Ventilación no invasiva con presión positiva (PPCVR o VNIPP a dos niveles) para el edema pulmonar cardiogénico
Resumen
Antecedentes
Ésta es una actualización de una revisión sistemática publicada previamente en 2008 acerca de la ventilación no invasiva con presión positiva (VNIPP). La ventilación no invasiva con presión positiva (VNIPP) ha sido utilizada ampliamente para aliviar los signos y síntomas de la dificultad respiratoria debida al edema pulmonar cardiogénico. La VNIPP previene el colapso alveolar y ayuda a redistribuir el líquido intraalveolar, mejora la distensibilidad pulmonar y se reduce la presión de la respiración.
Objetivos
Determinar la efectividad y la seguridad de la VNIPP en el tratamiento de adultos con edema pulmonar cardiogénico en etapa aguda.
Métodos de búsqueda
Se hicieron búsquedas en las siguientes bases de datos el 20 de abril 2011: CENTRAL y DARE, (The Cochrane Library, número 2 de 4, 2011); MEDLINE (Ovid, 1950 hasta abril de 2011); EMBASE (Ovid, 1980 hasta abril de 2011); CINAHL (1982 hasta abril del 2011); y en LILACS (1982 hasta abril de 2011). También se examinaron las listas de referencias de los estudios incluidos y se estableció contacto con expertos y fabricantes de equipamiento. No se aplicó ninguna restricción de idioma.
Criterios de selección
Se seleccionaron ensayos clínicos cegados y no cegados, aleatorios o cuasialeatorios, de pacientes adultos con edema pulmonar cardiogénico agudo o crónico con crisis agudas, en los que se comparó la VNIPP (presión positiva continua en las vías respiratorias [PPCVR]) o la VNIPP a dos niveles más la atención médica estándar con la atención médica estándar sola.
Obtención y análisis de los datos
Dos revisores, de forma independiente, seleccionaron los estudios y obtuvieron los datos con un formulario estándar de obtención de datos. Se evaluó la calidad del estudio haciendo hincapié en la ocultación de la asignación, la generación de la secuencia de asignación, las pérdidas durante el seguimiento, los evaluadores de resultado, el informe de resultado selectivo y el cumplimiento del principio de intención de tratar.
Resultados principales
Se incluyeron 32 estudios (2916 participantes) de riesgo de sesgo generalmente bajo o incierto. En comparación con la atención médica estándar, la VNIPP redujo significativamente la mortalidad en el hospital (CR 0,66; IC del 95%: 0,48 a 0,89) y la intubación endotraqueal (CR 0,52; IC del 95%: 0,36 a 0,75). No se encontraron diferencias en la duración de la estancia hospitalaria con VNIPP; sin embargo, la estancia en la unidad de cuidados intensivos se redujo en un día (DMP ‐0,89 días, IC del 95%: ‐1,33 a ‐0,45). Comparada con la atención médica estándar, no se observó un aumento significativo de la incidencia del infarto agudo de miocardio con la VNIPP durante su aplicación (RR 1,24; IC del 95%: 0,79 a 1,95) o después (RR 0,70; IC del 95%: 0,11 a 4,26). Se identificaron menos eventos adversos con el uso de VNIPP (en particular, dificultad respiratoria progresiva y trastorno neurológico [coma]) en comparación con la atención médica estándar.
Conclusiones de los autores
La VNIPP, especialmente la PPCVR, junto con la atención médica estándar es una intervención efectiva y segura para el tratamiento de los pacientes adultos con edema pulmonar cardiogénico agudo. Las pruebas hasta la fecha sobre el beneficio potencial de la VNIPP en cuanto a la reducción de la mortalidad provienen enteramente de ensayos pequeños y se necesitan ensayos adicionales a gran escala.
PICOs
Resumen en términos sencillos
Ventilación no invasiva con presión positiva para el edema pulmonar cardiogénico
La insuficiencia cardíaca aguda presenta una incidencia elevada en la población general y puede dar lugar a la acumulación de líquido en los pulmones, conocida como edema pulmonar cardiogénico agudo (EPCA). Esta revisión se propuso determinar la efectividad y la seguridad de la ventilación no invasiva con presión positiva (VNIPP) (presión positiva continua en las vías respiratorias [PPCVR] o VNIPP a dos niveles) más la atención médica estándar, comparada con la atención médica estándar sola en adultos con EPCA. Se incluyeron 32 estudios (2916 participantes) de riesgo de sesgo generalmente bajo o incierto. Los resultados de los ensayos controlados aleatorios indican que la VNIPP puede reducir significativamente la mortalidad así como la tasa de necesidad de intubación endotraqueal y el número de días en la unidad de cuidados intensivos sin aumentar el riesgo de sufrir un ataque cardíaco durante o después del tratamiento. Se identificaron menos eventos adversos con el uso de VNIPP (en particular, dificultad respiratoria progresiva y trastorno neurológico [coma]) en comparación con la atención médica estándar. En la comparación de la PPCVR y la VNIPP a dos niveles, la PPCVR puede considerarse la primera opción en la selección de la VNIPP debido a que existen pruebas más consistentes de su efectividad y seguridad y costos más reducidos en comparación con la VNIPP a dos niveles. Las pruebas hasta la fecha sobre el beneficio potencial de la VNIPP en cuanto a la reducción de la mortalidad provienen enteramente de ensayos pequeños y se necesitan ensayos adicionales a gran escala.
Authors' conclusions
Summary of findings
| Non‐invasive positive pressure ventilation (CPAP and bilevel NPPV) for cardiogenic pulmonary oedema | ||||||
| Patient or population: patients with cardiogenic pulmonary oedema | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Non‐invasive positive pressure ventilation (CPAP and bilevel NPPV) | |||||
| Hospital mortalityY | Study population1 | RR 0.66 | 1107 | ⊕⊕⊕⊕ | ||
| 204 per 1000 | 135 per 1000 | |||||
| Moderate1 | ||||||
| 200 per 1000 | 132 per 1000 | |||||
| Endotracheal intubation rate | Study population | RR 0.52 | 1261 | ⊕⊕⊝⊝ | ||
| 249 per 1000 | 130 per 1000 | |||||
| Moderate | ||||||
| 300 per 1000 | 156 per 1000 | |||||
| Incidence of acute myocardial infarction (During intervention) | Study population | RR 1.24 | 461 | ⊕⊕⊕⊝ | ||
| 153 per 1000 | 190 per 1000 | |||||
| Moderate | ||||||
| 169 per 1000 | 210 per 1000 | |||||
| Incidence of acute myocardial infarction (After intervention) | Study population | RR 0.7 | 154 | ⊕⊝⊝⊝ | ||
| 26 per 1000 | 18 per 1000 | |||||
| Moderate | ||||||
| 13 per 1000 | 9 per 1000 | |||||
| Intolerance to allocated treatment | Study population | RR 0.47 | 1848 | ⊕⊕⊕⊝ | ||
| 234 per 1000 | 110 per 1000 | |||||
| Moderate | ||||||
| 350 per 1000 | 165 per 1000 | |||||
| Hospital length of stay | The mean hospital length of stay in the intervention groups was | 542 | ⊕⊝⊝⊝ | |||
| Intensive care unit length of stay (Copy) | The mean intensive care unit length of stay (copy) in the intervention groups was | 222 | ⊕⊕⊝⊝ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The most studies have mixed populations (with different levels of severity and co‐morbidities). | ||||||
Background
This is an update of a systematic review previously published in 2008 about non‐invasive positive pressure ventilation (NPPV) in acute cardiogenic pulmonary oedema (ACPE) (Vital 2008).
Recent publications showed that about 5.7 million Americans and 15 million Europeans have heart failure (HF) (AHA 2012; Dickstein 2008). In the United States, the estimate annual rates per 1000 population of new HF events for white men are 15.2 for those 65 to 74 years of age, 31.7 for those 75 to 84 years of age, and 65.2 for those 85 years of age. For white women in the same age groups, the rates are 8.2, 19.8, and 45.6, respectively. For black men, the rates are 16.9, 25.5, and 50.6, and for black women, the estimated rates are 14.2, 25.5, and 44.0, respectively (AHA 2012). Acute HF is defined as the rapid onset of signs and symptoms secondary to abnormal cardiac function. HF may occur in the presence or absence cardiac disease and may culminate in acute cardiogenic pulmonary oedema (ACPE) manifested by severe respiratory distress. The most common causes of ACPE are ischaemia, acute coronary syndromes, hypertensive crisis, valvular dysfunction, acute arrhythmias, pericardial disease, increased filling pressures or elevated systemic resistance and dilated cardiomyopathy (Dickstein 2008; Gibelin 2002; Guimarães 2004; Nieminen 2005; Williams 1995). ACPE typically presents with severe respiratory distress, tachypnoea,orthopnoea, crackles on auscultation, and oxygen desaturation (Dickstein 2008; Nieminen 2005). Patients with acute HF have a poor prognosis. The one‐year mortality rate associated with acute myocardial infarction complicated by acute HF is nearly 30% (Nieminen 2005). Furthermore, the in‐hospital and one‐year mortality rates associated with ACPE are 12% and 40%, respectively (Nieminen 2005). The incidence and burden of illness imposed by acute HF and ACPE are substantial, underscoring the need for effective preventative and treatment strategies.
In ACPE, cardiac failure results in increased back pressure on the pulmonary circulation which in turn, precipitates extravasation of fluid into the alveoli. In addition to overwhelming the capacity of the lymphatics for fluid removal (Allison 1991), the oedema fluid dilutes surfactant and neutralises its lubricating properties. Consequently, the lungs become less compliant and the effort of breathing increases (Chadda 2002; Martínez 1995; Meyer 1998; Park 2001). In the upright patient, most oedema fluid collects at the lung bases. The oedema fluid causes intrapulmonary shunting and, consequently, ventilation‐perfusion (V‐Q) mismatch and redistribution of blood flow to the upper lobes. ACPE presents clinically with difficulty breathing, hypoxaemia and anxiety. The resulting sympathetic nervous system activation manifests clinically with tachycardia, hypertension, peripheral vasoconstriction and diaphoresis (Allison 1991).
Clinicians treat patients with ACPE with supplemental oxygen, diuretics, nitrates, nitroglycerin and morphine sulfate. Additional treatments may be required to address the etiology of ACPE and may include antihypertensive therapy, inotropic agents, thrombolysis or urgent surgery (Dickstein 2008; Guimarães 2004; Nieminen 2005; Remme 1997; Williams 1995). A subset of patients with ACPE may require endotracheal intubation and mechanical ventilation (Meyer 1998). Mechanical ventilation supports oxygenation by recruiting previously collapsed alveoli (Levitt 2001; Williams 1995) and thereby improves lung compliance. However, invasive ventilation increases the risk for complications including nosocomial infections (pneumonia, sinusitis) and tracheal injury (Gay 2009; Keenan 1997). Consequently, may prolong intensive care unit (ICU) and hospital stay (Pang 1998). Patients with ACPE can be supported with NPPV, which has been shown to improve mortality and endotracheal intubation rate (ETI) as an initial treatment for patients with acute exacerbations of chronic obstructive pulmonary disease (Ram 2004).
NPPV includes forms of ventilatory support applied without the use of an endotracheal tube, and is considered to include continuous positive airway pressure (CPAP), without or with inspiratory pressure support (bilevel NPPV or BiPAP® ‐ Respironics, Inc, Murrysville, PA). The goals of NPPV use in the treatment of ACPE are to improve oxygenation, reduce the effort of breathing and increase cardiac output (Evans 2001). CPAP achieves these goals by maintaining positive airway pressure throughout the respiratory cycle thereby preventing alveolar collapse at end‐expiration. CPAP increases lung compliance and decreases the effort of breathing, while decreasing cardiac preload and afterload (Allison 1991; Bersten 1991; Räsänen 1985). CPAP improves arterial oxygenation (PaO2) by increasing the functional residual capacity of the lungs and reducing intrapulmonary shunt (Räsänen 1985). With CPAP high mean airway pressures are avoided and lower mean intra‐thoracic pressures develop during inspiration with favourable effects on venous return and a lower risk of barotrauma (Covelli 1982; Kelly 1997). Treatment with CPAP has beneficial effects on haemodynamics by ameliorating hypertension and tachycardia (Väisänen 1987). Unlike CPAP, bilevel NPPV combines inspiratory positive airway pressure with positive end‐expiratory pressure. Consequently, bilevel NPPV differs from CPAP in providing inspiratory assistance to rest the muscles of respiration (Mehta 1997). CPAP and bilevel NPPV are applied with either a nasal or oronasal mask at the patient‐ventilator interface (Chadda 2002; Keenan 1997). Complications associated with NPPV use include air leaks, mask discomfort, skin breakdown, eye irritation, sinus congestion, oronasal drying and patient‐ventilator dyssynchrony. Pneumothoraces and pneumonias can occur with NPPV administration but are less frequent compared to invasive ventilation (Bach 1997; Gay 2009). NPPV may also delay endotracheal intubation with patient deterioration in the intervening time period (Wood 1998).
Over the past 30 years, numerous reports have appeared in the literature regarding the potential effectiveness of NPPV for patients with ACPE (Masip 2000; Park 2001; Räsänen 1985). The delay in uptake of the evidence may be due to the need for increased patient supervision, the experience of staff with NPPV, NPPV availability and concerns regarding potential adverse effects (Bersten 1991; Pang 1998). Evidence from at least one randomised trial supports that NPPV accelerates recovery of vital signs and blood gases while avoiding intubation (Park 2001). The role of bilevel NPPV in patients with ACPE remains controversial. In a comparison of CPAP with bilevel NPPV, the latter demonstrated more rapid recovery of respiratory and haemodynamic parameters (Lin 1991), but bilevel NPPV was also associated with an increased incidence of acute myocardial infarction (Mehta 1997).
In spite of the potential advantages of NPPV for the management of ACPE, there appears to be a lack of high quality clinical evidence to support use of these interventions. The purpose of this systematic review was to identify, critically appraise and summarise the evidence for NPPV use for ACPE.
Objectives
The primary objective of this review was to determine the effectiveness and safety of NPPV (CPAP and/or bilevel NPPV) plus standard medical care compared to standard medical care in adults with ACPE on hospital mortality and other clinically important outcomes. The second objective of this review was to directly compare the effects of CPAP and bilevel NPPV on clinically important outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We included blinded or unblinded randomised controlled trials (RCTs) or quasi‐randomised controlled trials (QRCTs) in our review. We defined QRCTs as those in which the allocation procedure was unlikely to be adequately concealed (i.e. randomisation according to odd or even numbers, day of the week, social security number, or medical record number).
Types of participants
We included trials reporting on adult patients (>18 years) with acute or acute‐on‐chronic cardiogenic pulmonary oedema. We used the criteria of the American Heart Association (AHA) and/or the European Society of Cardiology (ESC) to define ACPE (Nieminen 2005; Williams 1995). Alternatively, a diagnosis of acute HF was based upon symptoms and clinical signs including dyspnoea, shortness of breath, nonproductive or cough productive of sputum), pallor, cyanosis, normal or elevated blood pressure, rales on auscultation and the presence of cold clammy skin. The diagnosis had to be supported by a chest radiograph, electrocardiograms, serum biomarkers of acute myocardial infarction or acute HF or echocardiography.
We excluded trials investigating NPPV for patients with a primary diagnosis of pneumonia, alternative etiologies of respiratory failure and its use as a weaning strategy.
Types of interventions
The control group included any form of standard medical care (SMC) provided for the management of cardiogenic pulmonary oedema (Nieminen 2005), excluding NPPV or alternative methods of ventilatory support.
The intervention group was standard medical care for the management of cardiogenic pulmonary oedema plus NPPV (CPAP and/or bilevel NPPV) applied through a nasal or facemask.
Types of outcome measures
Primary outcomes
-
Hospital mortality
Secondary outcomes
-
ETI
-
Incidence of acute myocardial infarction during and after intervention (during the hospital stay)
-
Intolerance to the allocated treatment (patients with criteria for intubation, but this situation did not happen, because they could get another type of ventilatory support)
-
Hospital length of stay (between admission to hospital up to death event or hospital leaving)
-
Intensive care unit (ICU) length of stay (time length in between admission to ICU up to death event or ICU leaving)
-
Arterial blood gases (PaCO2, PaO2) and pH one‐hour post intervention
-
Vital signs: respiratory rate, heart rate, blood pressure 1‐hour post intervention
-
Treatment failure (the combination of mortality and intubation and intolerance to the allocated treatment)
-
Adverse events
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) and the Database of Abstracts of Reviews Effectiveness (DARE) on The Cochrane Library (Issue 2, 2011), MEDLINE (Ovid, 1950 to 20 April 2011), EMBASE (Ovid, 1980 to 20 April 2011), Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO, 1982 to 20 April 2011) and LILACS (1982 to 20 April 2011) . When the searches were run in 2009 we combined the search strategies with the highly sensitive search strategies to identify RCTs in MEDLINE (Dickersin 1994), EMBASE (Lefebvre 1996), and LILACS (Castro 1999). When the searches were re‐run in 2011 the MEDLINE and EMBASE searches used the most current highly sensitive search filters for RCTs (Lefebvre 2011).
The search strategies for each database are presented in Appendix 1 (and Appendix 2 (2009 and 2011 searches, respectively).
Searching other resources
We reviewed the bibliographies of retrieved articles to identify related published and unpublished studies.
We contacted authors of included RCTs, experts in NPPV and ventilator manufacturers to obtain information on other published and unpublished studies. No language restrictions were applied.
Data collection and analysis
We followed the recommendations outlined in the Cochrane Handbook for Systematic Reviews of Interventions in preparing this review (Higgins 2011).
Selection of studies
Two authors (FV and ML) independently screened citations and selected trials meeting the inclusion criteria. Disagreements were resolved by a third author (AA).
Data extraction and management
Two authors (FV and ML) abstracted data using a standardised data collection form which highlighted characteristics of the study (design, methods of randomisation, withdrawals and dropouts, intention‐to‐treat analysis (ITT), informed consent, place and multicenter study), participants (age, gender, number, diagnostic criteria, inclusion and exclusion criteria) and interventions (type of NPPV, timing and duration of therapy, co‐interventions, SMC (intervention and dose)) and the outcomes reported. We requested unpublished data from primary authors to supplement outcomes where needed. A third author (ANA) resolved disagreements. If consensus could not be reached, we contacted the author of the trial for additional information.
Assessment of risk of bias in included studies
We assessed the methodological quality of the included trials with emphasis on allocation concealment, generation of allocation sequence, detection bias, attrition bias, selective outcome reporting and adherence to ITT as follows (Higgins 2011; Schulz 1995):
Allocation concealment
-
Low risk of bias: randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study
-
Moderate risk of bias: randomisation stated but no information on method used is available
-
High risk of bias: method of randomisation used such as alternate medical record numbers or unsealed envelopes; any information in the study that indicated that investigators or participants could influence intervention group (quasi‐randomised studies)
Generation of allocation sequence
-
Low risk of bias: adequate sequence generation
-
Moderate risk of bias: not reported in the paper or by contacting authors
-
High risk of bias: not adequate (quasi‐randomised studies)
Detection bias
-
Low risk of bias: outcome assessors were independent from the individuals administering/supervising the assigned treatments
-
Moderate risk of bias: not reported in the paper or by contacting authors
-
High risk of bias: outcome assessors aware about the assigned treatments
Attrition bias
-
Low risk of bias: drop‐outs without substantial (statistically significant) difference between the two comparison groups and/or a substantial drop‐out rate within the sample as a whole
-
Moderate risk of bias: not reported in the paper or by contacting authors
-
High risk of bias: drop outs with substantial (statistically difference) between the two treatment groups and/or a substantial drop‐out rate within the sample as a whole
Selective outcome reporting
-
Low risk of bias: reports of the study free of suggestion of selective outcome reporting
-
Moderate risk of bias: not reported in the paper or by contacting authors
-
High risk of bias: reports of the study with suggestion of selective outcome reporting
Adherence to the intention‐to‐treat principle
-
Yes: specifically reported by authors that intention‐to‐treat analysis was undertaken and this was confirmed on study assessment
-
No: not reported and lack of intention‐to‐treat analysis confirmed on study assessment (patients who were randomised were not included in the analysis because they did not receive the study intervention, they withdrew from the study or were not included because of protocol violation)
We also noted whether informed consent was obtained and whether the study was blinded.
Assessment of heterogeneity
We assessed the impact of heterogeneity using the I2 test (Higgins 2011). This test illustrates the percentage of variability in effect estimates resulting from heterogeneity, as opposed to sampling error. In the presence of the significant heterogeneity (>50%) and a sufficient number of studies reporting the outcome of interest, we conducted exploratory analyses to investigate potential sources of heterogeneity (e.g. participants, treatments and study quality). We hypothesised that age, gender and comorbidities may represent potential sources of heterogeneity among participants. Heterogeneity may be related to the initial treatment(s) used, the levels of pressure applied with NPPV or treatment duration. We considered P values < 0.10 to be statistically significant.
Data synthesis
We pooled dichotomous and continuous variables using relative risk (RR) and weighted mean difference (WMD), respectively, using random‐effects models. We presented the number needed to treat (NNT) and risk difference (RD) for statistically significant results and assessed their robustness using a fixed‐effect model. We report summary estimates of treatment effect with their associated 95% confidence intervals (CIs). All analyses were performed using the Cochrane Collaboration's statistical software, (Review Manager 2013). We considered P values < 0.05 to be statistically significant.
Sensitivity analysis
We decided a priori to conduct sensitivity analysis to assess the impact of excluding QRCTs on the outcomes of mortality and endotracheal intubation. Finally, we constructed funnel plots to assess for publication bias (Egger 1997).
Quality of the evidence
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to assess the overall quality of evidence (Guyatt 2008; Higgins 2011).
Results
Description of studies
Study search
We identified 1248 citations from our electronic searches (250 in MEDLINE, 427 in EMBASE, 378 in CENTRAL, 137 in CINAHL, 37 in LILACS, and 19 in DARE). We identified two additional studies through searches in bibliographic references and through correspondence with authors. Two authors (FV, ML) pre‐selected 35 references for more detailed review through abstract and title screening (see Figure 1 and Figure 2). All disagreements were resolved by a third reviewer (ANA). We have included 11 new studies with 21 previously included studies, totaling 32 studies involving 2,916 participants (Agmy 2008; Bautin 2005; Bellone 2004; Bellone 2005; Bersten 1991; Crane 2004; Delclaux 2000; Ferrari 2007; Ferrari 2010; Ferrer 2003; Fontanella 2010; Frontin 2010; Gray 2008; Kelly 2002; Levitt 2001; L'Her 2004; Liesching 2003; Lin 1991; Lin 1995; Martin‐Bermudez 2002; Masip 2000; Mehta 1997; Moritz 2007; Nava 2003; Park 2001; Park 2004; Räsänen 1985; Sharon 2000; Takeda 1997; Takeda 1998; Thys 2002; Weitz 2007). This included 23 RCTs comparing NPPV plus standard medical care (CPAP and/or bilevel NPPV) to standard medical care and 14 RCTs comparing CPAP plus standard medical care and bilevel NPPV plus standard medical care directly. One of the studies was a mega trial (Gray 2008). Further details of the included studies are available in the section Characteristics of included studies and justifications for studies excluded are described in Characteristics of excluded studies.

