Intra‐articular corticosteroid for knee osteoarthritis
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial 3‐arm parallel‐group design Trial duration: 12 weeks | |
| Participants | 82 participants with knee osteoarthritis were randomised 73 participants were reported at baseline Number of females: 59 of 73 (81%) Mean age: 69.1 years | |
| Interventions | Experimental intervention 40 mg triamcinolone acetonide (1 ml) plus 20 mg bupivacaine (4 ml), single intra‐articular injection Control intervention 1 ml saline plus 20 mg bupivacaine (4 ml), single intra‐articular injection | |
| Outcomes | Extracted pain outcome: WOMAC pain Extracted function outcome: WOMAC function Maximum follow‐up: 12 weeks | |
| Notes | Funding: Boztepe State Hospital, Ordu, Republic of Turkey | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized by the closed‐envelope technique into three groups". Because the "closed‐envelope technique" was not further specified, the risk of selection bias was considered unclear |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomized by the closed‐envelope technique into three groups". Because the "closed‐envelope technique" was not further specified, the risk of selection bias was considered unclear |
| Blinding of participants? | Low risk | Quote: "Since the solutions were in different colors, sticker was used to cover injectors to hide to ensure blinding." |
| Blinding of health care provider(s) | Low risk | Quote: "Injections were administered by another blinded investigator." |
| Intention‐to‐treat analysis performed? Pain | High risk | 9 out of 82 participants were excluded because (quote) "they did not come for follow‐up" |
| Intention‐to‐treat analysis performed? Function | High risk | 9 out of 82 participants were excluded because (quote) "they did not come for follow‐up" |
| Methods | Randomised controlled trial 2‐arm parallel‐group design Trial duration: 24 weeks | |
| Participants | 104 participants with knee osteoarthritis were randomised 104 participants were reported at baseline Number of females: 79 out of 104 (76%) Mean age: 63.0 years | |
| Interventions | Experimental intervention 20 mg triamcinolone hexacetonide (1 ml) plus 6 ml hylan GF‐20, single intra‐articular injection Control intervention 6 ml hylan GF‐20 intra‐articularly, single intra‐articular injection Quote: "Patients with bilateral disease had both knees treated with the same drug, but only one knee (reported by the patient as the worst) was included in the study" | |
| Outcomes | Extracted pain outcome: WOMAC Pain Extracted function outcome: WOMAC Global Maximum follow‐up: 24 weeks | |
| Notes | Funding: São Paulo Research Foundation (FAPESP) (Sao Paulo, Brazil) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by a computer‐generated program (available at: http://www.randomization.com/)." |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Blinding of participants? | Low risk | Quote: "Patients were blinded (blocked from watching the procedures by the use of a windscreen sunshade and did not know to which group they were assigned)." |
| Blinding of health care provider(s) | Unclear risk | Physicians were not explicitly described as blinded, so the risk of performance bias was unclear |
| Intention‐to‐treat analysis performed? Pain | High risk | 5 of 52 participants excluded in experimental group, 6 of 52 participants excluded in control group |
| Intention‐to‐treat analysis performed? Function | High risk | 5 of 52 participants excluded in experimental group, 6 of 52 participants excluded in control group |
| Methods | Randomised controlled trial 5‐arm parallel‐group design Trial duration: 12.9 months | |
| Participants | 150 participants with knee osteoarthritis were randomised Unclear number of participants with knee osteoarthritis reported at baseline Number of females: 115 Mean age: 65.4 | |
| Interventions | Experimental intervention Triamcinolone acetonide (no dosage or unit specified) + joint lavage, single intra‐articular application Control intervention Joint lavage, single intra‐articular application | |
| Outcomes | Extracted pain outcome: WOMAC Pain Extracted function outcome: WOMAC Function Maximum follow‐up: 12.9 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Blinding of participants? | Unclear risk | It was unclear if method used to blind participants was appropriate |
| Blinding of health care provider(s) | Unclear risk | Physicians were not explicitly described as blinded, so the risk of performance bias was unclear |
| Intention‐to‐treat analysis performed? Pain | Low risk | All randomised participants included in the analysis |
| Intention‐to‐treat analysis performed? Function | Low risk | All randomised participants included in the analysis |
| Methods | Randomised controlled trial 2‐arm parallel‐group design Trial duration: 8 weeks | |
| Participants | 51 injections in 44 knees belonging to 44 participants with knee osteoarthritis were randomised Unclear number of participants reported at baseline Number of females: 41 of 44 (93.2%) Mean age: Not reported | |
| Interventions | Experimental intervention 50 mg prednisolone acetate (2 ml), single intra‐articular injection Control intervention 2 ml physiologic saline, single intra‐articular injection | |
| Outcomes | Extracted pain outcome: Patient global assessment | |
| Notes | Funding: Aktiebolaget Ferrosan, Malmö, Sweden | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The out‐patient department nurse decided which fluid was to be injected by tossing a coin" |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Blinding of participants? | Unclear risk | It was unclear if method used to blind participants was appropriate |
| Blinding of health care provider(s) | Unclear risk | Physicians were not explicitly described as blinded, so the risk of performance bias was unclear |
| Intention‐to‐treat analysis performed? Pain | Low risk | All randomised participants included in the analysis |
| Intention‐to‐treat analysis performed? Function | Unclear risk | Did not report extractable function outcome data |
| Methods | Randomised controlled trial 2‐arm parallel‐group design Trial duration: 12 weeks | |
| Participants | 79 participants with knee osteoarthritis were randomised 79 participants were reported at baseline Number of females: 2 of 79 (2.5%) Mean age: 64.3 years | |
| Interventions | Experimental intervention 40 mg triamcinolone acetonide (1 ml), single intra‐articular injection Control intervention 1 ml 0.9% saline, single intra‐articular injection | |
| Outcomes | Extracted pain outcome: WOMAC Pain Extracted function outcome: WOMAC Global Maximum follow‐up: 12 weeks | |
| Notes | Funding: National Skeletal Muscle Research Center, NIH Grant HD050837 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Blinding of participants? | Low risk | Quote: "Patients and assessors were blinded to treatment status" "Patients were then randomized to receive an injection of either (...) triamcinolone acetonide or (...) saline, which were drawn into a syringe covered with opaque tape prior to the patient encounter." |
| Blinding of health care provider(s) | High risk | Quote: "Injections were given (...) by a non‐blinded physician" |
| Intention‐to‐treat analysis performed? Pain | High risk | 9 of 40 participants excluded in experimental group, 9 of 39 participants excluded in control group |
| Intention‐to‐treat analysis performed? Function | High risk | 9 of 40 participants excluded in experimental group, 9 of 39 participants excluded in control group |
| Methods | Randomised controlled trial 3‐arm parallel‐group design Trial duration: 4 weeks | |
| Participants | 60 participants with knee osteoarthritis were randomised 60 participants were reported at baseline Mean age: 70.6 | |
| Interventions | Experimental interventions 40 mg methylprednisolone acetate and lidocaine hydrochloride, single intra‐articular injection + Horizontal therapy* locally (10 times over 2 weeks, each lasting 30 minutes) Control intervention Horizontal therapy* locally (10 times over 2 weeks, each lasting 30 minutes) Treatment duration: 4 weeks *Horizontal therapy was described as (quote): "Placement of 4 cutaneous electrodal pads (8 x 13 cm), one in center of the popliteal, one on the patella and two others at the posterior proximal site of the thighs, with a stimulation frequency oscillating at 100 Hz between 4400 and 12346 Hz for 30 minutes" Maximum follow‐up: 4 weeks | |
| Outcomes | Extracted pain outcome: Pain overall Extracted function outcome: WOMAC Function Maximum follow‐up: 4 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "(...) using a computer generated 1:1:1 allocation sequence." |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Blinding of participants? | High risk | No intra‐articular sham injection in the placebo group (local therapy only) |
| Blinding of health care provider(s) | High risk | No intra‐articular sham injection in the placebo group (local therapy only) |
