Anestésicos tópicos para el control del dolor durante la reparación de laceraciones dérmicas
Información
- DOI:
- https://doi.org/10.1002/14651858.CD005364.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 22 febrero 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Anestesia
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Conceiving the review: Daniel Carr (DC).
Co‐ordinating the original review and the 2011 update review: Anthony Eidelman(AE) and DC.
Co‐ordinating the current 2016 updated review: DC and Baraa Tayeb (BT).
Undertaking manual searches: BT.
Screening search results: BT, DC, AE, Cristy Eidelman (CE) and Ewan McNicol (EM).
Organizing retrieval of papers: BT.
Screening retrieved papers against inclusion criteria: BT, AE, CE and EM.
Appraising the quality of papers: DC, AE, CE, BT and EM.
Abstracting data from papers: DC, AE, CE, BT and EM.
Writing to authors of papers for additional information: AE and BT.
Providing additional data about papers: BT and AE.
Obtaining and screening data from unpublished studies: AE.
Managing data for the review: BT and EM.
Entering data into Review Manager and reviewing entered data (RevMan 5.3): BT and CE.
Analysing RevMan 5.3 statistical data: BT.
Performing other statistical analyses not using RevMan 5.3: BT, AE, CE and EM.
Interpreting data: AE, CE, EM, BT and DC.
Performing statistical analysis: BT and EM.
Writing the review: BT, EM, CE, AE and DC.
Securing funding for the original review: DC.
Performing previous work that served as the foundation of the present study: EM, CE, AE and DC.
Serving as guarantor for the review (one review author): BT.
Taking responsibility for reading and checking the review before submission: BT, EM, DC and CE.
Sources of support
Internal sources
-
None, Other.
External sources
-
No sources of support supplied
Declarations of interest
Baraa O Tayeb: none known.
Anthony Eidelman: none known.
Cristy L Eidelman: none known.
Ewan D McNicol: none known.
Daniel B Carr has served as an officer, committee member and lecturer for various professional organizations and community medical centres. None of these activities involved topical application of local anaesthetics. He had patents issued (2012 to 2016) that reflected his work before joining Javelin/Hospira. These patents relate to multi‐valent (e.g. opioid‐tachykinin) peptides. None of them relate to topical local anaesthetics applied for any purpose, nor does Dr. Carr have any financial interest in these or any other patents.
Acknowledgements
We would like to thank Jane Cracknell, Managing Editor of the Cochrane Anaesthesia, Critical and Emergency Care Collaborative Review Group, for help and editorial advice provided during preparation of this update of the systematic review. We also acknowledge the efforts of Karen Hovhannisyan in assisting with the literature search .
We would like to thank Mathew Zacharias, Marialena Trivella, Ronan O’Sullivan, Stephen Priestley, Sujesh Bansal and Nete Villebro for their help and editorial advice in the past, Dr Joseph Lau for providing advice on statistics, and Dr Mukhtar Zaidi for providing pharmacological consultation.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Feb 22 | Topical anaesthetics for pain control during repair of dermal laceration | Review | Baraa O Tayeb, Anthony Eidelman, Cristy L Eidelman, Ewan D McNicol, Daniel B Carr | |
| 2011 Jun 15 | Topical anaesthetics for pain control during repair of dermal laceration | Review | Baraa O Tayeb, Anthony Eidelman, Cristy L Eidelman, Ewan D McNicol, Daniel B Carr | |
| 2005 Jul 20 | Topical anaesthetics for repair of dermal laceration | Protocol | Anthony Eidelman, Jocelyn Weiss, Ikay K Enu, Joseph Lau, Ewan D McNicol, Daniel B Carr | |
Differences between protocol and review
We made the following changes to the published protocol (Eidelman 2005a).
-
We have changed the title from "Topical anaesthetics for repair of torn skin" to "Topical anaesthetics for pain control during repair of dermal laceration" for clarity.
-
The name of co‐review author Cristy L Baldwin has been changed to Cristy L Eidelman.
-
We have rephrased review objectives for clarity and simplification.