QUOROM statement flow diagram of 2005

Figure 4. PRISMA statement flow diagram of 2011
Study design
Of the 32 parallel design studies, 30 were RCTs and 2 were QRCTs (Bersten 1991; Weitz 2007). Twenty seven studies were reported in full and five studies were reported in abstract form (Agmy 2008; Bautin 2005; Fontanella 2010; Liesching 2003; Martin‐Bermudez 2002).
Population
The 32 studies were based in several countries including Finland, Australia, Taiwan, Japan, Spain, Israel, USA, Brazil, UK, Belgium, Egypt, Russian, Germany, France and Italy. Eight studies were multicentre studies (Crane 2004; Delclaux 2000; Ferrari 2010; Ferrer 2003; Gray 2008; L'Her 2004; Moritz 2007; Nava 2003). All studies reported on adult patients with acute or acute‐on‐chronic cardiogenic pulmonary oedema. The number of patients recruited in each study ranged from 8 to 1069 with similar numbers of patients in the intervention and control groups. We have included in the systematic review one mega trial (Gray 2008); however, only their adverse events and the outcome intolerance to the allocated treatment were recorded. The average age of participants was 74 years. The prevalence of cardiogenic pulmonary oedema for men and women was 47% and 53%, respectively.
While nine studies were conducted in the ICU (Agmy 2008; Bautin 2005; Delclaux 2000; Ferrer 2003; Fontanella 2010; Lin 1991; Räsänen 1985; Takeda 1997; Takeda 1998), four studies were undertaken in the emergency department and included patients referred to the ICU after being on a ward (Bersten 1991; Masip 2000; Mehta 1997; Thys 2002). Additionally, Park 2001 reported that it was conducted in the hospital without reference to a specific location, two studies did not report the venue (Liesching 2003; Martin‐Bermudez 2002), and two other studies reported that the interventions were initiated in a mobile ICU and continued in the emergency department (Sharon 2000; Weitz 2007). Frontin 2010 reported that the interventions were initiated in a mobile ICU and continued in ICU. The remaining studies were solely undertaken in the emergency department.
Intervention
Of the studies identified, ten compared CPAP to SMC (Bersten 1991; Delclaux 2000; Frontin 2010; Kelly 2002; L'Her 2004; Lin 1991; Lin 1995; Räsänen 1985; Takeda 1997; Takeda 1998), and eight compared bilevel NPPV to SMC (Bautin 2005; Ferrer 2003; Levitt 2001; Masip 2000; Nava 2003; Sharon 2000; Thys 2002; Weitz 2007 ). Additionally, there were five three‐arm studies comparing CPAP to bilevel NPPV and to SMC (Agmy 2008; Crane 2004; Gray 2008; Park 2001; Park 2004). Finally, nine studies directly compared CPAP with bilevel NPPV (Bellone 2004; Bellone 2005; Ferrari 2007; Ferrari 2010; Fontanella 2010; Liesching 2003; Martin‐Bermudez 2002; Mehta 1997; Moritz 2007).
In Table 1, we present, where reported, the different IPAP and EPAP levels used and the duration of NPPV administration. The choice of patient‐ventilator interface for NPPV administration varied among the included studies with three studies using nasal masks exclusively (Mehta 1997; Takeda 1997; Takeda 1998). Two studies offered a choice between nasal and face masks based on patient effort (Ferrer 2003; Levitt 2001). One study used nasal masks for bilevel NPPV administration and face masks for CPAP administration (Park 2001). Four studies did not report the patient‐ventilator interface used (Agmy 2008; Gray 2008; Liesching 2003; Sharon 2000). The remaining studies reported using face masks for NPPV administration.
| Study | IPAP level (cmH2O) | EPAP in bievel NPPV (cmH2O) | PEEP in CPAP (cmH2O) | Time of bilevel NPPV (hours) | Time of CPAP (hours) |
| 5.1 (SD 0.3) | 9.8 (SD 1.1) | ||||
| 5 | 10 | 1.6 (SD 0.6) | 1.7 (SD 0.7) | ||
| 5 | 10 | 3.41 (SD 1.1) | 3.6 (SD 1.3) | ||
| 10 | 9.3 (SD 4.9) | ||||
| Crane 2004 | 15 | 5 | 10 | ||
| Ferrari 2007 | 15 (SD 3.1) | 7 (SD 1.2) | 8.8 (SD 1.9) | 6.0 (SD 4.7) | 8.1 (SD 8.3) |
| Ferrari 2009 | 14 (SD 3.1) | 6.7 (SD 1.4) | 8.8 (SD 1.7) | 5.9 (SD 4.0) | 8.4 (SD 7.1) |
| Fontanella 2010 | 18 (SD 3) | 10 (SD 2) | 8 (SD 2) | ||
| Frontin 2010 | 10 | ||||
| Gray 2009 | 14 (SD 5) | 7 (SD 3) | 10 (SD 4) | 2.0 (SD 1.3) | 2.2 (SD 1.5) |
| Kelly 2002 | 7.5 | ||||
| L'Her 2004 | 7.5 | 8 (SD 6) | |||
| Liesching 2003 | 12 | 4 | 10 | ||
| Martin‐Bermudez 2002 | 1.3 (SD 0.8) | 1.8 (SD 1) | |||
| Masip 2000 | 15.2 (SD 2.4) | 5 | 4.2 (SD 1.5) | ||
| Mehta 1997 | 14.35 (SD 1.73) | 5 | 10.08 (SD 1.24) | 7.1 (SD 4.7) | 6.4 (SD 5.8) |
| Moritz 2007 | 12 (SD 3.2) | 4.9 (SD 0.9) | 7.7 (SD 2.1) | 2.8 | 2.3 |
| Nava 2003 | 14.5 (SD 21.1) | 6.1 (SD 3.2) | 11.4 (SD 3.6) | ||
| Park 2001 | 12 | 4 | 7.5 | 2.5 (SD 0.6) | 2.8 (SD 1.5) |
| Park 2004 | 17 (SD 2) | 11 (SD 2) | 11 (SD 2) | 2.0 (SD 1.0) | 1.7 (SD 0.6) |
| Rasanen 1985 | 10 | ||||
| Sharon 2000 | 9.3 (SD 2.3) | 4.2 (SD 3.1) | |||
| Takeda 1997 | 11.9 (SD 8.4) | ||||
| Thys 2002 | 16.5 (SD 3.3) | 6.1 (SD 1.5) | 1.2 (SD 0.2) | ||
| Weitz 2007 | 12.5 (SD 1.2) | 5 |
CPAP ‐ continuous positive airway pressure; EPAP ‐ expiratory positive airway pressure; IPAP ‐ inspiratory positive airway pressure; PEEP ‐ positive expiratory end pressure; SD ‐ standard deviation; * statistically significant.
In addition to supplemental oxygen, studies reported the use of pharmacologic treatments including furosemide, morphine, diamorphine, sodium nitroprusside, glyceryl trinitrate, nitrate, isosorbide dinitrate, nitroglycerin, digoxin, digitalis, dopamine, dobutamine, epinephrine, norepinephrine, diazepam, chlorpromazine and antibiotics. Generally the medical team was free to choose the type of medication and its dose for the management of pulmonary oedema. However, one study used high doses of isosorbide dinitrate in the control group (Sharon 2000).
Outcomes
Almost all studies reported hospital mortality, except for Frontin 2010, Gray 2008 and Liesching 2003. Frontin 2010 reported mortality after 30 days. Gray 2008 reported mortality after 7 and 30 days from the beginning of the intervention. In order to perform a meta‐analysis taking into considering the weight of the mega‐trial (which presented opposite results to the first version of this review), we included data on mortality in a separate meta‐analysis. However, we were unable to consider the total number after randomisation by group in this meta‐analysis since Gray 2008 did not provide this information, which precluded intention‐to‐treat analysis according to dichotomous outcomes expected. The remaining outcomes were variably reported by the included studies. All dichotomous outcomes were described in adherence with the intention‐to‐treat principle. All authors reported their studies using mean and standard deviations for continuous outcomes. We transformed units from kilo pascals to millimetres of mercury in pooling results reported for arterial blood gases in two studies (Crane 2004; Kelly 2002).
Risk of bias in included studies
The summary of risk of bias can be viewed illustratively in Figure 3 and Figure 4.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
All studies included stated that treatment allocation was made randomly. The following studies, which adequately reported the method of allocation concealment, were classified as "low risk of bias": Bellone 2004; Bellone 2005; Crane 2004; Delclaux 2000; Ferrari 2007; Ferrari 2010; Frontin 2010; Gray 2008; Kelly 2002; L'Her 2004; Martin‐Bermudez 2002; Masip 2000; Moritz 2007; Nava 2003; Park 2001; Park 2004; Räsänen 1985; Takeda 1998; Thys 2002. The following studies did not describe the method of allocation concealment and were classified as "moderate risk of bias": Agmy 2008; Bautin 2005; Ferrer 2003; Fontanella 2010; Levitt 2001; Liesching 2003; Lin 1991; Lin 1995; Mehta 1997; Sharon 2000; Takeda 1997. The following two studies had an inadequate (quasi‐randomised) method and were classified as "high risk of bias": Bersten 1991; Weitz 2007.
In 19 studies sequence generation was described in an appropriate way (Bellone 2004; Bellone 2005; Crane 2004; Delclaux 2000; Ferrari 2007; Ferrari 2010; Ferrer 2003; Frontin 2010; Gray 2008; Levitt 2001; L'Her 2004; Martin‐Bermudez 2002; Masip 2000; Mehta 1997; Moritz 2007; Nava 2003; Park 2001; Park 2004; Sharon 2000). Bersten 1991 and Weitz 2007 did not describe sequence generation.
Incomplete outcome data
Just two studies have presented significant losses during follow up: Mehta 1997 had lost 9 of the 36 (25%) randomised patients due to infections, exacerbation of COPD and delay in obtaining the informed permission or to initiate intervention; and Lin 1991 lost 35 of the 80 (44%) randomised patients for having fulfilled the exclusion criteria or having failed to receive the intervention.
Selective reporting
The majority of the studies planned and reported the outcomes analysed. Two studies omitted some information (Ferrari 2010; L'Her 2004). Ferrari 2010 planned to analyse the effect of NPPV on systemic blood pressure but did not report this outcome. L'Her 2004 did not present the results in relation to autonomy for activities of daily living, patient comfort and adverse effects.
Other potential sources of bias
Detection bias was the domain that generated more uncertainty in risk analysis of bias. The majority of studies did not report on blinding. Given the nature of the intervention, blinding of participants and personnel to the intervention was not feasible. Studies by Mehta and Thys were reported as being single‐blinded, but in these instances the patient was reportedly blinded to his/her intervention (Mehta 1997; Thys 2002), which appears inconsistent with blinding. Only Delclaux 2000, Gray 2008, Martin‐Bermudez 2002 and Moritz 2007 reported that the evaluators were independent.
Twenty‐one studies performed analysis by ITT. For our analysis, we performed the analysis of all dichotomous variables by intention‐to‐treat.
Five studies were stopped early due to preliminary analysis which demonstrated significant differences between groups (L'Her 2004; Mehta 1997; Park 2004; Sharon 2000; Thys 2002). For examples, differences were seen in Mehta 1997 and Sharon 2000 due to the incidence of acute myocardial infarction, in Thys 2002 due to a batch of failures of treatment and clinical decline, in L'Her 2004 due to a greater number in deaths and complications in the control group and in Park 2004 due to a difference in the frequency of intubations. Seven studies did not explicitly report that informed consent was obtained (Agmy 2008; Bautin 2005; Fontanella 2010; Liesching 2003; Lin 1995; Martin‐Bermudez 2002; Räsänen 1985).
Sharon's study used different doses of isosorbide dinitrate as standard treatment in the different randomised groups, which may have influenced its results.
The analysis of funnel plot for the outcome hospital mortality demonstrates the indicates asymmetry and the potential risk of publication bias (Figure 5).