| Intention‐to‐treat analysis performed? Pain | Low risk | All randomised participants included in the analysis |
| Intention‐to‐treat analysis performed? Function | Low risk | All randomised participants included in the analysis |
| Methods | Randomised controlled trial 2‐arm cross‐over design Trial duration: 2 weeks | |
| Participants | 24 knees belonging to 16 participants with knee osteoarthritis were randomised 24 knees belonging to 16 participants were reported at baseline Mean age: 65 Number of females: 13 out of 16 (81%) | |
| Interventions | Experimental intervention 20 mg triamcinalone hexacetonide (1 ml), single intra‐articular injection Control intervention 1 ml of saline, single intra‐articular injection Cross‐over after 1 week. Every participant received 1 injection (experimental and control) each | |
| Outcomes | Extracted pain outcome: Pain overall Maximum follow‐up: 1 week | |
| Notes | 2 trials were reported in the same paper. Trial A did not report pain outcomes seperately for treatment and intervention and was excluded. Trial B was included in the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Blinding of participants? | High risk | Quote: Described as "single‐blind, blind‐observer", implying that participants were not blinded |
| Blinding of health care provider(s) | High risk | Quote: Described as "single‐blind, blind‐observer", implying that healthcare providers were not blinded |
| Intention‐to‐treat analysis performed? Pain | Low risk | All randomised participants included in the analysis |
| Intention‐to‐treat analysis performed? Function | Unclear risk | Not applicable, no function outcome reported |
| Methods | Randomised controlled trial 2‐arm parallel‐group design Trial duration: 8 weeks | |
| Participants | 34 participants with knee osteoarthritis were randomised 34 participants were reported at baseline Number of females: Not reported Mean age: 60.0 years | |
| Interventions | Experimental intervention 20 mg triamcinolone hexacetonide, single intra‐articular injection Control intervention "Polysorbate, sorbitol solution, benzyl alcohol and water", single intra‐articular injection | |
| Outcomes | Extracted pain outcome: Pain overall Maximum follow‐up: 8 weeks | |
| Notes | Funding: Grant from the Eastern Pennsylvania Chapter of the Arthritis Foundation and by the Philadelphia Foundation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence of allocation was not clearly reported, so the risk of selection bias was unclear. Quote: "Half of the patients, selected according to a predetermined random schedule, were treated (...)." |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Blinding of participants? | Low risk | Quote: "During the time of [the injection] (...), the physician and patient were positioned so that neither could see the nurse's face nor the material she injected. Thus, neither had any direct information concerning what was injected and, practically speaking, had no contact with the only person who knew" |
| Blinding of health care provider(s) | Low risk | Quote: "The physician‐experimenter performed the arthrocentesis (...) a nurse‐assistant entered the room and performed the injection through the intraarticular needle, and left the room. During the time of this taking place, the physician and patient were positioned so that neither could see the nurse's face nor the material she injected. Thus, neither had any direct information concerning what was injected and, practically speaking, had no contact with the only person who knew" |
| Intention‐to‐treat analysis performed? Pain | Low risk | All randomised participants included in the analysis. Quote: "All patients were seen 1 wk, 4 wk, 6 wk and 8 wk post‐injection except those whose pain scores at any subsequent evaluation were the same as their pre‐treatment scores; they were not seen further. It was assumed that their scores would no longer improve and they were counted as remaining at their pre‐treatment level throughout the experiment". |
| Intention‐to‐treat analysis performed? Function | Unclear risk | Not applicable, no function outcome reported |
| Methods | Randomised controlled trial 2‐arm parallel‐group design Trial duration: 12 weeks | |
| Participants | 299 knees belonging to 205 participants with knee osteoarthritis were randomised 299 knees belonging to 205 participants were reported at baseline Number of females: 234 (78%) of 299 knees belonged to female participants Mean age: 67.0 years | |
| Interventions | Experimental intervention 40 mg triamcinolone acetonide plus lavage (3 L of cold (8°C) saline), single intra‐articular application Control intervention Lavage (3 L of cold (8°C) saline), single intra‐articular application | |
| Outcomes | Extracted pain outcome: Pain overall Maximum follow‐up: 12 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Blinding of participants? | Unclear risk | Although the authors stated "Glucocorticoid treatment with triamcinolone acetonide was always given on a blind basis", they also stated that this was an open trial (Quote: "The study was of the longitudinal, open, prospective, controlled type").The risk of performance bias was therefore considered unclear |
| Blinding of health care provider(s) | Unclear risk | Although the authors stated "Glucocorticoid treatment with triamcinolone acetonide was always given on a blind basis", they also stated that this was an open trial (Quote: "The study was of the longitudinal, open, prospective, controlled type"). The risk of performance bias was therefore considered unclear |
| Intention‐to‐treat analysis performed? Pain | High risk | 82 of 299 knees were excluded at 1 month, 51 of 299 knees were excluded at 3 months |
| Intention‐to‐treat analysis performed? Function | Unclear risk | Not applicable, no function outcome reported |
| Methods | Randomised controlled trial 2‐arm parallel‐group design Trial duration: 6 weeks | |
| Participants | 84 participants with knee osteoarthritis were randomised 84 participants were reported at baseline Number of females: 60 out of 84 (71%) Mean age: 67.0 years | |
| Interventions | Experimental intervention 20 mg triamcinolone hexacetonide (1 ml), single intra‐articular injection Control intervention 1 ml of 0.9% normal saline, single intra‐articular injection | |
| Outcomes | Extracted pain outcome: Pain overall Extracted function outcome: Other function composite Maximum follow‐up: 6 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Blinding of participants? | Low risk | Quote: "Although this study was not, by strict definition, double‐blinded, we attempted to ensure that patients were not aware of the treatment allocated to them, by shielding the identity of the treatment received from their view at the time of injection; only the injecting physician (IL) was aware of the nature of the injection administered." |
| Blinding of health care provider(s) | High risk | Quote: "Although this study was not, by strict definition, double‐blinded, we attempted to ensure that patients were not aware of the treatment allocated to them, by shielding the identity of the treatment received from their view at the time of injection; only the injecting physician (IL) was aware of the nature of the injection administered." |
| Intention‐to‐treat analysis performed? Pain | Unclear risk | 2 of 42 participants in control group withdrew. It was unclear whether all participants randomised were also analysed |
| Intention‐to‐treat analysis performed? Function | Unclear risk | 2 of 42 participants in control group withdrew. It was unclear whether all participants randomised were also analysed |
| Methods | Randomised controlled trial 2‐arm cross‐over design Trial duration: 8.6 weeks | |
| Participants | 40 participants with knee osteoarthritis were randomised 40 participants were reported at baseline Number of females: 27 out of 40 (67.5%) Mean age: 42.3 years | |
| Interventions | Experimental intervention 0.4 mg dexamethasonephosphate plus 20 mg sodium hyaluronate in 2 ml phosphate buffer, 5 intra‐articular injections, 1 weekly for 5 weeks Control intervention 20 mg sodium hyaluronate in 2 ml phosphate buffer, 5 intra‐articular injections, 1 weekly for 5 weeks | |
| Outcomes | Extracted pain outcome: Pain on activities other than walking Maximum follow‐up: 8.6 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Blinding of participants? | High risk | Quote: "The trial design was open and randomized." |
| Blinding of health care provider(s) | High risk | Quote: "The trial design was open and randomized." |
| Intention‐to‐treat analysis performed? Pain | Low risk | All randomised participants included in the analysis |
| Intention‐to‐treat analysis performed? Function | Unclear risk | Not applicable, no function outcome reported |
| Methods | Randomised controlled trial 2‐arm parallel‐group design Trial duration: 26 weeks | |
| Participants | 100 participants with knee osteoarthritis were randomised 100 participants were reported at baseline Number of females: 61 out of 100 (61%) Mean age: 63.4 years | |