-
We have rephrased review outcomes for clarity and simplification.
-
We have updated methods and data collection on the basis of updated Cochrane standards.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Anesthetics, Local [*administration & dosage, adverse effects, chemistry];
- Cocaine [administration & dosage, adverse effects];
- Drug Combinations;
- Epinephrine [administration & dosage, adverse effects];
- Lacerations [*surgery];
- Pain Measurement;
- Randomized Controlled Trials as Topic;
- Skin [*injuries];
- Sutures;
- Tetracaine [administration & dosage, adverse effects];
Medical Subject Headings Check Words
Adult; Child; Humans;
PICO

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
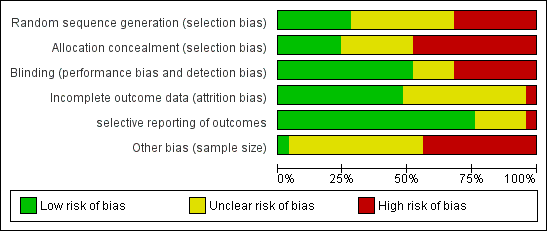
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
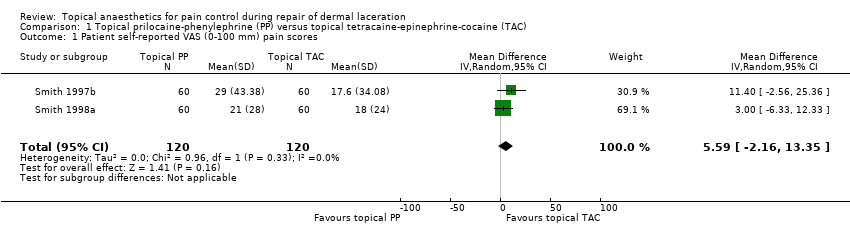
Comparison 1 Topical prilocaine‐phenylephrine (PP) versus topical tetracaine‐epinephrine‐cocaine (TAC), Outcome 1 Patient self‐reported VAS (0‐100 mm) pain scores.
| Pain control using topical local anaesthetics compared with infiltrated local anaesthetics or other topical agents for pain control during repair of dermal lacerations | ||||||
| Patient or population: adults and paediatric patients with dermal laceration Settings: any medical setting Intervention: topical local anaesthetics for pain control during repair of dermal laceration Comparison: infiltrated local anaesthetics or other topical agents for pain control during repair of dermal lacerations | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| (Infiltrated local anaesthetics or other topical agents) | (Topical local anaesthetics) | |||||
| Pain intensity measures Cocaine‐containing topical anaesthetics vs infiltrated local anaesthetics | See comment | See comment | Not estimable | 1006 | ⊕⊕⊝⊝ | Unable to mathematically combine results because of heterogeneity of outcome measures |
| Pain intensity measures Comparisons between different cocaine‐containing topical anaesthetics | See comment | See comment | Not estimable | 530 | ⊕⊕⊝⊝ | Unable to mathematically combine results because each topical anaesthetic comparison was limited to a single study |
| Pain intensity measures Cocaine‐free topical anaesthetics compared with infiltrated local anaesthetics | See comment | See comment | Not estimable | 543 | ⊕⊕⊝⊝ | Unable to mathematically combine results because of heterogeneity of outcome measures |
| Pain intensity measures Cocaine‐fee topical anaesthetics compared with cocaine‐containing topical anaesthetics | See comment | See comment | Not estimable | 1231 | ⊕⊕⊝⊝ | Two of the 11 trials studied a common topical anaesthetic and could be mathematically combined. |
| Pain intensity measures Comparisons between different cocaine‐free topical anaesthetics | See comment | See comment | Not estimable | 656 | ⊕⊕⊝⊝ | Trials could not be mathematically combined because each study compared a different cocaine‐free topical anaesthetic. |
| Anaesthetic‐related adverse effects | Study population | RR 0 (0 to 0) | 1686 | |||
| 1 per 1000 | 0 per 1000 | |||||