Funnel plot of comparison: 1 Hospital Mortality, outcome: 1.1 NPPV (CPAP and BILEVEL) x SMC.
Quality of the evidence
About 50% of the studies were considered as low risk of bias (Figure 3). The sole domain of quality that most studies did not report on was whether the analysis of outcomes was undertaken by the same person who applied the interventions (detection bias) (Figure 4). As such, we believe that the quality of the evidence included in this review is at moderate risk of bias.
More specifically, we defined seven relevant outcomes in order to assess the quality of evidence by GRADE (Guyatt 2008; Higgins 2011). The primary outcome (mortality) was supported by high quality evidence, two outcomes (incidence of acute myocardial infarction during intervention and intolerance to the allocated treatment) were considered to be supported by moderate quality evidence. Two outcomes (endotracheal intubation rate and ICU length of stay) were considered to be supported by low quality of evidence and for incidence of acute myocardial infarction after intervention and hospital length of stay were considered to be supported by very low quality evidence (summary of findings Table for the main comparison).
Effects of interventions
For studies comparing NPPV to SMC, we report our results as NPPV (CPAP and bilevel NPPV) versus SMC, CPAP alone versus SMC and bilevel NPPV alone versus SMC. In addition, we report results for studies directly comparing CPAP to bilevel NPPV. We report the pooled results for all primary and secondary analyses and sensitivity analyses conducted to explore potential sources of heterogeneity on the outcomes of hospital mortality and endotracheal intubation. The summary of our findings can be seen in summary of findings Table for the main comparison.
Hospital mortality
In 20 trials involving 1107 patients, we found a reduction in hospital mortality for patients treated with NPPV plus SMC compared with SMC care alone (RR 0.66, 95% CI 0.48 to 0.89). This translates into a RD of ‐ 0.07 or 7% (95% CI ‐0.12 to ‐0.02) and NNT of 14 (Analysis 1.1). Sensitivity analysis with the exclusion of quasi‐randomised studies (Bersten 1991; Weitz 2007), did not change the results (RR 0.65, 95% CI 0.46 to 0.91; Analysis 1.2). However if we consider Gray 2008 and Frontin 2010 that analysed the mortality in 7 and/or 30 days, our meta‐analysis of hospital mortality indicates that these studies reinforce the favourable use of NPPV in ACPE (RR 0.72, 95% CI 0.55 to 0.94 and RR 0.75, 95% CI 0.60 to 0.95, respectively Analysis 15.1 and Analysis 16.1).This result could have been anticipated using cumulative meta‐analysis techniques (Figure 6; MIX 2006).