| Interventions | Experimental intervention 40 mg methylprednisolone acetate (1 ml) dissolved in 4 ml of lidocaine hydrochloride, single intra‐articular injection + 12‐week exercise program Control intervention 1 ml isotonic saline mixed with 4 ml of lidocaine hydrochloride, single intra‐articular injection + 12‐week exercise program | |
| Outcomes | Extracted pain outcome: Other pain composite Extracted pain function: Other function composite Maximum follow‐up: 26 weeks | |
| Notes | Funding: Grants by: 10‐093704 from the Danish Council for Independent Research Medical Science, Oak Foundation, Association of Danish Physiotherapists, Lundbeck Foundation, Capital Region of Denmark | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated randomization sequence was produced before any patients were enrolled that allocated participants in permuted blocks of 2 to 6 to the corticosteroid or the placebo group (1:1)." |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization sequence was prepared by a biostatistician with no clinical involvement in the trial (R.C.). The allocation was concealed in a password‐protected computer file only accessible by the biostatistician. Individual allocations were held in sealed, opaque, consecutively numbered envelopes." |
| Blinding of participants? | Low risk | Quote: "To ensure blinding of the participants and the clinician performing the injections, the syringes were prepared by the study nurse in the absence of participants and blinded study staff. Because the corticosteroid liquid is milky white and the saline is clear, the syringes were masked with opaque tape to prevent disclosure of the content during the injection procedure." |
| Blinding of health care provider(s) | Low risk | Quote: "To ensure blinding of the participants and the clinician performing the injections, the syringes were prepared by the study nurse in the absence of participants and blinded study staff. Because the corticosteroid liquid is milky white and the saline is clear, the syringes were masked with opaque tape to prevent disclosure of the content during the injection procedure." |
| Intention‐to‐treat analysis performed? Pain | Low risk | All randomised participants included in the analysis |
| Intention‐to‐treat analysis performed? Function | Low risk | All randomised participants included in the analysis |
| Methods | Randomised controlled trial 2‐arm cross‐over design Trial duration: 16 weeks | |
| Participants | 59 participants with knee osteoarthritis were randomised 59 participants were reported at baseline Number of females: 37 out of 59 (63%) Mean age: 70.6 years | |
| Interventions | Experimental intervention 40 mg methyl prednisolone acetate (1 ml), single intra‐articular injection Control intervention 1 ml 0.9% saline, single intra‐articular injection Cross‐over after 8 weeks. Every participant received 1 injection (experimental and control) each | |
| Outcomes | Extracted pain outcome: Pain on activities other than walking Maximum follow‐up: 8 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Blinding of participants? | Unclear risk | Quote: "Each injection was given by a second operator, thus blinding both patient and assessor." No further description of blinding |
| Blinding of health care provider(s) | Unclear risk | Quote: "Each injection was given by a second operator, thus blinding both patient and assessor." No further description of blinding |
| Intention‐to‐treat analysis performed? Pain | High risk | Quotes: "As some data was missing due to patient withdrawal, all analyses were performed on a last measures carried forward, intention to treat basis", but still not all participants randomised were analysed. Quote: "One patient failed to enter the study and received no injection, leaving 59 patients available for the analysis." |
| Intention‐to‐treat analysis performed? Function | Unclear risk | Not applicable, no function outcome reported |
| Methods | Randomised controlled trial 2‐arm parallel‐group design Trial duration: 8.6 weeks | |
| Participants | 20 participants with knee osteoarthritis were randomised Unclear number of participants with knee osteoarthritis reported at baseline Number of females: 11 Mean age: 59.7 | |
| Interventions | Experimental intervention 80 mg methylprednisolone (2 ml) + 5 ml 1% lignocaine, single intra‐articular injection Control intervention 10 ml of 1% lignocaine, single intra‐articular injection | |
| Outcomes | Extracted pain outcome: Pain overall Extracted function outcome: Global disability score Maximum follow‐up: 8.6 weeks | |
| Notes | Funding: West London Research Network, Primary Care Scientist Award funded by the Department of Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Blinding of participants? | Unclear risk | It was unclear if method used to blind healthcare providers was appropriate |
| Blinding of health care provider(s) | High risk | Quote: "(The study) was single blind, with the principal investigator administering the treatment and also measuring outcome." |
| Intention‐to‐treat analysis performed? Pain | Low risk | All randomised participants included in the analysis |
| Intention‐to‐treat analysis performed? Function | Low risk | All randomised participants included in the analysis |
| Methods | Randomised controlled trial 5‐arm parallel‐group design Trial duration: 33.8 weeks | |
| Participants | 202 participants with knee osteoarthritis were randomised Unclear number of participants reported at baseline Number of females: 122 Mean age: not reported | |
| Interventions | Experimental intervention 50 mg of hydrocortisone (2 ml) + 8 ml of physiological normal saline, 5 intra‐articular injections, interval of 2 weeks Control intervention Physiological normal saline solution (no dosage), 5 intra‐articular injections, interval of 2 weeks | |
| Outcomes | Extracted pain outcome: Patients' global assessment Maximum follow‐up: 25.8 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Blinding of participants? | Unclear risk | It was unclear if method used to blind participants was appropriate |
| Blinding of health care provider(s) | Unclear risk | Physicians were not explicitly described as blinded, so the risk of performance bias was unclear |
| Intention‐to‐treat analysis performed? Pain | High risk | 21 of 202 participants were excluded |
| Intention‐to‐treat analysis performed? Function | Unclear risk | Not applicable, no function outcome reported |
| Methods | Randomised controlled trial 2‐arm parallel‐group design Trial duration: 12 weeks | |
| Participants | 79 participants with knee osteoarthritis were randomised 79 participants were reported at baseline Number of females: 3 out of 79 (4%) Mean age: 63.0 years | |
| Interventions | Experimental intervention 40 mg triamcinolone acetonide, single intra‐articular injection Control intervention 0.9% saline (no dosage), single intra‐articular injection | |
| Outcomes | Extracted pain outcome: WOMAC Pain Maximum follow‐up: 12 weeks | |
| Notes | Funding: University of California, San Diego | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Blinding of participants? | Unclear risk | It was unclear if method used to blind participants was appropriate |
| Blinding of health care provider(s) | Unclear risk | It was unclear if method used to blind healthcare providers was appropriate |
| Intention‐to‐treat analysis performed? Pain | High risk | 7 of 40 participants excluded in experimental group, 5 of 39 participants excluded in control group |
| Intention‐to‐treat analysis performed? Function | Unclear risk | Not applicable, no function outcome reported |
| Methods | Randomised controlled trial 2‐arm parallel‐group design Trial duration: 52 weeks | |
| Participants | 47 participants with knee osteoarthritis were randomised 40 participants were reported at baseline Number of females: 39 out of 47 (83%) Mean age: 58.0 years | |
| Interventions | Experimental intervention 40 mg triamcinolone acetonide (1 ml) plus 2 ml sodium hyaluronate. Sodium hyaluronate was administered in 3 intra‐articular injections in the first month and 3 intra‐articular injections during the sixth month, triamcinolone acid was added prior to the first and fourth application. Control intervention 2 ml sodium hyaluronate, 3 intra‐articular injections in the first month, and 3 intra‐articular injections during the sixth month | |
| Outcomes | Extracted pain outcome: WOMAC Pain Maximum follow‐up: 25.9 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned to one of the two treatment groups based on a table of randomly assorted digits: A and B." |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Blinding of participants? | Unclear risk | It was unclear if participants were blinded (trial described as "single blind" but no description of who was blinded) |
| Blinding of health care provider(s) | Unclear risk | It was unclear if healthcare providers were blinded (trial described as "single blind" but no description of who was blinded) |
