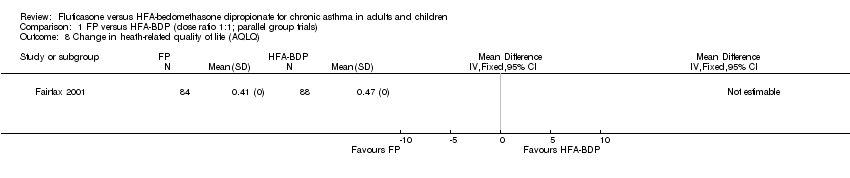
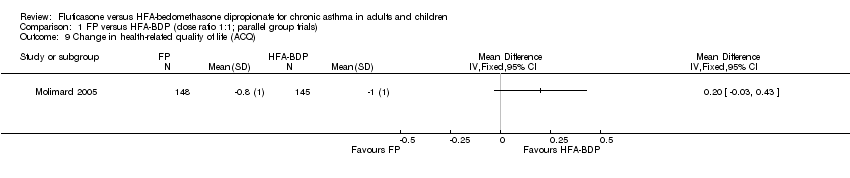
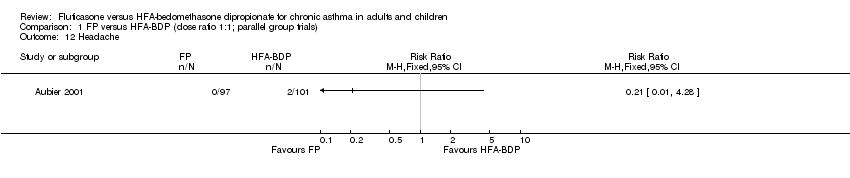
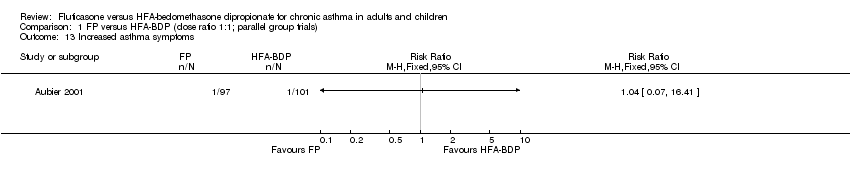
Contenido relacionado
Revisiones y protocolos relacionados
Toby J Lasserson, Christopher J Cates, Emma H Lasserson, John White | 19 abril 2006
Nick P Adams, Janine C Bestall, Toby J Lasserson, Paul Jones, Christopher J Cates | 8 octubre 2008
Nick P Adams, Toby J Lasserson, Christopher J Cates, Paul Jones | 17 octubre 2007
Pat Manning, Peter G Gibson, Toby J Lasserson | 23 abril 2008
Kayleigh M Kewa, Ella Flemynga, Bradley S Quon, Clarus Leung | 26 septiembre 2022
Nick P Adams, Janine C Bestall, Paul Jones | 21 enero 2002
Kayleigh M Kew, Charlotta Karner, Stephanie M Mindus, Giovanni Ferrara | 16 diciembre 2013
Nick P Adams, Janine C Bestall, Paul Jones, Toby J Lasserson, Benedict Griffiths, Christopher J Cates | 8 octubre 2008
Nick P Adams, Janine C Bestall, Paul Jones | 25 octubre 1999
Michael Smith, Shaikh Mohammed SI Iqbal, Brian H Rowe, Tracy N'Diaye | 20 enero 2003