| Medium‐risk population | ||||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aEach of the trials had high risk of bias in multiple domains or unclear risk of bias in three domains. bTwo of the four trials had at least one domain that was at high risk of bias. cTwo of the trials had unclear risk of bias in multiple domains, and the other two studies had high risk of bias in two domains. dSix of the studies had high risk of bias for at least one domain, and the other five studies had unclear risk of bias for one or more domains. eEach of the five trials had unclear risk of bias in one or more domains. However, no trials contained any domains that were clearly at high risk | ||||||
| Primary outcome subanalysis: pain intensity measures of topical prilocaine‐phenylephrine (PP) and topical tetracaine‐epinephrine‐cocaine (TAC) | ||||||
| Patient or population: treatment repair of dermal laceration Setting: any medical setting | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with topical tetracaine‐epinephrine‐cocaine (TAC) | Risk with topical prilocaine‐phenylephrine (PP) | |||||
| Participant self‐reported VAS (0‐100 mm) pain scores | Mean participant self‐reported VAS (0‐100 mm) pain score was 0. | Mean participant self‐reported VAS (0‐100 mm) pain scores in the intervention group was 5.59. | ‐ | 240 | Lowa | 5.59 (95% CI for effect estimate, 2.16 to 13.35) |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aEach of the trials had unclear risk of bias in one or more domains. However, no trials included any domains that were clearly at high risk. | ||||||
| Study | Anaesthetics | Participant self‐reported pain scores | Secondary outcome measures | Incidence of anaesthetic toxicity |
| Topical tetracaine‐epinephrine‐cocaine (TAC) vs infiltrated lidocaine | None | 1) Adequate initial anaesthesia (TAC = 89% vs infiltrated local anaesthetic = 79%; P = non‐significant) | Not reported | |
| Topical TAC vs infiltrated lidocaine | None | 1) Adequate initial anaesthesia for facial and scalp lacerations (topical TAC = 81% vs infiltrated local anaesthetic = 87%; P = 0.005). Adequate initial anaesthesia for extremity and trunk wounds (topical TAC = 43% vs infiltrated local anaesthetic = 89%; P < 0.0001) | 0/467 | |
| Topical TAC vs infiltrated lidocaine | None | 1) Verbal rating of anaesthetic efficacy (complete: TAC = 84% vs infiltrated local anaesthetic = 88%; P = not reported) | Not reported | |
| Topical TAC vs infiltrated lidocaine | Patient‐reported VAS (100 mm) pain scores (mean scores: topical TAC = 12.0 vs infiltrated local anaesthetic = 26.3; P = NS) | 1) Observer‐reported VAS pain scores | Not reported | |
| Topical TAC vs infiltrated lidocaine | None | 1) Observer‐reported VAS pain scores (suture technicians, research assistants, videotape reviewers) 2) Observer‐reported Lickert (1‐7) pain scores (parents, suture technicians) 3) Requirement for supplemental lidocaine infiltration (See Characteristics of included studies for data.) | Not reported | |
| Topical (epinephrine‐cocaine) AC vs infiltrated lidocaine | The study pooled patient‐reported VAS and Wong‐Baker Faces pain scores (mean score: topical AC = 4.50 vs infiltrated local anaesthetic = 4.40; P = NS) | 1) Physician‐rated VAS pain scores | 0/107 | |