| Intention‐to‐treat analysis performed? Pain | High risk | 7 of 23 participants excluded in experimental group, 0 of 24 participants excluded in control group |
| Intention‐to‐treat analysis performed? Function | Unclear risk | Not applicable, no function outcome reported |
| Methods | Randomised controlled trial 2‐arm parallel‐group design Trial duration: 26 weeks | |
| Participants | 98 participants with knee osteoarthritis were randomised 98 participants were reported at baseline Number of females: 56 out of 98 (57%) Mean age: 59.7 years | |
| Interventions | Experimental intervention 10 mg triamcinolone acetonide + hyaluronan solution (no dosage stated), 6 ml total, single intra‐articular injection Control intervention Hyaluronan solution (no dosage stated), single intra‐articular injection | |
| Outcomes | Extracted pain outcome: WOMAC Pain Extracted function outcome: WOMAC Function Maximum follow‐up: 26 weeks | |
| Notes | Funding: Carbylan Therapeutics | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization treatment was computer generated and was stratified by study center." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The randomization treatment was computer generated and was stratified by study center." |
| Blinding of participants? | Unclear risk | It was unclear if method used to blind participants was appropriate |
| Blinding of health care provider(s) | High risk | Quote: "An injecting physician delivered the randomized treatment and remained unblinded." |
| Intention‐to‐treat analysis performed? Pain | High risk | 2 of 33 participants excluded in experimental group, 1 of 33 participants excluded in control group |
| Intention‐to‐treat analysis performed? Function | High risk | 2 of 33 participants excluded in experimental group, 1 of 33 participants excluded in control group |
| Methods | Randomised controlled trial 5‐arm parallel‐group design Trial duration: 2.7 weeks | |
| Participants | 48 participants with knee osteoarthritis were randomised Unclear number of participants with knee osteoarthritis reported at baseline Number of females: 38 Mean age: 55 years | |
| Interventions | Experimental interventions Intervention (A): 40 mg triamcinolone, 3 intra‐articular injections, interval 1 week Intervention (B): 50 mg hydrocortisone, 3 intra‐articular injections, interval 1 week Control intervention Saline solution (no dosage stated), 2 intra‐articular injections, interval 1 week | |
| Outcomes | Extracted pain outcome: (A)‐(B): other algofunctional Extracted function outcome: (A)‐(B): other algofunctional Maximum follow‐up: 0.7 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Blinding of participants? | Unclear risk | It was unclear if method used to blind participants was appropriate |
| Blinding of health care provider(s) | Unclear risk | It was unclear if method used to blind healthcare providers was appropriate |
| Intention‐to‐treat analysis performed? Pain | Unclear risk | It was unclear whether all participants randomised were also analysed |
| Intention‐to‐treat analysis performed? Function | Unclear risk | It was unclear whether all participants randomised were also analysed |
| Methods | Randomised controlled trial 2 x 2 factorial design Trial duration: 24 weeks | |
| Participants | 98 participants with knee osteoarthritis were randomised 98 participants were reported at baseline Number of females: 66 out of 98 (67%) Mean age: 65.4 | |
| Interventions | Experimental interventions Intervention (A): 3.75 mg cortivazol (1.5 ml), single intra‐articular injection Intervention (B): Lavage, single intra‐articular application + 3.75 mg cortivazol (1.5 ml), single intra‐articular injection Control intervention Intervention (A): 1.5 ml 0.9% normal saline, single intra‐articular injection Intervention (B): Lavage, single intra‐articular application | |
| Outcomes | Extracted pain outcome: Pain overall Extracted function outcome: Lequesne index Maximum follow‐up: 24 weeks | |
| Notes | Funding: Société Française de Rhumatologie and the Direction de la Recherche Clinique (Assistance Publique ‐ Hôpitaux de Paris) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Blinding of participants? | Unclear risk | Quote: "The study was double‐blind in relation to the IA corticosteroid and open with regard to joint lavage." |
| Blinding of health care provider(s) | Unclear risk | Quote: "The study was double‐blind in relation to the IA corticosteroid and open with regard to joint lavage. However, the procedure (joint lavage and/or IA injection) was performed by a physician other than the blinded evaluator." |
| Intention‐to‐treat analysis performed? Pain | Low risk | All randomised participants included in the analysis. Quote: "The last observation–carried‐forward procedure was used to adjust for missing values." |
| Intention‐to‐treat analysis performed? Function | Low risk | All randomised participants included in the analysis. Quote: "The last observation–carried‐forward procedure was used to adjust for missing values." |
| Methods | Randomised controlled trial 2‐arm parallel‐group design Trial duration: 54 weeks | |
| Participants | 68 participants with knee osteoarthritis were randomised 68 participants were reported at baseline Number of females: 42 out of 68 (68%) Mean age: 63.2 years | |
| Interventions | Experimental intervention 40 mg triamcinolone acetonide (1 ml), 8 intra‐articular injections, interval 3 months, over 21 months Control intervention 1 ml saline intra‐articularly, 8 intra‐articular injections, interval 3 months, over 21 months | |
| Outcomes | Extracted pain outcome: WOMAC Pain. After end of treatment (during follow‐up) Extracted function outcome: WOMAC Function. After end of treatment (during follow‐up) Maximum follow‐up: 12.9 weeks | |
| Notes | Funding: Fonds de la recherche en santé du Québec | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to the IA steroid or IA saline group based on a table of randomly assorted digits." |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Blinding of participants? | Unclear risk | Study described as double‐blind but no description of method of blinding provided |
| Blinding of health care provider(s) | High risk | Study described as double‐blind. The following statements indicate that "double‐blind" in this trial means that only patients and outcome assessors were blinded: "In order to preserve the blind, the injections were given by a rheumatologist (DC or BH) other than the evaluators." "Investigators performed these evaluations in a blinded manner using validated measures." |
| Intention‐to‐treat analysis performed? Pain | High risk | 1 of 34 participants excluded in experimental group, 1 of 34 participants excluded in control group |
| Intention‐to‐treat analysis performed? Function | High risk | 1 of 34 participants excluded in experimental group, 1 of 34 participants excluded in control group |
| Methods | Randomised controlled trial 3‐arm parallel‐group design Trial duration: 8 weeks | |
| Participants | 16 participants with knee osteoarthritis were randomised Unclear number of participants with knee osteoarthritis reported at baseline Number of females: not reported Mean age: not reported | |
| Interventions | Experimental intervention 80 mg methylprednisolone, single intra‐articular injection Control intervention Saline (no dosage specified), single intra‐articular injection | |
| Outcomes | Extracted pain outcome: WOMAC Global Maximum follow‐up: 8 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Blinding of participants? | Unclear risk | It was unclear if method used to blind participants was appropriate |
| Blinding of health care provider(s) | Unclear risk | Physicians were not explicitly described as blinded, so the risk of performance bias was unclear |
| Intention‐to‐treat analysis performed? Pain | Unclear risk | It was unclear whether all participants randomised were also analysed |
| Intention‐to‐treat analysis performed? Function | Unclear risk | Not applicable, no function outcome reported |
| Methods | Randomised controlled trial 2‐arm parallel‐group design Trial duration: 24 weeks | |
| Participants | 77 participants with knee osteoarthritis were randomised 71 participants were reported at baseline Number of females: 27 out of 77 (35%) Mean age: 66.8 years | |
| Interventions | Experimental intervention 120 mg methylprednisolone acetate following joint lavage, single intra‐articular injection Control intervention Treatment duration: 1 day Normal saline (no dosage) following joint lavage, single intra‐articular injection | |
| Outcomes | Extracted pain outcome: WOMAC Pain Extracted function outcome: WOMAC Function Maximum follow‐up: 24 weeks | |
| Notes | Funding: National Health and Medical Research Council (Australia) Arthritis Foundation of Australia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was computer‐generated by a member of the hospital pharmacy department, who also prepared a blinded intra‐articular injection" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was computer‐generated by a member of the hospital pharmacy department, who also prepared a blinded intra‐articular injection" |