Cumulative meta‐analysis
Comparing CPAP plus SMC with SMC alone (13 trials, 699 patients) there was a lower hospital mortality rate in CPAP‐treated patients (RR 0.60, 95% CI 0.39 to 0.94; Analysis 1.6). The beneficial effect of CPAP translates into a RD of ‐11% (95% CI ‐0.18 to ‐0.04) and a NNT of 9. The effect of CPAP on hospital mortality remained significant after exclusion of a single QRCT (Bersten 1991) (RR 0.57, 95% CI 0.35 to 0.94; Analysis 1.7).
However, we found no benefit of bilevel NPPV plus SMC compared with SMC care alone on hospital mortality (11 trials, 506 patients, RR 0.65, 95% CI 0.39 to 1.09; Analysis 1.8), and after exclusion of quasi‐randomised studies (Bersten 1991; Weitz 2007) (Analysis 1.9). However, the similar effect size and wider confidence intervals for bilevel NPPV compared to the overall NPPV and CPAP‐specific results is noted.
We found no differences in hospital mortality comparing CPAP plus SMC and bilevel NPPV plus SMC directly (12 trials, 694 patients, RR 1.10, 95% CI 0.61 to 1.97; Analysis 1.12).
ETI rate
In 22 trials (1261 patients) we found a lower ETI rate for patients treated with NPPV plus SMC compared with SMC (RR 0.52, 95% CI 0.36 to 0.75; Analysis 2.1). This translates into a RD of ‐ 0.12 or 12% (95% CI ‐0.19 to ‐0.04) and a NNT of 8. Exclusion of two QRCTs (Bersten 1991; Weitz 2007) also revealed beneficial effect of NPPV compared to SMC (RR 0.53, 95% CI 0.37 to 0.78; Analysis 2.2). This remained in Gray 2008 and Frontin 2010 that analysed the frequency intubation in seven days (RR 0.55, 95% CI 0.38 to 0.78; Analysis 17.1).
When we compared CPAP plus SMC with SMC (14 trials, 825 patients) we found a significantly lower endotracheal intubation favouring CPAP‐treated patients (RR 0.47, 95% CI 0.33 to 0.67, RD ‐15%, 95% CI ‐0.24 to ‐0.06, NNT 7; Analysis 2.4). This result remained robust after exclusion of a single quasi‐randomised trial (Bersten 1991) (RR 0.48, 95% CI 0.34 to 0.67; Analysis 2.5).
We found no benefit of bilevel NPPV plus SMC compared with SMC alone on ETI rate (12 trials, 536 patients, RR 0.55, 95% CI 0.26 to 1.17; Analysis 2.6). However with significant heterogeneity and after exclusion of the QRCT (Weitz 2007) and Sharon 2000 (whose patients received different doses of drugs) this altered the results: lower ETI favouring bilevel NPPV‐treated patients (RR 0.45, 95% CI 0.26 to 0.80; Analysis 2.7).
In 13 trials (721 patients) comparing CPAP plus SMC to bilevel NPPV plus SMC care, there did not appear to be any difference between the two techniques in reducing ETI (RR 1.04, 95% CI 0.55 to 1.97; Analysis 2.10).
Incidence of acute myocardial infarction
In eight trials (461 patients) we found non‐significant differences in the occurrence of acute myocardial infarction during treatment with NPPV plus SMC compared to SMC (Analysis 3.1). A similar non‐significant differences were found in comparisons of CPAP plus SMC compared to bilevel NPPV plus SMC (Analysis 3.4), CPAP plus SMC care compared to SMC or bilevel NPPV plus SMC compared to SMC (Analysis 3.2; Analysis 3.3).
Only four studies (154 patients) comparing NPPV plus SMC to SMC alone reported no difference in the incidence of acute myocardial infarction after intervention (Analysis 4.1); two studies reported no difference after CPAP plus SMC care compared to SMC (RR 1.08, 95% CI 0.11 to 10.23; Analysis 4.2); three studies with bilevel NPPV plus SMC to SMC reported no difference in the incidence of acute myocardial infarction after intervention (RR 0.52, 95% CI 0.02 to 11.54; Analysis 4.3); two studies compared CPAP plus SMC to bilevel NPPV plus SMC and demonstrated no difference (RR 1.57, 95% CI 0.57 to 4.32; Analysis 4.4).
Intolerance to the allocated treatment
Thirteen studies (1848 patients) found a lower incidence of intolerance in the NPPV plus SMC (16%) compared to SMC (23%) (RR 0.47, 95% CI 0.29 to 0.77; Analysis 5.1), The grouping of the studies demonstrated relevant heterogeneity, which was eliminated with the exclusion of Gray 2008 (Analysis 5.2). We have not found plausible justification for this result. Nine studies (1304 patients) noted significantly lower intolerance with CPAP plus SMC compared to SMC (RR 0.55, 95% CI 0.36 to 0.85; Analysis 5.3); seven studies (995 patients) found no difference related with intolerance to bilevel NPPV plus SMC care compared to SMC (RR 0.58, 95% CI 0.24 to 1.42; Analysis 5.4). Three studies (894 patients) found no difference between CPAP plus SMC and bilevel NPPV plus SMC with respect to intolerance (RR 0.94, 95% CI 0.35 to 2.53; Analysis 5.5).
Length of hospital and ICU stay
Ten trials (542 patients) demonstrated no significant difference in hospital length of stay between NPPV plus SMC compared to SMC (WMD ‐0.80 days, 95% CI ‐2.10 to 0.51; Analysis 6.1). Though heterogeneity diminished after exclusion of one QRCT (Weitz 2007), it did not change the significance of our results (Analysis 6.2). Five studies (337 patients) demonstrated no differences CPAP plus SMC compared to SMC (WMD ‐0.51 days, 95% CI ‐1.69 to 0.67; Analysis 6.3). Seven studies (311 patients) showed no difference between bilevel plus SMC and SMC alone (WMD ‐1.38 days, 95% CI ‐3.38 to 0.62; Analysis 6.4), and similar results were obtained between CPAP plus SMC and bilevel NPPV plus SMC (WMD ‐0.46 days, 95% CI ‐1.99 to 1.07; Analysis 6.5).
Six studies (222 patients) comparing NPPV plus SMC to SMC alone demonstrated significantly shorter ICU stay favouring the NPPV group (WMD ‐0.89 days, 95% CI ‐1.33 to ‐0.45; Analysis 7.1). Three studies (169 patients) indicated a significantly shorter ICU stay for patients treated with CPAP plus SMC (WMD ‐1.09 days, 95% CI ‐1.63 to ‐0.56; Analysis 7.2). A further three studies (53 patients) showed no difference between bilevel NPPV plus SMC and SMC (WMD ‐0.65 days, 95% CI ‐1.37 to 0.06; Analysis 7.3) while no difference was seen between CPAP plus SMC and bilevel NPPV plus SMC (WMD 0.31 days, 95% CI ‐0.78 to 1.40; Analysis 7.4).
Vital signs one hour after intervention
Respiratory rate
Nine studies (438 patients) revealed significantly lower respiratory rates in patients on NPPV plus SMC in comparison with SMC (WMD ‐2.86 bpm, 95% CI ‐3.85 to ‐1.87; Analysis 8.1). Seven studies (300 patients) had lower respiratory rate on CPAP plus SMC compared to SMC (WMD ‐2.39 bpm, 95% CI ‐3.70 to ‐1.07; Analysis 8.2), while six studies (254 patients) showed lower respiratory rate in patients on bilevel NPPV plus SMC in comparison with those receiving SMC (WMD ‐3.52 bpm, 95% CI ‐4.80 to ‐2.23; Analysis 8.3). Five studies (218 patients) did not show a significant difference between CPAP plus SMC and bilevel NPPV plus SMC (WMD 0.57 bpm, 95% CI ‐1.00 to 2.13; Analysis 8.4).
Heart rate (HR)
Nine studies (438 patients) showed no significant difference between NPPV plus SMC and SMC in terms of heart rate (WMD ‐4.01 bpm, 95% CI ‐8.16 to 0.15; Analysis 9.1). Seven studies (300 patients) found no difference between CPAP plus SMC and SMC (WMD ‐4.45 bpm, 95% CI ‐10.81 to 1.92; Analysis 9.3). The grouping of the studies in these two analyses demonstrated heterogeneity, which was eliminated with the exclusion of Rasanen's and L'Her's studies and we have not found plausible justification for this result (Analysis 9.2 and Analysis 9.4). Six studies (254 patients) revealed lower heart rate in patients on bilevel NPPV plus SMC in comparison with SMC (WMD ‐4.21bpm, 95% CI ‐7.77 to ‐0.65; Analysis 9.5) and no significant results were seen in four studies (182 patients) comparing CPAP plus SMC and bilevel NPPV plus SMC (WMD 0.56 bpm, 95 %CI ‐5.22 to 4.11; Analysis 9.6).
Systolic blood pressure (SBP)
Six studies (182 patients) found no statistically significant difference between NPPV plus SMC and SMC groups in SBP (WMD ‐1.64 mmHg, 95% CI ‐7.83 to 4.56; Analysis 10.1). Six studies (211 patients) found no statistically significant difference between CPAP plus SMC and SMC groups in SBP (WMD ‐0.12 mmHg, 95% CI ‐6.56 to 6.81; Analysis 10.2). Similar non‐significant differences were seen in comparison of bilevel NPPV plus and SMC (WMD 1.93 mmHg, 95% CI ‐7.94 to 11.80; Analysis 10.3), and CPAP plus SMC versus bilevel NPPV plus SMC (WMD ‐1.17 mmHg, 95% CI ‐10.79 to 8.44; Analysis 10.4). However, the last analysis showed significant heterogeneity, which was resolved after removing Bellone 2004, which showed higher baseline SBP value than other studies (Analysis 10.5).
Diastolic blood pressure (DBP)
Five studies (138 patients) compared NPPV plus SMC and SMC care on DBP but found no difference (WMD ‐1.49 mmHg, 95% CI ‐6.43 to 3.45; Analysis 11.1). Five studies (167 patients) compared CPAP plus SMC and SMC on DBP but found no difference (WMD ‐0.92 mmHg, 95% CI ‐9.10 to 7.27; Analysis 11.2). However, heterogeneity was observed, which diminished after excluding studies Räsänen 1985 and Crane 2004 (there was a lower value for baseline DBP (mean and SD) in the latter studies in comparison with the others). After excluding those studies, the remaining three studies (107 patients) showed statistically significant lower DBP in patients on CPAP in comparison with those receiving SMC (WMD ‐7.32 mmHg, 95% CI ‐12.79 to ‐1.86; Analysis 11.3). No difference was seen when bilevel NPPV plus SMC care was compared to SMC (four studies; 87 patients) (WMD ‐0.96 mmHg, 95% CI ‐6.09 to 4.16; Analysis 11.4) or CPAP plus SMC vs bilevel NPPV plus SMC (WMD ‐2.60 mmHg, 95% CI ‐9.58 to 4.37; Analysis 11.5). A change was observed when Crane 2004 and Martin‐Bermudez 2002 were excluded, showing statistically significant better DBP in patients (n=62) on CPAP plus SMC in comparison with those receiving bilevel NPPV plus SMC, but we have not found plausible justification for this result (WMD ‐7.86 mmHg, 95% CI ‐13.03 to ‐2.70; Analysis 11.6).
Mean blood pressure (MBP)
Three studies (256 patients) found no statistically significant difference between NPPV plus SMC compared to SMC (WMD ‐2.41 mmHg, 95% CI ‐8.27 to 3.45; Analysis 12.1); similar non‐significance was seen in comparison of CPAP plus SMC and SMC (one study, 89 patients) (WMD 3.00 mmHg, 95% CI ‐5.31 to 11.31; Analysis 12.2), in bilevel NPPV plus SMC compared to SMC (two studies, 167 patients) (WMD ‐5.42 mmHg, 95%CI ‐11.60 to 0.76; Analysis 12.3), and in comparison of CPAP plus SMC and Bilevel NPPV plus standard medical care (one study, 80 patients) (WMD ‐4.40 mmHg, 95% CI ‐13.25 to 4.45; Analysis 12.4) .
Arterial blood gases and pH one hour after intervention
Four studies (140 patients) showed significantly higher PaO2 levels being found in NPPV plus SMC patients in comparison with those receiving SMC (WMD 10.04 mmHg, 95% CI 1.09 to 19.00; Analysis 13.1 ). Five studies (177 patients) found no difference in arterial oxygen levels (PaO2) between CPAP plus SMC and SMC alone (WMD ‐2.64 mmHg, 95% CI ‐25.87 to 20.59; Analysis 13.2). Pooling of these five studies showed significant heterogeneity. Exclusion of the Lin 1991 and Räsänen 1985 studies, which were conducted in ICU settings, showed significantly better PaO2 after one hour of intervention in SMC care patients in comparison with those receiving NPPV (WMD ‐22.09 mmHg, 95% CI ‐34.35 to ‐9.83; Analysis 13.3). Three studies (79 patients) comparing bilevel NPPV plus SMC versus SMCe did not show any significant difference between the groups (WMD 4.79 mmHg, 95% CI ‐11.11 to 20.69; Analysis 13.4). Three studies showed significantly higher PaO2 levels being found in bilevel NPPV plus SMC patients in comparison with those receiving CPAP plus SMCe (WMD ‐27.00 mmHg, 95% CI ‐44.75 to ‐9.25; Analysis 13.5).
We did not pool PaCO2 and pH one hour after intervention as these outcomes require assessment with respect to baseline values (normal for each patient). Pooling of these parameters may not be meaningful.
Treatment failure
We defined treatment failure as a composite of mortality, endotracheal intubation and intolerance to the allocated treatment. No study in this review reported this outcome.
Adverse events
Table 2 summarises the types and number of reported adverse events for both CPAP and bilevel NPPV, where they were reported, regardless of comparative intervention.
| Adverse events | Number of events (CPAP) | Total number CPAP | Number of event (bilevel NPPV) | Total number (bilevel NPPV) | RR (95% CI) |
| Skin damage | 2 | 472 | 17 | 458 | 0.06 (0.01, 0.43)* |
| Pneumonia | 0 | 40 | 1 | 76 | 0.63 (0.03, 15.03) |
| Pulmonary aspiration | 0 | 476 | 1 | 383 | 0.27 (0.01, 6.57) |
| Gastric distention | 5 | 178 | 8 | 51 | 0.18 (0.06, 0.52)* |
| GI bleeding | 0 | 11 | 3 | 85 | 1.02 (0.06, 18.63) |
| Vomitting | 7 | 381 | 9 | 419 | 0.86 (0.32, 2.27) |
| Pneumothorax | 0 | 440 | 1 | 430 | 0.33 (0.01, 7.97) |
| Asphyxia/claustrophobia | 0 | 20 | 1 | 65 | 1.05 (0.04, 24.76) |
| Mask discomfort | 15 | 364 | 22 | 440 | 0.82 (0.43, 1.57) |
| Sinusitis | 0 | 43 | 1 | 65 | 0.50 (0.02, 12.00) |
| Conjunctivitis | 0 | 70 | |||
| Eye irritation | 0 | 20 | |||
| Cardiac arrest | 6 | 333 | 13 | 410 | 0.57 (0.22, 1.48) |
| Stroke | 0 | 65 | |||
| Seizure | 0 | 65 | |||
| Hypotension | 36 | 332 | 37 | 346 | 1.01 (0.66, 1.56) |
| Arrhythmia requiring treatment | 12 | 332 | 25 | 345 | 0.50 (0.25, 0.98)* |
| Progressive respiratory distress | 17 | 333 | 21 | 346 | 0.84 (0.45, 1.57) |
| Increase breathing discomfort | 16 | 287 | 19 | 291 | 0.85 (0.45, 1.63) |
CI ‐ confidence interval; CPAP ‐ continuous positive airway pressure; RR ‐ relative risk; SD ‐ standard deviation; * statistically significant..
Fifteen studies reported adverse events with 232 events occurring in 1109 NPPV plus SMC treated patients and 154 events occurring in 751 SMC care treated patients. The adverse events reported were: skin damage, pneumothorax, pulmonary aspiration, gastric distension, vomiting, mask discomfort, hypotension, arrhythmia, progressive respiratory distress, gastrointestinal bleeding, asphyxia, conjunctivitis, sinusitis, eyes irritation, stroke, seizure, neurological failure (coma) and cardiorespiratory arrest. Participants receiving SMC had less skin damage and those treated with NPPV had less progressive respiratory distress and neurological failure (coma). Confining the analysis to trials published after 2000 did not alter these results. A detailed description of these complications can be found in Table 3, Analysis 14.1 and Analysis 14.2.
| Adverse events | Studies | NPPV group | SMC group | RR (95% CI) |
| Skin damage | 11 studies (Rasanen 1985, Bersten 1991, Takeda 1998, Masip 2000, Kelly 2002, Thys 2002, Nava 2003, Crane 2004, L'Her 2004, Park 2004, Bautin 2005) | 17/318 | 0/276 | 6.62 (1.20, 36.55)* |
| Pneumonia | 3 study (Lin 1991, Nava 2003, Bautin 2005) | 1/116 | 4/116 | 0.35 (0.05, 2.20) |
| Pulmonary aspiration | 5 studies (Rasanen 1985, Bersten 1991, Lin 1991, Kelly 2002, Gray 2008) | 1/787 | 0/468 | 1.58 (0.06, 38,61) |
| Gastrointestinal | 3 study (Takeda 1998, Masip 2000, Nava 2003) | 3/96 | 2/96 | 1,37 (0.27, 6.89) |
| Gastric distension | 8 studies (Rasanen 1985, Bersten 1991, Lin 1991, Takeda 1998, Thys 2002, Park 2004, L'Her 2004, Frotin 2010) | 13/253 | 0/231 | 13.26 ( 0.82, 215.12) |
| Vomiting | 5 studies (Masip 2000, Thys 2002, Crane 2004, Park 2004, Gray 2008) | 16/800 | 8/429 | 1.06 (0.46, 2.47) |
| Asphyxia | 2 study (Bersten 1991, Nava 2003) | 1/85 | 0/85 | 3.00 (0.12, 72.31) |
| Pneumothorax | 6 studies (Bersten 1991, Kelly 2002, Nava 2003, L'Her 2004, Gray 2008, Frotin 2010) | 1/894 | 1/580 | 0.72 (0.08, 6.89) |
| Conjunctivitis | 2 studies (Kelly 2002, L'Her 2004) | 0/70 | 0/77 | inestimable |
| Sinusitis | 2 study (Nava 2003, L'Her 2004) | 1/108 | 0/111 | 3.00 (0.12, 72.31) |
| Mask discomfort | 7 study (Masip 2000, Nava 2003, L'Her 2004, Park 2004, Bautin 2005, Weitz 2007, Frotin 2010) | 7/265 | 0/247 | 5.39 (0.97, 30.09) |
| Hypotension | 1 study (Gray 2008) | 73/678 | 46/352 | 0.82 (0.58, 1.16) |
| Arrhythmia | 1 study (Gray 2008) | 37/677 | 23/350 | 0.83 (0.50, 1.38) |
| Progressive respiratory distress | 2 studies (L'Her 2004, Gray 2008) | 39/722 | 36/400 | 0.58 (0.37, 0.89)* |
| Neurological failure (coma) | 1 study (L'Her 2004) | 1/44 | 11/46 | 0,10 (0.01, 0,71)* |
| Cardiorespiratory arrest | 3 studies (Nava 2003, L'Her 2004, Gray 2008) | 21/786 | 22/466 | 0.60 (0.29, 1.26) |
| Eye irritation | 1 study (Masip 2000) | 0/20 | 0/20 | inestimable |
| Stroke | 1 study (Nava 2003) | 0/65 | 0/65 | inestimable |
| Seizure | 1 study (Nava 2003) | 0/65 | 1/65 | 0.33 (0.01, 8.03) |
CI ‐ confidence interval; NPPV ‐ noninvasive positive pressure ventilation; RR ‐ relative risk; SMC ‐ standard medical care; * statistically significant.
Ten studies reported adverse events with 88 events complications in the 544 CPAP plus SMC treated patients and 146 events in the 572 SMC treated patients. The adverse events reported were: skin damage, pneumonia, pulmonary aspiration, gastrointestinal bleeding, gastric distension, vomiting, asphyxia, pneumothorax, conjunctivitis, sinusitis, mask discomfort, hypotension, arrhythmia, progressive respiratory distress, cardiorespiratory arrest and neurological failure (coma). The CPAP group had fewer progressive respiratory distress, cardiorespiratory arrest and neurological failure (coma). Details of adverse events in patients receiving CPAP and standard medical care are provided in Table 4 and Analysis 14.3.
| Adverse Events | Studies | CPAP group | SMC group | RR (95% CI) |
| Skin damage | 7 studies (Rasanen 1985, Bersten 1991, Takeda 1998, Kelly 2002, Crane 2004, L'Her 2004, and Park 2004) | 1/168 | 0/175 | 3.00 (0.13, 69.52) |
| Pneumonia | 1 study (Lin 1991) | 0/40 | 0/40 | inestimable |
| Pulmonary aspiration | 5 studies (Rasanen 1985, Bersten 1991, Lin 1991, Kelly 2002, Gray 2008) | 0/440 | 0/468 | inestimable |
| Gastrointestinal | 1 study (Takeda 1998) | 0/11 | 1/11 | 0.33 (0.02,7.39) |
| Gastric distension | 7 studies (Rasanen 1985, Bersten 1991, Lin 1991, Takeda 1998, Park 2004, L'Her 2004, Frotin 2010) | 5/221 | 0/226 | 11.00 ( 0.64, 189.65) |
| Vomiting | 3 studies (Crane 2004, Park 2004, Gray 2008) | 7/381 | 8/404 | 0.93 (0.34, 2.54) |
| Asphyxia | 1 study (Bersten 1991) | 0/20 | 0/20 | inestimable |
| Pneumothorax | 5 studies (Bersten 1991, Kelly 2002, L'Her 2004, Gray 2008, Frotin 2010) | 0/483 | 0/515 | inestimable |
| Conjunctivitis | 2 studies (Kelly 2002, L'Her 2004) | 0/70 | 0/77 | inestimable |
| Sinusitis | 1 study (L'Her 2004) | 0/43 | 0/46 | inestimable |
| Mask discomfort | 3 studies (L'Her 2004, Park 2004, Frotin 2010) | 0/130 | 0/135 | inestimable |
| Hypotension | 1 study (Gray 2008) | 36/332 | 46/352 | 0.83 (0.55, 1.25) |
| Arrhythmia | 1 study (Gray 2008) | 12/332 | 23/350 | 0.55 (0.28, 1.09) |
| Progressive respiratory distress | 2 studies (L'Her 2004, Gray 2008) | 18/376 | 36/400 | 0.53 (0.31, 0.92)* |
| Neurological failure (coma) | 1 study (L'Her 2004) | 1/44 | 11/46 | 0,10 (0.01, 0,71)* |
| Cardiorespiratory arrest | 2 studies (L'Her 2004, Gray 2008) | 8/376 | 21/401 | 0.41 (0.18, 0.91)* |
CI ‐ confidence interval; CPAP ‐ continuous positive airway pressure; RR ‐ relative risk; SMC ‐ standard medical care; * statistically significant.
Eight studies reported adverse events with 144 events occurring in 368 bilevel NPPV plus SMC care patients and 136 events occurring in 384 SMC. The adverse events reported were: skin damage, pneumonia, gastrointestinal bleeding, gastric distension, vomiting, pneumothorax, eyes irritation, sinusitis, mask discomfort, claustrophobia, cardiac arrest, stroke, seizure, gastric aspiration, hypotension, arrhythmia and progressive respiratory distress. Participants in the SMC group had a lower rate of skin damage Details of the adverse events in people receiving bilevel NPPV plus SMC are provided in Table 5 and Analysis 14.4.
| Adverse Events | Studies | Bilevel NPPV Group | SMC Group | RR (95% CI) |
| Skin damage | 6 studies (Masip 2000, Thys 2002, Nava 2003, Crane 2004, Park 2004, Bautin 2005) | 16/148 | 0/148 | 7.16 (1.27, 40.50)* |
| Pneumonia | 2 study (Nava 2003, Bautin 2005) | 1/76 | 4/76 | 0.35 (0.05, 2.20) |
| Gastrointestinal | 2 studies (Nava 2003, Masip 2000) | 3/85 | 1/85 | 2.32 (0.35, 15.42) |
| Gastric distension | 2 studies (Thys 2002, Park 2004) | 8/32 | 0/32 | 15.87 (0.96, 262.30) |
| Vomiting | 5 studies (Masip 2000, Thys 2002, Crane 2004, Park 2004, Gray 2008) | 9/419 | 8/429 | 1.21 (0.47, 3.11) |
| Pneumothorax | 2 study (Nava 2003, Gray 2008) | 1/411 | 1/421 | 1.01 (0.11, 9.63) |
