suture technicians, research assistants, videotape reviewers) 2) Observer‐reported Lickert (1‐7) pain scores (parents, suture technicians) 3) Requirement for supplemental lidocaine infiltration (See Characteristics of included studies for data.) | Not reported | |
| Prilocaine‐phenylephrine (PP), | VAS (100 mm) pain scores (mean score ± SD: PP = 29.0 ± 43.4, TP = 24.2 ± 37.2, TLP = 30.6 ± 40.3 vs TAC = 17.6 ± 34.1 (no significant differences between groups; P = 0.5) | 1) Oberver‐reported VAS (100 mm) pain scores 2) Oberver‐reported Likert (1‐7) pain scores 3) Suture technicians‐rated anaesthetic effectiveness | Not reported | |
| Prilocaine‐phenylephrine (PP) and bupivacaine‐phenylephrine (BP) vs TAC | VAS (100 mm) pain scores (mean score ± SD: PP = 21.0 ± 28.0 and BP = 41.0 ± 35.0 vs TAC = 18.0 ± 24.0) (no differences reported between groups; P = 0.07) | Observer‐reported VAS pain scores (suture technicians, research assistants and parents) | Not reported | |
| LAT vs TAC | Modified multi‐dimensional pain scale (0‐10) (mean ranked sum: LAT = 49.0 vs TAC = 46.9; P = 0.71) | 1) Physician‐rated modified multi‐dimensional pain scale (0‐10) 2) Percentage of sutures causing pain 3) Requirement for supplemental lidocaine infiltration | 0/95 | |
| LAT vs TAC | VAS (100 mm) pain scores (mean ranked sum: LET = 45.3 vs TAC = 50.8; P = 0.27) | 1) Physician‐reported VAS scores 2) Percentage of sutures causing pain | Not reported | |
| LAT vs TAC | None | 1) Adequacy of initial anaesthesia (LAT = 74.4% vs TAC = 79.5%; P = 0.46) 2) Anaesthetic effectiveness (complete anaesthesia: LAT = 82.4% vs topical TAC = 75.9%; P = 0.18) | 0/151 | |
| Lidocaine‐prilocaine (EMLA) vs TAC | VAS (100 mm) pain scores (mean score ± SD: EMLA = 46.0 ± 26.0 vs TAC = 40.0 ± 25.0; P = 0.50) | 1) Observer‐rated VAS pain scores 2) Requirement for supplemental lidocaine infiltration | Not reported | |
| Lidocaine‐epinephrine (LE) vs TAC | Faces pain scale (1‐9) scores (mean score ± SD: LE = 3.29 ± 1.92 vs TAC = 2.66 ± 1.78; P = 0.33) | Requirement for supplemental lidocaine infiltration | 0/35 | |
| Tetracaine‐epinephrine (TA) vs TAC | None | 1) Physician‐rating of anaesthetic effectiveness (complete anaesthesia: TA = 47.1% vs TAC = 75%' P < 0.05) 2) Requirement for rescue lidocaine infiltration (TA = 27.5% vs TAC = 8.9%; P = 0.01) | 0/107 | |
| Tetracaine (T) vs TAC | Numerical pain scale (0‐10) score (mean scores: tetracaine = 5.6 vs TAC = 3.53; P < 0.05; standard deviations not reported) | Requirement for supplemental lidocaine infiltration | Not reported | |
| AC: epinephrine (adrenaline) and cocaine; BN: bupivacaine‐noradrenaline; BP: blood pressure; cm: centimetre; c/w: compared with; ED: emergency department; EMLA: Eutectic Mixture of Local Anesthetics (lidocaine and prilocaine); EN: etidocaine‐noradrenaline; LAT: lidocaine, epinephrine and tetracaine (same as LET); LE: lidocaine and epinephrine; LET: same as LAT; LG: local gel; LI: local infiltration; MAC: bupivacaine 0.5%, epinephrine 1:2000, cocaine 10.0%; mm: milli‐metre; MN: mepivacaine‐noradrenaline; PN: prilocaine‐noradrenaline; N: number; NS: not significant; P = P value; PP: prilocaine, phenylephrine; RCT: randomized controlled trial; RICDRS: Restrained Infants and Children Distress Rating Scale; SD: standard deviation; SE: standard error; TA: tetracaine and epinephrine; TAC: tetracaine, epinephrine and cocaine; TLP: tetracaine, lidocaine and phenylephrine; TP: tetracaine and phenylephrine; VAS: visual analogue scale; vs: versus; w/w: weight per weight | ||||