| Blinding of participants? | Unclear risk | It was unclear if method used to blind participants was appropriate |
| Blinding of health care provider(s) | Unclear risk | It was unclear if method used to blind healthcare providers was appropriate |
| Intention‐to‐treat analysis performed? Pain | High risk | Quote: "In the event of relapse as defined above, the last documented outcome variables were carried forward". Still, 6 participants were excluded (those needing surgical intervention because of the arthroscopic findings at baseline) |
| Intention‐to‐treat analysis performed? Function | High risk | Quote: "In the event of relapse as defined above, the last documented outcome variables were carried forward". Still, 6 participants were excluded (those needing surgical intervention because of the arthroscopic findings at baseline) |
| Methods | Randomised controlled trial 3‐arm parallel‐group design Trial duration: 20 weeks | |
| Participants | 38 knees belonging to 25 participants with knee osteoarthritis were randomised Unclear number of participants with knee osteoarthritis reported at baseline Number of females: not stated Mean age: not stated | |
| Interventions | Experimental intervention Intervention (A): 25 mg hydrocortisone acetate (1 ml), 4 intra‐articular injections, interval 2 weeks over 6 weeks Intervention (B): 25 mg hydrocortisone tertiary‐butylacetate (1 ml), 4 intra‐articular injections, interval 2 weeks over 6 weeks Control intervention 1 ml of placebo, 4 intra‐articular injections, interval 2 weeks over 6 weeks Cross‐over design, every participant received 3 x 4 injections | |
| Outcomes | Only information on adverse events was extracted | |
| Notes | There was no extractable data on pain or function | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The order of courses in each patient was randomized from a master sheet in which names were entered consecutively." |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Blinding of participants? | Unclear risk | It was unclear if method used to blind participants was appropriate |
| Blinding of health care provider(s) | Unclear risk | Physicians were not explicitly described as blinded, so the risk of performance bias was unclear |
| Intention‐to‐treat analysis performed? Pain | Unclear risk | Did not report extractable pain outcome data |
| Intention‐to‐treat analysis performed? Function | Unclear risk | Did not report extractable function outcome data excluded in control group |
| Methods | Randomised controlled trial 4‐arm parallel‐group design Trial duration: 12 weeks | |
| Participants | 120 participants with knee osteoarthritis were randomised 120 participants were reported at baseline Number of females: 76 out of 120 (63%) Mean age: 60.0 years | |
| Interventions | Experimental intervention Intervention (A): 40 mg triamsinolon acetonate (1 ml), single intra‐articular injection Intervention (B): 3 mg betametazone disodium phosphate (1 ml), single intra‐articular injection Intervention (C): 40 mg methylprednisolone acetate (1 ml), single intra‐articular injection Control intervention 1 ml 0.9% sodium chloride, single intra‐articular injection | |
| Outcomes | Extracted pain outcome (A)‐(C): Pain overall Extracted function outcome (A)‐(C): Lequesne index Maximum follow‐up: 12 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "A total of 120 eligible patients with knee osteoarthritis were included (according to their admission date) and randomized into four groups." |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Blinding of participants? | Unclear risk | It was unclear if participants were blinded |
| Blinding of health care provider(s) | Unclear risk | Physicians were not explicitly described as blinded, so the risk of performance bias was unclear |
| Intention‐to‐treat analysis performed? Pain | Unclear risk | It was unclear whether all participants randomised were also analysed |
| Intention‐to‐treat analysis performed? Function | Unclear risk | It was unclear whether all participants randomised were also analysed |
| Methods | Randomised controlled trial 2‐arm parallel‐group design Trial duration: 4.3 weeks | |
| Participants | 41 knees belonging to 40 participants with knee osteoarthritis were randomised Unclear number of participants with knee osteoarthritis reported at baseline Number of females: 16 Mean age: 66.5 years | |
| Interventions | Experimental intervention 120 mg methylprednisolone acetate, single intra‐articular injection Control intervention Normal saline (no dosage stated), single intra‐articular injection | |
| Outcomes | Extracted pain outcome: WOMAC Global Extracted function outcome: Other function composite Maximum follow‐up: 4.3 weeks | |
| Notes | Funding: National Health and Medical Research Council, The Clive and Vera Ramaciotti Trust, The Rebecca L. Cooper Foundation, University of New South Wales, The Arthritis Foundation of Australia, The Royal Australasian College of Physicians | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal the random sequence of allocation was not reported, so the risk of selection bias was unclear |
| Blinding of participants? | Unclear risk | It was unclear if method used to blind participants was appropriate |
| Blinding of health care provider(s) | Unclear risk | Physicians were not explicitly described as blinded, so the risk of performance bias was unclear |
| Intention‐to‐treat analysis performed? Pain | Unclear risk | It was unclear whether all participants randomised were also analysed |
| Intention‐to‐treat analysis performed? Function | Unclear risk | It was unclear whether all participants randomised were also analysed |
| Methods | Randomised controlled trial 4‐arm parallel‐group design Trial duration: 12 weeks | |
| Participants | 209 knees belonging to 112 participants were randomised Unclear number of participants with knee osteoarthritis reported at baseline Number of females: not stated Mean age: not stated | |
| Interventions | Experimental intervention 20 mg triamcinolone acetonid plus 10 ml 0.5% procaine, single intra‐articular injection Control intervention 10 ml 0.5% procaine, single intra‐articular injection | |
| Outcomes | Extracted pain outcome: WOMAC Pain Maximum follow‐up: 12 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "joints were randomized by envelopes to one of 4 treatments" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "joints were randomized by envelopes to one of 4 treatments" |
| Blinding of participants? | Unclear risk | It was unclear if method used to blind participants was appropriate |
| Blinding of health care provider(s) | Unclear risk | Physicians were not explicitly described as blinded, so the risk of performance bias was unclear |
| Intention‐to‐treat analysis performed? Pain | Unclear risk | It was unclear whether all participants randomised were also analysed |
| Intention‐to‐treat analysis performed? Function | Unclear risk | Not applicable, no function outcome reported |
IA: intra‐articular
WOMAC: Western Ontario and McMaster Universities Arthritis Index
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Recent systematic review | |
| Wrong study design | |
| Wrong study design | |
| Recent systematic review | |
| Wrong study design | |
| Recent systematic review | |
| Active comparator | |
| Wrong study design: Abstract to relevant systematic review, no references listed | |
| Wrong study design: Abstract to relevant systematic review, no references listed | |
| Recent systematic review | |
| Wrong outcomes | |
| Recent systematic review | |
| Recent systematic review | |
| Wrong study design | |
| Recent systematic review | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong study population | |
| Wrong study design | |
| Recent systematic review | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design: Abstract to relevant systematic review | |
| Reason for exclusion | |
| Wrong study design | |
| Recent systematic review | |
| Wrong study design | |
| Wrong study design | |
| Reason for exclusion | |
| Reason for exclusion | |
| Wrong comparator | |
| Active comparator | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Active comparator | |
| Postsurgical setting | |
| Postsurgical setting | |
| Postsurgical setting | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design: Abstract to relevant systematic review | |
| Recent systematic review | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Duplicate reference | |
| Wrong study design | |
| Wrong intervention | |
| Postsurgical setting | |
| Postsurgical setting | |
| Postsurgical setting | |
| Wrong comparator | |
| Active comparator | |
| Wrong study design | |
| Wrong study design | |
| Wrong comparator | |
| Wrong study population | |
| Active comparator | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design: Abstract to relevant systematic review | |