| Eye irritation | 1 study (Masip 2000) | 0/20 | 0/20 | inestimable |
| Sinusitis | 1 study (Nava 2003) | 1/65 | 0/65 | 3.00 (0.12, 72.31) |
| Mask discomfort | 5 studies (Masip 2000, Nava 2003, Park 2004, Bautin 2005, Weitz 2007) | 7/135 | 0/139 | 5.39 (0.97, 30.09) |
| Claustrophobia | 1 study (Nava 2003) | 1/65 | 0/65 | 3.00 (0.12, 72.31) |
| Cardiac arrest | 2 study (Nava 2003, Gray 2008) | 13/410 | 17/420 | 0.96 (0.25, 3.61) |
| Stroke | 1 study (Nava 2003) | 0/65 | 0/65 | inestimable |
| Seizure | 1 study (Nava 2003) | 0/65 | 1/65 | 0.33 (0.01, 8.03) |
| Pulmonary aspiration | 1 studies (Gray 2008) | 1/347 | 0/357 | 3.09 (0.13, 75.50) |
| Hypotension | 1 study (Gray 2008) | 37/346 | 46/352 | 0.82 (0.54, 1.23) |
| Arrhythmia | 1 study (Gray 2008) | 25/345 | 23/350 | 1.10 (0.64, 1.90) |
| Progressive respiratory distress | 1 study (Gray 2008) | 21/346 | 35/354 | 0.61 (0.36, 1.03) |
CI ‐ confidence interval; NPPV ‐ noninvasive positive pressure ventilation; RR ‐ relative risk; SMC ‐ standard medical care; * statistically significant.
Five studies reported adverse events with 156 events occurring in 389 bilevel NPPV plus SMC treated patients and 126 events occurring in 376 CPAP plus SMC treated patients. The adverse events reported were: skin damage, pneumothorax, pulmonary aspiration, gastric distension, vomiting, mask discomfort, increased breathing discomfort, hypotension, arrhythmia, progressive respiratory distress, cardiorespiratory arrest, pharyngeal damage and cough. Patients treated with CPAP had fewer arrhythmias. A detailed description of the complications can be found in Table 6 and Analysis 14.5.
| Adverse events | Sstudies | Bilevel NPPV group | CPAP group | RR (95% CI) |
| Skin damage | 5 studies (Mehta 1997, Martin‐Bermudez 2002, Crane 2004, Park 2004, Gray 2008) | 4/400 | 6/390 | 0.64 (0.19, 2.16) |
| Pulmonary aspiration | 3 studies (Mehta 1997, Martin‐Bermudez 2002, Gray 2008) | 1/407 | 0/389 | 2.88 (0.12, 70.43) |
| Gastric distension | 3 studies (Mehta 1997, Martin‐Bermudez 2002, Park 2004) | 8/89 | 5/83 | 1.49 (0.56, 4.00) |
| Vomiting | 4 studies (Martin‐Bermudez 2002, Crane 2004, Park 2004, Gray 2008) | 11/437 | 11/420 | 0.97 (0.43, 2.19) |
| Pneumothorax | 2 studies (Mehta 1997, Gray 2008) | 1/365 | 0/350 | 2.89 (0.12, 70.63) |
| Mask discomfort | 4 studies (Mehta 1997, Martin‐Bermudez 2002, Park 2004, Gray 2008) | 17/375 | 16/360 | 1.03 (0.53, 2.00) |
| Increased breathing discomfort | 1 study (Gray 2008) | 16/291 | 11/285 | 1.42 (0.67, 3.02) |
| Hypotension | 2 studies (Martin‐Bermudez 2002, Gray 2008) | 41/387 | 40/371 | 0.98 (0.65, 1.48) |
| Arrhythmia | 1 study (Gray 2008) | 25/345 | 12/332 | 2.00 (1.02, 3.92)* |
| Progressive respiratory distress | 1 study (Gray 2008) | 21/346 | 17/333 | 1.19 (0.64, 2.21) |
| Cardiorespiratory arrest | 1 study (Gray 2008) | 10/345 | 6/333 | 1.61 (0.59, 4.38) |
| Pharyngeal damage | 1 study (Martin‐Bermudez 2002) | 1/41 | 1/39 | 0.95 (0.06, 14.69) |
| Cough | 1 study (Martin‐Bermudez 2002) | 1/41 | 1/39 | 0.32 (0.01, 7.57) |
CI ‐ confidence interval; CPAP ‐ continuous positive airway pressure; NPPV ‐ noninvasive positive pressure ventilation; RR ‐ relative risk; SMC ‐ standard medical care; * statistically significant.
Discussion
The inclusion of eleven new studies in this review did not change significantly the results for the effectiveness of the use of NPPV compared with SMC, but it was possible to identify modest differences between CPAP and bilevel NPPV.
Summary of main results
Our meta‐analysis demonstrates the effectiveness of NPPV and CPAP compared to SMC in reducing hospital mortality. Our results support that one death can be avoided for every 14 ACPE patients treated with NPPV. Similarly, one death can be prevented for every nine ACPE patients treated with CPAP. This means that 69 lives out of 1000 could be saved with the addition of NPPV to SMC. The reduction in mortality was supported by studies comparing CPAP to SMC but not by studies comparing bilevel NPPV to SMC or CPAP to bilevel NPPV. The effect size of bilevel NPPV was notably similar to CPAP compared to SMC, but these results were more imprecise. Potential reasons for these discordant findings may include the relatively small number of patients experiencing events in these studies leading to low power; incorporating more recent studies that may have led to reduced mortality from SMC; or a true difference between CPAP and bilevel NPPV, which is corroborated by the findings from studies that directly compare CPAP and bilevel NPPV in patients with ACPE. However, the mega trial published by Gray 2008 analysed mortality 7 and 30 days after the NPPV initiation in patients with ACPE and did not identify any important differences between the NPPV groups compared to the SMC. Nonetheless, when we include these data in a meta‐analysis of our primary outcome (in‐hospital mortality), we find a persistent benefit of NPPV in reducing mortality in patients with ACPE. We do identify limitations in Gray 2008 that may contribute with findings divergences. First, Gray 2008 reported that it used ITT analyses but its analysis did not include all randomised patients (follow‐up bias). Second, the study did not report the total distribution of randomisation by group, which impeded the meta‐analysis of various outcomes and could be considered as a potential source of heterogeneity (Higgins 2011). Third, the reporting of continuous variables included only mean value of the difference between zero and one hour after the intervention and not as mean and standard deviation. However, it has not been possible to obtain these data for our meta‐analysis.
We also found that NPPV significantly reduced endotracheal intubation compared to SMC. Only eight patients need to be treated with NPPV to avoid 1 intubation. We noted similar results in studies comparing CPAP to SMC where the NNT was 7. Although NPPV has demonstrated to effectively reduce the intubation rate, it was not possible to assess if NPPV could postpone this outcome, as suggested in the study of Wood 1998. This randomised study reported significant delay in intubation and mechanical ventilation of acute respiratory distress syndrome (ARDS) patients treated with bilevel NPPV compared with SMC. The difference however was not found to be statistically significant (P=0.06), perhaps due to a low event rate (seven in the bilevel NPPV group and five in the SMC group). Likewise, the outcomes of mortality and intubation rate did not show statistically significant difference between the groups. In contrast to Confaloniere`s study of patients with pneumonia, although bilevel NPPV reduced the intubation rate, no difference in the time to intubate and initiate mechanical ventilation was observed (Confalonieri 1999).
An additional important finding of our review was that the incidence of acute myocardial infarction was not increased either during or after NPPV use. This is an important finding, as some studies (Mehta 1997; Rusterholtz 1999; Sharon 2000) had noted an increased incidence of acute myocardial infarction in patients treated with bilevel NPPV. To this end, few studies clearly distinguished between acute myocardial infarction at randomisation or evolving during or after NPPV initiation. Consistent with our results, Bellone and colleagues applied stringent criteria to detect acute myocardial infarction (Bellone 2004) and noted non‐significant differences in acute myocardial infarction among CPAP and bilevel NPPV treated patients. Recent RCTs (Ferrari 2007; Moritz 2007; Gray 2008) have also shown no significant differences on the incidence of acute myocardial infarction and other relevant outcomes when compared CPAP to bilevel NPPV.
NPPV also appears to reduce the risk of progression of respiratory failure and neurological failure (coma) compared to SMC. These results were mainly influenced by studies conducted with CPAP, which additionally demonstrated a reduction in cardiovascular arrest and adverse events risk in general when compared to SMC. NPPV is associated with increased risk for skin damage, but this adverse event is not generally considered major. This adverse event outcome was primarily influenced by Nava's studies, which may be related to the particular time and use of NPPV mask in that study. CPAP demonstrated a lower risk of developing arrhythmias when compared to using bilevel NPPV. It is possible that this reduced risk of adverse events with the use of CPAP could be related to lower positive pressure levels than those used in the bilevel NPPV, which may influence haemodynamics and/or O2 consumption. Despite similar effect sizes between CPAP and bilevel NPPV in terms of our primary outcome, these safety data are notable.
Our review did not reveal a significant difference between NPPV and SMC in terms of hospital length of stay. However, our review supports about one‐day reduction in ICU length of study with NPPV compared to SMC, and CPAP, in particular, compared to SMC. These differences were not supported in comparisons of bilevel NPPV to SMC and CPAP to bilevel NPPV. Some studies such as Holt 1994 have shown a reduction in hospital length of stay in ACPE patients using CPAP. We postulate that the reductions in ICU length of stay may be related to the reduction in intubation, as Wysocki 1995 related the smaller intubation rate to shorter hospital stay in a post‐hoc analysis. Although our review found NPPV and CPAP to be better tolerated than SMC, future studies should assess whether a trade‐off exists between treatment intolerance and other important outcomes such as mortality. In Crane 2004, while a large number of CPAP treated patients were intolerant of treatment, patients in this group also had lower mortality compared to bilevel NPPV and SMC.
NPPV was introduced to emergency departments in the early 1990s and became a relatively common place by approximately 2001 (Bersten 1991). In our review we noted similar reductions in mortality in patients treated with NPPV in the emergency department (RR 0.55, 95% CI 0.36 to 0.86; Analysis 5.2) and in the ICU (RR 0.48, 95% CI 0.28 to 0.83; Analysis 5.3). This finding supports initiation of NPPV in patients with ACPE in the emergency department. Additionally in our analysis, a cross‐over RCTof 124 patients with out‐of‐hospital diagnosis of ACPE and use of CPAP demonstrated faster improvements in dyspnoea, faster improvements in PaO2, lower intubation rates, lower use of dobutamine, and lower hospital mortality (Plaisance 2007). These results reinforce the positive pressure concept considered as non‐pharmacologic treatment and not a simple measure of support in ACPE cases.
We did find consistent effects of NPPV compared SMC and bilevel NPPV compared CPAP on PaO2 in patients with ACPE in first hour. However, it would be interesting if future studies analyse the existence of a direct relation among an early PaO2 improvements with outcomes such as mortality and need for intubation.
Some studies have postulated that patients with ACPE and hypercapnia (increased amount of carbon dioxide in the blood > 45 mmHg) may benefit to a greater extent from bilevel NPPV treatment (Hoffmann 1999; Rusterholtz 1999; Wysocki 1995; Wysocki 1999). In a post‐hoc analysis, we pooled studies including ACPE patients with hypercapnia at admission (mean values of PaCO2) and compared NPPV to SMC on the outcomes of mortality and endotracheal intubation. Compared to all population with ACPE patients, we found significant reductions in mortality (RR 0.60, 95% CI 0.40 to 0.88; Analysis 1.5 and ETI rate (RR 0.44, 95% CI 0.25 to 0.77; Analysis 2.3) favouring hypercapnic patients although the between‐group differences were not statistically significant. Similarly, we found favourable reductions in mortality and intubation in among patients treated with bilevel NPPV versus standard medical care (RR 0.59, 95% CI 0.34 to 1.02 and RR 0.47, 95% CI 0.22 to 0.97, respectively; Analysis 1.10 and Analysis 2.8) and no difference in mortality (RR 0.98, 95% CI 0.45 to 2.14; Analysis 1.13 ) or intubation (RR 1.17, 95% CI 0.58 to 2.33; Analysis 2.11) in hypercapnic patients compared with all population with ACPE patients treated with bilevel NPPV versus CPAP; although the between group differences are not statistically significant. These analysis are reinforced by the meta‐analysis studies by Agmy 2008, Moritz 2007; Nava 2003 and Masip 2000 who realised the mortality analysis and/or endotracheal intubation rate in the subgroup of hypercapnics patients and the results were more favourable to the use of bilevel compared to SMC in mortality and intubation (RR 0.20, 95% CI 0.04 to 0.86 and RR 0.28, 95% CI 0.12 to 0.69, respectively; Analysis 1.11 and Analysis 2.9), but no significant differences between CPAP and bilevel (RR 1.06, 95% CI 0.33 to 3.37 and RR 0.92, 95% CI 0.26 to 3.21, respectively; Analysis 1.14 and Analysis 2.12). Though these comparisons didn't report significant difference to the meta‐analysis results conducted in patients with ACPE in general. Our analyses have demonstrated that patients with ACPE and hypercapnia seem to benefit even more from the usage of NPPV, particularly from bilevel NPPV.
Once the benefits of NPPV have been demonstrated, cost‐benefit analyses, like the one conducted by Newberry et al. showing that NPPV cost less than mechanical ventilation (Newberry 1995), are important. In another study, the cost of respiratory services were lower with NPPV compared with SMC, but total cost with NPPV was higher than in the SMC group (Kramer 1995).
Overall completeness and applicability of evidence
In this review, we pooled the results of 32 RCTs (27 comparing NPPV and SMC and 14 comparing CPAP and bilevel NPPV) in patients with ACPE on important clinical outcomes, or eleven studies additional than the first version of this systematic review. This update maintains the results of the previous version where both NPPV and CPAP reduce hospital mortality, endotracheal intubation, ICU length of stay and respiratory rate compared to SMC. We did not observe the same beneficial effects on important clinical outcomes among studies comparing bilevel NPPV to SMC and CPAP to bilevel NPPV, though these results were similar in their effect size yet more imprecise. We also found that NPPV, CPAP and bilevel NPPV were better tolerated than SMC alone. Importantly, compared to SMC, use of NPPV, bilevel NPPV and CPAP in patients with ACPE did not increase the incidence of acute myocardial infarction during or after intervention. However this update identified a lower incidence of important adverse events in patients receiving NPPV plus SMC, as lower risk of progression of respiratory failure and coma. If we consider only CPAP, also a lower risk of cardiorespiratory arrest is also demonstrated. Among the studies that made the direct comparison between CPAP and bilevel NPPV a significantly lower incidence of arrhythmias with CPAP was observed. The only adverse event significantly unfavourable to the use of NPPV identified was skin damage. Our results demonstrate the effectiveness and safety of NPPV, especially CPAP, as adjuvant therapy to SMC in patients with ACPE.
Quality of the evidence
We considered the quality of the evidence to be moderate, although there may be some potential for publication bias. We defined seven relevant outcomes in order to assess the quality of evidence by GRADE (Guyatt 2008; Higgins 2011), and the primary outcome of mortality was considered to supported by quality evidence, where incidence of acute myocardial infarction during intervention and intolerance to the allocated treatment were considered to supported by only moderate quality of evidence. The remaining (endotracheal intubation rate and ICU length of stay, incidence of acute myocardial infarction after intervention and hospital length of stay) were unsupported by adequate evidence.
Potential biases in the review process
The major weakness of our review is the comparatively small number of events within trials comparing bilevel NPPV to SMC and CPAP to bilevel NPPV, thus limiting the inferences that can be made from them. It is important to note that SMC varied widely among the studies and included different drugs at different dosages. This disparity may have influenced treatment effect across studies. For example, Sharon 2000 used high doses of isosorbide dinitrate, which was related to better results in important outcomes such as acute myocardial infarction incidence and need for intubations when compared with low doses of isosorbide. Another potential source of bias is the variable inclusion criteria across studies. Disparities in baseline characteristics may have influenced patient response to treatment. A final source of bias may be the different thresholds or criteria used for reintubation in the included studies.
Strengths of this review include methods to reduce bias (multimodal search strategy without language restrictions, duplicate independent screening and data abstraction and prespecified criteria for appraising methodologic quality) with the quantitative differential of the Cochrane reviews (Jadad 2000; McKibbon 2004). To this end, we obtained additional information from 17 authors (Agmy 2008; Bautin 2005; Bersten 1991; Bellone 2005; Crane 2004; Delclaux 2000; Ferrari 2007; Gray 2008; Kelly 2002; Martin‐Bermudez 2002; Masip 2000; Nava 2003; Park 2004; Räsänen 1985; Takeda 1998; Thys 2002; Weitz 2007). As a result, our meta‐analysis includes the largest number of trials exploring the role of NPPV in ACPE and consequently had increased power in pooling outcomes (Potts 2009). We analysed the impact of NPPV on a broad set of physiological and clinical outcomes. Although there was heterogeneity in some analyses, it was possible to observe that the exclusion of low quality studies did not influence the results (Bersten 1991; Weitz 2007). The generalisability of our findings is enhanced by the diverse nature of the trials included in our review, representing international experience with NPPV use for ACPE.
Agreements and disagreements with other studies or reviews
The first systematic review on the role of NPPV for ACPE reported a lower intubation rate with CPAP Pang 1998. Hess 2004 subsequently suggested that bilevel NPPV may benefit patients with ACPE with hypercapnia. Nadar 2005 then found that bilevel NPPV decreased intubation, improved oxygenation, and was better tolerated compared to CPAP in patients with ACPE. However, because of the concern regarding the potential for acute myocardial infarction, bilevel NPPV could not be recommended for treatment of ACPE. Our review supports the use of NPPV, and especially CPAP, in the treatment of ACPE. These findings are important as current ACPE management guidelines do not recommend NPPV use in the absence of A level of evidence (Nieminen 2005). The results of our review could make this recommendation possible.
However, newer meta‐analyses have found results similar to ours (Collins 2006; Ho 2006; Masip 2005; Peter 2006;Weng 201O; Winck 2006), although with small differences in methodological and included studies, all conclude that NPPV is effective in reducing mortality in patients with ACPE. However, a mega‐trial conducted in patients with ACPE was published and concluded that NPPV does not reduce mortality compared to SMC (Gray 2008); however, it is possible to observe several important differences in this study that differ from the others included in systematic reviews and meta‐analysis as: mortality was evaluated after 7 or 30 days from the beginning of the intervention, while other studies have analysed mortality during the period of hospitalisation. Gray 2008 excluded patients who were more seriously ill. Approximately one in five patients in the SMC arm crossed over to NPPV within two hours of randomisation in Gray 2008, suggesting contamination that would attenuate the apparent effect of NPPV on mortality. Gray 2008 also had a 40% lower event rate than found in our meta‐analysis, which may be due to differences in patient characteristics, SMC, or other unmeasured differences that could influence the size of the treatment effect of NPPV. The quality of the studies that had evaluated the primary outcome mortality was high, as of can be visualised in summary of findings Table for the main comparison, which strengthens our inference of the benefit of NPPV in reducing mortality in patients with ACPE.