| AC: epinephrine (adrenaline) and cocaine; BN: bupivacaine‐noradrenaline; BP: blood pressure; cm: centimetre; c/w:compared with; ED: emergency department; EMLA: Eutectic Mixture of Local Anesthetics (lidocaine and prilocaine); EN: etidocaine‐noradrenaline; LAT: lidocaine, epinephrine and tetracaine (same as LET); LE: lidocaine and epinephrine; LET: same as LAT; LG: local gel; LI: local infiltration; MAC: bupivacaine 0.5%, epinephrine 1:2000, cocaine 10.0%; mm: milli‐metre; MN: mepivacaine‐noradrenaline; PN: prilocaine‐noradrenaline; N: number; NS: not significant; P = P value; PP: prilocaine, phenylephrine; RCT: randomized controlled trial; RICDRS: Restrained Infants and Children Distress Rating Scale; SD: standard deviation; SE: standard error; TA: tetracaine and epinephrine; TAC: tetracaine, epinephrine and cocaine; TLP: tetracaine, lidocaine and phenylephrine; TP: tetracaine and phenylephrine; VAS: visual analogue scale; vs: versus; w/w: weight per weight. | ||||
| Study | Topical Anaesthetics | Patient self‐reported pain scores | Secondary outcome measures | Incidence anaesthetic toxicity |
| Bupivacaine‐adrenaline‐cocaine (MAC) vs tetracaine‐epinephrine‐cocaine (TAC) | 1) In children < 12 years of age: Wong‐Baker Faces (1‐9) Scale (mean score ± SD: topical MAC = 2.35 ± .50 vs topical TAC = 2.46 ± 2.34; P = 0.96) 2) Participants 12 years of age or older: VAS (100 mm) pain scale (mean score ± SD: topical MAC = 6.9 ± 10.9 vs topical TAC = 12.0 ± 14.5; P = 0.16) | 1) Adequacy of initial anaesthesia 2) Participant preference for topical anaesthesia in the future | 0/180 | |
| TAC vs adrenaline‐cocaine (AC) | None | 1) Physician calculated total number of 'sutures eliciting pain' (topical AC = 7/103 (4%) vs topical TAC = 7/151 (7%); P = not reported) | 0/55 | |
| TAC vs cocaine (C) | None | 1) Incidence of 'poor anaesthesia' (topical cocaine = 20% vs topical TAC = 12%; P = not reported) 2) Physician numerical rating of anaesthetic effectiveness (0 = least effective to 10 = most effective) (mean scores ± SD: topical cocaine = 6.44 ± 3.48 vs topical TAC = 7.74 ± 3.03; P = 0.005) | 0/139 | |
| TAC (two different strengths) vs tetracaine‐cocaine (TC) | None | Topical TAC 1 vs topical TC: 2) Requirement for second dose of topical anaesthetic (TAC 1 = 30% vs topical TC = 66%; P < 0.003) 3) Requirement for supplemental lidocaine infiltration (TAC 1 = 6% vs topical TC = 9%; P = not reported) Topical TAC 2 vs topical TC: 2) Requirement for second dose of topical anaesthetic (TAC 2 = 46% vs TC = 66%; P < 0.003) 3) Requirement for supplemental lidocaine infiltration (TAC 2 = 2% vs TC = 9%; P = not reported) | 1/156 (erythematous rash 1 day after application of standard topical TAC) |
| Study | Anaesthetics | Participant self‐reported pain scores | Secondary outcome measures | Incidence of anaesthetic toxicity |
| Topical lidocaine‐epinephrine‐tetracaine (LAT) vs infiltrated lidocaine | VAS (100 mm) pain scores (median values: topical LAT = 0 vs infiltrated local anaesthetic = 0; P = 0.48) | 1) Physician‐rated VAS pain scores 2) Requirement for supplemental lidocaine infiltration 3) Percentage of painful sutures | Not reported | |
| Topical lidocaine‐epinephrine (LE) vs infiltrated lidocaine | VAS (100 mm) pain scores (mean score ± SD: topical TLE = 0.16 ± 0.46 vs infiltrated lidocaine = 0.20 ± 0.49; P = 0.59) | 1) Amount of lidocaine required (mg) 2) Total number of topical anaesthetic applications | Not reported | |