| Wrong study design: Abstract to relevant systematic review | |
| Wrong study design | |
| Wrong study design: Abstract to relevant systematic review | |
| Wrong study design: Abstract to relevant systematic review | |
| Postsurgical setting | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Active comparator |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised controlled trial 2‐arm parallel‐group design Trial duration: 12 weeks |
| Participants | 16 participants with knee osteoarthritis were randomised |
| Interventions | Experimental intervention 3‐month exercise program plus 40 mg triamcinolone mixed with 4 ml 1% lidocaine, single intra‐articular injection Control intervention 3‐month exercise program plus 1 ml normal saline mixed with 4 ml 1% lidocaine, single intra‐articular injection |
| Outcomes | Maximum follow‐up: 12 weeks Outcome data (KOOS pain and function, WOMAC pain and function) not extractable |
| Notes |
| Methods | Unclear |
| Participants | Unclear |
| Interventions | Unclear |
| Outcomes | Outcome data not extractable |
| Notes |
| Methods | Randomised controlled trial 2‐arm parallel‐group design |
| Participants | 25 participants with knee osteoarthritis were randomised |
| Interventions | Experimental intervention 40 mg methylprednisolone acetate, single intra‐articular injection Control intervention saline, single intra‐articular injection Cross‐over design: Every participant received 1 injection each |
| Outcomes | Maximum follow‐up: 1 week Outcome data (WOMAC pain, pain overall, ICOAP questionnaire, ultrasound examination) not extractable |
| Notes |
| Methods | Randomised controlled trial 2‐arm parallel‐group design |
| Participants | 25 participants with knee osteoarthritis were randomised |
| Interventions | Experimental intervention 40 mg methylprednisolone acetate, single intra‐articular injection Control intervention saline, single intra‐articular injection Cross‐over design: Every participant received 1 injection each |
| Outcomes | Maximum follow‐up: 1 week Outcome data (WOMAC pain, pain overall, ICOAP questionnaire, ultrasound examination) not extractable |
| Notes |
| Methods | Measurement reliability study on participants later taking part in a randomised controlled trial for intra‐articular corticosteroid injection in knee osteoarthritis |
| Participants | 15 participants with knee osteoarthritis |
| Interventions | Unclear Data for the study was collected before the intra‐articular injection |
| Outcomes | Outcome data not extractable |
| Notes |
| Methods | Measurement reliability study on participants later taking part in a randomised controlled trial for intra‐articular corticosteroid injection in knee osteoarthritis |
| Participants | 15 participants with knee osteoarthritis |
| Interventions | Unclear Data for the study was collected before the intra‐articular injection |
| Outcomes | Outcome data not extractable |
| Notes |
| Methods | Open‐label clinical trial |
| Participants | 100 participants with knee osteoarthritis |
| Interventions | Experimental intervention Corticosteroid, single intra‐articular injection, type and dosage of corticosteroid unclear. The study analysed the changes in MRI scans before and after the intra‐articular corticosteroid injection. All participants received the experimental intervention, there was no control group. |
| Outcomes | Outcome data not extractable |
| Notes |
| Methods | Randomised controlled trial 2‐arm parallel‐group design |
| Participants | 80 participants with knee osteoarthritis were randomised |
| Interventions | Experimental intervention 40 mg triamcinolone hexacetonide, 8 intra‐articular injections, 3 months interval Control intervention Placebo, 8 intra‐articular injections, 3 months interval |
| Outcomes | Outcome data (pain overall, WOMAC) not extractable |
| Notes |
| Methods | Randomised controlled trial 2‐arm parallel‐group design |
| Participants | 104 participants with knee osteoarthritis were randomised |
| Interventions | Experimental intervention 20 mg of hexacetonide triamcinolone plus 6 ml of hylan GF‐20, single intra‐articular injection Control intervention 6 ml of hylan GF‐20, single intra‐articular injection |
| Outcomes | Maximum follow‐up: 24 weeks Outcome data (VAS, WOMAC, and Lequesne) not extractable |
| Notes |
| Methods | Unclear |
| Participants | Unclear |
| Interventions | Unclear |
| Outcomes | Outcome data not extractable |
| Notes |
ICOAP: Intermittent and Constant Osteoarthritis Pain
KOOS: Knee Injury and Osteoarthritis Outcome Score
MRI: magnetic resonance imaging
VAS: visual analogue scale
WOMAC: Western Ontario and McMaster Universities Arthritis Index
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
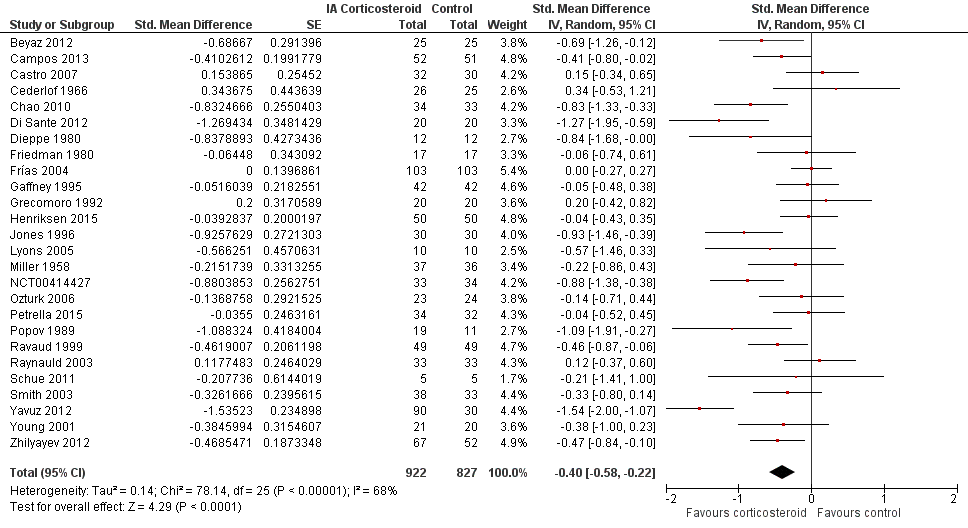
| 1 Pain ‐ Main Show forest plot | 26 | 1749 | Std. Mean Difference (Random, 95% CI) | ‐0.40 [‐0.58, ‐0.22] |
| Analysis 1.1 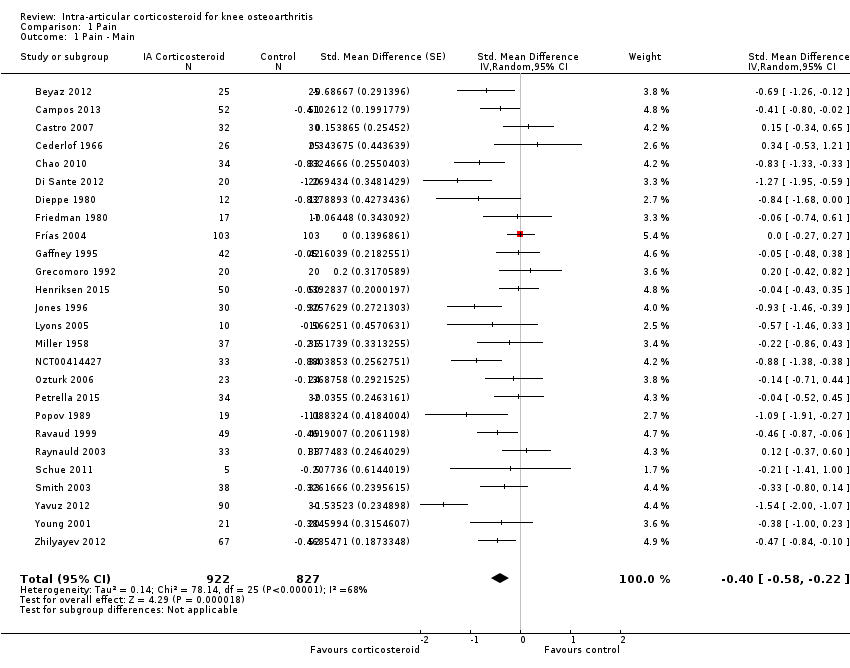 Comparison 1 Pain, Outcome 1 Pain ‐ Main. | ||||
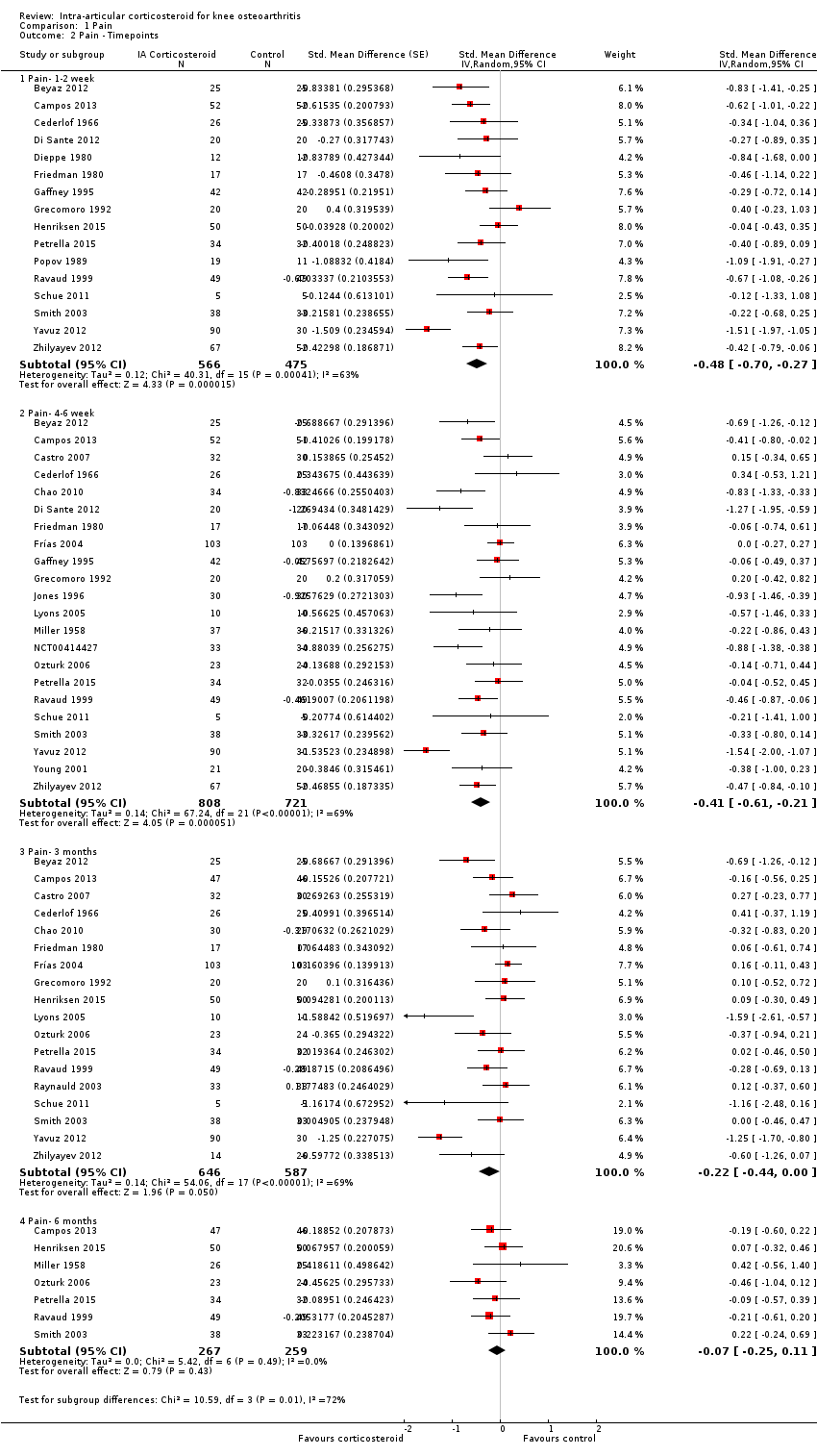
| 2 Pain ‐ Timepoints Show forest plot | 26 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| Analysis 1.2 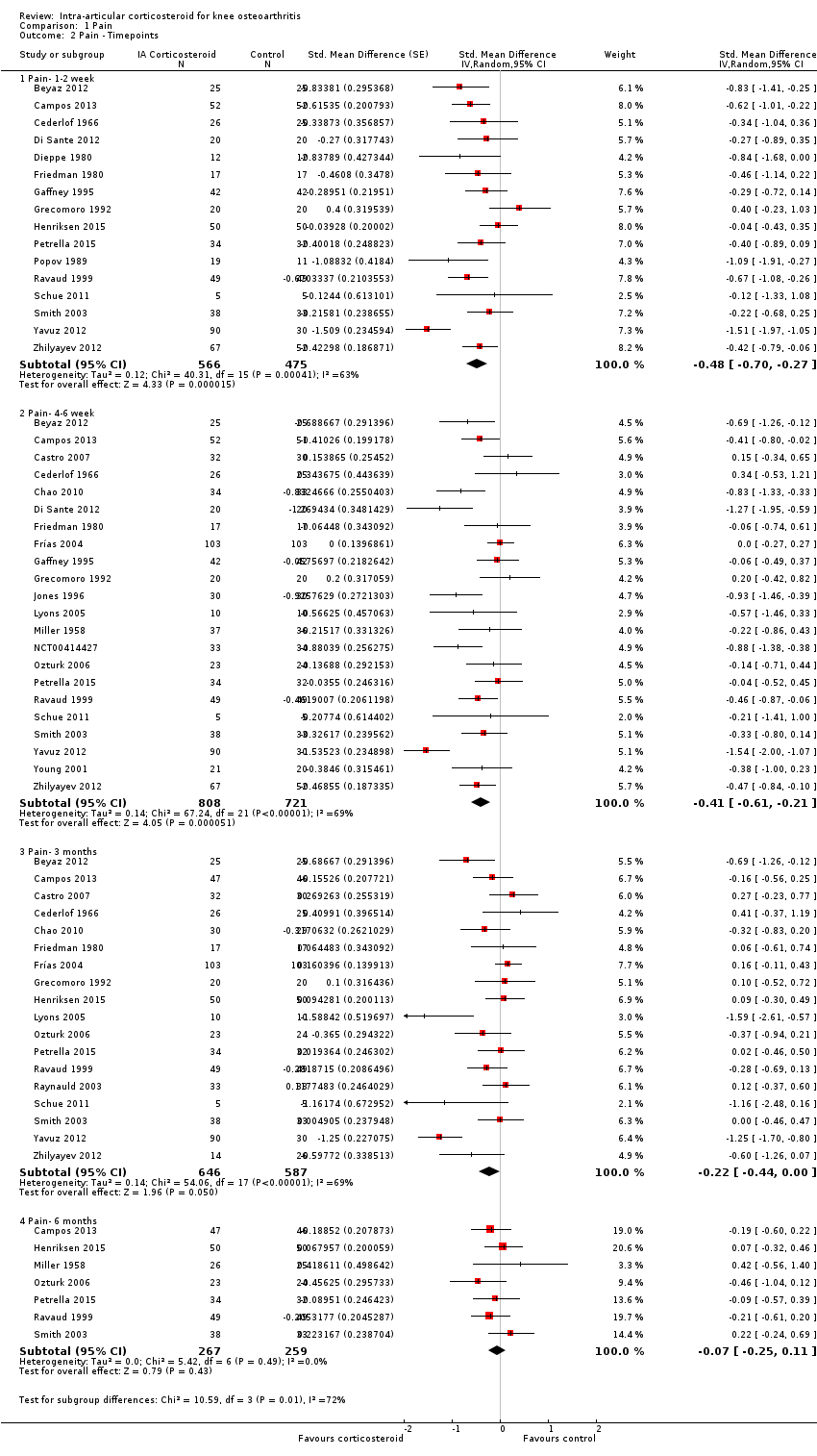 Comparison 1 Pain, Outcome 2 Pain ‐ Timepoints. | ||||
| 2.1 Pain‐ 1‐2 week | 16 | 1041 | Std. Mean Difference (Random, 95% CI) | ‐0.48 [‐0.70, ‐0.27] |
| 2.2 Pain‐ 4‐6 week | 22 | 1529 | Std. Mean Difference (Random, 95% CI) | ‐0.41 [‐0.61, ‐0.21] |
| 2.3 Pain‐ 3 months | 18 | 1233 | Std. Mean Difference (Random, 95% CI) | ‐0.22 [‐0.44, 0.00] |
| 2.4 Pain‐ 6 months | 7 | 526 | Std. Mean Difference (Random, 95% CI) | ‐0.07 [‐0.25, 0.11] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
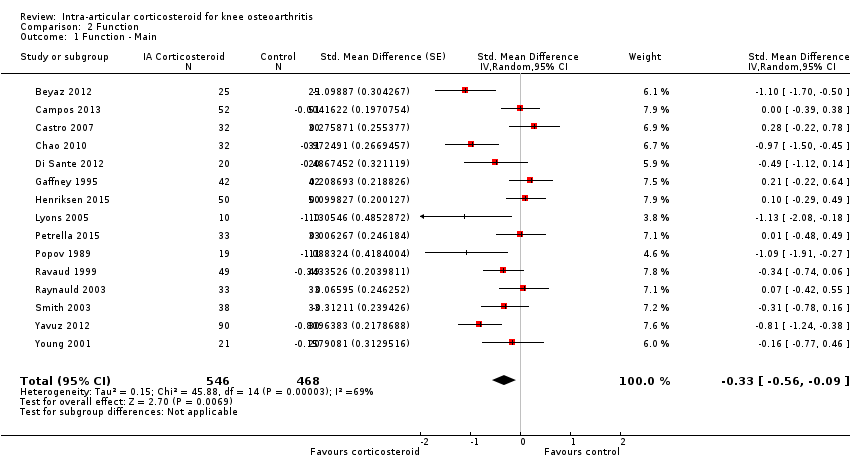
| 1 Function ‐ Main Show forest plot | 15 | 1014 | Std. Mean Difference (Random, 95% CI) | ‐0.33 [‐0.56, ‐0.09] |
| Analysis 2.1  Comparison 2 Function, Outcome 1 Function ‐ Main. | ||||
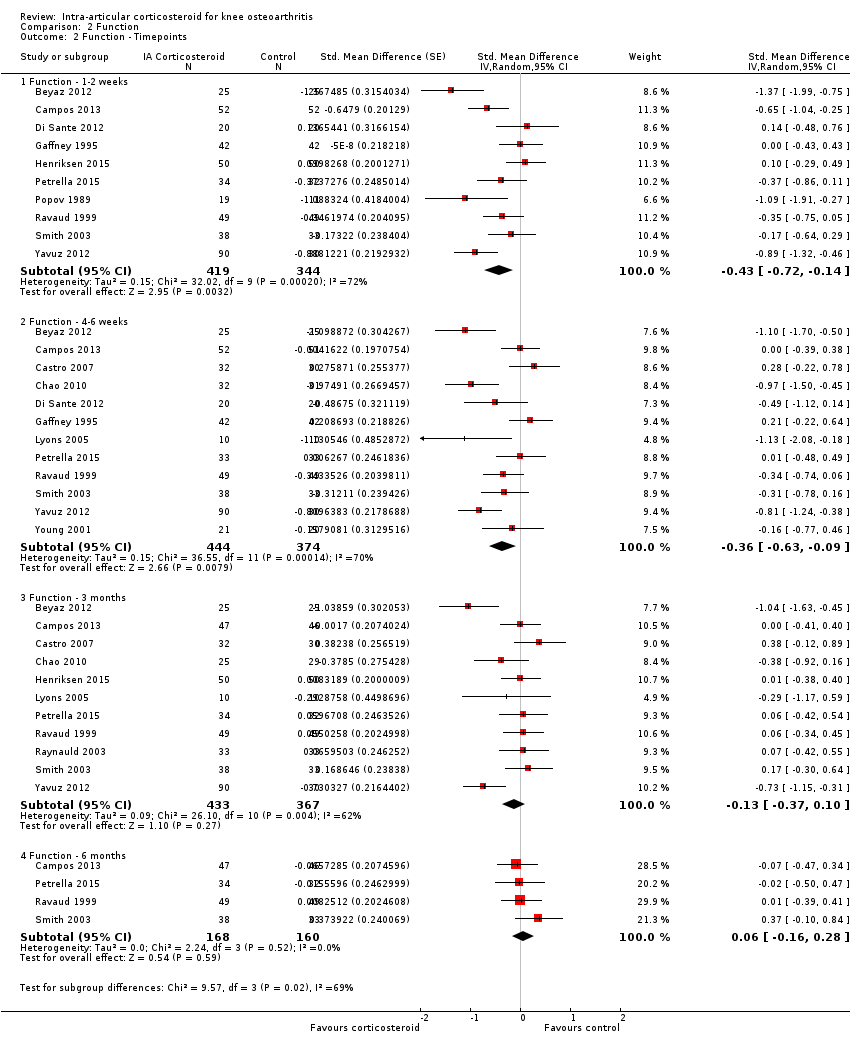
| 2 Function ‐ Timepoints Show forest plot | 15 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| Analysis 2.2 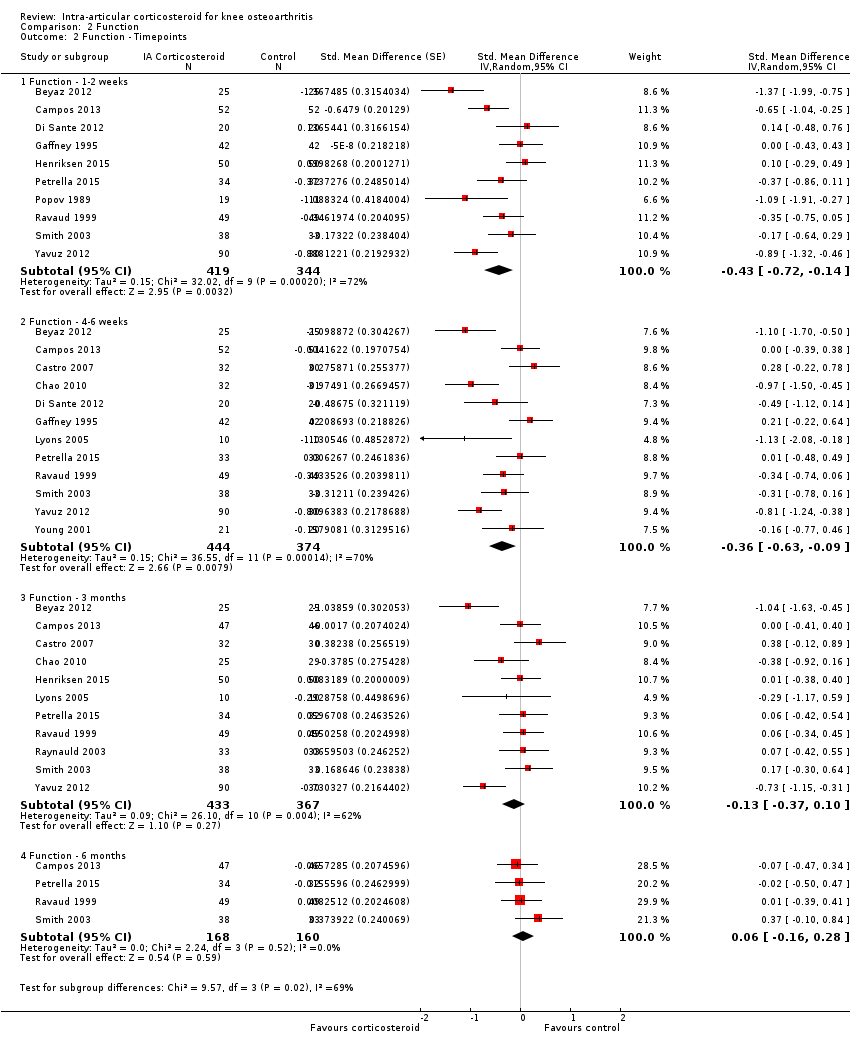 Comparison 2 Function, Outcome 2 Function ‐ Timepoints. | ||||
| 2.1 Function ‐ 1‐2 weeks | 10 | 763 | Std. Mean Difference (Random, 95% CI) | ‐0.43 [‐0.72, ‐0.14] |
| 2.2 Function ‐ 4‐6 weeks | 12 | 818 | Std. Mean Difference (Random, 95% CI) | ‐0.36 [‐0.63, ‐0.09] |
| 2.3 Function ‐ 3 months | 11 | 800 | Std. Mean Difference (Random, 95% CI) | ‐0.13 [‐0.37, 0.10] |
| 2.4 Function ‐ 6 months | 4 | 328 | Std. Mean Difference (Random, 95% CI) | 0.06 [‐0.16, 0.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Quality of life ‐ Main Show forest plot | 2 | 184 | Std. Mean Difference (Random, 95% CI) | ‐0.01 [‐0.30, 0.28] |
| Analysis 3.1 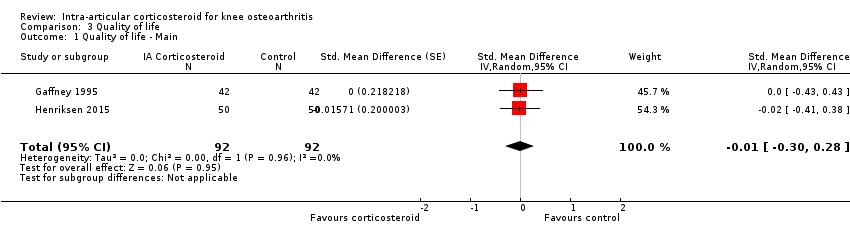 Comparison 3 Quality of life, Outcome 1 Quality of life ‐ Main. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
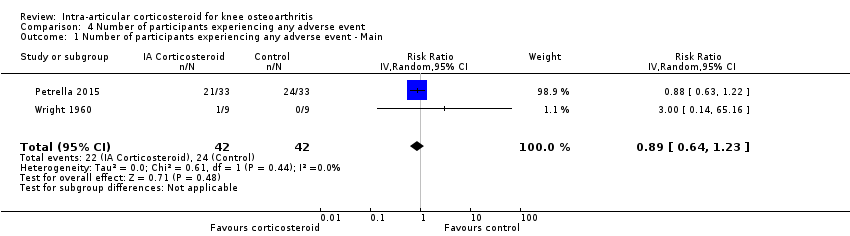
| 1 Number of participants experiencing any adverse event ‐ Main Show forest plot | 2 | 84 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.64, 1.23] |
| Analysis 4.1  Comparison 4 Number of participants experiencing any adverse event, Outcome 1 Number of participants experiencing any adverse event ‐ Main. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who with draw because of adverse events ‐Main Show forest plot | 2 | 204 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.05, 2.07] |
| Analysis 5.1  Comparison 5 Number of participants who withdraw because of adverse events, Outcome 1 Number of participants who with draw because of adverse events ‐Main. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants experiencing any serious adverse event ‐ Main Show forest plot | 5 | 331 | Risk Ratio (IV, Random, 95% CI) | 0.63 [0.15, 2.67] |
| Analysis 6.1 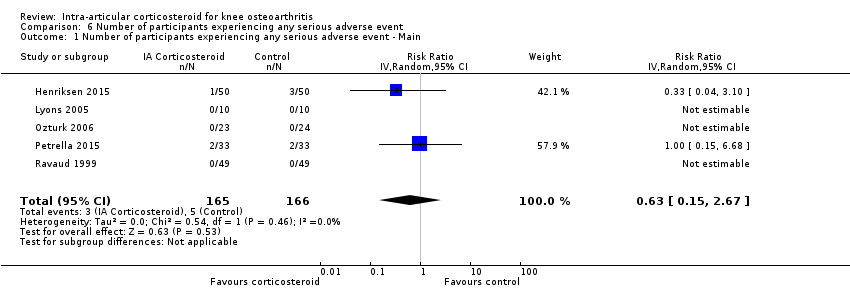 Comparison 6 Number of participants experiencing any serious adverse event, Outcome 1 Number of participants experiencing any serious adverse event ‐ Main. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Joint space narrowing ‐ Main Show forest plot | 1 | 68 | Std. Mean Difference (Random, 95% CI) | ‐0.02 [‐0.49, 0.46] |
| Analysis 7.1  Comparison 7 Joint space narrowing, Outcome 1 Joint space narrowing ‐ Main. | ||||
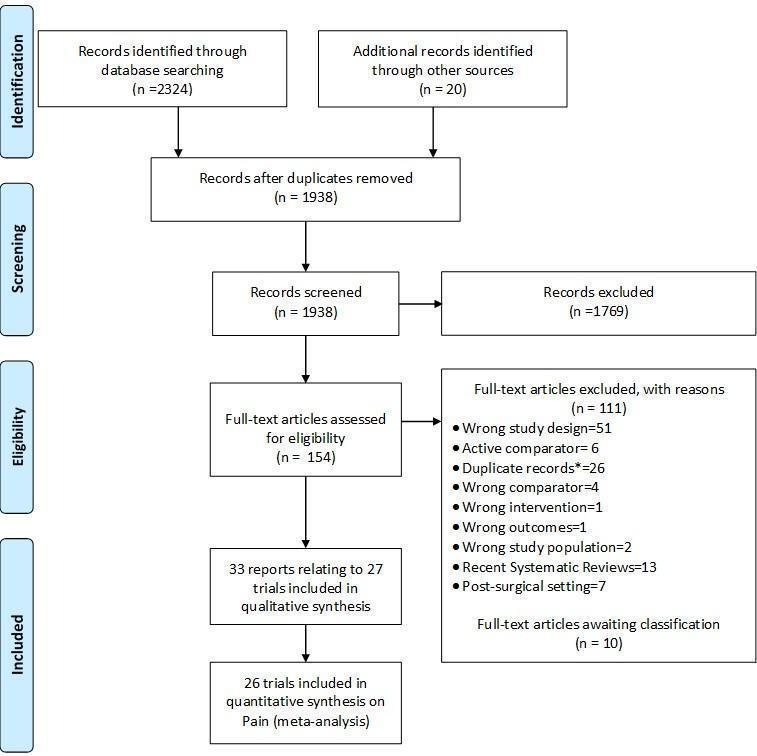
Study flow chart. *records with the exact same bibliographic information of another already‐screened record.
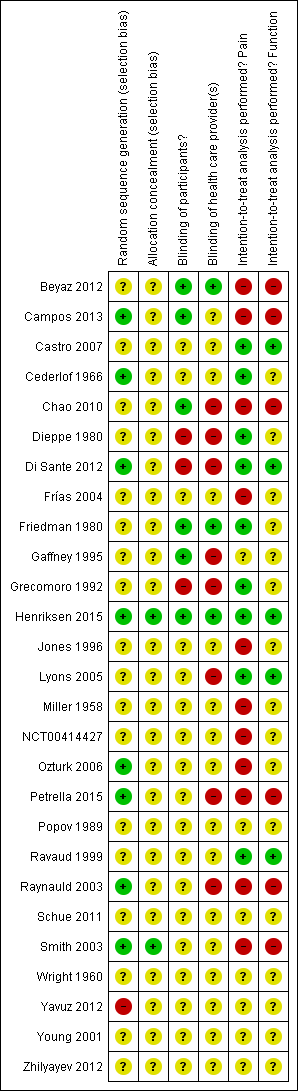
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Forest plot of comparison: 1 Pain, outcome: 1.1 Pain ‐ Main.

Contour‐enhanced funnel plot for effects on knee pain. Numbers on x axis refer to standardised mean differences (SMDs), on y axis to standard errors of SMDs
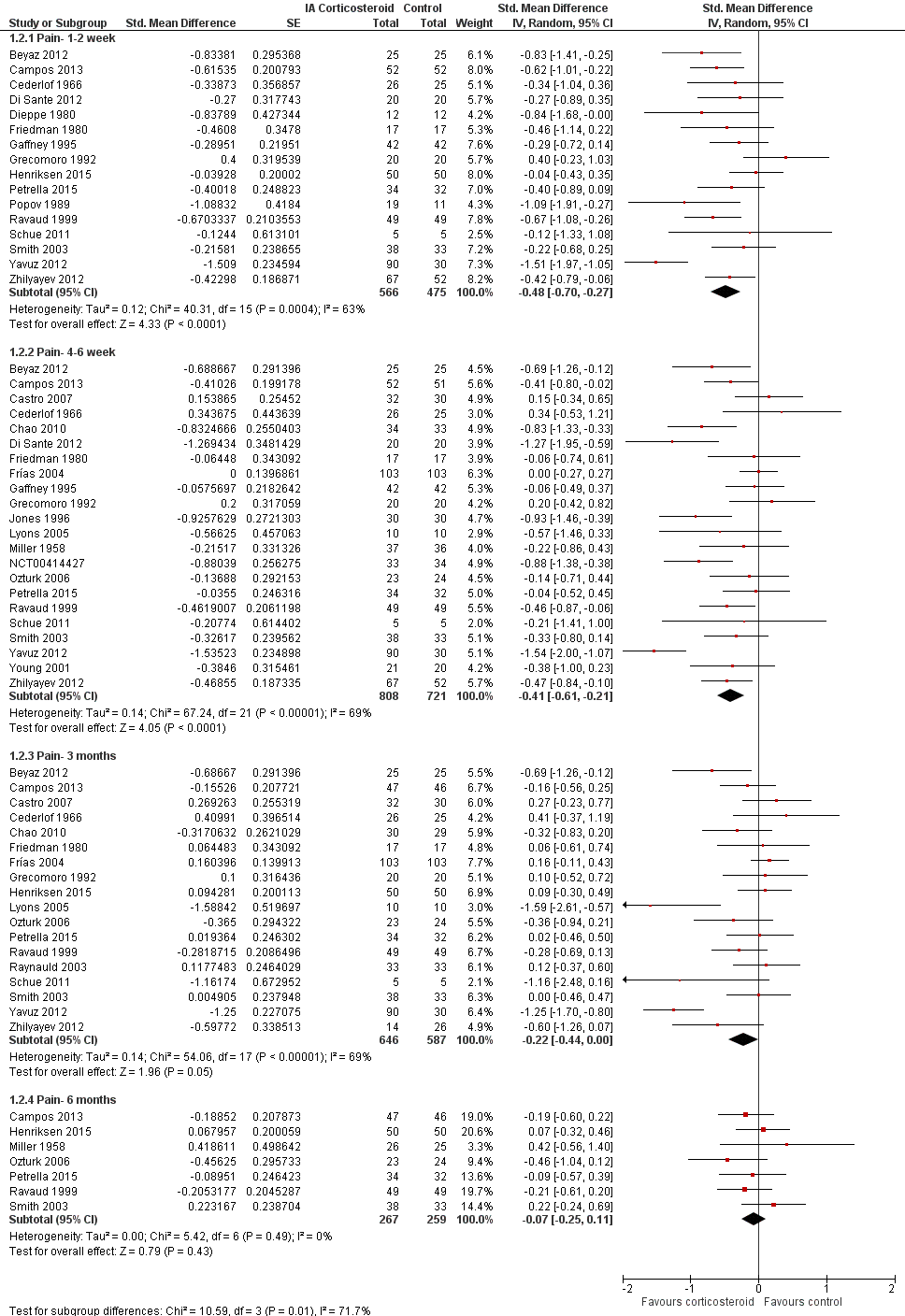
Forest plot of comparison: 1 Pain, outcome: 1.2 Pain ‐ Time points. P for trend = 0.001
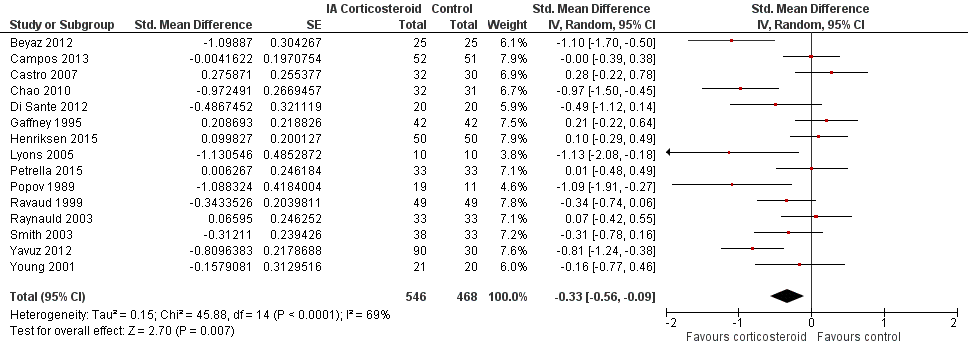
Forest plot of comparison: 2 Function, outcome: 2.1 Function ‐ Main.