QUOROM statement flow diagram of 2005

Figure 4. PRISMA statement flow diagram of 2011

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Funnel plot of comparison: 1 Hospital Mortality, outcome: 1.1 NPPV (CPAP and BILEVEL) x SMC.

Comparison 1 Hospital mortality, Outcome 1 NPPV (CPAP and BILEVEL) x SMC.

Comparison 1 Hospital mortality, Outcome 2 NPPV (CPAP and BILEVEL) X SMC ‐ sensitivity analysis.
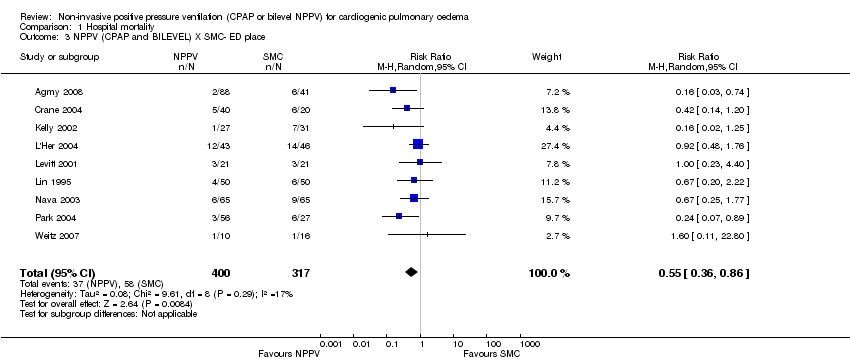
Comparison 1 Hospital mortality, Outcome 3 NPPV (CPAP and BILEVEL) X SMC‐ ED place.

Comparison 1 Hospital mortality, Outcome 4 NPPV (CPAP and BILEVEL) X SMC ‐ ICU place.

Comparison 1 Hospital mortality, Outcome 5 NPPV (CPAP and BILEVEL) X SMC ‐ in patients hypercanics ‐ baseline.

Comparison 1 Hospital mortality, Outcome 6 CPAP x SMC.

Comparison 1 Hospital mortality, Outcome 7 CPAP X SMC ‐ sensitivity analysis.

Comparison 1 Hospital mortality, Outcome 8 BILEVEL X SMC.

Comparison 1 Hospital mortality, Outcome 9 BILEVEL X SMC ‐ sensitivity analysis.

Comparison 1 Hospital mortality, Outcome 10 BILEVEL X SMC ‐ in patients hypercanics ‐ baseline.

Comparison 1 Hospital mortality, Outcome 11 BILEVEL X SMC ‐ in patients hypercanics.

Comparison 1 Hospital mortality, Outcome 12 CPAP X BILEVEL.

Comparison 1 Hospital mortality, Outcome 13 CPAP X BILEVEL ‐ in patients hypercanics ‐ baseline.

Comparison 1 Hospital mortality, Outcome 14 CPAP X BILEVEL ‐ in patients hypercanics.

Comparison 2 EETI rate, Outcome 1 NPPV (CPAP and BILEVEL) X SMC.

Comparison 2 EETI rate, Outcome 2 NPPV (CPAP and BILEVEL) X SMC ‐ sensitivity analysis.

Comparison 2 EETI rate, Outcome 3 NPPV (CPAP and BILEVEL) X SMC ‐ in patients hypercapnics ‐ baseline.

Comparison 2 EETI rate, Outcome 4 CPAP X SMC.

Comparison 2 EETI rate, Outcome 5 CPAP X SMC ‐ sensitivity analysis.

Comparison 2 EETI rate, Outcome 6 BILEVEL X SMC.

Comparison 2 EETI rate, Outcome 7 BILEVEL X SMC ‐ sensitivity analysis.

Comparison 2 EETI rate, Outcome 8 BILEVEL X SMC ‐ in patients hypercapnics ‐ baseline.

Comparison 2 EETI rate, Outcome 9 BILEVEL X SMC ‐ in patients hypercapnics.

Comparison 2 EETI rate, Outcome 10 CPAP X BILEVEL.

Comparison 2 EETI rate, Outcome 11 CPAP X BILEVEL ‐ in patients hypercapnics ‐ baseline.

Comparison 2 EETI rate, Outcome 12 CPAP X BILEVEL ‐ in patients hypercapnics.

Comparison 3 Incidence of acute myocardial infarction (during intervention), Outcome 1 NPPV (CPAP and BILEVEL) X SMC.

Comparison 3 Incidence of acute myocardial infarction (during intervention), Outcome 2 CPAP X SMC.

Comparison 3 Incidence of acute myocardial infarction (during intervention), Outcome 3 BILEVEL X SMC.

Comparison 3 Incidence of acute myocardial infarction (during intervention), Outcome 4 CPAP X BILEVEL.

Comparison 3 Incidence of acute myocardial infarction (during intervention), Outcome 5 BILEVEL X SMC ‐ heterogeneity analysis.

Comparison 4 Incidence of acute myocardial infarction (after intervention), Outcome 1 NPPV (CPAP and BILEVEL) X SMC.

Comparison 4 Incidence of acute myocardial infarction (after intervention), Outcome 2 CPAP X SMC.

Comparison 4 Incidence of acute myocardial infarction (after intervention), Outcome 3 BILEVEL X SMC.

Comparison 4 Incidence of acute myocardial infarction (after intervention), Outcome 4 CPAP X BILEVEL.

Comparison 5 Intolerance to allocated treatment, Outcome 1 NPPV (CPAP and BILEVEL) X SMC.

Comparison 5 Intolerance to allocated treatment, Outcome 2 NPPV (CPAP and BILEVEL) X SMC ‐ heterogeneity analysis.

Comparison 5 Intolerance to allocated treatment, Outcome 3 CPAP X SMC.

Comparison 5 Intolerance to allocated treatment, Outcome 4 BILEVEL X SMC.

Comparison 5 Intolerance to allocated treatment, Outcome 5 CPAP X BILEVEL.
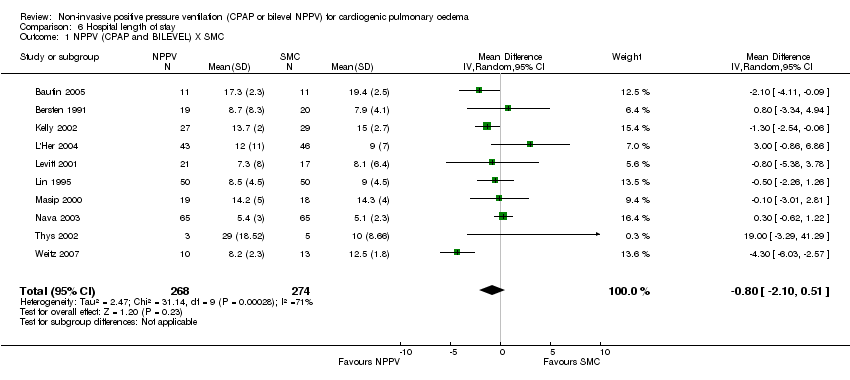
Comparison 6 Hospital length of stay, Outcome 1 NPPV (CPAP and BILEVEL) X SMC.

Comparison 6 Hospital length of stay, Outcome 2 NPPV (CPAP and BILEVEL) X SMC ‐ heterogeneity analysis.

Comparison 6 Hospital length of stay, Outcome 3 CPAP X SMC.

Comparison 6 Hospital length of stay, Outcome 4 BILEVEL X SMC.

Comparison 6 Hospital length of stay, Outcome 5 CPAP X BILEVEL.

Comparison 7 ICU length of stay, Outcome 1 NPPV (CPAP and BILEVEL) X SMC.

Comparison 7 ICU length of stay, Outcome 2 CPAP X SMC.

Comparison 7 ICU length of stay, Outcome 3 BILEVEL X SMC.

Comparison 7 ICU length of stay, Outcome 4 CPAP X BILEVEL.

Comparison 8 Breath rate after one hour, Outcome 1 NPPV (CPAP and BILEVEL) X SMC.

Comparison 8 Breath rate after one hour, Outcome 2 CPAP X SMC.

Comparison 8 Breath rate after one hour, Outcome 3 BILEVEL X SMC.

Comparison 8 Breath rate after one hour, Outcome 4 CPAP X BILEVEL.

Comparison 9 Heart rate after one hour, Outcome 1 NPPV (CPAP and BILEVEL) X SMC.
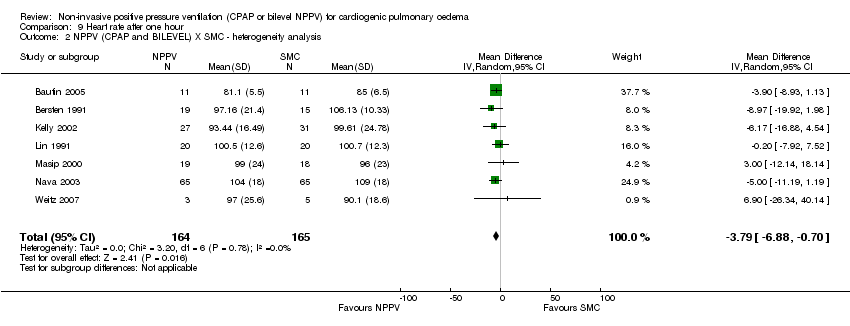
Comparison 9 Heart rate after one hour, Outcome 2 NPPV (CPAP and BILEVEL) X SMC ‐ heterogeneity analysis.

Comparison 9 Heart rate after one hour, Outcome 3 CPAP X SMC.

Comparison 9 Heart rate after one hour, Outcome 4 CPAP X SMC ‐ heterogeneity analysis.

Comparison 9 Heart rate after one hour, Outcome 5 BILEVEL X SMC.

Comparison 9 Heart rate after one hour, Outcome 6 CPAP X BILEVEL.

Comparison 10 Sistolic blood pressure after one hour, Outcome 1 NPPV (CPAP and BILEVEL) X SMC.

Comparison 10 Sistolic blood pressure after one hour, Outcome 2 CPAP X SMC.

Comparison 10 Sistolic blood pressure after one hour, Outcome 3 BILEVEL X SMC.

Comparison 10 Sistolic blood pressure after one hour, Outcome 4 CPAP X BILEVEL.

Comparison 10 Sistolic blood pressure after one hour, Outcome 5 CPAP X BILEVEL ‐ heterogeneity analysis.

Comparison 11 Diastolic blood pressure after one hour, Outcome 1 NPPV (CPAP and BILEVEL) X SMC.

Comparison 11 Diastolic blood pressure after one hour, Outcome 2 CPAP X SMC.

Comparison 11 Diastolic blood pressure after one hour, Outcome 3 CPAP X SMC ‐ heterogeneity analysis.

Comparison 11 Diastolic blood pressure after one hour, Outcome 4 BILEVEL X SMC.

Comparison 11 Diastolic blood pressure after one hour, Outcome 5 CPAP X BILEVEL.

Comparison 11 Diastolic blood pressure after one hour, Outcome 6 CPAP X BILEVEL ‐ heterogeneity analysis.

Comparison 12 Mean blood pressure after one hour, Outcome 1 NPPV (CPAP and BILEVEL) X SMC.

Comparison 12 Mean blood pressure after one hour, Outcome 2 CPAP X SMC.

Comparison 12 Mean blood pressure after one hour, Outcome 3 BILEVEL X SMC.

Comparison 12 Mean blood pressure after one hour, Outcome 4 CPAP X BILEVEL.

Comparison 13 PaO2 (mmHg) after one hour, Outcome 1 NPPV (CPAP and BILEVEL) X SMC.

Comparison 13 PaO2 (mmHg) after one hour, Outcome 2 CPAP X SMC.

Comparison 13 PaO2 (mmHg) after one hour, Outcome 3 CPAP X SMC ‐ heterogeneity analysis.

Comparison 13 PaO2 (mmHg) after one hour, Outcome 4 BILEVEL X SMC.

Comparison 13 PaO2 (mmHg) after one hour, Outcome 5 CPAP X BILEVEL.

Comparison 14 Adverse events, Outcome 1 NPPV (CPAP and BILEVEL) X SMC.

Comparison 14 Adverse events, Outcome 2 NPPV (CPAP and BILEVEL) X SMC ‐ AFTER 2000.

Comparison 14 Adverse events, Outcome 3 CPAP X SMC.

Comparison 14 Adverse events, Outcome 4 BILEVEL X SMC.

Comparison 14 Adverse events, Outcome 5 CPAP X BILEVEL.

Comparison 15 Hospital or 7‐day mortality, Outcome 1 NPPV (CPAP and BILEVEL) X SMC.

Comparison 16 Hospital or 30‐day mortality, Outcome 1 NPPV (CPAP and BILEVEL) X SMC.