| Study | Topical anaesthetics | Participant self‐reported pain scores | Secondary outcome measures | Incidence of anaesthetic toxicity |
| Bupivacaine‐norepinephrine (BN) vs etidocaine‐norepinephrine (EN) vs mepivacaine‐norepinephrine (MN) vs prilocaine‐norepinephrine (PN) | Patient‐reported VAS (100 mm) pain scores (mean scores: topical BN = 18.3 vs topical EN = 46.5 vs topical MN = 27.0 vs topical PN = 36.0) (no significant differences between any cocaine‐free topical groups) | 1) Observer‐reported VAS and Likert pain scale scores 2) Observer‐rated Restrained Infants and Children Disress Rating Scale 3) Suture technician‐rated anaesthetic effectiveness | Not reported | |
| Prilocaine‐phenylephrine (PP) vs | VAS (100 mm) pain scores (mean score ± SD: PP = 29.0 ± 43.4 vs TP = 24.2 ± 37.2 vs TLP = 30.6 ± 40.3) (no significant differences between groups; P = 0.5) | 1) Oberver‐reported VAS (100 mm) pain scores 2) Oberver‐reported Likert (1‐7) pain scores 3) Suture technicians rated anaesthetic effectiveness | Not reported | |
| Prilocaine‐phenylephrine (PP) vs bupivacaine‐phenylephrine (BP) | VAS (100 mm) pain scores (mean score ± SD: topical PP = 21.0 ± 28.0 vs topical BP = 41.0 ± 35.0; P = 0.07) | Observer‐reported VAS pain scores (suture technicians, research assistants and parents) | Not reported | |
| Lidocaine‐prilocaine (EMLA) vs lidocaine‐epinephrine‐tetracaine (LAT) | VAS (100 mm) pain scores were not significantly different between the 2 groups (mean pain scores not provided; P > 0.05). | 1) Observer‐reported VAS pain scores (legal guardian and physician) 2) Requirement for supplemental lidocaine infiltration | Not reported | |
| Topical LAT gel vs LAT solution | None | 1) Adequacy of initial anaesthesia (adequate anaesthesia: LAT solution = 84% vs LAT gel = 82%; P > 0.05) 2) Effectiveness of anaesthesia (complete anaesthesia: LAT solution = 76% vs LAT gel = 85%; P = 0.007) | 0/194 | |
| AC: epinephrine (adrenaline) and cocaine; BN: bupivacaine‐noradrenaline; BP: blood pressure; cm: centimetre; c/w: compared with; ED: emergency department; EMLA: Eutectic Mixture of Local Anesthetics (lidocaine and prilocaine); EN: etidocaine‐noradrenaline; LAT: lidocaine, epinephrine and tetracaine (same as LET); LE: lidocaine and epinephrine; LET: same as LAT; LG: local gel; LI: local infiltration; MAC: bupivacaine 0.5%, epinephrine 1:2000, cocaine 10.0%; mm: milli‐metre; MN: mepivacaine‐noradrenaline; PN: prilocaine‐noradrenaline; N: number; NS: not significant; P = P value; PP: prilocaine, phenylephrine; RCT: randomized controlled trial; RICDRS: Restrained Infants and Children Distress Rating Scale; SD: standard deviation; SE: standard error; TA: tetracaine and epinephrine; TAC: tetracaine, epinephrine and cocaine; TLP: tetracaine, lidocaine and phenylephrine; TP: tetracaine and phenylephrine; VAS: visual analogue scale; vs: versus; w/w: weight per weight | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Patient self‐reported VAS (0‐100 mm) pain scores Show forest plot | 2 | 240 | Mean Difference (IV, Random, 95% CI) | 5.59 [‐2.16, 13.35] |