Contour‐enhanced funnel plot for effects on knee function. Numbers on x axis refer to standardised mean differences (SMDs), on y axis to standard errors of SMDs
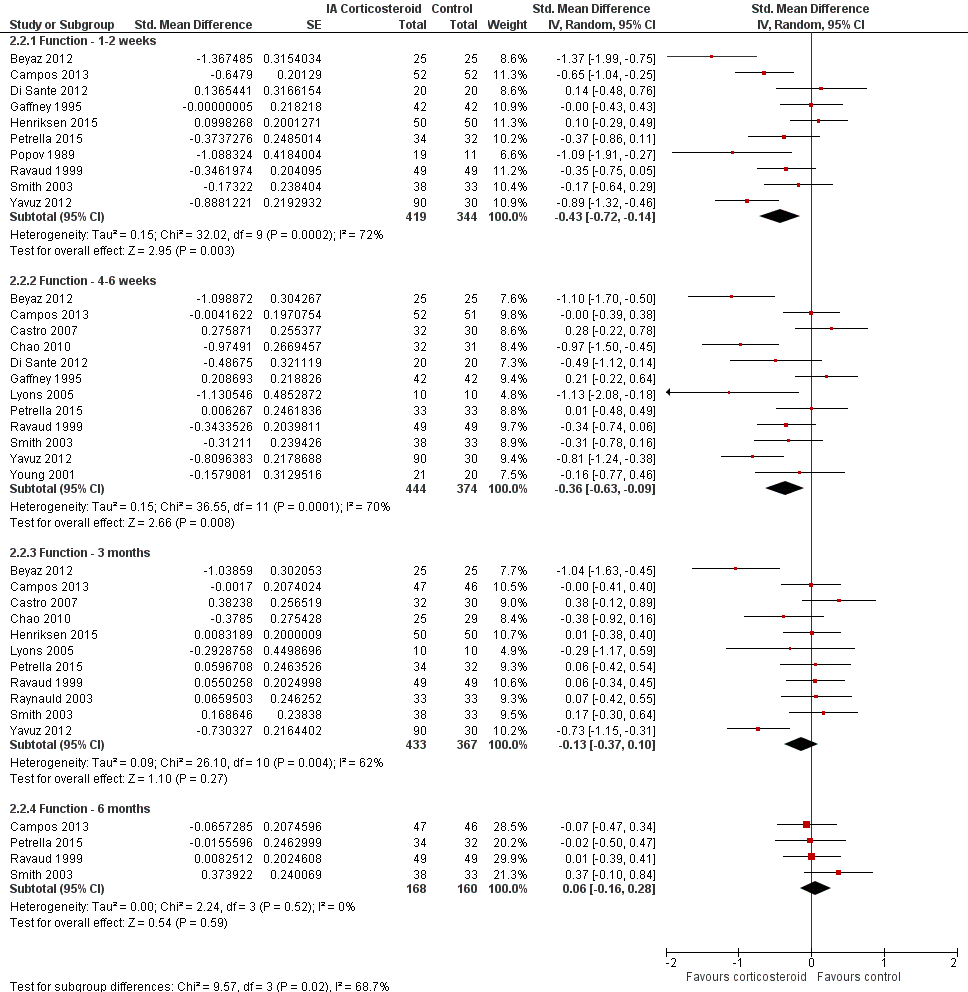
Forest plot of comparison: 2 Function, outcome: 2.2 Function ‐ Time points. P for trend = 0.011
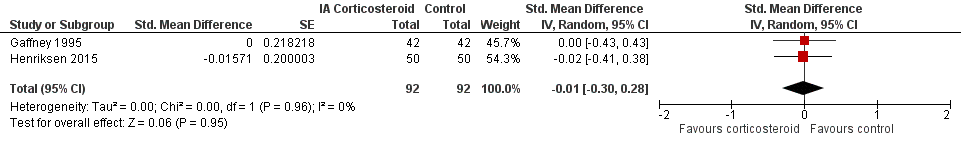
Forest plot of comparison: 3 Quality of life, outcome: 3.1 Quality of life ‐ Main.

Forest plot of comparison: 7 Joint space narrowing, outcome: 7.1 Joint space narrowing ‐ Main.

Forest plot of comparison: 4 Number of participants experiencing any adverse event, outcome: 4.1 Number of participants experiencing any adverse event ‐ Main.

Forest plot of comparison: 5 Number of participants who withdraw because of adverse events, outcome: 5.1 Number of participants who withdraw because of adverse events ‐Main.

Forest plot of comparison: 6 Number of participants experiencing any serious adverse event, outcome: 6.1 Number of participants experiencing any serious adverse event ‐ Main.

Comparison 1 Pain, Outcome 1 Pain ‐ Main.
Comparison 1 Pain, Outcome 2 Pain ‐ Timepoints.
Comparison 2 Function, Outcome 1 Function ‐ Main.
Comparison 2 Function, Outcome 2 Function ‐ Timepoints.

Comparison 3 Quality of life, Outcome 1 Quality of life ‐ Main.
Comparison 4 Number of participants experiencing any adverse event, Outcome 1 Number of participants experiencing any adverse event ‐ Main.
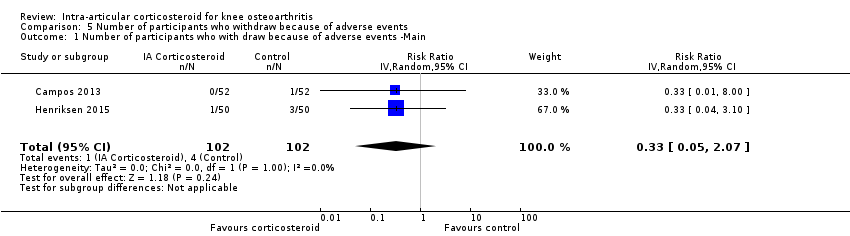
Comparison 5 Number of participants who withdraw because of adverse events, Outcome 1 Number of participants who with draw because of adverse events ‐Main.
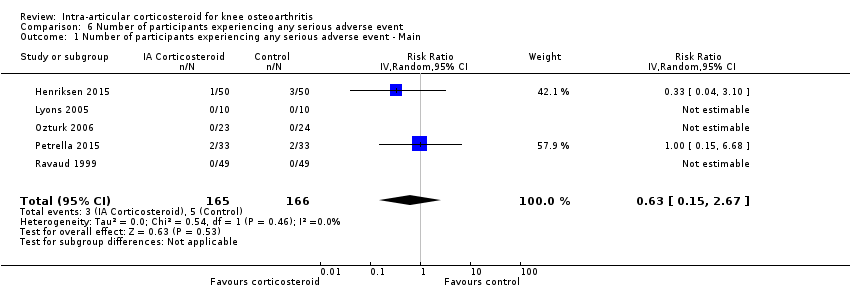
Comparison 6 Number of participants experiencing any serious adverse event, Outcome 1 Number of participants experiencing any serious adverse event ‐ Main.
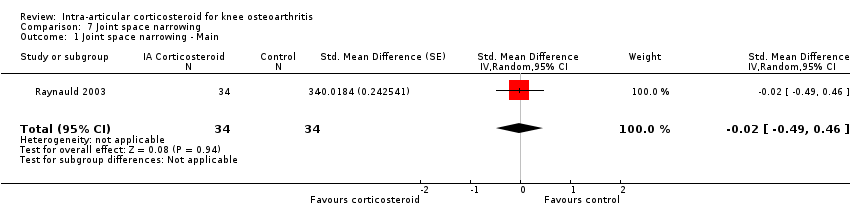
Comparison 7 Joint space narrowing, Outcome 1 Joint space narrowing ‐ Main.