Comparison 17 General or 7‐day ETI rate, Outcome 1 NPPV (CPAP and BILEVEL) X SMC.
| Non‐invasive positive pressure ventilation (CPAP and bilevel NPPV) for cardiogenic pulmonary oedema | ||||||
| Patient or population: patients with cardiogenic pulmonary oedema | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Non‐invasive positive pressure ventilation (CPAP and bilevel NPPV) | |||||
| Hospital mortalityY | Study population1 | RR 0.66 | 1107 | ⊕⊕⊕⊕ | ||
| 204 per 1000 | 135 per 1000 | |||||
| Moderate1 | ||||||
| 200 per 1000 | 132 per 1000 | |||||
| Endotracheal intubation rate | Study population | RR 0.52 | 1261 | ⊕⊕⊝⊝ | ||
| 249 per 1000 | 130 per 1000 | |||||
| Moderate | ||||||
| 300 per 1000 | 156 per 1000 | |||||
| Incidence of acute myocardial infarction (During intervention) | Study population | RR 1.24 | 461 | ⊕⊕⊕⊝ | ||
| 153 per 1000 | 190 per 1000 | |||||
| Moderate | ||||||
| 169 per 1000 | 210 per 1000 | |||||
| Incidence of acute myocardial infarction (After intervention) | Study population | RR 0.7 | 154 | ⊕⊝⊝⊝ | ||
| 26 per 1000 | 18 per 1000 | |||||
| Moderate | ||||||
| 13 per 1000 | 9 per 1000 | |||||
| Intolerance to allocated treatment | Study population | RR 0.47 | 1848 | ⊕⊕⊕⊝ | ||
| 234 per 1000 | 110 per 1000 | |||||
| Moderate | ||||||
| 350 per 1000 | 165 per 1000 | |||||
| Hospital length of stay | The mean hospital length of stay in the intervention groups was | 542 | ⊕⊝⊝⊝ | |||
| Intensive care unit length of stay (Copy) | The mean intensive care unit length of stay (copy) in the intervention groups was | 222 | ⊕⊕⊝⊝ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The most studies have mixed populations (with different levels of severity and co‐morbidities). | ||||||
| Study | IPAP level (cmH2O) | EPAP in bievel NPPV (cmH2O) | PEEP in CPAP (cmH2O) | Time of bilevel NPPV (hours) | Time of CPAP (hours) |
| 5.1 (SD 0.3) | 9.8 (SD 1.1) | ||||
| 5 | 10 | 1.6 (SD 0.6) | 1.7 (SD 0.7) | ||
| 5 | 10 | 3.41 (SD 1.1) | 3.6 (SD 1.3) | ||
| 10 | 9.3 (SD 4.9) | ||||
| Crane 2004 | 15 | 5 | 10 | ||
| Ferrari 2007 | 15 (SD 3.1) | 7 (SD 1.2) | 8.8 (SD 1.9) | 6.0 (SD 4.7) | 8.1 (SD 8.3) |
| Ferrari 2009 | 14 (SD 3.1) | 6.7 (SD 1.4) | 8.8 (SD 1.7) | 5.9 (SD 4.0) | 8.4 (SD 7.1) |
| Fontanella 2010 | 18 (SD 3) | 10 (SD 2) | 8 (SD 2) | ||
| Frontin 2010 | 10 | ||||
| Gray 2009 | 14 (SD 5) | 7 (SD 3) | 10 (SD 4) | 2.0 (SD 1.3) | 2.2 (SD 1.5) |
| Kelly 2002 | 7.5 | ||||
| L'Her 2004 | 7.5 | 8 (SD 6) | |||
| Liesching 2003 | 12 | 4 | 10 | ||
| Martin‐Bermudez 2002 | 1.3 (SD 0.8) | 1.8 (SD 1) | |||
| Masip 2000 | 15.2 (SD 2.4) | 5 | 4.2 (SD 1.5) | ||
| Mehta 1997 | 14.35 (SD 1.73) | 5 | 10.08 (SD 1.24) | 7.1 (SD 4.7) | 6.4 (SD 5.8) |
| Moritz 2007 | 12 (SD 3.2) | 4.9 (SD 0.9) | 7.7 (SD 2.1) | 2.8 | 2.3 |
| Nava 2003 | 14.5 (SD 21.1) | 6.1 (SD 3.2) | 11.4 (SD 3.6) | ||
| Park 2001 | 12 | 4 | 7.5 | 2.5 (SD 0.6) | 2.8 (SD 1.5) |
| Park 2004 | 17 (SD 2) | 11 (SD 2) | 11 (SD 2) | 2.0 (SD 1.0) | 1.7 (SD 0.6) |
| Rasanen 1985 | 10 | ||||
| Sharon 2000 | 9.3 (SD 2.3) | 4.2 (SD 3.1) | |||
| Takeda 1997 | 11.9 (SD 8.4) | ||||
| Thys 2002 | 16.5 (SD 3.3) | 6.1 (SD 1.5) | 1.2 (SD 0.2) | ||
| Weitz 2007 | 12.5 (SD 1.2) | 5 | |||
| CPAP ‐ continuous positive airway pressure; EPAP ‐ expiratory positive airway pressure; IPAP ‐ inspiratory positive airway pressure; PEEP ‐ positive expiratory end pressure; SD ‐ standard deviation; * statistically significant. | |||||
| Adverse events | Number of events (CPAP) | Total number CPAP | Number of event (bilevel NPPV) | Total number (bilevel NPPV) | RR (95% CI) |
| Skin damage | 2 | 472 | 17 | 458 | 0.06 (0.01, 0.43)* |
| Pneumonia | 0 | 40 | 1 | 76 | 0.63 (0.03, 15.03) |
| Pulmonary aspiration | 0 | 476 | 1 | 383 | 0.27 (0.01, 6.57) |
| Gastric distention | 5 | 178 | 8 | 51 | 0.18 (0.06, 0.52)* |
| GI bleeding | 0 | 11 | 3 | 85 | 1.02 (0.06, 18.63) |
| Vomitting | 7 | 381 | 9 | 419 | 0.86 (0.32, 2.27) |
| Pneumothorax | 0 | 440 | 1 | 430 | 0.33 (0.01, 7.97) |
| Asphyxia/claustrophobia | 0 | 20 | 1 | 65 | 1.05 (0.04, 24.76) |
| Mask discomfort | 15 | 364 | 22 | 440 | 0.82 (0.43, 1.57) |
| Sinusitis | 0 | 43 | 1 | 65 | 0.50 (0.02, 12.00) |
| Conjunctivitis | 0 | 70 | |||
| Eye irritation | 0 | 20 | |||
| Cardiac arrest | 6 | 333 | 13 | 410 | 0.57 (0.22, 1.48) |
| Stroke | 0 | 65 | |||
| Seizure | 0 | 65 | |||
| Hypotension | 36 | 332 | 37 | 346 | 1.01 (0.66, 1.56) |
| Arrhythmia requiring treatment | 12 | 332 | 25 | 345 | 0.50 (0.25, 0.98)* |
| Progressive respiratory distress | 17 | 333 | 21 | 346 | 0.84 (0.45, 1.57) |
| Increase breathing discomfort | 16 | 287 | 19 | 291 | 0.85 (0.45, 1.63) |
| CI ‐ confidence interval; CPAP ‐ continuous positive airway pressure; RR ‐ relative risk; SD ‐ standard deviation; * statistically significant.. | |||||
| Adverse events | Studies | NPPV group | SMC group | RR (95% CI) |
| Skin damage | 11 studies (Rasanen 1985, Bersten 1991, Takeda 1998, Masip 2000, Kelly 2002, Thys 2002, Nava 2003, Crane 2004, L'Her 2004, Park 2004, Bautin 2005) | 17/318 | 0/276 | 6.62 (1.20, 36.55)* |
| Pneumonia | 3 study (Lin 1991, Nava 2003, Bautin 2005) | 1/116 | 4/116 | 0.35 (0.05, 2.20) |
| Pulmonary aspiration | 5 studies (Rasanen 1985, Bersten 1991, Lin 1991, Kelly 2002, Gray 2008) | 1/787 | 0/468 | 1.58 (0.06, 38,61) |
| Gastrointestinal | 3 study (Takeda 1998, Masip 2000, Nava 2003) | 3/96 | 2/96 | 1,37 (0.27, 6.89) |
| Gastric distension | 8 studies (Rasanen 1985, Bersten 1991, Lin 1991, Takeda 1998, Thys 2002, Park 2004, L'Her 2004, Frotin 2010) | 13/253 | 0/231 | 13.26 ( 0.82, 215.12) |
| Vomiting | 5 studies (Masip 2000, Thys 2002, Crane 2004, Park 2004, Gray 2008) | 16/800 | 8/429 | 1.06 (0.46, 2.47) |
| Asphyxia | 2 study (Bersten 1991, Nava 2003) | 1/85 | 0/85 | 3.00 (0.12, 72.31) |
| Pneumothorax | 6 studies (Bersten 1991, Kelly 2002, Nava 2003, L'Her 2004, Gray 2008, Frotin 2010) | 1/894 | 1/580 | 0.72 (0.08, 6.89) |
| Conjunctivitis | 2 studies (Kelly 2002, L'Her 2004) | 0/70 | 0/77 | inestimable |
| Sinusitis | 2 study (Nava 2003, L'Her 2004) | 1/108 | 0/111 | 3.00 (0.12, 72.31) |
| Mask discomfort | 7 study (Masip 2000, Nava 2003, L'Her 2004, Park 2004, Bautin 2005, Weitz 2007, Frotin 2010) | 7/265 | 0/247 | 5.39 (0.97, 30.09) |
| Hypotension | 1 study (Gray 2008) | 73/678 | 46/352 | 0.82 (0.58, 1.16) |
| Arrhythmia | 1 study (Gray 2008) | 37/677 | 23/350 | 0.83 (0.50, 1.38) |
| Progressive respiratory distress | 2 studies (L'Her 2004, Gray 2008) | 39/722 | 36/400 | 0.58 (0.37, 0.89)* |
| Neurological failure (coma) | 1 study (L'Her 2004) | 1/44 | 11/46 | 0,10 (0.01, 0,71)* |
| Cardiorespiratory arrest | 3 studies (Nava 2003, L'Her 2004, Gray 2008) | 21/786 | 22/466 | 0.60 (0.29, 1.26) |
| Eye irritation | 1 study (Masip 2000) | 0/20 | 0/20 | inestimable |
| Stroke | 1 study (Nava 2003) | 0/65 | 0/65 | inestimable |
| Seizure | 1 study (Nava 2003) | 0/65 | 1/65 | 0.33 (0.01, 8.03) |
| CI ‐ confidence interval; NPPV ‐ noninvasive positive pressure ventilation; RR ‐ relative risk; SMC ‐ standard medical care; * statistically significant. | ||||
| Adverse Events | Studies | CPAP group | SMC group | RR (95% CI) |
| Skin damage | 7 studies (Rasanen 1985, Bersten 1991, Takeda 1998, Kelly 2002, Crane 2004, L'Her 2004, and Park 2004) | 1/168 | 0/175 | 3.00 (0.13, 69.52) |
| Pneumonia | 1 study (Lin 1991) | 0/40 | 0/40 | inestimable |
| Pulmonary aspiration | 5 studies (Rasanen 1985, Bersten 1991, Lin 1991, Kelly 2002, Gray 2008) | 0/440 | 0/468 | inestimable |
| Gastrointestinal | 1 study (Takeda 1998) | 0/11 | 1/11 | 0.33 (0.02,7.39) |
| Gastric distension | 7 studies (Rasanen 1985, Bersten 1991, Lin 1991, Takeda 1998, Park 2004, L'Her 2004, Frotin 2010) | 5/221 | 0/226 | 11.00 ( 0.64, 189.65) |
| Vomiting | 3 studies (Crane 2004, Park 2004, Gray 2008) | 7/381 | 8/404 | 0.93 (0.34, 2.54) |
| Asphyxia | 1 study (Bersten 1991) | 0/20 | 0/20 | inestimable |
| Pneumothorax | 5 studies (Bersten 1991, Kelly 2002, L'Her 2004, Gray 2008, Frotin 2010) | 0/483 | 0/515 | inestimable |
| Conjunctivitis | 2 studies (Kelly 2002, L'Her 2004) | 0/70 | 0/77 | inestimable |
| Sinusitis | 1 study (L'Her 2004) | 0/43 | 0/46 | inestimable |
| Mask discomfort | 3 studies (L'Her 2004, Park 2004, Frotin 2010) | 0/130 | 0/135 | inestimable |
| Hypotension | 1 study (Gray 2008) | 36/332 | 46/352 | 0.83 (0.55, 1.25) |
| Arrhythmia | 1 study (Gray 2008) | 12/332 | 23/350 | 0.55 (0.28, 1.09) |
| Progressive respiratory distress | 2 studies (L'Her 2004, Gray 2008) | 18/376 | 36/400 | 0.53 (0.31, 0.92)* |
| Neurological failure (coma) | 1 study (L'Her 2004) | 1/44 | 11/46 | 0,10 (0.01, 0,71)* |
| Cardiorespiratory arrest | 2 studies (L'Her 2004, Gray 2008) | 8/376 | 21/401 | 0.41 (0.18, 0.91)* |
| CI ‐ confidence interval; CPAP ‐ continuous positive airway pressure; RR ‐ relative risk; SMC ‐ standard medical care; * statistically significant. | ||||
| Adverse Events | Studies | Bilevel NPPV Group | SMC Group | RR (95% CI) |
| Skin damage | 6 studies (Masip 2000, Thys 2002, Nava 2003, Crane 2004, Park 2004, Bautin 2005) | 16/148 | 0/148 | 7.16 (1.27, 40.50)* |
| Pneumonia | 2 study (Nava 2003, Bautin 2005) | 1/76 | 4/76 | 0.35 (0.05, 2.20) |
| Gastrointestinal | 2 studies (Nava 2003, Masip 2000) | 3/85 | 1/85 | 2.32 (0.35, 15.42) |
| Gastric distension | 2 studies (Thys 2002, Park 2004) | 8/32 | 0/32 | 15.87 (0.96, 262.30) |
| Vomiting | 5 studies (Masip 2000, Thys 2002, Crane 2004, Park 2004, Gray 2008) | 9/419 | 8/429 | 1.21 (0.47, 3.11) |
| Pneumothorax | 2 study (Nava 2003, Gray 2008) | 1/411 | 1/421 | 1.01 (0.11, 9.63) |
| Eye irritation | 1 study (Masip 2000) | 0/20 | 0/20 | inestimable |
| Sinusitis | 1 study (Nava 2003) | 1/65 | 0/65 | 3.00 (0.12, 72.31) |
| Mask discomfort | 5 studies (Masip 2000, Nava 2003, Park 2004, Bautin 2005, Weitz 2007) | 7/135 | 0/139 | 5.39 (0.97, 30.09) |
| Claustrophobia | 1 study (Nava 2003) | 1/65 | 0/65 | 3.00 (0.12, 72.31) |
| Cardiac arrest | 2 study (Nava 2003, Gray 2008) | 13/410 | 17/420 | 0.96 (0.25, 3.61) |
| Stroke | 1 study (Nava 2003) | 0/65 | 0/65 | inestimable |
| Seizure | 1 study (Nava 2003) | 0/65 | 1/65 | 0.33 (0.01, 8.03) |
| Pulmonary aspiration | 1 studies (Gray 2008) | 1/347 | 0/357 | 3.09 (0.13, 75.50) |
| Hypotension | 1 study (Gray 2008) | 37/346 | 46/352 | 0.82 (0.54, 1.23) |
| Arrhythmia | 1 study (Gray 2008) | 25/345 | 23/350 | 1.10 (0.64, 1.90) |
| Progressive respiratory distress | 1 study (Gray 2008) | 21/346 | 35/354 | 0.61 (0.36, 1.03) |
| CI ‐ confidence interval; NPPV ‐ noninvasive positive pressure ventilation; RR ‐ relative risk; SMC ‐ standard medical care; * statistically significant. | ||||
| Adverse events | Sstudies | Bilevel NPPV group | CPAP group | RR (95% CI) |
| Skin damage | 5 studies (Mehta 1997, Martin‐Bermudez 2002, Crane 2004, Park 2004, Gray 2008) | 4/400 | 6/390 | 0.64 (0.19, 2.16) |
| Pulmonary aspiration | 3 studies (Mehta 1997, Martin‐Bermudez 2002, Gray 2008) | 1/407 | 0/389 | 2.88 (0.12, 70.43) |
| Gastric distension | 3 studies (Mehta 1997, Martin‐Bermudez 2002, Park 2004) | 8/89 | 5/83 | 1.49 (0.56, 4.00) |
| Vomiting | 4 studies (Martin‐Bermudez 2002, Crane 2004, Park 2004, Gray 2008) | 11/437 | 11/420 | 0.97 (0.43, 2.19) |
| Pneumothorax | 2 studies (Mehta 1997, Gray 2008) | 1/365 | 0/350 | 2.89 (0.12, 70.63) |
| Mask discomfort | 4 studies (Mehta 1997, Martin‐Bermudez 2002, Park 2004, Gray 2008) | 17/375 | 16/360 | 1.03 (0.53, 2.00) |
| Increased breathing discomfort | 1 study (Gray 2008) | 16/291 | 11/285 | 1.42 (0.67, 3.02) |
| Hypotension | 2 studies (Martin‐Bermudez 2002, Gray 2008) | 41/387 | 40/371 | 0.98 (0.65, 1.48) |
| Arrhythmia | 1 study (Gray 2008) | 25/345 | 12/332 | 2.00 (1.02, 3.92)* |
| Progressive respiratory distress | 1 study (Gray 2008) | 21/346 | 17/333 | 1.19 (0.64, 2.21) |
| Cardiorespiratory arrest | 1 study (Gray 2008) | 10/345 | 6/333 | 1.61 (0.59, 4.38) |
| Pharyngeal damage | 1 study (Martin‐Bermudez 2002) | 1/41 | 1/39 | 0.95 (0.06, 14.69) |
| Cough | 1 study (Martin‐Bermudez 2002) | 1/41 | 1/39 | 0.32 (0.01, 7.57) |
| CI ‐ confidence interval; CPAP ‐ continuous positive airway pressure; NPPV ‐ noninvasive positive pressure ventilation; RR ‐ relative risk; SMC ‐ standard medical care; * statistically significant. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 NPPV (CPAP and BILEVEL) x SMC Show forest plot | 20 | 1107 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.48, 0.89] |
| 2 NPPV (CPAP and BILEVEL) X SMC ‐ sensitivity analysis Show forest plot | 18 | 1023 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.46, 0.91] |
| 3 NPPV (CPAP and BILEVEL) X SMC‐ ED place Show forest plot | 9 | 717 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.36, 0.86] |
| 4 NPPV (CPAP and BILEVEL) X SMC ‐ ICU place Show forest plot | 7 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.28, 0.83] |
| 5 NPPV (CPAP and BILEVEL) X SMC ‐ in patients hypercanics ‐ baseline Show forest plot | 9 | 603 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.40, 0.88] |
| 6 CPAP x SMC Show forest plot | 13 | 699 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.39, 0.94] |
| 7 CPAP X SMC ‐ sensitivity analysis Show forest plot | 12 | 659 | Risk Difference (M‐H, Random, 95% CI) | ‐0.12 [‐0.19, ‐0.04] |
| 8 BILEVEL X SMC Show forest plot | 11 | 506 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.39, 1.09] |
| 9 BILEVEL X SMC ‐ sensitivity analysis Show forest plot | 9 | 458 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.37, 1.09] |
| 10 BILEVEL X SMC ‐ in patients hypercanics ‐ baseline Show forest plot | 7 | 401 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.34, 1.02] |
| 11 BILEVEL X SMC ‐ in patients hypercanics Show forest plot | 2 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.04, 0.86] |
| 12 CPAP X BILEVEL Show forest plot | 12 | 694 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.61, 1.97] |
| 13 CPAP X BILEVEL ‐ in patients hypercanics ‐ baseline Show forest plot | 9 | 518 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.45, 2.14] |
| 14 CPAP X BILEVEL ‐ in patients hypercanics Show forest plot | 2 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.33, 3.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 NPPV (CPAP and BILEVEL) X SMC Show forest plot | 22 | 1261 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.36, 0.75] |
| 2 NPPV (CPAP and BILEVEL) X SMC ‐ sensitivity analysis Show forest plot | 20 | 1195 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.37, 0.78] |
| 3 NPPV (CPAP and BILEVEL) X SMC ‐ in patients hypercapnics ‐ baseline Show forest plot | 9 | 621 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.25, 0.77] |
| 4 CPAP X SMC Show forest plot | 14 | 825 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.33, 0.67] |
| 5 CPAP X SMC ‐ sensitivity analysis Show forest plot | 13 | 785 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.34, 0.67] |
| 6 BILEVEL X SMC Show forest plot | 12 | 536 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.26, 1.17] |
| 7 BILEVEL X SMC ‐ sensitivity analysis Show forest plot | 10 | 470 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.26, 0.80] |
| 8 BILEVEL X SMC ‐ in patients hypercapnics ‐ baseline Show forest plot | 7 | 401 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.22, 0.97] |
| 9 BILEVEL X SMC ‐ in patients hypercapnics Show forest plot | 3 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.12, 0.69] |
| 10 CPAP X BILEVEL Show forest plot | 13 | 721 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.55, 1.97] |
| 11 CPAP X BILEVEL ‐ in patients hypercapnics ‐ baseline Show forest plot | 9 | 518 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.58, 2.33] |