| Topical bupivacaine‐norepinephrine (BN), topical etidocaine‐norepinephrine (EN), topical mepivacaine‐norepinephrine (MN) and topical prilocaine‐norepinephrine (PN) vs infiltrated lidocaine | VAS (100 mm) pain scores (mean scores: BN = 18.3, EN = 46.5, MN = 27.0, PN = 36.0 vs infiltrated anaesthetic = 26.3, standard deviations not reported) (no significant difference between any of the cocaine‐free topical agents and infiltrated lidocaine) | 1) Observer‐reported VAS pain scores 2) Observer‐reported Likert pain scores 3) Oberver‐rated Restrained Infants and Children Disress Rating Scale 4) Suture technician‐rated anaesthetic effectiveness | Not reported | |
| Topical mepivacaine‐norepinephrine (MN) vs infiltrated lidocaine | None | 1) Observer‐reported VAS pain scale scores 2) Observer‐reported Lickert pain scores 3) Requirement for supplemental lidocaine infiltration (See characteristics of included studies for data) | Not reported | |
| Topical anaesthetic putty (containing 4.94% w/w lidocaine hydrochloride, equivalent to 4% w/w lidocaine base) vs lidocaine infiltration (1% w/v) | Mean pain score was 0.78 + 1.12 (SD) after lidocaine infiltration, 1.49 + 1.76 after topical anaesthetic putty. | 1) Need for rescue anaesthesia 2) Wound evaluation score 7‐10 days after treatment 3) Wound infection 4) Wound dehiscence 5) Adverse effects (inflamed wound or resuturing). | No anaesthetic toxicity reported | |
| Topical anaesthetic lidocaine, adrenaline and tetracaine (LAT) (4% lidocaine, 1:2 000 adrenaline, 1% tetracaine) vs lidocaine infiltration. Dosage of neither group was reported. | LAT gel group reported mean (± SE) pain intensity of 2.5 (0.52) vs 2.6 (0.58) for the lidocaine infiltration group. Pain during LAT application was 1.5 (0.40) vs 2.6 (0.58) during lidocaine infiltration (P ≤ 0.01). | 1) Pain score by parents or clinicians (intended to be gathered for children < 10 years old but such data were not reported) 2) Wound complications (infection, dehiscence, missing sutures) | None reported | |
| AC: epinephrine (adrenaline) and cocaine; BN: bupivacaine‐noradrenaline; BP: blood pressure; cm: centimetre; c/w: compared with; ED: emergency department; EMLA: Eutectic Mixture of Local Anesthetics (lidocaine and prilocaine); EN: etidocaine‐noradrenaline; LAT: lidocaine, epinephrine and tetracaine (same as LET); LE: lidocaine and epinephrine; LET: same as LAT; LG: local gel; LI: local infiltration; MAC: bupivacaine 0.5%, epinephrine 1:2000, cocaine 10.0%; mm: milli‐metre; MN: mepivacaine‐noradrenaline; PN: prilocaine‐noradrenaline; N: number; NS: not significant; P = P value; PP: prilocaine, phenylephrine; RCT: randomized controlled trial; RICDRS: Restrained Infants and Children Distress Rating Scale; SD: standard deviation; SE: standard error; TA: tetracaine and epinephrine; TAC: tetracaine, epinephrine and cocaine; TLP: tetracaine, lidocaine and phenylephrine; TP: tetracaine and phenylephrine; VAS: visual analogue scale; vs: versus; w/w: weight per weight. | ||||
| Study | Topical anaesthetics | Participant self‐reported pain scores | Secondary outcome measures | Incidence of anaesthetic toxicity |
| Bupivacaine‐norepinephrine (BN), etidocaine‐norepinephrine (EN), mepivacaine‐norepinephrine (MN) and prilocaine‐norepinephrine (PN) vs tetracaine‐epinephrine‐cocaine (TAC) | Participant‐reported VAS (100 mm) pain scores (mean scores: BN = 18.3, EN = 46.5, MN, PN = 36.0 vs TAC = 12.0, standard deviations not reported) (TAC significantly outperformed EN; no significant differences between any other groups) | 1) Observer‐reported VAS and Likert pain scale scores 2) Observer‐rated Restrained Infants and Children Disress Rating Scale 3) Suture technician‐rated anaesthetic effectiveness | Not reported | |