| Intra‐articular corticosteroid compared with sham injection for osteoarthritis of the knee | ||||||
| Patient or population: participants with osteoarthritis of the knee Settings: various orthopaedic or rheumatology clinics Intervention: intra‐articular corticosteroid Comparison: sham injection | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Sham injection | Intra‐articular corticosteroid | |||||
| Pain intensity Various pain scales. (median follow‐up: 12 weeks) | ‐1.8 cm change on 10‐cm VAS1 | ‐2.8 cm change 46% improvement | SMD ‐0.40 (‐0.58 to ‐0.22) Predictive interval (‐1.20 to 0.40) | 1749 (26) | ⊕⊕⊝⊝ | NNTB 8 (95% CI 6 to 13)4 |
| Function Various function scales. (median follow‐up: 12 weeks) | ‐1.2 units on WOMAC (range 0 to 10)1 | ‐1.9 units on WOMAC 34% improvement | SMD ‐0.33 (‐0.56 to ‐0.09) Predictive interval (‐1.19 to 0.54) | 1014 (15) | ⊕⊕⊝⊝ | NNTB 10 (95% CI 7 to 33)7 |
| Number of participants experiencing any adverse event (median follow‐up: 17 weeks) | 150 per 1000 participant‐years8 | 134 per 1000 participant‐years | RR 0.89 (0.64 to 1.23) | 84 (2) | ⊕⊕⊝⊝ | Little evidence of harmful effect (NNTB not statistically significant) |
| Number of participants who withdraw because of adverse events (median follow‐up: 25 weeks) | 17 per 1000 participant‐years8 | 6 per 1000 participant‐years | RR 0.33 (0.05 to 2.07) | 204 (2) | ⊕⊕⊝⊝ | Little evidence of harmful effect (NNTB not statistically significant) |
| Number of participants experiencing any serious adverse event (median follow‐up: 26 weeks) | 4 per 1000 participant‐years8 | 3 per 1000 participant‐years | RR 0.63 (0.15 to 2.67) | 331 (5) | ⊕⊕⊝⊝ | Little evidence of harmful effect (NNTB not statistically significant) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence 1 Median reduction as observed across placebo groups in large osteoarthritis trials (see methods section, Nüesch 2009). 5 SMDs were back‐transformed onto a standardised Western Ontario and McMaster Universities Arthritis Index (WOMAC) disability score ranging from 0 to 10 on the basis of a typical pooled SD of 2.1 in trials that assessed function using WOMAC disability scores and expressed as change based on an assumed standardised reduction of 0.58 SD units in the control group. 9 Downgraded (2 levels) because: Most studies that reported this outcome are of high or unclear risk of bias, and statistical heterogeneity is large. 10 Downgraded (3 levels) because: 50% or more of the studies that reported this outcome are of high or unclear risk of bias, and the confidence interval of the pooled estimate is wide and includes the null effect. | ||||||
| Variable | Number of studies | N of participants corticosteroids | N of participants control | Pain intensity SMD (95% CI) | Heterogeneity I2 (%) | P value* |
| All trials | 26 | 922 | 827 | ‐0.40 (‐0.58 to ‐0.22) | 68% | |
| Allocation concealment | 0.15 | |||||
| Adequate | 2 | 88 | 83 | ‐0.16 (‐0.46 to 0.14) | 0% | |
| Inadequate or unclear | 24 | 834 | 744 | ‐0.42 (‐0.62 to ‐0.22) | 69% | |
| Blinding of participants | 0.64 | |||||
| Adequate | 6 | 220 | 218 | ‐0.34 (‐0.61 to ‐0.06) | 49% | |
| Inadequate or unclear | 20 | 702 | 609 | ‐0.42 (‐0.65 to ‐0.19) | 72% | |
| Blinding of therapists | 0.45 | |||||
| Adequate | 3 | 92 | 92 | ‐0.24 (‐0.66 to 0.17) | 44% | |
| Inadequate or unclear | 23 | 830 | 735 | ‐0.42 (‐0.62 to ‐0.22) | 70% | |
| Intention‐to‐treat analysis | 0.29 | |||||
| Yes | 9 | 236 | 233 | ‐0.26 (‐0.57 to 0.06) | 59% | |
| No or unclear | 17 | 686 | 594 | ‐0.47 (‐0.69 to ‐0.24) | 71% | |
| Type of control intervention | 0.08 | |||||
| Sham injection | 19 | 614 | 526 | ‐0.50 (‐0.72 to ‐0.28) | 65% | |
| No intervention | 7 | 284 | 280 | ‐0.18 (‐0.47 to 0.11) | 63% | |
| Funding independent of industry | 0.80 | |||||
| Yes | 11 | 341 | 333 | ‐0.37 (‐0.55 to ‐0.18) | 26% | |
| No or unclear | 15 | 581 | 494 | ‐0.41 (‐0.70 to ‐0.12) | 78% | |
| Trial size | 0.05 | |||||
| ≥ 50 per trial group | 3 | 205 | 204 | ‐0.13 (‐0.37 to 0.12) | 34% | |
| < 50 per trial group | 23 | 717 | 623 | ‐0.44 (‐0.65 to ‐0.24) | 67% | |
| Trial size | 0.013 | |||||
| ≥ 100 per trial group | 1 | 103 | 103 | 0.00 (‐0.27 to 0.27) | N/A | |
| < 100 per trial group | 25 | 819 | 724 | ‐0.42 (‐0.61 to ‐0.23) | 66% | |
| Publication type | 0.93 | |||||
| Full journal article | 22 | 785 | 706 | ‐0.40 (‐0.61 to ‐0.20) | 70% | |
| Other type or unpublished material | 4 | 137 | 121 | ‐0.38 (‐0.84 to ‐0.08) | 65% | |
| Ultrasound guidance of injections | 0.71 | |||||
| Yes | 2 | 70 | 70 | ‐0.62 (‐1.83 to 0.58) | 89% | |
| No or unclear | 24 | 852 | 757 | ‐0.39 (‐0.57 to ‐0.20) | 67% | |
| Use of local anaesthetic | 0.41 | |||||
| Yes | 5 | 172 | 157 | ‐0.55 (‐0.93 to ‐0.16) | 62% | |
| No or unclear | 21 | 750 | 670 | ‐0.36 (‐0.57 to ‐0.15) | 70% | |
| Concomitant viscosupplementation | 0.08 | |||||
| Yes | 4 | 129 | 127 | ‐0.16 (‐0.42 to 0.09) | 4% | |
| No or unclear | 22 | 793 | 700 | ‐0.46 (‐0.67 to ‐0.25) | 71% | |
| Concomitant joint lavage | ≤ 0.001 | |||||
| Yes | 4 | 197 | 187 | ‐0.06 (‐0.26 to 0.15) | 0% | |
| No or unclear | 26 | 725 | 640 | ‐0.57 (‐0.78 to ‐0.35) | 72% | |
| Use of crystalline preparation | 0.82 | |||||
| Yes | 18 | 623 | 562 | ‐0.47 (‐0.69 to ‐0.24) | 72% | |
| No or unclear | 12 | 299 | 265 | ‐0.52 (‐0.90 to ‐0.14) | 76% | |
| Prednisolone equivalence dose | 0.53 | |||||
| ≥ 50 mg | 17 | 520 | 470 | ‐0.55 (‐0.85 to ‐0.25) | 80% | |
| < 50 mg | 13 | 402 | 357 | ‐0.43 (‐0.66 to ‐0.20) | 56% | |
| Number of randomised comparisons are shown in "number of studies" for stratified analyses according to use of lavage as co‐intervention, crystalline preparation, prednisolone equivalence. *P value for interaction. N/A: not available. CI: confidence interval | ||||||
| Variable | Number of studies | N of participants corticosteroids | N of participants control | Function SMD (95% CI) | Heterogeneity I2 (%) | P value* |
| All trials | 15 | 546 | 468 | ‐0.33 (‐0.56 to ‐0.09) | 69% | |
| Allocation concealment | 0.25 | |||||
| Adequate | 2 | 88 | 83 | ‐0.09 (‐0.49 to 0.32) | 43% | |
| Inadequate or unclear | 13 | 458 | 385 | ‐0.37 (‐0.64 to ‐0.10) | 72% | |
| Blinding of participants | 0.97 | |||||
| Adequate | 5 | 201 | 199 | ‐0.32 (‐0.82 to 0.18) | 83% | |
| Inadequate or unclear | 10 | 345 | 269 | ‐0.33 (‐0.59 to ‐0.07) | 58% | |
| Blinding of therapists | 0.78 | |||||
| Adequate | 2 | 75 | 75 | ‐0.48 (‐1.65 to 0.70) | 91% | |
| Inadequate or unclear | 13 | 471 | 393 | ‐0.31 (‐0.55 to ‐0.06) | 66% | |
| Intention‐to‐treat analysis | 0.49 | |||||
| Yes | 5 | 161 | 159 | ‐0.21 (‐0.59 to 0.17) | 62% | |
| No or unclear | 10 | 385 | 309 | ‐0.38 (‐0.69 to ‐0.07) | 73% | |
| Type of control intervention | 0.031 | |||||
| Sham injection | 11 | 409 | 334 | ‐0.45 (‐0.74 to ‐0.15) | 73% | |
| No intervention | 4 | 137 | 134 | ‐0.01 (‐0.27 to 0.25) | 13% | |
| Funding independent of industry | 0.73 | |||||
| Yes | 9 | 310 | 302 | ‐0.36 (‐0.66 to ‐0.07) | 68% | |
| No or unclear | 6 | 236 | 166 | ‐0.27 (‐0.71 to 0.16) | 76% | |
| Trial size | 0.023 | |||||
| ≥ 50 per trial group | 2 | 102 | 101 | 0.05 (‐0.23 to 0.32) | 0% | |
| < 50 per trial group | 13 | 444 | 367 | ‐0.40 (‐0.67 to ‐0.13) | 70% | |
| Trial size | N/A | |||||
| ≥ 100 per trial group | 0 | 0 | 0 | N/A | N/A | |
| < 100 per trial group | 15 | 546 | 468 | ‐0.33 (‐0.56 to ‐0.09) | 69% | |
| Publication type | 0.023 | |||||
| Full journal article | 14 | 514 | 438 | ‐0.37 (‐0.61 to ‐0.13) | 68% | |
| Other type or unpublished material | 1 | 32 | 30 | 0.28 (‐0.22 to 0.78) | N/A | |
| Ultrasound guidance of injections | 0.49 | |||||
| Yes | 2 | 70 | 70 | ‐0.14 (‐0.70 to 0.43) | 58% | |
| No or unclear | 13 | 476 | 398 | ‐0.36 (‐0.62 to ‐0.09) | 71% | |
| Use of local anaesthetic | 0.34 | |||||
| Yes | 4 | 105 | 105 | ‐0.60 (‐1.25 to 0.05) | 78% | |
| No or unclear | 11 | 441 | 363 | ‐0.25 (‐0.51 to 0.00) | 68% | |
| Concomitant viscosupplementation | 0.06 | |||||
| Yes | 2 | 85 | 84 | ‐0.00 (‐0.30 to 0.30) | 0% | |
| No or unclear | 13 | 461 | 384 | ‐0.39 (‐0.66 to ‐0.12) | 72% | |
| Concomitant joint lavage | 0.18 | |||||
| Yes | 3 | 94 | 84 | ‐0.13 (‐0.55 to 0.28) | 48% | |
| No or unclear | 16 | 452 | 384 | ‐0.46 (‐0.71 to ‐0.21) | 70% | |
| Use of crystalline preparation | 0.66 | |||||
| Yes | 12 | 365 | 319 | ‐0.37 (‐0.66 to ‐0.08) | 73% | |
| No or unclear | 7 | 181 | 149 | ‐0.47 (‐0.83 to ‐0.11) | 61% | |
| Prednisolone equivalence dose | 0.16 | |||||
| ≥ 50 mg | 12 | 328 | 277 | ‐0.52 (‐0.83 to ‐0.20) | 74% | |
| < 50 mg | 7 | 218 | 191 | ‐0.22 (‐0.48 to 0.05) | 47% | |
| Number of randomised comparisons are shown in "number of studies" for stratified analyses according to use of lavage as co‐intervention, crystalline preparation, prednisolone equivalence. *P value for interaction. N/A: not available. CI: confidence interval | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain ‐ Main Show forest plot | 26 | 1749 | Std. Mean Difference (Random, 95% CI) | ‐0.40 [‐0.58, ‐0.22] |
| 2 Pain ‐ Timepoints Show forest plot | 26 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 2.1 Pain‐ 1‐2 week | 16 | 1041 | Std. Mean Difference (Random, 95% CI) | ‐0.48 [‐0.70, ‐0.27] |
| 2.2 Pain‐ 4‐6 week | 22 | 1529 | Std. Mean Difference (Random, 95% CI) | ‐0.41 [‐0.61, ‐0.21] |
| 2.3 Pain‐ 3 months | 18 | 1233 | Std. Mean Difference (Random, 95% CI) | ‐0.22 [‐0.44, 0.00] |
| 2.4 Pain‐ 6 months | 7 | 526 | Std. Mean Difference (Random, 95% CI) | ‐0.07 [‐0.25, 0.11] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Function ‐ Main Show forest plot | 15 | 1014 | Std. Mean Difference (Random, 95% CI) | ‐0.33 [‐0.56, ‐0.09] |
| 2 Function ‐ Timepoints Show forest plot | 15 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 2.1 Function ‐ 1‐2 weeks | 10 | 763 | Std. Mean Difference (Random, 95% CI) | ‐0.43 [‐0.72, ‐0.14] |
| 2.2 Function ‐ 4‐6 weeks | 12 | 818 | Std. Mean Difference (Random, 95% CI) | ‐0.36 [‐0.63, ‐0.09] |
| 2.3 Function ‐ 3 months | 11 | 800 | Std. Mean Difference (Random, 95% CI) | ‐0.13 [‐0.37, 0.10] |
| 2.4 Function ‐ 6 months | 4 | 328 | Std. Mean Difference (Random, 95% CI) | 0.06 [‐0.16, 0.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Quality of life ‐ Main Show forest plot | 2 | 184 | Std. Mean Difference (Random, 95% CI) | ‐0.01 [‐0.30, 0.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants experiencing any adverse event ‐ Main Show forest plot | 2 | 84 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.64, 1.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who with draw because of adverse events ‐Main Show forest plot | 2 | 204 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.05, 2.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants experiencing any serious adverse event ‐ Main Show forest plot | 5 | 331 | Risk Ratio (IV, Random, 95% CI) | 0.63 [0.15, 2.67] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Joint space narrowing ‐ Main Show forest plot | 1 | 68 | Std. Mean Difference (Random, 95% CI) | ‐0.02 [‐0.49, 0.46] |