| 12 CPAP X BILEVEL ‐ in patients hypercapnics Show forest plot | 2 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.26, 3.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 NPPV (CPAP and BILEVEL) X SMC Show forest plot | 8 | 461 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.79, 1.95] |
| 2 CPAP X SMC Show forest plot | 3 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.37, 2.24] |
| 3 BILEVEL X SMC Show forest plot | 7 | 356 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.78, 2.49] |
| 4 CPAP X BILEVEL Show forest plot | 7 | 409 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.39, 1.10] |
| 5 BILEVEL X SMC ‐ heterogeneity analysis Show forest plot | 6 | 316 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.69, 1.88] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 NPPV (CPAP and BILEVEL) X SMC Show forest plot | 4 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.11, 4.26] |
| 2 CPAP X SMC Show forest plot | 2 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.11, 10.23] |
| 3 BILEVEL X SMC Show forest plot | 3 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.02, 11.54] |
| 4 CPAP X BILEVEL Show forest plot | 2 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.57, 4.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 NPPV (CPAP and BILEVEL) X SMC Show forest plot | 13 | 1848 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.29, 0.77] |
| 2 NPPV (CPAP and BILEVEL) X SMC ‐ heterogeneity analysis Show forest plot | 12 | 692 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.30, 0.58] |
| 3 CPAP X SMC Show forest plot | 9 | 1304 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.36, 0.85] |
| 4 BILEVEL X SMC Show forest plot | 7 | 995 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.24, 1.42] |
| 5 CPAP X BILEVEL Show forest plot | 3 | 894 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.35, 2.53] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 NPPV (CPAP and BILEVEL) X SMC Show forest plot | 10 | 542 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐2.10, 0.51] |
| 2 NPPV (CPAP and BILEVEL) X SMC ‐ heterogeneity analysis Show forest plot | 9 | 519 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐1.35, 0.58] |
| 3 CPAP X SMC Show forest plot | 5 | 337 | Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.69, 0.67] |
| 4 BILEVEL X SMC Show forest plot | 7 | 311 | Mean Difference (IV, Random, 95% CI) | ‐1.38 [‐3.38, 0.62] |
| 5 CPAP X BILEVEL Show forest plot | 6 | 402 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐1.99, 1.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 NPPV (CPAP and BILEVEL) X SMC Show forest plot | 6 | 222 | Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.33, ‐0.45] |
| 2 CPAP X SMC Show forest plot | 3 | 169 | Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐1.63, ‐0.56] |
| 3 BILEVEL X SMC Show forest plot | 3 | 53 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.37, 0.06] |
| 4 CPAP X BILEVEL Show forest plot | 3 | 159 | Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.78, 1.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 NPPV (CPAP and BILEVEL) X SMC Show forest plot | 9 | 438 | Mean Difference (IV, Random, 95% CI) | ‐2.86 [‐3.85, ‐1.87] |
| 2 CPAP X SMC Show forest plot | 7 | 300 | Mean Difference (IV, Random, 95% CI) | ‐2.39 [‐3.70, ‐1.07] |
| 3 BILEVEL X SMC Show forest plot | 6 | 254 | Mean Difference (IV, Random, 95% CI) | ‐3.52 [‐4.80, ‐2.23] |
| 4 CPAP X BILEVEL Show forest plot | 5 | 218 | Mean Difference (IV, Random, 95% CI) | 0.57 [1.00, 2.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 NPPV (CPAP and BILEVEL) X SMC Show forest plot | 9 | 438 | Mean Difference (IV, Random, 95% CI) | ‐4.01 [‐8.16, 0.15] |
| 2 NPPV (CPAP and BILEVEL) X SMC ‐ heterogeneity analysis Show forest plot | 7 | 329 | Mean Difference (IV, Random, 95% CI) | ‐3.79 [‐6.88, ‐0.70] |
| 3 CPAP X SMC Show forest plot | 7 | 300 | Mean Difference (IV, Random, 95% CI) | ‐4.45 [‐10.81, 1.92] |
| 4 CPAP X SMC ‐ heterogeneity analysis Show forest plot | 5 | 191 | Mean Difference (IV, Random, 95% CI) | ‐4.10 [‐8.93, 0.72] |
| 5 BILEVEL X SMC Show forest plot | 6 | 254 | Mean Difference (IV, Random, 95% CI) | ‐4.21 [‐7.77, ‐0.65] |
| 6 CPAP X BILEVEL Show forest plot | 4 | 182 | Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐5.22, 4.11] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 NPPV (CPAP and BILEVEL) X SMC Show forest plot | 6 | 182 | Mean Difference (IV, Random, 95% CI) | ‐1.64 [‐7.83, 4.56] |
| 2 CPAP X SMC Show forest plot | 6 | 211 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐6.56, 6.81] |
| 3 BILEVEL X SMC Show forest plot | 4 | 87 | Mean Difference (IV, Random, 95% CI) | 1.93 [‐7.94, 11.80] |
| 4 CPAP X BILEVEL Show forest plot | 4 | 182 | Mean Difference (IV, Random, 95% CI) | ‐1.17 [‐10.79, 8.44] |
| 5 CPAP X BILEVEL ‐ heterogeneity analysis Show forest plot | 3 | 136 | Mean Difference (IV, Random, 95% CI) | 2.57 [‐4.30, 9.44] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 NPPV (CPAP and BILEVEL) X SMC Show forest plot | 5 | 138 | Mean Difference (IV, Random, 95% CI) | ‐1.49 [‐6.43, 3.45] |
| 2 CPAP X SMC Show forest plot | 5 | 167 | Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐9.10, 7.27] |
| 3 CPAP X SMC ‐ heterogeneity analysis Show forest plot | 3 | 107 | Mean Difference (IV, Random, 95% CI) | ‐7.32 [‐12.79, ‐1.86] |
| 4 BILEVEL X SMC Show forest plot | 4 | 87 | Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐6.09, 4.16] |
| 5 CPAP X BILEVEL Show forest plot | 4 | 182 | Mean Difference (IV, Random, 95% CI) | ‐2.60 [‐9.58, 4.37] |
| 6 CPAP X BILEVEL ‐ heterogeneity analysis Show forest plot | 2 | 62 | Mean Difference (IV, Random, 95% CI) | ‐7.86 [‐13.03, ‐2.70] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 NPPV (CPAP and BILEVEL) X SMC Show forest plot | 3 | 256 | Mean Difference (IV, Random, 95% CI) | ‐2.41 [‐8.27, 3.45] |
| 2 CPAP X SMC Show forest plot | 1 | 89 | Mean Difference (IV, Random, 95% CI) | 3.00 [‐5.31, 11.31] |
| 3 BILEVEL X SMC Show forest plot | 2 | 167 | Mean Difference (IV, Random, 95% CI) | ‐5.42 [‐11.60, 0.76] |
| 4 CPAP X BILEVEL Show forest plot | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐4.40 [‐13.25, 4.45] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 NPPV (CPAP and BILEVEL) X SMC Show forest plot | 4 | 140 | Mean Difference (IV, Random, 95% CI) | 10.04 [1.09, 19.00] |
| 2 CPAP X SMC Show forest plot | 5 | 177 | Mean Difference (IV, Random, 95% CI) | ‐2.64 [‐25.87, 20.59] |
| 3 CPAP X SMC ‐ heterogeneity analysis Show forest plot | 3 | 117 | Mean Difference (IV, Random, 95% CI) | ‐22.09 [‐34.35, ‐9.83] |
| 4 BILEVEL X SMC Show forest plot | 3 | 79 | Mean Difference (IV, Random, 95% CI) | 4.79 [‐11.11, 20.69] |
| 5 CPAP X BILEVEL Show forest plot | 3 | 136 | Mean Difference (IV, Random, 95% CI) | ‐27.00 [‐44.75, ‐9.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 NPPV (CPAP and BILEVEL) X SMC Show forest plot | 15 | 11329 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.63, 1.16] |
| 1.1 Skin damage | 11 | 594 | Risk Ratio (M‐H, Random, 95% CI) | 6.62 [1.20, 36.55] |
| 1.2 Pneumonia | 3 | 232 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.05, 2.20] |
| 1.3 Pulmonary aspiration | 5 | 1255 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.06, 38.61] |
| 1.4 Gastrointestinal Bleeding | 3 | 192 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.27, 6.89] |
| 1.5 Gastric distention | 8 | 484 | Risk Ratio (M‐H, Random, 95% CI) | 13.26 [0.82, 215.12] |
| 1.6 Vomiting | 5 | 1229 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.46, 2.47] |
| 1.7 Asphyxia | 2 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.31] |
| 1.8 Pneumothorax | 6 | 1474 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.08, 6.89] |
| 1.9 Conjunctivitis | 2 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.10 Sinusitis | 2 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.31] |
| 1.11 Disconfort with mask | 7 | 512 | Risk Ratio (M‐H, Random, 95% CI) | 5.39 [0.97, 30.09] |
| 1.12 Hypotension | 1 | 1030 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.58, 1.16] |
| 1.13 Arrhythmia | 1 | 1027 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.50, 1.38] |
| 1.14 Progressive respiratory distress | 2 | 1122 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.37, 0.89] |
| 1.15 Cardiorespiratory arrest | 3 | 1252 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.29, 1.26] |
| 1.16 Neurological failure (coma) | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 0.71] |
| 1.17 Eye irritation | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.18 Stroke | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.19 Seizures | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.03] |
| 2 NPPV (CPAP and BILEVEL) X SMC ‐ AFTER 2000 Show forest plot | 11 | 10703 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.63, 1.19] |
| 2.1 Skin damage | 8 | 492 | Risk Ratio (M‐H, Random, 95% CI) | 6.62 [1.20, 36.55] |
| 2.2 Pneumonia | 2 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.05, 2.20] |
| 2.3 Pulmonary aspiration | 2 | 1095 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.06, 38.61] |
| 2.4 Gastrointestinal Bleeding | 2 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 2.32 [0.35, 15.42] |
| 2.5 Gastric distention | 4 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 13.26 [0.82, 215.12] |
| 2.6 Vomiting | 5 | 1229 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.46, 2.47] |
| 2.7 Asphyxia | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.31] |
| 2.8 Pneumothorax | 5 | 1434 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.08, 6.89] |
| 2.9 Conjunctivitis | 2 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.10 Sinusitis | 2 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.31] |
| 2.11 Disconfort with mask | 7 | 512 | Risk Ratio (M‐H, Random, 95% CI) | 5.39 [0.97, 30.09] |
| 2.12 Hypotension | 1 | 1030 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.58, 1.16] |
| 2.13 Arrhythmia | 1 | 1027 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.50, 1.38] |
| 2.14 Progressive respiratory distress | 2 | 1122 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.37, 0.89] |
| 2.15 Cardiorespiratory arrest | 3 | 1252 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.29, 1.26] |
| 2.16 Neurological failure (coma) | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 0.71] |
| 2.17 Eye irritation | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.18 Stroke | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.19 Seizures | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.03] |
| 3 CPAP X SMC Show forest plot | 10 | 7133 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.45, 0.87] |
| 3.1 Skin damage | 7 | 343 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 69.52] |
| 3.2 Pneumonia | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Pulmonary aspiration | 5 | 908 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Gastrointestinal Bleeding | 1 | 22 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.02, 7.39] |
| 3.5 Gastric distention | 7 | 447 | Risk Ratio (M‐H, Random, 95% CI) | 11.00 [0.64, 189.65] |
| 3.6 Vomiting | 3 | 785 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.34, 2.54] |
| 3.7 Asphyxia | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.8 Pneumothorax | 5 | 998 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.9 Conjunctivitis | 2 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.10 Sinusitis | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.11 Disconfort with mask | 3 | 265 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.12 Hypotension | 1 | 684 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.55, 1.25] |
| 3.13 Arrhythmia | 1 | 682 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.28, 1.09] |
| 3.14 Progressive respiratory distress | 2 | 776 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.31, 0.92] |
| 3.15 Cardiorespiratory arrest | 2 | 777 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.18, 0.91] |
| 3.16 Neurological failure (coma) | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 0.71] |
| 4 BILEVEL X SMC Show forest plot | 8 | 6823 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.76, 1.46] |
| 4.1 Skin damage | 6 | 296 | Risk Ratio (M‐H, Random, 95% CI) | 7.16 [1.27, 40.50] |
| 4.2 Nosocomial pneumonia | 2 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.05, 2.20] |
| 4.3 Disconfort with mask | 5 | 274 | Risk Ratio (M‐H, Random, 95% CI) | 5.39 [0.97, 30.09] |
| 4.4 Gastrointestinal bleeding | 2 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 2.32 [0.35, 15.42] |
| 4.5 Gastric dilatation | 2 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 15.87 [0.96, 262.30] |
| 4.6 Vomiting | 5 | 848 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.47, 3.11] |
| 4.7 Claustrophobia | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.31] |
| 4.8 Pneumothorax | 2 | 832 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.11, 9.63] |
| 4.9 Eye irritation | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.10 Sinusitis | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.31] |
| 4.11 Cardiac arrest | 2 | 830 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.25, 3.61] |
| 4.12 Stroke | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.13 Seizures | 1 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.03] |
| 4.14 Gastric aspiration | 1 | 704 | Risk Ratio (M‐H, Random, 95% CI) | 3.09 [0.13, 75.50] |
| 4.15 Hypotension | 1 | 698 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.54, 1.23] |
| 4.16 Arrhythmia | 1 | 695 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.64, 1.90] |
| 4.17 Progressive respiratory distress | 1 | 700 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.36, 1.03] |
| 5 CPAP X BILEVEL Show forest plot | 5 | 7593 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.94, 1.48] |
| 5.1 Skin damage | 5 | 790 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.19, 2.16] |
| 5.2 Pneumothorax | 2 | 715 | Risk Ratio (M‐H, Random, 95% CI) | 2.89 [0.12, 70.63] |
| 5.3 Pulmonary aspiration | 3 | 796 | Risk Ratio (M‐H, Random, 95% CI) | 2.88 [0.12, 70.43] |
| 5.4 Gastric distension | 3 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.56, 4.00] |
| 5.5 Vomiting | 4 | 857 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.43, 2.19] |
| 5.6 Disconfort with mask | 4 | 735 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.53, 2.00] |
| 5.7 Increased breathing discomfort | 1 | 576 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.67, 3.02] |
| 5.8 Hypotension | 2 | 758 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.65, 1.48] |
| 5.9 Arrhythmia | 1 | 677 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [1.02, 3.92] |
| 5.10 Progressive respiratory distress | 1 | 679 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.64, 2.21] |
| 5.11 Cardiorespiratory arrest | 1 | 678 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.59, 4.38] |
| 5.12 Pharyngeal damage | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.06, 14.69] |
| 5.13 Cough | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.57] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 NPPV (CPAP and BILEVEL) X SMC Show forest plot | 21 | 2263 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.55, 0.94] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 NPPV (CPAP and BILEVEL) X SMC Show forest plot | 22 | 2387 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.60, 0.95] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 NPPV (CPAP and BILEVEL) X SMC Show forest plot | 23 | 2417 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.38, 0.78] |