| Mepivacaine‐norepinephrine (MN) vs | None | 1) Observer‐reported VAS pain scores (suture technicians, research assistants, videotape reviewers) 2) Observer‐reported Lickert (1‐7) pain scores (parents, suture technicians) 3) Requirement for supplemental lidocaine infiltration (See Characteristics of included studies for data.) | Not reported | |
| Prilocaine‐phenylephrine (PP), | VAS (100 mm) pain scores (mean score ± SD: PP = 29.0 ± 43.4, TP = 24.2 ± 37.2, TLP = 30.6 ± 40.3 vs TAC = 17.6 ± 34.1 (no significant differences between groups; P = 0.5) | 1) Oberver‐reported VAS (100 mm) pain scores 2) Oberver‐reported Likert (1‐7) pain scores 3) Suture technicians‐rated anaesthetic effectiveness | Not reported | |
| Prilocaine‐phenylephrine (PP) and bupivacaine‐phenylephrine (BP) vs TAC | VAS (100 mm) pain scores (mean score ± SD: PP = 21.0 ± 28.0 and BP = 41.0 ± 35.0 vs TAC = 18.0 ± 24.0) (no differences reported between groups; P = 0.07) | Observer‐reported VAS pain scores (suture technicians, research assistants and parents) | Not reported | |
| LAT vs TAC | Modified multi‐dimensional pain scale (0‐10) (mean ranked sum: LAT = 49.0 vs TAC = 46.9; P = 0.71) | 1) Physician‐rated modified multi‐dimensional pain scale (0‐10) 2) Percentage of sutures causing pain 3) Requirement for supplemental lidocaine infiltration | 0/95 | |
| LAT vs TAC | VAS (100 mm) pain scores (mean ranked sum: LET = 45.3 vs TAC = 50.8; P = 0.27) | 1) Physician‐reported VAS scores 2) Percentage of sutures causing pain | Not reported | |
| LAT vs TAC | None | 1) Adequacy of initial anaesthesia (LAT = 74.4% vs TAC = 79.5%; P = 0.46) 2) Anaesthetic effectiveness (complete anaesthesia: LAT = 82.4% vs topical TAC = 75.9%; P = 0.18) | 0/151 | |
| Lidocaine‐prilocaine (EMLA) vs TAC | VAS (100 mm) pain scores (mean score ± SD: EMLA = 46.0 ± 26.0 vs TAC = 40.0 ± 25.0; P = 0.50) | 1) Observer‐rated VAS pain scores 2) Requirement for supplemental lidocaine infiltration | Not reported | |
| Lidocaine‐epinephrine (LE) vs TAC | Faces pain scale (1‐9) scores (mean score ± SD: LE = 3.29 ± 1.92 vs TAC = 2.66 ± 1.78; P = 0.33) | Requirement for supplemental lidocaine infiltration | 0/35 | |
| Tetracaine‐epinephrine (TA) vs TAC | None | 1) Physician‐rating of anaesthetic effectiveness (complete anaesthesia: TA = 47.1% vs TAC = 75%' P < 0.05) 2) Requirement for rescue lidocaine infiltration (TA = 27.5% vs TAC = 8.9%; P = 0.01) | 0/107 | |
| Tetracaine (T) vs TAC | Numerical pain scale (0‐10) score (mean scores: tetracaine = 5.6 vs TAC = 3.53; P < 0.05; standard deviations not reported) | Requirement for supplemental lidocaine infiltration | Not reported | |
| AC: epinephrine (adrenaline) and cocaine; BN: bupivacaine‐noradrenaline; BP: blood pressure; cm: centimetre; c/w: compared with; ED: emergency department; EMLA: Eutectic Mixture of Local Anesthetics (lidocaine and prilocaine); EN: etidocaine‐noradrenaline; LAT: lidocaine, epinephrine and tetracaine (same as LET); LE: lidocaine and epinephrine; LET: same as LAT; LG: local gel; LI: local infiltration; MAC: bupivacaine 0.5%, epinephrine 1:2000, cocaine 10.0%; mm: milli‐metre; MN: mepivacaine‐noradrenaline; PN: prilocaine‐noradrenaline; N: number; NS: not significant; P = P value; PP: prilocaine, phenylephrine; RCT: randomized controlled trial; RICDRS: Restrained Infants and Children Distress Rating Scale; SD: standard deviation; SE: standard error; TA: tetracaine and epinephrine; TAC: tetracaine, epinephrine and cocaine; TLP: tetracaine, lidocaine and phenylephrine; TP: tetracaine and phenylephrine; VAS: visual analogue scale; vs: versus; w/w: weight per weight | ||||
| Study | Topical anaesthetics | Participant self‐reported pain scores | Secondary outcome measures | Incidence of anaesthetic toxicity |
| Bupivacaine‐norepinephrine (BN) vs etidocaine‐norepinephrine (EN) vs mepivacaine‐norepinephrine (MN) vs prilocaine‐norepinephrine (PN) | Patient‐reported VAS (100 mm) pain scores (mean scores: topical BN = 18.3 vs topical EN = 46.5 vs topical MN = 27.0 vs topical PN = 36.0) (no significant differences between any cocaine‐free topical groups) | 1) Observer‐reported VAS and Likert pain scale scores 2) Observer‐rated Restrained Infants and Children Disress Rating Scale 3) Suture technician‐rated anaesthetic effectiveness | Not reported | |
| Prilocaine‐phenylephrine (PP) vs | VAS (100 mm) pain scores (mean score ± SD: PP = 29.0 ± 43.4 vs TP = 24.2 ± 37.2 vs TLP = 30.6 ± 40.3) (no significant differences between groups; P = 0.5) | 1) Oberver‐reported VAS (100 mm) pain scores 2) Oberver‐reported Likert (1‐7) pain scores 3) Suture technicians rated anaesthetic effectiveness | Not reported | |
| Prilocaine‐phenylephrine (PP) vs bupivacaine‐phenylephrine (BP) | VAS (100 mm) pain scores (mean score ± SD: topical PP = 21.0 ± 28.0 vs topical BP = 41.0 ± 35.0; P = 0.07) | Observer‐reported VAS pain scores (suture technicians, research assistants and parents) | Not reported | |
| Lidocaine‐prilocaine (EMLA) vs lidocaine‐epinephrine‐tetracaine (LAT) | VAS (100 mm) pain scores were not significantly different between the 2 groups (mean pain scores not provided; P > 0.05). | 1) Observer‐reported VAS pain scores (legal guardian and physician) 2) Requirement for supplemental lidocaine infiltration | Not reported | |
| Topical LAT gel vs LAT solution | None | 1) Adequacy of initial anaesthesia (adequate anaesthesia: LAT solution = 84% vs LAT gel = 82%; P > 0.05) 2) Effectiveness of anaesthesia (complete anaesthesia: LAT solution = 76% vs LAT gel = 85%; P = 0.007) | 0/194 | |
| AC: epinephrine (adrenaline) and cocaine; BN: bupivacaine‐noradrenaline; BP: blood pressure; cm: centimetre; c/w: compared with; ED: emergency department; EMLA: Eutectic Mixture of Local Anesthetics (lidocaine and prilocaine); EN: etidocaine‐noradrenaline; LAT: lidocaine, epinephrine and tetracaine (same as LET); LE: lidocaine and epinephrine; LET: same as LAT; LG: local gel; LI: local infiltration; MAC: bupivacaine 0.5%, epinephrine 1:2000, cocaine 10.0%; mm: milli‐metre; MN: mepivacaine‐noradrenaline; PN: prilocaine‐noradrenaline; N: number; NS: not significant; P = P value; PP: prilocaine, phenylephrine; RCT: randomized controlled trial; RICDRS: Restrained Infants and Children Distress Rating Scale; SD: standard deviation; SE: standard error; TA: tetracaine and epinephrine; TAC: tetracaine, epinephrine and cocaine; TLP: tetracaine, lidocaine and phenylephrine; TP: tetracaine and phenylephrine; VAS: visual analogue scale; vs: versus; w/w: weight per weight | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Patient self‐reported VAS (0‐100 mm) pain scores Show forest plot | 2 | 240 | Mean Difference (IV, Random, 95% CI) | 5.59 [‐2.16, 13.35] |


